Abstract
BACKGROUND
Focal seizures in temporal lobe epilepsy (TLE) are associated with widespread brain network perturbations and neurocognitive problems.
OBJECTIVE
To determine whether brainstem connectivity disturbances improve with successful epilepsy surgery, as recent work has demonstrated decreased brainstem connectivity in TLE that is related to disease severity and neurocognitive profile.
METHODS
We evaluated 15 adult TLE patients before and after (>1 yr; mean, 3.4 yr) surgery, and 15 matched control subjects using magnetic resonance imaging to measure functional and structural connectivity of ascending reticular activating system (ARAS) structures, including cuneiform/subcuneiform nuclei (CSC), pedunculopontine nucleus (PPN), and ventral tegmental area (VTA).
RESULTS
TLE patients who achieved long-term postoperative seizure freedom (10 of 15) demonstrated increases in functional connectivity between ARAS structures and fronto-parietal-insular neocortex compared to preoperative baseline (P = .01, Kruskal–Wallis), with postoperative connectivity patterns resembling controls’ connectivity. No functional connectivity changes were detected in 5 patients with persistent seizures after surgery (P = .9, Kruskal–Wallis). Among seizure-free postoperative patients, larger increases in CSC, PPN, and VTA functional connectivity were observed in individuals with more frequent seizures before surgery (P < .05 for each, Spearman's rho). Larger postoperative increases in PPN functional connectivity were seen in patients with lower baseline verbal IQ (P = .03, Spearman's rho) or verbal memory (P = .04, Mann–Whitney U). No changes in ARAS structural connectivity were detected after successful surgery.
CONCLUSION
ARAS functional connectivity disturbances are present in TLE but may recover after successful epilepsy surgery. Larger increases in postoperative connectivity may be seen in individuals with more severe disease at baseline.
Keywords: Brainstem, Epilepsy surgery, Functional connectivity, Postoperative, Temporal lobe epilepsy
ABBREVIATIONS
- ALFF
amplitude low-frequency fluctuation
- ARAS
ascending reticular activating system
- BOLD
blood oxygenation level-dependent
- CSC
cuneiform/subcuneiform nuclei
- DTI
diffusion tensor imaging
- EEG
electroencephalography
- FICS
focal impaired consciousness seizure
- fMRI
functional MRI
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PPN
pedunculopontine nucleus
- SD
standard deviation
- SUDEP
sudden unexpected death in epilepsy
- TE
echo time
- TLE
temporal lobe epilepsy
- TR
repetition time
- VTA
ventral tegmental area
Temporal lobe epilepsy (TLE) is a debilitating disorder, and seizures are often medication resistant.1-4 Whereas seizures typically begin in the hippocampus, TLE engenders widespread brain problems that cannot be alone explained by abnormalities in this focal region, including broad neurocognitive deficits, gray matter atrophy, and connectivity pertubations.5-8 We hypothesize that recurrent seizures may cause abnormalities in deep brain regions important for arousal, leading to reduced connectivity between these structures and neocortex, which may contribute to neuropsychological problems. Recent work reported reductions in connectivity between brainstem ascending reticular activating system (ARAS) nuclei and neocortex in TLE that were related to disease severity and neurocognitive deficits.9,10 It remains unknown how ARAS connectivity might be influenced by epilepsy treatment.
In TLE, surgery leads to seizure freedom in approximately two thirds of patients.3,11 Postoperative seizure freedom often promotes improved quality of life,12 improved neurocognition,13,14 and decreased mortality risk.15 Are these benefits accompanied by connectivity reorganization? Few have evaluated postoperative connectivity in TLE,16,17 and, to our knowledge, connectivity of arousal structures has not been evaluated. Here, we examine brainstem connectivity in 15 TLE patients before and after surgery, alongside 15 controls. We focus on networks previously found to be most perturbed prior to surgery,9,10 including connections between ARAS structures and fronto-parietal-insular neocortex. In postoperative patients, we analyze brainstem ARAS connectivity changes before and after surgery to determine the effects of epilepsy surgery on connectivity in TLE.
METHODS
Participants
We evaluated 15 adult TLE patients who presented for epilepsy surgery evaluation from 2012 to 2016, received surgery, and>1-yr postoperative follow-up. TLE diagnosis was established with our institution's standard multidisciplinary process, including neurologists, neurosurgeons, and neuropsychologists. This included analyzing patient history, seizure semiology, magnetic resonance imaging (MRI), video electroencephalography (EEG), positron emission tomography (PET), language/memory localization by functional MRI (fMRI) or Wada, and neuropsychological testing. Then, the multidisciplinary committee diagnosed TLE and recommended proceeding to surgery without intracranial EEG for all 15 patients.18 Postoperative patients were reimaged 33.6 ± 11.6 (mean ± standard deviation [SD]) mo after surgery. The 15 healthy control participants were individually matched to patients by age, sex, and handedness, except for one control who was not handedness matched (Table). Informed consent for this study was obtained from all participants, and all procedures were approved by the Vanderbilt University Institutional Review Board. STROBE checklist was implemented.
TABLE.
Patient and Control Subject Demographics
| Patients | Controls | P value | |
|---|---|---|---|
| Age, yr | 39.4 ± 14.2 | 40.2 ± 13.6 | .85 |
| Gender, female | 8 (53.3) | 8 (53.3) | .99 |
| Handedness, right | 13 (86.6) | 14 (93.3) | .99 |
| Epilepsy duration, yr | 22.6 ± 16.5 | ||
| Seizure frequency, monthly | |||
| FACS | .2 ± .6 | ||
| FICS | 3.0 ± 2.5 | ||
| FBTC | .3 ± 1.1 | ||
| History of FBTC, yes | 7 (46.6) | ||
| Epileptogenic side, right | 10 (66.6) | ||
| MTS on MRI, yes | 12 (80.0) | ||
| Nonlesional on MRI, yes | 3 (20.0) | ||
| Time between preoperative MRI and surgery, mo | 1.87 ± 3.5 | ||
| Range time between preoperative MRI and surgery, mo (min, max) | (0, 14) | ||
| Time between surgery and postoperative MRI, mo | 33.6 ± 11.6 | ||
| Range time between surgery and postoperative scan, mo (min, max) | (14, 52) | ||
| Surgery type | |||
| SAH | 8 (53.3) | ||
| ATL | 5 (33.3) | ||
| Laser | 2 (13.3) | ||
| Operative specimen pathology | |||
| MTS | 11 (73.3) | ||
| Gliosis | 2 (13.3) | ||
| No specimen | 2 (13.3) | ||
| Seizure free after surgery | 10 (66.6) |
ATL, anterior temporal lobectomy; FACS, focal aware conscious seizures; FBTC, focal to bilateral tonic clonic (secondarily generalized) seizures; FICS, focal impaired consciousness seizures; Laser, laser ablation of amygdala and hippocampus; max, maximum; min, minimum; MRI, magnetic resonance imaging; MTS, mesial temporal sclerosis; SAH, selective amygdalohippocampectomy.
