Abstract
Hyperactivity of the NOTCH pathway is associated with tumor growth and radiotherapy resistance in lung cancer, and NOTCH/γ‐secretase inhibitors (GSIs) are a potential therapeutic target. The therapeutic outcome, however, is often restricted by the dose‐limiting toxicity of combined treatments on the surrounding healthy tissue. The NOTCH signaling pathway is also crucial for homeostasis and repair of the normal airway epithelium. The effects of NOTCH/γ‐secretase inhibition on the irradiation of normal lung epithelium are unknown and may counteract antitumor activity. Here we, therefore, investigated whether normal tissue toxicity to radiation is altered upon NOTCH pathway inhibition. We established air‐liquid interface pseudostratified and polarized cultures from primary human bronchial epithelial cells and blocked NOTCH signaling alone or after irradiation with small‐molecule NOTCH inhibitor/GSI. We found that the reduction in proliferation and viability of bronchial stem cells (TP63+) in response to irradiation is rescued with concomitant NOTCH inhibition. This correlated with reduced activation of the DNA damage response and accelerated repair by 24 hours and 3 days postirradiation. The increase in basal cell proliferation and viability in GSI‐treated and irradiated cultures resulted in an improved epithelial barrier function. Comparable results were obtained after in vivo irradiation, where the combination of NOTCH inhibition and irradiation increased the percentage of stem cells and ciliated cells ex vivo. These encourage further use of normal patient tissue for toxicity screening of combination treatments and disclose novel interactions between NOTCH inhibition and radiotherapy and opportunities for tissue repair after radiotherapy.
Keywords: air‐liquid interface system, NOTCH signaling, primary bronchial epithelial cells, radiation‐induced lung injury, radiotherapy
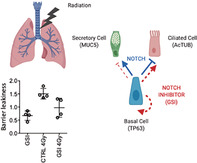
Significance statement.
Radiation‐induced lung injury is a dose‐limiting toxicity that limits the effective dose that should be administered and forces the interruption of the treatment. The NOTCH signaling pathway is a potential therapeutic target for lung cancer because its inhibition reduces tumor growth and synergizes with radiotherapy and chemotherapy in preclinical models. However, the effect of inhibiting NOTCH in irradiated normal lung tissue is not known and could impact the therapeutic benefit of combination treatments. This study demonstrates that small‐molecule inhibitors of the NOTCH pathway enhance the survival of irradiated primary human and murine bronchial epithelial lung stem cells. This finding may be beneficial in lung cancer treatment with radiotherapy and NOTCH inhibitors by protecting normal lung tissue while increasing tumor control.
1. INTRODUCTION
Radiotherapy‐induced lung injury is a dose‐limiting complication resulting in loss of pulmonary function occurring in as many as 30% of patients with thoracic cancer. It negatively affects the quality of life and requires de‐escalation treatment, which lowers the probability of tumor control. 1
Radiation therapy induces DNA damage response (DDR), production of reactive oxygen species (ROS), and cell death and leads to the initiation of an acute inflammatory response called radiation pneumonitis, which can persist and progress into a late irreversible and progressive remodeling leading to a reduction of lung volume and lung function. 2 Lung irradiation leads to a dose‐dependent decrease of the lung epithelial colony‐forming ability, suggesting a functional impairment of lung stem cell renewal and repair after radiotherapy. 3 Surviving lung stem cells repair DNA damage through the error‐prone nonhomologous end joining (NHEJ) pathway. Thus, subsets of surviving airway progenitors may accumulate mutations contributing to pulmonary disease and oncogenic transformation. 4
The human bronchial airway epithelium is a pseudostratified epithelium composed of a luminal layer, containing the differentiated ciliated and secretory cells, and a basal layer containing the bronchial stem cells. 5 The integrity of the bronchial luminal lining is important for the mucociliary clearance of the lung, where mucous and ciliated cells move trapped pathogens out of the lung as air moves in, while the stem cells retain the self‐renewal capacity and promote the replacement of damaged cells. 6 The epithelial cells have a low turnover, however, after chemical or pathogen injury, lung stem cells proliferate to repopulate the damaged area. 7 , 8 , 9 An imbalance in the percentage of these cell types gives rise to progressive diseases such as chronic obstructive pulmonary disease (COPD), asthma, and emphysema. 10
There is increasing evidence that self‐renewal and differentiation in the lung epithelium are dependent on NOTCH signaling. 11 NOTCH receptors are transmembrane glycoproteins that interact with membrane‐bound ligands on neighboring cells. The mammalian genome encodes for four NOTCH receptors and five NOTCH ligands (JAGGED1 and JAGGED2 or DELTA1, DELTA3, and DELTA4). 12 Upon ligand binding on adjacent cells, NOTCH receptors undergo two consecutive cleavages. The first cleavage releases the NOTCH ectodomain and is followed by the intramembranous and rate‐limiting cleavage by the γ‐secretase complex. 13 The γ‐secretase releases the NOTCH intracellular domain from the cell membrane, which translocates into the nucleus and binds the DNA‐bound protein CSL (Suppressor of Hairless, Lag‐1, also called RBP‐Jk), and together with the Mastermind (MAML) coactivators, forms the NOTCH transcriptional complex.
NOTCH receptors are expressed during fetal lung development and regulate cell fate determination and branching along the proximal‐distal axis. 14 In the postnatal lung, NOTCH restricts basal cells to secretory lineages, suppressing ciliated cell differentiation. Furthermore, the maintenance of secretory cells requires NOTCH signaling as blocking JAGGED1/2‐NOTCH pathway can transdifferentiate secretory into ciliated cells without proliferation. 15 NOTCH receptors have different roles in the homeostasis of the adult airway epithelium. NOTCH1 is essential for club cell regeneration through activation of its downstream targets HES5 and PAX6, 16 and it is involved in lung development upon injury. NOTCH2 contributes to the alveolar integrity of epithelial and smooth muscle layers and leads to predetermined heterogeneity among basal cells of the mouse tracheal epithelium that regulates the differentiation of the progenitors into ciliated cells, 17 , 18 NOTCH3 regulates pulmonary endocrine cell fate 17 and as well as the pool of progenitor cells available for neuroendocrine differentiation. 19
NOTCH signaling is deregulated and mutated in many types of cancers including lung cancer. 20 High expression of NOTCH1 and NOTCH3, ligands, and target genes is correlated with worse survival in resected nonsmall cell lung cancer (NSCLC). 21 , 22 , 23 Preclinical studies showed that NOTCH1 stimulated NSCLC tumor growth and survival through upregulation of the insulin‐like growth factor 1 receptor 24 and survivin 25 in hypoxia. Constitutive NOTCH signaling promotes tumor growth of NSCLC xenografts and resistance to radiotherapy in part by promoting the survival of the treatment resistance hypoxic tumor fraction, 23 inducing radiation sensitivity to small‐molecule NOTCH inhibitor/γ‐secretase inhibitor (GSI). 26 NOTCH signaling has recently been shown to directly regulate the DDR. NOTCH1 binds to ATM ataxia telangiectasia mutated through direct competition with FOXO3 which is necessary for ATM phosphorylation and activation, preventing apoptosis of T‐cell leukemia. 27 , 28 These results suggest that NOTCH inhibition and radiation therapy may be effectively combined in the treatment of NSCLC.
However, the effects of NOTCH inhibition on the homeostasis and pathological response of the normal lung epithelium when combined with irradiation are unknown and could counteract any positive therapeutic effect on tumor control. Here, we investigated the effect of NOTCH inhibition on the proliferation and differentiation of irradiated primary human and murine bronchial epithelium using air‐liquid interface (ALI) culture systems.
