Highlights
-
•
The COVID-19 pandemic causes unprecedented impact on cancer patients.
-
•
Cancer patients are greater susceptible to COVID-19 infection and intense symptoms.
-
•
A rethink of the care pathway is required to reduce the risk of contamination.
-
•
Oncologists must detect and manage drug-related problems with anti-COVID-19 drugs.
-
•
Caution is needed about interactions between anticancer and viral experimental drugs.
Keywords: COVID-19, Immune depression, Drug safety, Drug-drug interactions, Herb-Drug interactions, Drug-related problems, Clinical trials
Abstract
The Coronavirus disease (COVID-19) pandemic is disrupting our health environment. As expected, studies highlighted the great susceptibility of cancer patients to COVID-19 and more severe complications, leading oncologists to deeply rethink patient cancer care.
This review is dedicated to the optimization of care pathways and therapeutics in cancer patients during the pandemic and aims to discuss successive issues.
First we focused on the international guidelines proposing adjustments and alternative options to cancer care in order to limit hospital admission and cytopenic treatment in cancer patients, most of whom are immunocompromised.
In addition cancer patients are prone to polypharmacy, enhancing the risk of drug-related problems as adverse events and drug-drug interactions. Due to increased risk in case of COVID-19, we reported a comprehensive review of all the drug-related problems between COVID-19 and antineoplastics.
Moreover, in the absence of approved drug against COVID-19, infected patients may be included in clinical trials evaluating new drugs with a lack of knowledge, particularly in cancer patients. Focusing on the several experimental drugs currently being evaluated, we set up an original data board helping oncologists and pharmacists to identify promptly drug-related problems between antineoplastics and experimental drugs.
Finally additional and concrete recommendations are provided, supporting oncologists and pharmacists in their efforts to manage cancer patients and to optimize their treatments in this new era related to COVID-19.
Introduction
Severe Acute Respiratory Syndrom-CoronaVirus-2 (SARS-CoV-2) has spread worldwide since late 2019, causing coronavirus disease 19 (COVID-19) in humans, involving severe acute respiratory syndrome associated with a multi-symptom presentation. From Wuhan in the Hubei province of China [1], [2], the virus spread rapidly in all continents, leading the World Health Organization, on March 11, 2020, to upgrade it as a pandemic [3]. There are currently so far nor approved preventive neither curative treatments. The global situation is inducing medical teams to conduct clinical trials to evaluate approved drugs for other indications. A concept rapidly emerged of patients at risk of severity. These include the particular population of cancer patients, in whom the immunosuppression and infection risk inherent to disease and treatment necessitates specific prevention and treatment measures.
The increased susceptibility of cancer patients to viral respiratory infection is well known, implicating both disease and treatment [4], [5]. Risk is higher in lymphopenia affecting cell immune function and antibody-mediated immunity [6]. Impaired lymphocyte function [7] and neutropenia [5] are further risk factors, as are monoclonal antibody treatment against lymphocyte antigens and above all hematopoietic stem-cell (HSC) allograft [7]. Cancer patients, especially lung cancer patients with associated respiratory disorder or ischemic cardiopathy, are also at high risk of severe infection [8]. For influenza, rates of hospital admission and mortality are respectively 4- and 10-fold higher in cancer patients than in the general population [9]. Coronavirus patients (excluding SARS-CoV-2) show significantly greater 30-day mortality in case of cancer (24.4% vs 3.0%, p < 0.001) [10]. Cancer was shown to be the main factor in 30-day mortality on multivariate analysis (p < 0.001), ahead of age > 65 years (p = 0.026), bacterial superinfection (p = 0.031), and initial state of shock (p = 0.042) [10].
The first data from a multicenter Chinese cohort of 1590 COVID-19 positive (COVID-19+) patients showed comorbidities to be a risk factor for contamination, severe manifestations and early death [11]. Patients with history of or active cancer showed higher risk of infection (1.13% versus 0.29% in the general population). Most of all, cancer patients (n = 18) deteriorated more rapidly (hazard ratio (HR) = 3.56 (95%CI, 1.65–7.69)) [8]. Allowing for the limitations of the study and notably the small sample, the authors considered cancer patients’ immune depression to be the prime cause of poor prognosis. A second Chinese series of 1276 patients in the Wuhan region found 2.2% prevalence of SARS-CoV-2 in cancer patients (28 infected), and 28.6% mortality: i.e., 1.7-fold greater prevalence (95%CI, 1.2–2.4) and 10-fold greater mortality than in the general population. Patients with anticancer treatment during the previous 14 days showed greater risk of mortality (HR = 4.08, 95%CI, 11–15.3, p = 0.037) [12]. Among 1524 patients followed for cancer in a Wuhan hospital, there was SARS-CoV-2 contamination in 12 cases (0.79%; 95%CI, 0.3–1.2%), higher than Wuhan population (0.37%). More than half the COVID-19+ patients (58.3%) had lung cancer and age > 60 years was a factor for extra risk (4.3% versus 1.8%) [13]. Other factors reported to aggravate COVID-19 infection in cancer patients include comorbidity such as type-2 diabetes, chronic kidney failure [14] or cardiovascular disease [15]. Most of the epidemiological studies are based on Chinese cohorts and caution is needed for the extrapolation to other countries with different comorbidity prevalence. An Italian study reports a prevalence of 20.3% (n = 72) patients with active cancer among 355 patients who died from COVID-19 [16]. Moreover, such patients are often polymedicated [17], [18], receiving drugs with narrow therapeutic index (antineoplastics, immunosuppressants) exposing COVID-19+ patients with symptoms to increased risk of drug interactions and potentialization of adverse effects.
Moreover, other risks, related to quarantine, need to be taken into account in managing cancer and its complications: Wang et al. notably report problems of access to health-care, drug shortages and delay in diagnosis of treatment toxicity [19]. The poor prognosis of cancer patients admitted with COVID-19 infection was also due to the decision not to admit certain (metastatic) patients to intensive care.
On the basis of these preliminary data, at least 3 consequences of the pandemic for the management of cancer patients can be raised:
-
–
Cancer patients seem more susceptible to SARS-CoV-2 infection, and complications (hospital admission, intensive care, death) are probably more frequent than in the general population [8], [12].
-
–
COVID-19+ cancer patients show greater risk of iatrogenic drug-related problems, due to polymedication [20].
-
–
The pandemic is tying up considerable human and economic resources and care activity is frequently rescheduled or cancelled, temporarily modifying cancer patient management. The lack of large clinical studies in cancer patients facing COVID-19 lead scientific societies and expert groups guidelines to advocate postponing surgery and drug treatment in some cases. The delay of cancer diagnoses has to be assessed in the months to come [21].
The aim of the present guidelines is to review and report a summary of the various recommendations/expert consensus published by scientific societies or oncology groups for the management of cancer patients in the context of the COVID-19 pandemic. We will focus on drug-related problems facing a polymedicated and fragile population (drug interactions, potentialization of symptoms and adverse effects), including molecules undergoing clinical trials. Finally, the ability of clinical oncology pharmacy to limit the risk of contamination and to manage drug-related problems in cancer patients will be assessed.
Cancer patient management in the context of the COVID-19 pandemic
There are two emerging risks for cancer patients: a 3-fold greater infection risk than in the general population (associated with poor prognosis and greater mortality) and the risk of delayed access to diagnosis, current care and therapeutic innovations. The challenge is thus to assess, on a case-by-case basis, the trade-off between delayed diagnosis and treatment of cancer and exposure to SARS-CoV-2.
Limiting the risk of COVID-19 infection in cancer patients
To limit the risk of SARS-CoV-2 contamination and the risks associated with cancer treatments, there have been several reports of experience and recommendations according to tumor location by expert groups and scientific societies [22], [23], [24] and summary are now available [25]. Cancer patients are more liable to get infected by SARS-CoV-2 due to immune depression induced by the cancer and treatments such as chemotherapy and surgery [26], [27]. Organizational measures and treatment adaptations can be proposed to reduce such risks.
Organizational measures
For cancer patients, the difficulty of social distancing is aggravated by limited contact with health-care centers. Any organizational measure reducing the number and duration of consultations, chemotherapy sessions and admissions are welcome. Vigilance is to be increased regarding risk factors: lymphopenia, neutropenia, age > 65 years, and comorbidities [28]. The overall strategy to avoid contact between cancer patients and COVID-19+ patients involves separating COVID-19+ and COVID-19-free pathways in care centers or organizing care in a separate structure. For outpatients receiving oral antineoplastics, in-hospital follow-up (medical consultations, pharmaceutic interviews, therapeutic drug monitoring) are to be suspended whenever possible in favor of telemedicine [24]. In-hospital drug delivery (immunomodulators (IMiD), recent tyrosine kinase inhibitors (TKI), drugs under temporary use authorization) can be sent to a corresponding community pharmacy near to the patient’s home.
Adapting anticancer treatment strategy
The management of cancer patient’s treatment continues to be discussed case by case into the management case conferences and clinicians are required to take decisions for protecting patients regarding the emerging risk. The lack of published data or conducted clinical studies makes it difficult to take the right therapeutic decision. Because of the emergency situation, the summary of recommendations cannot be based solely on the highest evidence, clinical trials and meta-analyses. Adaptations as modified, delayed or discontinued anticancer treatments were suggested mainly based on expert consensus. In addition the first Chinese series are lacking of consistency for demonstrating the significant risk in cancer patients [29]. Regarding the association with severity of COVID-19, however, a recent analysis from the Montefiore Medical Center in New York among 218 cancer patients suggested an association between cancer patients and COVID-19 related fatality in particular for hematologic malignancies (case fatality rate of 37% versus 25% for solid malignancies) [30]. The authors conclude that there is a need for proactive strategies to reduce likelihood of infection and improve early identification in this vulnerable patient population.