For continuous variables, data are mean ± standard deviation, and the statistical test is Mann–Whitney U. For categorical variables, data are N (%), and the statistical test is Chi-square. N = 15 patients and 15 controls.
Imaging
MRI was performed using Philips Achieva 3T scanner (Philips Healthcare, Best, the Netherlands) and 32-channel head coil. Images acquired included (1) 3-dimensional, T1-weighted, whole-brain images for interparticipant normalization and tissue segmentation (gradient echo, repetition time (TR) = 9.1 ms, echo time (TE) = 4.6 ms, 192 shots, flip angle = 8°, and matrix = 256 × 256, 1 × 1 × 1 mm3); (2) 2-dimensional, T1-weighted axial images for functional to structural images coregistration (1 × 1 × 4 mm3); (3) two 10-min, T2*-weighted blood oxygenation level-dependent (BOLD) fMRI images at rest with eyes closed (field of view = 240 mm, TE = 35 ms, TR = 2 s, 34 axial slices, slice thickness = 3.5 mm/0.5 mm gap, and matrix = 80 × 80, 3 × 3 ×4 mm3), 300 volumes acquired during each 10-min acquisition; and (4) diffusion-weighted imaging (b = 1600 s/mm2, 92 directions, and 2.5 × 2.5 × 2.5 mm3). Physiological respiratory and cardiac rates were recorded at 500 Hz.
Connectivity Regions
Regions for connectivity analyses included 3 ARAS structures (cuneiform/subcuneiform nuclei: CSC, pedunculopontine nucleus: PPN, and ventral tegmental area: VTA) from Harvard Ascending Arousal Network Atlas (https://www.martinos.org/resources/aan-atlas)19 and 105 cortical/subcortical regions from Harvard-Oxford Atlas (http://www.fmrib.ox.ac.uk/fsl/). Atlas coregistration details were previously reported.10
Functional Connectivity Analysis
SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and MATLAB 2016a (MathWorks, Natick, Massachusetts) were used to preprocess fMRI. fMRI preprocessing included slice-timing correction, segmentation into white matter, gray matter, and cerebrospinal fluid, and spatial normalization to Montreal Neurological Institute template. Signal fluctuations from movement and physiological noise were minimized using standard protocols across all participants. Motion correction was accomplished by framewise displacement correction, and physiological noise correction was accomplished using a retrospective image correction (RETROICOR)20 algorithm. We used SPM to normalize and coregister fMRI through T1 images to cortical/subcortical atlas. Finally, fMRI images were band-pass filtered between 0.0067 and 0.1 Hz. For each of the 2 fMRI sessions in each participant, functional connectivity was computed between each ARAS region (CSC, PPN, and VTA) and each of 105 cortical/subcortical areas by partial Pearson correlation between each region's time series, with 6 motion time series and mean white matter BOLD signal serving as confounds. Fisher Z scores for each participant were averaged across both fMRI sessions. We evaluated functional connectivity differences in patients before and after surgery between ARAS and frontal, parietal, and insular neocortical regions, which showed large decreases in TLE patients in prior work.9,10 These “frontoparietal” cortical regions (a term used henceforth) included bilateral inferior frontal gyrus pars opercularis and pars triangularis, precentral gyrus, postcentral gyrus, superior parietal lobule, and insula. We visualized ARAS functional connectivity differences between participant groups with CONN toolbox 17 (https://www.nitrc.org/projects/conn/).21 Patients’ functional connectivity image laterality was oriented according to epileptogenic side, and images of matched controls were flipped accordingly.
Amplitude of Low-Frequency Fluctuations Measurements
Functional connectivity measurements between 2 regions do not allow insight into which of the regions (if any) is “driving” connectivity differences. To further understand ARAS and frontoparietal fMRI signal fluctuations, we measured amplitude of low-frequency fluctuations (ALFF) from fMRI in ARAS structures and frontoparietal regions in patients who achieved postoperative seizure freedom alongside matched controls. fMRI preprocessing proceeded as above (low-pass filter, 0.1 Hz). ALFF was calculated by transforming time series BOLD signal to frequency domain using MATLAB Fourier transform function. Then, we measured averaged square root of the absolute value of the transformed signal in 0.01 to 0.08 Hz frequency band22 and divided by mean ALFF of the brain (equation 1).
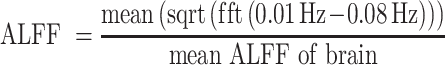 |
(1) |
Structural Connectivity Analysis
Diffusion tensor imaging (DTI) was processed with FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), and estimates of voxel-wise diffusion were measured using BEDPOSTX algorithm Bayesian approach.23 PROBTRACKX, a probabilistic fiber-tracking algorithm with crossing fibers, was used to examine tracts seeded from each of 3 ARAS structures (CSC, PPN, and VTA) to 105 cortical/subcortical targets. PROBTRACKX used 5000 trials from all voxels in each seed region and tracked a streamline until exceeding limits set for number of steps per sample = 2000 steps; step length = 0.5 mm; or curvature = 0.2 or ± 80°. Structural connectivity tractography was corrected for distance from ARAS seed and calculated as the sum of all tracts from all voxels in seed region that went through each target region. In patients achieving postoperative seizure freedom, structural connectivity was compared to preoperative baseline between ARAS structures and (1) frontoparietal regions, defined above, and (2) the 10 areas of greatest structural connectivity decreases in patients vs controls, which included thalamus, caudate, putamen, pallidum, posterior cingulate, precuneus, middle frontal gyrus, frontal pole, supplementary motor area, and precentral gyrus. One patient and matched control were excluded from structural connectivity analysis given absent postoperative patient DTI; both patient and control were included in all other analyses. In example participants, we employed BrainSuite Diffusion Pipeline (http://brainsuite.org)24 to visualize deterministic diffusion tractography seeded from the 3 ARAS regions.
