Abstract
Idiopathic ventricular fibrillation is diagnosed in patients who survived a ventricular fibrillation episode without any identifiable structural or electrical cause after extensive investigations. It is a common cause of sudden death in young adults. The study reviews the diagnostic value of systematic investigations and the new insights provided by detailed electrophysiological mapping. Recent studies have shown the high incidence of microstructural cardiomyopathic areas, which act as the substrate of ventricular fibrillation re-entries. These subclinical alterations require high-density endo- and epicardial mapping to be identified using electrogram criteria. Small areas are involved and located individually in various sites (mostly epicardial). Their characteristics suggest a variety of genetic or acquired pathological processes affecting cellular connectivity or tissue structure, such as cardiomyopathies, myocarditis, or fatty infiltration. Purkinje abnormalities manifesting as triggering ectopy or providing a substrate for re-entry represent a second important cause. The documentation of ephemeral Purkinje ectopy requires continuous electrocardiography monitoring for diagnosis. A variety of diseases affecting Purkinje cell function or conduction are potentially at play in their pathogenesis. Comprehensive investigations can therefore allow the great majority of idiopathic ventricular fibrillation to ultimately receive diagnoses of a cardiac disease, likely underlain by a mosaic of pathologies. Precise phenotypic characterization has significant implications for interpretation of genetic variants, the risk assessment, and individual therapy. Future improvements in imaging or electrophysiological methods may hopefully allow the identification of the subjects at risk and the development of primary prevention strategies.
Key Words: J-wave syndromes, sudden cardiac death, unexplained death, ventricular fibrillation
Abbreviations and Acronyms: ARVD, arrhythmogenic right ventricular dysplasia; BrS, Brugada syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia; ECG, electrocardiography; ICD, implantable-cardioverter defibrillator; IVF, idiopathic ventricular fibrillation; LV, left ventricular; PVC, premature ventricular contraction; RV, right ventricular; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia
Highlights
-
•
VF can be unexplained despite extensive investigations, notably in the young.
-
•
The use of high-density electrophysiological mapping detects causes in the great majority of victims.
-
•
Microstructural cardiomyopathies are the main causes, likely underlain by multiple pathological processes.
-
•
The phenotypic characterization of substrate is critical to develop therapy and interpret genetic results.
Central Illustration
Sudden cardiac death (SCD) remains a major health problem on all continents. Estimates vary around 350,000 victims per year in the United States and in Europe, and are even higher in Southeast Asia. Coronary artery disease and cardiomyopathies are the main causes in older persons (1, 2, 3, 4, 5, 6). However, in victims younger than 35 years of age, a common finding is the absence of structural heart disease at autopsy, which is reported in 29% to 40% of cases in recent population-based studies, particularly in young male patients (7, 8, 9, 10, 11, 12). In the patients surviving after resuscitation maneuvers, ventricular fibrillation (VF) is consistently the lethal heart rhythm disorder identified at the time of event.
Experimental studies have demonstrated that, once initiated, VF is maintained by the continuous formation of re-entrant waves, underlain by depolarization or repolarization heterogeneities. In cardiomyopathic post-transplant human hearts, re-entry has been shown to self-perpetuate for many cycles by anchoring to a myocardial scar or structural heterogeneity (13, 14, 15).
Over the last 20 years, considerable efforts have been devoted to the search for electrocardiographic, structural, and genetic anomalies in survivors (1,4,10). Despite these progresses, unexplained SCD, defined as no apparent structural heart disease after extensive investigations, remains frequent in young adults (16, 17, 18, 19, 20, 21, 22, 23, 24, 25). In this review, we report the current knowledge on idiopathic VF (IVF), defined as VF with no apparent structural or electrical heart disease after extensive investigations (e.g., no apparent phenotype). We discuss the value of systematic clinical testing for confirmation of IVF diagnosis and exclusion of other causes of SCD. The results of individual phenotypic characterization based on high-density electrophysiological mapping will be analyzed, with their implications for genetic interpretation and therapeutic approach.
Criteria Identifying IVF
Definition of IVF
The Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society expert consensus statements on inherited primary arrhythmia syndromes define IVF as a resuscitated VF victim who had known cardiac, respiratory, metabolic, and toxicological causes have been excluded through clinical investigations (4,5). The diagnosis is based on the absence of a substrate for VF by exclusion of ischemic and structural cardiac diseases (i.e., arrhythmogenic right ventricular dysplasia [ARVD], hypertrophic and dilated cardiomyopathy, myocarditis, cardiac sarcoidosis, congenital heart disease) and “primary arrhythmia syndromes” (i.e., Brugada syndrome [BrS], catecholaminergic polymorphic ventricular tachycardia [CPVT], long QT syndrome, short QT syndrome, and early repolarization syndrome). Whether a heart is considered normal depends obviously on the resolution of examinations and their timing relative to the clinical event (26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45).
IVF accounts for 14% to 23% of SCD in young adults. There is a relative paucity of specific recommendations for the clinical management of IVF, but recent reviews and new data have become available (4, 5, 6,22, 23, 24, 25).
Systematic clinical testing
Noncardiac causes of IVF, including accidental, cerebral, or respiratory causes, and drug abuse or intoxication or electrolyte abnormality, are easily eliminated by simple investigations and will not be discussed here.
Figure 1 shows a diagnostic flow chart illustrating the systematic assessment of patients who survived SCD, with each investigation being important for differential diagnosis of IVF.
Figure 1.
Flow Chart
Diagnostic flow chart for investigations (blue) for patients surviving a sudden cardiac death and their results (yellow). CPVT = catecholaminergic polymorphic ventricular tachycardia; LV = left ventricle; MRI = cardiac magnetic resonance; PVC = premature ventricular contraction; RV = right ventricle; SAECG = signal-averaged electrocardiogram; SR = sinus rhythm.
Electrocardiography-telemetry
Twelve-lead electrocardiography (ECG) should be performed using standard and high precordial lead position, and include recordings of spontaneous or induced changes in the QRS and J-wave or ST-segment patterns, including circadian changes, post-pause, Valsalva, stress test, or pharmacological tests (Figures 1 and 2). Exercise test and signal-averaged ECG are recommended.
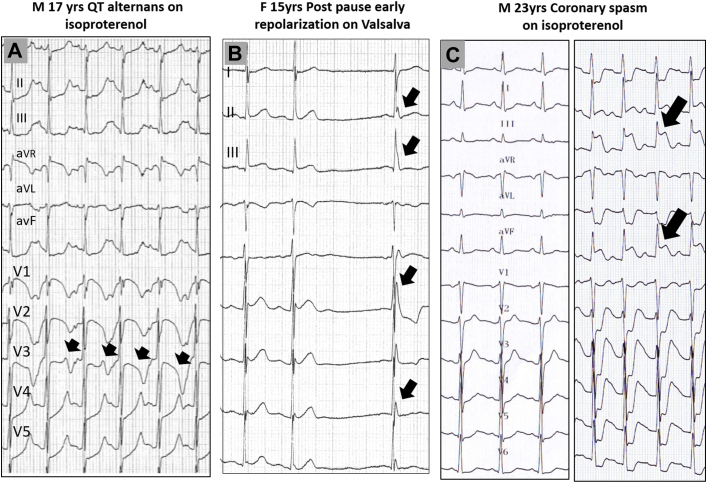
Figure 2.
Repolarization Changes During Provocative Tests, in Patients Initially Referred for Idiopathic Ventricular Fibrillation, Which Showed Evidence for Other Diagnosis
(A) Isoproterenol testing producing abrupt QT changes (alternans) in a patient with apparently normal QT interval; a KCNQ1 mutation (long QT1 syndrome) was later evidenced. (B) Valsalva maneuver producing a prominent inferolateral J-wave after a sinus pause. (C) Isoproterenol testing inducing ST-segment elevation and coronary spasm, which was proven during a repeat test with coronary angiography. The arrows indicate the repolarization change.
The 12-lead ECG recordings of premature ventricular contraction (PVC) patterns (coupling, morphologies) should include the initial days in the intensive care unit, in which the PVCs may be considered normal in the context of recent VF and are therefore often underdocumented.
