Abstract
Background
Individuals with diabetes are at a greater risk of hospitalization and mortality resulting from viral, bacterial, and fungal infections. The coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread quickly to more than 213 countries and claimed 395,779 lives as of June 7, 2020. Notably, in several studies, diabetes is one of the most reported comorbidities in patients with severe COVID-19.
Scope of review
In this review, I summarize the clinical data on the risk for infectious diseases in individuals with diabetes while highlighting the mechanisms for altered immune regulation. The focus is on coronaviruses. Based on the new clinical data obtained from COVID-19 patients, a discussion of mechanisms, such as cytokine storm, pulmonary and endothelial dysfunction, and hypercoagulation, that may render individuals with diabetes more vulnerable to COVID-19 is provided.
Major conclusions
Epidemiological studies show that poorly controlled diabetes is a risk factor for various infectious diseases. Given the global burden of diabetes and the pandemic nature of coronaviruses, understanding how diabetes affects COVID-19 severity is critical to designing tailored treatments and clinical management of individuals affected by diabetes.
Keywords: Diabetes, Infection, Coronavirus, COVID-19, SARS-CoV-2
1. Diabetes and infection risk
Diabetes is a chronic disease characterized by abnormally high blood glucose levels resulting from an impairment in insulin action and/or insulin secretion. More than 425 million individuals have diabetes worldwide and projections show this number rising to 629 million by 2045 [1]. While type 1 diabetes (T1D) is characterized by autoimmune mediated destruction of insulin producing β-cells, type 2 diabetes (T2D) results from a combination of insulin resistance and β-cell insulin secretory defect, in the long-term resulting β-cell exhaustion and eventually destruction [2]. Diabetes is the leading noncommunicable, chronic pandemic disease worldwide and is associated with complications. Over time, high blood glucose can damage tiny and large blood vessels, causing an increased risk for microvascular and macrovascular complications [3]. A research study with more than 1.3 million participants showed that 98% of adults with type 2 diabetes have at least one comorbid chronic disease and almost 90% have at least two [4,5]. The most common conditions in patients with T2D included hypertension (82.1%), overweight/obesity (78.2%), hyperlipidemia (77.2%), chronic kidney disease (24.1%), and cardiovascular disease (21.6%) [4]. In a longitudinal study including 915 individuals with T1D and 3,590 children in the reference cohort, incidences of six chronic diseases were significantly higher in T1D children [6]. T1D was associated with an increased risk (HR; 95% CI) of hospitalization for any comorbidity (3.7; 2.5 to 5.5), thyroid disease (14.2; 6.7 to 31.0), non-infectious enteritis and colitis (5.9; 3.0 to 11.5), cardiovascular disorders (3.1; 2.3 to 4.2), mental disorders (2.0; 1.4 to 3.1), epilepsy (2.0; 1.1 to 3.7), and (obstructive) pulmonary disease (1.5; 1.2 to 2.0). Observational studies have also shown that the cerebrovascular mortality rate is elevated at all ages in patients with T1D [7].
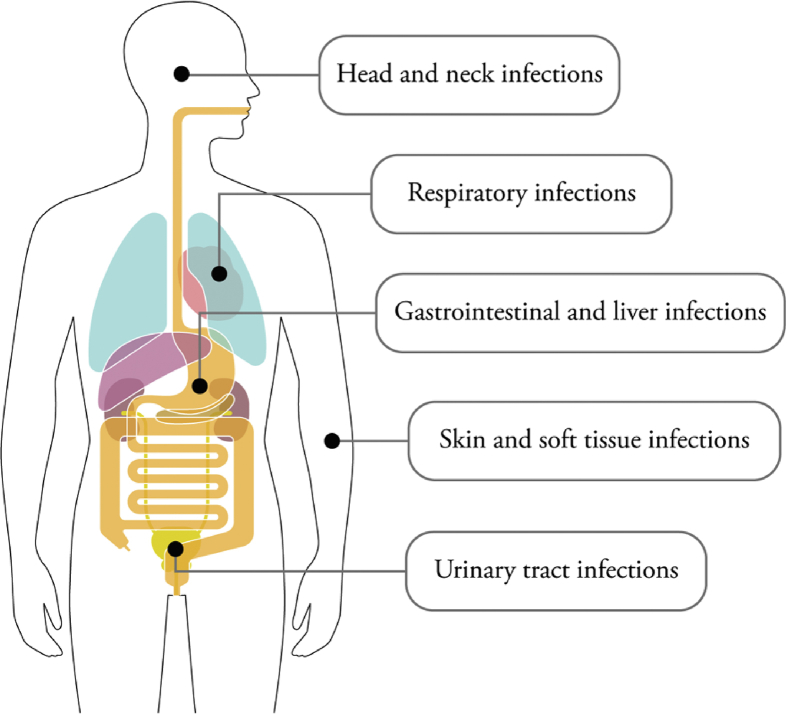
Poorly controlled diabetes increases the risk for skin, bone, eye, ear, gastrointestinal, urinary tract, and respiratory infections, among others, with significantly increased hospitalization and mortality rates [[8], [9], [10], [11]] (Figure 1). Seshasai et al. examined the risk of infection-related deaths in more than 800,000 participants and showed that infectious diseases substantially reduced life expectancy of individuals with diabetes [12]. The hazard ratio for a person with diabetes dying from any infection was 2.39. More recent epidemiological studies have explored relationships between poorer control of diabetes and infections, using English primary care databases with more than 85,000 individuals with diabetes [8,13]. The data demonstrated that poor glycemic control is powerfully associated with serious infections. In these studies, authors have also compared the infection risk between individuals with T1D and T2D and found that infection-related hospitalizations and deaths were higher in individuals with T1D. For instance, Carey at al. showed that the incidence rate ratio for infection-related hospitalizations was 3.71 (95% CI 3.27–4.21) for individuals with T1D and 1.88 (95% CI 1.83–1.92) for individuals with T2D [13]. In the follow-up study by the same group, Critchley et al. showed that poor diabetes control increased hospitalizations in individuals with T1D or T2D [8]. However, in individuals with T1D and poor control, this risk was even greater.
Figure 1.
Main infectious diseases associated with diabetes. Head and neck infections (e.g., invasive external otitis), respiratory infections (e.g., Streptococcus pneumoniae, influenza, H1N1, tuberculosis), skin and soft tissue infections (e.g., foot infection, gangrene), gastrointestinal and liver infections (e.g., Helicobacter pylori, hepatitis B, hepatitis C, enteroviruses), and urinary tract infections (bacteriuria, cystitis) are more frequent in individuals with diabetes.
