Abstract
BACKGROUND
Recurrent atypical and malignant meningiomas have poor outcomes with surgical therapy alone. Neither adjuvant chemotherapy nor postoperative radiation therapy remedies this problem.
OBJECTIVE
To evaluate our experience with the treatment of 15 patients treated with I-125 or Cs-131 brachytherapy radiation seeds as an adjuvant in these difficult cases.
METHODS
Patients with high-grade recurrent meningioma who underwent resection and intraoperative placement of brachytherapy seeds at our institution from 2002 to 2014 were identified and studied by retrospective chart review.
RESULTS
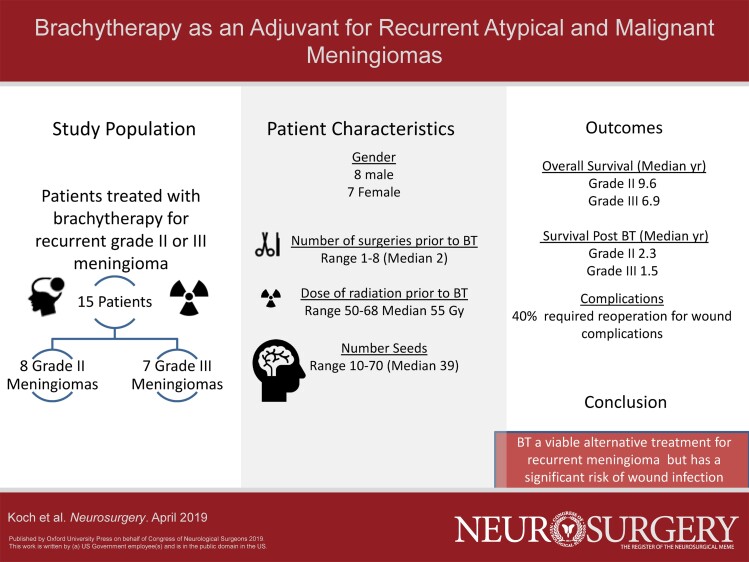
Fifteen patients with median age of 68.8 yr were treated with I-125 (n = 13) or Cs-131 (n = 2) brachytherapy seeds for cases of recurrent, grade II (n = 8), or grade III (n = 7) meningioma at our institution from 2002 to 2014. These lesions originated from a variety of locations including, convexity (3), falcine (3), frontal (2), occipital (1), parietal (2), 2 sphenoid wing (2), and temporal (2), based recurrent meningiomas. Patients had a median of 2 prior open surgical interventions and received local radiation therapy with a median dose of 55 Gy prior to brachytherapy. Survival at 2.5 yr was 56% for grade II and 17% for grade III lesions. Survival was significantly associated with patient age but not tumoral pathology. Forty percent of patients required reoperations for wound complications following brachytherapy.
CONCLUSION
Brachytherapy with implantation of permanent radiation seeds provides a viable alternative treatment for recurrent meningioma while carrying a significant clinical risk of wound infection and need for reoperation.
Keywords: Radiosurgery, Radiation therapy, Brachytherapy, Malignant meningioma, Atypical meningioma
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- BT
brachytherapy
- CT
computed tomography
- EBRT
external beam radiation therapy
- GTR
gross total resection
- RT
radiation therapy
Despite maximal resection and adjuvant radiation therapy (RT), atypical (grade II) and malignant (grade III) meningiomas have a high recurrence rate.1,2 In the recurrent setting, treatment options are limited with currently no effective chemotherapy or targeted therapy options. These patients undergo additional surgical resections that are often limited by anatomic restrictions and brain parenchymal invasion. Furthermore, repeat irradiation with external beam radiation therapy (EBRT) delivered to the point of recurrence is limited by maximal safe dosages.3-5
Brachytherapy (BT), or radiation delivered with radioactive implants, provides an alternative means of delivering radiation with a sharp dose fall-off, thereby permitting focal treatment of the resection bed while minimizing exposure to the surrounding healthy tissues.6,7 Initial reports of BT in the setting of recurrent atypical and malignant meningiomas suggest a therapeutic benefit.8,9
Objectives
Herein, we report our results and patient outcomes of 15 patients with recurrent atypical and malignant meningiomas treated with adjuvant iodine-125 (I-125) or cesium-131 (Cs-131) BT radiation seeds at the time of surgical re-resection over a 12-yr period.
