Abstract
SARS-CoV-2, the agent of COVID-19, shares a lineage with SARS-CoV-1, and a common fatal pulmonary profile but with striking differences in presentation, clinical course, and response to treatment. In contrast to SARS-CoV-1 (SARS), COVID-19 has presented as an often bi-phasic, multi-organ pathology, with a proclivity for severe disease in the elderly and those with hypertension, diabetes and cardiovascular disease. Whilst death is usually related to respiratory collapse, autopsy reveals multi-organ pathology. Chronic pulmonary disease is underrepresented in the group with severe COVID-19. A commonality of aberrant renin angiotensin system (RAS) is suggested in the at-risk group. The identification of angiotensin-converting-enzyme 2 (ACE2) as the receptor allowing viral entry to cells precipitated our interest in the role of ACE2 in COVID-19 pathogenesis.
We propose that COVID-19 is a viral multisystem disease, with dominant vascular pathology, mediated by global reduction in ACE2 function, pronounced in disease conditions with RAS bias toward angiotensin-converting-enzyme (ACE) over ACE2. It is further complicated by organ specific pathology related to loss of ACE2 expressing cells particularly affecting the endothelium, alveolus, glomerulus and cardiac microvasculature. The possible upregulation in ACE2 receptor expression may predispose individuals with aberrant RAS status to higher viral load on infection and relatively more cell loss. Relative ACE2 deficiency leads to enhanced and protracted tissue, and vessel exposure to angiotensin II, characterised by vasoconstriction, enhanced thrombosis, cell proliferation and recruitment, increased tissue permeability, and cytokine production (including IL-6) resulting in inflammation. Additionally, there is a profound loss of the “protective” angiotensin (1–7), a vasodilator with anti-inflammatory, anti-thrombotic, antiproliferative, antifibrotic, anti-arrhythmic, and antioxidant activity. Our model predicts global vascular insult related to direct endothelial cell damage, vasoconstriction and thrombosis with a disease specific cytokine profile related to angiotensin II rather than “cytokine storm”. Our proposed mechanism of lung injury provides an explanation for early hypoxia without reduction in lung compliance and suggests a need for revision of treatment protocols to address vasoconstriction, thromboprophylaxis, and to minimize additional small airways and alveolar trauma via ventilation choice. Our model predicts long term sequelae of scarring/fibrosis in vessels, lungs, renal and cardiac tissue with protracted illness in at-risk individuals. It is hoped that our model stimulates review of current diagnostic and therapeutic intervention protocols, particularly with respect to early anticoagulation, vasodilatation and revision of ventilatory support choices.
Introduction
SARS-CoV-2, the agent of COVID-19, shares a lineage with SARS-CoV-1 disease (SARS), and a common fatal pulmonary profile but with striking differences in presentation, clinical course, and response to treatment [1], [2], [3], [4]. In contrast to SARS, COVID-19 has presented as an often bi-phasic, multi-organ pathology, with a proclivity for severe disease in the elderly and those with hypertension, diabetes and cardiovascular disease. Whilst death is almost universally related to respiratory collapse with multiple systems revealed to be failing at autopsy, the co-morbidity of chronic pulmonary disease is surprisingly relatively underrepresented in the group with severe COVID-19 [4]. COVID-19 has not responded consistently to immunosuppressive therapy, antimicrobial agents or invasive ventilation [5]. There is evolving evidence for a mechanism of lung injury that is not always typical of Acute Respiratory Distress Syndrome (ARDS) [2]. There is a need to revise the disease model for COVID-19 away from historical pulmonary-centric septic disease states typically characterised by lymphopaenia and cytokine storm with secondary bacterial sepsis. The diagnosis, assessment and management of COVID-19 cannot be based entirely on previous SARS, MERS or Influenza pandemics. Our disease hypothesis suggests a revision of our use of diagnostic tools (to better capture atypical disease presentations) and consider early treatment and/or prevention of microvascular thrombosis and constriction in identified groups at high risk of severe or fatal disease. Our hypothesis provides a mechanism of pulmonary insult, that warrants revision of indications for invasive ventilation particularly in view of poor outcomes [1], [6], with higher mortality for invasively ventilated patients than that recorded in SARS [1], [4], [6].
A multi-system disease
Whilst the respiratory system is almost universally involved in clinical presentation and dominates current therapy in COVID-19, those with more severe disease have been observed to have comorbidities in the vascular, renal, and cardiovascular systems [7], [8]. Gastrointestinal (GI) symptoms are common and often reported earlier in disease. This observation would support a primary respiratory or GI point of viral infection, with the clinical impact of viral infection focussed initially in the lungs, with ongoing infection and severe disease involving the vascular, renal and cardiac systems. This might suggest that COVID-19 is a multi-organ pathology related to viral infection with SARS-CoV-2 [7], [8], [9].