During the 2009 H1N1 influenza virus pandemic, numerous clinical studies suggested that individuals with diabetes were a key susceptibility group for severe H1N1 infections [[14], [15], [16], [17], [18]]. Overall, hospitalization of patients with diabetes due to influenza virus or flu-like infections is up to six times more likely to occur compared to healthy individuals [16]. For example, in the United States, of the 272 patients were hospitalized in 2009 for the H1N1 flu, diabetes was present in 15% of hospitalized patients [15]. In Canada, diabetes tripled the risk of hospitalization after infection with the 2009 H1N1 virus and quadrupled the risk of admission to the intensive care unit [14]. Similarly, in Germany, diabetes doubled the risk of a fatal outcome after infection with the 2009 virus [18]. Fasting plasma glucose was also shown as an independent predictor for severity of H1N1 pneumonia [17].
2. Altered immune function in individuals with diabetes
Based on the epidemiological evidence, individuals with diabetes are more prone to acquiring selected types of (rare) infections and are more susceptible to certain complications while infected with pathogens [8,19,20]. Important mechanisms related to specific infections such as skin ulcers and lesions with poor wound healing are the result of comorbid neuropathy or increased urinary tract infections and are linked to a decrease in the antibacterial activity of urine [21]. Recent data also suggest that microbial dysbiosis in individuals with hyperglycemia may play a role in the spread of enteric pathogens. For example, the microbial tissue profile of individuals with T2D differs from that of individuals with normoglycemia, with enhanced levels of typically opportunistic Enterobacteriaceae in various tissues [22]. Given the reported decrease in intestinal barrier function with hyperglycemia and concomitant increase in systemic spread of enteric pathogens [23], individuals with hyperglycemia may be more susceptible to the entry of bacteria into their system. Despite the individual specific mechanisms for increased infectious disease prevalence in individuals with diabetes, the common pathogenic mechanisms are associated with immune dysfunction. Here, I will highlight our current knowledge on the altered immune cell function in individuals with diabetes (Table 1).
Table 1.
Major immune cell function alterations in individuals with diabetes.
The immune system can be divided into the innate and adaptive-humoral or adaptive-cellular immune systems. Innate immunity refers to non-specific initial defense mechanisms triggered upon pathogen recognition; adaptive immunity refers to antigen-specific responses designed to eliminate and memorize the specific non-self-pathogens. While humoral immunity does not seem to be significantly altered in individuals with diabetes, there is significant number of reports showing alterations in cell-mediated immunity, such as chemotaxis, phagocytosis, and cytokine secretion in both T1D and T2D [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. For instance, a significantly lower degree of chemotaxis has been found in the polymorphonuclear leukocytes (PMNs) of individuals with T1D and T2D compared to controls [26,33]. An inverse correlation between hyperglycemia and chemotaxis was also shown [30], suggesting that poor glycemic control may contribute to this impairment. In addition, blood neutrophils of diabetic mice showed a decreased migratory response to CXCL2/MIP-2 in vitro and in vivo compared to nondiabetic mice [32].
PMNs from individuals with diabetes have also demonstrated lower phagocytic capacity compared to PMNs from individuals without diabetes [26,28,29]. In these studies, the phagocytic response was reported to be worse in patients with increased HbA1c levels and poorer glucose control. A reduction in phagocytic activity, which is essential to contain and kill pathogens and process them for antigen presentation may partly explain the increased infection severity in individuals with diabetes. By contrast, serum antibody concentrations in individuals with diabetes are normal, and they respond to vaccinations, such as to pneumococcal vaccine similar to reference control individuals [35]. No differences have been shown in the immune response to intramuscular hepatitis B vaccine between children with T1D and controls [36]. Furthermore, the antibody response to influenza vaccination is not impaired in individuals with T1D or T2D [37,38]. Thus, humoral immune responses, at least based on these studies, seem to be fairly unaffected by diabetes. In the next section, the most current evidence on altered innate-mediated and cell-mediated adaptive immunity in individuals with diabetes is discussed in more detail (Table 2).
Table 2.
Major immune cell types with altered function in individuals with diabetes.
| Cell Type | Alteration | References |
|---|---|---|
| Natural Killer Cells | Reduced activity in T2D | [26,39,40] |
| Macrophages | Differential subtypes in obesity | [[41], [42], [43]] |
| Neutrophils | Both an increase and a decrease in numbers are reported in T1D | [45]-increase [27,46,47]-decrease |
| T cells | ||
|
Abnormal differentiation of T cells in T2D Increased Th17 subpopulation, higher percentage of CD8+, and lower percentage CD39+ T cells in T2D Decreased FOX Treg cell pool in T1D |
[[52], [53], [54], [55], [56], [57]]-T2D [58,59]-T1D |
2.1. Innate immunity
2.1.1. Natural killer (NK) cells
Natural killer cells are effector lymphocytes of the innate immune system and rapidly kill virus-infected and tumor cells without prior sensitization while remaining tolerant of normal cells. Despite the discrepancies among studies, accumulating evidence suggests that NK cell activity is reduced in individuals with T2D. Delemaire et al. reported a decrease in NK cells in obese patients with elevated fasting blood glucose levels [26]. Another study provided more evidence that NK cell populations were altered in obese humans with an increase in low cytotoxic CD56bright and a decrease in the number of high cytotoxic CD56dim NK cell subsets in obese subjects [39]. A subsequent study demonstrated that NK cell activity was lower in T2D patients and significantly related to glucose control [40]. Although more studies suggest that poorly controlled diabetes NK activity is reduced in patients with diabetes, larger population studies are warranted to more closely examine the association between NK cell activity and glucose control.
2.1.2. Myeloid cells
Myeloid cells include monocytes, macrophages, neutrophils, basophils, erythrocytes, megakaryocytes, and platelets. Myeloid cells play major roles in innate immunity, where they are rapidly recruited into local tissues, upon pathogen invasion, via various chemokine receptors, for phagocytosis, as well as secretion of inflammatory cytokines. Macrophage subtypes were reported to be differentially present in the adipose tissue of obese patients [41]. Although adipose tissue macrophages generally express more M2 markers, mice fed a high-fat-diet exhibited macrophage populations with high pro-inflammatory M1 gene expression markers [42]. Kratz et al. showed that classical macrophage activation markers are absent in the adipose tissue macrophages of obese humans, and metabolic dysfunction is a driver of a distinct pro-inflammatory phenotype in adipose tissue macrophages [43]. Many studies have also implied that neutrophils are involved in the initiation and perpetuation of autoimmune diabetes [44]. In addition, studies have reported an alteration in neutrophil numbers in individuals with T1D. Although some of the earlier studies reported an increased number of neutrophils in T1D [45], subsequent studies have shown a decrease [27,46,47]. The stages of diabetes and ethnic background might explain some of the differences. Thus, longitudinal studies might help clarify the discrepancies.
2.2. Adaptive cellular immunity
2.2.1. T cells
Multiple studies have demonstrated that T2D is associated with overactivated T cells and the activation of inflammatory pathways [[48], [49], [50], [51]]. Low-grade chronic inflammation in individuals with either T1D or T2D has been described [24,50,51]. Although CD8+ T cells are essential for the adaptive immune response against infections by secreting pro-inflammatory cytokines, such as IFN-γ and TNF-α, CD4+ T cells are critical for multiple functions, from the activation of innate immune system cells, including B-lymphocytes and cytotoxic T cells, to the suppression of immune reaction. T cells have been reported to be abnormally differentiated in individuals with T2D [[52], [53], [54]].