The goal is to recognize the site of origin of PVCs, whether they originate from the Purkinje system, right ventricle (RV), or left ventricle (LV), and whether they are mono-or polymorphic (29, 30, 31, 32, 33, 34). PVCs of ventricular origin have a wider duration and initial slower deflection than do Purkinje ectopy. The presence of frequent PVCs gives a potential indication of an abnormal (concealed) myocardial area, particularly when they do not have the common morphological patterns from right or left outflow tracts, the latter being commonly associated with normal myocardium. Purkinje ectopies have a narrower QRS duration, particularly when they originate from the left Purkinje system (<120 ms), where they exhibit a right bundle branch block morphology. Purkinje ectopies from the right Purkinje arborization have a left bundle branch block morphology, and a wider QRS duration (130 to 150 ms) but an initially rapid deflection (Figure 3). The narrower left Purkinje ectopies are probably due to the dense left arborization, allowing simultaneous capture of a greater part of the LV.
Figure 3.
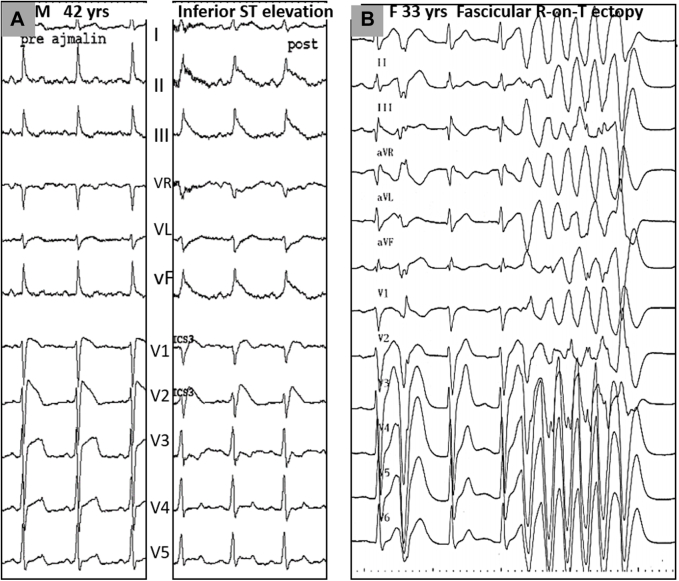
Different Patterns of Purkinje Activity in 4 Patients With Idiopathic Ventricular Fibrillation
(A) Purkinje ectopy from the right Purkinje system, exhibiting a left bundle branch block morphology; note the initially rapid deflection (arrows). (B) Typical Purkinje ectopy from the left Purkinje system (asterisks) with narrow QRS duration, right bundle branch block morphology, and short coupling intervals. The earliest activity preceding ectopic beat is found in the distal left posterior fascicle, with different activation sequences associated with the 2 different electrocardiography morphologies. (C) Purkinje ectopy with long coupling interval in the aftermath of ventricular fibrillation, with no ventricular fibrillation recurrences after catheter ablation of ectopy. (D) Spontaneous polymorphic ventricular tachycardia associated with 1-to-1 Purkinje activity. LV = left ventricle.
Short coupling (R-on-T) PVCs are clearly in favor of Purkinje origin but can also be seen in ventricular origin (18,35) and vice versa, Purkinje ectopy may have a long coupling interval (>360 ms). It is essential to document the presence of Purkinje ectopy by 12-lead ECG, as it can be the unique abnormality underlying VF, and can be treated by catheter ablation.
Structural investigations
Patients classified as having IVF should have no identifiable structural heart disease demonstrated by normal echocardiographic and delayed gadolinium–enhanced cardiac magnetic resonance, no detectable coronary artery disease upon coronary angiography or exercise testing. Ergonovine or acetylcholine provocative tests are performed to exclude coronary artery spasm in the context of pain or ST-segment elevation (27). However, the presence of minor anatomical “defects-variants” in coronary artery or valve apparatus (mitral valve prolapse) raises a problem in establishing their implication (26). Ventricular biopsy can be performed in selected cases (suspected myocarditis, sarcoidosis, ARVD). Ideally, the biopsy location should be guided by electrogram or imaging abnormality to increase diagnostic yield and potentially provide new insights in IVF (28).
Pharmacological tests
Pharmacological tests have the objective to reveal an arrhythmogenic marker like a distinct ECG phenotype or to provoke an arrhythmia in susceptible patients. Their diagnostic value has been specified in prior reports by Krahn et al. (19) and Visser et al. (22). Pharmacological tests have a high yield for their specific diagnostic purpose, but they may also reveal “unexpected” responses that provide clues for other causes. An abnormal response to a drug test can be the only abnormality detected by the screening protocol. Standard protocols are used for Class Ia drugs and catecholamine infusion as described previously (19, 20, 21, 22).
Pharmacological testing with infusion of a sodium-channel blocker (ajmaline, pilsicainide, flecainide, or procainamide) is performed to exclude BrS. The sensitivity of ajmaline is higher than flecainide or procainamide in diagnosing BrS (22,25,36,37). In addition, a J-point ST-segment elevation may be observed in the sole inferolateral leads (Figure 4A), which appears specific of an abnormal depolarization area in the inferior part of ventricles; such patients often have an SCN5A variant (38,39). The use of sodium-channel blockers may also produce unexpected responses, revealing specific causes of VF. In a multicenter group of 16 IVF patients, we have observed the induction of R-on-T ectopy, mostly from Purkinje tissue, during administration of Na+ blockers (Figure 4B). There was no concomitant BrS or inferior J-point elevation. The provocation of R-on-T ectopy was reproducible in all 9 patients who underwent a repeat test. In 4 patients, the test was the only means to reveal R-on-T ectopy, while other patients had also spontaneous episodic ectopy. Four patients developed VF or polymorphic ventricular tachycardia (VT) during the test. These 4 patients who had syncope as the only symptom received an implantable-cardioverter defibrillator (ICD). A spontaneous VF was then observed in 3 of them (at a follow-up of 28 months), suggesting a high clinical value of the drug-testing response (40).
Figure 4.
Uncommon Responses to Sodium-Channel Blocker Testing
(A) ST-segment elevation in the inferior leads during ajmaline testing in association with a Brugada syndrome. Mapping showed an abnormal depolarization area in the inferior part of the right ventricle. (B) Idiopathic ventricular fibrillation patient: the induction of R-on-T ectopy during administration of ajmaline was the only abnormality found during the systematic assessment. The provocation of R-on-T ectopy was reproducible on a repeated ajmaline test during electrophysiological study, and proven to originate from left Purkinje tissue. Spontaneous Purkinje ectopy were later documented during a recurrence of ventricular fibrillation.
Catecholamine infusion tests are performed to exclude stress- or effort-induced VT, or CPVT (the latter in association with genetic testing). Adrenaline is also recommended to confirm the absence of long QT syndrome. However, false positive tests are frequent in healthy subjects and QT variations, and measurements may be of ambiguous interpretation. QTc prolongation provoked by physical (abrupt or brisk standing position) or mental stress appears more specific (22,25,41,42).
Isoproterenol testing has a different impact than adrenaline testing. It may similarly show long QT syndrome (Figure 2A) or CPVT, but its high beta-agonist action is particularly interesting to reveal the arrhythmogenicity of (discrete or overt) structural heart diseases. We use it in an infusion of 3 min at a dose of 45 μg/min (43,44), which produces a mean peak sinus rate of 152 ± 18/min (85% of theoretical maximum heart rate on exercise). The isoproterenol test has a higher arrhythmogenic power than exercise testing, inducing nonsustained VTs (≥3 beats) in 74% to 85% of ARVD (PVCs dominantly negative in V1) and 42% of dilated cardiomyopathies (PVCs dominantly positive in V1). In contrast, nonsustained VTs are induced in only in 2% to 2.7% of control patients with PVCs and normal hearts at echocardiography (43,44). In IVF patients presumed to be free of structural heart disease, the isoproterenol test has a significant value when it induces repetitive forms of monomorphic or polymorphic PVCs (Figure 5), as this response often indicates the presence of concealed myocardial alterations that will be demonstrated with high-density electrogram mapping (see results detailed subsequently). Such vulnerability to catecholaminergic arrhythmias is also obviously a potential indication for beta-blocker treatment.
