Version Changes
Revised. Amendments from Version 1
We have updated the manuscript to provide the clarifications and additional information requested by the reviewers. For example, we have added two new boxes (“Key data needs and questions that require additional economic evaluations”, and “Guidance for future economic evaluations of schistosomiasis interventions”). We have also added more information and clarifications on the studies time horizons and the lack of adjustments for inflation. We have added the additional reference by Salari et al.
Abstract
Background: Schistosomiasis is one of the most prevalent neglected tropical diseases (NTDs) with an estimated 229 million people requiring preventive treatment worldwide. Recommendations for preventive chemotherapy strategies have been made by the World Health Organization (WHO) whereby the frequency of treatment is determined by the settings prevalence. Despite recent progress, many countries still need to scale up treatment and important questions remain regarding optimal control strategies. This paper presents a systematic review of the economic evaluations of human schistosomiasis interventions.
Methods: A systematic review of the literature was conducted on 22nd August 2019 using the PubMed (MEDLINE) and ISI Web of Science electronic databases. The focus was economic evaluations of schistosomiasis interventions, such as cost-effectiveness and cost-benefit analyses. No date or language stipulations were applied to the searches.
Results: We identified 53 relevant health economic analyses of schistosomiasis interventions. Most studies related to Schistosoma japonicum followed by S. haematobium. Several studies also included other NTDs. In Africa, most studies evaluated preventive chemotherapy, whereas in China they mostly evaluated programmes using a combination of interventions (such as chemotherapy, snail control and health education). There was wide variation in the methodology and epidemiological settings investigated. A range of effectiveness metrics were used by the different studies.
Conclusions: Due to the variation across the identified studies, it was not possible to make definitive policy recommendations. Although, in general, the current WHO recommended preventive chemotherapy approach to control schistosomiasis was found to be cost-effective. This finding has important implications for policymakers, advocacy groups and potential funders. However, there are several important inconsistencies and research gaps (such as how the health benefits of interventions are quantified) that need to be addressed to identify the resources required to achieve schistosomiasis control and elimination.
Keywords: Schistosomiasis, NTDs, Economic evaluations, Cost-benefit, Cost-effectiveness, Cost per DALY averted, Preventive chemotherapy, MDA
Introduction
Schistosomiasis (also known as bilharzia) is an acute and chronic parasitic disease caused by blood flukes of the genus Schistosoma. People become infected when larval forms of the parasite (released by freshwater snails) penetrate the skin during contact with infested water. It is one of the most prevalent neglected tropical diseases (NTDs) with an estimated 229 million people requiring preventive treatment worldwide 1. There are two main forms of human schistosomiasis: urogenital caused by S. haematobium, and intestinal caused by S. mansoni, S. guineensis, S. intercalatum, S. japonicum, and S. mekongi. Schistosomiasis can result in anaemia, chronic pain, diarrhea, and malnutrition, causing poor school performance and lower fitness 2. It is estimated that 89.3% of those requiring treatment for schistosomiasis live in Africa 1.
In Africa, schistosomiasis control mainly focuses on mass school-based or community-wide preventive chemotherapy using praziquantel 3: the large-scale distribution of drugs to eligible populations, without diagnosing or testing individuals for current infection. The World Health Organization’s (WHO) recommended guidelines for preventive chemotherapy are currently dependent on the prevalence of infection in school-aged children (SAC; 5–14 years old) prior to preventive chemotherapy 4. Although SAC are generally the focus using targeted school-based preventive chemotherapy, treatment of adults is also recommended depending on the endemicity. Specifically, in low-risk communities (below 10% baseline prevalence in SAC), treatment of SAC once every 3 years is recommended, along with treatment of suspected cases 4. For moderate-risk communities (10–50% baseline prevalence in SAC), biennial treatment of SAC and at-risk adults is recommended 4. For high-risk communities (>50% baseline prevalence in SAC), annual treatment of SAC and at-risk adults is recommended 4. Outside of Africa, it is more common for preventive chemotherapy to be complemented by other interventions, such as health education and snail control 5– 8.
Merck KGaA have committed to donate 250 million tablets of praziquantel annually for the treatment of schistosomiasis, primarily for SAC in Africa 9. The current WHO goals of morbidity control and elimination as a public health problem 10, focus on preventive chemotherapy to reduce the prevalence of heavy-intensity infections in SAC and furthermore the interruption of transmission in selected settings (reducing the incidence of infections to zero) 11, 12 ( Box 1). The post-2020 WHO goals are currently being developed, along with revisions to the current preventive chemotherapy guidelines.