Bogdan et al. showed that Th17, a subset of CD4+ T cells, and IL-17A levels were elevated in individuals with newly diagnosed T2D, which probably promoted chronic inflammation [55]. In a more recent study, Garidou et al. analyzed cytokine production from the peripheral blood mononuclear cells (PBMCs) of individuals with T2D and investigated the differentiation of different CD4+ T cell subsets [52]. Similar to the previous study, they showed that Th17 cell population was increased in individuals with T2D.
Other studies have provided evidence for increased inflammation in individuals with T2D, based on the presence of a higher percentage of CD8+ T cells, an increase in the Th1/Th2 ratio, and elevated levels of cytokines (e.g., IL-4, IL-10, IL-13, IFN-γ, and TNF-α) [53,54,56]. Notably, CD39+ Treg cells, a subpopulation of cells implicated in suppressing Th17 cells, were also shown to be in lower percentage in the PBMCs from individuals with T2D [57]. Autoreactive CD4+ and CD8+ T cells play fundamental roles in the destruction of insulin-producing β-cells in T1D pathogenesis. In addition, the FOXP3+ Treg cell pool has been shown to have an essential role in regulating immune homeostasis, and autoimmunity is compromised in T1D [58,59]. Thus, the combination of increased T cell activation, elevated inflammatory cytokine profile, and decreased Treg function may contribute to dysregulated immune function observed in individuals with diabetes.
3. Coronaviruses
Coronaviruses are a large family of enveloped viruses with a positive-sense single-stranded RNA genome and a nucleocapsid of helical symmetry. They possess one of the largest genomes, approximately 26.4 kb–31.7 kb, among all known RNA viruses [60]. The Latin name corona (“crown” or “wreath”) describes the characteristic appearance of virions by electron microscopy, with large, bulbous surface projections resembling a crown. Coronaviruses primarily infect upper respiratory and gastrointestinal tracts of mammals and birds, and human coronaviruses vary significantly in disease severity [61].
Of the seven known human coronaviruses, four (HCoV-OC43, HCoV-HKU1, HCoV-229E, HCoV-NL63) cause mild to moderate symptoms of the common cold in humans, and three can cause more serious, even fatal, disease: severe acute respiratory syndrome (SARS, caused by the SARS-CoV virus), Middle East respiratory syndrome, (MERS, caused by the MERS-CoV virus), and COVID-2019 (caused by the SARS-CoV-2 virus). The genetic sequence of SARS-CoV-2 shows more than an 80% shared identity with SARS-CoV and 50% with MERS-CoV [62,63]. Coronavirus entry into host cells is mediated by the transmembrane spike (S) glycoprotein that forms the homotrimers protruding from the viral surface, which mediates receptor binding and proteolysis, leading to virus-cell fusion [64]. Angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV and SARS-CoV-2; MERS-CoV uses dipeptidyl peptidase 4 (DPP4) as its cellular receptor [63,65].
3.1. Outbreaks
SARS-CoV originated in the early 2000s and caused the 2002–2004 SARS outbreak. At the end of the epidemic in 2004, the incidence was 8422 cases with a case fatality rate (CFR) of 11% [66]. MERS is caused by the MERS coronavirus (MERS-CoV); was identified in September 2012; and continues to cause sporadic, localized outbreaks. Until January 2020, 2519 cases of MERS were reported with a CFR of 34% [67]. SARS-CoV-2 causes COVID-19, the third novel coronavirus to emerge in this century, and was declared a pandemic on March 11, 2020, by the World Health Organization. As of June 6, 2020, more than six million cases have been reported, and the estimated fatality rate is 0.5%–3.5% [68].
3.2. Clinical characteristics
All three human coronaviruses—SARS-CoV, MERS-CoV, SARS-CoV-2—replicate in the lower respiratory tract and can thus cause pneumonia that in severe cases leads to severe hypoxia, respiratory failure, multiorgan failure, shock, and death [69]. Studies have reported that the most common symptoms of COVID-19 are fever (82.2%) and cough (61.7%) [[70], [71], [72], [73]]. The age of most patients has been between 30 and 79 years, and the majority are male. Two patterns are that the severity increases with age and the presenting symptoms are broadly consistent with the epidemiology of SARS-CoV and MERS-CoV infections. Infection with SARS-CoV-2 has been shown to reach a peak viral load 4–7 days after symptom onset; this peak is earlier than SARS-CoV, which peaks at approximately 10 days after symptom onset [[70], [71], [72], [73]]. Notably, SARS-CoV-2 has been found in upper respiratory tract secretions of asymptomatic individuals [74].
Epidemiological studies from all three coronavirus related outbreaks (SARS, MERS, COVID-19) have identified diabetes and other comorbid illnesses such as hypertension and cardiovascular and cerebrovascular diseases as risk factors for severe or lethal infections [[75], [76], [77], [78], [79], [80], [81]]. Booth et al. analyzed clinical features of 144 patients infected with SARS-CoV and showed an increase in mortality and morbidity with diabetes [76]; six out of eight patients who died had diabetes. Another study showed that plasma glucose levels and diabetes were independent predictors for mortality and morbidity in patients with SARS [82]. Alqahtani et al. showed that the prevalence of death was 50% in patients with diabetes compared to the overall mortality rate of 20% in 281 confirmed MERS-CoV cases [83].
In the current pandemic with SARS-CoV-2, clinical reports also show distinctive comorbidities with diabetes. For example, the demographics of 52 patients in Wuhan with severe SARS-CoV-2 pneumonia showed that 20% had diabetes [81]. In a detailed clinical investigation of 140 hospitalized cases, Zhang et al. showed that diabetes (12.1%) was one of the most common comorbidities after hypertension (30.0%) [84]. The incidence of patients in intensive care having diabetes is twofold higher compared to that of non-intensive care patients with COVID-19 [85]. Similarly, mortality is approximately threefold higher in individuals with diabetes compared to the general mortality of COVID-19 in China [86,87]. Among the 5,700 patients hospitalized with COVID-19 in the New York Area, diabetes (33.8%) was also reported to be one of the most common morbidities [88]. Why are people with diabetes at increased risk for disease severity and mortality due to COVID-19 infection?
Until now, clinical studies reporting on the characteristics of COVID-19 patients had not indicated whether the enrolled individuals with diabetes had T1D or T2D. A very recent retrospective, multicentered study of 7,337 cases of COVID-19 in China investigated the severity and mortality among 952 individuals with pre-existing T2D [89]. The authors reported that although individuals with T2D required more medical interventions and had significantly higher mortality rates compared to patients without diabetes, well-controlled blood glucose levels were associated with improved outcomes. Poorly controlled glucose levels were associated with hospitalizations resulting from various types of infection in individuals with T1D and T2D. Additionally, preliminary analyses from the National Health Service England showed that the risk of COVID-19 mortality in either T1 or T2 diabetes is independently associated with the level of hyperglycemia [90]. Although large-scale, independent, multicenter studies are required to understand the impact of pre-existing T1 or T2 diabetes on SARS-CoV2 infection, it is plausible to suspect that in individuals with either T1 and T2 diabetes, well-controlled blood glucose levels would correlate with improved outcomes.
