Abstract
Cytokines produced by immune cells have been demonstrated to act on muscle stem cells (MuSCs) and direct their fate and behavior during muscle repair and regeneration. Nevertheless, it is unclear whether and how MuSCs can also in turn modulate the properties of immune cells. Here, we showed that in vitro expanded MuSCs exhibited a potent anti‐inflammatory effect when infused into mice suffering from inflammatory bowel disease (IBD). Supernatant conditioned by MuSCs similarly ameliorated IBD. This beneficial effect of MuSCs was not observed when macrophages were depleted. The MuSC supernatant was found to greatly attenuate the expression of inflammatory cytokines but increase the expression of programmed death‐ligand 1 in macrophages treated with lipopolysaccharide and interferon gamma. Further analysis revealed that MuSCs produce a large amount of insulin‐like growth factor‐2 (IGF‐2) that instructs maturing macrophages to undergo oxidative phosphorylation and thus acquire anti‐inflammatory properties. Interestingly, the IGF‐2 production by MuSCs is much higher than by mesenchymal stem cells. Knockdown or neutralization of IGF‐2 abrogated the anti‐inflammatory effects of MuSCs and their therapeutic efficacy on IBD. Our study demonstrated that MuSCs possess a strong anti‐inflammatory property and the bidirectional interactions between immune cells and MuSCs have important implications in muscle‐related physiological and pathological conditions.
Keywords: anti‐inflammation, IBD, IGF‐2, macrophages, muscle stem cells, OXPHOS
Immune cells are known to direct the fate of muscle stem cells (MuSCs) during tissue repair and regeneration. MuSCs can also act on maturing macrophages and confer them with anti‐inflammatory properties via insulin‐like growth factor‐2, thus ameliorating dextran sulfate sodium‐induced colitis.

Significance statement.
Inflammatory macrophages are known to promote the expansion of activated muscle stem cells (MuSCs) during the tissue repair process by retaining MuSCs in a proliferative and undifferentiated state. This study revealed that MuSCs could also endow maturing macrophages with anti‐inflammatory properties, by producing insulin‐like growth factor‐2 that dictates the metabolic preference of macrophages.
1. INTRODUCTION
Skeletal muscle accounts for 30%~50% of human body weight, and is essential for posture maintenance, breathing, movement, and the metabolism of glucose and lipid.1 In addition to multinucleated myofibers, there are abundant leukocytes in skeletal muscle. It is estimated that there are approximately 109 leukocytes per liter of skeletal muscle in adult mammals.2 Although the intramuscular leukocytes comprise diverse types of immune cells, monocytes and macrophages are the overwhelming majority, and primarily reside in the muscle stem cell (MuSC; also known as satellite cell) niche.3 Their synchronized functional transformations and proper representation in the MuSC niche during muscle repair and regeneration directly determine the fate of MuSCs and affect the repair and regeneration outcomes.4, 5, 6, 7 However, it is unclear how MuSCs regulate the functions of immune cells.
Recent studies have shown that the skeletal muscle tissue also possesses a major immunoregulatory function and produces various immune regulatory molecules that are believed to contribute to tissue homeostasis and some physiological functions.8 The proinflammatory cytokine interleukin‐6 (IL‐6) released from exercising muscle blunts endotoxin‐induced inflammatory responses.9 Meteorin‐like produced from the skeletal muscle tissue can induce “browning” of white adipose tissue and guide IL‐4‐dependent macrophage polarization.10 Muscle‐derived IL‐15 was shown to promote the proliferation of naive T cells, enhance natural killer cell development and cytotoxicity, reduce lipid deposition, and protect against visceral adiposity.11, 12, 13 Although mounting evidence exists for muscle‐mediated immunomodulation, whether the immunoregulatory factors are derived from MuSCs and which factors are activated during pathophysiological processes14 remains to be determined.
Increasing evidences started to reveal that metabolic reprogramming is associated with divergent functional fates of macrophages.15 Proinflammatory macrophages (the traditionally defined M‐pro macrophages) primarily rely on glycolysis and have reduced oxidative phosphorylation (OXPHOS) capacity. Within this metabolic rewiring, succinate in M‐pro macrophages inhibits the activity of prolyl hydroxylases enzymes, thereby stabilizing hypoxia responsive element‐1α (HIF‐1α), and consequently inducing IL‐1β production.16 In contrast, anti‐inflammatory macrophages (the traditionally defined M‐anti macrophages) are fueled by OXPHOS. M‐anti macrophages are characteristic of higher expression of anti‐inflammatory cytokine IL‐10, and decreased productions of nitric oxide and tumor necrosis factor‐α (TNF‐α).17 Recent studies showed that tissue stem cells can program the fate of macrophages through metabolic rewiring. For example, insulin‐like growth factor‐2 (IGF‐2), released from mesenchymal stem cells (MSCs) under hypoxia conditions, can endow maturing macrophages with OXPHOS preference, thus shaping anti‐inflammatory properties of macrophages in experimental autoimmune encephalomyelitis (EAE).18 Transplanted neuron stem cells (NSCs) were also shown to scavenge inflammatory metabolite succinate and consequently reduce the infiltration of mononuclear phagocytes during chronic neuroinflammation.19 Based on these new advances, we speculated that MuSCs may also modulate the metabolic preferences in macrophages and consequently influence their function, and thus be exploited for the treatment of inflammatory disorders.
In this study, we investigated the immunomodulatory function of MuSCs. We showed that transplanted MuSCs could dampen the pathological conditions in mice with inflammatory bowel disease (IBD). The MuSC secretome was found to attenuate the inflammatory response of macrophages in an OXPHOS‐dependent manner. Importantly, we found that IGF‐2 was responsible for the anti‐inflammatory effect of MuSCs. Our study uncovered a novel IGF‐2 dependent immunoregulatory property of MuSCs, which may be exploited for the management of inflammatory diseases.
2. MATERIALS AND METHODS
2.1. Animals
C57BL/6J mice were purchased from Charles River Experimental Animal Technology Co., Ltd. (Beijing, China), and maintained in a specific pathogen‐free facility of the Laboratory Animal Center of Soochow University. Male mice used for experimental models were 8 to 10 weeks of age. These healthy mice were maintained in individually ventilated cages at 21°C to 23°C with a 12:12 hour light/dark cycle. The relative humidity in the animal facility was between 40% and 60%. The mice were fed ad libitum with irradiated food from Jiangsu Xie Tong Pharmaceutical Bioengineering Co., Ltd. (Nanjing, China). All animal experiment protocols presented in this study were approved by the Institutional Animal Care and Use Committee of Soochow University.