Box 1. Glossary.
Cost-benefit analysis: A type of economic evaluation in which both the costs and the resulting outcomes (i.e. health benefits) of the interventions in question, are expressed in monetary terms. The results are typically expressed by a benefit-cost ratio.
Cost-effectiveness analysis: A type of economic evaluation in which the cost of the interventions in question are compared to the quantity of a non-monetary effectiveness measure (such as the number of deaths or cases averted). This avoids the challenges associated with monetising the benefits of healthcare interventions. The results are expressed as a cost per unit of outcome (see cost-effectiveness ratio).
Cost-effectiveness ratio: A statistic used to summarise the cost-effectiveness of an intervention. It is calculated by dividing the cost of an intervention by its effectiveness measure, such as a cost per disability-adjusted life year (DALY) averted. An incremental cost-effectiveness ratio is calculated by dividing the difference in costs by the difference in effectiveness outcomes of two alternative options (it summarises the ‘extra cost per additional unit of effect gained’).
Cost-utility analysis: A specific subtype of cost-effectiveness analysis, where the effectiveness of the intervention is measured using a "utility-based" unit (such as disability-adjusted life years (DALYs) averted and quality-adjusted life years (QALYs) gained).
Disability-adjusted life years (DALYs): A measure of disease burden that is calculated as the sum of the years of life lost due to premature mortality and the years of healthy life lost due to disability. The number of years of healthy life lost due to disability is calculated using a disability weight factor between 0 and 1, that reflects the severity of the disease/disability (with 0 representing perfect health and 1 representing death). One DALY can be thought of as one year of "healthy" life lost.
Economic costs: These define the cost of a resource as its value in its next best alternative use that has been forgone (also known as an opportunity cost). This is a broader conceptualization of a resource’s value than its financial cost, as it recognizes that using a resource makes it unavailable for productive use elsewhere. The rationale behind economic costs is that they are intended to represent the full value of the resources used for an intervention, and they account for the fact that resources can have a value that is not (fully) captured by their financial costs (such as the ‘free’ use of building space provided by Ministries of Health, and the unpaid time devoted to mass preventive chemotherapy programmes by volunteers). This is particularly important when considering issues related to the sustainability and replicability of interventions.
Economic evaluation: Economic evaluations are a specific type of health economic analysis that formally evaluates the costs and benefits of two (or more) alternative courses of action.
Elimination as a public health problem: Goal defined by achieving <1% prevalence of heavy-intensity infections in school-aged children 10.
Financial costs: The actual expenditure (i.e. the amount paid) for the goods, resources and services that are purchased.
Heavy-intensity infections: For intestinal schistosomiasis caused by S. mansoni, heavy-intensity infections are defined as greater than 400 eggs per gram of stool and for urogenital schistosomiasis caused by S. haematobium, this is defined as over 50 eggs per 10 mL of urine 10.
Interruption of transmission (breaking transmission): End goal defined by reducing the incidence of infections to zero 10.
Morbidity control: Goal defined by achieving <5% prevalence of heavy-intensity infections in school-aged children 10.
Prevalence settings: Defined as low with below 10% baseline (prior to treatment) prevalence in school-aged children; moderate with 10–50% baseline prevalence in school-aged children; high with above 50% baseline prevalence in school-aged children 4, 10.
Time horizon: The time horizon of an economic evaluation determines the duration over which the outcomes (i.e. the effectiveness) and costs are calculated.
Mass preventive chemotherapy: The large-scale distribution of drugs to eligible populations, without diagnosing or testing individuals for current infection 3.
Quality-adjusted life year (QALY): A outcome measure used to quantify the effectiveness of a particular intervention. QALYs have been designed to capture both gains in quality and quantity of life. The utility weights used to estimate the gains in quality of life are measured on a scale where perfect health is valued as 1 and death as 0.
The treatment coverage of SAC has increased notably over the last decade, reaching 61.2% in 2018 1. However, despite the WHO recommendations, adults are often missed, and their coverage is markedly lower (18.2%) 1. Although most treatment programmes currently rely solely on preventive chemotherapy, additional operational components, such as the provision of potable water and adequate sanitation, hygiene education, behaviour change and snail control may become essential when moving towards elimination of schistosomiasis 13.