METHODS
Study Design
We performed an observational, retrospective, single-center study of recurrent atypical and malignant meningiomas treated with BT.
Settings
With institutional review board approval as a consent exempt study, records contained in the radiation oncology database within the study period between January 2002 and December 2014 were reviewed.
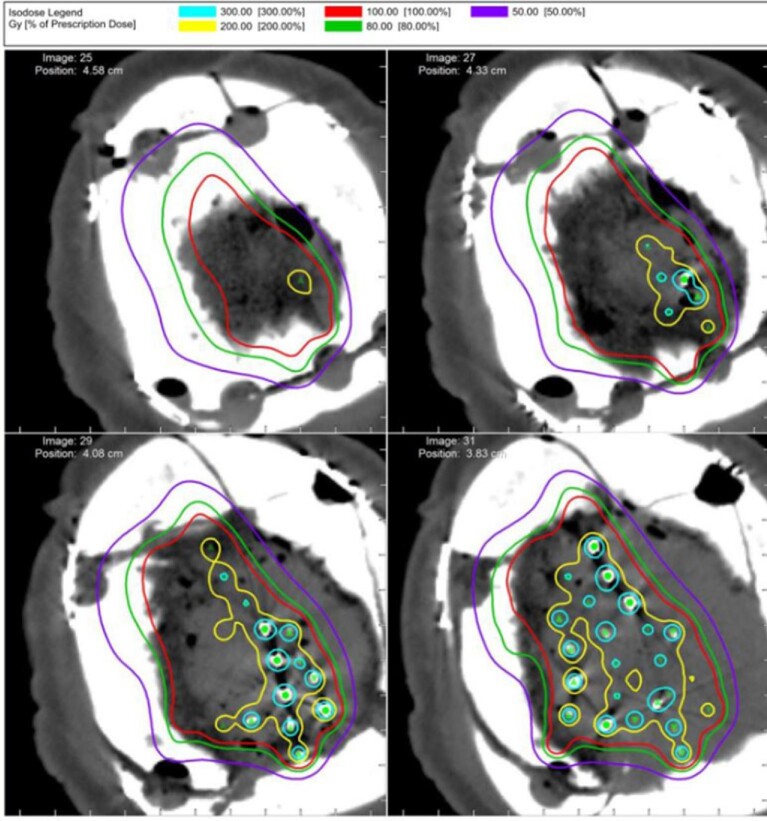
Patients were offered BT as a treatment modality following a multi-disciplinary discussion regarding optimal treatment of recurrent lesions despite maximal resection and RT precluding further EBRT. All surgeries were performed in cooperation with the radiation oncologists and physicists who were present for seed placement. Following maximal safe resection, radiation seeds were placed within the resection cavity, covered with Surgicel (Ethicon, Somerville, New Jersey), and fixed in place with fibrin sealant. Seeds are placed up to exposed dura and are not placed above the dura or dura substitute. Dural substitute is then used and the bone flap replaced. Two types of radioactive seeds were utilized for the procedures, Iodine-125 (Oncura, GE Medical, Chicago, Illinois) and Cesium-131 (IsoRay Medical, Richland, Washington). The seeds were encased in a strand of suture material with 1.0 cm spacing between the centers of each seed. Each strand contained 10 seeds. The activity of the I-125 seeds utilized was 0.5 mCi/seed assayed for the day of the implant. The activity of the Cs-131 seeds utilized was 4.09 mCi/seed assayed for the day of the implant. Endpoint dose for the I-125 procedures were 100 and 80 Gy for the Cs-131 procedures. Strands were cut to size and placed into the tumor bed. Spacing between strands was kept as close to 1.0 cm as possible yielding a 1.0-cm matrix of seeds encompassing the tumor bed. Postoperative computed tomography (CT) imaging was performed to assess for the spacing and placement of the seeds for dosimetry (Figure 1).
FIGURE 1.
Postoperative CT scan with associated radiation dose following placement of brachytherapy seeds for a convexity meningioma.
Participants and Study Size
Selection criteria included patients who had undergone intraoperative BT at time of recurrent surgery for atypical or malignant meningioma. Pathological grade was based on WHO criterion at the time of surgery. Grade II lesions included atypical, clear cell, and chordoid subtypes, while grade III lesions included malignant, rhabdoid, and papillary subtypes. In total, 15 patients were identified.