It appears reasonable to limit hospital visit for cancer patients including for receiving anticancer treatment but, in the other hand, it seems challenging to suggest discontinuing an active anticancer treatment after several months of treatment. Hanna et al. have provided an outstanding commentary regarding the precautionary principle in the context of COVID-19 [31]. Because countries and hospitals are undergoing the pandemic curve at different time and intensity, they define three scenarios: the case when health systems are (1) preparedness (with no confirmed cases); (2) moderate health-care resource limitation; (3) severe health-care resource limitation. Based on a conceptual framework for prioritizing the use of radiotherapy and systemic treatments during the COVID-19 pandemic, they define nine categories classified from low (Alternative treatments exist or delay does not affect outcomes) to high risk (Imminent risk of early mortality). Although the authors emphasize the need to prioritize cancer treatment, they recommend to take into account one of each scenario and to use the different prioritizations above in accordance. The absence of evidence avoids adaptations of therapies in a global approach. The place for multidisciplinary case conferences has also to be highlighted. Hanna et al. took the examples of decreased survival for patients receiving adjuvant chemotherapy for colorectal cancer and breast cancer [32], [33]. On the other hand, the replacement of fluorouracil by capecitabin for adjuvant colorectal cancer patients is based on evidence [34].
The following factors need to be taken into account: curative or palliative objective; patient age (with increased risk from 60 or and 70 years, depending on the report, in lung [13] or digestive/colorectal cancer [35] and others [36]); life-expectancy; and number of treatment lines [31], [22]. For comparable efficacy, oral antineoplastics compatible with home therapy are preferable to parenteral (i.e. capecitabine instead of 5-fluorouracile). A pause in treatment can be considered in slow-progression cancer under control for several months. According to the expert opinion of the European Society for Blood and Marrow Transplantation [37], patients scheduled for HSC transplantation or chimeric antigen receptor T-cell (CAR-T) therapy must be screened for SARS-CoV-2 before conditioning therapy; if positive, treatment should be postponed for at least 3 months according to the guidelines of the European Centre for Disease Prevention and Control [38]. In such clinical emergencies without alternative treatment, the risk/benefit ratio may indicate continuation of treatment, in which case it is primordial to check COVID-19 status ahead of immunosuppressive conditioning or lymphodepleting treatment [37].
Depending on tumor location, the scientific societies and expert groups advise dose adaptation or replacement of certain anticancer treatments. Risk is considered low for radiation therapy, moderate for single-agent treatment and oncologic surgery. Lymphopenia is likely to be associated with more severe COVID-19 outcomes. Risk seems very high in case of polychemotherapy, especially in case of <600/mm3 lymphopenia and more especially if this is persistent with associated long-course corticosteroids. A recent systematic review focusing on risk factors associated to mortality in patients with COVID-19, showed that patients in the non-survival group were more likely to have a lower lymphocyte count (p < 0.00001) [39]. However the significance of neutropenia is less clear and the question of risks arises with cytopenic TKIs (dasatinib, imatinib, palbociclib, abemaciclib, olaparib and others [40]). Awaiting further studies, clinicians should also consider cytopenic TKIs as a potential risk factor of severe COVID infection.
Immune checkpoint inhibitors (anti-PD-1 and anti-PD-L1 monotherapy) do not induce immunosuppression and preliminary available analyses did not shown detrimental effect of immunotherapy compared to other anticancer treatment in the Thoracic cancERs international coVid 19 cOLlaboraTion (TERAVOLT cohort) [41] and depending of the temporal relationship between treatment exposure and diagnosis of COVID-19 [42]. Caution is nevertheless required due to possible cumulative risk with COVID-19 symptoms, with rare but severe interstitial pneumopathy aggravating the pulmonary damage [43]. Such cumulative risk may also occur with certain oral antineoplastics such as everolimus, crizotinib, and trametinib [44]. Some authors also suggested cumulative risk of cytokine release syndrome between immune checkpoint inhibitors or CAR-T cells and cytokine storm in severe COVID-19 infection [43]. Expert opinion favors postponing immunotherapy if possible, especially in case of associations such as nivolumab-ipilimumab and in stable diseases [35], [45]. In the case where immune checkpoint inhibitors have to be initiated or continued, half-reduced frequency administration has to be considered for nivolumab and pembrolizumab by double the dose [47.48]. Because of an elimination half-life of 27 days, a reduced frequency from Q3W to Q4W should also be considered for atezolizumab (anti-PD-L1) by increasing the dose from 1200 mg to 1680 mg [41]. Despite elimination half-life of 12 days for durvalumab (anti-PD-L1) the same reduced frequency schedule from Q3W to Q4W should be considered by increasing the dose from 1200 mg to 1500 mg [41].
A summary of expert guidelines regarding this issue are shown in Table 1 [35], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]. For example, carboplatin should replace cisplatin, being quick to administer and less toxic without compromise on efficacy; treatments with risk of pulmonary toxicity, such as bleomycin, can be changed. G-CSF (granulocyte-colony stimulating factor) use should be encouraged in case of risk of neutropenia. Although scientific societies have not declared a consensus, the prevalence of cardiac involvement with COVID-19 [76] casts doubt on the use of cardiotoxic anticancer drugs such as anthracyclines or trastuzumab if there are alternatives available. And, overall, delays in implementing surgery, radiation therapy or grafting (notably for allografts) may require stop-gap antineoplastics in order to control tumor progression. Finally we report in the Table 1 the expert consensus proposals regarding immune checkpoint inhibitors use in advanced lung cancer patients. Beyond the need to reduced administration frequency in order to limits, several expert groups suggest treatment discontinuation in responders but the treatment duration varies from 1 [41], [45] to 2 years [48], [51]. In the study by Santini et al. among 482 advanced NSCLC patients, n = 68 of them stopped ICI due to irAE (median time of the first irAE was 69–73 days). For n = 38 patients with rechallenge, the median time from detection of the initial irAE to retreatment was 32 days (range 7–177) and the median duration of ICI from the start of retreatment was 9.2 months (range 23 days–34 months). The estimated 2-year survival from diagnosis was 64% [77]. The one prospective trial which focused on this point was the CheckMate-153 comparing the effect of nivolumab discontinuation after 1 year (in case of partial response or stable disease) versus continuous nivolumab in patients with advanced NSCLC which shown a clear benefit to continuation strategy in term of progression-free survival (PFS) (not reach vs. 10.3 month, respectively, hazard ratio = 0.42 [95% CI: 0.25–0.71]) but not in term of OS (not reached vs. 23.2 months, respectively, HR 0.63 [95% CI: 0.33–1.20]) [78]. Based on this result and out of the COVID-19 context, the recent commentary by Metro & Signorelli reminds us that treatment duration in patients who are benefiting from an ICI should be certainly >1 year [79]. The Table 1 reports this suggestion of at least 2 years of treatment to consider discontinuation.
Table 1.
Summary of International and National guidelines during COVID-19 according to the different cancer type and tumoral localisation.*
| Cancer type and/or localisation | Stage | Antineoplastic protocol | Therapeutic adjustment options in the limits and rules of the national regulatory agencies, and as per local guidelines and practice | Reference | |
|---|---|---|---|---|---|
| Lung cancer | NSCLC | Adjuvant | Cisplatin – Paclitaxel | Carboplatin - Paclitaxel + G-CSF | [48], [49] |
| Locally advanced | Durvalumab | Reduced frequency Q4W and double the dose | [45], [50] | ||
| Metastatic | Nivolumab | Discontinuation of immunotherapy after 2 years of treatment should be considered. Stop (>2year) or reduced frequency Q4W and double the dose | [41], [45], [46], [48], [51] | ||
| Metastatic | Pembrolizumab | Discontinuation of immunotherapy after 2 years of treatment should be considered. Stop (>2year) or reduced frequency Q6W and double the dose | [41], [45], [47], [48], [51] | ||
| Metastatic | TKI targeting EGFr | Treatment continuation and monitoring using telemedicine | [48], [51] | ||
| SCLC | Local | Cisplatin – Etoposide (± atezolizumab or durvalumab) | Carboplatin – Etoposide + G-CSF (± atezolizumab or durvalumab) | [48], [53] | |
| Skin cancer | Melanoma | Adjuvant or advanced | Nivolumab Q2W Pembrolizumab Q3W |
Reduced frequency (nivolumab Q4W and pembrolizumab Q6W) and double the dose | [46], [47], [52] |
| Genitourinary | Prostate | Metastatic first line | Docetaxel | Androgen deprivation + abiraterone/enzalutamide (expert consensus) | [54], [55], [56] |
| Prostate | Metastatic pre-treated with second generation hormonotherapy | Docetaxel | Avoid or reduce the number of docetaxel cycles + G-CSF (expert consensus) | [54], [55] | |
| Seminoma | Metastatic with intermediate risk | BEP Protocol | Avoid bleomycin (VIP + G-CSF) (expert consensus) | [54], [55] | |
| Bladder | Metastatic first line | Intensive MVAC Protocol | Cisplatin – Gemcitabine + G-CSF (expert consensus) | [54], [55] | |
| Kidney | Metastatic with high or intermediate risk | Ipilimumab-Nivolumab | TKI sunitinib or pazopanib (expert consensus) | [54], [55] | |
| Digestive | Colic | Adjuvant | FOLFOX | CapOx or capecitabine monotherapy (low risk) or no treatment (frail patients) (expert consensus) | [35], [57] |
| Colorectal | Metastatic unresectable | FOLFOX or FOLFIRI ± targeted therapy | Capecitabine or CapOx or CapIri ± targeted therapy (expert consensus) | [35], [57] | |
