Abstract
The outbreak of coronavirus infectious disease-2019 (COVID-19) pneumonia raises the concerns of effective deactivation of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in medical wastewater by disinfectants. In this study, we evaluated the presence of SARS-CoV-2 viral RNA in septic tanks of Wuchang Cabin Hospital and found a striking high level of (0.5–18.7) × 103 copies/L after disinfection with sodium hypochlorite. Embedded viruses in stool particles might be released in septic tanks, behaving as a secondary source of SARS-CoV-2 and potentially contributing to its spread through drainage pipelines. Current recommended disinfection strategy (free chlorine ≥0.5 mg/L after at least 30 min suggested by World Health Organization; free chlorine above 6.5 mg/L after 1.5-h contact by China Centers for Disease Control and Prevention) needs to be reevaluated to completely remove SARS-CoV-2 viral RNA in non-centralized disinfection system and effectively deactivate SARS-CoV-2. The effluents showed negative results for SARS-CoV-2 viral RNA when overdosed with sodium hypochlorite but had high a level of disinfection by-product residuals, possessing significant ecological risks.
Keywords: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Disinfection, Cabin hospital, Disinfection by-products
Graphical abstract
Highlights
-
•
First report on disinfection performance for SARS-CoV-2 in medical wastewater
-
•
Incomplete removal of SARS-CoV-2 RNA under WHO guideline
-
•
High level of DBPs when SARS-CoV-2 is completed removed.
1. Introduction
The outbreak of coronavirus infectious disease-2019 (COVID-19) pneumonia since 2019 is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Lai et al., 2020; Li et al., 2020; Ralph et al., 2020), and it has rapidly spread throughout 202 countries around the world. Till 20th June 2020, there are over 8.0 million confirmed cases and around 450,000 deaths globally, and the number is still increasing rapidly (WHO, 2020a). There is clear evidence of human-to-human transmission of SARS-CoV-2 (Chan et al., 2020; Chang et al., 2020; Li et al., 2020; Poon and Peiris, 2020). Besides direct contact and respiratory routes (Carlos et al., 2020; Lai et al., 2020; Wu et al., 2020), stool transmission might be an alternative route owing to the survival of SARS-CoV-2 in patient's stools (Holshue et al., 2020; Ling et al., 2020; Tian et al., 2020; Xiao et al., 2020; Xing et al., 2020; Zhang et al., 2020c). As municipal wastewater pipe network receives huge amounts of wastewater from asymptomatic patients and treated sewage from hospitals, SARS-CoV-2 from non- or inefficient-disinfected wastewater might persist for a prolonged time in pipe network, becoming a secondary spreading source (Zhang et al., 2020a). It brings urgent requirement and careful consideration of disinfection strategies to prevent SARS-CoV-2 from entering drainage pipe network.
Disinfection is of great importance to eliminate or deactivate pathogenic microorganisms. Traditional disinfection strategies include ultraviolet germicidal irradiation and biocidal agents, e.g., gaseous ozone, alcohol, formaldehyde, hydrogen peroxide, peroxyacetic acid, povidone iodine and chlorine-based disinfectant (Kampf et al., 2020; Tseng and Li, 2008; Tseng and Li, 2007; Walker and Ko, 2007). Some disinfectants are intensively used in hospitals for nurse personal care (Dumas et al., 2019; Ioannou et al., 2017). Among them, chlorine-based disinfectants are widely used for their broad sterilization spectrum, high inactivation efficiency, low price, and easy decomposition with little residue (How et al., 2017). Nevertheless, overuse of chlorine-based disinfectants brings concerns of disinfection by-products (DBPs) which are harmful to ecosystems and human health (Bull et al., 2011; Richardson et al., 2007; Wang et al., 2014). More than 600 kinds of DBPs have been observed (Richardson, 2011), such as trihalomethanes (THMs), haloacetic acids (HAAs), halogen acetonitriles (HANs), halonitromethanes (HNMs) and haloacetamides (HAcAms) (Ding et al., 2020; Kozari et al., 2020; Luo et al., 2020; Zhai et al., 2014). Some of them are reported attributable for bladder cancer (Evlampidou et al., 2020; Li and Mitch, 2018) and adverse reproductive outcomes (Nieuwenhuijsen et al., 2000). For effective centralized disinfection, World Health Organization (WHO) has suggested free chlorine ≥0.5 mg/L after at least 30 min of contact time at pH<8.0 (WHO, 2020b). Additionally, China has launched a guideline for emergency treatment of medical sewage containing SARS-CoV-2 on 1st February 2020, requiring free chlorine of ≥6.5 mg/L and contact time of ≥1.5 h in disinfection units (China-MEE, 2020). Unfortunately, the performance of chlorine-based disinfectants on SARS-CoV-2 in real medical wastewater treatment system is not clear yet.