Cardiovascular disease and T2D are strongly associated with obesity. Although the initial studies reporting comorbidities in patients with COVID-19 have not provided information on body mass index (BMI) of patients, accumulating data suggest a high prevalence of obesity in patients with severe COVID-19. In a retrospective cohort study with 124 SARS-CoV-2 positive patients admitted to intensive care in a single French center, obesity (BMI >30 kg/m2) and severe obesity (BMI >35 kg/m2) were present in 47.6% and 28.2% of cases, respectively [91]. The need for invasive mechanical ventilation was significantly associated with BMI. In a retrospective study from New York City, analysis of BMI stratified by age in 3615 COVID-19 positive symptomatic patients showed obesity as a risk factor for hospital admission and need for critical care, especially for patients aged <60 years [92]. Similarly, from a cohort of 265 patients with COVID-19 admitted to intensive care units at university hospitals in the United States (Johns Hopkins, University of Cincinnati, New York University, University of Washington, Florida Health, and University of Pennsylvania), there was a significant inverse correlation between age and BMI, in which younger individuals admitted to hospital were more likely to be obese [93]. Recent data from a very large cohort of 20,133 hospital inpatients with COVID-19 in the United Kingdom showed that obesity was an independent risk factor for mortality, with hazard ratio of 1.33 (95% confidence interval 1.19 to 1.49, p < 0.001) [94]. Together, these data show major implications for the clinical care of COVID-19 patients with obesity and warrant independent longitudinal studies to assess hospitalization duration and mortality to implement improved health care policies.
3.3. Pathogenesis of COVID-19
The pathology of SARS-CoV-2 infection is similar to SARS-CoV with overactivated inflammatory responses strongly associated with damage to the airways [95]. The SARS-CoV-2 infection can be roughly divided into three stages [96,97]: stage I is the early infection phase, stage II is the pulmonary phase, and stage III is the hyperinflammation phase. In the early stages of the disease, patients present generally with symptoms such as fever, dry cough, and headache. Clinical signs include lymphopenia, as well as elevated IL-6, prothrombin, and D-dimer and mild LDH levels. In the pulmonary phase, some patients develop shortness of breath and abnormal chest images, and elevated transaminases are present. In the advanced, more severe phase, some patients develop a so-called “cytokine storm,” leading to other complications including acute respiratory distress syndrome, shock, multiorgan failure, and death. Elevated inflammatory markers are clinical hallmarks of this phase (CRP, LDH, IL-6, D-dimer, ferritin, Troponin, NT—proBNP). These findings are in line with SARS and MERS in that the presence of lymphopenia and “cytokine storm” may have a major role in the pathogenesis of COVID-19 [[85], [86], [87]]. Therefore, disease severity in patients is due to not only the viral infection but also the host response.
The knowledge on the cellular events following SARS-CoV-2 infection is based on data obtained from studies that have reported the immunological characteristics of moderate and severe cases of COVID-19 [87,98,99]. Infection of epithelial cells in the airways and subsequent replication of the virus in these tissues probably causes high levels of virus-linked apoptosis/pyroptosis, triggering inflammatory responses marked by the activation of pro-inflammatory cytokines or chemokines. Subsequently, macrophages and monocytes are recruited to the site; T cells and B cells are activated, and the infection is resolved in most cases.
However, in some individuals, immune dysregulation induces an insufficient type I interferon (IFN) response, aberrant pro-inflammatory cytokine secretion by alveolar macrophages, and subsequent CD4+ and CD8+ T cell dysfunction [96,98]. A successful type I interferon (IFN) response is critical to suppress viral replication, dissemination at an early stage, and induction of effective adaptive immune response. In the severe or lethal cases of SARS-CoV or MERS-CoV infection, increased neutrophil and monocyte/macrophage influx is consistently observed, and the response to viral infection by type I IFN is suppressed. In a mouse model of SARS-CoV infection, dysregulated type I IFN and inflammatory monocyte-macrophages are the main causes of lethal pneumonia [100]. Data from patients with SARS-CoV-2 also indicate an increase in total neutrophils, reduction in total lymphocytes, and increase in serum IL-6 and c-reactive protein (CRP) [101]. Increased neutrophils and decreased lymphocytes also correlate with disease severity and death. Furthermore, patients needing intensive care had higher plasma levels of many pro-inflammatory cytokines, namely, IP-10, MCP-1, MIP-1A, and TNFα, as well as fewer CD4+ and CD8+ T cells [86,87]. Thus, SARS-CoV2, akin to SARS-CoV and MERS-CoV infections, seems to induce a “cytokine storm” associated with disease severity and outcome. However, investigation of innate and adaptive immune cell subsets during different phases of the SARS-CoV2 infection may identify additional mechanisms that drive immune dysregulation in some patients.
4. Mechanisms for increased SARS-CoV-2 infection risk/severity for individuals with diabetes
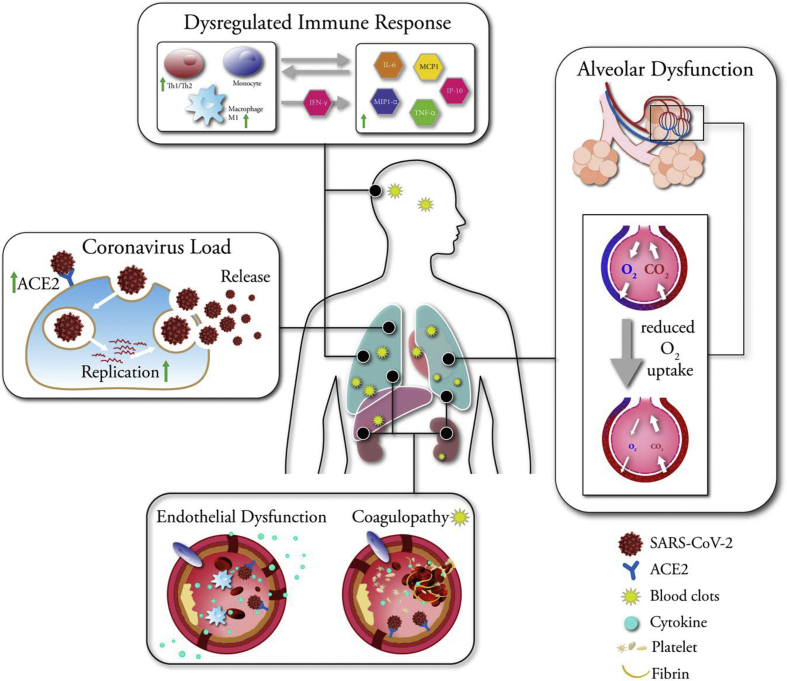
Studies have identified diabetes as a risk factor for many infections [8,13]. In the COVID-19 pandemic, diabetes is one of the leading comorbidities associated with infection severity [81,84,88]. Other comorbidities include older age, being male, and underlying medical conditions such as chronic lung disease, cardiovascular disease, and hypertension. Late diabetes complications such as diabetic kidney disease and ischemic heart disease may complicate the situation for individuals with diabetes, making them frailer, and further increasing the severity of COVID-19 disease, leading to kidney or heart failure. However, plasma glucose levels and diabetes were independent predictors for mortality and morbidity in patients with SARS [82]. Additionally, during the 2009 H1N1 pandemic, when adjusted for other illnesses, diabetes increased infection severity [14]. Moreover, mouse models have demonstrated that diabetes increased the severity of infections with influenza and MERS-CoV viral strains [102,103]. Thus, the mechanisms by which diabetes can increase infection severity cannot be explained alone with the associated comorbidities (Figure 2).