Figure 5.
Polymorphic Ventricular Tachycardia on Isoproterenol Testing
In 2 idiopathic ventricular fibrillation patients, isoproterenol testing induced nonsustained polymorphic ventricular tachycardia having a relatively slow rate. Premature ventricular beats are dominantly (A) positive or (B) negative in the V1 lead. In both patients, high-density electrogram mapping revealed the presence of localized myocardial alterations, in the left (case shown in Figure 9) and right ventricles, respectively. LBBB = left bundle branch block; RBBB = right bundle branch block
In rare cases, the isoproterenol test has been reported to induce ST-segment elevation and coronary spasm (45). We have seen 2 such cases initially considered IVF, with 1 of them shown in Figure 2C. The role of strong sympathetic stimulation with parasympathetic nervous system is believed to play a role in the pathogenesis of coronary spasm induction (45).
Finally, there are other conditions associated with the clinical occurrence of VF as strong vagal drive or dynamic changes in J-wave or QT intervals, for which no test is currently available to reproduce them in the hospital environment. The development of provocation tests specific for these diverse conditions would be desirable to improve the diagnostic yield of clinical work-up and risk stratification.
Role of Invasive Electrophysiological Study
As IVF patients meet criteria for ICD insertion, an electrophysiological study for the sole reason of inducing VF is not currently indicated for risk stratification, particularly as the VF inducibility rate is relatively low (5,19). Invasive testing has been reported in selected patients to determine whether a specific substrate, such as a monomorphic VT or supraventricular arrhythmia causing hemodynamic collapse, is the cause of VF (46,47).
However, an electrophysiological study may have an important role for diagnosis of subclinical myocardial alterations when the initial imaging work-up is negative, by relying on sole electrophysiological criteria. This has been demonstrated in a recent study combining invasive and noninvasive mapping (Figure 6), which showed that noninvasively detected re-entries during VF were often localized in distinct altered areas in patients with IVF (24). This point will be developed subsequently.
Figure 6.
Colocalization of VF Re-Entries With the Areas of Abnormal Electrograms
The body surface maps in 2 spontaneous ventricular fibrillation (VF) episodes show the location of VF re-entries by the blue curves and red areas, during the initial 4 s of VF. The re-entries are dominant in the anterior right ventricle (RV) (anteroposterior [AP] view) and their location is reproducible in the inferior RV, and the high septum–left ventricular (LV) outflow tract area; the latter seen in the left anterior oblique (LAO) view. Low-voltage fragmented electrograms recorded by epicardial electrodes are present during normal sinus rhythm within the white-dotted contour, in contiguity with the areas of VF re-entries. All other ventricular regions show narrow signals indicating healthy underlying tissue. LAD = left anterior descending artery.
VF induction
In our practice, electrophysiological study is performed using 2 10-electrode catheters positioned on RV and LV sides of the septum, and an additional duodecapolar mapping catheter. Standard induction protocol is performed using pacing from the RV apex then from the LV (near the distal posterior fascicle) using a pulse width of 2 ms at 20 mA. The latter is used instead of the conventional “double diastolic threshold” in order to achieve shorter extrastimulation coupling intervals and increase the rate of VF inducibility. Programmed ventricular stimulation is performed using 2 basic cycle lengths (600 and 400 ms) with up to 3 extrastimuli until the refractory period was met. During the induction protocol, noninvasive body surface mapping can be used to detect the ventricular wavefronts during the initial organized VF, and to locate the main driver areas (Figure 6). We use an array of 252 body surface electrodes combined to computed tomography–based geometry (ECVUE, Medtronic, Minneapolis, Minnesota) (24).
Electrogram mapping in sinus rhythm
A 20-pole catheter with 2-mm interelectrode distances is used for endocardial (PENTARAY, Biosense Webster, Diamond Bar, California) and epicardial (PENTARAY or LASSO [Biosense Webster]) measurements. A transseptal or retroaortic approach is performed to access the endocardial LV and a subxyphosternal approach to access into the pericardial space. Electroanatomical mapping of the endocardium and epicardium is performed using magnetic localization (CARTO 3 system [Biosense Webster]). The objective is to perform high-density recordings (>3,000 sites in epicardium) and to determine whether electrogram characteristics identify areas of abnormal conduction indicative of localized myocardium alterations. Electrogram criteria abnormalities are identical to those defining fibrotic and cardiomyopathic tissue during mapping of ischemic or dilated cardiomyopathies. Areas of low-amplitude electrograms were defined in bipolar (<1 mV) and unipolar modes (<8.3 and 5.5 mV in LV and RV, respectively). Because low-amplitude electrograms can be due to normal fat tissue on the epicardium, epicardial electrograms were considered abnormal if they harbored fragmented signals with a duration superior to 70 ms (onset to offset) or more than 3 components or split or late potentials (48, 49, 50). It is important to underline that the 70-ms criterion is based on prior small-sized population studies using large interelectrode distances. The current use of 2-mm bipoles (or smaller electrodes) improving detection of the near-field component likely requires adjusting reference cutoffs for electrogram mapping, also taking into account other variables influencing signal characteristics (51, 52, 53).
Patient Population: Results of High-Density Mapping
Our experience of IVF includes 172 patients (1994 to 2019), of whom 64 were referred specifically for VF trigger ablation. VF triggers originated from Purkinje cells in 57 (RV in 29, LV in 22, both in 6) and ventricular myocardium in 7 (RV outflow tract in 5, others in 2). Interestingly, in this (Caucasian) patient cohort, there was a strong association between the patient gender and the origin of Purkinje triggers. Right Purkinje ectopy was observed dominantly in men (n = 24 of 29, 82.7%) and left Purkinje ectopy in women (n = 19 of 22, 86%), which was significant (p = 0.001 on chi-square test).
From this cohort, we describe in detail the last 50 patients (39 men, 11 women) who had comprehensive investigations (as shown in Figure 1) were systematically performed. They survived the index VF episode at 33 ± 11 years of age, which was diagnosed as idiopathic VF after the initial evaluation protocol. No patient had any ECG showing short or long QT syndrome, BrS, or J-wave pattern. Structural imaging and pharmacological testing were negative. This IVF population had clinical characteristics (male predominance, young age, occurrence at rest) similar to those of wider groups previously published (11). Table 1 summarizes the clinical findings in this cohort. The results of high-density electrophysiological studies are detailed subsequently.
Table 1.
Clinical Results in 50 Patient Survivors of IVF, With Comparison of Those With or Without Myocardial Conduction Abnormality on High-Density Mapping
| Population (N= 50) | IVF With Localized Myocardial Alteration (n = 34) | IVF Without Myocardial Abnormality (n = 16)∗ | p Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, yrs | 33 ± 11 | 33 ± 12 | 33 ± 12 | 0.968 |
| Male | 39 (78) | 29 (85) | 10 (62.5) | 0.11† |
| Family history of SCD | 6 (12) | 4 (11) | 2 (12.5) | 1.00 |
| Activity at index VF (sleep/effort/other) | 11/7/32 | 7/5/22 | 4/2/10 | 1.00 |
| 12-lead ECG | ||||
| PR interval, ms | 166 ± 28 | 169 ± 30 | 160 ± 26 | 0.335 |
| QRS duration, ms | 95 ± 10 | 100 ± 9 | 86 ± 5 | <0.001† |
| QT interval, ms | 390 ± 28 | 394 ± 27 | 384 ± 30 | 0.325 |
| QTc interval, ms | 413 ± 31 | 411 ± 27 | 416 ± 37 | 0.668 |
| Documented Purkinje ectopy | 14 (28.0) | 6 (17.6) | 8 (50.0) | NR |
| VT runs (>3 beats) on isoproterenol | 11 (22) | 10 (26) | 1 (6) | 0.080† |
| Electrophysiological study | ||||
| Inducible VF | 26 (54.1)‡ | 19 (55.9)‡ | 7 (43.7) | 0.37 |
| VF inducibility with 1/2/3 extrastimuli | 0/10/16 | 0/8/11 | 0/2/5 | 0.668 |
| Electrogram mapping (epicardial sites) | 3,842 ± 1,684 | 4,068 ± 1,886 | 3,357± 1,103 | 0.282 |
| RV endocardial sites | 718 ± 599 | 824 ± 692 | 506 ± 289 | 0.190 |
| LV endocardial sites | 970 ± 907 | 995 ± 1,051 | 907 ± 489 | 0.835 |
Values are mean ± SD and n (%). Continuous variables were compared with a 2-sided Student’s t-test. Categorical variables were compared with the Fisher exact test.