A subgroup of people with severe and critical COVID-19 have demonstrated a profile of deterioration and mortality consistent with overwhelming viral sepsis with cytokine storm and ARDS. This is not a typical profile of COVID-19. This group may be considered for additional immune-targeted disease therapy and may be identified by clinical features including unabating fever, ARDS, cytopenias, and biochemical markers of escalating inflammation, including interleukin-6 (IL-6) and ferritin, as suggested by data gathered in Wuhan [10].
A new disease hypothesis
Appreciating the clear differences between SARS and COVID-19 in presentation, poor prognostic indicators related to individuals’ co-morbid status, and biochemical and radiologic profiles, a novel disease model may assist in: 1) the early recognition of atypical (non-respiratory) presentations of disease; 2) early prophylactic treatment intervention for individuals at risk of severe and critical disease which could take place in the community; 3) revised management of pulmonary complications including those related to prone posturing and ventilation protocols; 4) allowing better utilisation of data collated at a global level in the absence of an evidence-based disease model at this time; 5) identification of different markers of disease progression in at-risk individuals.
The hypothesis of COVID-19 as a viral functional angiotensin-converting-enzyme 2 (ACE2) deficiency disorder, with ACE2 related multi-organ cell loss, comes some way to addressing this need. This model is supported by global data and observations, including that gathered phenotypically, biochemically, radiologically, and at autopsy. It evolved from the early observation of hypoxic pulmonary insult occurring without reduction in pulmonary compliance and often in the absence of fever. This is not consistent with ARDS, documented in historical pulmonary septic disease states with cytokine storm complicated by hypoxia. A pulmonary vascular pathology was sought in parallel with a possible disruption of surfactant function. Thrombosis and vasoconstriction were implied, with the former supported by consistent, but not specific radiologic findings of ground glass change. Thrombosis has now been confirmed in late disease and at autopsy [11]. The commonality of an aberrant renin angiotensin system (RAS), was identified in the group at risk of severe disease, rather than the presence of underlying lung disease. The biochemical markers in early disease suggested coagulation, renal, cardiac, and respiratory pathology in evolution. With the identification of ACE2 as the receptor for SARS-CoV-2 entry, the role of and distribution of ACE2 in the human body was investigated. The hypothesis evolved, providing an explanation for pathophysiologic commonality in the at-risk group in COVID-19, a mechanism for hypoxic pulmonary pathology and the unexpected degree of lung injury consequent to ventilation as well as vascular, renal and cardiac complications. It also predicts that presentation may be atypical and that long term sequelae related to endothelial, renal, pulmonary and cardiac involvement may occur. The hypothesis also predicts a cytokine profile driven by ACE2 deficiency in the RAS, rather than viral-related immune modulation. The immunologic effects of the RAS are well documented [12], favouring IL-6 production and affecting CD4+ and CD8+ cell populations.
Angiotensin-converting-enzyme 2
ACE2 is a membrane-associated aminopeptidase expressed in the endothelium, renal and cardiovascular tissue, and epithelia of the small intestine and testes [13]. ACE2 is also expressed in the lung, kidney, and GI tract according to PCR analysis – tissues shown to harbor SARS-CoV-1 [13], [14]. The entry receptor utilized by SARS-CoV-2 in COVID-19 is ACE2, and the enzyme utilized for S protein priming is transmembrane protease serine 2 (TMPRSS2) [15] or the controversial poly-basic furin cleavage site.