2. MATERIALS AND METHODS
2.1. Primary bronchial epithelial cells
Primary human bronchial epithelial cells (PBECs) were kindly provided by the Primary Lung Culture facility MUMC+ (Maastricht, the Netherlands). Lung tissue used for the isolation of PBECs was obtained from the Maastricht Pathology Tissue Collection (MPTC) and originated from tissue resected during lobectomies or pneumonectomies of patients who underwent surgery for lung cancer (six different donors PUL# 112, 119, 109, 123, 127, 152). Collection, storage, and use of tissue and patient data were performed in agreement with the “Code for Proper Secondary Use of Human Tissue in the Netherlands” (http://www.fmwv.nl). The scientific board of the MPTC approved the use of materials for this study under MPTC2010‐019. In addition, formal permission was obtained from the local Medical Ethic Committee code 2017‐0087, and patients have provided written consent to the use of the material for research. PBECs passage 1 was grown in keratinocyte serum‐free medium supplemented with 1 mM isoproterenol, mycozap, 2.5 ng epidermal growth factor and bovine pituitary extract. Cells were seeded in fibronectin‐coated flasks. Soybean trypsin inhibitors from Glycine max were used to resuspend the cells after trypsinization. Cells were collected by centrifugation, 5 minutes at 150 RCF and counted with an automatic counter (Beckman Coulter).
2.2. Airway epithelium differentiation in ALI culture
Isolated PBECs were seeded onto 12‐mm transwell membranes with 0.4‐μm pore polyester membrane inserts (Corning Incorporated, Corning, NY) (90 000 cells/transwell in 500 μL) in stimulation medium. Stimulation medium contained bronchial epithelial cell growth medium (BEGM) (Lonza ee‐3171) and Dulbecco's modified Eagle medium (no glucose) (Gibco 11 366‐025), supplemented with Pen/Strep, HEPES, BEGM Single Quot Kit (Lonza 4175), and bovine serum albumin (BSA). PBECs were submerged by adding 500 μL of cells in the insert and 1.5 mL of stimulation medium at the bottom. PBECs were cultured in the stimulation medium at 37°C in 5% CO2 humidified incubator. Stimulation medium was replaced every 2 days until cells reached confluence. After cells reached confluence, the medium was removed from the insert and only supplied in the basal chamber. Retinoic acid (RA), in a final concentration of 50 nM, was supplemented to the BEGM. Cells received ALI treatment by only adding stimulation medium (+RA) to the basal chamber of each well (1 mL/well).
2.3. Mice studies
C57Bl/6 mice were used in this study. Animal work was performed in accordance with national guidelines and approved protocols (# 2014‐116). Animals were randomized (n = 12) across no irradiation or whole thorax irradiation with a single dose of 2 or 5 Gy (dose rate 3 Gy/min) using the X‐RAD 225Cx small animal irradiator (PXI, 250 KeV, 12 mA, 0.3‐mm copper filter). Two opposite and parallel beams were used to deliver the dose in a 40‐mm2 collimator with main target the trachea. Mice were sacrificed (n = 6) 24 hours after radiotherapy (RT) or 7 days after RT, tracheas were isolated and PBECs harvested and seeded in the ALI system.
The remaining materials and methods used in the manuscript are described in Data S1.
3. RESULTS
3.1. Human PBEC differentiation in ALI
To investigate the combined effects of irradiation and NOTCH inhibition on primary human lung epithelium in vitro, we established ALI cultures from PBECs from at least three human donors. We fully characterized PBEC cultures by investigating the expression of basal (TP63, CK5) and suprabasal differentiation markers for secretory cells (MUC5A, MUC1) and ciliated cells (Acetylated Tubulin [Ac‐TUB]) and proliferation (5‐ethynyl‐2‐deoxyuridine [EdU]) for a period of 28 days after airlift by Western blotting and immunofluorescence. At the start of PBEC cultures, all cells express the basal makers TP63 and CK5 and around 10% of TP63+ cells are proliferating (Figure 1A,C). Western blot for TP63 and CK5 markers showed that basal stem cells decrease during differentiation until day 28 (Figure 1A). Differentiated mucous cells appear 1 week after airlift and ciliated cells 2 weeks after airlift and cultures are fully differentiated at day 21 (Figure 1A). A similar pattern was observed in two other donors (Figure S1A). Costaining of TP63 and MUC5A showed that at day 0 no differentiated cells are present while at day 28, 20% of the cells are positive for MUC5A, 30% percent positive for Ac‐TUB, and 30% positive for TP63 (Figure 1B,C). At the time of airlift, 10% of cells proliferate with a mild increase in the first 7 days. Proliferation ceases on day 21 when the cultures are completely differentiated (Figure 1B,C). All the EdU+ cells were TP63+ suggesting that only the basal stem cell proliferates. Immunofluorescence and Western blot analysis on protein extracts at the same time points showed the same trend in marker expression for at least three independent donors.
FIGURE 1.
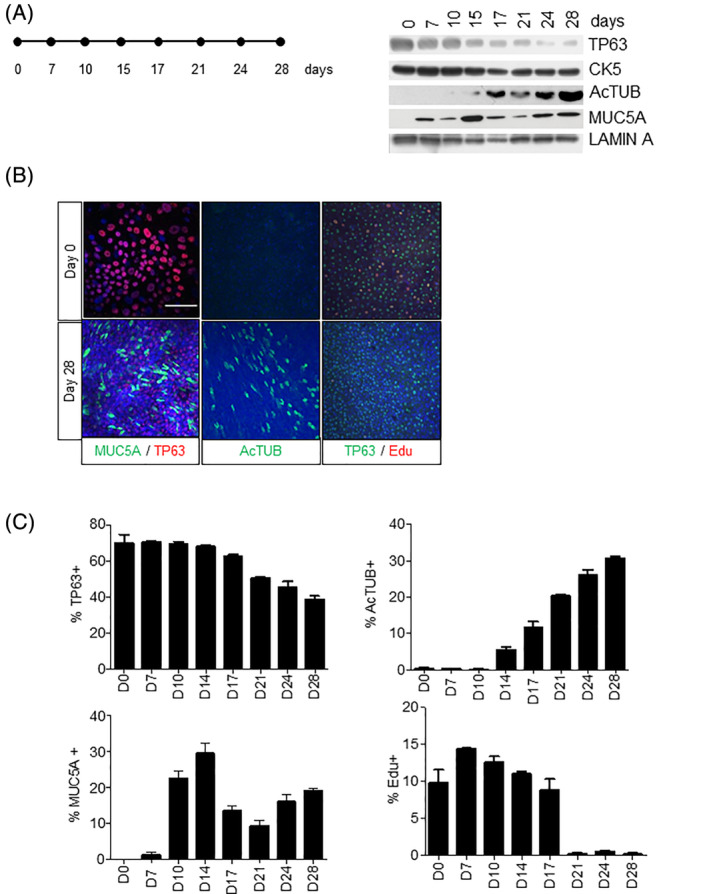
Proliferation and differentiation of human PBECs in ALI culture. A, Western blot of the time‐dependent expression (days) of basal stem cell markers (TP63 and CK5) and differentiation markers Ac‐TUB (ciliated cells) and MUC5A (mucous cells) after airlift in ALI culture. Lamin A was used as loading control. B, Immunofluorescent costaining of PBECs at day 0 and day 28 for TP63, MUC5A; Ac‐TUB, and proliferation with EdU. C, Quantification of TP63+, Ac‐TUB+, MUC5A+ and EdU+ cells in ALI system. For each staining condition, we randomly selected five different fields. The cells in these five fields were then counted to obtain a total of 500‐1000 cells per condition (100‐200 cells per image). Stainings with TP63, Ac‐TUB, MUC5, and EdU were captured using a ×20 objective. The Z‐stack was used as the image in the paper. Image‐J was used to count the positive cells and the foci in the nucleus. Comparable results were obtained in at least three independent donors. All scale bars are 1 μm. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; PBECs, primary human bronchial epithelial cells
3.2. NOTCH inhibition increases TP63+ basal stem cells
Next, we asked how NOTCH signaling affected cell fate decisions in differentiated PBECs by assessing the expression of basal and differentiated bronchial epithelial cell markers at several time points after incubation with the pan‐NOTCH inhibitor/GSI dibenzazepine (DBZ) 21 days after airlift. We observed a temporal increase in the expression of TP63, CK5 (basal cells), and Ac‐TUB (ciliated cells) protein levels with concomitant downregulation of MUC5A (secretory cells) in ALI protein extracts (Figure 2A). Similar results were obtained in two different donors (Figure S2A). To address if these changes were caused by a change in protein expression or cell numbers, we conducted immunostaining for the same markers and found that TP63+ and ciliated cell numbers significantly increased after NOTCH inhibition while MUC5A+ cells decreased (Figure 2B,C). To address if the increased percentage of TP63+ cells was due to increased proliferation or decreased cell death, we performed EdU/TP63 costaining to identify actively proliferating cells. We found that NOTCH inhibition significantly increased the number of TP63+ cells in a time‐dependent manner compared with vehicle controls (Figure 2D,E). At day 28, we observed an average of fivefold increase in proliferating TP63+ cells compared with control cell cultures that had completely ceased proliferation. The absolute number of PBECs in these cultures was increased by GSI treatment (Figure S2B). No EDu+ cells were observed that were also positive for MUC5A or Ac‐TUB differentiation markers. Similar results were observed for three donors (Figure S2B). Taken together, our results show that multipotent human TP63+ cells, in fully differentiated primary cultures, can be restimulated to proliferate and generate ciliated cells and block the formation of MUC5A+ secretory cells when NOTCH signaling is blocked.