Figure 2.
Mechanisms associated with increased COVID-19 severity in individuals with diabetes. Coronavirus Load: SARS-CoV-2 infects the lung tissue via entry through ACE2 receptor. Individuals with diabetes have increased ACE2 receptor expression. Medications such as ACE inhibitors, GLP-1 agonists, and statins may increase ACE2 levels further. Increased glucose levels may allow SARS-CoV-2 replication. Dysregulated Immune Response: Individuals with diabetes have low chronic inflammation, which can lead to exaggerated macrophage and monocyte and T cell recruitment, promoting further inflammation in a feedback loop. Overproduction of pro-inflammatory cytokines may eventually damage the lung infrastructure. The resulting cytokine storm may initiate multiple systemic coagulation. Alveolar Dysfunction: Diabetes is associated with numerous structural changes to the lung including augmented permeability of the vasculature and reduced gas exchange. Impaired respiratory function present in individuals with diabetes may aggravate pulmonary complications, causing an increased need for mechanical ventilation in diabetes patients. Endothelial Dysfunction: In diabetes, endothelium shows markers of inflammation with increased immune cells, cytokines, potentially exacerbating the cytokine storm and pulmonary lesions. SARS-CoV2 can directly infect endothelial cells via the ACE2 receptors present on the endothelial cells. Change of vascular tone toward more vasoconstriction in diabetes patients can aggravate the subsequent organ ischemia, tissue edema, and a procoagulant state during COVID-19 infection. Coagulopathy: Individuals with diabetes have significant upregulation of hypercoagulation and fibrinolysis markers, and increased platelet activity and adhesion to endothelial wall, creating a favorable environment for thromboembolic events to occur under hyperinflammatory conditions such as SARS-CoV-2 infection. Blood clots can be detected in multiple organs.
4.1. Viral load
One possible mechanism through which diabetes can increase infection risk is increased viral load due to efficient virus entry. The entry receptor for SARS-CoV-2, ACE2, is expressed in various tissues including the lung, heart, kidney tubules, the luminal surface of the small intestine, and blood vessels [104]. Increased ACE2 expression in the lung, kidney, and heart was shown in mouse models of diabetes [105]. In addition, many medications prescribed to diabetes patients such as GLP-1 agonists and anti-hypertension medications such as ACE inhibitors and statins upregulate ACE2 expression [106]. Thus, because of increased ACE2 receptor expression in multiple tissues in diabetes, the severity of COVID-19 might be higher. Furthermore, ACE2 expression is reported within the exocrine and endocrine pancreas [104]. It remains to be determined whether pancreatic inflammation reported in some individuals with SARS-CoV-2 infection may lead to the exacerbation or development of diabetes in some patients. Elevated blood glucose levels can increase glucose concentrations in the airway secretions and exposure of pulmonary epithelial cells to elevated glucose concentrations have significantly increased influenza virus infection and replication [107,108]. Although, it remains to be determined whether hyperglycemia increases SARS-CoV2 replication in vivo, this is a possible explanation of the prolonged recovery of COVID-19 patients with diabetes.
4.2. Dysregulated immune response and cytokine storm
In all viral infections, successful clearance of viral load heavily depends on the orchestrated actions of the innate and adaptive immune system. As discussed in detail above, patients with T1D or T2D have a dysregulated immune system with abnormal cytokine responses and disproportionate immune cell numbers. Elevated glucose levels may also suppress the antiviral response [109]. In the context of COVID-19, a severe disease progression is described by a delay in interferon gamma response with a prolonged hyperinflammatory state and lower CD4+ and CD8+ cell numbers [69]. Individuals with diabetes have been described to have alterations in innate immune system components. For example, initial cellular events to recognize and kill pathogens such as chemotaxis and phagocytosis are impaired in individuals with diabetes [27,28]. NK cell activity is reduced in individuals with diabetes and more pro-inflammatory M1 macrophages are present in T2D [40,43]. Furthermore, T cell activity is skewed. Individuals with diabetes are in a more chronic low-level pro-inflammatory state, with a described Th1/Th2 imbalance [24,53]. Regardless of the involvement of the endothelial cells, the initial delay in interferon gamma response together with the hyperinflammatory response in individuals with diabetes may exacerbate the “cytokine storm” and increase COVID-19 severity.
4.3. Alveolar dysfunction
A recent case series of 5,700 patients hospitalized with COVID-19 in the New York City area reported that of the patients who died, those with diabetes were more likely to have received invasive mechanical ventilation in the intensive care than those without diabetes [88]. Other studies have shown an association between T2D and a significant increase in the occurrence of ventilator-associated pneumonia in mechanically ventilated adult trauma patients [110]. These observations suggest that individuals with T2D may have impairments in their alveolar function. Indeed, earlier studies have shown that pulmonary function parameters such as forced vital capacity, total lung capacity, alveolar membrane permeability, and alveolar gas exchange were significantly reduced in individuals with T2D [111]. Individuals with T1D were also reported to have a lower forced vital capacity, which was predicted to be related to poor glycemic control [112]. In animal models, diabetes was associated with numerous structural changes to the lung including augmented permeability of the vasculature and a collapsed alveolar epithelium [113]. Notably, endothelial capillary basal lamina and alveolar epithelia were described to be significantly thicker in individuals with diabetes than in controls [114]. Thus, the impaired respiratory function present in individuals with diabetes, in conjunction with the propensity of SARS-CoV-2 to infect lung tissue cells may aggravate pulmonary complications of COVID-19.
4.4. Endothelial dysfunction
Virus-induced pyroptosis is associated with vascular damage and inflammation, as observed in patients with SARS-CoV [115]. In all pathologies observed due to SARS, MERS, and COVID-19, damage was not limited to lung and occurred in multiple organs including the heart and kidneys, suggesting that SARS-CoV-2 can infect vascular endothelial cells and circulate to other organs [116]. Indeed, ACE2 is expressed in blood vessels, and Monteil et al. recently demonstrated that SARS-CoV-2 could directly infect blood vessel cells in engineered human capillary organoids [104]. Equally important, Varga et al. showed the presence of viral elements and accumulation of inflammatory cells within endothelial cells of COVID-19 patients, with evidence of endothelial and inflammatory cell death [117]. These data suggest that SARS-CoV-2 infection could initiate endothelial inflammation in several organs and that pyroptosis may have an important role in endothelial cell injury and host inflammatory response.