2.2. MuSC isolation, expansion, and differentiation
MuSCs were isolated and expanded as previously described.20, 21 Briefly, hind limb skeletal muscles of C57BL/6J mice were dissected, gently and rapidly minced with scissors, and then incubated with collagenase II (750 U/mL, from Gibco, Life Technologies, San Francisco, California) solution for 1 hour at 37°C on a shaker. Digested tissues were then washed once with cold Hanks' balanced salt solution (HBSS) and centrifuged at 500g for 5 minutes. Second incubation was then performed by adding collagenase II (100 U/mL) and dispase (11 U/mL, Gibco) solution for 30 minutes at 37°C on a shaker. Digested tissues were then filtered through a 40 μm cell strainer to generate a mononucleated cell suspension ready for an antibody staining. Resuspended cells were stained using antibodies: PE‐conjugated rat antimouse CD31, PE‐conjugated rat antimouse CD45, APC‐conjugated rat antimouse Sca1 and Pacific Blue‐conjugated rat antimouse VCAM1 (both from Biolegend, San Diego, California). All antibodies were used at ~1 μg per 107 cells. The staining samples were incubated with antibodies for 40 minutes at 4°C. MuSCs marked as VCAM1+CD31−CD45−Sca1− were obtained by fluorescence‐activated cell sorting.
Sorted MuSCs were serially expanded every 2 days in myogenic cell proliferation medium containing F10 medium containing 20% fetal bovine serum (FBS), 5 ng/mL IL‐1α, 5 ng/mL IL‐13, 10 ng/mL interferon gamma (IFN‐γ) and 10 ng/mL TNF‐α, 2.5 ng/mL bFGF and 1% penicillin‐streptomycin (both from Gibco). Supernatant was concentrated 10‐fold using 3 kD centrifugal filtration unit to IBD therapies. In addition, cultured MuSCs were differentiated in myogenic cell differentiation medium containing Dulbecco's modified Eagle's medium (DMEM) with 5% horse serum (both from Gbico) for 3 days. All details regarding the characterization of cultured MuSCs were shown in Figure S1.
2.3. IBD induction and experimental therapies
To induce colitis, 4% dextran sulfate sodium (DSS, MP Biomedicals, Santa Ana, California) in drinking water was provided ad libitum for 7 days. MuSCs (1 × 106) were i.v. administered to treat IBD mice on day 2 after the beginning of DSS treatment. Some mice were treated with concentrated MuSC supernatant injected ip daily during IBD induction. Clodronate liposomes (1 mg/mice, from Yesen, Shanghai) were ip administered to IBD mice on days 1 and 4 after the beginning of DSS treatment for macrophage deletion. IGF‐2 neutralizing antibodies (20 μg/mice, from R&D Systems, Minneapolis, Minnesota) were ip administered to IBD mice daily during IBD induction to block the function of IGF‐2 in MuSC secretome. Control group mice received normal drinking water. Body weight of each mouse was monitored daily during IBD induction. All experimental mice were euthanized at the end of the 7‐day DSS treatment, and the colon samples were collected for further processing.
2.4. Colitis severity analysis
The disease activity index (DAI) was evaluated at the end of the 7‐day DSS treatment by scoring the body weight loss (grades, 0‐4:0, none; 1, <10% loss of the initial body weight; 2, 10‐15% loss of the initial body weight; 3, 15‐20% loss of the initial body weight; 4, > 20% loss of the initial body weight), stool consistency (grades, 0‐2:0, none; 1, mild diarrhea; 2, moderate to severe diarrhea), rectal bleeding (grades, 0‐2:0, none; 1, mild bleeding; 2, moderate to severe bleeding), and general activity (grades, 0‐2:0, normal; 1, mildly depressed; 2, moderately to severely depressed).22
2.5. H&E staining and histological analysis
Colon tissues from IBD mice were collected and fixed in 4% paraformaldehyde for 3 days. The colon tissues were then dehydrated through sequentially treatment with 75% ethanol (overnight), 85% ethanol (1 hour), 95% ethanol (1 hour) and 100% ethanol (1 hour). The samples were treated with xylene for 20 minutes twice before embedded in paraffin. Finally, the samples were cut into 3 μm sections.
Histological analysis was performed using standard hematoxylin and eosin (H&E) staining process. The severity of IBD symptoms was evaluated by scoring the extent of bowel wall thickening (grades, 0‐3:0, none; 1, mucosa; 2, mucosa and submucosa; 3, transmural), the damage of crypt (grades, 0‐3:0, none; 1, loss of goblet cells; 2, only surface epithelium intact; 3, loss of entire crypt and epithelium), and the infiltration of inflammatory cells (grades, 0‐2:0, none; 1, mild to moderate; 2, severe) through H&E colon sections.22
For macrophage immunostaining, colon sections were immunostained with anti‐F4/80 antibody after deparaffinization, rehydration, and antigen unmasking. The samples were incubated in biotinylated antirabbit secondary antibody followed by 3,3′‐diaminobenzidine tetrahydrochloride. Finally, the slides were counterstained with hematoxylin.
2.6. Isolation of colonic inflammatory cells and flow cytometric analysis
The colon samples were excised, rubbed off any visible fat and rinsed in phosphate‐buffered saline (PBS). They were then cut into 1 cm pieces, and immersed in warm HBSS containing 5% FBS, 2 mM DL‐Dithiothreitol (DTT, Sigma‐Aldrich, St. Louis, Missouri) and 5 mM ethylenedinitrilotetraacetic acid (EDTA, Sigma‐Aldrich) at 37°C for 20 minutes on a shaker. The tissue samples were then digested in DMEM medium containing 10% FBS, collagenase I (1 mg/mL), and dispase (11 U/mL) at 37°C for 1 hour on a shaker. Digested tissues were then passed through a 70 μm cell strainer to generate a mononucleated cell suspension ready for 80%/40% Percoll separation. Finally, collected cells were stained using antibodies: FITC‐conjugated rat antimouse CD11b, APC‐conjugated rat antimouse F4/80, Pacific Blue‐conjugated rat antimouse PD‐L1 (both from Biolegend). Flow cytometry data were analyzed using FlowJo (BD Biosciences, Franklin Lake, New Jersey).