Variables
Detailed medical history including patient age, gender, tobacco use, and medical comorbidities were collected. Relevant surgical data included timing of operations, extent of resection, BT type, and number of seeds. Post-treatment data was collected at the time of death or last available information. The United States Social Security Death Index was used to confirm patient mortality events where unclear.
Statistical Analyses
Descriptive statistics were calculated for clinical and radiographic factors, using the median and/or mean as a measure of central tendency. Kaplan–Meier functions were used to perform survival analyses and a Cox proportional hazard model was used to determine statistical significance with a P value of <.05 noted to be significant (R foundation for statistical computing, Vienna, Austria 2017).
RESULTS
Participants
As summarized in Table 1, BT was used as a treatment in 15 patients with grade II (8) and grade III (7) meningiomas.
TABLE 1.
Demographic and Lesion Characteristics of Patients Treated with Brachytherapy for Recurrent Meningioma
| Gender | 9—Male6—Female |
| Grade | 9—Grade 26—Grade 3 |
| Median age at brachytherapy in years (range) | 66 (40-89) |
| Median number of implanted seeds (range) | 39 (10-70) |
| Location | 3—Parafalcine3—Convexity9—Skull base |
| Gross total resection at initial surgery | 8—GTR7—Subtotal |
Descriptive Data
Eight (53%) of patients were male and the median age was 68.8 yr (range 40.3-89.8 yr). All patients were on steroids at the time of BT but comprehensive data regarding the duration of steroid treatment were not available. All patients were of atypical and malignant subtypes. There were no patients with clear cell, chordoid, rhabdoid, or papillary subtypes.
Tumor locations varied amongst the cohort (Table 1). No single location dominated although convexity and falcine lesions were most frequent. Three of the grade II and one of the grade III lesions began as grade I lesions that recurred with malignant transformation.
Outcome Data
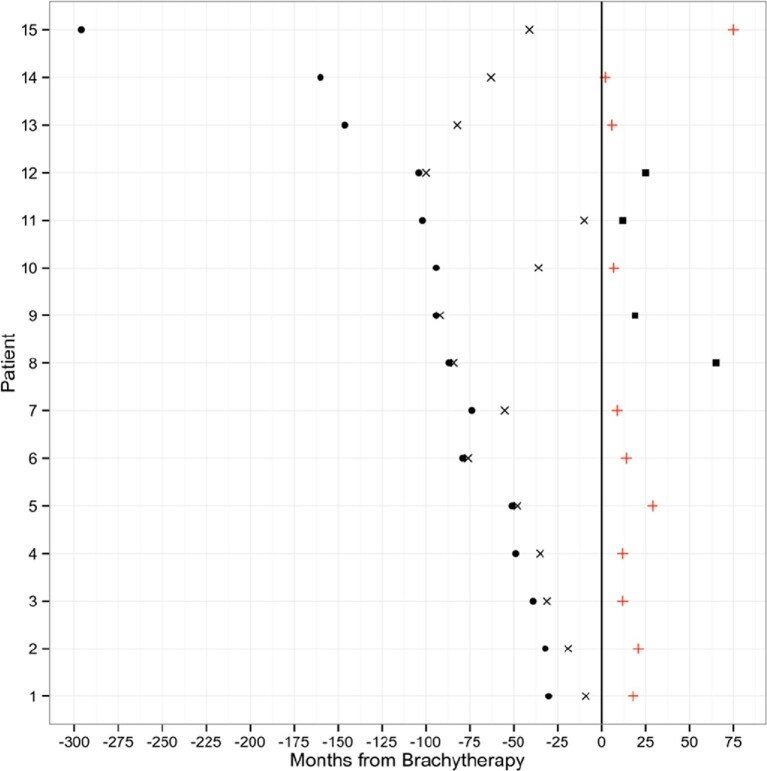
The median number of operations prior to BT was 1 in grade II tumors and 2 in grade III lesions (Table 2). All patients received some form of fractionated EBRT prior to BT treatment with an average dose of 60 Gy. One patient received stereotactic photon radiosurgery only prior to BT. Figure 2 demonstrates the intervals of time between original surgery, RT, BT, and follow-up for all patients included in the study. The median time to BT from first surgery was 14 mo (range, 2-255 mo) with a trend towards a smaller interval in recently treated patients. The majority of patients (13 of 15) received I-125 implant seeds with a range of 10 to 70 seeds placed into the resection cavity (median 39 seeds placed). The other 2 patients received 17 and 20 Cesium-131 implant seeds, respectively. Two more recent grade II patients underwent implantation of Cs-131. Furthermore, BT tended to be performed after a shorter time interval in more recently treated patients.