Despite recent progress, important questions remain regarding optimal schistosomiasis strategies 14. Economic evaluations have an important role in informing strategies and policy decisions. The aim of this paper is to provide a descriptive overview of the economic evaluations that have been conducted, the areas of research focus and the key findings for human schistosomiasis interventions. Based on these findings, we describe important areas of uncertainty, drivers of variation and remaining research gaps that require further attention within future economic evaluations for schistosomiasis.
Methods
A systematic review of the literature was conducted on 22 nd August 2019 using the PubMed (MEDLINE) and ISI Web of Science electronic databases. The focus was economic evaluations of schistosomiasis interventions, such as cost-effectiveness and cost-benefit analyses. Although not technically an economic evaluation, studies reporting estimated economic benefits of schistosomiasis interventions were also included. Variants of the following search terms were used to find relevant papers: schistosomiasis and either cost-benefit, cost-effectiveness, economic(s), or economic evaluation. No specific date or language stipulations were applied to the searches. A more detailed summary of the search terms and the PRISMA checklist are supplied as extended data. Studies on schistosomiasis interventions that were identified as a relevant type of economic analysis were included. Studies related to health economic evaluation of schistosomiasis diagnostics/monitoring, cost of illness and willingness to pay for treatment were excluded.
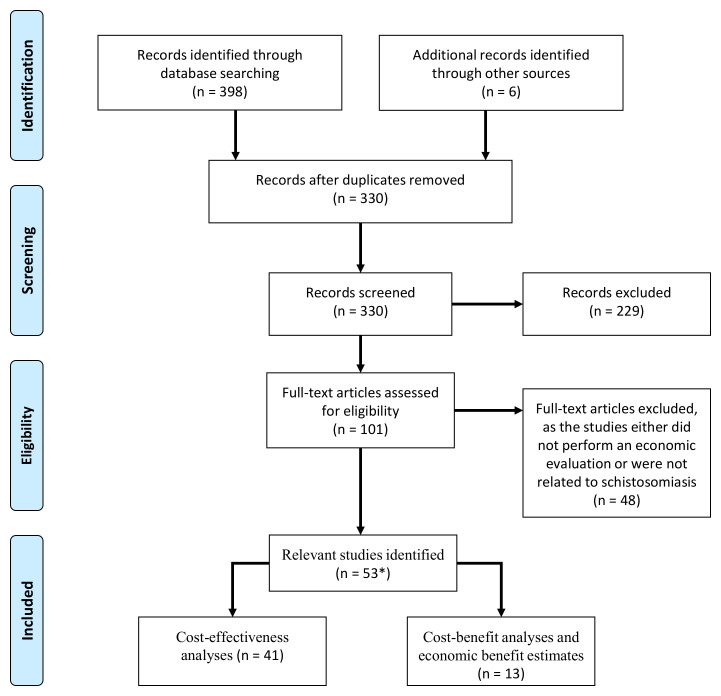
The titles and abstracts of the identified papers were examined initially for relevance by two independent reviewers [HCT and JT]. The full texts for potentially relevant articles were then reviewed to determine eligibility for inclusion. The bibliographies of papers suitable for inclusion were then scanned for studies not originally retrieved from the databases. Discrepancies were solved by consensus among the reviewers. The full selection process is outlined in Figure 1.
Figure 1. Flow diagram outlining the inclusion and exclusion of the identified studies.
*Some studies reported both cost-benefit and cost-effectiveness estimates. A PRISMA checklist is supplied as extended data 15.
The identified health economic studies were grouped into two broad types:
Cost-effectiveness analyses and cost-utility analyses: Cost-effectiveness analysis is a form of economic analysis that compares the relative costs and effectiveness of different courses of action. The effectiveness of the interventions under investigation is measured in terms of natural units (such as life years gained, cases averted, or heavy cases averted). Cost-utility analysis is a specific subtype of cost-effectiveness analysis, where the effectiveness of the intervention is measured using a "utility-based" unit (such as disability-adjusted life years (DALYs) averted and quality-adjusted life years (QALYs) gained) ( Box 1).
Cost-benefit analyses and estimates of economic benefits: Cost-benefit analysis is a form of economic analysis that compares the relative costs and benefits of different courses of action ( Box 1). Unlike, cost-effectiveness analyses, the benefits of an intervention are expressed in monetary terms, i.e. compares the cost of an intervention to its monetary benefits. We also included studies that only estimated the economic benefits of schistosomiasis interventions.