2.7. Preparation of bone marrow‐derived macrophages
Bone marrow cells from femurs and tibias of male C57BL6/J mice were isolated and cultured in DMEM/F12 medium containing 10% FBS and 20% L929 conditioned medium for 7 days. Fresh differentiation medium was added on day 4. MuSC supernatant was added on days 1, 3, and 5. To test the role of OXPHOS in the regulation of inflammatory response in macrophages, MuSC‐educated macrophages were treated with 50 ng/mL lipopolysaccharide (LPS Sigma‐Aldrich), 20 ng/mL IFN‐γ, and 1 μM oligomycin A (Selleck, Houston, Texas) for 24 hours. For IGF‐2 blockage, bone marrow cells were treated with 5 μg/mL IGF‐2 antibodies (or 5 μg/mL isotype antibodies as control) following MuSC supernatant.
2.8. MSC isolation and expansion
MSCs were isolated and expanded as previously described.23 Briefly, tibia and femur bone marrow of C57BL/6J mice were harvested. Cells were plated in DMEM medium supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin. The nonadherent cells were removed after 24 hours, and adherent cells were retained. Medium was changed every 3 days. To obtain MSC clones, cells at confluence were harvested and seeded into 96‐well plates by limited dilution. Individual clones were then picked and expanded. MuSCs and MSCs in same passage were compared for IGF‐2 expression.
2.9. Real‐time PCR
Total RNA from each sample was extracted using the Trizol (Invitrogen, Carlsbad, California) reagents and reverse‐transcribed using PrimeScript RT Master Mix (Invitrogen) following the manufacturer's instructions. Gene expressions were measured by QuantStudio 6 Flex System (Applied Biosystems, Foster City, California) using SYBR Green Master Mix (Invitrogen). Total amount of mRNA was normalized to endogenous β‐actin mRNA. The primers of the target genes were listed as shown in Table S1.
2.10. Western blotting analysis
Cells were harvested and lysed in the RIPA buffer containing 1 mM phenylmethanesulfonyl fluoride (PMSF, from Beyotime, Shanghai, China) for 30 minutes on ice. Lysates were clarified by centrifugation at 14 000g for 20 minutes. Protein samples were diluted in 5× sodium dodecyl sulfate (SDS, Sigma‐Aldrich) loading buffer and fractionated in a 12% SDS‐polyacrylamide gel. Proteins were transferred onto a polyvinylidene fluoride (PVDF, from Millipore, Temecular, California) and incubated for 1 hour in 5% nonfat dry milk dissolved in tris buffered saline (TBST; 150 mM NaCl, 50 mM tris‐HCl, pH 7.5, 0.05% Tween 20) at room temperature. The blotting membranes were incubated with primary antibodies overnight at 4°C, extensively washed in TBST, incubated with hP‐conjugated secondary antibody for 2 hours at room temperature, and washed again with TBST. The blotting membranes were developed with chemiluminescent reagents.
2.11. IGF‐2 knockdown using shRNA
To achieve IGF‐2 knockdown, we transduced MuSCs with IGF‐2‐targeting shRNA carried on a lentivirus vector (PGLV3/H1/GFP/Puro, from GenePharma, Shanghai, China). The shRNA target sequence for IGF‐2 was 5′‐GGAGCTTGTTGACACGCTTCA‐3′. The scrambled shRNA target sequence was 5′‐TTCTCCGAACGTGTCACGT‐3′. MuSCs were incubated with lentivirus and polybrene (5 μg/mL, GenePharma) for 12 hours. Puromycin (5 μg/mL, Gbico) was added to the culture medium to screen out transduced cells.
2.12. Statistical analysis
Statistical analysis of all experiments was analyzed using the Prism software (version 8.0, La Jolla, California). For two‐group comparison, two‐tailed unpaired t tests were performed. For multiple group comparison, one‐way analysis of variance test was performed. All data were represented as the mean and SEM. P values less than .05 were considered statistically significant.
3. RESULTS
3.1. MuSCs ameliorate DSS‐induced colitis
To investigate whether MuSCs could modulate inflammation in vivo, we treated mice suffering from DSS‐induced IBD with in vitro expanded MuSCs. We found that a single intravenous injection of MuSCs significantly improved clinical parameters such as body weight and DAI as compared to control mice treated with PBS (Figure 1A,B). MuSC‐treated IBD mice also had significantly less reduction in colon length (Figure 1C). Moreover, MuSCs reduced the extent of bowel wall thickening, crypt damage and the infiltration of inflammatory cells in colons as shown by histological examination (Figure 1D). In particular, there was a reduced macrophage infiltration in colitic mice after MuSC treatment (Figure S2A). More importantly, MuSCs decreased the IL‐6 level in serum (Figure 1E), an important indicator for colitis progression.24
Figure 1.

Muscle stem cells (MuSCs) alleviate inflammatory bowel disease (IBD). A‐C, Body weight, disease activity index, and colon length of IBD mice treated with phosphate‐buffered saline (PBS) and MuSCs on day 2 post‐IBD induction. D, Representative H&E‐stained colon sections and histological scores of IBD mice treated with MuSCs. Scale bars, 50 μm. E, Interleukin‐6 (IL‐6) in the serum of IBD mice treated with MuSCs was assayed by enzyme linked immunosorbent assay (ELISA). F‐H, Body weight, disease activity index, and colon length of IBD mice treated with F10 medium and MuSC supernatant (MuSC‐S) daily post‐IBD induction. I, Representative H&E‐stained colon sections and histological scores of IBD mice treated with MuSC‐S. Scale bars, 75 μm. J, IL‐6 in the serum of IBD mice treated with MuSC‐S was assayed by ELISA. Data are presented as mean ± SEM. *P < .05, **P < .01, ****P < .0001. DSS, dextran sulfate sodium
To determine whether the beneficial effect of MuSCs is through secretory factors, we treated IBD mice with concentrated supernatant conditioned by cultured MuSCs (MuSC‐S). The supernatant exhibited a therapeutic efficacy similar to that with MuSCs. The MuSC supernatant dramatically ameliorated clinical parameters and increased colon length in IBD mice (Figure 1F‐H). Moreover, histopathological examination confirmed the beneficial effect of MuSC‐S (Figure 1I and S2B). Strikingly, the MuSC supernatant‐treated mice had lower IL‐6 serum level (Figure 1J). Collectively, these results suggested that MuSCs as well as their secretome possess anti‐inflammatory effects.