TABLE 2.
Pretreatment and Posttreatment Outcomes for Patients Treated with Brachytherapy Based on Grade
| Grade 2 | Grade 3 | |
|---|---|---|
| GTR at first surgery | 3/9 | 5/6 |
| Time from first surgery to EBRT in months (median and range) | 4 (2-255) | 39.5 (13-92) |
| Number of operations prior to brachytherapy (median and range) | 1 (1-4) | 2 (2-8) |
| Time to progression after brachytherapy in months (median and range) | 8.5 (2-40) | 4.5 (3-7) |
| Survival time from brachytherapy in months (median and range) | 13 (2-75) | 12 (7-21) |
| Overall survival in months (median and range) | 89 (51-371) | 61 (48-158) |
FIGURE 2.
Combination diagram demonstrating each patient's treatment course prior to and following brachytherapy. The time of brachytherapy marks the 0 time point, circle is the point of first surgery, X is the point of RT, and + is the point of death, and the black square represents the last clinical follow-up.
At the end of the study period, all but 2 grade II patients had recurrence of their disease. Of the entire cohort, 10 patients had died as a result of their disease: 6 grade III and 4 grade II patients. The overall survival from initial diagnosis ranged from 3 to 30 yr for the grade II lesions and from 4 to 13 yr for the grade III lesions.
Reoperations occurred in 6 of 15 (40%) of those patients who underwent BT for recurrent high-grade meningioma (Table 3). All of the reoperations occurred as a result of infection or wound dehiscence. Among the 6 patients experiencing a wound complication, the number of reoperations ranged from 1 to 14. Of note, 1 patient required 14 reoperations secondary to resection of a planum sphenoidale grade II meningioma and subsequent development of a cerebrospinal fluid leak and wound infection following BT. This required numerous endonasal and cranial wound debridements and eventual skull base repair. Two of the 6 patients with wound complications received bevacizumab postoperatively. Six patients developed symptomatic radiation necrosis, which was treated with steroids and bevacizumab. Univariate analysis (Table, Supplemental Digital Content) demonstrated no significant association of wound complications with tumor grade, gender, age at time of BT, or number of radioactive implants. Following BT, 6 patients (3 grade II and 3 grade III) received bevacizumab off-label for treatment of radiation necrosis. Two patients received hydroxyurea, 1 patient received ironetecan, and another patient was treated with parseotide.
TABLE 3.
Characteristics of Each Case Requiring Reoperation Following Brachytherapy
| Lesion location | Tumor grade | No. of reoperations | Time to first reoperation | Reason for reoperation |
|---|---|---|---|---|
| Convexity | 2 | 3 | 6 yr | Dehiscence |
| Anterior fossa | 2 | 14 | 68 d | CSF leak, dehiscence, infection |
| Convexity | 3 | 1 | 33 d | Dehiscence |
| Convexitya | 3 | 3 | 70 d | Purulence |
| Sphenoid Winga | 2 | 2 | 66 d | Edema, subdural hematoma/purulence |
| Convexity | 3 | 2 | 5 d | Purulence |
aReceived Avastin following brachytherapy.
Main Results
On univariate analysis, only age at BT demonstrated significant correlation with overall survival (P = .05) while tumor grade, gender, resection status, and number of seeds did not (Table, Supplemental Digital Content). This was further demonstrated on multivariate analysis of age and grade with both having significant correlation with overall survival (P = .02 and .03, respectively). Time to progression was significantly different between grade II and grade III patients on univariate and multivariate analysis (P = .04 and .04, respectively). Log-rank test for time to progression further demonstrated a significant difference based upon lesion grade (Figure 3). Survival from BT was noted to be significantly associated with age (P = .05, but not significantly different based on lesion grade, P = .12). Figure 4 illustrates the survival curve from BT, where survival at 2.5 yr was 56% for grade II lesions and 17% for grade III lesions, with no statistically significant difference based on tumor grade (P = .11). Gross total resection at initial surgical treatment occurred in 3 grade II and 4 grade III patients with no observed difference in survival (P = .27).