Disease Measures and Neurocognitive Testing
Participant demographics and patient disease measures including seizure frequency, epilepsy duration, history of focal to bilateral tonic-clonic (secondarily generalized) seizures, epileptogenic side, and MRI results were collected from epileptologists’ assessments (Table). Seizure outcomes were defined at the time of postoperative MRI using Engel classification, Engel 1 indicating freedom from disabling seizures, and Engel 2-4 indicating persistent seizures.25
A licensed neuropsychologist administered a standardized battery of neurocognitive examinations to preoperative patients. Given that previous work suggested relationships between ARAS connectivity disturbances and verbal ability in TLE,9,10 we related increases in postoperative connectivity in seizure-free patients to preoperative verbal IQ and memory. Verbal IQ was established using verbal comprehension index, Wechsler Adult Intelligence Scale, Fourth Edition, and verbal memory was established using California Verbal Learning Test, part II, and Wechsler Memory Scale. Verbal memory performance was categorized as average/above average (40-100th percentile) or below average (0-40th percentile), as compared to a standard normative population.
Statistical Analyses
Nonparametric tests were employed for non-normally distributed data determined using the Anderson–Darling test.26 Participant demographics were compared with Mann–Whitney U-test for continuous variables and chi-square for categorical variables. Kruskal–Wallis, with post hoc Dunn when appropriate, was used to compare functional connectivity, ALFF, and structural connectivity between groups: preoperative patients, postoperative patients, and controls. For all groups compared with Kruskal–Wallis, Levene's test was used to ensure homogeneity of variances between groups prior to statistical comparison.27 Spearman's rho was used to compare continuous disease measures and verbal IQ to functional connectivity differences between postoperative and preoperative values. Verbal memory testing performance and categorical disease parameters were dichotomized and compared with Mann–Whitney U-test. Statistical analyses were performed with MATLAB 2016a and SPSS23 (Armonk, New York). Significance was prospectively defined as P < .05 for all tests, and Bonferroni–Holm correction was used for multiple comparisons where indicated.
RESULTS
ARAS Functional Connectivity in TLE Patients Increases After Successful Surgery
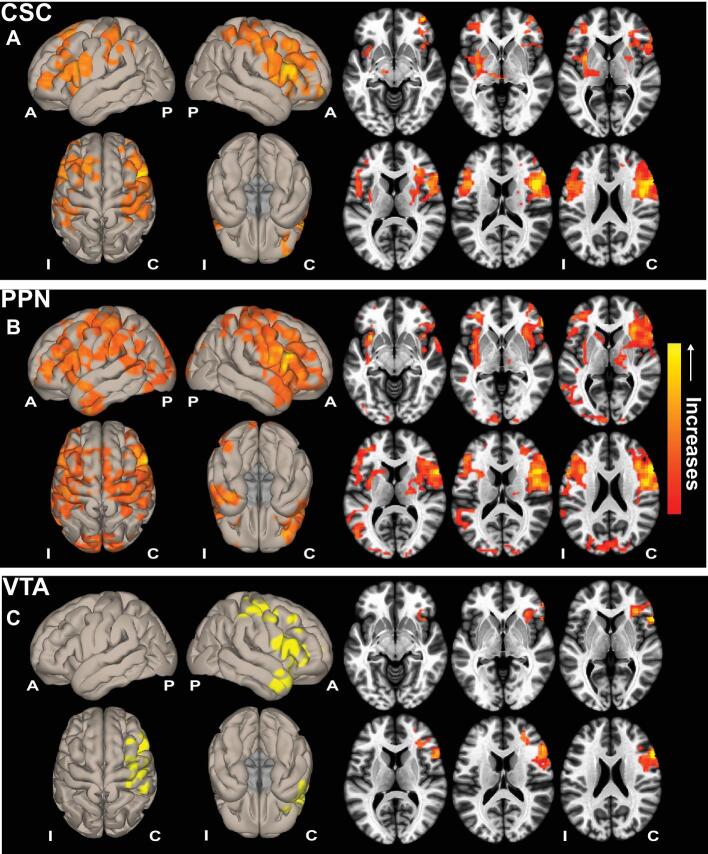
At the time of postoperative scan (>1 yr after surgery), 10 patients had achieved seizure freedom and 5 patients continued to experience seizures. For the 10 seizure-free patients, 7 patients were Engel 1A, and 1 patient each had Engel 1B, 1C, and 1D outcome. As previous work demonstrated functional connectivity decreases between ARAS and fronto-parietal-insular neocortex in TLE9,10; we examined whether this connectivity changes after successful epilepsy surgery. Compared to preoperative baseline, patients who achieved postoperative seizure freedom (10 of 15) demonstrated connectivity increases between ARAS structures and bilateral fronto-parietal-insular cortical regions on voxel-wise, whole-brain connectivity maps (Figure 1). Connectivity increases seeded from PPN appeared most prominent (Figure 1B), followed by CSC (Figure 1A) and VTA (Figure 1C). Next, we specifically analyzed ARAS functional connectivity to frontoparietal cortex.
Figure 1.
ARAS functional connectivity increases in seizure-free TLE patients after surgery. Cortical surface (left) and axial slice (right) views are shown, demonstrating functional connectivity increases in patients with TLE who achieved seizure freedom after surgery, seeded from CSC A, PPN B, and VTA C. Data represent seed-to-voxel functional connectivity (bivariate correlation) maps comparing postoperative vs preoperative fMRI (paired t-test, cluster threshold level P < .05, FDR correction) generated using the CONN toolbox (https://www.nitrc.org/projects/conn/). Positive contrasts are shown, and no connectivity decreases were observed on negative contrasts. Images are oriented across all patients with respect to the epileptogenic side. N = 10 patients before surgery and > 1 yr after surgery. A, anterior; ARAS, ascending reticular activating system; C, contralateral; CSC, cuneiform/subcuneiform nuclei; FDR, false discovery rate; I, ipsilateral; P, posterior; PPN, pedunculopontine nucleus; VTA, ventral tegmental area.