FIGURE 2.
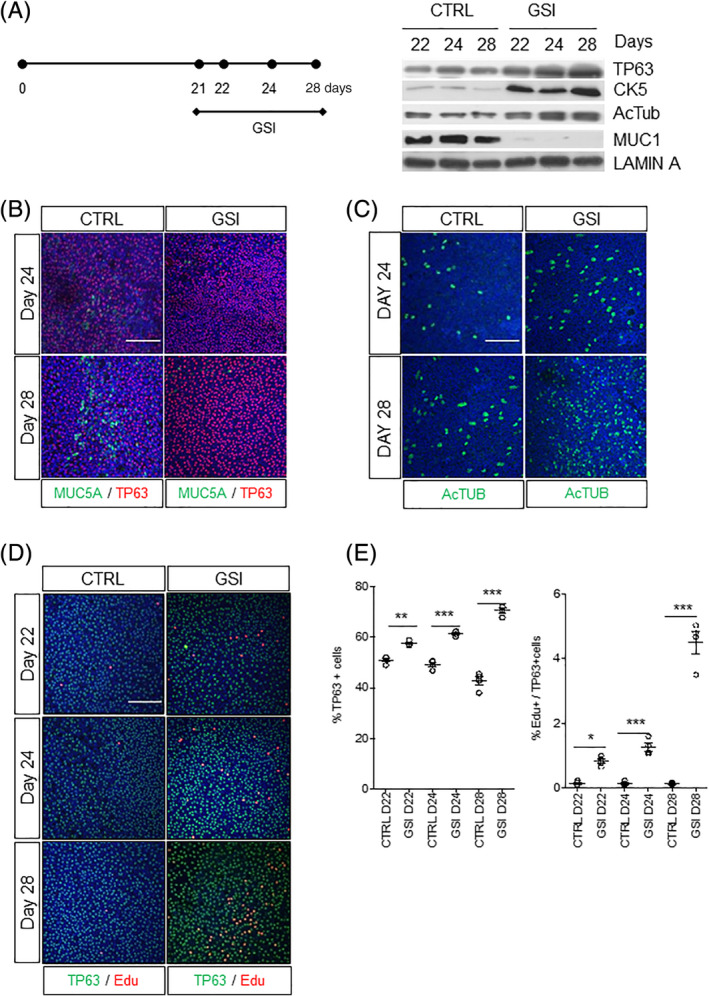
NOTCH inhibition promotes basal and ciliated phenotypes in ALI cultures. A, Increase of basal stem cell markers (TP63 and CK5) and ciliated (Ac‐TUB) and decrease of secretory (MUC1) cells at days 22, 24, and 28 upon NOTCH/γ‐secretase inhibition for 1, 3, and 7 days, respectively. Lamin A was used as loading control. Immunofluorescent staining of PBECs at days 24 and 28 stained for, B, TP63 and MUC5A or for, C, Ac‐TUB with and without GSI. D, Immunofluorescent staining of PBECs at days 22, 24, and 28 stained for TP63 and EdU with and without GSI. E, Quantification graphs of the percentage of TP63+ and EdU+/TP63+ cells at days 22, 24, and 28 with and without GSI. N = 3 biological repeats per donor (three donors). One‐way ANOVA: *P < .05; **P < .001; ***P < .0001. All scale bars are 1 μm. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; ANOVA, analysis of variance; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
3.3. NOTCH inhibition prevents the loss of stem cells upon radiation
To address the role of the NOTCH signaling in cell fate decisions in response to ionizing radiation, we investigated the expression of the different cell type‐specific markers on protein level at different time points after irradiation in differentiated ALI cultures. For this purpose, we conducted different treatment schedules of GSI treatment in combination with irradiation at 4 Gy. This dose of radiation was chosen because 4 Gy irradiation significantly affects the self‐renewal of irradiated human and murine basal cells in monolayer culture. 29 As shown before, GSI consistently increased the expression of TP63, CK5, and Ac‐TUB in nonirradiated ALI cultures. After 4 Gy irradiation, a reduction in the expression of TP63 and CK5 was observed at 24 and 72 hours after irradiation (Figure 3A). The expression of the ciliated cell marker was not affected up to 72 hours postirradiation. When PBECs were cultured with GSI 2 days prior to irradiation, the reduction in TP63+ expression was strongly blocked at all time points as shown by Western blotting (Figure 3A) and immunofluorescence (Figure 3B) indicating that GSI increased the survival of basal TP63 expressing cells after irradiation. Furthermore, mucous cells were drastically reduced upon GSI treatment alone and when combined with irradiation (Figure 3C). We quantified these differences and found that there was a rescue and significant increase of TP63 basal stem cells in 3D stratified ALI cultures when NOTCH signaling was blocked (Figure 3D).
FIGURE 3.