3.2. The anti‐inflammatory effects of MuSCs are mediated by macrophages
Since recent studies have shown that macrophages are critically involved in the pathogenesis of IBD,22, 25, 26 we determined whether the therapeutic effect of MuSCs is also related to their regulation of the pro‐ or anti‐inflammatory properties of macrophages. Hence, clodronate liposomes were used to deplete macrophages in IBD mice (Figure S3). We found that as expected, IBD was greatly alleviated by macrophage depletion. Interestingly, in clodronate liposome‐treated IBD mice, MuSC supernatant had no detectable therapeutic efficacy, indicating that the beneficial effect of MuSCs in IBD mice requires macrophages (Figure 2A‐C). Taken together, these data suggested that MuSCs exert their anti‐inflammatory effects by acting on macrophages.
Figure 2.

Muscle stem cells (MuSCs) ameliorate inflammatory bowel disease (IBD) via macrophages. A, Colon length of IBD mice treated with clodronate liposome and MuSC supernatant (MuSC‐S). B and C, Representative H&E‐stained colon sections and histological scores of IBD mice treated with clodronate liposome and MuSC‐S. Scale bars, 100 μm. Data are presented as mean ± SEM. *P < .05, ****P < .0001. DSS, dextran sulfate sodium
3.3. MuSCs confer macrophages anti‐inflammatory properties during their maturation
To explore whether MuSCs could guide the polarization of macrophages, we treated bone marrow‐derived monocytes with MuSC supernatant during their maturation. The mRNA levels of M‐anti‐related genes, including Arg‐1, Chil3, CD206, and transforming growth factor beta (TGF‐β), were remarkably increased in macrophages educated by MuSC supernatant (Figure 3A). These macrophages also exhibited lower expression levels of IL‐1β and IL‐6 upon LPS plus IFN‐γ stimulation (Figure 3B,C). Importantly, anti‐inflammatory molecule programmed death‐ligand 1 (PD‐L1), which is known to attenuate immune responses and maintain immune homeostasis,27, 28 was significantly upregulated by the administration of MuSC supernatant (Figure 3D). In vivo, MuSC supernatant was shown to reduce the number of infiltrated mononuclear cells in colons (Figure 3E). Tissue‐infiltrated macrophages, in particular, were declined after MuSC secretome treatment, as shown by flow cytometry (Figure 3F). Furthermore, these macrophages were also shown to express higher levels of PD‐L1 in IBD mice receiving MuSC supernatant (Figure 3G). Together, these data demonstrated that MuSCs are capable of promoting macrophage polarization toward anti‐inflammatory phenotypes during their differentiation from monocytes.
Figure 3.

Muscle stem cells (MuSCs) confer maturing macrophages anti‐inflammatory properties. F10 medium or MuSC supernatant (MuSC‐S) was supplied during the maturation of bone marrow‐derived monocytes. A, Expression levels of anti‐inflammatory genes in matured macrophages were assayed by quantitative real time polymerase chain reaction (qRT‐PCR). B, Expression levels of interleukin (IL)‐1β and IL‐6 in macrophages treated with lipopolysaccharide (LPS) (50 ng/mL) plus interferon gamma (IFN‐γ) (20 ng/mL) for 24 hours were assayed by qRT‐PCR. C, The concentration of IL‐1β and IL‐6 in the supernatant of macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) for 48 hours were assayed by enzyme linked immunosorbent assay (ELISA). D, Gene and protein expression levels of PD‐L1 in macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) were respectively assayed by qRT‐PCR and flow cytometry. E, Infiltration of mononuclear cells into colon lamina propria in inflammatory bowel disease (IBD) mice treated with MuSC‐S or F10 medium, as determined by flow cytometric analysis. F, Infiltration of CD11b+F4/80+ macrophages into colon lamina propria in IBD mice treated with MuSC‐S or F10 medium, as determined by flow cytometric analysis. G, Flow cytometric analysis of the expression of PD‐L1 in infiltrated CD11b+F4/80+ macrophages in IBD colons. Data are presented as mean ± SEM. *P < .05, **P < .01, ***P < .001, ****P < .0001. MFI, mean fluorescence intensity; N.S., no significance
3.4. Attenuation of the inflammatory activities in macrophages requires OXPHOS
Recent studies have revealed that metabolic reprogramming is essential for shaping the function and phenotypes of macrophages.29, 30, 31 Glycolysis determines the proinflammatory responses, while OXPHOS defines the anti‐inflammatory properties of macrophages.15, 16, 17 Indeed, lactate accumulation was found to be decreased in macrophages treated with MuSC supernatant (Figure 4A). Consistently, the mRNA levels of glycolytic enzymes, including hexokinase‐2 (HK2) and glucose transporter 1 (GLUT1) in maturing macrophages, were reduced by MuSC supernatant. Conversely, peroxisome proliferator‐activated receptor‐γ (PPAR‐γ), a M‐anti‐related metabolic molecule, was upregulated in macrophages treated with MuSC supernatant (Figure 4B). Interestingly, the production of mitochondrial superoxides in macrophages in response to proinflammatory stimulation was reduced by MuSC supernatant (Figure 4C). Moreover, MuSC‐preprogrammed macrophages possessed a higher mitochondria membrane potential (Figure 4D). These results suggested that MuSCs may have impacted on the mitochondria in the macrophages. To further determine the role of OXPHOS in the modulation of the maturing macrophages by MuSCs, we added oligomycin A, an adenosine triphosphate (ATP) synthase inhibitor that interrupts the mitochondrial coupling process and abrogates OXPHOS, during the treatment of macrophages by LPS plus IFN‐γ and found that the suppression of IL‐6 expression and the induction of PD‐L1 expression by MuSC supernatant were respectively blunted (Figure 4E). Taken together, these results confirmed that MuSCs could endow maturing macrophages OXPHOS‐dependent anti‐inflammatory properties.
Figure 4.