In this work, we studied the presence of SARS-CoV-2 viral RNA in septic tanks of Wuchang Fangcang (Cabin) Hospital (Wuhan, China) to evaluate the disinfection performance and optimize disinfection strategies to prevent SARS-CoV-2 from spreading through drainage pipelines. Further analysis of DBPs evaluated the potential ecological risks in the effluents.
2. Materials and methods
2.1. Wuchang Cabin Hospital and disinfection strategy
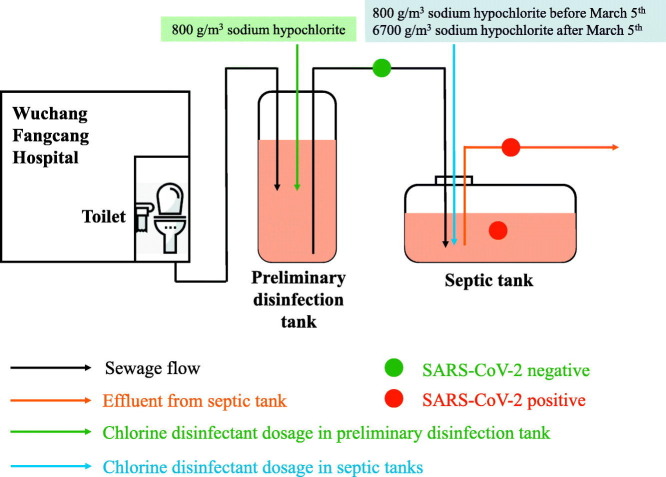
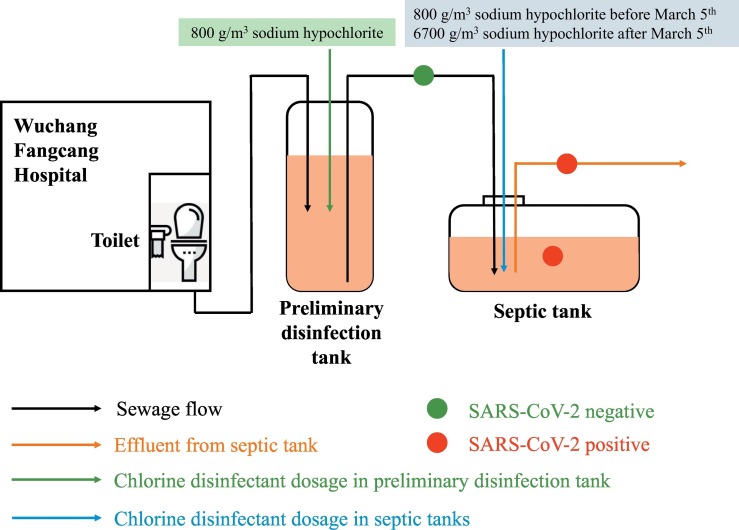
Wuchang Cabin Hospital was a temporary hospital designated for COVID-19 patients, formerly Wuchang Stadium located in Wuhan (longitude, E114°20′05″; latitude, N30°32′46″). It was open from 5th February as the first cabin hospital in Wuhan and closed on 10th March, in total receiving 1124 COVID-19 patients (Fig. 1 ). It had eight separate toilets and all sewage from toilets and showers were combined, with daily volume of wastewater ranging from 60 to 200 m3. The wastewater was firstly pumped from toilets and showers into the first preliminary disinfection tank, and sodium hypochlorite was unregularly added to a final concentration of 800 g/m3 when it was 90% full. Afterwards, the first preliminary disinfection tank was temporarily enclosed and the wastewater was then pumped into another disinfection tank and disinfected in the same way. On each day, septic tanks outside the hospital were emptied at 8 am to discharge the effluents into pipe network and wastewater treatment plants and received wastewater from preliminary tanks at 10 am. Sodium hypochlorite was supplemented for 1.5 h contact and the mixing was conducted by continuous pumping and stirring. Wastewater was then pumped into three septic tanks. Before March 5th, the dosage of sodium hypochlorite in septic tanks was 800 g/m3 and it increased to 6700 g/m3 after 5th March to secure complete deactivation of SARS-CoV-2.