FIGURE 3.
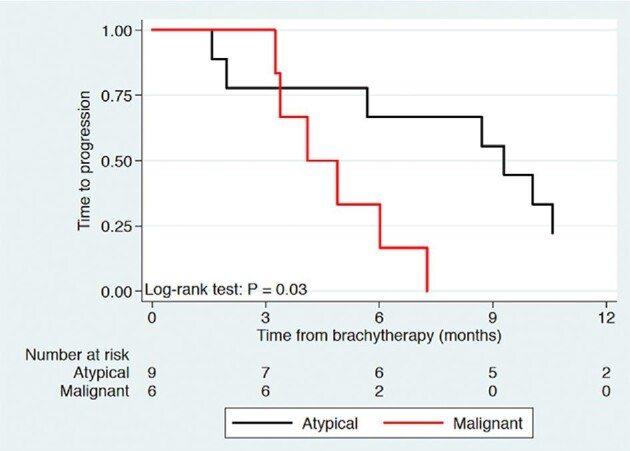
Kaplan–Meier curve of time to progression following brachytherapy of atypical and malignant meningiomas.
FIGURE 4.
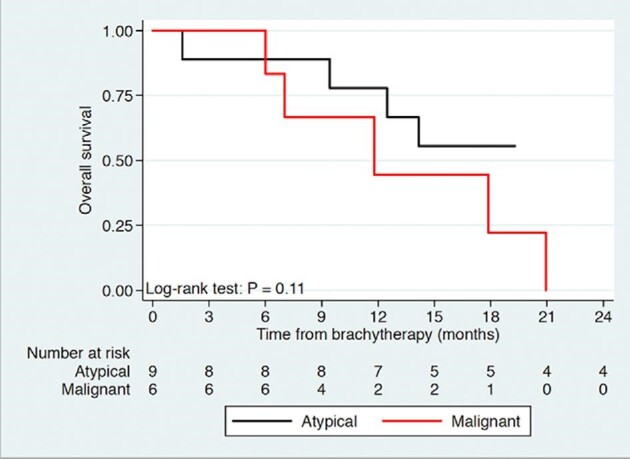
Kaplan–Meier curve of overall survival following brachytherapy of atypical and malignant meningiomas.
DISCUSSION
Key Results
We found that BT presents a reasonable option of further salvage therapy for recurrent high-grade meningiomas. Optimal treatment of grade II and III meningiomas remains an area of active study. Prior research demonstrates a progression-free survival benefit with gross total resection.2-4,8,10 Further retrospective studies indicate that postoperative RT, prior to recurrence, of atypical and malignant lesions improves survival. Currently, RTOG 0539 and EORTC 22042 directly aim to address the question whether all patients should receive radiation postoperatively or only at recurrence.1,11 Regardless, treatment strategies at re-recurrence remain problematic. As illustrated in our series, a variety of systemic agents have been used or are undergoing investigation as adjuvant therapies.12 Unfortunately, none of these therapies offers definitive benefit. Presently, this again leaves only radiation and surgery as putative therapies. Surgery can debulk the lesion alleviating mass effect but does not affect the underlying tumor biology. Although RT can slow the time to recurrence, repeat EBRT is not usually feasible due to cutaneous and local brain parenchymal reirradiation toxicities.3,13 BT is a compromise, a way to maximize the benefits of surgery while eliminating the entrance dosage associated with EBRT.