Muscle stem cells (MuSCs) modulate glycolysis and mitochondrial function in maturing macrophages. F10 medium or MuSC supernatant (MuSC‐S) was supplied during the maturation of bone marrow‐derived monocytes. A, Analysis of 24‐hours lactate production in the supernatant of matured macrophages. B, Expression levels of metabolic related genes in matured macrophages. C, The accumulation of mitochondrial reactive oxygen species (ROS) in macrophages treated with lipopolysaccharide (LPS) (50 ng/mL) plus interferon gamma (IFN‐γ) (20 ng/mL) for 6 hours was measured by MitoSox Red. D, Matured macrophages were subjected to JC‐1 staining to measure their mitochondria membrane potential. E, Interleukin‐6 (IL‐6) and PD‐L1 mRNA expression were determined in macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) in the presence or absence of oligomycin A. Data are represented as mean ± SEM. *P < .05, **P < .01, ***P < .001. MFI, mean fluorescence intensity
3.5. IGF‐2 is responsible for the anti‐inflammatory property of MuSCs
We further determined the factors that drive OXPHOS and thereby endow the maturing macrophages an anti‐inflammatory phenotype. IGF‐2 was recently shown to preprogram maturing macrophages to acquire OXPHOS‐dependent anti‐inflammatory properties.18 We thus tested whether IGF‐2 was involved by depleting IGF‐2 in MuSCs with IGF‐2 shRNA (Figure 5A). Importantly, with IGF‐2 being knocked down, the ability of MuSCs to upregulate PD‐L1 in macrophages was greatly compromised (Figure 5B). IGF‐2‐shRNA MuSCs also failed to suppress IL‐6 expression in macrophages upon proinflammatory stimulation (Figure 5C). In addition, the expressions of HK‐2 (M‐pro metabolic regulator) and PPAR‐γ (M‐antimetabolic regulator) were reverted in IGF‐2‐shRNA MuSC‐preprogrammed macrophages (Figure 5D).
Figure 5.

Insulin‐like growth factor‐2 (IGF‐2) is responsible for anti‐inflammatory phenotypes in muscle stem cells (MuSC)‐preprogrammed macrophages. A, Efficiency of shRNA‐mediated IGF‐2 knockdown in MuSCs. B‐D, Ctrl‐shRNA or IGF‐2‐shRNA MuSC supernatant (MuSC‐S) was supplied during the maturation of bone marrow‐derived monocytes (BMDMs). B, Expression level of PD‐L1 in matured macrophages treated with lipopolysaccharide (LPS) (50 ng/mL) plus interferon gamma (IFN‐γ) (20 ng/mL) for 24 hours was assayed by quantitative real time polymerase chain reaction (qRT‐PCR). C, The concentration of interleukin‐6 (IL‐6) in the supernatant of macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) for 48 hours was assayed by enzyme linked immunosorbent assay (ELISA). D, Expression levels of HK‐2 and PPAR‐γ in macrophages were assayed by qRT‐PCR. E‐H, MuSC‐S was incubated with IGF‐2 neutralizing antibodies or isotype control antibodies and then preprogramed BMDMs during their maturation. Expression levels of PD‐L1 and IL‐6 in macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) for 24 hours were assayed by qRT‐PCR (E and F). G, The concentration of IL‐6 in the supernatant of macrophages treated with LPS (50 ng/mL) plus IFN‐γ (20 ng/mL) for 48 hours was assayed by ELISA. H, Expression levels of metabolic related genes in matured macrophages were assayed by qRT‐PCR. Data are represented as mean ± SEM. *P < .05, **P < .01, ***P < .001. Ctrl‐shRNA, scrambled shRNA control; N.S., no significance
Consistently, IGF‐2 neutralization also eliminated the M‐anti‐polarizing effects of MuSCs on maturing macrophages. The expression of PD‐L1 in MuSC‐preprogrammed macrophages was reduced to control levels when IGF‐2 neutralizing antibodies were applied (Figure 5E). The decrease in IL‐6 expression was also abolished in MuSC‐preprogrammed macrophages using the neutralization antibodies upon proinflammatory stimulation (Figure 5F,G). IL‐6 expression level was even higher in macrophages treated with IGF‐2 neutralizing antibodies than those treated with F10 medium, suggesting that the antibodies may even bind to IGF‐2 in serum, thus further reducing the IGF‐2 level in culture medium. Furthermore, IGF‐2 ligation affected HK2 and PPAR‐γ expression switch in MuSC‐treated macrophages (Figure 5H). Taken together, these results demonstrated that IGF‐2 mediated the anti‐inflammatory effects of MuSCs.
We previously reported that MSCs cultured under low oxygen also conferred maturing macrophages anti‐inflammatory properties via the production of IGF‐2.18 We next compared the expression levels of IGF‐2 in MuSCs and MSCs. Interestingly, the expression level of IGF‐2 was much higher in MuSCs than in MSCs under normal culture condition (Figure S4A,B). The IGF‐2 protein level was still higher in MuSCs than in MSCs even when they were subjected to low‐oxygen condition (Figure S4C). These results indicate that MuSCs are potentially superior to MSCs when used as a therapeutic for inflammatory diseases.
3.6. IGF‐2 is required for the IBD alleviating effect of MuSCs
We next tested whether IGF‐2 produced by MuSCs accounted for the beneficial effects in vivo. IBD mice were treated with supernatants from Ctrl‐shRNA‐ and IGF‐2‐shRNA‐transduced MuSCs, respectively. The supernatant from IGF‐2‐shRNA MuSCs failed to prevent the decrease in colon length in IBD mice (Figure 6A). Parameters in bowel wall thickening, crypt damage and the infiltration of inflammatory cells in colons were similar to those in the IBD control group (Figure 6B). Furthermore, the IGF‐2‐shRNA MuSC supernatant was no longer effective in suppressing IL‐6 expression in colitic mice (Figure 6C).
Figure 6.