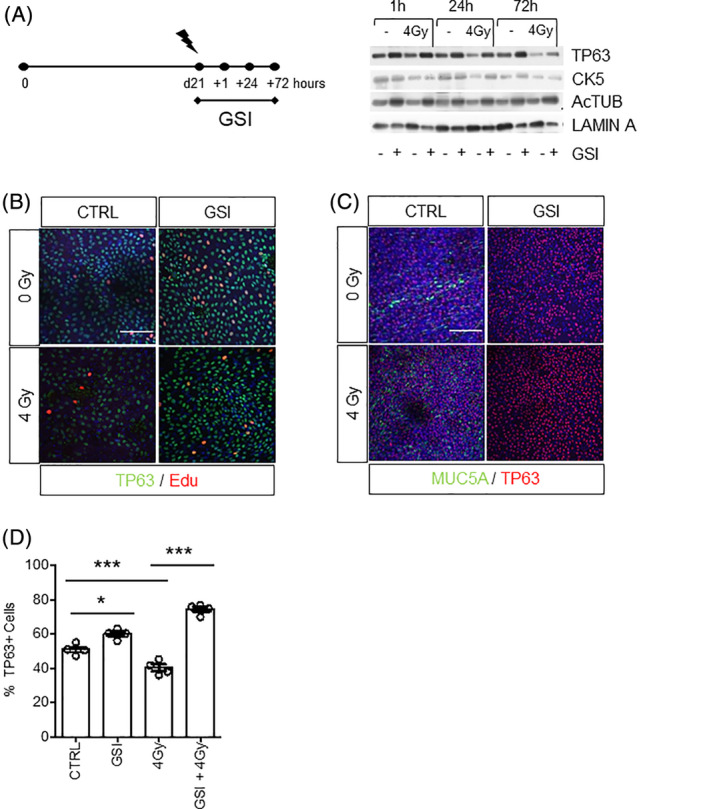
NOTCH inhibition prevents the loss of TP63+ basal stem cells after radiation. A, Expression of TP63, CK5, Ac‐TUB 1 hour, 24 hours, and 3 days after RT of PBECs upon continuous vehicle or GSI treatment by Western blot. Lamin A was used as loading control. B, Immunofluorescent costaining of TP63 and EdU showed increased proliferation of TP63+ basal cells 3 days after irradiation upon GSI treatment. C, Immunofluorescent costaining of TP63 and MUC5A in control and GSI‐treated samples with and without irradiation. D, Quantification graph of the percentage of TP63+ cells in irradiated samples upon GSI treatment. N = 3 biological repeats per donor. One‐way ANOVA: *P < .05; ***P < .0001. All scale bars are 1 μm. Ac‐TUB, Acetylated Tubulin; ANOVA, analysis of variance; EdU, 5‐ethynyl‐2‐deoxyuridine; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
3.4. Long‐term effects of NOTCH inhibition on self‐renewal and differentiation of bronchial epithelium
Next, we asked whether the short‐term increase in survival of irradiated bronchial stem cells upon NOTCH inhibition also translated into a long‐term in vitro and in vivo ability to repopulate and differentiate in ALI cultures. In 2 and 4 Gy irradiated cultures at 14 days after irradiation (Figure 4A), we observed a dose‐dependent decrease in the expression of TP63, CK5, and Ac‐TUB and the secretory marker MUC1 (Figure 4A). In contrast, in irradiated PBECs in which NOTCH signaling was blocked, a robust radiation dose‐dependent increase in the expression of basal markers TP63, CK5 was observed comparable to short‐term effects. We demonstrated a NOTCH‐dependent increase in Ac‐TUB expression and a decrease in MUC1 expression. Next, we sought to address if NOTCH inhibition would also affect long‐term survival of in vivo irradiated bronchial epithelial stem cells. We used conformal thoracic irradiation with two opposite beams to deliver a 2 and 5 Gy dose to the whole lung of C57Bl/6 mice and isolated PBEC from tracheas at 1 and 2 days after RT and cultured them in ALI. 29 We asked whether NOTCH inhibition using GSI, before‐after airlift or continuously affected the response of lung epithelial cells to RT (Figure S3A). Regardless of the timing or the dose of GSI, we found that NOTCH inhibition significantly promoted the expansion of irradiated TP63+ basal stem cells (Figure 4B) and ciliated cells (Figure S3B). When using immunofluorescence and confocal staining of ALI epithelia to quantify the TP63+ cell numbers upon 2‐Gy irradiation, we observed that the TP63+ cell numbers increased from 40% in CTRL samples to 50% in GSI‐CTRL, 66% in CTRL‐GSI, and 70% when GSI was given continuously (P < .001). Similar results were observed in the ALI's derived from 5‐Gy irradiated mice (Figure 4C). The increased percentage of murine basal stem cells upon NOTCH inhibition was also observed in ALI system cultures derived from nonirradiated control mice like for the human PBECs (Figure S3C).
FIGURE 4.
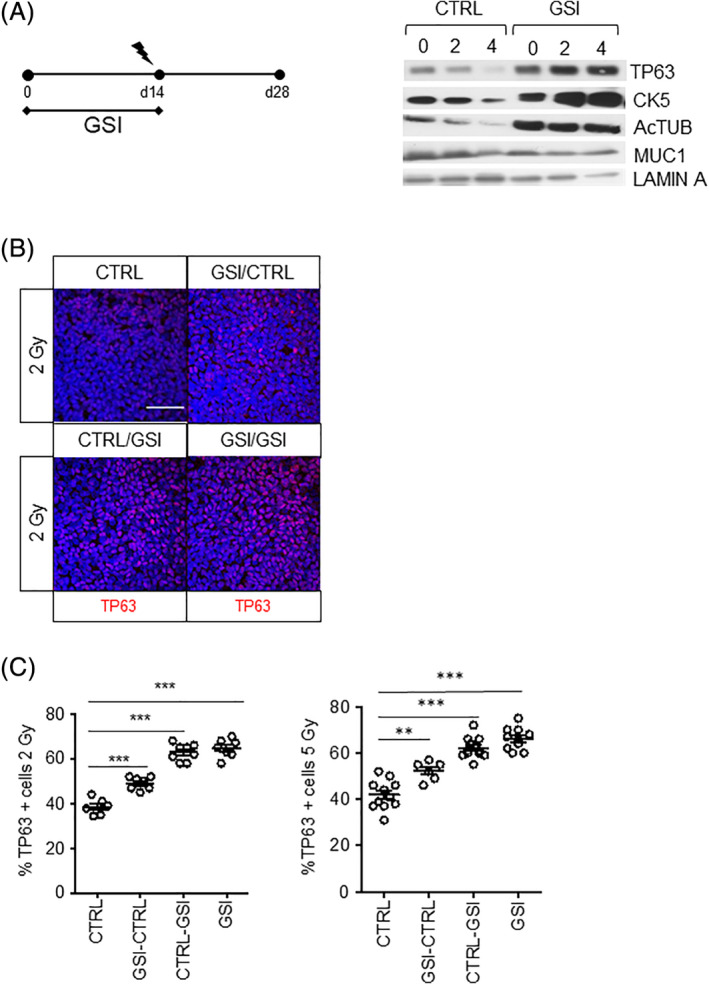
NOTCH inhibition increases the proliferation of irradiated TP63+ cells. A, PBECs were grown in ALI in the presence of vehicle or GSI for 14 days prior to 2‐ or 4‐Gy irradiation at day 14. Cell collection was at 28 days after airlift. No GSI or vehicle was added after irradiation. Protein expression of epithelial lung cell markers TP63, Ac‐TUB, and MUC1. Lamin A was used as loading control. B, Ac‐TUB showed increased percentage of TP63 when NOTCH was inhibited in primary murine stem cells after in vivo irradiation. This effect was time‐dependent. Longer incubation with NOTCH inhibitors resulted in increased percentage of TP63+ cells. C, Quantification of TP63+ cells in irradiated mice 2 and 5 Gy. N = 3 biological repeats per donor. Statistical analysis was one‐way ANOVA: **P < .001; ***P < .0001. All scale bars are 1 μm. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; ANOVA, analysis of variance; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
3.5. NOTCH inhibition increases the percentage of living cells and reduces cell death
We next asked if the rescue of radiation‐induced loss of TP63 basal stem cells by NOTCH inhibition could be explained by reduced cell death. We, therefore, measured the percentage of living cells 3 and 7 days after irradiation at day 21 in culture when all cells are postmitotic (Figure 5A). A dose‐dependent decrease of living cells was observed upon irradiation which increased from three (from 36% at 0 Gy to 28% at 4 Gy, P < .001) to 7 days post‐RT (from 40% at 0 Gy to 20% at 4 Gy, P < .001). No significant difference was observed between nonirradiated samples whereas a significant increase in living cells was observed when NOTCH inhibition was combined with irradiation at both 3‐day and 7‐day postirradiations (from 24% to 32% at 2 Gy and from 20% to 26% at 4 Gy, P < .001) (Figure 5A). To address whether this increase in the total number of living cells was linked to reduced apoptotic cell death, we analyzed expression and activation of the proapoptotic proteins cleaved CASPASE‐3 by Western blot 24 hours and 3 days after irradiation. We found that NOTCH inhibition suppressed both basal‐ and radiation‐induced cleaved CASPASE‐3 (Figure 5B) and inhibited the induction of tumor suppressor protein TP53 and its downstream target cyclin‐dependent kinase p21CIP (Figure 5C). Comparable results were obtained in two other independent donors (Figure S4A). To investigate whether the decrease in proapoptotic signaling translated into increased survival and self‐renewal, ALI cultures were treated with GSI, irradiated at day 21 and reseeded thereafter in 2D. Cell growth was measured upon repeated passaging. Consistently, 99% of cells are positive for TP63 and negative for differentiation markers (Ac‐TUB and MUC5AC). We observed a significant increase of living cells in the first and second reseeding when NOTCH was inhibited and combined with irradiation (2‐4 Gy) compared with control cells (P < .05) (Figure 5D). After passage 3, however, there was a significant reduction in self‐renewal capacity and significant differences between control and GSI‐irradiated cultures were lost. These results suggest that irradiated TP63+ cells are capable of increased self‐renewal upon NOTCH inhibition.
FIGURE 5.