Interpretation
Prior case series illustrate that BT represents a viable option in the treatment of meningiomas.8,9 Our series, similar to other recent series, demonstrates the feasibility and associated outcomes of BT for recurrent lesions with a median survival from BT of 2.3 and 1.5 yr for grade II and III lesions, respectively. The overall median survival of grade II patients at 9.6 yr and grade III at 6.9 yr compares favorably with other recent studies.14,15 Age and tumor grade were significantly associated with time to progression but only age demonstrated significant association with survival from BT. Although our study is not powered to assess this significant association further, older patients frequently have more comorbidities and generally worse recovery from surgical procedures.16 This, in addition to the association of age with all-cause mortality, likely leads to this significant survival difference. The lack of effect of tumor grade is likely due to both the underpowered nature of this retrospective cohort as well as the biased nature of this cohort for particularly recalcitrant aggressive lesions of both pathologic subtypes. Furthermore, the wide range in time to BT, the number of surgeries prior to BT, and nonstandardized prior RT complicate interpretation of patient outcomes and may account for the more modest effect of BT observed when compared to other surgical series. Particularly, the timing of RT following the first surgical resection, especially in grade II patients, may have an underlying effect on our outcomes that overshadows the benefits of BT.11
This patient population is at particularly high risk for wound complications because of the history of prior surgeries, prior RT, and current BT. In addition, chronic steroids are often used in these cases, which also lead to poor wound healing. We believe that all modifiable wound risk factors must be addressed. Steroids should be weaned off if possible and the nutritional state should be optimized preoperatively. Vitamins A, C, and E and oral zinc have potential benefit in this setting and should be considered.17-21 In our series, supplemental vitamin use was based on surgeon preference and only 1 patient received supplemental vitamin C. Finally, risk factors such as smoking, diabetes, and immunosuppression must be addressed prior to surgery and can be a relative contraindication to re-resection and/or BT.
Besides systemic factors that might affect wound healing, surgical techniques and selection must be appropriate for this setting. Intraoperatively, the neurosurgeon must follow fundamental principles of wound handling such as maximizing viability of the skin edges. Pericranial flaps are useful for vascularized coverage but are most often previously used or scarred in the recurrent setting. Therefore, the closest autologous flaps that can be used are generally vascularized rotational flaps such as temporalis muscle or galeal flaps. In the absence of any local flaps, tension-free closure is absolutely critical. Here, back-cuts with scalp rotation are the gold standard (with perpendicular galeal scoring as necessary), but again can be limited due to fibrosis from prior irradiation. A review of all flap techniques is beyond the scope of this discussion and hence our emphasis on involving the reconstructive team early. It is not unreasonable to discuss complex meningioma cases with reconstruction colleagues at the first recurrence from the index surgery. In our series, discussions were held with our craniofacial plastic surgery colleagues but ultimately modified primary closure techniques were chosen over frank rotational or pedicled free flaps. Ultimately, in our population, 40% of patients had either wound breakdown or infection postoperatively, which is admittedly high, but consistent with prior literature and the significant risk factors involved.14,15 Although the post BT dosimetery demonstrates a negligible radiation dose to the scalp, whether this further plays a role in the high rate of wound complications remains a concern.
Generalizability
Presently, medical therapy plays a minor role in the management of patients with high-grade meningiomas and mainly focuses on mitigating tumoral edema and or radiation necrosis. Among currently available chemotherapeutics and biologics, bevacizumab is occasionally used prior to RT as a sensitizer, and post RT to treat brain swelling associated with radiation necrosis. Larger trials and studies of bevacizumab as an adjuvant radiosensitizer in patients with gliomas have not yielded significant survival benefits, but the use of this agent in meningiomas has not been explored.22 In our series, 6 patients received bevacizumab to treat post-BT radiation necrosis. All treatments were initiated at least 2 mo after BT. The effects of this treatment were beyond the scope of our study but warrant further investigation given the high risk of radiation necrosis associated with this particular patient population.23 The ability to treat edema without the side effects of chronic steroid use is appealing, but the detrimental impact of bevacizumab on wound healing limits its early application.24
Limitations
There are several factors and limitations of our present study that prevent full extrapolations of the effect of BT. The small number of patients involved in the study and the wide variety in treatment timing and relation to other therapies, as demonstrated in Figure 1, highlight that these findings are hypothesis-generating. The patients within this study were selected for therapy based on the aggressive clinical course of their tumors despite maximal therapy indicating that even the “lower grade” lesions may act like a higher grade lesion. Additionally, identifying local BT failure vs elsewhere disease progression and differentiating local failure from radiation necrosis further complicate the interpretation of post-BT treatment outcomes. This longitudinal retrospective study had pathology reports spanning several WHO tumor grading iterations. Newer WHO-grading guidelines increased the percentage of lesions demarcated as grade II lesions and may have upstaged some of our grade II lesions to grade III. Regardless, the study does demonstrate the feasibility and potential pitfalls of the use of BT within this tumor subset of aggressive recurrent meningiomas.