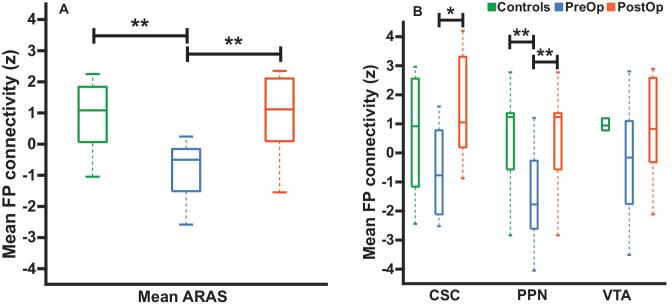
Mean connectivity between ARAS structures and frontoparietal cortex was higher in postoperative patients who achieved seizure freedom, with postoperative connectivity more closely resembling controls (Figure 2A). Postoperative connectivity increases were observed for PPN and CSC, whereas no increase was observed for VTA (Figure 2B). Notably, increase in postoperative ARAS-frontoparietal connectivity was comparable in patients who stopped or reduced preoperative epilepsy medications (n = 6; 1.38 ± 1.78, mean ± SD) vs those continuing similar medication regimens (n = 4; 2.44 ± 1.29; P = .35, Mann–Whitney U-test) at postoperative scan. Motion during fMRI has been shown to introduce an artifact; therefore, we analyzed maximum translation and rotation. In the seizure-free group and controls (n = 10), no difference in maximum translation was detected among preoperative patients (0.77 ±0.36 mm, mean ± SD), postoperative patients (0.65 ± 0.22 mm), or controls (0.53 ± 0.23 mm; P = .32, Kruskal–Wallis). We detected no difference in maximum rotation for preoperative patients (0.74° ± 0.32°), postoperative patients (0.62° ± 0.21°), or controls (0.44° ± 0.23°; P = .06, Kruskal–Wallis). These analyses suggest that postoperative connectivity increases detected were not driven primarily by medication changes or motion artifacts.
Figure 2.
ARAS-frontoparietal functional connectivity in TLE patients before and after surgery and controls. A, Mean functional connectivity between ARAS and frontoparietal and insular neocortex is reduced in preoperative patients with TLE compared to controls. However, connectivity in the same TLE patients is increased > 1 yr after surgery, resembling connectivity in controls. B, Examining ARAS regions individually, increases in frontoparietal connectivity are seen after surgery in CSC and PPN, but not VTA. n = 10 patients before surgery and > 1 yr after surgery, who ultimately achieved seizure freedom vs 10 matched controls. *P = .05, Kruskal–Wallis with post hoc Dunn; **P value range = .01-.04, Kruskal–Wallis with post hoc Dunn. Central bar shows median, bottom and top edges of box indicate 25th and 75th percentiles, and whiskers indicate data extremes. ARAS, ascending reticular activating system; CSC, cuneiform/subcuneiform nuclei; FP, frontoparietal; PostOp, postoperative patients; PPN, pedunculopontine nucleus; PreOp, preoperative patients; VTA, ventral tegmental area.
Examination of patients with continued postoperative seizures was limited by sample size (n = 5); outcomes were Engel 2C in 2 patients and Engel 3A in 3 patients at the time of postoperative scan. In these individuals, we detected no differences in ARAS-frontoparietal postoperative connectivity (2.82 ± 2.24, mean ± SD) compared to preoperative baseline (2.71 ± 3.14; P = .59, Kruskal–Wallis). With analysis of voxel-wise connectivity maps seeded from CSC, PPN, or VTA, we did not detect any altered connectivity postoperatively compared to preoperatively (data not shown). We performed motion analysis for the nonseizure-free group (n = 5), and maximum translation did not differ between preoperative patients (0.89 ± 0.47 mm), postoperative patients (0.61 ± 0.25 mm), or controls (0.43 ± 0.11 mm; P = .28, Kruskal–Wallis). There was also no detectable difference in maximum rotation among preoperative patients (0.65° ± 0.36°), postoperative patients (0.57° ± 0.23°), or controls (0.35° ± 0.15°; P = .26, Kruskal–Wallis). Overall, these results suggest that ARAS-frontoparietal connectivity may increase in patients who achieve postoperative seizure freedom.
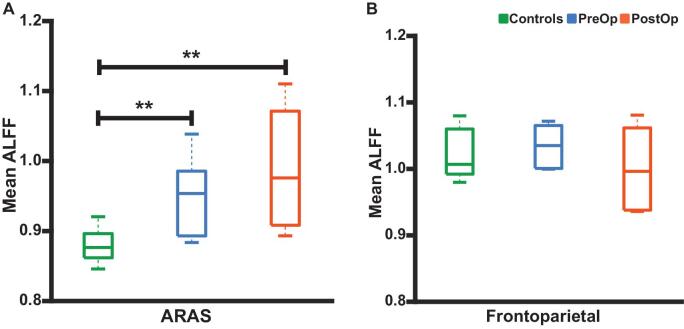
ARAS ALFF is Altered in Patients but Does Not Change After Surgery
We also examined ALFF in ARAS structures and frontoparietal cortex in patients who achieved seizure freedom to better understand ARAS-frontoparietal connectivity. Mean ARAS ALFF was higher in TLE patients (both pre- and postoperative) compared to controls (Figure 3A). However, no differences in frontoparietal ALFF were noted between controls, preoperative patients, and postoperative patients (Figure 3B). We observed no differences in ARAS or frontoparietal ALFF in postoperative patients compared to preoperative baseline (Figures 3A and 3B). These results suggest that ARAS-frontoparietal connectivity disturbances in epilepsy may be driven more by ARAS alterations than frontoparietal changes.28 However, unlike ARAS functional connectivity, ARAS ALFF does not appear to change after successful surgery.
Figure 3.
ALFF in ARAS, but not frontoparietal neocortex, differs between patients and controls. A, Differences in mean ALFF values in ARAS between control subjects and both preoperative and postoperative patients. No ARAS ALFF differences are noted between preoperative and postoperative patients. B, No differences in mean ALFF values in bilateral frontoparietal/insular neocortical regions are observed between controls, preoperative patients, or postoperative patients. N = 10 patients before surgery and > 1 yr after surgery, who ultimately achieved seizure freedom vs 10 matched controls. **P < .05, Kruskal–Wallis with post hoc Dunn. Central bar shows median, bottom, and top edges of box indicate 25th and 75th percentiles, and whiskers indicate data extremes. ALFF, amplitude of low-frequency fluctuations, ARAS, ascending reticular activating system; CSC, cuneiform/subcuneiform nuclei; PostOp, postoperative patients; PPN, pedunculopontine nucleus; PreOp, preoperative patients; VTA, ventral tegmental area.