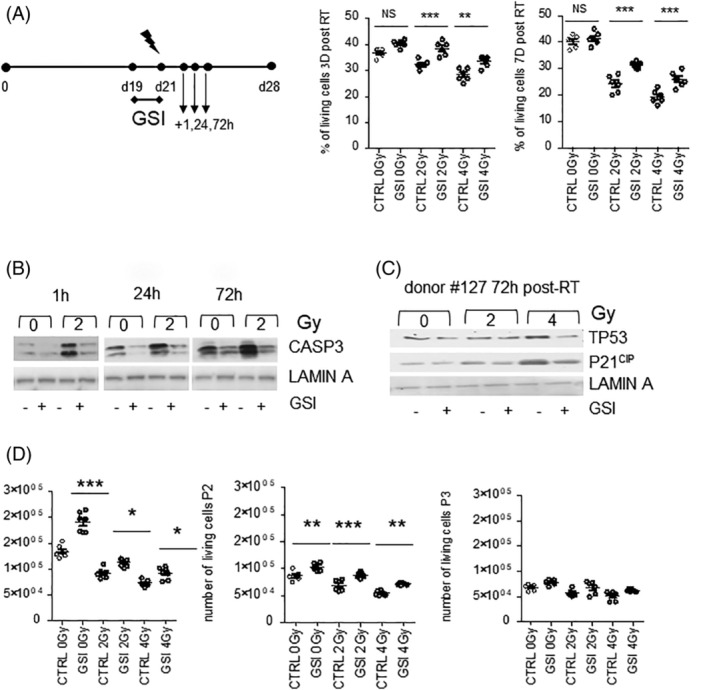
NOTCH inhibition increases the percentage of living cells and reduces Caspase 3, TP53, P21CIP levels. A, PBECs were grown for 28 days in ALI and treated with dimethyl sulfoxide (DMSO) and GSI, from day 19 to 21, for 48 hours. Cells were irradiated with 2 and 4 Gy at 21 days. The percentage of living cells was measured after 3 and 7 days. B, Caspase 3 Western blot 1, 24, and 72 hours after RT. C, TP53 and P21CIP blot 72 hours post‐RT. D, 50 000 cells at the 21st day after airlift were harvested and seeded for three passages and the number of living cells was measured. N = 3 biological repeats per donor. Statistics for one‐way ANOVA: *P < .05; **P < .001; ***P < .0001. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; ANOVA, analysis of variance; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
3.6. NOTCH inhibition enhances the DDR and decreases double‐stranded DNA breaks
Radiation triggers activation of the DDR which can provoke a TP53‐dependent growth arrest and cell death. Activation of the DDR through phosphorylation of ATM triggers the phosphorylation of 53BP1, which is recruited to the double‐strand breaks in the DNA. We observed that irradiation of PBEC cultures after 21 days in cultures led to an immediate induction of phosphorylated ATM and activated checkpoint signaling. This increase in pATM/ATR was further increased when cells were cultured in the presence of GSI (Figure 6A). Consistently, the activation of downstream kinases pCHK2 and pCHK1 was observed at 24 hours and continued up to 3 days after irradiation in addition to the GSI induced rescue of TP63 expression (Figure 6A). We also noted increased expression of the homologous recombination (HR) protein RAD51 showing that ongoing double‐stranded DNA break repair is enhanced upon NOTCH inhibition (Figure 6A). We next determined the induction of 53BP1 at two different time points after irradiation, 24 hours and 3 days after irradiation. Confocal imaging of 53BP1 foci in the nucleus demonstrated that upon irradiation, there was a dose‐dependent increase in nuclear 53BP1 foci in TP63+ progenitor cells and reduced 53BP1 when irradiation was combined with NOTCH inhibition (Figure 6B) at 24 hours and maintained at least 3 days after irradiation (Figure 6C). The accumulated observations suggest that irradiated basal stem cells in a multilayer epithelium are better protected against the damaging effects of radiation in part by enhanced activation of the DDR and DNA repair.
FIGURE 6.
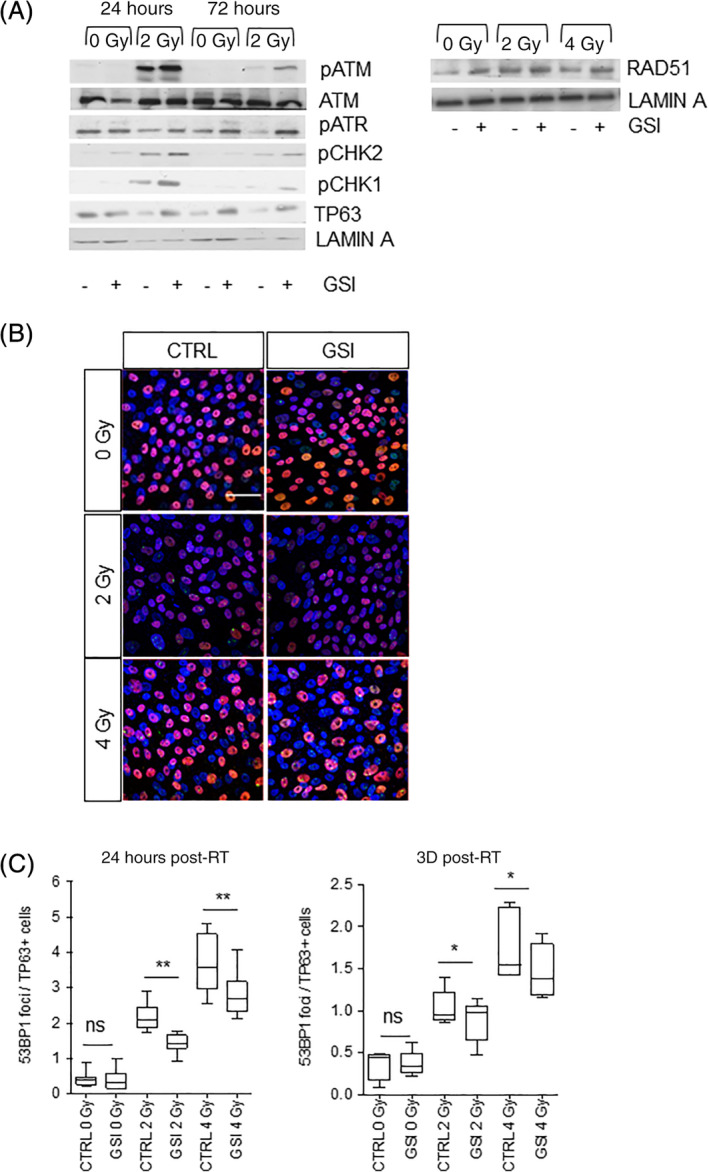
NOTCH inhibition decreases the 53BP1 foci in irradiated samples. A, Western blotting for ATM, pATM, pATR, pCHK1, pCHK2, and TP63 24 and 72 hours after RT in PBECs treated with and without GSI from day 19 to day 21, 48 hours prior to irradiation. Lamin A was used as loading control. B, 53BP1‐TP63 costaining in 0, 2, and 4 Gy samples treated with and without GSI, 2 days prior to irradiation. C, Quantification of 53BP1 staining in TP63+ cells, 24 hours and 3 days after RT in presence or absence of NOTCH inhibition at day 19 to 21, 48 hours prior RT (2‐4 Gy). N = 3 biological repeats per donor. Statistics for one‐way ANOVA: *P < .05; **P < .001. All scale bars are 1 μm. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; ANOVA, analysis of variance; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
3.7. NOTCH inhibition improves the integrity of irradiated lung epithelium
To investigate if the increased number of TP63+ basal stem cells upon irradiation when NOTCH signaling is blocked also improved protection of the epithelium cells from the radiation damage, we measured the transfer of dextran‐FITC through the ALI epithelium as a measure of epithelial barrier integrity. Dextran‐FITC transfer from the top to the bottom of the culture was enhanced in irradiated cell cultures indicating epithelial leakage. However, when irradiated cultures were pretreated with NOTCH inhibitors, the transfer of dextran‐FITC was significantly reduced (Figure 7A). These results suggest that GSI‐treated cultures may result in less epithelium damage when compared with irradiated cultures, resulting in less dextran transfer. We performed Western blots for ZO1, CD2AP, and AFADIN in three different donors to evaluate the effect of NOTCH inhibition on the expression of tight junction markers at 0‐, 2‐, and 4 Gy irradiation. We observed upregulation of ZO1, CD2AP, AFADIN when NOTCH was inhibited which sustained when combined with irradiation. Taken together these results show that NOTCH inhibition can prevent the loss of basal stem cells after irradiation and that, under these conditions, the epithelium is more effectively repaired through repopulation of the damaged areas, covering the denuded areas.