In individuals with T1D or T2D, endothelial dysfunction is a consistent finding and precedes microvascular disease [118]. Increased vascular lesions, endothelial inflammation, and vasoconstriction associated with endothelial dysfunction put individuals with diabetes at greater risk for endotheliitis in several organs. Change of vascular tone toward more vasoconstriction can lead to subsequent organ ischemia, tissue edema, and a procoagulant state [117]. However, further research is necessary to assess if the organ failure observed in COVID-19 is due to direct viral infection of the vasculature and other organs. Moreover, endothelial dysfunction observed in individuals with diabetes may contribute to the cytokine storm and pulmonary lesions. Glycemic oscillations have been reported to induce endothelial cytokine and adhesion molecule production, which is in turn predicted to have caused uncontrolled extravasation of leukocytes in the alveolus during influenza virus infection, leading to lung damage and impairment in respiratory function [[119], [120], [121]]. Whether cytokine production from endothelial cells contributes to pulmonary lesions in COVID-19 patients and whether this is aggravated in individuals with diabetes remains to be investigated.
4.5. Coagulopathy
More recent data indicate that a significant number of COVID-19 patients in intensive care units show hypercoagulation in multiple organs with elevated D-dimer levels and fibrin/fibrinogen degradation products, which are associated inversely with overall survival rates [87,97,122]. Severe COVID-19 has also been associated with a significantly increased risk for developing deep vein thrombosis and pulmonary embolism [123,124]. Multiple systemic coagulation is known to be activated in response to infectious complications, triggering host inflammatory reactions and activation of coagulation [125]. Hypercoagulation occurs due to profound inflammatory response, probably due to “cytokine storm” observed in some COVID-19 patients. Because individuals with diabetes have an increased risk for a more pronounced inflammatory response, they may be at a greater risk to suffer from coagulation abnormalities. Notably, individuals with either T1D or T2D have significant upregulation of hypercoagulation and fibrinolysis markers in plasma [126,127].
Moreover, hyperglycemia was shown to exaggerate coagulation, and hyperinsulinemia attenuated fibrinolytic activity during systemic inflammation [128]. In this study by Stegenga et al. the authors applied clamp techniques in healthy volunteers and increased either glucose, insulin, both or none, and administered a defined dose of LPS to induce a systemic inflammatory response [128]. After various time points, the inflammatory response and activation of coagulation/fibrinolysis were evaluated. The results demonstrated that hyperglycemia led to more pronounced activation of coagulation and neutrophil degranulation was diminished. Taken together, individuals with diabetes may be more vulnerable to thrombotic events during inflammatory states. It remains unclear whether individuals with diabetes experience more coagulation during the course of COVID-19. Although SARS-CoV-2 does not appear to cause intrinsic procoagulant effects, clarification of whether SARS-CoV-2 or the consequences of cytokine storm precipitate the onset of systemic coagulation in COVID-19 patients will be critical to design prevention and/or intervention therapies.
5. Conclusions
A growing number of studies have demonstrated diabetes as an important risk factor affecting the clinical severity of a wide range of infections. Dysregulated immune cell populations and activity observed in individuals with diabetes play a critical role in aggravating the severity. Notably, diabetes is one of the comorbidities associated with morbidity and mortality of COVID-19. A combination of underlying chronic conditions such as hypertension, obesity, and cardiovascular diseases together with altered ACE2 receptor expression, immune dysregulation, alveolar and endothelial dysfunction, and increased systemic coagulation may put individuals with diabetes at risk for COVID-19 severity. Furthermore, there is insufficient knowledge as to how glucose-lowering medications may modulate the host immune response in individuals with diabetes. Thus, understanding the clinical course and how glucose-lowering medications affect the severity of SARS-CoV-2 infection is of critical importance for disease management. Humanized mouse models of SARS-CoV-2 infections and more clinical data may provide insights into disease course. Moreover, studies to compare COVID-19 severity between individuals with T1D and T2D and identify additional clinical and/or biochemical parameters may help health care providers identify individuals at greater risk and make timely tailored therapeutic recommendations as the pandemic evolves.
Acknowledgments
I thank Dr. Maria Glavas and Dr. Tobias Schafmeier for providing feedback on this manuscript, and Dr. Sami Caner for support with graphic illustrations. I gratefully acknowledge funding support from JDRF International.
Conflict of interest
None.
References
- 1.International Diabetes Federation . 8th ed. 2017. IDF Diabetes Atlas. [Google Scholar]
- 2.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 3.Beckman J.A., Creager M.A. Vascular complications of diabetes. Circulation Research. 2016;118(11):1771–1785. doi: 10.1161/CIRCRESAHA.115.306884. [DOI] [PubMed] [Google Scholar]
- 4.Iglay K., Hannachi H., Joseph Howie P., Xu J., Li X., Engel S.S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Current Medical Research and Opinion. 2016;32(7):1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 5.Long A.N., Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. Journal of Clinical Hypertension. 2011;13(4):244–251. doi: 10.1111/j.1751-7176.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazeli Farsani S., Souverein P.C., van der Vorst M.M., Knibbe C.A., de Boer A., Mantel-Teeuwisse A.K. Chronic comorbidities in children with type 1 diabetes: a population-based cohort study. Archives of Disease in Childhood. 2015;100(8):763–768. doi: 10.1136/archdischild-2014-307654. [DOI] [PubMed] [Google Scholar]
- 7.Laing S.P., Swerdlow A.J., Carpenter L.M., Slater S.D., Burden A.C., Botha J.L. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke. 2003;34(2):418–421. doi: 10.1161/01.str.0000053843.03997.35. [DOI] [PubMed] [Google Scholar]
- 8.Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41(10):2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 9.Hine J.L., de Lusignan S., Burleigh D., Pathirannehelage S., McGovern A., Gatenby P. Association between glycaemic control and common infections in people with Type 2 diabetes: a cohort study. Diabetic Medicine. 2017;34(4):551–557. doi: 10.1111/dme.13205. [DOI] [PubMed] [Google Scholar]
- 10.Kornum J.B., Thomsen R.W., Riis A., Lervang H.H., Schonheyder H.C., Sorensen H.T. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30(9):2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 11.Mor A., Dekkers O.M., Nielsen J.S., Beck-Nielsen H., Sorensen H.T., Thomsen R.W. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. American Journal of Epidemiology. 2017;186(2):227–236. doi: 10.1093/aje/kwx049. [DOI] [PubMed] [Google Scholar]
- 12.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N. Diabetes mellitus, fasting glucose, and risk of cause-specific death. New England Journal of Medicine. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey I.M., Critchley J.A., DeWilde S., Harris T., Hosking F.J., Cook D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- 14.Allard R., Leclerc P., Tremblay C., Tannenbaum T.N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care. 2010;33(7):1491–1493. doi: 10.2337/dc09-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S., Kamimoto L., Bramley A.M., Schmitz A.M., Benoit S.R., Louie J. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. New England Journal of Medicine. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 16.Peleg A.Y., Weerarathna T., McCarthy J.S., Davis T.M. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metabolism Research Review. 2007;23(1):3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Chen H., Li Q., Qiu B., Wang J., Sun X. Fasting plasma glucose is an independent predictor for severity of H1N1 pneumonia. BMC Infectious Diseases. 2011;11:104. doi: 10.1186/1471-2334-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilking H., Buda S., von der Lippe E., Altmann D., Krause G., Eckmanns T. Mortality of 2009 pandemic influenza A(H1N1) in Germany. Euro Surveillance. 2010;15(49) doi: 10.2807/ese.15.49.19741-en. [DOI] [PubMed] [Google Scholar]