Relating ARAS Functional Connectivity Changes to Disease and Neurocognitive Variables
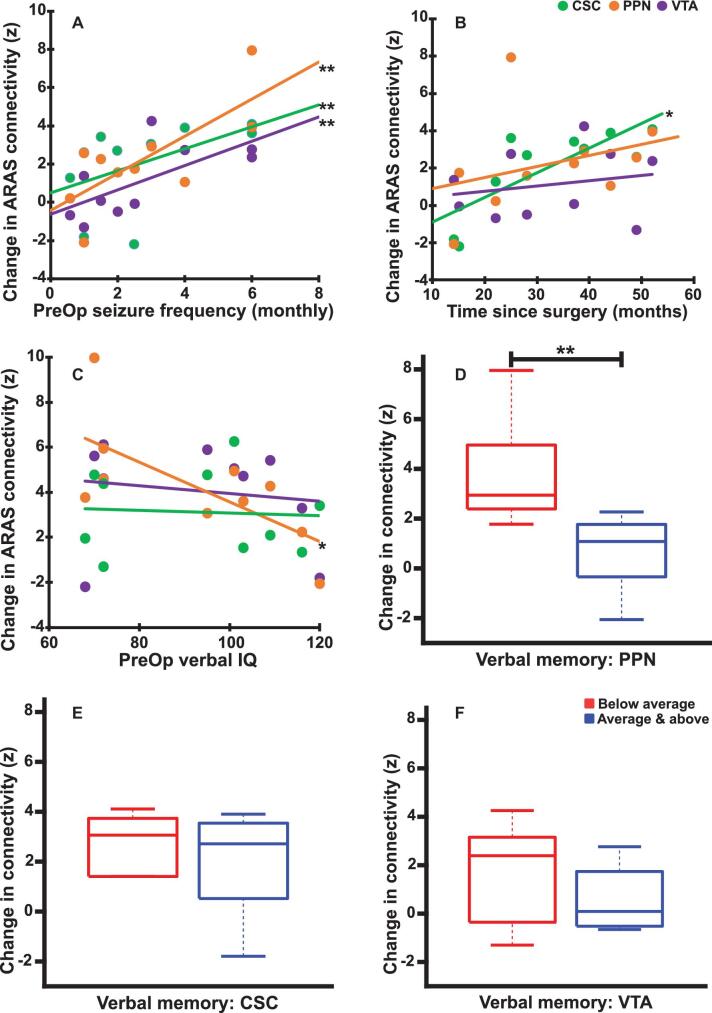
Prior work demonstrated larger ARAS functional connectivity reductions in patients with more frequent focal impaired consciousness seizures (FICS).9 We asked whether preoperative FICS frequency was related to postoperative connectivity change in seizure-free patients. Larger postoperative connectivity increases between CSC, PPN, and VTA and frontoparietal cortex were observed in patients with more frequent preoperative FICS (Figure 4A). This suggests that individuals with greater disease burden have larger connectivity increases after successful surgery. Examining whether these connectivity changes were related to time between surgery and postoperative MRI, we observed a marginal relationship between CSC connectivity and time, and no relationship for PPN or VTA (Figure 4B). No relationship was observed between postoperative ARAS-frontoparietal connectivity change and epilepsy duration in these individuals (rho = 0.17-0.56; P = .63-.09 for CSC, PPN, and VTA).
Figure 4.
Relationships between ARAS postoperative functional connectivity changes and disease measures in seizure-free patients. A, Larger increases in functional connectivity between each ARAS structure (CSC, PPN, and VTA) and frontoparietal neocortex are associated with higher preoperative focal impaired consciousness seizure frequency. B, Larger increases in functional connectivity between CSC and frontoparietal neocortex are associated with longer time between surgery and the postoperative scan, whereas no similar relationship is observed with changes in PPN or VTA connectivity. C, Patients with lower preoperative verbal IQ before surgery demonstrate a larger postoperative increase in functional connectivity between PPN and frontoparietal neocortex, although no similar relationship is noted for CSC or VTA. D-F, Patients with worse preoperative verbal memory performance show a larger increase in PPN postoperatively D, but no such relationship is noted for CSC E or VTA F. N = 10 patients, who ultimately achieved seizure freedom after surgery. *P value range = .02-.03, uncorrected, **P value range = .03-.04 after Bonferroni-Holm correction for Spearman's rho A-C or Mann–Whitney U-test D-F. Central bar shows median, bottom and top edges of box indicate 25th and 75th percentiles, and whiskers indicate data extremes. ARAS, ascending reticular activating system; CSC, cuneiform/subcuneiform nuclei; PPN, pedunculopontine nucleus; PreOp, preoperative patients; VTA, ventral tegmental area.
Previous studies reported that ARAS connectivity reductions are associated with impaired verbal performance in preoperative TLE patients.9,10 Therefore, we evaluated potential relationships between preoperative verbal IQ and memory and postoperative change in ARAS-frontoparietal connectivity in seizure-free patients. A marginal relationship was observed between lower preoperative verbal IQ and increase in postoperative PPN functional connectivity, whereas no relationships were seen for CSC or VTA (Figure 4C). Furthermore, compared to individuals with average or above verbal memory, patients with preoperative below average verbal memory experienced larger increases in postoperative PPN connectivity (Figure 4D), but not CSC (Figure 4E) or VTA (Figure 4F) functional connectivity. This suggests that individuals with worse verbal IQ and memory may experience greater increases in postoperative PPN functional connectivity.