FIGURE 7.
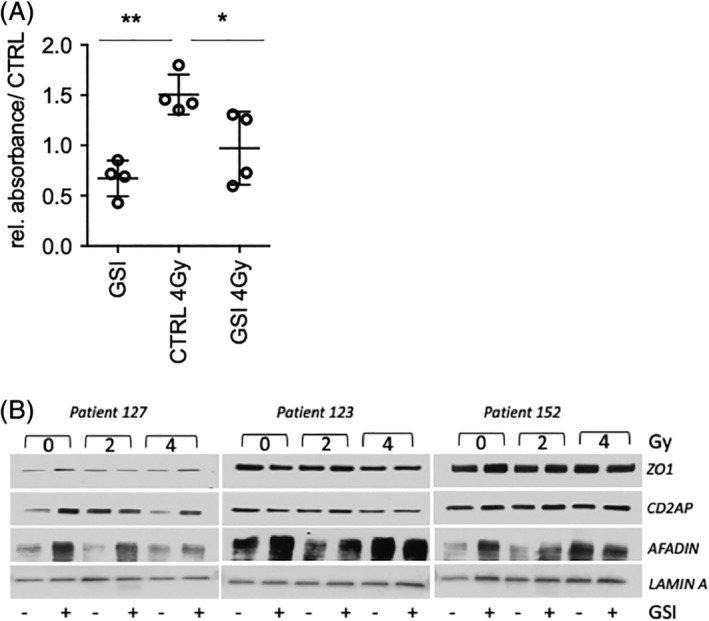
NOTCH inhibition improves the epithelial integrity in irradiated samples Quantification graph of dextran absorbance in samples treated with and without GSI, 2 days prior RT performed at day 21. N = 3 biological repeats per donor. Statistics for one‐way ANOVA: *P < .05; **P < .001. Western blot for ZO1, CD2AP, and AFADIN in three donors. Upregulation of ZO1, CD2AP, and AFADIN in the samples where NOTCH was inhibited at 0, 2, and 4 Gy. Ac‐TUB, Acetylated Tubulin; ALI, air‐liquid interface; ANOVA, analysis of variance; GSI, γ‐secretase inhibitor; PBECs, primary human bronchial epithelial cells
4. DISCUSSION
In the present study, we used patient‐derived primary lung epithelial cultures to study the role of NOTCH signaling in the response of stem cells and differentiated bronchial epithelium to irradiation. Our results show that physiological NOTCH signaling in PBECs and murine bronchial epithelial cells attenuates the DDR and the proliferative capacity and viability of irradiated lung progenitors in an ALI culture system of primary cells. NOTCH inhibition promotes the survival of irradiated basal stem cells, retains their capacity to differentiate, and improves epithelial barrier integrity after irradiation.
The importance of investigating the effects of NOTCH inhibitors on lung model systems arises from the need to develop approaches to reduce adverse effects in lung cancer patients treated with radiotherapy as part of their first‐line treatment. Radiation‐related toxicity is also a strong dose‐limiting factor in terms of long‐term survival and quality of life in patients receiving thoracic irradiation as part of their cancer treatment. 2 Most lung cancer patients often suffer from comorbidities such as chronic obstructive pulmonary disease (COPD), which further reduces their tolerance to combination treatment and probability for tumor control. 30 Treatments reducing or preventing stem cell loss or approaches that allow repair of the damaged epithelium post‐treatment may reduce these radiation side effects.
NOTCH is a master regulator of cell fate in embryonic, fetal, and adult tissues controlling proliferation cell death and differentiation. 31 , 32 The NOTCH pathway is essential at multiple stages of development and controls stem cell numbers and activity in the context of age‐related tissue regeneration and repair. 33 Finn et al showed that the Delta‐like 1 (DLK1)‐dependent temporal modulation of the NOTCH signaling pathway is necessary for the alveolar epithelium after Pseudomonas aeruginosa induced injury. Thus, DLK1 and NOTCH signaling components represent potential therapeutic targets for accelerating repair of the alveolar epithelium and resolving acute lung injury. 34 Inactivation of NOTCH in the intestinal epithelium of mice results in the loss of the proliferative crypt compartment through inhibition of Bmi1. NOTCH and Bmi‐1 contribute to the proliferation and self‐renewal of local progenitor cells by regulating the cell cycle progression via the p16INK4a/p19ARF pathway. 35 Furthermore, there is mounting evidence that the NOTCH pathway is deregulated in various types of lung diseases including lung cancer 36 , 37 and influences treatment response to systemic treatments 38 as well as radiotherapy 39 making it an attractive therapeutic target in lung cancer. High NOTCH activity in NSCLC is associated with worse disease‐free survival in patients and increased tumor cell proliferation, greater hypoxic fraction, and radiation resistance in vivo models. 23 NOTCH drives tumorigenesis by cell cycle progression and inhibiting apoptosis. NOTCH1 and NOTCH3 induce cyclin D1 expression, mediated by JAGGED1 binding to promote proliferation. 40
In our study, we found that the small‐molecule inhibitor targeting NOTCH/γ‐secretase (DBZ) induced cell proliferation and survival of bronchial stem cells and ciliary cells while reducing the number of secretory mucous‐producing cells. We observed a differential radiation sensitivity of the main cell types in the ALI system. The stem cells were the most sensitive cell types, while the mucous cells seemed to increase upon irradiation and the ciliated cells remained unaffected.
This is interesting as in the intestine NOTCH suppresses goblet cell differentiation at the expense of absorptive cells, 31 a phenotype dependent of KLF4, which induces goblet cell differentiation. 41 , 42 In line with our study, others have observed a similar role of NOTCH in regulating stem cell function and hierarchy. Specifically, genetic inhibition of NOTCH increased mammary stem cell activity, leading to anomalies in the mammary tissue such as the formation of aberrant end buds. This suggests a role of endogenous NOTCH signaling in restricting mammary stem cell expansion. 43
We previously showed that NOTCH signaling is actively expressed in human and murine basal stem cells and when combined with irradiation NOTCH inhibition provokes a G1‐S phase cell cycle arrest. Furthermore, NOTCH inhibition in irradiated lung stem cells results into a greater activation of the DNA damage checkpoint kinases pATM and pCHK2 and in an increased level of residual 53BP1 foci in irradiated lung basal stem cells, reducing their self‐renewal capacity. 29 In our previous study, however, we did not investigate the combined effects of irradiation and NOTCH inhibition in a more complex polarized and stratified bronchial epithelium where primary basal stem cells reside, renew, and give rise to differentiated functional daughter cells. We found that in the postmitotic bronchial epithelium, NOTCH signaling promotes radiation‐induced apoptotic cell death of lung stem cells resulting in a decrease in epithelial barrier integrity. Blockade of NOTCH signaling can cause irradiated stem cells to reenter the cell cycle, activate the DDR pathway, and suppress the proapoptotic pathways to promote their survival and enable their differentiation. RAD51 recruitment suggests that NOTCH blockade in irradiated lung epithelial cells provokes faster DNA repair. This is the first study to show that inhibiting NOTCH signaling enhances lung stem cell repair. Recent studies have shown that blocking NOTCH signaling in basal PBECs can prolong their viability to improve in vitro expansion and ALI culture; however, these expanded basal cell populations were increasingly impaired in their ability to promote ciliary differentiation. 44
Prior studies have shown that expansion of progenitor/stem cell polls in tissues can play an important role as a mechanism to prevent damage by radiation. 45 Although the lung is a relatively quiescent organ, it shows great ability to proliferate and to repair after exposure to DNA damaging agents, demonstrating the existence of a highly active lung stem cell population. 46 After injury, progenitor/stem cells are capable of restoring normal tissue architecture by inducing the generation of the different cell types within a tissue. NOTCH pathway inhibition disrupted the normal pattern of lineage segregation upon injury. 47 After SO2‐mediated airway epithelial injury, the secretory cell lineage is stimulated via NOTCH signaling, which induces progenitor cell self‐renewal and differentiation. 48 , 49 Other studies have seen that ROS trigger the NOTCH signaling pathway by activating NRF2. Therefore, ROS and NOTCH together mediate progenitor cell self‐renewal through regulating their proliferative capacity. 50 We observed that the loss of TP63 basal stem cells induced by a single dose of radiation was counteracted by NOTCH inhibition. Furthermore, the increased proliferating cells derived from the combination of NOTCH inhibitors and irradiation retain stemness capability after two‐cell passaging. Radiotherapy mostly targets dividing cells, such as the progenitor/basal cells along the upper airway epithelium. To our knowledge, this is the first study to test the consequences of administering NOTCH inhibition therapy on irradiated normal lung tissue. As regeneration upon irradiation ensues, we show that PBECs following anti‐NOTCH treatment produce massive amounts of progenitor cells, whereas the other cell types remain more stable. Thus, we suggest that basal cells contribute to regeneration and repair of irradiated normal lung tissue via regulation of the NOTCH pathway.