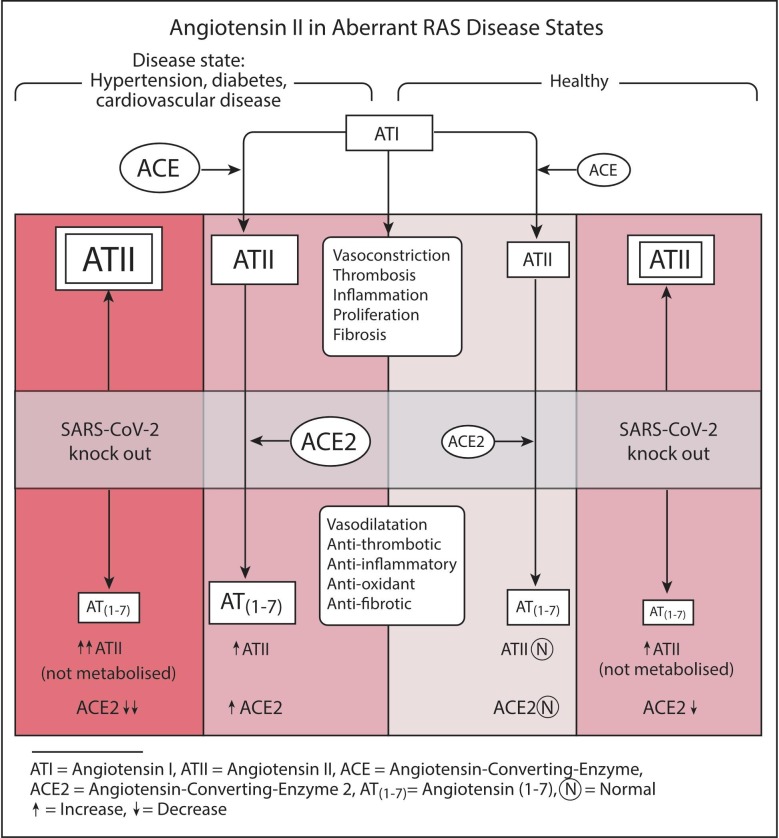
We propose that COVID-19 is a multiorgan pathology with dominant morbidity related to respiratory insult that is compounded by a more generalised disease driver that leads to a poorer prognosis and higher mortality in an identified at-risk group. We propose that the disease driver is relative ACE2 deficiency related to viral destruction of ACE2 expressing tissues, with more severe disease manifesting in a cohort with abnormality in the RAS favouring the angiotensin-converting-enzyme (ACE) arm. This is supported by the observed frequency of hypertension, cardiovascular disease and diabetes as co-morbidities in the group with severe and fatal disease [16], [17]. These disease entities have been well studied, with documentation of RAS abnormality characterised by increased ACE and angiotensin II (ATII) levels (Fig. 1 ). We hypothesise that in these conditions, there is a greater dependence on ACE2 to abrogate the effects of ATII and increase angiotensin (1–7) (AT(1–7)). An upregulation of ACE2 expressing cells related to chronic ATII elevation [18] or treatment with ACE-inhibitors [19], may increase the infective potential of SARS-CoV-2 in this group as a consequence of the duality of ACE2 functioning as both a receptor for viral entry to cells and as an enzyme. With infection related cell damage and loss, enzymatic ACE2 activity would be globally compromised. Organ specific loss of ACE2 expressing cells (endothelial, alveolus in lungs, proximal tubule and glomerulus in kidneys, pericytes in heart) is likely to also contribute to the pathophysiology of COVID-19.
Fig. 1.
Effects of SARS-CoV-2 related reduction in ACE2 expression in aberrant RAS disease states, increasing ATII effects and reducing AT(1–7) effects.
Angiotensin II and angiotensin (1–7) activity
These conditions are by no means the only ones that may confer escalated risk of severe or critical disease. We suggest that the pathogenesis is mediated by enhanced and protracted tissue, and vessel exposure to ATII, characterised by vasoconstriction, enhanced thrombosis, cell proliferation and recruitment, increased tissue permeability, and cytokine production (including IL-6) resulting in inflammation. A recent study noted serum levels of ATII were elevated in COVID-19 patients and correlated with viral load and lung injury [20]. ATII is generated by both ACE and non-ACE pathways, but is the dominant substrate for ACE2, being converted to AT(1–7) [21], a vasodilator with anti-inflammatory, anti-thrombotic, antiproliferative, antifibrotic, anti-arrhythmic, and antioxidant activity [12], [22]. The viral destruction of ACE2 expressing cells may lead to the profound loss of the “protective” AT(1–7) effects in an environment of ATII effect dominance (Fig. 1).
Enhanced thrombosis may occur via several ATII driven pathways, including increased thrombin [23], procoagulant endothelial cell activity via bradykinin [24], [25], immune effects via T cells (CD4+, CD8+) [12], [26], platelet aggregation [27], and reduced fibrinolysis via bradykinin and endothelin. The prothrombotic effects of ATII appear to extend to the microvasculature in murine models [23]. Platelet aggregation and endothelial damage in the septic setting may contribute further to thrombosis.