| Pancreas | Local | FOLFIRINOX | FOLFOX or FOLFIRINOX without 5-FU bolus and cap irinotecan at 150 mg/m2; add G-CSF | [35], [57], [58], [59] | |
| Gastric | Local | FLOT perioperative | FLOT + G-CSF or CapOx (if no dysphagia) | [35], [57], [60] | |
| Oesogastric | Metastatic | FOLFOX ± trastuzumab | CapOx ± trastuzumab (if HER2+++) (expert consensus) | [61] | |
| Anal cancer | Metastatic | 5FU-cisplatin or DCF protocol | CapOx or carboplatin - capecitabine (expert consensus) | [35] | |
| GIST | Adjuvant post-operative | TKI targeting bcr-abl | TKI continuation and monitoring using telemedicine (expert consensus) | [35] | |
| Breast | Breast | Metastatic | CDK4/6 inhibitors | Adapt doses or postpone CDK4/6 inhibitors to avoid neutropenia (expert consensus) | [62], [63] |
| Upper Aero-digestive Tract | Head and Neck | Metastatic | TPEx | Adapt schedule from Q3W to Q2W with reduced doses of cisplatin and docetaxel (both 40 mg/m2) and cetuximab 500 mg/m2 (expert consensus) | [64] |
| Neuro-oncology | Glioma IDH-wt MGMT-methylated |
High grade | Chemo-radiotherapy with temozolomide | Treatment continuation (expert consensus) | [65], [66] |
| Glioma IDH-wt | High grade | Bevacizumab Q2W | Bevacizumab Q6W to Q8W (expert consensus) | [65] | |
| Glioma IDH-mutated | Oligo-symptomatic | Procarbazine, lomustine, vincristine | Consider to report for 6 months or more (expert consensus) | [65] | |
| Hematology | Follicular lymphoma | Induction | Immuno-chemotherapy anti-CD20-based | Anti-CD20 alone If chemotherapy is necessary, prefer R-CHOP to R-Bendamustine (expert consensus) |
[67], [68] |
| Follicular lymphoma Mantle cell lymphoma |
Maintenance | Anti-CD20 | Consider to report or remove maintenance cycles | [67], [68], [69] | |
| Chronic Lymphocytic Leukaemia | Induction | Rituximab and venetoclax | Avoid anti-CD20 and venetoclax. Prefer alternative therapies (expert consensus) | [70] | |
| Lymphoblastic Acute Leukaemia | Maintenance | POMP | Stop vincristine and corticosteroids and maintain methotrexate and 6-mercaptopurine (expert consensus) | [71] | |
| Multiple Myeloma | Induction | VRD following by ASCT | Report ASCT as possible; replace by 6 or 8 cycles of VRD according to the risk stratification (expert consensus) | [72], [73] | |
| Induction/consolidation or relapsed/refractory | Dexamethasone 40 mg weekly | Decrease dexamethasone to 20 mg weekly or avoid if possible | [72], [73], [74] | ||
| Relapsed and/or refractory | Carfilzomib D1-2 Daratumumab Q2W |
Reduce carfilzomib frequency D1 Daratumumab monthly frequency until cycle 3 (expert consensus) |
[72], [73], [75] |
ASCT: Autologous Stem Cell Transplant; BEP: Bleomycin, Etoposide, CapOx: Capecitabine, Oxaliplatin; CapIri: Capecitabine, Irinotecan; Cisplatin; COVID-19: Coronavirus Disease-19; EGFr: Epidermal Growth Factor receptor; FLOT: Docetaxel, 5-fluorouracile, oxaliplatin; FOLFOX: Oxaliplatin, 5-fluorouracile; FOLFIRI: Irinotecan, 5-fluorouracile; FOLFIRINOX; Oxaliplatin, Irinotecan, 5-fluorouracile; G-CSF: Granulocyte-Colony Stimulating Factors; GIST: Gastro-Intestinal Stromal Tumor; GRAALL: Group for Research on Adul Acute Lymhoblastic Leukemia; IDH: Isocitrate deshydrogenase; MGMT: O-6-methylguanine-DNAmethyltransferase; MVAC: Methotrexate, Vinblastine, Doxorubicin, Cisplatin; NSCLC: Non-Small Cell Lung Cancer; POMP: Methotrexate, Vincristine, Prednisone, Mercaptopurine; Q2W: Every 2 weeks; Q3W: Every 3 weeks; Q6W: Every 6 weeks; Q8W: Every 8 weeks; R-CHOP: Rituximab, Cyclophosphamide, Doxorubicine, Vincristine, Prednisone; TPEx: Docetaxel, Cisplatin, Cetuximab; VRD: Velcade, Revlimid, Dexamethasone; wt: Wild Type; SCLC: Small Cell Lung Cancer; TKI: Tyrosine Kinase Inhibitor; VIP: Vinblastine, Ifosfamide, Cisplatin;
The fact that COVID-19 emerged so recently means that data are controversial. It has been suggested that non-steroidal anti-inflammatory drugs (NSAIDs) may exacerbate the risk of respiratory and cardiac complications in various situations of infection, and thus likely in severe forms of COVID-19 [80]. Regarding corticosteroids, a meta-analysis performed by the WHO following the SARS-CoV epidemic found no harmful effects [81]; in COVID-19, clinical trials using corticosteroids are ongoing especially in the early stage of respiratory distress syndrome. In the absence of firm evidence, corticotherapy should be avoided whenever possible, and any prescription of high-dose corticosteroids, including for anticancer purposes, needs to be assessed on a case-by-case basis as seen in Table 1 for dexamethasone in multiple myeloma patients [72]. The use of corticosteroid as antiemetic does not reach a consensus [35], [82].
Treatment of COVID-19+ cancer patients: Anticancer treatment and risks incurred by anti-COVID-19 treatment (excluding intensive care condition)
In cancer patients, diagnosis of COVID-19+ indicates admission to a specialized COVID-19 unit, or transfer for oncology inpatients. In oncology centers, dedicated COVID-19 units, including palliative care units, are planned, depending on the number of such patients in the catchment. Management is complex, taking into account of the potentially life-threatening prognosis in COVID-19, loss of chance due to cancer treatment discontinuation in progressive cancer, and the immunosuppression risk incurred by both diseases and possibly by the required drugs. The risk of developing severe forms of COVID-19 infection argues for interrupting, in most cases, the anticancer treatment while the COVID-19 infection is being treated. Nevertheless, cancer patients will have likely received drug treatment in the days preceding COVID-19 diagnosis and require supplementary protection against the anti-COVID-19 regimen. The iatrogenic issue is double: anticipating the immunosuppressive impact of certain anti-COVID-19 drugs with risk of symptom aggravation; and managing drug interaction between anti-COVID-19 drug and antineoplastic agents. This double risk has to be taken into account in low-symptom COVID-19 infection concomitant to progressive cancer, where the ongoing anticancer treatment may be continued or else adapted.
In Europe, the antiviral anti-COVID-19 armamentarium mainly comprises association lopinavir/ritonavir, hydroxychloroquine, remdesivir and azithromycin. These drugs are use off-label with a facilitating regulatory framework. In the DisCoVeRy clinical trial (NCT04315948), interferon beta-1a is also used. When anticancer treatment is continued in a COVID-19+ patient, or for the case of antineoplastics with long elimination half-life, the infection treatment may give rise to drug interactions or potentialize adverse effects. Expected interactions between COVID-19 treatments, cancer treatments and the support treatments frequently used for cancer patients have therefore been reviewed (Table 2A, Table 2B, Table 2C and Table 3 ), based on the summaries of product characteristics (SPC) of each and several specialized drugs-interactions screening database: Theriaque® (Centre National Hospitalier d’Information sur le Médicament, https://www.theriaque.org/), Drugs.com (Food and Drug Administration https://www.drugs.com/), HIV Drug Interactions (University of Liverpool, https://www.hiv-druginteractions.org/), QT Drugs List (Arizona Center for Education and Research on Therapeutics, https://www.crediblemeds.org/), DDI-Predictor (Claude Bernard University, Lyon 1, https://www.ddi-predictor.org/), GPR-ICAR Department (Paris Hospitals Board (AP-HP), http://sitegpr.com/fr/), and the Oncolien® data-base (SFPO, https://oncolien.sfpo.com/).
Table 2A.
Pharmacokinetic interactions related to lopinavir/ritonavir and oral antineoplastics (variation in plasma concentration and exposure of antineoplastic – cytochromes and transporters involved).
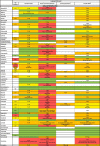 |
 : No data available or degree of interaction not measurable.
: No data available or degree of interaction not measurable.
 : 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
: 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
 : Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
: Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
 : Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
: Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
 : Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), AUC Ratio ≥ 10 (DDI Predictor).
: Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), AUC Ratio ≥ 10 (DDI Predictor).
↗ Increase of chemotherapy/immunosuppressive/support treatment concentration.
↘ Decrease of chemotherapy/immunosuppressive/support treatment concentration.
▲ Increase of lopinavir/ritonavir concentration.
▾ Decrease of lopinavir/ritonavir concentration.
AUC Ratio = area under the substrate concentration in blood or plasma over the dosing interval at a steady-state/AUC of the substrate given with an inhibitor an inducer.
NA: Not Applicable (cytochrome CYP is not a relevant pathway).
pNET: primitive Neuro Ectodermal Tumor.
GIST: Gastro Intestinal Stroma Tumor.
Table 2B.
Pharmacokinetic interactions related to lopinavir/ritonavir and parenteral antineoplastics, immunosuppressive drugs and most used support treatment.
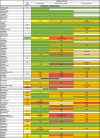 |
 : No data available or degree of interaction not measurable.
: No data available or degree of interaction not measurable.
 : 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
: 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
 : Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
: Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
 : Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
: Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
 : Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), AUC Ratio ≥ 10 (DDI Predictor).
: Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), AUC Ratio ≥ 10 (DDI Predictor).
↗ Increase of chemotherapy/immunosuppressive/support treatment concentration.
↘ Decrease of chemotherapy/immunosuppressive/support treatment concentration.
▲ Increase of lopinavir/ritonavir concentration.
▾ Decrease of lopinavir/ritonavir concentration.
AUC Ratio = area under the substrate concentration in blood or plasma over the dosing interval at a steady-state/AUC of the substrate given with an inhibitor an inducer.
BCRP: Breast Cancer Resistance Protein.
MATE: Multidrug and Toxin Extrusion Protein.