Due to the number of studies identified and the range of research questions investigated ( Figure 1), it was not possible to provide a detailed summary of the results of every study. Instead in line with the aim of the paper, we provide an overview of the studies that have been done and discuss key overarching findings and research gaps that need to be addressed. Due to their significance to policy makers, we outline further details of the studies reporting the cost per DALY averted related to preventive chemotherapy.
The studies estimates were kept in their original cost year and not adjusted to a single reference year.
Results
We identified 53 relevant health economic analyses of schistosomiasis interventions. An overview of the studies is presented in Table 1. Interestingly, there were notably more economic evaluations of schistosomiasis than those identified in previous reviews for other helminth infections of human health importance 16– 18.
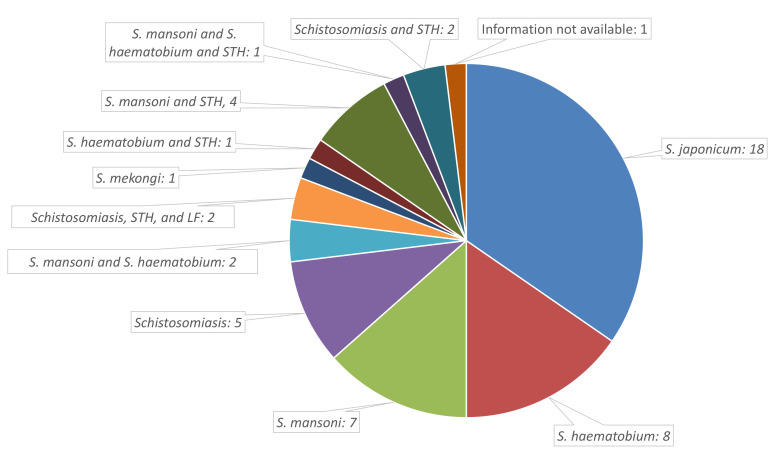
The number of economic evaluations performed on schistosomiasis interventions has gradually increased over time, although a notable number were published pre-2000 ( Table 1). Most studies were related to S. japonicum, followed by S. haematobium, and S. mansoni ( Figure 2). Only one study related to S. mekongi ( Table 1). Several studies also included other NTDs, particularly soil-transmitted helminths (STH). In Africa, most studies evaluated mass preventive chemotherapy, whereas in China they mostly evaluated programmes using a combination of interventions together ( Table 1). Few studies were performed for other settings ( Table 1).
Table 1. Summary of the identified studies.
| Study | Publication
year |
Species | Intervention(s) | Research focus |
|---|---|---|---|---|
| Cost-effectiveness analyses | ||||
| 19 | 1987 | Schistosomiasis | PC | The optimal choice of PC strategy (selective vs mass). |
| 20 | 2006 | Schistosomiasis | PC | The cost-effectiveness of treating school-age children for
schistosomiasis. |
| 21 | 2011 | Schistosomiasis | PC | The cost-effectiveness of PC for schistosomiasis control. |
| 22 | 1977 | S. haematobium | Chemotherapy and snail control
(separately and in combination) |
The cost-effectiveness of alternative disease control
measures within the Khuzestan Province, Iran. |
| 23 | 1986 | S. haematobium | Selective chemotherapy | The cost-effectiveness of selective chemotherapy;
1) metrifonate - 3 dose regimen, fortnightly intervals vs 2) praziquantel - one dose regimen |
| 24 | 1994 | S. haematobium | Alternative treatment strategies | The cost-effectiveness of alternative treatment strategies;
1) Mass treatment by a mobile team, 2) Reagent strip testing by schoolteachers with referral to the dispensary for treatment, 3) Passive testing and treatment at the dispensary |
| 25 | 2011 | S. haematobium | PC | The cost-effectiveness of PC for schistosomiasis control in
Niger. |
| 26 | 2013 | S. haematobium | PC | The potential cost-effectiveness of schistosomiasis treatment
for reducing HIV transmission in Africa. |
| 27 | 2013 | S. haematobium | Provision of clean water,
sanitation, and health education with administration of praziquantel to school-aged children |
The cost-effectiveness of a community-based intervention for
reducing the transmission of S. haematobium and HIV in Africa. |
| 28 | 2018 | S. haematobium | PC and/or snail control | The cost-effectiveness of PC, focal chemical-based snail
control, and combined strategies against schistosomiasis. |
| 29 | 2000 | S. japonicum | Selective chemotherapy | The cost-effectiveness of mass indirect hemagglutination
screening vs. a questionnaire followed by indirect hemagglutination testing. |
| 30 | 2001 | S. japonicum | Selective chemotherapy vs PC | The cost-effectiveness of selective chemotherapy (using
water contract surveys) vs PC in Hunan province, China. |
| 31 | 2002 | S. japonicum | Chemotherapy | The cost-effectiveness of three chemotherapy schemes
against S. japonicum in Dongting Lake region, China; 1) Treatment to those with contact with infected water and/or symptoms of infection; 2) mass chemotherapy-treatment; and 3) treatment prescribed to positive cases after Kato-Katz examination |