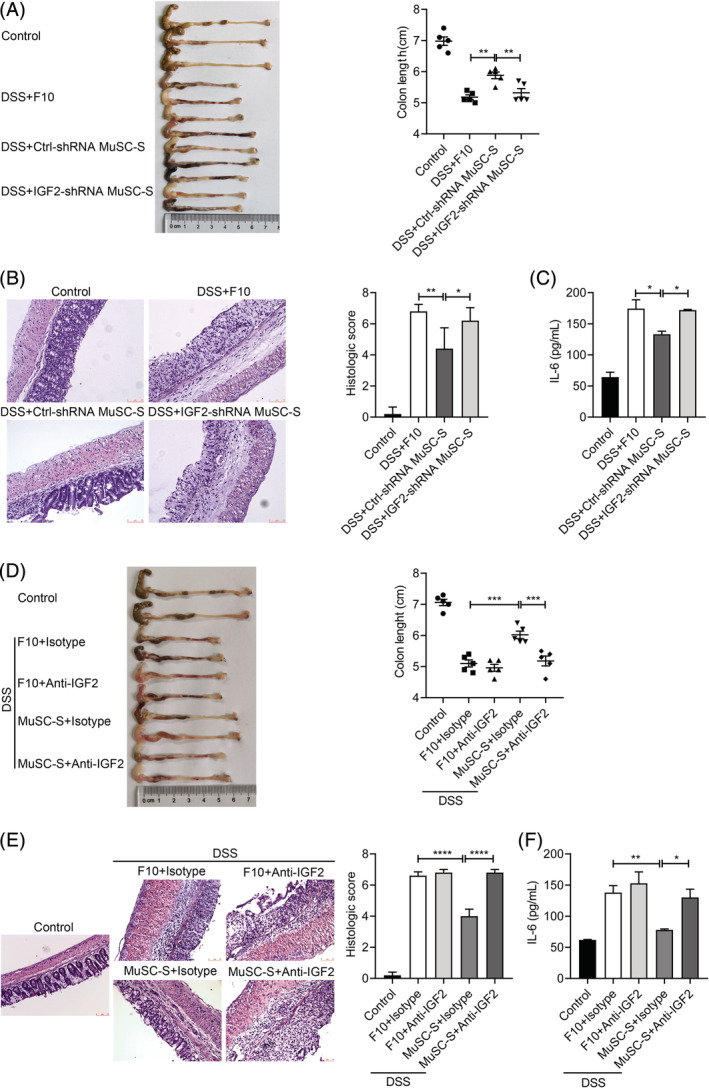
Insulin‐like growth factor‐2 (IGF‐2) depletion or inhibition abrogates the beneficial effects of muscle stem cells (MuSCs) on inflammatory bowel disease (IBD). A, Colon length of IBD mice treated with Ctrl‐shRNA or IGF‐2‐shRNA MuSC supernatant (MuSC‐S) daily post‐IBD induction. B, Representative H&E‐stained colon sections and histological scores of IBD mice treated with Ctrl‐shRNA or IGF‐2‐shRNA MuSC‐S daily post‐IBD induction. Scale bars, 75 μm. C, IL‐6 in the serum of IBD mice treated with Ctrl‐shRNA or IGF‐2‐shRNA MuSC‐S daily post‐IBD induction was assayed by enzyme linked immunosorbent assay (ELISA). D, Colon length of IBD mice treated with MuSC‐S combined with IGF‐2 neutralizing antibodies or isotype control antibodies. E, Representative H&E‐stained colon sections and histological scores of IBD mice treated with MuSC‐S combined with IGF‐2 neutralizing antibodies or isotype control antibodies. Scale bars, 75 μm. F, IL‐6 in the serum of IBD mice treated with MuSC‐S combined with IGF‐2 neutralizing antibodies or isotype control antibodies was assayed by ELISA. Data are represented as mean ± SEM. *P < .05, **P < .01, ***P < .001, ****P < .0001. Ctrl‐shRNA, scrambled shRNA control; DSS, dextran sulfate sodium
We also injected IGF‐2 neutralizing antibodies along with the MuSC supernatant into IBD mice. As expected, IGF‐2 neutralization abolished the therapeutic effects of MuSC secretome on IBD mice (Figure 6D,E). In addition, IGF‐2 neutralization greatly restored IL‐6 level in serum in MuSC supernatant‐treated mice (Figure 6F). Collectively, these data demonstrated that the beneficial effects of MuSCs in IBD mice depend on IGF‐2.
4. DISCUSSION
Upon tissue damage, activated stem cells are believed to exert their proregenerative effects through differentiating into new functional cells that replace the injured or lost cells, a process also termed as “cell replacement.” However, recent studies showed that MSCs can produce immunomodulatory factors, including cytokines, chemokines, and growth factors, that can alter the fate of tissue progenitor cells, change the dynamic of inflammation, remodel extracellular matrix (ECM) and promote angiogenesis. Such actions of MSCs during tissue repair and regeneration have been termed as “cell empowerment.”32, 33, 34 Transplanted NSCs were also found to engage in multifaceted stem cell to immune cell communication programs by orchestrating local innate and adaptive immune responses in inflammatory central nervous system (CNS) disorders.35, 36, 37 MuSCs were shown to secrete exosomes, which regulate collagen expression in fibrogenic cells, and prevent excessive ECM deposition.38 We showed here that MuSCs could orchestrate an anti‐inflammatory microenvironment by endowing maturing macrophages with anti‐inflammatory properties. Thus, MuSCs do not only respond to inflammatory cues in their microenvironment but may also act on immune cells to alter or shape the microenvironment. Given skeletal muscle as a major immune regulatory organ8 and MuSCs as the central myogenic cells in regulating tissue development and regeneration,39, 40 we speculated that MuSCs may also provide cues that instruct the immune cells to participate in tissue repair. If the MuSCs do possess immunomodulatory effects, it is expected that intravenously infused MuSCs may also be able to modulate inflammatory conditions other than in skeletal muscle, as MSCs do in various inflammatory conditions. Indeed, we found that transplanted MuSCs significantly ameliorated the disease in IBD mice, which was accompanied by a decrease in serum IL‐6 and improvement of pathological conditions. This result indicates that MuSCs can exert a potent immunomodulatory effect in vivo beyond the context of muscle repair and regeneration.
Many studies have verified the potential of stem cells to differentiate into functional descendants that replace damaged resident cells, and thereby promote tissue regeneration in various diseases, such as multiple sclerosis and acute renal failure.41, 42 However, in the case of nervous tissue repair, only very few of the transplanted NSCs (approximately 1%) survived, migrated to, and integrated within the brain and spinal cord in EAE model.19 Beyond the physical replacement of injured resident cells, inflammation‐licensed stem cells also orchestrate the remodeling of tissue microenvironment by producing immunoregulatory factors that regulate immune cells, as well as growth factors that dictate the functions of other progenitor cells.23, 43, 44, 45 Notably, we found that MuSC secretome possessed a therapeutic efficacy equivalent to that of MuSCs for IBD. These findings support that MuSCs can promote tissue repair indirectly, in a way known as “cell empowerment.”