Human lungs constantly deal with insults from inhaled pollutants that cause DNA damage. Therefore, lung stem cells need to efficiently respond to exogenous DNA damage for the proper repair of the epithelial airway after injury. Stem cells require the full DDR machinery including nucleotide excision repair, base excision repair, HR, and NHEJ to repair these lesions. 51 In response to ionizing radiation, lung basal stem cells repair induced DNA DSBs by activating the error‐prone NHEJ pathway, allowing basal cells to proliferate after injury. 4 Weeden et al found that NHEJ activation was strongly induced after radiation exposure; however, DSBs were resolved 24 hours postirradiation. Thereby, residual 53BP1 foci were quantified to analyze the DNA damage induced by radiation at later time points. Our 53BP1 labeling demonstrated that DBZ‐treated PBECs are more proficient than control cells in repairing radiation‐induced DSBs although we provided no direct evidence for this. The association between 53BP1 activation with DNA repair suggested a model whereby DSB‐repair efficiency is normally limited by NOTCH activity in progenitor cells. In contradiction with this model, we observed an increased amount of phosphorylated ATM, which mediates 53BP1 activation, in cells treated with DBZ. Recent investigations have found that NOTCH suppresses the activation of the DDR by blocking the activation/phosphorylation of ATM. Furthermore, NOTCH blockade promoted ATM‐dependent apoptosis in irradiated leukemia cells that rely on the NOTCH signaling for their survival. 28 Further investigations will be required to unravel the specific role of NOTCH on regulating the DDR responsible for the protective effects of adding GSI to irradiated PBECs. For example, our study did not address which NOTCH paralog is driving these cell fate decisions and protects against radiation‐induced cell loss, since NOTCH inhibitor/GSI, including DBZ, efficiently target all four NOTCH receptors. Current models suggest that NOTCH1 and NOTCH2 signaling promote goblet cell fate while NOTCH3 is more involved in basal cell renewal. 19
More recently multipotent stem cells have been identified at the bronchiolar alveolar junction stem cells (BASCs) that are involved in the response to epithelial injury. 52 , 53 It is not known if these BASCs have the ability to repair radiation‐induced damage of the bronchial and pulmonary epithelium and whether the primary human TP63+ cells identified here have similar properties.
In conclusion, our study demonstrates a key role of NOTCH signaling in determining the fate of human lung stem cells in a cultured polarized epithelium in response to radiotherapy. NOTCH inhibition promotes proliferation, stem cell renewal, and differentiation by suppressing cell death, while increasing the DDR improves epithelial barrier integrity. While our bronchial epithelial cell model is a simplified model of the human bronchus, our results suggest that temporal NOTCH inhibition would be favorable to the repair of the irradiated epithelium. Further research is needed if NOTCH targeting could be exploited on opposite sides of the same coin, namely, to inhibit anticancer activity and promote treatment resistance while protecting normal tissue. As such, NOTCH targeting in lung cancer could enhance the therapeutic benefit of anticancer treatments due to its dual activity as an antitumor agent and its protective effect on normal tissue.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
L.G.: conceived the ideas, performed all the experiments, analyzed the data, wrote, edited, and approved the manuscript; E.M.R., A.G., L.D.: provided technical help, revised, and approved the manuscript; C.W.: provided animal work help, revised, and approved the manuscript; A.W.D.B.: provided help with the DNA damage experiments, revised, and approved the manuscript; M.V.: conceived the ideas, analyzed the data, wrote, edited, and approved the manuscript.
Supporting information
Data S1: Materials and methods: NOTCH blockade and irradiation; Western blotting; whole mount immunostaining and confocal microscopy; click‐it EdU Alexa Fluor 555 Imaging kit; image analysis; trypan blue staining; reseeding of PBECs; dextran assay; and statistical analysis.
Figure S1 NOTCH inhibition affects cell fate specification in human PBECs Western blot for TP63, CK5, Ac‐TUB, MUC1 of PBECs seeded in ALI system for 28 days (donor 119 and 106). Lamin A was used as loading control.
Figure S2 NOTCH blockade causes increase of TP63+ basal and ciliary expression, A, Western blot for TP63, CK5, Ac‐TUB, MUC1 in samples treated with and without GSI at day 22, 24 and 28 after the airlift (Donor # 119‐106). B) GSI treatment significantly increases the absolute number of cells. Statistics for one‐way ANOVA: *P < .05; ***P < .0001.
Figure S3 NOTCH blockade causes increase of TP63+ basal and ciliary expression in murine cultures, A, Representative scheme of the treatment plan. The epithelial cells were peeled off from the trachea of the (0‐2‐5 Gy) irradiated mice and seeded in air‐liquid interface system. The cells have been treated with NOTCH inhibitors at different time points: 2 days prior airlift, after airlift until day 14th and before and after airlift. B, Confocal staining of differentiated cultures treated with or without GSI respectively before airlift, after airlift, before and after airlift. Immunofluorescent staining for TP63 and Ac‐TUB of murine ALI system at 15 days after airlift and relative quantification, C. Statistics for one‐way ANOVA: *P < .05; ***P < .0001. All scale bars are 1um
Figure S4 NOTCH inhibition decreases TP53, P21CIP protein levels. Western Blot of TP53, P21CIP in 72 hours post irradiation in human ALI treated with and without GSI (1uM) in donor 152 and 123.
ACKNOWLEDGMENTS
We thank Nico Kloosterboer and Mieke Dentener of the Primary Lung Culture facility (MUMC+) for donor cells, advice, and protocols. Funding was received from the H2020 research and innovation program Marie Curie Sklodowska ITN (grant # 642623).