Respiratory involvement
We hypothesise that ACE2 expressing cells are rapidly infected by SARS-CoV-2. Initially, this is by ingestion and/or aspiration. Early respiratory symptomatology is consistent with previously described SARS-CoV-1 infection via ACE2 receptors in the ciliated respiratory epithelial cells, with apical release of replicated virus [28] rapidly infecting the downstream ACE2 expressing cells – including type 2 alveolar cells. As opposed to SARS-CoV-1 infection, significant hypoxia in COVID-19 is not accompanied in early disease by a reduction in pulmonary compliance [6], [29]. This suggests possible microvascular thrombotic disease and/or inappropriate vasoconstriction of the pulmonary vascular bed. In the absence of early lung biopsy, evidence is lacking. Early thrombosis, however, is supported in the context of hypoxia by D-dimer elevation and ground glass change on CT scans. Fat embolism has a similar radiologic appearance, supporting early thrombosis over hypoxia related vasoconstriction alone. The preservation of lung compliance, early in disease, is not consistent with diffuse alveolar damage (DAD), or ARDS contributing to the hypoxic insult.
Advancing microvascular thrombosis may be supported again by the observation at autopsy of marked right ventricular dilatation reflecting elevated pulmonary vascular pressure [11]. This occurred in the absence of detectable left ventricular pathology. As disease progresses, more drivers of coagulation are likely to be recruited and disseminated intravascular coagulation (DIC) may ensue. Autopsy findings of pulmonary small vessel thrombosis was reported by Fox and colleagues [11]. Tang and colleagues reported that abnormal coagulation parameters, particularly D-dimer and fibrin degradation products (FDPs), were associated with poor prognosis in COVID-19 [30]. An association with anticoagulation and improved survival after adjustment for ventilation was noted in a large United States cohort [31].
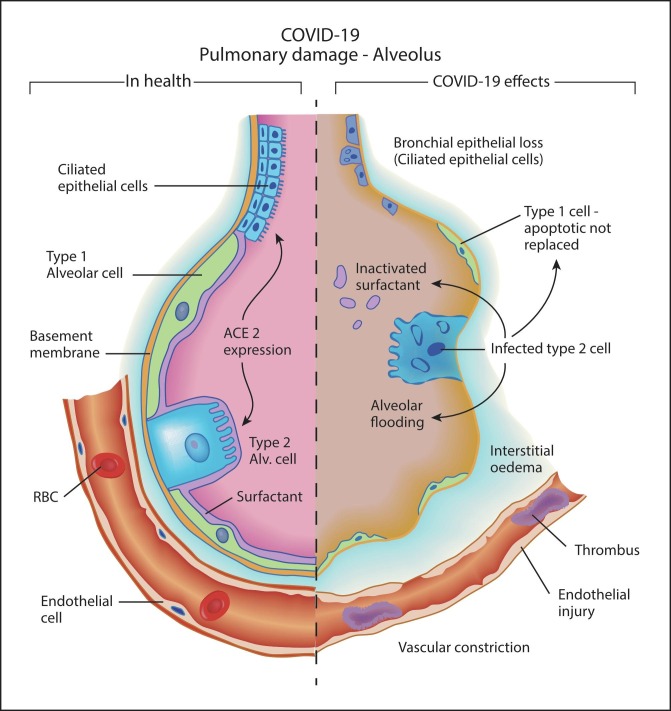
Possible ATII driven thrombosis and vasoconstriction are likely to be compounded by alveolar damage via the viral loss of ACE2-rich type 2 alveolar cells. These cells in health are tasked with surfactant production, maintenance of alveolar homeostasis, and transdifferentiation into type 1 alveolar cells, which comprise 90% of the lining of the alveolus. The demise of these cells is likely to lead to hypoxia via surfactant loss, alveolar flooding, and apoptosis of type 1 alveolar cells without replacement via transdifferentiation from type 2 alveolar cells (Fig. 2 ).
Fig. 2.
COVID-19 Pulmonary damage model demonstrating effects of loss of ACE2 expressing cells (ciliated respiratory epithelial cells and type 2 alveolar cells), and vascular effects of ACE2 deficiency with endothelial injury, vasoconstriction, thrombosis, and interstitial oedema.
Our hypothesis suggests that as disease progresses, the destruction of alveoli leads to DAD, rather than ARDS. The destruction of alveoli is evident at autopsy, with DAD [11] and radiologic appearances consistent with DAD are documented in advanced disease [6], [32].