We showed that the anti‐inflammatory effect of MuSCs was mediated by macrophages. Indeed, after macrophage depletion, MuSC secretome failed to ameliorate DSS‐induced colitis in mice. More importantly, MuSC secretome did not only reduce mononuclear cell infiltration in the affected colons, but also, upregulated PD‐L1 in inflammatory macrophages. In vitro, we also demonstrated that MuSC secretome shaped the anti‐inflammatory properties of maturing macrophages, as indicated by the expression of M‐anti‐related genes, Arg‐1, Chil3, CD206, and TGF‐β and the inhibition of inflammatory responses stimulated by LPS plus IFN‐γ. We recently showed that IGF‐2 released from MSCs exposed to low‐oxygen endows maturing macrophages with OXPHOS‐dependent anti‐inflammatory properties.18 IGF‐2 is known to play a critical role in the development of skeletal muscle in mammals.46 Interestingly, activated MuSCs possess high levels of IGF‐2.47 Because the IGF‐2 produced by MuSCs can dictate an anti‐inflammatory phenotypes of maturing macrophages, it can be speculated that in addition to passively responding to the environmental cues, the activated MuSCs may also actively participate in the making or remodeling of an extracellular environment that is conducive to their differentiation along the route of muscle regeneration, via the secretion of IGF‐2 and other unknown factors.
5. CONCLUSION
In the present study, our work identified a novel anti‐inflammatory function of MuSCs that is based on their production of IGF‐2. By endowing maturing macrophages an OXPHOS‐dependent anti‐inflammatory property, the IGF‐2‐producing MuSCs can thus orchestrate anti‐inflammatory microenvironments favorable for tissue repair and regeneration. These findings provide novel insights into how MuSCs act on macrophages and modulate inflammatory responses under pathological conditions. Because MuSCs can produce more IGF‐2 than the commonly used MSCs in cell therapy, MuSCs potentially have a more potent therapeutic efficacy for some inflammatory diseases.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.K.F., S.C.Z.: collection and/or assembly of data, data analysis and interpretation, and manuscript writing; L.J.C., Z.H.L., P.B.H., Y.J.C., Y.Y.Z., X.L.L., R.L., Y.S.P.: collection and/or assembly of data, data analysis and interpretation; Q.W.S., Z.Y.Z., L.S., Y.N.L., Z.L.F., L.Y.L.: provision of study material or patients; G.M., Y.W., C.S.S., Y.F.S.: conception and design, financial support, final approval of manuscript.
Supporting information
FIGURE S1. Characterization of MuSCs and their differentiation in vitro. A, Characterization of cultured MuSC surface markers by flow cytometric analysis. B, Characterization of nuclear factor PAX7 in cultured MuSCs by flow cytometric analysis. C, Representative images of the differentiation potentials of cultured MuSCs. Red indicated myosin heavy chain (MyHC) staining; Hoechst indicated nuclei staining; Merge indicated merged images of MyHC and Hoechst staining. Scale bar, 50 μm.
FIGURE S2. MuSCs lead to reduced macrophage infiltration in colitic mice. A, Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with and without MuSCs. B, Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with and without MuSC supernatant (MuSC‐S). Scale bars, 75 μm.
FIGURE S3. Clodronate liposomes deplete tissue‐infiltrated macrophages in colitic mice. Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with clodronate liposomes and MuSC supernatant (MuSC‐S). Scale bars, 75 μm.
FIGURE S4. IGF‐2 expression in MSCs and MuSCs. A, Gene expression levels of IGF‐2 in MSCs and MuSCs were assayed by quantitative real time polymerase chain reaction (qRT‐PCR). B, Protein expression levels of IGF‐2 in MSCs and MuSCs were assayed by western blot. C, Protein expression levels of IGF‐2 in MSCs and MuSCs under normoxia or hypoxic condition were assayed by western blot. Data are presented as mean ± SEM. ****P < .0001.
Table S1. Gene‐specific primers for qRT‐PCR.
ACKNOWLEDGMENTS
This study was supported by grants from the Ministry of Science and Technology of China (2018YFA0107500), National Natural Science Foundation of China (81530043, 81930085 and 31900635), the Suzhou Science and Technology Program (SZS201616) and Social Development Project of Jiangsu Province (BE2016671), and the State Key Laboratory of Radiation Medicine and Protection, Soochow University (GZN1201804 and GZN1201903).
Fang J, Zhang S, Liu Z, et al. Skeletal muscle stem cells confer maturing macrophages anti‐inflammatory properties through insulin‐like growth factor‐2. STEM CELLS Transl Med. 2020;9:773–785. 10.1002/sctm.19-0447
Jiankai Fang and Shengchao Zhang contributed equally to this study.
Funding information State Key Laboratory of Radiation Medicine and Protection, Soochow University, Grant/Award Numbers: GZN1201903, GZN1201804; Social Development Project of Jiangsu Province, Grant/Award Number: BE2016671; Suzhou Science and Technology Program, Grant/Award Number: SZS201616; National Natural Science Foundation of China, Grant/Award Numbers: 31900635, 81930085, 81530043; Ministry of Science and Technology of China, Grant/Award Number: 2018YFA0107500
Contributor Information
Changshun Shao, Email: shaoc@suda.edu.cn.