OCT: Organic Cation Transporter.
UGT: Uridine Glucuronyl Transferase.
NA: Not Applicable (cytochrome CYP is not a relevant pathway).
Table 2C.
Pharmacokinetic interactions related to hydroxychloroquine, azithromycin and remdesivir.
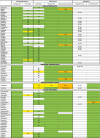 |
 : No data available or degree of interaction not measurable.
: No data available or degree of interaction not measurable.
 : 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
: 1 < AUC ratio ≤ 1.4 (DDI Predictor), No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque).
 : Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
: Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque), 1.5 ≤ AUC ratio ≤ 1.9 (DDI Predictor).
 : Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
: Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), 2 ≤ AUC Ratio ≤ 9.9 (DDI) or 0.4 < AUC Ratio AUC < 0.6 (DDI Predictor).
AUC Ratio = area under the substrate concentration in blood or plasma over the dosing interval at a steady-state/AUC of the substrate given with an inhibitor an inducer.
▲ Increase of lopinavir/ritonavir concentration.
▾ Decrease of lopinavir/ritonavir concentration.
Table 3.
Pharmacodynamics interactions related to anti-COVID-19 drugs and antineoplastics, immunosuppressive drugs and most used support treatment.
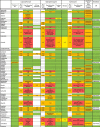 |
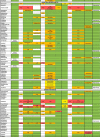 |
 : No data available or degree of interaction not measurable.
: No data available or degree of interaction not measurable.
 : No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque, QT Drugs List).
: No interaction expected (Drugs Information Database, HIV Drugs Interaction, Theriaque, QT Drugs List).
 : Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque).
: Potential Weak Interaction (HIV Drug Interaction), Minor Interaction (Drugs Information Database), To be taken into account or precaution for use (Theriaque).
 : Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), possible risk of torsades de Pointes (QT Drugs List).
: Potential Interaction (HIV Drug Interaction), Moderate Interaction (Drugs Information Database), Avoid association (Theriaque), possible risk of torsades de Pointes (QT Drugs List).
 : Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), known risk of torsades de pointes (QT Drugs List).
: Contra-indicated (Theriaque), Major Interaction (Drugs Information Database), Do Not Coadminister (HIV Drug Interaction), known risk of torsades de pointes (QT Drugs List).
↗ Increase of chemotherapy/immunosuppressive/support treatment concentration.
↘ Decrease of chemotherapy/immunosuppressive/support treatment concentration.
SPC: Summary of Product Characteristics.
Association lopinavir/ritonavir
The protease inhibitor association lopinavir and ritonavir is commonly used to treat HIV-1 infection. Some in-vitro and clinical data demonstrated its activity on SARS-CoV-2 virus. The dosage used for COVID-19 is the same as HIV-1 treatment: 400/100 mg, twice daily for 7–14 days.
Pharmacokinetic interactions
Lopinavir and ritonavir are strong inhibitors of cytochrome P450 isoform CYP3A, thus increasing the concentration of numerous CYP-substrate drugs. They are also efflux protein P-gp inhibitors leading to increased concentration of P-gp substrates. Interactions are numerous, notably with narrow-window treatments. Among oral antineoplastics, Liver cytochrome metabolized Kinase Inhibitors (including TKIs and others) should be avoided in association to lopinavir/ritonavir. If TKIs continuation is needed, dose reduction and monitoring of TKI-related adverse events are advised (Table 2A). It is important to note that drug-interaction impact may be different according to the database. Some support treatments such as hydroxyzine, oxycodone and zopiclone, often prescribed for cancer patients should be avoided or at least closely monitored when associated to 3A4 inhibitor or antiretroviral drugs with cardiac toxicity. In hematologic cancer patients with HSC graft, the benefit of lopinavir and ritonavir needs weighing against the increased risk of post-graft immunosuppressive treatment imbalance and resultant complications such as graft-versus-host disease (GVHD). Lopinavir and ritonavir are also CYP3A4 substrates, and concomitant moderate or powerful inducers require special vigilance. Dabrafenib and enzalutamide can continue to impair the efficacy of antiretrovirals for several days after discontinuation, with risk of failure of COVID-19 treatment.
Pharmacodynamic interactions, additive adverse effects
Cardiotoxicity by QT prolongation is frequent with lopinavir and ritonavir (Table 3). Many TKIs, doxorubicin and ondansetron seriously increase QT interval and should not be associated to lopinavir or ritonavir (Table 3). The same is true for serotonin reuptake inhibitors, frequently prescribed for depression in cancer patients [83]. Autoimmune diseases, and notably hepatitis and pancreatitis, have been reported with lopinavir and ritonavir, which should not be used if immune checkpoint inhibitors are continued.
Hydroxychloroquine
Hydroxychloroquine is used to prevent systemic lupus episodes and other autoimmune and inflammatory diseases. Its activity involves viral infection inhibition (experimental) and immunomodulation contributing to control of the cytokine storm that occurs in the late stage of COVID-19 infection. A loading dose at Day 1 followed up by 400 mg daily dose for 9 days is usually reported in studies. The loading dose is within the maximal 600 mg/day specified in the market authorization (400 mg twice daily only in clinical trials).
Pharmacokinetic interactions
Hydroxychloroquine is a weak inhibitor of CYP2D6 and of the P-gp efflux protein, requiring intensified monitoring when used concomitantly with narrow-window drugs having this metabolic pathway: gefitinib, tamoxifen (Table 2C). A few clinically relevant pharmacokinetic drug-interactions are reported with hydroxychloroquine.
Pharmacodynamic interactions, additive adverse effects
Hydroxychloroquine incurs a risk of QT prolongation and torsade de pointe occurrence when associated to other drugs with the same toxicity profile. ECG monitoring is widely established and has to be systematically done in case of certain antineoplastics (see Table 3) such as cabozantinib, ceritinib, crizotinib, nilotinib, osimertinib, vandetanib, vemurafenib, oxaliplatin and arsenic trioxide [84].
Attention should also be paid to support treatments. Risk of torsade de pointe contraindicates association to ondansetron (>8mg) or domperidone (replace by metomimazine). Antidepressants such as (es)citalopram widely prescribed for cancer patients are likewise contraindicated [85]. All torsade de pointe risks such as hypokalemia (with corticosteroids, cisplatin or other drug when associated with digestive disorders) and bradycardia (e.g., with thalidomide) should also be taken into account.
Hydroxychloroquine also incurs a risk of peripheral neuropathy. Vigilance is required for patients treated by platinum salts, anthracyclines, taxanes and immunomodulators, and even more in case of comorbidities such as diabetes mellitus or chronic alcohol abuse. Regarding the ocular toxicity of hydroxychloroquine, attention should also be required when associated with MEK inhibitors for example [86].
Remdesivir
Remdesivir is a nucleoside analog of adenine used against Ebola with an orphan drug status approval in the United Stated but not in Europe. It has undergone several clinical trials, in the light of in-vitro efficacy against Coronaviruses (SARS-CoV, MERS-CoV). For COVID-19, the most used dosage in clinical trials is 200 mg on day 1 then 100 mg daily by parenteral route for 10 days.
Pharmacokinetic interactions
The interaction profile is favorable, with neither induction nor inhibition of cytochrome P450 and of the main transporters in preclinical studies so no impact is expected in this way. As a CYP3A4 substrate, the association to an inductor or inhibitor drug requires close monitoring for adverse effects (Table 2C).
Pharmacodynamic interactions, additive adverse effects
The safety profile includes nephrotoxicity and hepatotoxicity requiring dose adaptation when reduced glomerular filtration rate or transaminase elevation, respectively. Association to nephrotoxic or hepatotoxic drugs requires monitoring. Recent clinical trials reported diarrhea and hypokalemia up to 9% and 12%, respectively [87], [88]. Attention should be payed to serum potassium levels in patients taking diarrhea and/or hypokalemia-induced medicines. As anemia and thrombocytopenia can also be observed, association with antineoplastic agents with significant hematologic toxicities should be used with caution (Table 3).
Azithromycin
Azithromycin is a macrolide antibiotic used for COVID-19 to prevent bacterial super-infection. Clinical practices report mainly schedules based on 500 mg on day 1 followed by a daily dose of 250 mg the next four days, associated, or not, to hydroxychloroquine.
Pharmacokinetic interactions
Azithromycin appears to inhibit efflux protein P-gp leading to increased concentration of P-gp substrates. Caution should be taken particularly with narrow therapeutic index drugs such as cyclosporine in immunocompromised patients. Blood concentration of cyclosporine should be closely monitored.
Pharmacodynamic interactions, additive adverse effects
The major risk concerns the cardiac sphere with QT prolongation and risk of torsade de pointe. This effect is particularly feared considering the frequent association of azithromycin and hydroxychloroquine. The use of this association must be avoided with some TKIs, arsenic trioxide and antidepressant.
Interferon beta-1a
Interferon beta-1a is indicated in multiple sclerosis and is evaluated in DisCoVeRy trial at 44 µg at days 1, 3 and 6. It shows a very favorable interaction profile, with no cytochrome- or transporter-mediated effects. Some rare cases of autoimmune hepatitis or severe liver failure were reported. Liver monitoring should be intensified in case of association to potentially hepatotoxic drugs such as crizotinib or lenalidomide (Table 3). In case of at-risk associations, the Drugs.com data-base advises treatment adaptation if the alanine transaminase threshold exceeds 5 times the normal value. There is also a risk of lowering the epileptogenic threshold, as association to tramadol is strongly contraindicated, especially in at-risk patients (epilepsy, advanced age, brain tumor).