ARAS Structural Connectivity Does Not Change After Epilepsy Surgery
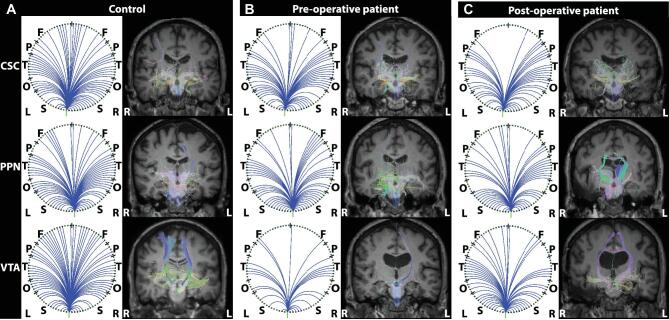
We next asked whether ARAS structural connectivity changes after surgery, as prior work demonstrated ARAS functional and structural connectivity decreases in TLE.10 Diffusion tractography in example participants (Figure 5) reveals fewer tracts reaching targets seeded from CSC, PPN, and VTA in patients compared to controls (Figure 5A). No obvious differences between patient structural connectivity patterns before surgery (Figure 5B) vs after surgery (Figure 5C) were observed. When evaluating 10 regions of greatest ARAS structural connectivity decreases in TLE patients (3.3 × 105 ± 1.0 × 105 tracts, mean ± SD) compared to controls (4.5 × 105 ± 1.1 × 105 tracts), no changes in structural connectivity were observed after surgery in patients who achieved seizure freedom (3.3 × 105 ± 7.8 × 104 tracts; P > .99, Kruskal–Wallis with post hoc Dunn). Furthermore, no differences in structural connectivity from ARAS to frontoparietal neocortex were observed between preoperative patients (2.1 × 105 ± 1.0 × 105 tracts), postoperative patients (2.4 × 105 ± 8.3 × 104 tracts), or controls (2.3 × 105 ± 7.9 × 104 tracts; P = .63, Kruskal–Wallis). Thus, unlike functional connectivity, structural connectivity alterations in TLE may not change after successful surgery.
Figure 5.
Example ARAS structural connectivity. Diffusion tractography seeded from CSC (top row), PPN (middle row), and VTA (bottom row) in an example matched control A, preoperative patient B, and the same patient postoperative C for each region. Figures are generated using the BrainSuite Diffusion Pipeline (BDP; http://brainsuite.org). On the left in each column A-C are circle graphs that summarize projections seeded from ARAS regions to cortical and subcortical regions in BrainSuite SVReg Atlas. On the right in each column A-C are estimated diffusion tensors overlaid onto T1-weighted coronal anatomical images using a rigid mutual information-based registration. Overall, for the 3 ARAS seed regions, the most tracts are seen in the controls A compared to patients B and C. Additionally, visually, there are no differences in estimated tracts between preoperative patients B and postoperative patients C. BrainSuite settings: 1 seed per voxel, step-size = 0.25 mm, maximum steps = 500, angle-threshold = 10°, fractional anisotropy threshold = 0.05, orientation distribution function sampling = 20, and generalized fraction anisotropy/lambda 2 threshold = 0.01. CSC, cuneiform/subcuneiform nuclei; F, frontal; L, left; O, occipital; P, parietal; PPN, pedunculopontine nucleus; R, right; S, subcortical; T, temporal; VTA, ventral tegmental area.
DISCUSSION
Previous investigations suggested recurrent seizures may lead to decreased ARAS connectivity, which may contribute to broad neurocognitive problems in TLE.9,10 Might these connectivity perturbations “improve” in patients who achieve seizure freedom after epilepsy surgery? Our present findings suggest that postoperative ARAS-frontoparietal functional connectivity may increase after successful surgery (10 of 15 patients), more closely resembling connectivity in controls. Although we did not see functional connectivity increases in patients with continued postoperative seizures, only 5 individuals were included in this analysis. Why does diminished ARAS functional connectivity in TLE matter? ARAS connectivity reductions may contribute to neurocognitive deficits or be related to sudden unexpected death in epilepsy (SUDEP). SUDEP has been proposed to involve dysfunction of ARAS networks,29 and risk of SUDEP decreases after epilepsy surgery.30
Why might ARAS-frontoparietal functional connectivity be disturbed in TLE, and why might it recover after successful surgery? In our working model (Figure 6), built upon work by Blumenfeld,31,32 normal cortical activation is maintained during interictal baseline through normal connectivity from subcortical activating structures (Figure 6A). During the ictal period, seizure activity begins in the hippocampus (Figure 6B) and may spread to subcortical activating structures (Figure 6C), resulting in focal seizures with impaired consciousness (FICS) and neocortical depression given absent subcortical excitation (Figure 6C). This transient network inhibition is associated with sleep-like neocortical rhythms and diminished cortical blood flow in TLE patients33-35 and is supported by rodent studies showing reduced neocortical activity and behavioral arrest that only occurs if limbic seizure activity propagates to subcortical activating structures.36-38 Although neocortical activation transiently recovers after postictal period (Figures 6A and 6C), it is possible that, over time, recurrent FICS lead to decreased connectivity between subcortical activating structures and neocortex that persists during interictal, resting state (Figure 6D). This may reflect an evolutionarily advantageous phenomenon preventing secondary generalization of seizure activity or may result from cumulative damage to neural networks from seizures. After successful epilepsy surgery, FICS cessation may allow normalization of ARAS-frontoparietal functional connectivity (Figure 6E). Prior work supporting this hypothesis observed larger functional connectivity decreases in TLE patients with more frequent FICS, and we now observe that these individuals may also have larger functional connectivity increases after successful surgery.
Figure 6.
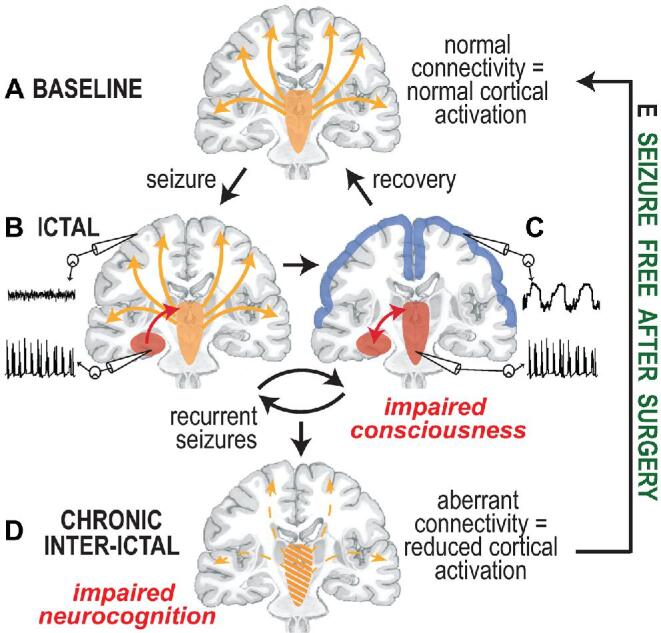
Model for subcortical-cortical connectivity disturbances and recovery in TLE. A, At wakeful baseline, neocortical activation is maintained via direct and indirect excitatory projections from subcortical activating structures, including ARAS, intralaminar thalamus, and basal forebrain. B, During the transition to the ictal period, seizure activity begins in the mesial temporal lobe and may remain confined there without disturbing cortical activity, generating a small consciousness-sparing focal seizure, or aura. C, When seizure activity spreads to involve subcortical activating structures, the normal excitatory input from the subcortical regions to the neocortex is perturbed, and the neocortex defaults to a sleep-like inhibited state, resulting in a consciousness-impairing focal seizure. D, Over time, recurrent consciousness-impairing focal seizures may lead to progressive dysfunction of subcortical activating structures and aberrant connectivity between these regions and the neocortex, leading to a chronic state of reduced neocortical activation and impaired neurocognition. E, Seizure freedom after successful epilepsy surgery may allow recovery of certain subcortical-cortical functional connectivity pertubations. Adapted from Blumenfeld and Taylor,46 with permission and courtesy of Hal Blumenfeld.