ECG = electrocardiography; IVF = idiopathic ventricular fibrillation; LV = left ventricular; NR = not relevant; PK = Purkinje; RV = right ventricular; SCD = sudden cardiac death; VF = ventricular fibrillation.
Includes 8 with documented PK ectopy, 2 with inducible PK activity, and 6 unexplained IVF.
A trend or a significant result (p < 0.05).
2 patients refused.
Purkinje-related IVF
An abnormality affecting the Purkinje system was the unique finding in 10 patients. It was evidenced as either spontaneous ectopy or by repetitive Purkinje activity inducible by programmed stimulation. None of these patients had abnormal myocardial areas upon endocardial-epicardial mapping.
The presence of Purkinje ectopy was documented in 8 patients, by continuous ECG telemetry in the days following VF, with short coupling intervals (falling on the T-wave) in 7 and long coupling intervals in 1. Their role in VF initiation was demonstrated in 4 patients. The ectopy originated from the distal left Purkinje system in 4 patients, the right Purkinje system in 3 patients, and both in 1 patient (Figure 3).
Another Purkinje abnormality was found during programmed stimulation—in 2 of the previous patients with Purkinje ectopy and 2 without Purkinje ectopy—resulting in a total of 10 patients (20%) who had IVF was associated with a Purkinje abnormality. In these 4 patients, repetitive activity was consistently inducible within the distal Purkinje network by programmed stimulation, particularly by pacing close to the distal left Purkinje fascicle (54). The activity consisted of polymorphic VT (median 7 beats; range 3 to 17), with all beats preceded by distal Purkinje potentials (Figures 7A and 7B). The left bundle branch potential was slower or absent, excluding a bundle branch re-entry. Termination of polymorphic VT was preceded by slowing or disappearance of Purkinje potentials (Figure 7C). In 2 cases, polymorphic Purkinje VT degenerated into VF requiring direct current cardioversion. We have considered these inducible activities as abnormal responses, as they have not been reported in a unique study evaluating LV programmed stimulation (55). Furthermore, short Purkinje coupling intervals (<160 ms) were occasionally recorded between subsequent Purkinje activities, well below the normal refractory period of Purkinje cells.
Figure 7.
Inducible Repetitive Activity in Peripheral Purkinje
(A) Twelve-lead electrocardiography: induction of polymorphic ventricular tachycardia (VT) with programmed stimulation in a 15-year-old girl. Pacing is performed from the left ventricle (LV) close to the left posterior Purkinje system. (B) Endocardial recordings with a multipolar catheter along the distal left posterior fascicle show a more rapid cycle length (mean 202 ms; range 156 to 240 ms) between Purkinje activities (asterisks) than in the LV (217 ms) or right ventricle (RV) (220 ms). The exact values are indicated beat to beat. Each ventricular beat is preceded by Purkinje activity, with a progressively longer timing. The induction of polymorphic Purkinje-driven VT was consistently reproducible, and led to sustained ventricular fibrillation in 1 instance. (C) Another episode of induced polymorphic VT showing that VT termination is clearly preceded by slowing of Purkinje activity (asterisks). CL = cycle length.
Microstructural cardiomyopathic alterations
Myocardial areas manifesting low amplitude and fractionated electrograms were found in 34 of 50 (68%) patients, providing electrophysiological evidence of local conduction alteration (Figures 8 and 9). They were located in the RV in 22 of 34 (64.7%) patients, the LV in 7 of 34 (20.6%), or both ventricles in 5 of 34 (14.7%), and covered a small size representing 5 ± 3% of the total ventricular surface (24). The comparison of endocardial and epicardial recordings at the same locations showed that the abnormal electrograms were recorded in 1 side, mostly epicardial, indicating an alteration affecting a part of ventricular wall. The cardiac magnetic resonance data focusing on the abnormal areas failed to identify any structural abnormalities. All patients were also retested using ajmaline infusion to exclude BrS. The term microstructural alteration refers to this nontransmural lesion and the inability to perceive it by current imaging techniques. In the patients who had VF inducible by programmed stimulation, the main VF drivers (on noninvasive mapping) were anchored to abnormal myocardial areas in 85% of cases (24) (Figure 6). Finally, it is noteworthy that a subset of patients with microstructural cardiomyopathy (17.6%) also had Purkinje ectopy, as commonly seen in a variety of structural heart disease (SHD), where they may act as VF triggers (56).
Figure 8.
Electrocardiography and Examples of Abnormal Epicardial Electrograms During Sinus Rhythm in the RV in 2 Patients With Idiopathic Ventricular Fibrillation
(A) The abnormal electrograms are present within the black contour of epicardial voltage map in the right ventricular (RV) apex. (B) The abnormal electrograms are present within 2 areas (black contour) of the epicardial anterior RV. Despite prolonged and late epicardial electrograms, note the absence of late potentials in high-amplification electrocardiography. Both patients were negative for a panel of 31 genes, including SCN5A and arrhythmogenic RV cardiomyopathy–related genes. The arrows indicate the abnormal electrograms.
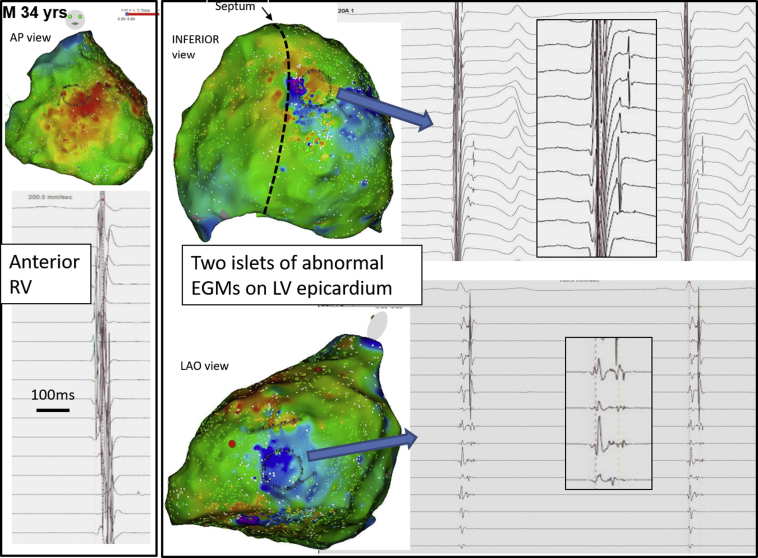
Figure 9.
Examples of Abnormal Epicardial EGMs During Sinus Rhythm in 2 Small Areas in the LV
The 2 areas are located in the lateral and inferior left ventricle (LV), with the abnormal signals amplified in the inlet; the recording circular catheter is visible on the electroanatomical activation maps. EGM = electrogram; LAO = left anterior oblique; RV = right ventricular.
Distinctive factors
The results of clinical examinations in patients with or without myocardial abnormality (34 vs. 16) were evaluated (Table 1). Patients with myocardial abnormality were dominantly men (29 of 34 vs. 10 of 16; p = 0.11) and had a wider QRS duration (100 ± 9 ms vs. 86 ± 5 ms; p < 0.001) after excluding cases with ventricular conduction disturbance (bundle branch block or fascicular block), and even after adjusting for body weight. In addition, they have a trend for a higher incidence of VT runs (≥3 beats) during catecholamine testing: 10 of the 11 patients with isoproterenol-induced VT runs had myocardial abnormality (10 of 34 vs. 1 of 16; p = 0.08). Figure 5 shows 2 examples of nonsustained polymorphic VT appearing on isoproterenol (without ECG or genetic criteria of CPVT or long QT syndrome) who had an area of myocardial abnormality later found in the epicardium. These preliminary results warrant confirmation in a wider patient population.