Alternative and complementary medicine
Complementary and Alternative Medicines (CAM) are widespread in cancer patients [89], and has to be taken into account in the present crisis. Prevailing uncertainties concerning the prevention and treatment of COVID-19 stimulate use to CAM [90], [91]. The potential toxicity of certain plants can cumulate with anti-COVID-19 drug toxicity and pharmacokinetic drug-interactions can occur, and necessitates screening. Notable risks include [92], [93]:
-
-
QT interval prolongation: Boldo, Fucus, Asian Ginseng, Seville orange, Passion flower, Dandelion
-
-
Increased immunosuppression: Olive tree, Curcuma, Licorice
-
-
Immunity reinforcement: Echinacea, Ginseng
-
-
Nephrotoxicity: Licorice, White willow
-
-
Hepatotoxicity: Ruscus, Horsetail
-
-
Laxative effect: Rhubarb, Senna, Ispaghul
-
-
Strong CYP3A4 inhibition: Cranberry, Fenugreek, Ginger, Grapefruit, Seville orange, Aloe Vera, Curcuma, Gingko biloba
-
-
CYP3A4 induction: St John’s wort, Garlic, Hawthorn, Coneflower, Kava Kava, Green mint, Sage
COVID-19 and clinical research: Taking account of the cancer patient’s situation
Clinical research on COVID-19 treatment
In the context of the COVID-19 pandemic, clinical research is crucial. The prime aim is to develop treatment drugs. The second is to launch fast-track controlled trials [94], without methodological short-cuts and able to assess relevant efficacy of potentially interesting drugs from early stage of infection to advanced intensive care. A major point is the implementation of adaptive trials able to provide rapid intermediate results. In France, research is piloted by the INSERM’s REACting (RESearch and ACtion targeting emerging infectious diseases) consortium. The Flash call for projects by the ANR national research agency selected 86 projects with a €14.5 M funding budget. In Europe, €48.5 m has been allocated to 18 projects by the European Commission – Coronavirus Research and Innovation. A recent example of the ongoing dynamic is the phase III DisCoVeRy clinical trial comparing several adjuncts (remdesivir, lopinavir/ritonavir, interferon beta-1A, hydroxychloroquine) to standard care, beginning March 22. It should finally include 3200 patients in Europe, including 800 in France. The WHO’s Solidarity trial, currently running in >20 countries across four continents (North and South America, Africa, Asia and Europe), is also based on an adaptive methodology; comparing adding hydroxychloroquine or remdesivir to standard care, the protocol will be enriched by other treatment arms such as favipiravir [95].
The aim is a quick and easy inclusion of several thousands of patients worldwide so as to obtain emergency data on the pandemic. The Table 4 reports the ten most frequent investigated medicines in clinical trials currently underway and their potential risk for cancer patients [96], [97], [98], [99], [100], [101]. Many of these trials use closed approach in a competitive strategy. They include several anticancer or associated drugs such as bevacizumab, nivolumab, ruloxitinib, thalidomide, methylprednisolone and tocilizumab. Tocilizumab is an anti-interleukin-6 (IL-6) monoclonal antibody initially indicated for rheumatic disease and more recently applied in severe cytokine release syndrome induced by CAR-T cell administration [102] for malignant hematopathy; application to COVID-19 is based on the same rationale, inhibit IL-6 pathway and consequently a part of pro-inflammatory cytokines [103]. For other drugs under assessment, a literature search was conducted (Table 3) to identify points of vigilance for COVID-19 patients with cancer included in the registered trials: drug interactions, cumulative risk of cytopenia, contraindications in immune depression. An exhaustive review of the investigational medicines product ongoing clinical trials is available in Appendix.
Table 4.
Ten most represented Investigational Medicine Products (IMP) on ongoing clinical trials for the treatment of COVID-19 and potential impact for cancer patients.
| IMP | NCT identifier | Documented/hypothetical mechanism of action | Potential impact for cancer patients |
|---|---|---|---|
| Allogeneic Mesenchymal Cells | NCT04366271, NCT04361942, NCT04339660, NCT04252118 NCT04313322, NCT04348461, NCT04336254, NCT04348435 NCT04315987, NCT04273646, NCT04366830, NCT04346368 NCT04288102, NCT04293692, NCT04352803, NCT04366063 NCT04366323, NCT04349631, NCT04377334, NCT04345601 NCT04355728, NCT04382547, NCT04333368, NCT04302519 NCT04371601, NCT04341610, NCT04269525, NCT04276987 |
Immunosuppressive and tissue repair properties | No potential impact [96] |
| Angiotensin-converting-enzyme inhibitors Angiotensin receptor blockers |
NCT04329195, NCT04335136, NCT04375046, NCT04287686 NCT04345406, NCT04353596, NCT04364893, NCT04337008 NCT04351581, NCT04330300, NCT04338009, NCT04332666 NCT04375124, NCT04335123, NCT04312009, NCT04311177 NCT04340557, NCT04366050, NCT04355936, NCT04360551, NCT04335786 |
Anti-hypertensive agents inhibiting virus entry into the host cell (mediated by ACE2 receptor) | No potential impact |
| Bacille Calmette-Guérin Vaccine | NCT04327206, NCT04328441, NCT04379336, NCT04362124 NCT04350931, NCT04369794, NCT04373291, NCT04348370, NCT04384549 |
Live vaccine | Risk of live attenuated vaccine |
| Chloroquine Hydroxychloroquine |
NCT04362332, NCT04359537, NCT04328272, NCT04351919 NCT04346329, NCT04329611, NCT04345653, NCT04351516 NCT04370262, NCT04352933, NCT04347889, NCT04350450 NCT04364815, NCT04369742, NCT04363866, NCT04382625 NCT04340544, NCT04329923, NCT04351620, NCT04333225 NCT04345692, NCT04323631, NCT04331834, NCT04371926 NCT04385264, NCT04315896, NCT04354870 NCT04342221, NCT04353271, NCT04372017, NCT04358068 NCT04330495, NCT04318444, NCT04330144, NCT04318015 NCT04381988, NCT04261517, NCT04379492, NCT04341441 NCT04370015, NCT04354441, NCT04328961, NCT04363827 NCT04334967, NCT04363450, NCT04332991, NCT04361461 NCT04384380, NCT04334148, NCT04342169, NCT04342156 NCT04321278, NCT04371523, NCT04333654, NCT04374942 NCT04325893, NCT04353037, NCT04316377, NCT04308668 NCT04303507, NCT04336748, NCT04349371, NCT04333732 NCT04333628, NCT04328493, NCT04344951, NCT04331600 NCT04353336, NCT04360759, NCT04346667, NCT04342650 NCT04323527, NCT04328467, NCT04304053, NCT04351191 NCT04349228, NCT04352946, NCT04349228 |
Increase of lysosomes pH Membrane fusion and endocytosis inhibition |
Elimination half-life of 10–30 days [97] CYP2D6 inhibitor [98] Cardiac toxicity (QT prolongation) Ocular toxicity (e.g. MEK inhibitors) |
| Colchicine | NCT04350320, NCT04375202, NCT04355143, NCT04367168 NCT04360980, NCT04326790, NCT04328480, NCT04322565 NCT04363437, NCT04322682 |
Anti-inflammatory and antimitotic | CYP3A4 substrate [99] |
| Convalescent Plasma Therapy | NCT04346446, NCT04345679, NCT04383548, NCT04344535 NCT04355897, NCT04338360, NCT04374487, NCT04343261 NCT04345991, NCT04354831, NCT04356534, NCT04372979 NCT04321421, NCT04345523, NCT04343755, NCT04347681 NCT04376788, NCT04385043, NCT04348877, NCT04384497 NCT04372368, NCT04344015, NCT04333355, NCT04348656 NCT04365439, NCT04361253, NCT04380935, NCT04353206 NCT04363034, NCT04342182, NCT04358211, NCT04352751 NCT04384588, NCT04374149, NCT04374370, NCT04385199 NCT04381858, NCT04355767, NCT04374526, NCT04373460 NCT04375098, NCT04377568, NCT04383535, NCT04366245 NCT04360486, NCT04364737, NCT04356482, NCT04340050 NCT04374565, NCT04358003, NCT04323800, NCT04332380 NCT04357106, NCT04332835, NCT04359810, NCT04327349 NCT04385186, NCT04376034, NCT04358783, NCT04377672 NCT04374539, NCT04325672, NCT04333251, NCT04380532 NCT04346589, NCT04264858 |
Passive immunity | No data available |
| Nitric Oxyde Gas | NCT04290858, NCT03331445, NCT04338828, NCT04383002 NCT04305457, NCT04312243, NCT04290871, NCT04337918 NCT04306393, NCT04358588 |
Vasodilatation | No potential impact |
| Ruxolitinib | NCT04348071, NCT04355793, NCT04362137, NCT04359290 NCT04354714, NCT04377620, NCT04338958, NCT04337359 NCT04334044, NCT04331665 |
JAK2 inhibitor | CYP3A4 and CYP2C9 substrate (no clinically relevant) [100] |
| Tinzaparin Enoxaparin |
NCT04344756, NCT04366960, NCT04345848, NCT04362085 NCT04359277, NCT04377997, NCT04373707, NCT04360824 NCT04367831, NCT04354155 |
Anticoagulant | No potential impact |
| Tocilizumab | NCT04345445, NCT04317092, NCT04377750 NCT04331795, NCT04377659, NCT04361032, NCT04346355 NCT04320615, NCT04372186, NCT04363736, NCT04335071 NCT04377503, NCT04363853, NCT04356937, NCT04370834 NCT04315480, NCT04361552, NCT04331808 |
Anti-IL-6 | Induction of cytochromes P450 and increase elimination of associated substrates [101] |
IL: Interleukin; IMP: Investigational Medicine Product; SPC: Summary of Product Characteristics.
Note: the trials summarized were these referenced on ClinicalTrial.gov until May, 15th 2020. An exhaustive table of ongoing trials evaluating medical strategy is displayed in Appendix. The Cochrane France organization provides a website (https://covid-nma.com/) dedicated to map current researches on the prevention and treatment of COVID-19 (weekly update) and to review study results as soon as they are available (daily updated).