Fig. 1.
Schematic disinfection process of septic tanks of Wuchang Cabin Hospital.
2.2. Sampling and chemical analysis
Influent and effluent samples were collected from septic tanks of Wuchang Cabin Hospital on 26th February, 1st March and 10th March 2020 (Table 1 ). On these sampling days, the number of COVID-19 patients in Wuchang Cabin Hospital was stable, ranging from 200 to 400. Around 2.0 L of water was directly collected in a plexiglass sampler and transferred into a sterile plastic bag for biological analysis and a brown glass bottle for DBPs analysis. As we found obvious settlement of stool particles and suspended solids in septic tanks, a stratified plexiglass sampler was used to obtain samples from different layers of septic tanks to assess the levels of viruses and DBPs along depth, designated as top-layer (0–50 cm) and bottom-layer (50–100 cm) water.
Table 1.
SARS-CoV-2 viral RNA copy numbers and free chlorine in the effluents of septic tanks of Wuchang Cabin Hospital. Disinfectants were added at 10 am every day into the septic tanks.
| Dates | Samples | SARS-CoV-2 (copies/L) | Free chlorine (mg/L)c |
|---|---|---|---|
| 26th February | Influent of septic tank | NDa (0/1)b | ND |
| Effluent of septic tank | (14.7 ± 2.2) × 103 (1/1) | ND | |
| 1st March | Influent of septic tank | ND (0/1) | ND |
| Effluent of septic tank | (7.5 ± 2.8) × 103 (6/6) | ND | |
| 10th March | Influent of septic tank | ND (0/2) | ND |
| Effluent of septic tank | ND (0/2) | 23.0 ± 2.0 |
ND, non-detected.
Fraction in bracket is number of positive samples to number of total samples.
Free chlorine in septic tank 12-h after sodium hypochlorite addition.
Free chlorine was measured on site using PCII58700–00 (HACH, USA). DBPs measurements were carried out on a GCMS-QP2020 (Shimadzu, Japan) equipped with Atomx purge, trap autosampler (Teledyne Tekmar, USA) and an SH-RTX-5MS [(5%)-phenyl-(95%)-methylpolysiloxane] fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness). The autosampler operating conditions were as follows: purge for 11 min at 30 °C with high-purity nitrogen gas at a flow rate of 40 mL/min, dry purge for 1.0 min at 20 °C with the flow rate of 40 mL/min, pre-desorption at 180 °C and desorption for 2.0 min at 190 °C, and bake for 6.0 min at 200 °C.