Our hypothesis supports a possible mechanism for disappointing outcomes in ventilated patients, particularly related to those requiring or receiving high positive end expiratory pressure (PEEP) and the observed autopsy findings [6], [11], [33]. The advantage of prone posturing and PEEP application is well documented in ARDS [34]. Advantage in ARDS was felt to be largely related to the distribution of oedema, where the mass of the diseased lung may be increased to 300% of the normal [35]. In normal subjects, prone posturing improved alveolar ventilation, however, the addition of 10cmH2O PEEP in ventilated patients reduced ventilation/perfusion matching via perfusion changes in dependent lung [36]. It is possible that prior to the evolution of pulmonary oedema in COVID-19 (when lung compliance is near normal), escalating PEEP in response to deteriorating hypoxia may be self-defeating. In addition to this, our disease model identifies that the at-risk group characteristic of aberrant RAS status favouring ACE activity may be more susceptible to volume induced lung injury related to PEEP. Decreased ACE2 activity with increased ACE activity contributed to lung injury [7] in in vitro studies of cyclical stretch of human lung epithelial cells and to volume induced lung injury in animal models [6], [33].
Viraemic spread
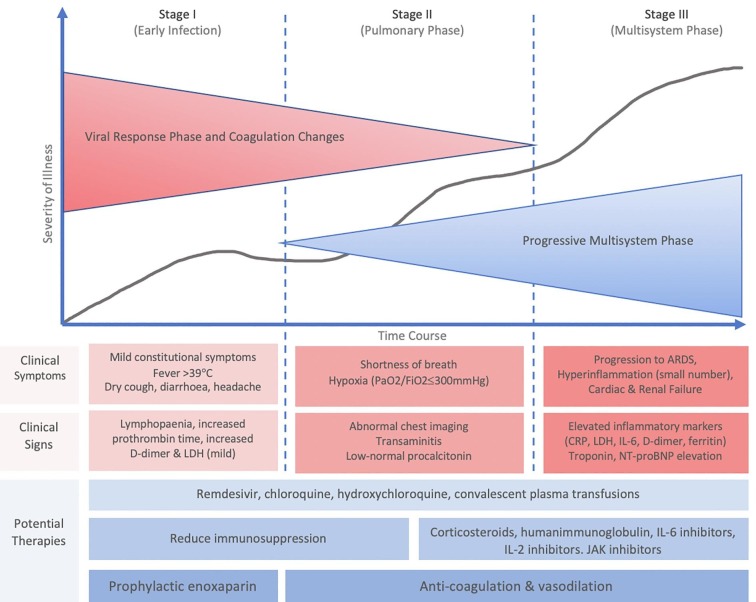
Viral spread to the vascular system may occur via pulmonary capillaries given the intimacy of the pulmonary capillary bed to the infected alveoli. We postulate the viraemia to lead to vascular, renal and myocardial infection at this time, supported in a remote manner by the somewhat unpredictable timing of fever, and the reports of biphasic clinical decline that involves shortness of breath, hypoxia (PaO2/FiO2 < 300 mmHg), transaminitis, low-normal procalcitonin, and abnormal chest imaging (Fig. 3 ) [32].
Fig. 3.
The progressive phase model of COVID-19 with the addition of anticoagulation to prevent and treat microthrombotic disease, as advanced by the authors of the current paper. Modified and redrawn from [32].
Renal involvement
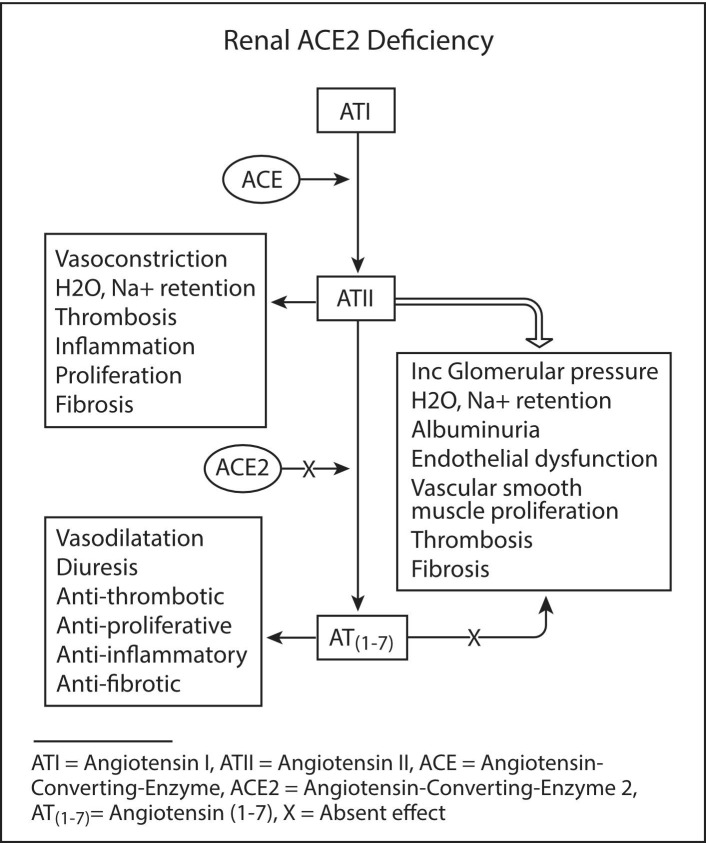
In hypertension and renal disease, particularly diabetic nephropathy, an aberrant RAS is well documented, with a bias to higher ACE/ACE2 in renal biopsy [37], [38], [39]. We suggest that ACE2 deficiency leads to increased and unabating ATII activity resulting in vasoconstriction, water and sodium retention, cell proliferation, thrombosis, inflammation, oxidative stress, and fibrosis. In addition to the damaging effects of ATII, the deficiency in ACE2 leads to a reduction in AT(1–7), with loss of its vasodilatory, diuretic, antiproliferative, antioxidant, and antithrombotic effects (via bradykinin) [22]. In the acute setting, renal injury is likely due initially to the sustained effects of ATII, including vasoconstriction that favours the efferent over afferent vessels, resulting in increased glomerular pressures, water and sodium retention, albuminuria, and possible microvascular thrombosis (Fig. 4 ). This is supported by documentation of albuminuria and elevated creatine kinase [16], [40]. The physical loss of ACE2 expressing glomerular cells may further add to the renal insult.