CONCLUSION
BT coupled with surgery is an option for salvage treatment of select patients with recurrent atypical and malignant meningioma. Patient selection and counseling should factor the significant risk of wound breakdown and infection with the potential benefit of delayed disease progression.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content. Table. Univariate cox analysis of overall survival and infection with respect to tumor grade, gender, age, prior resection and number of brachytherapy seeds. Multivariate of tumor grade and age at brachytherapy.
REFERENCES
- 1.Walcott BP, Nahed BV, Brastianos PK, Loeffler JS. Radiation treatment for WHO grade II and III meningiomas. Front. Oncol. 2013;3:227. doi: 10.3389/fonc.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghi MK, Carter BS, Cosgrove GRet al.. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56-60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 3.Aizer AA, Bi WL, Kandola MSet al.. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015;121(24):4376-4381. [DOI] [PubMed] [Google Scholar]
- 4.Wang W-H, Lee C-C, Yang H-Cet al.. Gamma knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2016;87:557-564. [DOI] [PubMed] [Google Scholar]
- 5.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas: treatment results on the basis of a 22-year experience. Cancer. 2012;118(4):1048-1054. [DOI] [PubMed] [Google Scholar]
- 6.Kumar PP, Patil AA, Syh HW, Chu WK, Reeves MA. Role of brachytherapy in the management of the skull base meningioma treatment of skull base meningiomas. Cancer. 1993;71(11):3726-3731. [DOI] [PubMed] [Google Scholar]
- 7.Vuorinen V, Heikkonen J, Brander Aet al.. Interstitial radiotherapy of 25 parasellar/clival meningiomas and 19 meningiomas in the elderly. Analysis of short-term tolerance and responses. Acta Neurochir (Wien). 1996;138(5):495-508. [DOI] [PubMed] [Google Scholar]
- 8.Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57-61. [DOI] [PubMed] [Google Scholar]
- 9.Magill ST, Theodosopoulos PV, McDermott MW. Resection of falx and parasagittal meningioma: complication avoidance. J Neurooncol. 2016;130(2):253-262. [DOI] [PubMed] [Google Scholar]
- 10.Hardesty DA, Wolf AB, Brachman DGet al.. The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg. 2013;119(2):475-481. [DOI] [PubMed] [Google Scholar]
- 11.Rogers L, Zhang P, Vogelbaum MAet al.. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaley TJ, Wen P, Schiff Det al.. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur G, Sayegh ET, Larson Aet al.. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16(5):628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magill ST, Lau D, Raleigh DR, Sneed PK, Fogh SE, McDermott MW. Surgical resection and interstitial iodine-125 brachytherapy for high-grade meningiomas: a 25-year series. Neurosurgery. 2017;80(3):409-416. [DOI] [PubMed] [Google Scholar]
- 15.Abou Al-Shaar H, Almefty KK, Abolfotoh Met al.. Brachytherapy in the treatment of recurrent aggressive falcine meningiomas. J Neurooncol. 2015;124(3):515-522. [DOI] [PubMed] [Google Scholar]
- 16.Polanczyk CA, Marcantonio E, Goldman Let al.. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637-643. [DOI] [PubMed] [Google Scholar]
- 17.Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7 (1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85(3):604-608. [DOI] [PubMed] [Google Scholar]
- 19.Jagetia GC, Rajanikant GK, Rao SK. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat Res. 2003;159(3):371-380. [DOI] [PubMed] [Google Scholar]
- 20.Levenson SM, Gruber CA, Rettura G, Gruber DK, Demetriou AA, Seifter E. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984;200(4):494-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin LC, Que J, Lin LK, Lin FC. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2006;65(3):745-750. [DOI] [PubMed] [Google Scholar]
- 22.Wick W, Gorlia T, Bendszus Met al.. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954-1963. [DOI] [PubMed] [Google Scholar]
- 23.Delishaj D, Ursino S, Pasqualetti Fet al.. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9(4):273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon CR, Rojavin Y, Patel Met al.. A review on bevacizumab and surgical wound healing. Ann Plast Surg. 2009;62(6):707-709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.