- 19.Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clinical Infectious Diseases. 2005;41(3):281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 20.Shah B.R., Hux J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 21.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiological Reviews. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 22.Anhê F.F., Jensen B.A.H., Varin T.V., Servant F., Van Blerk S., Richard D. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nature Metabolism. 2020;2(3):233–242. doi: 10.1038/s42255-020-0178-9. [DOI] [PubMed] [Google Scholar]
- 23.Thaiss C.A., Levy M., Grosheva I., Zheng D., Soffer E., Blacher E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 24.Alexandraki K.I., Piperi C., Ziakas P.D., Apostolopoulos N.V., Makrilakis K., Syriou V. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. Journal of Clinical Immunology. 2008;28(4):314–321. doi: 10.1007/s10875-007-9164-1. [DOI] [PubMed] [Google Scholar]
- 25.Davidson N.J., Sowden J.M., Fletcher J. Defective phagocytosis in insulin controlled diabetics: evidence for a reaction between glucose and opsonising proteins. Journal of Clinical Pathology. 1984;37(7):783–786. doi: 10.1136/jcp.37.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delamaire M., Maugendre D., Moreno M., Le Goff M.C., Allannic H., Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Medicine. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 27.Huang J., Xiao Y., Zheng P., Zhou W., Wang Y., Huang G. Distinct neutrophil counts and functions in newly diagnosed type 1 diabetes, latent autoimmune diabetes in adults, and type 2 diabetes. Diabetes Metabolism Research Review. 2019;35(1) doi: 10.1002/dmrr.3064. [DOI] [PubMed] [Google Scholar]
- 28.Lecube A., Pachon G., Petriz J., Hernandez C., Simo R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PloS One. 2011;6(8) doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marhoffer W., Stein M., Maeser E., Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256–260. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 30.Mowat A.G., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with rheumatoid arthritis. Journal of Clinical Investigation. 1971;50(12):2541–2549. doi: 10.1172/JCI106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlou S., Lindsay J., Ingram R., Xu H., Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunology. 2018;19(1):24. doi: 10.1186/s12865-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiller F., Carlos D., Souto F.O., de Freitas A., Soares F.S., Vieira S.M. alpha1-Acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Diabetes. 2012;61(6):1584–1591. doi: 10.2337/db11-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tater D., Tepaut B., Bercovici J.P., Youinou P. Polymorphonuclear cell derangements in type I diabetes. Hormone and Metabolic Research. 1987;19(12):642–647. doi: 10.1055/s-2007-1011899. [DOI] [PubMed] [Google Scholar]
- 34.Trevelin S.C., Carlos D., Beretta M., da Silva J.S., Cunha F.Q. Diabetes mellitus and sepsis: a challenging association. Shock. 2017;47(3):276–287. doi: 10.1097/SHK.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 35.Lederman M.M., Schiffman G., Rodman H.M. Pneumococcal immunization in adult diabetics. Diabetes. 1981;30(2):119–121. doi: 10.2337/diab.30.2.119. [DOI] [PubMed] [Google Scholar]
- 36.Li Volti S., Caruso-Nicoletti M., Biazzo F., Sciacca A., Mandara G., Mancuso M. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Archives of Disease in Childhood. 1998;78(1):54–57. doi: 10.1136/adc.78.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pozzilli P., Gale E.A., Visalli N., Baroni M., Crovari P., Frighi V. The immune response to influenza vaccination in diabetic patients. Diabetologia. 1986;29(12):850–854. doi: 10.1007/BF00870139. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan P.A., Paich H.A., Handy J., Karlsson E.A., Schultz-Cherry S., Hudgens M. The antibody response to influenza vaccination is not impaired in type 2 diabetics. Vaccine. 2015;33(29):3306–3313. doi: 10.1016/j.vaccine.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahr I., Jahn J., Zipprich A., Pahlow I., Spielmann J., Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunologic Research. 2018;66(2):234–244. doi: 10.1007/s12026-018-8989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J.H., Park K., Lee S.B., Kang S., Park J.S., Ahn C.W. Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. Journal of Diabetes Investigation. 2019;10(5):1223–1228. doi: 10.1111/jdi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutens L., Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lumeng C.N., DelProposto J.B., Westcott D.J., Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metabolism. 2014;20(4):614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J., Xiao Y., Xu A., Zhou Z. Neutrophils in type 1 diabetes. Journal of Diabetes Investigation. 2016;7(5):652–663. doi: 10.1111/jdi.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collier A., Jackson M., Bell D., Patrick A.W., Matthews D.M., Young R.J. Neutrophil activation detected by increased neutrophil elastase activity in type 1 (insulin-dependent) diabetes mellitus. Diabetes Research. 1989;10(3):135–138. [PubMed] [Google Scholar]
- 46.Harsunen M.H., Puff R., D'Orlando O., Giannopoulou E., Lachmann L., Beyerlein A. Reduced blood leukocyte and neutrophil numbers in the pathogenesis of type 1 diabetes. Hormone and Metabolic Research. 2013;45(6):467–470. doi: 10.1055/s-0032-1331226. [DOI] [PubMed] [Google Scholar]
- 47.Valle A., Giamporcaro G.M., Scavini M., Stabilini A., Grogan P., Bianconi E. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62(6):2072–2077. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inacio D.P., Amado T., Silva-Santos B., Gomes A.Q. Control of T cell effector functions by miRNAs. Cancer Letters. 2018;427:63–73. doi: 10.1016/j.canlet.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Stentz F.B., Kitabchi A.E. Activated T lymphocytes in Type 2 diabetes: implications from in vitro studies. Current Drug Targets. 2003;4(6):493–503. doi: 10.2174/1389450033490966. [DOI] [PubMed] [Google Scholar]
- 50.Nicholas D.A., Proctor E.A., Agrawal M., Belkina A.C., Van Nostrand S.C., Panneerseelan-Bharath L. Fatty acid metabolites combine with reduced beta oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metabolism. 2019;30(3):447–461. doi: 10.1016/j.cmet.2019.07.004. e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of the American Medical Association. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 52.Garidou L., Pomie C., Klopp P., Waget A., Charpentier J., Aloulou M. The gut microbiota regulates intestinal CD4 T cells expressing RORgammat and controls metabolic disease. Cell Metabolism. 2015;22(1):100–112. doi: 10.1016/j.cmet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Menart-Houtermans B., Rutter R., Nowotny B., Rosenbauer J., Koliaki C., Kahl S. Leukocyte profiles differ between type 1 and type 2 diabetes and are associated with metabolic phenotypes: results from the German Diabetes Study (GDS) Diabetes Care. 2014;37(8):2326–2333. doi: 10.2337/dc14-0316. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 55.Jagannathan-Bogdan M., McDonnell M.E., Shin H., Rehman Q., Hasturk H., Apovian C.M. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. The Journal of Immunology. 2011;186(2):1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sireesh D., Dhamodharan U., Ezhilarasi K., Vijay V., Ramkumar K.M. Association of NF-E2 related factor 2 (Nrf2) and inflammatory cytokines in recent onset type 2 diabetes mellitus. Scientific Reports. 2018;8(1):5126. doi: 10.1038/s41598-018-22913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortez-Espinosa N., Cortes-Garcia J.D., Martinez-Leija E., Rodriguez-Rivera J.G., Barajas-Lopez C., Gonzalez-Amaro R. CD39 expression on Treg and Th17 cells is associated with metabolic factors in patients with type 2 diabetes. Human Immunology. 2015;76(9):622–630. doi: 10.1016/j.humimm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Tang Q., Adams J.Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xufre C., Costa M., Roura-Mir C., Codina-Busqueta E., Usero L., Pizarro E. Low frequency of GITR+ T cells in ex vivo and in vitro expanded Treg cells from type 1 diabetic patients. International Immunology. 2013;25(10):563–574. doi: 10.1093/intimm/dxt020. [DOI] [PubMed] [Google Scholar]