Fig. 4.
Renal effects of ACE2 deficiency in an ACE/ACE2 biased renin-angiotensin system as a mechanism for glomerular dysfunction, thrombosis, and fibrosis.
As disease progresses, a more diffuse renal impairment develops with the absence of overt septic inflammation in autopsy specimens [38]. An abnormal renal ACE/ACE2 ratio is also seen in diabetics without established nephropathy, IgA nephropathy, and subtotal nephrectomy [37]. This group of patients may well carry higher risk for COVID-19 renal disease. The hypothesis also predicts that renal scarring is likely to occur in survivors particularly related to vessel and glomerular damage.
Cardiac pathology
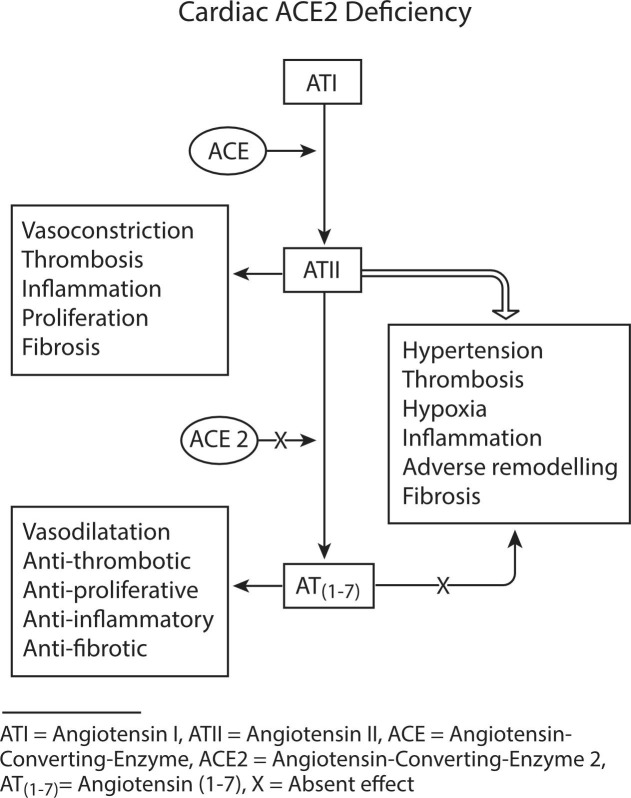
Myocardial damage is suggested by the elevation in hypersensitive troponin I (TnI) and dysrhythmia in a small series (7.2% and 16.7%, respectively) [16]. With the disease model proposed here, elevated ATII activity with suboptimal AT(1–7) activity may lead in the predisposed heart to microvascular thrombosis, vasoconstriction related hypoxia, and cell/fibroblast proliferation, with both acute and intermediate term sequelae of progressive heart failure, and adverse remodelling and fibrosis (Fig. 5 ). Right ventricular dysfunction is likely to reflect the pulmonary vascular burden of thrombosis and vasoconstriction.
Fig. 5.
Cardiac effects of ACE2 deficiency in an ACE/ACE2 biased renin-angiotensin system as a mechanism for thrombosis, adverse remodelling, and fibrosis.