Clinical research in oncology in the COVID-19 pandemic era
Clinical research in oncology is the keystone for management of cancer patients and the prime topic in clinical research in France. The French national drug safety agency, ANSM [104], and most oncology groups have taken positions on the subject. On the European level, EMA and several task forces work on the “Guidance on the management of clinical trials during the COVID-19 pandemic”. A summary of different national and international guidelines in this field has been recently published [94]. New limitations and constraints have emerged with the pandemic, including the need to limit hospital admission of clinical trial participants. Four questions emerge: the appropriateness of initiating a new clinical trial, patients eligible for inclusion, patients already included, and included patients contracting COVID-19 infection.
Regarding new inclusions in the clinical trials currently ongoing, the guidelines from expert consensus recommend suspension, to which most scientific societies have agreed (except for clinical context without conventional therapies and taking into account the benefit-risk regarding COVID-19). For patients already included in a trial, the issue is to ensure safety. Clinical trials require frequent presence and supplementary examinations on top of standard care, increasing the risk of COVID-19 infection [105]. For the hospital pharmacist, this firstly implies anticipating supply restrictions on experimental drugs. For trials based on drugs already on the market (e.g., parenteral antineoplastics) but not supplied by the sponsor, with its agreement it should be possible to use the same drug. Follow-up needs to be continued, and drug interactions need to be anticipated [106]. The ANSM advocates limiting patient travel to the center and making treatment available elsewhere [107]. The French Society of Pharmacy Oncology guidelines recommend that the activity related to clinical trials be reduced as much as possible (recommendation n°18) [89]. The appropriateness of initiating new trials should be assessed by the sponsor and the principal investigator, with priority given to trials related to the management of patients infected with SARS-CoV-2. Early-phase clinical trials in oncology, especially of drugs for which the benefit to the patient has not yet been demonstrated, should not be started.
For patients contracting COVID-19 while included in a trial, global guidelines does not foresee automatic withdrawal, unlike the International Gynecologic Cancer Society [108]. Here again, the pharmacists can refer to the trial protocol to identify iatrogenic risk between COVID-19 treatment and the experimental treatment. The risk of staff exposure to COVID-19 (e.g., return of unused units) should be managed in the same way as for other drugs.
Guidelines for the practice of oncology clinical pharmacy under the COVID-19 pandemic
Since the start of the crisis, hospital pharmacists have been closely involved with the medical and care teams in supplying the care and intensive care units dedicated to COVID-19. They are also involved in drawing up fast-changing treatment protocols, including alternative strategies to cope with shortages and fast-track trials assessing treatment strategies in infected patients [109]. Also, the crisis situation and mobilization of human and material resources entails a risk of inattention to cancer patients and iatrogenic risk due to distraction [21].
Prompt implementation of clinical pharmacy in COVID-19+ units contributes to treatment optimization in the face of a theoretical risk of drug-related iatrogenesis in this novel population – risk also run by cancer patients in the COVID-19 context. The benefit of pharmaceutical expertise in cancer is well-established [110], [111], [112], and the pandemic has led to adaptation of oncology clinical pharmacy activity: an organizational approach to limit contact-time in the care units (pharmaceutical care including consultations, medication reconciliation) and a pharmacologic approach (medication prescription analysis). Neither approach needs to be short-term with a return to business-as-usual envisaged within a matter of weeks: medium and even long-term perspectives are possible. This notably involves adapting oncology clinical pharmacy so as to set up COVID-19 pathways in hospital, including for cancer patients. This publication must be considered as a support for pharmacist to be able to act effectively for cancer patients, who tend to be polymedicated and immunodepressed. The present document provides keys for anticipating drug interactions, possible adverse effects as well as poor medication adherence in concomitant treatment of cancer patients with COVID-19. Finally, hospital and community pharmacists need to be especially alert to use of over-the-counter treatments (OTC) and CAM, as the pandemic encourages such self-medication, leading to drug-related problems and herb-drug interactions.
Guidelines and opinions on pharmacy practice in the COVID-19 context have been published [109], [113], [114], but none focusing on oncology pharmacy.
In the present state of knowledge, the French Society of Oncology Pharmacy (SFPO) advocates the following proposals for the management of cancer patients during the pandemic:
-
–
Maintaining and reinforcing oncology clinical pharmacy activity (prescription analysis, dose adaptation, medication reconciliation, educational follow-up, etc.), prioritization (of patients at highest iatrogenic risk) and reorganization according to local capacity.
-
–
Deploying new oncology clinical pharmacy expertise centered on prevention of drug-related problems (adverse effects, interactions) with anti-COVID-19 treatment in current care (Table 2A, Table 2B, Table 2C, Table 3).
-
–
Ensuring cancer patients’ access to innovations in case of COVID infection, by setting up real-time literature watch on cancer patients’ risks related to drugs assessed in clinical trials, based on the present Table 4, “Ongoing clinical trials of COVID-19 treatments and implications for cancer patients”.
-
–
Maintaining pharmacy interviews and reminders of the current distancing and safety measures.
-
–
Maintaining active involvement of pharmacists in the multidisciplinary case conferences under the same conditions as for the other participants, as advised by the French national cancer institute [115], and in any dedicated COVID-19 multidisciplinary team meetings.
-
–
Expertise and close collaboration with medical oncology teams in treatment strategy decision-making. This involves selection of chemotherapy protocols when the initial strategy is no longer feasible, associated recommendations (dose equivalences between parenteral and oral forms), creation of new adapted protocols (flat dose and cycle timing in immunotherapy), and providing subcutaneous forms for monoclonal antibodies (rituximab, trastuzumab). It also involves active help with support drugs, especially those leading to drug interactions (Table 2A, Table 2B, Table 2C).
-
–
Deployment of telemedicine (teleconsultation and telemonitoring) to reduce face-to-face pharmacy work (follow-up of patients under oral therapy) [116].
-
–
Exchanges with community health professionals (community pharmacists, family doctors, etc.) in a community-hospital network (switch from parenteral to oral chemotherapy, oral anticancer drugs, etc.), if possible via secure hospital-community platforms.
Conclusion
The present pandemic requires significant prompt adaptation. The present guidelines are intended to accompany hospital pharmacists in this perspective, in a situation in which all pharmaceutical activities are changing. Oncology clinical pharmacy needs to be protected, as it concerns frail patients and needs to be able to adapt to new iatrogenic risks in a rapidly changing context of knowledge about COVID-19 disease and treatment strategies, notably in clinical research. The authors wish to spotlight delay in diagnosis and treatment, rescheduling and interruption of chemotherapy cycles and the considerable obstacles to cancer patients’ access to innovation throughout the pandemic. The impact of reorganizing care in this unprecedented crisis for cancer patients has to be assessed and shared. The learning experience should make it possible to adapt and improve the recommendations put forward by the health authorities and scientific societies with a necessary view to improving cancer treatment in a health crisis. We need also to bear in mind the difficulty of foreseeing the health situation in the months to come. Rapid changes are to be expected in oncology, at the very least involving setting up dedicated care pathways in health structures. Pharmaceutic teleconsultations and remote interviews are being developed and will continue, requiring long-term rethinking in telemedicine. Finally, ongoing clinical trials may yet identify and validate curative strategies against COVID-19, and, probably in the longer term, to identify candidate vaccines. New guidelines for cancer patients will then be welcome. The exceptional context of this year 2020 entails deep changes in the fight against cancer in which pharmacists play a strategic role. Oncology clinical pharmacy now has to take on a new risk, with all the concerns that entails, and to respond on an emergency footing with new expertise that is already opening up opportunities to optimize treatment for cancer patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
Guidelines of the French Society for Oncology Pharmacy (SFPO): cancer patients and the coronavirus 19 (COVID-19) pandemic
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctrv.2020.102063.
Contributor Information
Florian Slimano, Email: florianslimano@gmail.com.
Amandine Baudouin, Email: amandine.baudouin@chu-lyon.fr.
Jérémie Zerbit, Email: jeremiezerbit@gmail.com.
Anne Toulemonde-Deldicque, Email: anne.deldicque@gmail.com.
Audrey Thomas-Schoemann, Email: schoemann.audrey@gmail.com.
Régine Chevrier, Email: regine.chevrier@clermont.unicancer.fr.
Mikaël Daouphars, Email: mikael.daouphars@chb.unicancer.fr.
Isabelle Madelaine, Email: isabelle.madelaine@aphp.fr.
Bertrand Pourroy, Email: bertrand.pourroy@ap-hm.fr.
Jean-François Tournamille, Email: jf.tournamille@sfpo.com.
Alain Astier, Email: prof.astier@gmail.com.
Florence Ranchon, Email: florence.ranchon@chu-lyon.fr.
Jean-Louis Cazin, Email: jean-louis.cazin@univ-lille.fr.
Christophe Bardin, Email: christophe.bardin@aphp.fr.
Catherine Rioufol, Email: catherine.rioufol@chu-lyon.fr.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F., Tan Wenjie. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [accessed April, 5th 2020].
- 4.Martino R., Porras R.P., Rabella N., Williams J.V., Rámila E., Margall N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2005;11(10):781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaly R.F., Vigil K.J., Saad M., Vilar-Compte D., Cornejo-Juarez P., Perez-Jimenez C. A multicenter study of pandemic influenza A (H1N1) infection in patients with solid tumors in 3 countries: early therapy improves outcomes. Cancer. 2012;118(18):4627–4633. doi: 10.1002/cncr.27447. [DOI] [PubMed] [Google Scholar]
- 6.Nichols W.G., Guthrie K.A., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 7.Issa N.C., Fishman J.A. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48(6):772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 8.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitterman R., Eliakim-Raz N., Vinograd I., Zalmanovici Trestioreanu A., Leibovici L., Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018;2:CD008983. doi: 10.1002/14651858.CD008983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y.-J., Lee E.S., Lee Y.-S. High mortality from viral pneumonia in patients with cancer. Infect Dis. 2019;51(7):502–509. doi: 10.1080/23744235.2019.1592217. [DOI] [PubMed] [Google Scholar]
- 11.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 Transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Extance A. Covid-19 and long term conditions: what if you have cancer, diabetes, or chronic kidney disease? BMJ. 2020 Mar;25(368):m1174. doi: 10.1136/bmj.m1174. [DOI] [PubMed] [Google Scholar]
- 15.French High Council for Public Health, Provisional statement: Recommendations on prevention and management of Covid-19 in patients at risk of severe forms. Rapport de l’HCSP. Paris: Haut Conseil de la Santé Publique; 2020 Mar. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=799 [accessed April, 23th 2020].