2.3. RNA extraction and RT-qPCR
Collected water samples were placed in 4 °C ice-boxes and immediately transferred into laboratory for RNA extraction. After centrifugation at 3000 rpm to remove suspended solids, the supernatant was subsequently supplemented with NaCl (0.3 mol/L) and PEG-6000 (10%), settled overnight at 4 °C, and centrifuged at 10,000g for 30 min. Viral RNA in pellets was extracted using the EZ1 virus Mini kit (Qiagen, Germany) according to the manufacturer's instructions. SARS-CoV-2 RNA was quantified by RT-qPCR using AgPath-ID™ One-Step RT-PCR Kit (Life Technologies, USA) on a LightCycler 480 Real-time PCR platform (Roche, USA) in duplicates. Two target genes simultaneously amplified were open reading frame lab (CCDC-ORF1, forwards primer: 5′-CCCTGTGGGTTTTACACTTAA-3′; reverse primer: 5′-ACGATTGTGCATCAGCTGA-3′; fluorescence probe: 5′-FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′) and nucleocapsid protein (CCDC-N, forwards primer: 5′-GGGGAACTTCTCCTGCTAGAAT-3′; reverse primer: 5′-CAGACATTTTGCTCTCAAGCTG-3′; fluorescence probe: 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′). RT-qPCR amplification for CCDC-ORF1 and CCDC-N was performed in 25 μL reaction mixtures containing 12.5 μL of 2 × RT-PCR Buffer, 1 μL of 25 × RT-PCR Enzyme Mix, 4 μL mixtures of forward primer (400 nM), reverse primer (400 nM) and probe (120 nM), and 5 μL of template RNA. Reverse transcription was conducted at 45 °C for 10 min (1 cycle), followed by initial denaturation at 90 °C for 10 min (1 cycle) and 40 thermal cycles of 60 °C for 45 s and 90 °C for 15 s. Quantitative fluorescent signal for each sample was normalized by ROX™ passive reference dye provided in 2 × RT-PCR buffer. For each RT-qPCR run, both positive and negative controls were included. The copy numbers of SARS-CoV-2 was obtained from a standard calibration curve by a 10-fold serial dilution of genes encoding nucleocapsid protein with an amplification efficiency of 102.6%, calculated as copies = 10[−(Cq-39.086)/3.262] (R2 = 0.991). For quality control, a reagent blank and extraction blank were included for RNA extraction procedure and no contamination was observed.
2.4. Data analysis
One-way ANOVA was used to compare the difference between samples and p-value less than 0.05 refers to significant difference. All data were presented in mean ± standard deviation (n = 3).
3. Results and discussions
For all influents of septic tanks received from Wuchang Cabin Hospital, there was no positive result for SARS-CoV-2 viral RNA. On 26th February and 1st March, SARS-CoV-2 viral RNA was (14.7 ± 2.2) × 103 and (7.5 ± 2.8) × 103 copies/L in the effluents of septic tanks, respectively (Table 1). Although there was no significant difference between these two sampling days, there were more SARS-CoV-2 RNA in top-layer waters [(10.0 ± 5.3) × 103 copies/L] than in bottom-layer waters [(5.1 ± 2.1) × 103 copies/L, p=0.019].
On 26th February and 1st March, free chlorine in the effluents was above 6.5 mg/L after 1.5 h contact with 800 g/m3 of sodium hypochlorite, meeting well with the guideline for emergency treatment of medical sewage containing SARS-CoV-2 suggested by China Centers for Disease Control and Prevention (China CDC). Twelve hours after sodium hypochlorite addition, free chlorine declined to nondetectable (total chlorine 4.0–8.8 mg/L) when SARS-CoV-2 viral RNA were detectable (Table 1). It hinted an unexpected presence of SARS-CoV-2 viral RNA after disinfection and a dosage of 800 g/m3 of sodium hypochlorite could not completely remove SARS-CoV-2 viral RNA. To improve disinfection performance and secure a complete deactivation of SARS-CoV-2, the dosage of sodium hypochlorite was increased to 6700 g/m3 since 6th March and free chlorine in the effluents on 10th March ranged from 21.0 to 25.0 mg/L at 12-h after supplementing sodium hypochlorite. Eventually, the effluents of septic tanks were negative for SARS-CoV-2 viral RNA. However, trichlormethane, tribromomethane, bromodichloromethane and dibromochloromethane was 332 ± 122 (97.8% of total DBPs), 1.9 ± 1.0 (0.5% of total DBPs), 5.1 ± 3.1 (1.5% of total DBPs) and 0.6 ± 0.5 (0.2% of total DBPs) μg/L in the effluents, respectively. There was no significant difference in DBPs levels between top- and bottom-layer waters.