ACE2 is expressed by cardiac myocytes, fibroblasts, and endothelial cells. ACE2 expression is increased in human heart failure and may reflect a predisposition to cardiac disease susceptibility in the context of COVID-19 [41], [42]. Chen and colleagues identified that the pericyte, with very high ACE2 expression, may be the entry point for myocardial infection [41]. In the autopsy series from New Orleans, Fox and colleagues acknowledged that the finding of scattered individual myocyte necrosis without evidence of viral myocarditis would be consistent with microvascular dysfunction related to pericyte infection [11], as hypothesized by Chen and colleagues [41]. Chen’s findings are consistent with our belief that cardiac infection follows viraemia, with a delayed declaration of pathological involvement. All subjects had gross right ventricular dilatation. None of the 4 autopsy subjects had known cardiac disease.
More recently, Peng and colleagues described echocardiographic features of severe COVID-19 [43]. This included left ventricular segmental contraction abnormalities (Takotsubo cardiomyopathy), right ventricular dilatation and systolic dysfunction, and, finally, global systolic and diastolic heart failure. These findings are not inconsistent with cardiac microvascular dysfunction and ischaemia with, as described previously, the right ventricular failure reflecting the pulmonary vascular burden of thrombosis and vasoconstriction.
TnI elevation and echocardiographic assessment remain useful tools for assessing disease progression or prognosis, particularly in subjects with existing cardiac failure [10], [43], [44], [45]. The hypothesis predicts adverse remodelling of the myocardium as a consequence of microvascular cardiac disease.
Discussion
Our hypothesis supports the revision of use of diagnostic tools with reference to the possibility of atypical (non-respiratory) disease presentations. Tissues with high ACE2 expression may be targeted for sampling in early or protracted infection to minimise community spread and false negative results. Faecal or urine PCR testing for viral infection may be reconsidered if respiratory sampling is unhelpful.
We propose that identified high risk groups be considered for early treatment or prevention of microvascular thrombosis and constriction, given the likelihood of progression to multisystem failure and the possibility of vascular, pulmonary, myocardial, and renal scarring sequelae in survivors [44], [45]. We suggest consideration of low molecular weight heparin (LMWH) or unfractionated heparin as these agents are likely to have both adequate anti-coagulant and additional anti-inflammatory effects (via reduction in IL-6). In a recent retrospective analysis of LMWH anticoagulation, Shi and colleagues reported a reduction in levels of D-dimer, FDPs, and IL-6, with an improvement in lymphocyte percentages when compared to a control group [46].
Sildenafil, with a number of studies in progress, may be considered as a vasodilator for efficacy of administration and cost in parallel with long term safety data and well-defined recommendations for monitoring of side effects. Sildenafil may also have positive effects on cilial function in COVID-19 [47].
Reconsideration of parameters for invasive ventilation and multitargeted therapy may be in order given poor outcomes [1], [6], with higher mortality for invasively ventilated patients than that recorded in SARS [1], [4], [6], and the possible alternative mechanism of pulmonary insult proposed – thrombosis, vasoconstriction, surfactant loss, and alveolar destruction via viral loss of type 2 alveolar cells. PEEP < 10cmH2O with prone posturing and supplemental oxygen should be considered in patients requiring respiratory support in the absence of ARDS.
With respect to individuals with only mild or moderate disease, we propose that future treatment study protocols consider prophylactic level anticoagulation in at-risk individuals with a low threshold for escalation to full anticoagulation and vasodilation if disease progression is supported by measures of clinical state (particularly hypoxia), and well documented pathology parameters (including D-dimer, FDPs, CRP, neutrophil, lymphocyte and platelet levels, TnI, and liver and renal function profiles, including albuminuria and IL-6).
Prevention of thrombosis may be commenced in the community in symptomatic infected patients and at-risk populations with mild disease or positive exposure, given their increased risk of venous thromboembolism and the widespread safety data for LMWH and heparin use to establish efficacy. Whilst thrombosis may not be universal, the intervention is low risk and may abrogate one of the irreversible elements of this disease. In areas with high disease prevalence, prophylactic-level anti-coagulation could be administered and monitored by local medical officers in the outpatient setting and in residential care facilities.
Emerging results of single-target therapies will necessarily impact on the evolution of disease models like ours, with autopsy data unfortunately offering our best evidence for end stage disease description, but little guidance for early therapeutic options.
Conclusion
In conclusion, we present a COVID-19 disease hypothesis with the intention of stimulating discussion and further research on diagnostic and therapeutic intervention protocols, particularly with respect to early anticoagulation, vasodilatation and revision of ventilatory support choices. This may benefit a large group of people who are at risk of manifesting significant pulmonary complications of both disease and treatment and multisystem disease sequelae.