IVF: A Spectrum of Concealed Diseases
The previous results show that a systematic set of investigations reveals areas of altered myocardial conduction in 68% of IVF patients. Purkinje abnormality alone (without associated myocardial abnormality) is observed in 20% of patients, a value possibly overestimated by a referral bias. No abnormality could be observed in 12% of patients. These results indicate that extending the phenotypic investigations by endocardial-epicardial mapping allows identifying a certain or probable cause in the great majority of IVF patients. Such invasive mapping may be performed during the index clinical presentation; it will also address the questions raised by the patient and his family on the tragic event of unexplained VF. It may alternatively be performed in the case of recurrent VF for the prospect of substrate identification and ablation.
The most frequent abnormality is the presence of localized areas of slow-conducting myocardium. The abnormal areas were associated with anchoring of re-entries during VF on noninvasive mapping, suggesting a pathophysiological link. These abnormal areas likely indicate microstructural alterations of the myocardium because of fibrosis, fatty tissue or inflammatory infiltration, or cellular or intercellular (gap junctions) pathologies. Their incidence as observed here by electrogram mapping, is much higher than that reported in autopsy studies of IVF patients, which relates to the different methods used (11,25). The electrogram patterns that define the abnormality give no indication on the specific disease, although a more formal characterization may provide useful information in the future. However, the varying anatomical and transmural location is suggestive of a large spectrum of potential pathologies. The alterations in the epicardial RV are similar to those encountered in BrS, or ARVD; those in the posterolateral LV are similar to those encountered in myocarditis, sarcoidosis, or genetic or acquired cardiomyopathies (57, 58, 59, 60, 61, 62, 63, 64). A subendocardial alteration may be a damage after coronary spasm. IVF, in these cases, may represent genetically negative or spatially limited forms (negative imaging) of myocardial diseases, or other unknown affections (Central Illustration). The results are in keeping with the increasing detection of gene variants associated with myocardial structure that are reported in IVF (4,5,11,21,25).
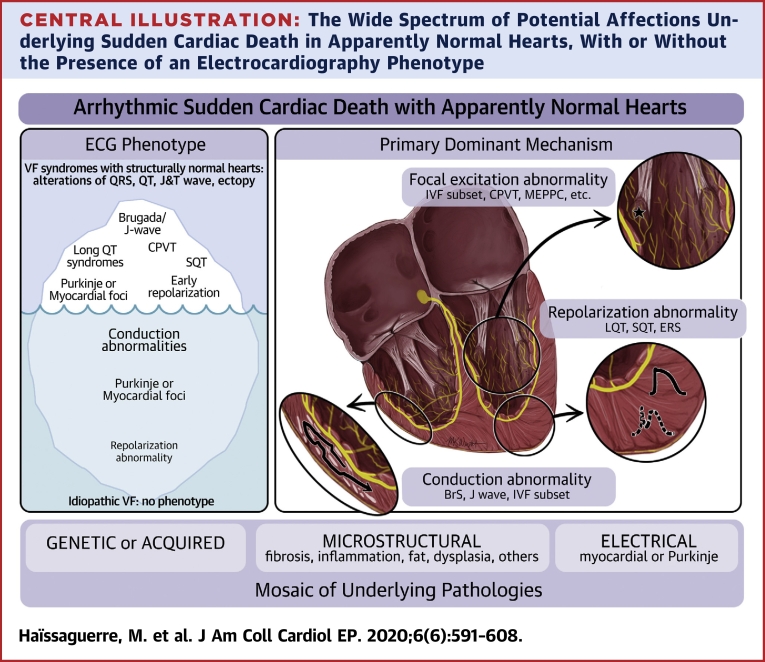
Central Illustration.
The Wide Spectrum of Potential Affections Underlying Sudden Cardiac Death in Apparently Normal Hearts, With or Without the Presence of an Electrocardiography Phenotype
Although the pathophysiology of these affections is likely a complex mosaic at a cellular scale, the individual substrate when mapped during electrophysiology exploration shows abnormalities dominantly affecting depolarization, or repolarization, or excitation.
The abnormalities affecting the Purkinje system were less frequent. They were evidenced as triggering ectopy or by Purkinje repetitive activity inducible by programmed stimulation. Purkinje ectopy was documented by ECG and endocardial mapping; 4 of them had a documented VF initiation. In others, the pathogenic role of Purkinje system could only be inferred, as it was the unique apparent abnormality. The ability of inducing Purkinje repetitive activity in 4 patients was considered an abnormal response, as it is rarely observed in our experience of VF inductions and not reported in a prior study (55). However, further investigations are needed to confirm its significance, and whether it may reveal subclinical Purkinje abnormalities (“Purkinjopathy”). Unfortunately, major limitations are currently present in the clinical capabilities to investigate Purkinje function and structure, which restrict their characterization. Multiple arrhythmic expressions with the phenotype of Purkinje ectopy (short-coupling, CPVT, multifocal ectopic Purkinje premature contractions [MEPPC]) have been previously described and some genetic abnormalities have been reported so far and were recently reviewed (23). An alteration in intracellular calcium handling is likely key to the genesis of spontaneous ectopy, particularly by triggered activity based on delayed afterdepolarizations. The ability of inducing Purkinje re-entrant activity may alternatively be due to altered conduction properties (56), which would relate more to sodium current or gap junctional alterations.
Finally, no myocardial or Purkinje abnormality could be evidenced in a minority of IVF patients (“true” idiopathic VF). Unexplained IVF may have resulted from nondetected abnormalities of conduction, or of repolarization (long QT syndrome, early repolarization), or undocumented ectopic trigger; possibly associated with transient variables (as fever, hypokalemia, drugs, autonomic neural system [SNA] variations). Such causes may be diagnosed later during a recurrent VF episode, or after genetic testing.
High-density electrophysiological mapping can therefore discriminate not only between the different arrhythmogenic substrates within IVF patients (who have no clinical phenotype), but also within patients who exhibit a same phenotypic expression. In recent studies, the inferolateral J-wave phenotype has been demonstrated to be the expression of distinct mechanisms—either early repolarization or late depolarization—with strong implications in patient management (39,65,66). In a specific biomedical research program we have the opportunity to perform ex vivo human heart investigations in patients who died of VF. Using high-resolution mapping and imaging methods as well as targeted cellular biology, we could confirm that a mosaic of different mechanisms manifesting a similar clinical phenotype can be responsible for SCD associated with an apparently normal heart (67). Pending further investigations, it may be expected that VF substrates will be more precisely characterized and, in combination with deeper genotyping characterization, will be categorized using more appropriate terminology.
Genotypic And Phenotypic Characterization
In the past, a variety of arrhythmias initially classified as IVF have been secondarily recognized as distinct diseases after genetic testing, in correlation with the phenotype of 12 lead-ECGs at baseline or during arrhythmia. The identification of a genetic disorder is essential for patient care as it has implications for the management of the disease but also for the family of the affected individual. The 2017 consensus statements recommend genetic testing “for arrhythmia syndromes in young patients (<40 years of age) with unexplained SCD, or unexplained near drowning” (5). The yield of genetic testing is reported as higher if a family history of SCD at a young age is present. In addition, familial phenotypic screening should be performed in first degree relatives, as shown by Behr et al. (21). This included resting ECG, exercise testing, and echocardiography. In selected cases, Holter and signal-averaged ECGs, cardiac magnetic resonance, and pharmacologic testing can be performed (21,22,25).
In inherited arrhythmia syndromes, the yield for a given phenotype varies from about 20% (BrS) to 60% (CPVT) or 75% (long QT syndrome), and several mutations can be associated with a same phenotype (68,69). In IVF, extensive genetic testing—using whole-exome sequencing and next-generation sequencing approaches—is currently not recommended because of the low yield and high cost. These large data have allowed the detection of a plethora of candidate genes for IVF associated with cardiac electrical function or myocardial structure. However, the results need to be evaluated against the “genetic background noise,” and the task for interpretation of multiple detected variants is challenging (68, 69, 70, 71). In addition, most novel variants have not been evaluated using functional studies to validate their arrhythmogenic impact. These variants often concern a specific family or geographic region (as the common DPP6 in the Netherlands), which emphasizes the large spectrum of genetic factors involved in IVF and arrhythmic syndromes.