The absence of SARS-CoV-2 viral RNA in the influents of septic tanks suggested that preliminary disinfection in Wuchang Cabin Hospital was satisfactory to remove SARS-CoV-2 in aqueous phase after 1.5-h contact. In septic tanks, disinfection achieved free chlorine >6.5 mg/L for 1.5 h when the dosage of sodium hypochlorite was 800 g/m3, meeting well with the guideline for emergency treatment of medical sewage containing SARS-CoV-2 suggested by China CDC. However, SARS-CoV-2 viral RNA was surprisingly positive in the effluents after 12 h when free chlorine declined to nondetectable. It might be explained by the release of embedded SARS-CoV-2 viral RNA from stool particles in septic tanks. SARS-CoV-2 has been found in patients' stools in many previous studies (Wu et al., 2020; Xing et al., 2020), which could escape from disinfection and slowly release into aqueous phase. Suspended particles as small as 7 mm can protect viruses from UV exposure and dwindle their vulnerability to direct sunlight inactivation, and 0.3-mm sized particles can shield viruses from disinfection for their prolonged survival (Templeton et al., 2005). As stools are rich in organic compounds and form numerous suspended solids containing SARS-CoV-2, they are of high risk as the source releasing viruses in septic tanks. It is also evidenced by more SARS-CoV-2 RNA in upper-layer waters, explained by more stool residuals and suspended solids in the bottom of septic tanks absorbing SARS-CoV-2 from aquatic water. Our results suggested that current recommended disinfection dosage could effectively remove aqueous SARS-CoV-2 viruses in wastewater containing limited suspended solids, like wastewater treatment plants (La Rosa et al., 2020; Zhang et al., 2020b), but it might be not enough for embedded viruses in suspended solids and require higher level of chlorine-based disinfectant supplement. Septic tanks can behave as a long-term source to release SARS-CoV-2 viral RNA into waters when disinfection is not sufficient and challenges public health via potentially spreading viruses in drainage pipelines.
The surprising presence of SARS-CoV-2 viral RNA after disinfection with sodium hypochlorite suggested that free chlorine >0.5 mg/L after 1.5-h contact time cannot completely remove SARS-CoV-2 viral RNA, and 800 g/m3 dosage of sodium hypochlorite might be not enough to secure a complete disinfection of medical wastewaters, particularly for those from cabin, temporary or non-centralized hospitals containing huge amount of stool debris and suspended solids. From the negative results of SARS-CoV-2 viral RNA (Table 1), the complete deactivation of SARS-CoV-2 was achieved when the dosage of sodium hypochlorite was 6700 g/m3. As the number of COVID-19 patients was similar on the three sampling days, they produced similar wastewater discharge and viral load. The change of SARS-CoV-2 in effluents was therefore attributing to the increasing sodium hypochlorite supplement for disinfection. Nevertheless, it was an over-dosage and resulted in a significant level of DBPs in the effluents, which was about 15 times higher than other hospital wastewater (Luo et al., 2020). The four detected chloroform and trihalomethanes accounted to the majority of total DBPs in wastewaters (Furst et al., 2019) and their composition was similar as previous studies (Luo et al., 2020; Zhong et al., 2019). They show high ecological risks and challenge the surrounding environment receiving disinfected medical wastewater, possessing threats to ecological system and human health (Ding et al., 2020; Li et al., 2019). Additionally, applying high level of chlorine-based and other disinfectants have lasted for three months in Wuhan since the outbreak of COVID-19, and further studies are suggested to carefully evaluate its ecological risks. It is therefore important to consider alternative strategies to both improve disinfection performance and reduce DBPs. One possible solution is to segregate wastewater and suspended solids from preliminary disinfection tanks, preventing the prolonged release of SARS-CoV-2 from stool particles into aqueous phase. The recommended 800 g/m3 dosage of sodium hypochlorite is then enough to secure negative viral presence and produce fewer DBPs in the effluents, and the suspended solids can be treated separately as medical wastes (Wang et al., 2020).