Sources of support
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Marcus Cremonese, Medical Illustration (http://www.medicalillustration.com.au/), and Danielle Santarelli, Medical Writing (Genesis Research Services).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.ICNARC report on COVID-19 in critical care. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. 2020, Accessed 28 March 2020.
- 2.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a typical acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau A.-C.-W., Yam L.Y.-C., So L.K.-Y. Management of critically ill patients with severe acute respiratory syndrome (SARS) Int J Med Sci. 2004:1–10. doi: 10.7150/ijms.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ and Vander Heide RS. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans, Cold Spring Harbor Laboratory (2020). [DOI] [PMC free article] [PubMed]
- 12.Crowley S.D., Rudemiller N.P. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017;28:1350–1361. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12. [DOI] [PMC free article] [PubMed]
- 14.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B., Ferguson C., White J.T. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J., Tong Z., Guan X., Du B., Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner AJ. Chapter 25 - ACE2 Cell Biology, Regulation, and Physiological Functions In: The Protective Arm of the Renin Angiotensin System (RAS). T Unger, UM Steckelings and RAS dos Santos, Editors. Academic Press, Boston (2015), pp. 185-189.
- 19.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donoghue M., Hsieh F., Baronas E. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87 doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 22.Wosten-van Asperen R.M., Lutter R., Specht P.A. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 23.Senchenkova E.Y., Russell J., Esmon C.T., Granger D.N. Roles of coagulation and fibrinolysis in angiotensin II-enhanced microvascular thrombosis. Microcirculation. 2014;21:401–407. doi: 10.1111/micc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celi A., Cianchetti S., Dell'Omo G., Pedrinelli R. Angiotensin II, tissue factor and the thrombotic paradox of hypertension. Expert Review of Cardiovascular Therapy. 2010;8:1723–1729. doi: 10.1586/erc.10.161. [DOI] [PubMed] [Google Scholar]
- 25.Kimura S., Tsuji H., Nishimura H. Bradykinin enhances in vitro procoagulant and antifibrinolytic properties of rat vascular endothelial cells. Thromb Res. 2002;106:41–50. doi: 10.1016/s0049-3848(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 26.Senchenkova E.Y., Russell J., Kurmaeva E., Ostanin D., Granger D.N. Role of T lymphocytes in angiotensin IIâMediated microvascular thrombosis. Hypertension. 2011;58:959–965. doi: 10.1161/HYPERTENSIONAHA.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henrion D., Kubis N., LeÌvy B.I. Physiological and pathophysiological functions of the AT 2 subtype receptor of angiotensin II. Hypertension. 2001;38:1150–1157. doi: 10.1161/hy1101.096109. [DOI] [PubMed] [Google Scholar]
- 28.Jia H.P., Look D.C., Shi L. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24 doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplantation. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R., Pan Y., Fanelli V. Mechanical stress and the induction of lung fibrosis via the midkine signaling pathway. Am J Respir Crit Care Med. 2015;192:315–323. doi: 10.1164/rccm.201412-2326OC. [DOI] [PubMed] [Google Scholar]
- 34.Guerin C., Reignier J., Richard J.C. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 35.Gattinoni L., Caironi P. Prone positioning: beyond physiology. Anesthesiology. 2010;113:1262–1264. doi: 10.1097/ALN.0b013e3181fcd97e. [DOI] [PubMed] [Google Scholar]
- 36.Petersson J., Ax M., Frey J., Sanchez-Crespo A., Lindahl S.G., Mure M. Positive end-expiratory pressure redistributes regional blood flow and ventilation differently in supine and prone humans. Anesthesiology. 2010;113:1361–1369. doi: 10.1097/ALN.0b013e3181fcec4f. [DOI] [PubMed] [Google Scholar]
- 37.Mizuiri S., Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4:74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakahara S., Konoshita T., Mizuno S. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148:2453–2457. doi: 10.1210/en.2006-1287. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Q.-Y., Wang X.-T., Zhang L.-N. Using echocardiography to guide the treatment of novel coronavirus pneumonia. Crit Care. 2020;24 doi: 10.1186/s13054-020-02856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical management of severe acute respiratory infection when COVID-19 is suspected: Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. 2020, Accessed 28 March 2020.
- 46.Shi C., Wang C., Wang H. Cold Spring Harbor Laboratory; 2020. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnett C.F., Machado R.F. Sildenafil in the treatment of pulmonary hypertension. Vascular Health Risk Manage. 2006;2:411–422. doi: 10.2147/vhrm.2006.2.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.