Next, previous studies reported that relationships exist between ARAS connectivity decreases and diminished verbal abilities, and here, we observe larger PPN functional connectivity increases in patients with worse preoperative verbal IQ and memory. This may suggest greater potential for improvement in those with more significant preoperative neurocognitive deficits. Interestingly, PPN deep brain stimulation in Parkinson disease has been associated with neurocognitive improvements,39,40 and PPN stimulation in rats can help prevent deleterious neocortical and behavioral effects of limbic seizures.41
Prior work noted diminished ARAS structural connectivity in TLE patients, albeit to different structures than functional connectivity changes.10 It is also known that functional connections can exist absent direct axonal connections, presumably because functional connectivity may reflect indirect/polysynaptic pathways.42,43 Perhaps it is, therefore, not surprising that we did not observe postoperative ARAS structural connectivity increases, as new axonal growth is not likely the source of functional connectivity improvements. Lack of postoperative ARAS structural change may be further supported by our observation that whereas fMRI ALFF in ARAS was altered in TLE patients compared to controls, ARAS ALFF did not change after successful surgery. Further comparison of functional connectivity, structural connectivity, and ALFF in TLE may improve the understanding of disease-related and treatment-related network alterations.
This study has other limitations worth discussing. This work must be considered a preliminary analysis of postoperative ARAS connectivity changes because of the small sample size and heterogeneous patient population. The study is insufficiently powered to evaluate potential confounders, including pathology results and surgery type, using multivariate analysis. Our results must be validated in a larger cohort with appropriate subgroup analyses in future studies. Nevertheless, this is the first study to evaluate brainstem arousal connectivity changes with epilepsy surgery and include long-term postoperative imaging. Additionally, of the 2 ARAS regions in which we observed increases in postoperative functional connectivity increased connectivity was observed in 9 of 10 patients for PPN and 8 of 10 patients for CSC. We also note limitations of statistical tests used in this study and that lack of significant differences between participant groups does not imply that groups are equal. We utilized nonparametric tests given that the Anderson–Darling test suggested our data were non-normally distributed, and results may differ using various statistical tests. Repeating our analyses using parametric tests (eg, analysis of variance with post hoc Fisher's least significant difference procedures, not shown), our findings remained consistent.
Another limitation is that long-term postoperative neuropsychological assessments were unavailable. Prior work demonstrated that patients with long-term postoperative seizure freedom often have improved neurocognition.14,44,45 Although we hypothesize that connectivity improvements may be accompanied by improvements in certain neurocognitive domains in seizure-free patients, this could not be tested in this study. Although the goal of this preliminary study was to first determine whether postoperative ARAS connectivity may improve toward control values and relate connectivity changes to preoperative clinical variables, future studies should include long-term postoperative neuropsychological assessments to relate connectivity to postoperative cognitive changes. Finally, another future direction will be acquiring serial connectivity measurements at various postoperative time points to determine potential effects of evolving seizure outcome and time since last the seizure(s) on connectivity.
CONCLUSION
In summary, connectivity perturbations of certain subcortical arousal networks are present in TLE and may be related to disease severity and neurocognitive function. After successful epilepsy surgery, some brainstem functional connectivity patterns may recover and more closely resemble connectivity in healthy control. These findings may have important implications for treatment selection and timing and for future investigations into neuromodulation targets, neuropsychological outcomes, and risk of SUDEP in TLE.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. This work was supported in part by the National Institutes of Health Grants R00 NS097618 (to Dr Englot), R01 NS075270 (to Dr Morgan), T32 EB021937 (to Dr González), and T32 GM07347 (to Dr González).
Acknowledgements
We thank Justin Blaber for technical assistance, and we also thank biostatistician Hakmook Kang for discussion aiding the selection of statistical tests. For atlases and toolboxes used in this study, we thank the Harvard Center of Morphometric Analysis (Harvard-Oxford Atlas), the Martinos Center for Biomedical Imaging (Harvard Ascending Arousal Network Atlas), the McGovern Institute for Brain Research (CONN toolbox), and the Leahy/Shattuck neuroimaging collaboration (BrainSuite).
Notes
North American Neuromodulation Society Annual Meeting, January 12, 2018, Las Vegas, Nevada. Poster presentation title: “Disturbances of brainstem functional connectivity in temporal lobe epilepsy patients may recover after successful epilepsy surgery.”
American Society for Stereotactic and Functional Neurosurgery Biennial Meeting, June 5, 2018, Denver, Colorado. Plenary presentation title “The effects of epilepsy surgery on deep arousal structure functional connectivity in temporal lobe epilepsy.”
COMMENT
Epilepsy is associated with widespread functional and structural alterations that might contribute to neurocognitive problems, but it is not known whether successful epilepsy surgery normalizes these changes. To address this issue, the authors of this study examined pre- and postoperative brainstem connectivity in 15 patients with temporal lobe epilepsy and 15 matched control participants using resting-state functional magnetic resonance imaging connectivity analysis and diffusion tensor imaging to evaluate functional and structural connectivity, respectively. They found that functional connectivity between brainstem and bilateral frontal-parietal-insular cortex significantly improved in the 10 patients who were seizure free at 1 yr, particularly involving the pedunculopontine nucleus and cuneiform/subcuneiform cortex, and this was not seen in the 5 patients who did not improve. The increase in connectivity was directly proportional to preoperative seizure frequency and inversely proportional to preoperative verbal intelligence quotient. There were no differences observed in structural connectivity. The authors conclude that epilepsy surgery is able to restore normal functional connectivity that is lost because of seizures, and this may have important implications for cognitive outcome.