- 16.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA; 2020 [in press]. https://doi.org/10.1001/jama.2020.4683. [DOI] [PubMed]
- 17.Lees J., Chan A. Polypharmacy in elderly patients with cancer: clinical implications and management. Lancet Oncol. 2011;12(13):1249–1257. doi: 10.1016/S1470-2045(11)70040-7. [DOI] [PubMed] [Google Scholar]
- 18.Maggiore R.J., Dale W., Gross C.P., Feng T., Tew W.P., Mohile S.G. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriatr Soc. 2014;62(8):1505–1512. doi: 10.1111/jgs.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020 Apr;21(4):e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G., Zhang H., Yang Y. Challenges and countermeasures of integrative cancer therapy in the epidemic of COVID-19. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420912811. 1534735420912811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortiula F., Pettke A., Bartoletti M., Puglisi F., Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31(5):553–555. doi: 10.1016/j.annonc.2020.03.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinelli A., Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020;107(7):785–787. doi: 10.1002/bjs.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuech J.-J., Gangloff A., Di Fiore F., Michel P., Brigand C., Slim K. Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg. 2020;157(3S1):S7–S12. doi: 10.1016/j.jviscsurg.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You B., Ravaud A., Canivet A., Ganem G., Giraud P., Guimbaud R. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21(5):619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burki T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamboj M., Sepkowitz K.A. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10(6):589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 27.Longbottom E.R., Torrance H.D.T., Owen H.C., Fragkou P.C., Hinds C.J., Pearse R.M. Features of postoperative immune suppression are reversible with interferon gamma and independent of interleukin-6 pathways. Ann Surg. 2016;264(2):370–377. doi: 10.1097/SLA.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson A.G., Gyawali B., Evans G. COVID-19 and cancer: do we really know what we think we know? Nat Rev Clin Oncol. 2020;17(7):386–388. doi: 10.1038/s41571-020-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A. Fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0516. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17(5):268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biagi J.J., Raphael M.J., Mackillop W.J., Kong W., King W.D., Booth C.M. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review of meta-analysis. JAMA. 2011;305(22):2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 33.Raphael M.J., Biagi J.J., Kong W., Mates M., Booth C.M., Mackillop W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;160(1):17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 34.André T., Vernerey D., Mineur L., Bennouna J., Desrame J., Faroux R. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized open-label, international duration evaluation of adjuvant (IDEA) France. Phase III Trial J Clin Oncol. 2018;36(15):1469–1477. doi: 10.1200/JCO.2017.76.0355. [DOI] [PubMed] [Google Scholar]
- 35.Di Fiore F., Bouché O., Lepage C., Sefriou D., Gangloff A., Schwarz L. COVID-19 epidemic: proposed alternatives in the management of digestive cancers: a French Intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR) Dig Liver Dis. 2020;52(6):597–603. doi: 10.1016/j.dld.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grellety T, Ravaud A, Canivet A, Ganem G, Giraud P, Guimbaud R, et al. [SARS-CoV-2/COVID 19 infection and solid cancers: synthesis of recommendations for health professionals]. Bull Cancer; 2020 [in press]. https://doi.org/10.1016/j.bulcan.2020.03.001. [DOI] [PMC free article] [PubMed]
- 37.Ljungman P., Mikulska M., de la Camara R., Basak G.W., Chabannon C., Corbacioglu S. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donrs, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;13:1–6. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Centre for Disease Prevention and Control. Coronavirus disease 2019 (COVID-19) and supply of substances of human origin in the EU/EEA [Internet]. European Centre for Disease Prevention and Control. 2020 Available from: https://www.ecdc.europa.eu/en/publications-data/coronavirus-disease-2019-covid-19-and-supply-substances-human-origin-eueea [accessed April, 23th 2020].
- 39.Tian W., Jiang W., Yao J., Nocholson C.J., Rebecca H.L., Sigurslid H.H. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fachi M.M., Tonin F.S., Leonart L.P., Rotta I., Fernandez-Llimos F., Pontarolo R. Haematological adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukaemia: a network meta-analysis. Br J Clin Pharmacol. 2019;85(10):2280–2291. doi: 10.1111/bcp.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dingerman A.C., Soo R.A., Jazieh A.R., Sice S.J., Kim Y.T., Teo L.L. Treatment guidance for lung cancer patients during the COVID-19 pandemic. J Thorac Oncol. 2020;15(7):1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo J., Rivzi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020:CD-20-0596. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020 doi: 10.2217/imt-2020-0067. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah R.R. Tyrosine kinase inhibitor-induced interstitial lung disease: clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016;39(11):1073–1091. doi: 10.1007/s40264-016-0450-9. [DOI] [PubMed] [Google Scholar]
- 45.Girard N., Greillier L., Zalcman G., Cadranel J., Moro-Sibilot D., Mazières J. Proposals for managing patients with thoracic malignancies during COVID-19 pandemic. Respir Med Res. 2020 doi: 10.1016/j.resmer.2020.100769. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long G.V., Tykodi S.S., Schneider J.G., Garbe C., Gravis G., Rashford M. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29(11):2208–2213. doi: 10.1093/annonc/mdy408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lala M., Li T.R., de Alwis D.P., Sinha V., Mayawala K., Yamamoto N. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68–75. doi: 10.1016/j.ejca.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 48.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/lung-cancer-in-the-covid-19-era [accessed April, 26th 2020]. [DOI] [PMC free article] [PubMed]
- 49.Griesinger F., Korol E.E., Kayaniyil S., Varol N., Ebner T., Goring S.M. Efficacy and safety of first-line carboplatine-versus cisplatin-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer. 2019;135:196–204. doi: 10.1016/j.lungcan.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Antonia S., Goldberg S.B., Balmanoukian A., Chaft J.E., Sanborn R.E., Gupta A. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh A.P., Berman A.T., Marmarelis M.E., Haas A.R., Feigenberg S.J., Braun J. Management of lung cancer during the COVID-19 pandemic. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00286. [in press] [DOI] [PubMed] [Google Scholar]
- 52.Rossi A., Di Maio M., Chiodini P., Rudd R.M., Okamoto H., Skarlos D.V. Chemotherapy in fisrt-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30(14):1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 53.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Melanoma. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/melanoma-in-the-covid-19-era [accessed April, 26th 2020].
- 54.Méjean A., Rouprêt M., Rozet F., Bensalah K., Murez T., Game X. Recommendations CCAFU on the management of cancers of the urogenital system during an epidemic with Coronavirus COVID-19. Prog Urol. 2020;30(5):221–231. doi: 10.1016/j.purol.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fizazi pour K. les membres du bureau du Groupe d’étude des tumeurs uro-génitales. Therapeutic options for genitourinary cancers during the epidemic period of COVID-19. Bull Cancer. 2020;107(4):395–397. doi: 10.1016/j.bulcan.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V. Androgene-deprivation therapies for prostate cancer and risk of infection by SARS-CoV- 2: a population-based study (n=4532) Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.479. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Colorectal cancer (CRC). Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/gastrointestinal-cancers-colorectal-cancer-crc-in-the-covid-19-era [accessed April, 26th 2020]. [DOI] [PMC free article] [PubMed]
- 58.Mahaseth H., Brutcher E., Kauh J., Hawk N., Kim S., Chen Z. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adecnocarcinoma. Pancreas. 2013;42(8):1311–1315. doi: 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 59.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 60.Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomized, phase 2–3 trial. Lancet. 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 61.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Gastro-oesophageal tumours. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/gastrointestinal-cancers-gastro-oesophageal-tumours-in-the-covid-19-era [accessed April, 26th 2020].
- 62.de Azambuja E., Trapani D., Loibl S., Delaloge S., Senkus E., Criscitiello C. ESMO management and treatment adapted recommendations in the COVID-19 era: breast cancer. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gligorov J., Bachelot T., Pierga J.Y., Antoine E.C., Balleyguier C., Barranger E. COVID-19 and people followed for breast cancer: French guidelines for clinical practice of nice-St-Paul De Vence, in collaboration with the Collège National Des Gynécologues Et Obstétriciens Français (CNGOF), the Société d’Imagerie De La Femme (SIFEM), the Société Française De Chirurgie Oncologie (SFCO), the Société Française de Sénologie et Pathologie Mammaire (SFSPM) and the French Breast Cancer Intergroup-UNICANCER (UCBG) Bull Cancer. 2020;107(5):528–537. doi: 10.1016/j.bulcan.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.French Society of Otolaryngology and Head-and-Neck Surgery. Available from: https://ressources-aura.fr/wp-content/uploads/2020/04/covid-19-et-orl-propositions-onco-UNICANCER-_26-03-2020-1.pdf [accessed April, 26th 2020].
- 65.Mohile N.A., Blakeley J.O., Gatson N.T.N., Hottinger A.F., Lassman A.B., Ney D.E. Urgent considerations for the neuro-oncologic treatment of patients with gliomas during the COVID-19 pandemic. Neuro-Oncol. 2020 doi: 10.1093/neuonc/noaa090/5818980. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Primary brain tumor. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/primary-brain-tumours-in-the-covid-19-era [accessed 20 May 2020].
- 67.American Society of Hematology. COVID-19 and Indolent Lymphomas - Hematology.org. Available from: https://www.hematology.org:443/covid-19/covid-19-and-indolent-lymphomas [accessed April, 26th 2020].
- 68.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Indolent B-NHL (Follicular Lymphoma, Marginal Zone Lymphoma, Waldenström’s Macroglobulinaemia). Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/haematological-malignancies-indolent-b-nhl-in-the-covid-19-era [accessed April, 26th 2020].