Giuranno L, Roig EM, Wansleeben C, et al. NOTCH inhibition promotes bronchial stem cell renewal and epithelial barrier integrity after irradiation. STEM CELLS Transl Med. 2020;9:799–812. 10.1002/sctm.19-0278
Funding information Marie Curie Sklodowska ITN, Grant/Award Numbers: 642623, H2020
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Prasanna PG, Stone HB, Wong RS, et al. Normal tissue protection for improving radiotherapy: where are the gaps? Transl Cancer Res. 2012;1(1):35‐48. [PMC free article] [PubMed] [Google Scholar]
- 2. Verma V, Simone CB 2nd, Werner‐Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally‐advanced non‐small cell lung cancer. Cancers. 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farin AM, Manzo ND, Kirsch DG, Stripp BR. Low‐ and high‐LET radiation drives clonal expansion of lung progenitor cells in vivo. Radiat Res. 2015;183(1):124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weeden CE, Chen Y, Ma SB, et al. Lung basal stem cells rapidly repair DNA damage using the error‐prone nonhomologous end‐joining pathway. PLoS Biol. 2017;15(1):e2000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BLM. IL‐6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA. 2014;111(35):E3641‐E3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20(8):822‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hogan BL, Barkauskas CE, Chapman HA, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rawlins EL, Ostrowski LE, Randell SH, Hogan BLM. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104(2):410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106(31):12771‐12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61(5):448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BLM. Notch‐dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8(6):639‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Tetering G, Vooijs M. Proteolytic cleavage of Notch: "HIT and RUN". Curr Mol Med. 2011;11(4):255‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsao PN, Chen F, Izvolsky KI, et al. Gamma‐secretase activation of Notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283(43):29532‐29544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lafkas D, Shelton A, Chiu C, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528(7580):127‐131. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Z, Zhou L, Yang X, et al. Notch‐RBP‐J‐independent marginal zone B cell development in IgH transgenic mice with VH derived from a natural polyreactive antibody. PloS One. 2012;7(6):e38894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139(23):4365‐4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsao PN, Matsuoka C, Wei SC, et al. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc Natl Acad Sci USA. 2016;113(29):8242‐8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori M, Mahoney JE, Stupnikov MR, et al. Notch3‐jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142(2):258‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aster JC, Pear WS, Blacklow SC. The varied roles of Notch in cancer. Annu Rev Pathol. 2017;12:245‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hassan KA, Wang L, Korkaya H, et al. Notch pathway activity identifies cells with cancer stem cell‐like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19(8):1972‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin MM, Ye Y‐Z, Qian Z‐D, Zhang Y‐B. Notch signaling molecules as prognostic biomarkers for non‐small cell lung cancer. Oncol Lett. 2015;10(5):3252‐3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Theys J, Yahyanejad S, Habets R, et al. High NOTCH activity induces radiation resistance in non small cell lung cancer. Radiother Oncol. 2013;108(3):440‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hensley CT, Faubert B, Yuan Q, et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164(4):681‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450(7171):893‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizugaki H, Sakakibara‐Konishi J, Ikezawa Y, et al. Gamma‐secretase inhibitor enhances antitumour effect of radiation in Notch‐expressing lung cancer. Br J Cancer. 2012;106(12):1953‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adamowicz M, Vermezovic J, d'Adda di Fagagna F. NOTCH1 inhibits activation of ATM by impairing the formation of an ATM‐FOXO3a‐KAT5/Tip60 complex. Cell Rep. 2016;16(8):2068‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vermezovic J, Adamowicz M, Santarpia L, et al. Notch is a direct negative regulator of the DNA‐damage response. Nat Struct Mol Biol. 2015;22(5):417‐424. [DOI] [PubMed] [Google Scholar]
- 29. Giuranno L, Wansleeben C, Iannone R, et al. NOTCH signaling promotes survival of irradiated basal airway stem cells. Am J Physiol Lung Cell Mol Physiol. 2019;317:L414‐L423. [DOI] [PubMed] [Google Scholar]
- 30. Raviv S, Hawkins KA, DeCamp MM Jr, Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med. 2011;183(9):1138‐1146. [DOI] [PubMed] [Google Scholar]
- 31. Vooijs M, Ong CT, Hadland B, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP‐CRE. Development. 2007;134(3):535‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9(4):377‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bigas A, Espinosa L. The multiple usages of Notch signaling in development, cell differentiation and cancer. Curr Opin Cell Biol. 2018;55:1‐7. [DOI] [PubMed] [Google Scholar]
- 34. Finn J, Sottoriva K, Pajcini KV, et al. Dlk1‐mediated temporal regulation of Notch signaling is required for differentiation of alveolar type II to type I cells during repair. Cell Rep. 2019;26(11):2942‐2954. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez‐Arribillaga E, Rodilla V, Pellegrinet L, et al. Bmi1 regulates murine intestinal stem cell proliferation and self‐renewal downstream of Notch. Development. 2015;142(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 36. Westhoff B, Colaluca IN, D'Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci USA. 2009;106(52):22293‐22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol. 2012;727:89‐98. [DOI] [PubMed] [Google Scholar]
- 38. Sosa Iglesias V, Giuranno L, Dubois LJ, Theys J, Vooijs M. Drug resistance in non‐small cell lung cancer: a potential for NOTCH targeting? Front Oncol. 2018;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yahyanejad S, Theys J, Vooijs M. Targeting Notch to overcome radiation resistance. Oncotarget. 2016;7(7):7610‐7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ling H, Sylvestre JR, Jolicoeur P. Notch1‐induced mammary tumor development is cyclin D1‐dependent and correlates with expansion of pre‐malignant multipotent duct‐limited progenitors. Oncogene. 2010;29(32):4543‐4554. [DOI] [PubMed] [Google Scholar]
- 41. Real PJ, Ferrando AA. NOTCH inhibition and glucocorticoid therapy in T‐cell acute lymphoblastic leukemia. Leukemia. 2009;23(8):1374‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng H, Pritchard DM, Yang X, et al. KLF4 gene expression is inhibited by the Notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G490‐G498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bouras T, Pal B, Vaillant F, et al. Notch signaling regulates mammary stem cell function and luminal cell‐fate commitment. Cell Stem Cell. 2008;3(4):429‐441. [DOI] [PubMed] [Google Scholar]
- 44. Eenjes E, Mertens TCJ, Buscop‐van Kempen MJ, et al. A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture. Sci Rep. 2018;8(1):7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26(10):2595‐2601. [DOI] [PubMed] [Google Scholar]
- 46. Weeden CE, Asselin‐Labat ML. Mechanisms of DNA damage repair in adult stem cells and implications for cancer formation. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):89‐101. [DOI] [PubMed] [Google Scholar]
- 47. Pardo‐Saganta A, Law BM, Tata PR, et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16(2):184‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xing Y, Li A, Borok Z, Li C, Minoo P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem Cells. 2012;30(5):946‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108(52):E1475‐E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paul MK, Bisht B, Darmawan DO, et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2‐dependent Notch signaling. Cell Stem Cell. 2014;15(2):199‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hang B. Formation and repair of tobacco carcinogen‐derived bulky DNA adducts. J Nucleic Acids. 2010;2010:709521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Q, Liu K, Cui G, et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar‐duct junction. Nat Genet. 2019;51(4):728‐738. [DOI] [PubMed] [Google Scholar]
- 53. Salwig I, Spitznagel B, Vazquez‐Armendariz AI, et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Materials and methods: NOTCH blockade and irradiation; Western blotting; whole mount immunostaining and confocal microscopy; click‐it EdU Alexa Fluor 555 Imaging kit; image analysis; trypan blue staining; reseeding of PBECs; dextran assay; and statistical analysis.
Figure S1 NOTCH inhibition affects cell fate specification in human PBECs Western blot for TP63, CK5, Ac‐TUB, MUC1 of PBECs seeded in ALI system for 28 days (donor 119 and 106). Lamin A was used as loading control.
Figure S2 NOTCH blockade causes increase of TP63+ basal and ciliary expression, A, Western blot for TP63, CK5, Ac‐TUB, MUC1 in samples treated with and without GSI at day 22, 24 and 28 after the airlift (Donor # 119‐106). B) GSI treatment significantly increases the absolute number of cells. Statistics for one‐way ANOVA: *P < .05; ***P < .0001.
Figure S3 NOTCH blockade causes increase of TP63+ basal and ciliary expression in murine cultures, A, Representative scheme of the treatment plan. The epithelial cells were peeled off from the trachea of the (0‐2‐5 Gy) irradiated mice and seeded in air‐liquid interface system. The cells have been treated with NOTCH inhibitors at different time points: 2 days prior airlift, after airlift until day 14th and before and after airlift. B, Confocal staining of differentiated cultures treated with or without GSI respectively before airlift, after airlift, before and after airlift. Immunofluorescent staining for TP63 and Ac‐TUB of murine ALI system at 15 days after airlift and relative quantification, C. Statistics for one‐way ANOVA: *P < .05; ***P < .0001. All scale bars are 1um
Figure S4 NOTCH inhibition decreases TP53, P21CIP protein levels. Western Blot of TP53, P21CIP in 72 hours post irradiation in human ALI treated with and without GSI (1uM) in donor 152 and 123.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