The phenotypic characterization of IVF patients has an important role to establish causality of genetic variants. Microstructural myocardial affections or Purkinje conduction alterations would be more likely associated with mutations in genes coding for the sodium channels, connexins, and structural proteins. Mutations associated with spontaneous Purkinje ectopy could be more related to calcium handling. However, the heterogeneous nature and pathogenesis of mutations, and the ubiquity of mutated proteins in the heart, reduce both the mutation and tissue specificity. Mutations in SCN5A have many clinical expressions. Mutations in RYR2 have been associated with CPVT perceived as a primary electrical disease, or ARVD manifesting as structural myocardial abnormalities. An example of a complex phenotype is shown in a patient with pathogenic DPP6 mutation. Although this mutation was deemed to selectively increase the Purkinje cell transient outward potassium current, we also observed severe alterations on the RV epicardial myocardium by electrogram mapping (Figure 10). High-density mapping allowing deeper phenotypic characterization may possibly show more complex phenotypes than expected in other unexplored electrical syndromes as the long QT syndrome or CPVT, with implications for their early detection and therapy.
Figure 10.
Complex Phenotype in a Patient With Pathogenic DPP6 Mutation
This patient was referred for ablation of ventricular fibrillation triggers originating from the right Purkinje system (left). However, epicardial mapping also revealed an extensive damage on the right ventricular (RV) epicardial structure shown by voltage mapping (purple indicating ≥1 mV) and prolonged fragmented electrograms (right). ant = anterior; LV = left ventricular.
Despite the complexity in genetic interactions and phenotypic expressions, idiopathic VF and other VF associated with apparently normal hearts can be tentatively classified according to the dominant primary substrate. Depolarization/conduction abnormalities associated with uncoupled and delayed electrograms are the most prevalent affections (48,57,72). They can be associated with secondary repolarization changes (73). They include BrS where abnormal depolarization is present in the RV outflow tract, the subset of inferolateral J waves with abnormal depolarization in the inferior part of the (right or left) ventricles, and the subset of IVF associated with abnormal depolarization in a variety of areas. Repolarization abnormalities include long QT syndrome, short QT syndrome, and the subset of inferolateral J waves due to early repolarization (66). Excitation abnormalities include IVF due to Purkinje or PVC triggers, or VF due to multiple focal excitations (e.g., CPVT or MEPPC); “accidental” VF due to external excitation (commotion cordis, electrocution) may also be added.
Although mixed conditions are obviously present (as Purkinje trigger and depolarization abnormality, or repolarization changes secondary to depolarization abnormality), the groups share a common type of primary substrate and mode of therapy. The abnormal depolarization electrograms can be diagnosed and targeted for ablation with the same method in BrS, late depolarization J-wave, or IVF. The abnormal repolarization group appears more an electrical dysfunction amenable to drug therapy. In the abnormal excitation group, the ablation of abnormal foci enables effective prevention (Central Illustration).
Therapy In IVF
Insertable cardioverter-defibrillator
In patients with IVF (or idiopathic polymorphic VT), an ICD insertion is recommended “if meaningful survival > 1 year is expected.” ICD insertion in patients with IVF is justified by the high recurrence rate of ventricular arrhythmias, varying from 11% to 45% (74).
Drug therapy
The use of pharmacotherapy is to provide a reduction of ICD interventions or symptomatic relief (frequent PVCs) in patients considered to have no substrate for catheter ablation or reluctant to invasive therapy. Beta-blockers are indicated in few selected patients notably those with arrhythmias upon stress or effort. Verapamil has an excellent effect on patients with frequent Purkinje triggers and polymorphic VTs; however, the short half-life of this medication makes it more consistently efficient for immediate therapy using intravenous administration than for long-term oral administration (17,18). Quinidine has been also shown to be beneficial in IVF. It results in a significant reduction of ICD shocks (from 7.5 ± 12 to 0.9 ± 1.7 over 34 months), and drug interruption is frequently associated with breakthrough events (75). Further research will be needed to evaluate whether specific IVF substrates as described previously would be most receptive to quinidine or other therapies.
Catheter ablation
Catheter ablation is currently recommended “for patients with recurrent episodes of IVF initiated by PVCs with a consistent QRS morphology.” This statement indicates that the triggers are the main ablation target in consensus recommendations (5). The presence of localized depolarization substrate, in a significant part of IVF patients, represent a potential novel target for ablation. In keeping with the success of substrate-based ablations in patients with BrS or overt structural heart diseases, IVF patients displaying microstructural myocardial substrate are likely to benefit from this type of strategy.
Trigger ablation
The technique has been detailed in prior articles including the maneuvers to elicit PVCs that are suppressed with sedation (18,30, 31, 32). The site of earliest ventricular activation during spontaneous PVCs is the target of choice. Multielectrode catheters are useful for mapping over a wide area of ventricular myocardium with higher spatial sampling and resolution. Special care should be taken during Purkinje mapping to avoid inadvertent bumping of the right bundle, with the left 1 being much less vulnerable. In patients without clinical PVCs, PVCs can be inducible by pacing maneuvers (atrial or ventricular) or drug infusion like isoproterenol (1 to 2 μg/kg/min) or Class I drugs. Noninvasive electrocardiographic mapping imaging can be used to indicate the area of interest in patients with rare or polymorphic PVCs. In the absence of ectopy, ablation can target the local Purkinje potentials or the site of best matched morphology (PVCs documented in the ward) by pace-mapping. Last, intracardiac echocardiography can be helpful in the complex anatomical regions like the papillary muscles.
Ablation is facilitated by the use of irrigated tip catheters. In most cases, ablation is extended approximately 1 to 2 cm around the target site, particularly in Purkinje triggers, in order to reach additional foci and to “prune” the Purkinje arborization to minimize re-entries. During ablation, it is common to have exacerbation of the arrhythmia (multiple PVCs leading to polymorphic VT and, more rarely, to VF) before the eradication of premature beats. The occurrence of QRS widening during ablation indicates potential catheter displacement toward the more proximal conduction system and ablation should be stopped (18).
Substrate ablation
An irrigated tip ablation catheter is used with the target of eliminating fragmented electrograms, as described for VT in structural heart disease or BrS (48,49,76, 77, 78, 79). Radiofrequency energy is delivered with a power of 35 to 45 W and duration varying from 10 to 30 s guided by impact on the local electrogram. Radiofrequency lesions are delivered point-by-point at the area covering abnormal electrograms using serial applications. We have reported a mean duration of 18 ± 5 min of radiofrequency energy application in our initial series of 12 patients, with 10 patients free of recurrences at a median follow-up of 14 months (24). Besides the usual risks of catheter ablation, the high prevalence of epicardial abnormalities requires specific precautions, notably to avoid damaging the coronary arteries and phrenic nerve, as recently reviewed in consensus documents (79). Preoperative imaging of these structures can be integrated to periprocedural 3-dimensional mapping system and is useful to reduce these risks (80). New ablative energies are emerging that may demonstrate advantages of safety or efficacy upon standard radiofrequency ablation (81).
Summary
Idiopathic VF is a puzzling diagnosis defined by negativity of current medical investigations. This condition accounts for a significant part of SCD in young adults. Detailed phenotypic investigations demonstrate that a majority of IVF can be elucidated, particularly using endocardial-epicardial mapping, by showing an important role for microstructural myocardial or Purkinje abnormalities. The various locations and patterns of abnormalities strongly suggest that a mosaic of pathologies—acquired or genetically determined—can potentially underlie their genesis.
Further advances in phenotypic investigations and genetic screening will likely allow a more specific characterization of various substrates, a more adequate terminology, and hopefully, an early detection of patients at risk for the development of primary prevention.