Owing to the operational limitations during the COVID-19 outbreak in Wuhan and restricted sample transport to other laboratories, this study did not demonstrate viral viability after disinfection by viral culture. Additionally, disinfection operations in septic tanks of Wuchang Cabin Hospital were strictly controlled by government officials that we could not collect more samples or explore the appropriate dosage of sodium hypochlorite for complete removal of SARS-CoV-2 with minimal DBPs. The suggested dosage of sodium hypochlorite is based on the complete destruction of viral RNA and might overestimate the level of required chlorine-based disinfectants or bring extra risks of DBPs. Further studies are suggested to evaluate optimal disinfection dosage considering the reduction of viral viability. This work attempts to give initial information about the potential risks of viral spread and DBPs residues during disinfection processes for medical wastewater containing SARS-CoV-2.
4. Conclusion
Our study for the first time reported an unexpected presence of SARS-CoV-2 viral RNA in septic tanks after disinfection with 800 g/m3 of sodium hypochlorite and current disinfection guideline by WHO and China CDC might not secure a complete removal of SARS-CoV-2 in medical wastewater. SARS-CoV-2 might be embedded in patient's stools, protected by organic matters from disinfection, and slowly release when free chlorine declines. Septic tanks in non-centralized disinfection system of cabin hospitals or isolation points potentially behave as a secondary source spreading SARS-CoV-2 in drainage pipelines for a prolonged time. Disinfection strategy is of great urgency to improve and the ecological risks of DBPs need to be carefully considered.
CRediT authorship contribution statement
Dayi Zhang:Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing.Haibo Ling:Investigation.Xia Huang:Conceptualization, Writing - original draft.Jing Li:Investigation.Weiwei Li:Investigation.Chuan Yi:Investigation.Ting Zhang:Investigation.Yongzhong Jiang:Investigation.Yuning He:Writing - original draft.Songqiang Deng:Data curation.Xian Zhang:Data curation.Xinzi Wang:Data curation.Yi Liu:Conceptualization, Writing - original draft.Guanghe Li:Conceptualization, Writing - original draft.Jiuhui Qu:Conceptualization, Writing - original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Damia Barcelo
Contributor Information
Dayi Zhang, Email: zhangdayi@tsinghua.edu.cn.
Jiuhui Qu, Email: jhqu@tsinghua.edu.cn.
References
- Bull R.J., Reckhow D.A., Li X.F., Humpage A.R., Joll C., Hrudey S.E. Potential carcinogenic hazards of non-regulated disinfection by-products: Haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology. 2011;286:1–19. doi: 10.1016/j.tox.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S.F., Kok K.H., To K.K.W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China-MEE . 2020. Technical Solution for Emergency Treatment of Medical Sewage Contaminated by Novel Coronavirus. [Google Scholar]
- Ding X.L., Zhu J.Y., Zhang J., Dong T.Y., Xia Y.K., Jiao J.D. Developmental toxicity of disinfection by-product monohaloacetamides in embryo-larval stage of zebrafish. Ecotoxicol. Environ. Saf. 2020;189 doi: 10.1016/j.ecoenv.2019.110037. [DOI] [PubMed] [Google Scholar]
- Dumas O., Varraso R., Boggs K.M., Quinot C., Zock J.P., Henneberger P.K. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evlampidou I., Font-Ribera L., Rojas-Rueda D., Gracia-Lavedan E., Costet N., Pearce N. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ. Health Perspect. 2020;128 doi: 10.1289/EHP4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst K.E., Coyte R.M., Wood M., Vengosh A., Mitch W.A. Disinfection byproducts in Rajasthan, India: are trihalomethanes a sufficient indicator of disinfection byproduct exposure in low-income countries? Environmental Science & Technology. 2019;53:12007–12017. doi: 10.1021/acs.est.9b03484. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How Z.T., Kristiana I., Busetti F., Linge K.L., Joll C.A. Organic chloramines in chlorine-based disinfected water systems: a critical review. J. Environ. Sci. 2017;58:2–18. doi: 10.1016/j.jes.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Ioannou S., Andrianou X.D., Charisiadis P., Makris K.C. Biomarkers of end of shift exposure to disinfection byproducts in nurses. J. Environ. Sci. 2017;58:217–223. doi: 10.1016/j.jes.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozari A., Paloglou A., Voutsa D. Formation potential of emerging disinfection by-products during ozonation and chlorination of sewage effluents. Sci. Total Environ. 2020;700 doi: 10.1016/j.scitotenv.2019.134449. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.F., Mitch W.A. Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environmental Science & Technology. 2018;52:1681–1689. doi: 10.1021/acs.est.7b05440. [DOI] [PubMed] [Google Scholar]