- 60.Woo P.C., Huang Y., Lau S.K., Yuen K.Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Current Topics in Microbiology and Immunology. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8(Suppl):S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization . 2019. Mers situation update. [Google Scholar]
- 68.World Health Organization . 2020. Coronavirus disease (COVID-19) situation report - 138. [Google Scholar]
- 69.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology. 2020 doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J.Y., Ko J.H., Kim Y., Kim Y.J., Kim J.M., Chung Y.S. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. Journal of Korean Medical Science. 2020;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet Infectious Diseases. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England Journal of Medicine. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ling Z., Xu X., Gan Q., Zhang L., Luo L., Tang X. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. European Journal of Radiology. 2020;126:108956. doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Journal of the American Medical Association. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 77.Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. The Lancet Infectious Diseases. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivers C.M., Majumder M.S., Lofgren E.T. Risks of death and severe disease in patients with Middle East respiratory syndrome coronavirus, 2012-2015. American Journal of Epidemiology. 2016;184(6):460–464. doi: 10.1093/aje/kww013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y.M., Hsu C.Y., Lai C.C., Yen M.F., Wikramaratna P.S., Chen H.H. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Scientific Reports. 2017;7(1):11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiration Medicine. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetic Medicine. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 83.Alqahtani F.Y., Aleanizy F.S., Ali El Hadi Mohamed R., Alanazi M.S., Mohamed N., Alrasheed M.M. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiology and Infection. 2018:1–5. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 85.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Research in Cardiology. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 87.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu L., She Z.G., Chen X., Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabolism. 2020;31(6):1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holman N.K., P, Kar P., O'Keefe J., Curley M., Weaver A. 2020. Type 1 and Type 2 diabetes and COVID-19 related mortality in England: a cohort study in people with diabetes. pre-print NHS England. [Google Scholar]
- 91.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinology. 2020 doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death & Differentiation. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Journal of the American Medical Association. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. Journal of Clinical Investigation. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marshall R.J., Armart P., Hulme K.D., Chew K.Y., Brown A.C., Hansbro P.M. Glycemic variability in diabetes increases the severity of influenza. mBio. 2020;11(2) doi: 10.1128/mBio.02841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. International Journal of Molecular Sciences. 2017;18(3) doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocrine Reviews. 2020;41(3) doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Philips B.J., Meguer J.X., Redman J., Baker E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Medicine. 2003;29(12):2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 108.Kohio H.P., Adamson A.L. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444(1–2):301–309. doi: 10.1016/j.virol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 109.Reading P.C., Allison J., Crouch E.C., Anders E.M. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? Journal of Virology. 1998;72(8):6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darvishi-Khezri H., Alipour A., Emami Zeydi A., Firouzian A., Mahmudi G., Omrani-Nava M. Is type 2 diabetes mellitus in mechanically ventilated adult trauma patients potentially related to the occurrence of ventilator-associated pneumonia? Journal of Research in Medical Sciences. 2016;21:19. doi: 10.4103/1735-1995.179887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anandhalakshmi S., Manikandan S., Ganeshkumar P., Ramachandran C. Alveolar gas exchange and pulmonary functions in patients with type II diabetes mellitus. Journal of Clinical and Diagnostic Research. 2013;7(9):1874–1877. doi: 10.7860/JCDR/2013/6550.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Komatsu W.R., Barros Neto T.L., Chacra A.R., Dib S.A. Aerobic exercise capacity and pulmonary function in athletes with and without type 1 diabetes. Diabetes Care. 2010;33(12):2555–2557. doi: 10.2337/dc10-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popov D., Simionescu M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. European Respiratory Journal. 1997;10(8):1850–1858. doi: 10.1183/09031936.97.10081850. [DOI] [PubMed] [Google Scholar]
- 114.Weynand B., Jonckheere A., Frans A., Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration. 1999;66(1):14–19. doi: 10.1159/000029331. [DOI] [PubMed] [Google Scholar]
- 115.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Frontiers in Microbiology. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228) doi: 10.1016/S0140-6736(20)30558-4. e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Avogaro A., Albiero M., Menegazzo L., de Kreutzenberg S., Fadini G.P. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285–S290. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hulme K.D., Gallo L.A., Short K.R. Influenza virus and glycemic variability in diabetes: a killer combination? Frontiers in Microbiology. 2017;8:861. doi: 10.3389/fmicb.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perrone L.A., Plowden J.K., Garcia-Sastre A., Katz J.M., Tumpey T.M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathogens. 2008;4(8) doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Short K.R., Kroeze E., Fouchier R.A.M., Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. The Lancet Infectious Diseases. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 122.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antoniak S. The coagulation system in host defense. Research Practitioner Thrombosis & Haemostasis. 2018;2(3):549–557. doi: 10.1002/rth2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carr M.E. Diabetes mellitus: a hypercoagulable state. Journal of Diabetic Complications. 2001;15(1):44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 127.Kearney K., Tomlinson D., Smith K., Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovascular Diabetology. 2017;16(1):34. doi: 10.1186/s12933-017-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stegenga M.E., van der Crabben S.N., Blumer R.M., Levi M., Meijers J.C., Serlie M.J. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112(1):82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]