Footnotes
This work was supported by the National Research Agency (ANR-10-IAHU04-LIRYC) and the European Research Council (SYMPHONY). Dr. Haissaguerre has received research grants from Biosense Webster and Medtronic. Dr. Sacher has received honoraria and consulting fees from Biosense Webster. Dr. Jais has received speaker fees from Boston Scientific and Biosense Webster. Dr. Nademanee has received research grant support from Medtronic and Biosense Webster; and has received royalties from Biosense Webster. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Clinical Electrophysiologyauthor instructions page.
References
- 1.Fishman G.I., Chugh S.S., DiMarco J.P. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S. American Heart Association Statistics Committee; Stroke Statistics Committee. Heart Disease and Stroke Statistics—2016 Update - a report from the American Heart Association. Circulation. 2015;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Murakoshi N., Aonuma K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ J. 2013;77:2419–2431. doi: 10.1253/circj.cj-13-1129. [DOI] [PubMed] [Google Scholar]
- 4.Priori S.G., Blomström-Lundqvist C., Mazzanti A. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann V., Bougouin W., Karam N. Paris-SDEC investigators. Characteristics and clinical assessment of unexplained sudden cardiac arrest in the real world setting: Focus on idiopathic ventricular fibrillation. Eur Heart J. 2018;39:1981–1987. doi: 10.1093/eurheartj/ehy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckart R., Shry E.A., Burke A.P. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Winkel B.G., Holst A.G., Theilade J. Nationwide study of sudden cardiac death in persons aged 1-35 years. Eur Heart J. 2011;32:983–990. doi: 10.1093/eurheartj/ehq428. [DOI] [PubMed] [Google Scholar]
- 9.Semsarian C., Ingles J., Wilde A.A.M. Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J. 2015;36:1290–1296. doi: 10.1093/eurheartj/ehv063. [DOI] [PubMed] [Google Scholar]
- 10.Neilan T.G., Farhad H., Mayrhofer T. Late gadolinium enhancement among survivors of sudden cardiac arrest. J Am Coll Cardiol Img. 2015;8:414–423. doi: 10.1016/j.jcmg.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnall R.D., Weintraub R.G., Ingles J. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687. [DOI] [PubMed] [Google Scholar]
- 12.Torkamani A., Muse E.D., Spencer E.G., Rueda M., Wagner G.N., Lucas J.R. Molecular autopsy for sudden unexpected death. JAMA. 2016;316:1492–1494. doi: 10.1001/jama.2016.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray R.G., Pertsov A.M., Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 14.Nash M.P., Mourad A., Clayton R.H. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–542. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- 15.Nair K., Umapathy K., Farid T. Intramural activation during early human ventricular fibrillation. Circ Arrhythm Electrophysiol. 2011;4:692–703. doi: 10.1161/CIRCEP.110.961037. [DOI] [PubMed] [Google Scholar]
- 16.Viskin S, Belhassen B. Idiopathic ventricular fibrillation. Am Heart J 1990;120:661–71. [DOI] [PubMed]
- 17.Leenhardt A., Glaser E., Burguera M., Nürnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 18.Haissaguerre M., Shoda M., Jais P. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 19.Krahn A.D., Healey J.S., Chauhan V. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 20.Obeyesekere M.N., Klein G.J., Modi S. How to perform and interpret provocative testing for the diagnosis of Brugada syndrome, long QT syndrome, and catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:958–964. doi: 10.1161/CIRCEP.111.965947. [DOI] [PubMed] [Google Scholar]
- 21.Behr E.R., Dalageorgou C., Christiansen M. Sudden arrhythmic death syndrome: Familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 2008;29 doi: 10.1093/eurheartj/ehn219. 1670–168. [DOI] [PubMed] [Google Scholar]
- 22.Visser J., van der Heijden J.F., Doevendans P.A., Loh P., Wilde A.A., Hassink R.J. Idiopathic ventricular fibrillation: the struggle for definition, diagnosis, and follow-up. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003817. [DOI] [PubMed] [Google Scholar]
- 23.Wilde A.A.M., Garan H., Boyden P.A. Role of the Purkinje system in heritable arrhythmias. Heart Rhythm. 2019;16:1121–1126. doi: 10.1016/j.hrthm.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 24.Haïssaguerre M., Hocini M., Cheniti G. Localized structural alterations underlying a subset of unexplained sudden cardiac death. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray B., Ackerman M.J., Semsarian C., Behr E.R. Evaluation after sudden death in the young: a global approach. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007453. [DOI] [PubMed] [Google Scholar]
- 26.Basso C., Perazzol O., Marra M. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 27.Zaya M., Mehta P.K., Bairey Merz C.N. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol. 2014;63:103–109. doi: 10.1016/j.jacc.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J.J., Hebl V.B., DeSimone C.V. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. J Am Coll Cardiol HF. 2014;2:466–473. doi: 10.1016/j.jchf.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda T., Shimizu W., Taguchi A. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol. 2005;46:1288–1294. doi: 10.1016/j.jacc.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 30.Knecht S., Sacher F., Wright M. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 31.Nogami A. Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation: trigger elimination and substrate modification. J Cardiovasc Electrophysiol. 2015;26:110–115. doi: 10.1111/jce.12547. [DOI] [PubMed] [Google Scholar]
- 32.Van Herendael H., Zado E.S., Haqqani H. Catheter ablation of ventricular fibrillation: importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm. 2014;11:566–573. doi: 10.1016/j.hrthm.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Santoro F., Di Biase L., Hranitzky P. Ventricular fibrillation triggered by PVCs from papillary muscles: clinical features and ablation. J Cardiovasc Electrophysiol. 2014;25:1158–1164. doi: 10.1111/jce.12478. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T.T.S., Schaeffer B., Tanigawa S. Substrate modification for ablation of recurrent polymorphic ventricular tachycardia/fibrillation. Heart Rhythm. 2018;15:S284–S387. [Google Scholar]
- 35.Viskin S., Rosso R., Rogowski O., Belhassen B. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912–916. doi: 10.1111/j.1540-8167.2005.50040.x. [DOI] [PubMed] [Google Scholar]
- 36.Tadros R., Nannenberg E.A., Lieve K.V. Yield and pitfalls of ajmaline testing in the evaluation of unexplained cardiac arrest and sudden unexplained death: Single-center experience with 482 families. J Am Coll Cardiol EP. 2017;3:1400–1408. doi: 10.1016/j.jacep.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Cheung C.C., Mellor G., Deyell M.W. Comparison of ajmaline and procainamide provocation tests in the diagnosis of Brugada syndrome. J Am Coll Cardiol EP. 2019;5:504–512. doi: 10.1016/j.jacep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Potet F., Mabo P., Le Coq G. Novel Brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J Cardiovasc Electrophysiol. 2003;14:200–203. doi: 10.1046/j.1540-8167.2003.02382.x. [DOI] [PubMed] [Google Scholar]
- 39.Rollin A., Sacher F., Gourraud J.B. Prevalence, characteristics, and prognosis role of type 1 ST elevation in the peripheral ECG leads in patients with Brugada syndrome. Heart Rhythm. 2013;10:1012–1018. doi: 10.1016/j.hrthm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Escande W, Gourraud JB, Duchateau J, et al. Malignant Purkinje ectopy induced by sodium-channel blockers. Paper presented at: Heart Rhythm Society 40th Annual Scientific Sessions; May 8 to 11, 2019; San Francisco, California. [DOI] [PubMed]
- 41.Viskin S., Postema P.G., Bhuiyan Z.A. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955–1961. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churet M., Luttoo K., Hocini M., Haïssaguerre M., Sacher F., Duchateau J. Diagnostic reproducibility of epinephrine drug challenge interpretation in suspected long QT syndrome. J Cardiovasc Electrophysiol. 2019;30:896–901. doi: 10.1111/jce.13926. [DOI] [PubMed] [Google Scholar]