- 69.European Society for Medical Oncology. ESMO management and treatment adapted recommendations in the COVID-19 era: Diffuse large B-cell lymphoma, Mantle cell lymphoma and Aggressive T-cell lymphomas. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/haematological-malignancies-dlbcl-mcl-and-aggressive-t-cell-lymphoma-in-the-covid-19-era [accessed April, 26th 2020].
- 70.American Society of Hematology. COVID-19 and CLL – Hematology.org. Available from: https://www.hematology.org:443/covid-19/covid-19-and-cll [accessed April, 26th 2020].
- 71.Société Française d’Hématologie (SFH). COVID-19 et prise en charge des maladies d’hématologie. Available from: https://sfh.hematologie.net/sites/sfh.hematologie.net/files/medias/documents/covid-19_propostion_sfh_17-03-2020_0.pdf [accessed April, 26th 2020].
- 72.Francophone Myeloma Intergroup. Recommandations IFM. Available from: https://www.myelome.fr/PressRoom/50/2020-03-25-Flash-info-COVID-19.html [accessed April, 26th 2020].
- 73.Al Saleh A.S., Sher T., Gertz M.A. Multiple myeloma in the time of COVID-19. Acta Haematol. 2020 doi: 10.1159/000507690. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar S.K., Berdeja J.G., Niesvizky R., Lonial S., Laubach J.P., Hamadani M. Ixazomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma: long-term follow-up including ixazomib maintenance. Leukemia. 2019;33(7):1736–1746. doi: 10.1038/s41375-019-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreau P., Mateos M.-V., Berenson J.R., Weisel K., Lazzaro A., Song K. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):935–964. doi: 10.1016/S1470-2045(18)30354-1. [DOI] [PubMed] [Google Scholar]
- 76.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [in press] [DOI] [PubMed] [Google Scholar]
- 77.Santini F.C., Rizvi H., Plodkowski A.J., Ni A., Lacoutuer M.E., Gambarin-Gelwan M. Safety and efficacu of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–1099. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spigel D.R., McLeod M., Hussein M.A., Waterhouse D.M., Einhorn L., Horn L. 1297O – Randomized results of fixed-duraction (1-yr) vs continuous nivolumab in patients with advanced non-small cell lung cancer. Ann Oncol. 2017;28(Suppl. 5):v461. doi: 10.1093/annonc/mdx380.002. [DOI] [Google Scholar]
- 79.Metro G., Signorelli D. Immune checkpoint inhibitors rechallenge in non-small-cell lung cancer: different scenarios with different solutions? Lung Cancer. Manage. 2020;8(4):LMT18. doi: 10.2217/lmt-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;27(368):m1185. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 81.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Haar J., Hoes L.R., Coles C.E., Seamon K., Fröhling S., Jäger D. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 83.Morganroth J., Shah R.R., Scott J.W. Evaluation and management of cardiac safety using the electrocardiogram in oncology clinical trials: focus on cardiac repolarization (QTc interval) Clin Pharmacol Ther. 2010;87(2):166–174. doi: 10.1038/clpt.2009.214. [DOI] [PubMed] [Google Scholar]
- 84.Cautela J., Lalevée N., Ammar C., Ederhy S., Peyrol M., Debourdeau P. Management and research in cancer treatment-related cardiovascular toxicity: challenges and perspectives. Int J Cardiol. 2016;224:366–375. doi: 10.1016/j.ijcard.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 85.Binkhorst L., Mathijssen R.H.J., van Herk-Sukel M.P.P., Bannink M., Jager A., Wiemer E.A.C. Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer Res Treat. 2013;139(3):923–929. doi: 10.1007/s10549-013-2585-z. [DOI] [PubMed] [Google Scholar]
- 86.Stjepanovic N., Velazquez-Martin J.P., Bedard P.L. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol. 2016;27(6):998–1005. doi: 10.1093/annonc/mdw100. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y., Zhang D., Du G., Du R., Jianping Z., Jin Y. Remdesivir in adults with severe COVID- 19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Eng J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pourroy B., Tournamille J., Bardin C., Slimano F., Chevrier R., Rioufol C. Providing oncology pharmacy services during the coronavirus pandemic: french society for oncology pharmacy (Société Francaise de Pharmacie Oncologique-SFPO) guidelines. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00295. [in press] [DOI] [PubMed] [Google Scholar]
- 90.Brown J.D. Cannabidiol as prophylaxis for SARS-CoV-2 and COVID-19? Unfounded claims versus potential risks of medications during the pandemic. Res Soc Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.03.020. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gray P.E., Belessis Y. The use of Traditional Chinese Medicines to treat SARS-CoV-2 may cause more harm than good. Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104776. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grenoble University. Hedrine : Herb Drug Interaction Database. Available from: https://hedrine.univ-grenoble-alpes.fr/ [accessed April, 23th 2020].
- 93.Memorial Sloan Kettering Cancer Center. About Herbs, Botanicals & Other Products. Available from: https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs [accessed April, 23th 2020].
- 94.de Paula B.H.R., Araújo I., Bandeira L., Barreto N.M.P.B., Doherty G.J. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):624–627. doi: 10.1016/S1470-2045(20)30226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Solidarity 2019 coronavirus drug clinical trial. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [accessed April, 10th 2020].
- 96.Schweizer M.T., Wang H., Bivalacqua T.J., Partin A.W., Lim S.J., Chapman C. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Transl Med. 2019;8(5):441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Electronic Medicines Compendium. Avloclor 250 mg tablets. Available from: https://www.medicines.org.uk/emc/medicine/2272 [accessed May, 25th 2020].
- 98.Somer M., Kallio J., Pesonen U., Pyykkö K., Huupponen R., Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol. 2000;49(6):549–554. doi: 10.1046/j.1365-2125.2000.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Electronic Medicines Compendium. Colchicine 500 µg tablets. Available from: https://www.medicines.org.uk/emc/product/6415/smpc [accessed May, 25th 2020].
- 100.European Medicines Agency. Jakavi–European Public Assessment Report; 2020. Available from: https://www.jakavi.com/globalassets/assets1/emea-combined-h-2464-en.pdf [accessed May, 25th 2020].
- 101.European Medicines Agency. RoActemra–European Public Assessment Report; 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_fr.pdf [accessed May, 25th 2020].
- 102.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Michot J.M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.French national drug safety agency. COVID-19 – Clinical Trials underway. Available from: https://www.ansm.sante.fr/Activites/Essais-cliniques/COVID-19-Essais-cliniques-en-cours/(offset)/0 [accessed April, 6th 2020].
- 105.Kutikov A., Weinberg D.S., Edelman M.J., Horwitz E.M., Uzzo R.G., Fisher R.I. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ranchon F., Vial T., Rioufol C., Hénin E., Falandry C., Freyer G. Concomitant drugs with low risks of drug-drug interactions for use in oncology clinical trials. Crit Rev Oncol Hematol. 2015;94(2):189–200. doi: 10.1016/j.critrevonc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 107.French national drug safety agency. COVID-19 – Clinical Trials underways – What is the recommended pathway in case of home medicines delivery? Available from: https://www.ansm.sante.fr/Activites/Essais-cliniques/COVID-19-Essais-cliniques-en-cours/COVID-19-Essais-cliniques-en-cours-Quel-est-le-circuit-preconise-en-cas-de-delivrance-a-domicile [accessed April, 10th 2020].
- 108.Ramirez Pedro T, Chiva Luis, Eriksson Ane Gerda Z, Frumovitz Michael, Fagotti Anna, Gonzalez Martin Antonio, Jhingran Anuja, Pareja Rene. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Cancer. 2020;30(5):561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- 109.Li H., Zheng S., Liu F., Liu W., Zhao R. Fighting against COVID- 19: innovative strategies for clinical pharmacists. Res Soc Adm Pharm. 2020;30(5):561–563. doi: 10.1016/j.sapharm.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herledan C., Baudouin A., Larbre V., Gahbiche A., Dufay E., Alquier I. Clinical and economic impact of medication reconciliation in cancer patients: a systematic review. Support Care Cancer. 2020;28(8):3557–3569. doi: 10.1007/s00520-020-05400-5. [DOI] [PubMed] [Google Scholar]
- 111.Petit-Jean E., Correard F., Maillan G., de Crozals F., Bertrand B., Regnier V. Pharmaceutical consultations in oncology: French Society for Oncology Pharmacy (Société Francaise de Pharmacie Oncologique – SFPO) guidelines. Eur J Oncol Pharm. 2019;2(2) doi: 10.1097/OP9.0000000000000011. [DOI] [Google Scholar]
- 112.Chevrier R., Basuyau F., Benard-Thiery I., Bertrand C., Devys C., Divanon F. Apports des consultations pharmaceutiques dans la prise en charge des anticancéreux oraux. Innov Ther Oncol. 2018;4(1):45–51. doi: 10.1684/ito.2018.0108. [DOI] [Google Scholar]
- 113.Gross A.E., MacDougall C. Roles of the clinical pharmacist during the COVID-19 pandemic. J Am Coll Clin Pharm. 2020;3:564–566. doi: 10.1002/jac5.1253. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.International Pharmaceutical Federation. FIP Covid-19 Information Hub - FIP. Available from: https://www.fip.org/coronavirus [accessed April, 23th 2020].
- 115.Guidelines for tumor board meetings in the context of Covid-19. Available from: https://www.e-cancer.fr/Professionnels-de-sante/Coronavirus-COVID-19/Conseils-sur-l-organisation-des-reunions-de-concertation-pluridisciplinaire-RCP-en-cancerologie-dans-le-contexte-de-l-epidemie-au-Covid-19 [accessed April, 6th 2020].
- 116.Portnoy J., Waller M., Elliott T. Telemedicine in the Era of COVID-19. J Allergy Clin Immunol Pract. 2020;8(5):1489–1491. doi: 10.1016/j.jaip.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