| 32 | 2002 | S. japonicum | Information not available | Information not available. |
| 33 | 2003 | S. japonicum | Information not available | The cost-effectiveness of a rapid control strategy in new hilly
endemic areas of schistosomiasis in Taoyuan County, China. |
| 34 | 2003 | S. japonicum | A combination of interventions | The cost-effectiveness and benefit analysis of World Bank
Loan on schistosomiasis control in Qiangjiang City, China. |
| 35 | 2005 | S. japonicum | A combination of interventions | The cost-effectiveness of the national schistosomiasis control
programme in China from 1992 to 2000. |
| 36 | 2009 | S. japonicum | A combination of interventions | The cost-effectiveness of a more intensive strategy vs routine
interventions in the Poyang Lake region, China. |
| 37 | 2011 | S. japonicum | Snail control - environmental
modification |
The cost-effectiveness of a snail control project using
environmental modification in hilly regions, China. |
| 38 | 2013 | S. japonicum | A combination of interventions | The cost-effectiveness of a comprehensive
Schistosomiasis
japonica control programme in the Poyang Lake region, China. |
| 39 | 2013 | S. japonicum | Information not available | The cost-effectiveness of schistosomiasis comprehensive
control in Lushan County, China from 2007 to 2012. |
| 40 | 2014 | S. japonicum | A combination of interventions | The cost-effectiveness of comprehensive control measures
carried out in schistosomiasis endemic inner embankment of marshland and lake regions from 2006 to 2010 in Jiangling County, China. |
| 41 | 2017 | S. japonicum | A combination of interventions | The cost-effectiveness of a comprehensive schistosomiasis
control strategy with a focus on cattle and sheep removal in Junshan District, China. |
| 42 | 2018 | S. japonicum | Snail control – molluscicides | The cost-effectiveness of three molluscicides in Yangtze
River, China. |
| 43 | 1977 | S. mansoni | Snail control, chemotherapy,
and provision of water supplies |
The cost-effectiveness of three different schistosomiasis
interventions. |
| 44 | 1984 | S. mansoni | Chemotherapy with
oxamniquine with and without snail control |
The cost-effectiveness of different ways of controlling
intestinal schistosomiasis in the mining region of Maniema in Eastern Zaire. |
| 45 | 1995 | S. mansoni | Vaccination (hypothetical) | The desirable characteristics of a schistosomiasis vaccine. |
| 46 | 1997 | S. mansoni | Vaccination (hypothetical) | The target product profile of a schistosomiasis vaccine. |
| 47 | 1998 | S. mansoni | PC | An investigation into the interaction between drug efficacy
and drug price of praziquantel in determining the cost- effectiveness of school-based schistosomiasis treatment. |
| 48 | 2000 | S. mansoni | Treatment at a primary health care
centre |
The cost-effectiveness of different treatment strategies at
primary health care centres in Burundi; 1) screening all symptomatic patients (with Kato-Katz) and treating positive cases; 2) treating all symptomatic patients or 3) treating only those presenting with symptoms of severe diarrhoea. |
| 49 | 2010 | S. mekongi | PC | The cost-effectiveness of the schistosomiasis control
programme in Cambodia. |
| 50 | 1993 | Schistosomiasis and
STH |
PC | The cost-effectiveness of school-based treatment (via a
mobile team) for schistosomiasis and STH. |
| 51 | 2000 |
S. mansoni and
S. haematobium |
PC | The impact of school attendance on the unit cost and
effectiveness of school-based schistosomiasis chemotherapy programmes. |
| 52 | 2001 |
S. haematobium and
STH |
PC | The impact and cost-effectiveness of school-based
anthelmintic treatments in reducing anaemia in children in the United Republic of Tanzania. |
| 53 | 2004 | S. mansoni and STH | PC | The cost-effectiveness of a Kenyan school-based PC project. |
| 54 | 2008 | Schistosomiasis and
STH |
PC | The cost-effectiveness of nationwide school-based helminth
control in Uganda: intra-country variation and effects of scaling-up. |
| 55 | 2011 |
S. mansoni and
S. haematobium |
PC | A life path analysis to estimate the incremental cost-