- Li Z.G., Liu X.Y., Huang Z.J., Hu S.Y., Wang J.J., Qian Z.Y. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China. Water Res. 2019;157:247–257. doi: 10.1016/j.watres.2019.03.072. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020;133:1039–1143. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Feng L., Liu Y., Zhang L. Disinfection by-products formation and acute toxicity variation of hospital wastewater under different disinfection processes. Sep. Purif. Technol. 2020;238 [Google Scholar]
- Nieuwenhuijsen M.J., Toledano M.B., Eaton N.E., Fawell J., Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup. Environ. Med. 2000;57:73–85. doi: 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat. Med. 2020;26:317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph R., Lew J., Zeng T.S., Francis M., Xue B., Roux M. 2019-nCoV (Wuhan virus), a novel coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. Journal of Infection in Developing Countries. 2020;14:3–17. doi: 10.3855/jidc.12425. [DOI] [PubMed] [Google Scholar]
- Richardson S.D. Disinfection by-products: formation and occurrence in drinking water. Encyclopedia of Environmental Health. 2011:110–136. [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutation Research-Reviews In Mutation Research. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Templeton M.R., Andrews R.C., Hofmann R. Inactivation of particle-associated viral surrogates by ultraviolet light. Water Res. 2005;39:3487–3500. doi: 10.1016/j.watres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.C., Li C.S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J. Occup. Environ. Hyg. 2007;4:400–405. doi: 10.1080/15459620701329012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C., Li C. Inactivation of surface viruses by gaseous ozone. J. Environ. Health. 2008;70:56–62. [PubMed] [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environmental Science & Technology. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Wang W., Qian Y.C., Li J.H., Moe B., Huang R.F., Zhang H.Q. Analytical and toxicity characterization of halo-hydroxyl-benzoquinones as stable halobenzoquinone disinfection byproducts in treated water. Anal. Chem. 2014;86:4982–4988. doi: 10.1021/ac5007238. [DOI] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y.J., Yang W.J. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19) Situation Report. [Google Scholar]
- WHO . 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19: Technical Brief. [Google Scholar]
- Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Li C., He J., Hong Z. 2020. Evidence for Gastrointestinal Infection of SARS-CoV-2. medRxiv. (2020.02.17.20023721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Ni W., Wu Q., Li W., Li G., Tong J. 2020. Prolonged Presence of SARS-CoV-2 in Feces of Pediatric Patients During the Convalescent Phase. medRxiv. (2020.03.11.20033159) [Google Scholar]
- Zhai H.Y., Zhang X.R., Zhu X.H., Liu J.Q., Ji M. Formation of brominated disinfection byproducts during chloramination of drinking water: new polar species and overall kinetics. Environmental Science & Technology. 2014;48:2579–2588. doi: 10.1021/es4034765. [DOI] [PubMed] [Google Scholar]
- Zhang D., Yang Y., Huang X., Jiang J., Li M., Zhang X. 2020. SARS-CoV-2 Spillover Into Hospital Outdoor Environments. medRxiv. (2020.05.12.20097105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhang X., Ma R., Deng S., Wang X., Zhang X. 2020. Ultra-fast and Onsite Interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Environmental Specimens Via Surface Enhanced Raman Scattering (SERS) medRxiv. (2020.05.02.20086876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Gan W.H., Du Y., Huang H., Wu Q.Y., Xiang Y.Y. Disinfection byproducts and their toxicity in wastewater effluents treated by the mixing oxidant of ClO2/Cl-2. Water Res. 2019;162:471–481. doi: 10.1016/j.watres.2019.07.012. [DOI] [PubMed] [Google Scholar]