- 43.Haissaguerre M., Le Métayer P., D'Ivernois C., Barat J.L., Montserrat P., Warin J.F. Distinctive response of arrhythmogenic right ventricular disease to high dose isoproterenol. Pacing Clin Electrophysiol. 1990;13:2119–2126. doi: 10.1111/j.1540-8159.1990.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 44.Denis A., Sacher F., Derval N. Diagnostic value of isoproterenol testing in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2014;7:590–597. doi: 10.1161/CIRCEP.113.001224. [DOI] [PubMed] [Google Scholar]
- 45.Kumar N., Aksoy I., Phan K., Vainer J., Timmermans C. Coronary spasm during cardiac electrophysiological study following isoproterenol infusion. Interv Med Appl Sci. 2014;6:183–186. doi: 10.1556/IMAS.6.2014.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanck Z., Dhala A., Deshpande S., Sra J., Jazayeri M., Akhtar M. Bundle branch reentrant ventricular tachycardia: cumulative experience in 48 patients. J Cardiovasc Electrophysiol. 1993;4:253–262. doi: 10.1111/j.1540-8167.1993.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 47.Stein K.M., Euler D.E., Mehra R., Jewel AF Worldwide Investigators Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol. 2002;40:335–340. doi: 10.1016/s0735-1097(02)01957-5. [DOI] [PubMed] [Google Scholar]
- 48.Hsia H.H., Callans D.J., Marchlinski F.E. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 49.Soejima K., Stevenson W.G., Sapp J.L., Selwyn A.P., Couper G., Epstein L.M. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–1841. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 50.Cano O., Hutchinson M., Lin D. Electroanatomic substrate and ablation outcome for suspected epicardial left ventricular tachycardia in non-ischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Takigawa M., Relan J., Martin R. Detailed analysis of the relation between bipolar electrode spacing and far- and near-field electrograms. J Am Coll Cardiol EP. 2019;5:66–77. doi: 10.1016/j.jacep.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Glashan C.A., Tofig B.J., Tao Q. Multisize electrodes for substrate identification in ischemic cardiomyopathy: validation by integration of whole heart histology. J Am Coll Cardiol EP. 2019;5:1130–1140. doi: 10.1016/j.jacep.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Ustunkaya T., Desjardins B., Wedan R. Epicardial conduction speed, electrogram abnormality, and computed tomography attenuation associations in arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol EP. 2019;5:1158–1167. doi: 10.1016/j.jacep.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haissaguerre M., Cheniti G., Escande W. Idiopathic ventricular fibrillation associated with inducible repetitive activity within the distal Purkinje system Heart. Rhythm. 2019;16:1268–1272. doi: 10.1016/j.hrthm.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehdirad A.A., Keim S., Rist K., Mazgalev T., Tchou P. Asymmetry of retrograde and reentry within the His-Purkinje system: a comparative analysis of left and right ventricular stimulation. J Am Coll Cardiol. 1994;24:177–184. doi: 10.1016/0735-1097(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 56.Haissaguerre M., Vigmond E., Stuyvers B., Hocini M., Bernus O. Ventricular arrhythmias and the His-Purkinje system. Nat Rev Cardiol. 2016;13:155–166. doi: 10.1038/nrcardio.2015.193. [DOI] [PubMed] [Google Scholar]
- 57.Martini M., Nava A., Thiene G. Ventricular fibrillation without apparent structural heart disease. Am Heart J. 1989;118:1203–1209. doi: 10.1016/0002-8703(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 58.Nademanee K., Raju H., de Noronha S.V. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Junttila M.J., Holmström L., Pylkäs K. Primary myocardial fibrosis as an alternative phenotype pathway of inherited cardiac structural disorders. Circulation. 2018;137:2716–2726. doi: 10.1161/CIRCULATIONAHA.117.032175. [DOI] [PubMed] [Google Scholar]
- 60.Kazmirczak F., Chen K.A., Adabag S. Assessment of the 2017 AHA/ACC/HRS Guideline Recommendations for Implantable Cardioverter-Defibrillator Implantation in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynge T.H., Nielsen T.S., Gregers Winkel B., Tfelt-Hansen J., Banner J. Sudden cardiac death caused by myocarditis in persons aged 1-49 years: a nationwide study of 14 294 deaths in Denmark. Forensic Sci Res. 2019;4:247–256. doi: 10.1080/20961790.2019.1595352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadakia R.S., Link M.S., Dominic P., Morin D.P. Sudden cardiac death in nonischemic cardiomyopathy. Prog Cardiovasc Dis. 2019;62:235–241. doi: 10.1016/j.pcad.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Goff Z.D., Calkins H. Sudden death related cardiomyopathies - arrhythmogenic right ventricular cardiomyopathy, arrhythmogenic cardiomyopathy, and exercise-induced cardiomyopathy. Prog Cardiovasc Dis. 2019;62:217–226. doi: 10.1016/j.pcad.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Miles C., Finocchiaro G., Papadakis M. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haissaguerre M., Nademanee W., Hocini M. Depolarization versus repolarization abnormality underlying inferolateral J wave syndromes: new concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm. 2019;16:781–790. doi: 10.1016/j.hrthm.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nademanee W., Haissaguerre M., Hocini M. Mapping and ablation of ventricular fibrillation associated with early repolarization syndrome. Circulation. 2019;140:1477–1490. doi: 10.1161/CIRCULATIONAHA.118.039022. [DOI] [PubMed] [Google Scholar]
- 67.Bernus O, Michel C, Benoist D, et al. A wide spectrum of substrates underlie J-wave syndromes in humans. Poster presented at: Heart Rhythm Society 38th Annual Scientific Sessions; May 10 to 13, 2017; Chicago, Illinois. Heart Rhythm 2017;14(5).
- 68.Gray B., Behr E.R. New insights into the genetic basis of inherited arrhythmia syndromes. Circ Cardiovasc Genet. 2016;9:569–577. doi: 10.1161/CIRCGENETICS.116.001571. [DOI] [PubMed] [Google Scholar]
- 69.Ackerman M.J. Genetic purgatory and the cardiac channelopathies: exposing the variants of uncertain/unknown significance issue. Heart Rhythm. 2015;12:2325–2331. doi: 10.1016/j.hrthm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Offerhaus J.A., Bezzina C.R., Wilde A.A.M. Epidemiology of inherited arrhythmias. Nat Rev Cardiol. 2019;17:205–215. doi: 10.1038/s41569-019-0266-2. [DOI] [PubMed] [Google Scholar]
- 71.Gray B., Hasdemir C., Ingles J. Lack of genotype-phenotype correlation in Brugada syndrome and sudden arrhythmic death syndrome families with reported pathogenic SCN1B variants. Heart Rhythm. 2018;15:1051–1057. doi: 10.1016/j.hrthm.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 72.Coronel R., Casini S., Koopmann T.T. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 73.Hoogendijk M.G., Potse M., Vinet A., de Bakker J.M., Coronel R. ST segment elevation by current-to-load mismatch: an experimental and computational study. Heart Rhythm. 2011;8:111–118. doi: 10.1016/j.hrthm.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 74.Ozaydin M., Moazzami K., Kalantarian S., Lee H., Mansour M., Ruskin J.N. Long-term outcome of patients with idiopathic ventricular fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2015;26:1095–1104. doi: 10.1111/jce.12737. [DOI] [PubMed] [Google Scholar]
- 75.Malhi N., Cheung C.C., Deif B. Challenge and impact of Quinidine access in sudden death syndromes: a national experience. J Am Coll Cardiol EP. 2019;5:376–382. doi: 10.1016/j.jacep.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Nademanee K., Veerakul G., Chandanamattha P. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 77.Jais P., Maury P., Khairy P. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 78.Pappone C., Brugada J., Vicedomini G. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005053. [DOI] [PubMed] [Google Scholar]
- 79.Cronin E.M., Bogun F.M., Maury P. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Arrhythm. 2019;35:323–484. doi: 10.1002/joa3.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berte B., Cochet H., Dang L. Image-guided ablation of scar-related ventricular tachycardia: towards a shorter and more predictable procedure. J Interv Card Electrophysiol. 2019 Dec 19 doi: 10.1007/s10840-019-00686-w. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Guandalini G.S., Liang J.J., Marchlinski F.E. Ventricular tachycardia ablation: past, present, and future perspectives. J Am Coll Cardiol EP. 2019;5:1363–1383. doi: 10.1016/j.jacep.2019.09.015. [DOI] [PubMed] [Google Scholar]