It is well known that chronic seizures are associated with changes in connectivity that are proportional to seizure frequency, so it is unsurprising that a reduction in seizures (by surgery or any other means) will reverse these changes. It is also intuitive that resection of tissue that does not include the epileptogenic zone will not have this effect. The changes observed in this study are therefore likely to be a byproduct of less overall ictal activity, although increased connectivity might also have resulted from removal of brain tissue itself, decreased interictal activity, or effects of epilepsy medication, and it is not possible to discern the relative contribution of each of these. Also, without postoperative neurocognitive data, the functional significance of these findings is unclear, as it is not possible to draw any conclusions about the relationship of connectivity changes to actual postoperative neurocognitive outcome. Nevertheless, the results of this study confirm that successful epilepsy surgery results in widespread effects on brain physiology that might impact postoperative outcome.
Jonathan P. Miller
Cleveland, Ohio
REFERENCES
- 1. Engel J. What can we do for people with drug-resistant epilepsy? Neurology. 2016;87(23):2483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi H, Sell RL, Lenert L et al.. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy. JAMA. 2008;300(21):2497-2505. [DOI] [PubMed] [Google Scholar]
- 3. Engel J Jr, McDermott MP, Wiebe S et al.. Early surgical therapy for drug-resistant temporal lobe epilepsy. JAMA. 2012;307(9):922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tellez-Zenteno JF, Hernandez-Ronquillo L.. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Witt JA, Helmstaedter C.. Cognition in epilepsy. Curr Opin Neurol. 2017;30(2):174-179. [DOI] [PubMed] [Google Scholar]
- 6. Alvim MK, Coan AC, Campos BM et al.. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia. 2016;57(4):621-629. [DOI] [PubMed] [Google Scholar]
- 7. Aparicio J, Carreno M, Bargallo N et al.. Combined 18 F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage Clin. 2016;12:976-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016;57(10):1546-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Englot DJ, D’Haese PF, Konrad PE et al.. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2017;88(11):925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Englot DJ, Gonzalez HFJ, Reynolds BB et al.. Relating structural and functional brainstem connectivity to disease measures in epilepsy. Neurology. 2018;91(1):e67-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921-928; discussion 928. [DOI] [PubMed] [Google Scholar]
- 12. Macrodimitris S, Sherman EM, Williams TS, Bigras C, Wiebe S. Measuring patient satisfaction following epilepsy surgery. Epilepsia. 2011;52(8):1409-1417. [DOI] [PubMed] [Google Scholar]
- 13. Giovagnoli AR, Parente A, Didato G, Deleo F, Villani F. Expanding the spectrum of cognitive outcomes after temporal lobe epilepsy surgery: a prospective study of theory of mind. Epilepsia. 2016;57(6):920-930. [DOI] [PubMed] [Google Scholar]
- 14. Wachi M, Tomikawa M, Fukuda M et al.. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001;42(s6):4-8. [PubMed] [Google Scholar]
- 15. Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. 2016;86(21):1938-1944. [DOI] [PubMed] [Google Scholar]
- 16. Liao W, Ji GJ, Xu Q et al.. Functional connectome before and following temporal lobectomy in mesial temporal lobe epilepsy. Sci Rep. 2016;6(1):23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maccotta L, Lopez MA, Adeyemo B et al.. Postoperative seizure freedom does not normalize altered connectivity in temporal lobe epilepsy. Epilepsia. 2017;58(11):1842-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Englot DJ. A modern epilepsy surgery treatment algorithm: incorporating traditional and emerging technologies. Epilepsy Behav. 2018;80:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edlow BL, Takahashi E, Wu O et al.. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71(6):531-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162-167. [DOI] [PubMed] [Google Scholar]
- 21. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141. [DOI] [PubMed] [Google Scholar]
- 22. Yang H, Long XY, Yang Y et al.. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36(1):144-152. [DOI] [PubMed] [Google Scholar]
- 23. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790. [DOI] [PubMed] [Google Scholar]
- 24. Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Analysis. 2002;6(2):129-142. [DOI] [PubMed] [Google Scholar]
- 25. Engel J, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J, Pedley TA, eds. Epilepsy: A Comprehensive Textbook. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2479-2486. [Google Scholar]
- 26. Glantz S. Primer of Biostatistics. 6th ed.New York, NY: McGraw-Hill; 2005: https://www.r2library.com/Resource/Title/0071435093. Accessed August 17, 2018. [Google Scholar]
- 27. Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583-621. [Google Scholar]
- 28. Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci. 2013;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(suppl 1):28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perry MS, Duchowny M. Surgical versus medical treatment for refractory epilepsy: outcomes beyond seizure control. Epilepsia. 2013;54(12):2060-2070. [DOI] [PubMed] [Google Scholar]
- 31. Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res. 2009;177:147-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3(3):219-231. [DOI] [PubMed] [Google Scholar]
- 33. Englot DJ, Yang L, Hamid H et al.. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133(12):3764-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63(6):1015-1021. [DOI] [PubMed] [Google Scholar]
- 35. Blumenfeld H, McNally KA, Vanderhill SD et al.. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14(8):892-902. [DOI] [PubMed] [Google Scholar]
- 36. Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28(36):9066-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29(41):13006-13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Motelow JE, Li W, Zhan Q et al.. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85(3):561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alessandro S, Ceravolo R, Brusa L et al.. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289(1-2):44-48. [DOI] [PubMed] [Google Scholar]
- 40. Fischer J, Schwiecker K, Bittner V et al.. Modulation of attentional processing by deep brain stimulation of the pedunculopontine nucleus region in patients with parkinsonian disorders. Neuropsychology. 2015;29(4):632-637. [DOI] [PubMed] [Google Scholar]
- 41. Kundishora AJ, Gummadavelli A, Ma C et al.. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 2017;27(3):1964-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Honey CJ, Sporns O, Cammoun L et al.. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106(6):2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang H, Ding M. Linking functional connectivity and structural connectivity quantitatively: a comparison of methods. Brain Connect. 2016;6(2):99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sherman EM, Wiebe S, Fay-McClymont TB et al.. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52(5):857-869. [DOI] [PubMed] [Google Scholar]
- 45. Giovagnoli AR, Parente A, Didato G et al.. The course of language functions after temporal lobe epilepsy surgery: a prospective study. Eur J Neurol. 2016;23(12):1713-1721. [DOI] [PubMed] [Google Scholar]
- 46. Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9(5):301-310. [DOI] [PubMed] [Google Scholar]