Yufang Shi, Email: yfshi@suda.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18‐88 yr. J Appl Physiol (1985). 2000;89:81‐88. [DOI] [PubMed] [Google Scholar]
- 2. Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17:165‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Honda H, Kimura H, Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272‐277. [PMC free article] [PubMed] [Google Scholar]
- 4. Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuswanto W, Burzyn D, Panduro M, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin‐33‐dependent accumulation of regulatory T cells. Immunity. 2016;44:355‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mounier R, Theret M, Arnold L, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251‐264. [DOI] [PubMed] [Google Scholar]
- 8. Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age‐related multi‐morbidity? Nat Rev Immunol. 2019;19:563‐572. [DOI] [PubMed] [Google Scholar]
- 9. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL‐6 infusion inhibit endotoxin‐induced TNF‐alpha production in humans. FASEB J. 2003;17:884‐886. [DOI] [PubMed] [Google Scholar]
- 10. Rao RR, Long JZ, White JP, et al. Meteorin‐like is a hormone that regulates immune‐adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace DL, Berard M, Soares MV, et al. Prolonged exposure of naive CD8+ T cells to interleukin‐7 or interleukin‐15 stimulates proliferation without differentiation or loss of telomere length. Immunology. 2006;119:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Attar A, Presnell SR, Clasey JL, et al. Human body composition and immunity: visceral adipose tissue produces IL‐15 and muscle strength inversely correlates with NK cell function in elderly humans. Front Immunol. 2018;9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen AR, Hojman P, Erikstrup C, et al. Association between interleukin‐15 and obesity: interleukin‐15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008;93:4486‐4493. [DOI] [PubMed] [Google Scholar]
- 14. Bazgir B, Fathi R, Rezazadeh Valojerdi M, Mozdziak P, Asgari A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell J. 2017;18:473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL‐1beta through HIF‐1alpha. Nature. 2013;496:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doyle AG, Herbein G, Montaner LJ, et al. Interleukin‐13 alters the activation state of murine macrophages in vitro: comparison with interleukin‐4 and interferon‐gamma. Eur J Immunol. 1994;24:1441‐1445. [DOI] [PubMed] [Google Scholar]
- 18. Du L, Lin L, Li Q, et al. IGF‐2 Preprograms maturing macrophages to acquire oxidative phosphorylation‐dependent anti‐inflammatory properties. Cell Metab. 2019;29:1363‐1375. e1368. [DOI] [PubMed] [Google Scholar]
- 19. Peruzzotti‐Jametti L, Bernstock JD, Vicario N, et al. Macrophage‐derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22:355‐368. e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence‐activated cell sorting. Nat Protoc. 2015;10:1612‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu X, Xiao J, Wei Y, et al. Combination of inflammation‐related cytokines promotes long‐term muscle stem cell expansion. Cell Res. 2015;25:655‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song WJ, Li Q, Ryu MO, et al. TSG‐6 secreted by human adipose tissue‐derived mesenchymal stem cells ameliorates DSS‐induced colitis by inducing M2 macrophage polarization in mice. Sci Rep. 2017;7:5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141‐150. [DOI] [PubMed] [Google Scholar]
- 24. Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL‐6 and TNF‐alpha ameliorates intestinal permeability in DSS‐induced colitis. Cytokine. 2016;83:189‐192. [DOI] [PubMed] [Google Scholar]
- 25. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531‐543. [DOI] [PubMed] [Google Scholar]
- 26. Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:1992‐1998. [DOI] [PubMed] [Google Scholar]
- 27. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD‐L1 checkpoint. Immunity. 2018;48:434‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trabattoni D, Saresella M, Pacei M, et al. Costimulatory pathways in multiple sclerosis: distinctive expression of PD‐1 and PD‐L1 in patients with different patterns of disease. J Immunol. 2009;183:4984‐4993. [DOI] [PubMed] [Google Scholar]
- 29. Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24:807‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanchez‐Lopez E, Zhong Z, Stubelius A, et al. Choline uptake and metabolism modulate macrophage IL‐1beta and IL‐18 production. Cell Metab. 2019;29:1350‐1362. e1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giannakis N, Sansbury BE, Patsalos A, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019;20:626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 33. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493‐507. [DOI] [PubMed] [Google Scholar]
- 34. Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bacigaluppi M, Russo GL, Peruzzotti‐Jametti L, et al. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT‐1 in astrocytes. J Neurosci. 2016;36:10529‐10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bacigaluppi M, Pluchino S, Peruzzotti‐Jametti L, et al. Delayed post‐ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239‐2251. [DOI] [PubMed] [Google Scholar]
- 37. Pluchino S, Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61:1379‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell. 2017;20:56‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell. 2018;46:135‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol. 2016;17:267‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688‐694. [DOI] [PubMed] [Google Scholar]
- 42. Qian H, Yang H, Xu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial‐like cells. Int J Mol Med. 2008;22:325‐332. [PubMed] [Google Scholar]
- 43. Cusimano M, Biziato D, Brambilla E, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han X, Yang Q, Lin L, et al. Interleukin‐17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014;21:1758‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao W, Yang Y, Wang Z, et al. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273‐284. [DOI] [PubMed] [Google Scholar]
- 46. Younis S, Schonke M, Massart J, et al. The ZBED6‐IGF2 axis has a major effect on growth of skeletal muscle and internal organs in placental mammals. Proc Natl Acad Sci U S A. 2018;115:E2048‐E2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Charville GW, Cheung TH, Yoo B, et al. Ex vivo expansion and in vivo self‐renewal of human muscle stem cells. Stem Cell Reports. 2015;5:621‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Characterization of MuSCs and their differentiation in vitro. A, Characterization of cultured MuSC surface markers by flow cytometric analysis. B, Characterization of nuclear factor PAX7 in cultured MuSCs by flow cytometric analysis. C, Representative images of the differentiation potentials of cultured MuSCs. Red indicated myosin heavy chain (MyHC) staining; Hoechst indicated nuclei staining; Merge indicated merged images of MyHC and Hoechst staining. Scale bar, 50 μm.
FIGURE S2. MuSCs lead to reduced macrophage infiltration in colitic mice. A, Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with and without MuSCs. B, Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with and without MuSC supernatant (MuSC‐S). Scale bars, 75 μm.
FIGURE S3. Clodronate liposomes deplete tissue‐infiltrated macrophages in colitic mice. Representative immunostaining of F4/80‐positive macrophages in the colons from healthy and colitic mice treated with clodronate liposomes and MuSC supernatant (MuSC‐S). Scale bars, 75 μm.
FIGURE S4. IGF‐2 expression in MSCs and MuSCs. A, Gene expression levels of IGF‐2 in MSCs and MuSCs were assayed by quantitative real time polymerase chain reaction (qRT‐PCR). B, Protein expression levels of IGF‐2 in MSCs and MuSCs were assayed by western blot. C, Protein expression levels of IGF‐2 in MSCs and MuSCs under normoxia or hypoxic condition were assayed by western blot. Data are presented as mean ± SEM. ****P < .0001.
Table S1. Gene‐specific primers for qRT‐PCR.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