effectiveness of using double treatment (given 2 to 8 weeks after the first dose) instead of single annual treatment in school-based and community-wide PC programmes. |
| 56 | 2015 |
S. mansoni and
S. haematobium and STH |
PC | A comparison of the cost-effectiveness of school-based vs
community-wide PC for schistosomiasis and STH. |
| 57 | 2016 | S. mansoni and STH | PC | An assessment of the global guidelines for PC strategies
against schistosomiasis and STH. |
| 58 | 2018 | Schistosomiasis,
STH, and LF |
PC | The cost-effectiveness of PC in Madagascar. |
| 59 | 1996 | Information not
available |
Vaccination (hypothetical) | The potential cost-effectiveness of a vaccine against
schistosomiasis. |
| Cost-benefit analyses and estimates of economic benefits | ||||
| 60 | 2000 | Schistosomiasis | Various preventive schistosomiasis
interventions |
The cost-benefit of different preventive schistosomiasis
interventions in Kenya. |
| 61 | 2017 | Schistosomiasis | PC | The socioeconomic benefit of achieving the WHO 2020
targets for schistosomiasis. |
| 62 | 1974 | S. haematobium | Not applicable | The potential economic benefits of eliminating mortality
attributed to schistosomiasis in Zanzibar. |
| 63 | 1987 | S. japonicum | Mass chemotherapy and snail
control (molluscicides) |
The investigation of a theoretical model to determine the
most cost-efficient combination of preventive and curative measures in China. |
| 35 | 2005 | S. japonicum | A combination of interventions | An economic evaluation of the national schistosomiasis
control programme in China from 1992 to 2000. |
| 36 | 2009 | S. japonicum | A combination of interventions | The cost-benefit of a more intensive strategy vs routine
interventions in the Poyang Lake region, China. |
| 64 | 2011 | S. japonicum | Snail control – forest
environment |
The eco-economical benefit of snail control and
schistosomiasis prevention in mountainous regions in Yunnan Province, China. |
| 65 | 2012 | S. japonicum | Snail control – environmental
modification vs molluscicides |
The cost-benefit of snail control by environmental
modification (such as building low dykes, ploughing and planting) vs. molluscicide use in Jiaobei Beach of Zhenjiang City, China. |
| 66 | 1972 | S. mansoni | Snail control and mass
diagnosis/treatment campaign |
A cost-benefit analysis of an
S. mansoni control programme
on an irrigated sugar estate in northern Tanzania. |
| 53 | 2004 |
S. mansoni and
STH |
PC | The economic benefits of a school-based PC project in Kenya. |
| 67 | 2016 |
S. mansoni and
STH |
PC | The economic benefits of a school-based PC project in Kenya. |
| 58 | 2018 | Schistosomiasis,
STH, and LF |
PC | The financial, and education gains of investing in preventive
chemotherapy in Madagascar. |
LF: Lymphatic filariasis, STH: Soil-transmitted helminths PC: Preventive chemotherapy.
Figure 2. Overview of the number of studies done and the investigated species.
LF: Lymphatic filariasis, STH: Soil-transmitted helminths.
The methods used by the studies to parameterise the costs of preventive chemotherapy were variable: some used primary cost data whereas others performed rough calculations or used assumed crude benchmark values ( Table 2). There was also variation regarding the use of financial or economic costs within the studies ( Box 1). The economic cost of an intervention is typically higher than the financial cost ( Table 2). If praziquantel was assumed to be purchased it would be counted as a financial cost. Depending on the perspective of the analysis, the value of any donated praziquantel would be included as an economic cost. It should be noted that the shift towards drug distribution being integrated within school systems reduced the delivery costs associated with preventive chemotherapy. Consequently, the results of many of the earlier studies cannot be directly generalised to current control programmes ( Table 2).
Table 2. Cost-utility analyses of preventive chemotherapy for schistosomiasis.
| Study | Setting | Time horizon for
the effectiveness |
Intervention | Assumed
average costs of preventive chemotherapy |
Cost-effectiveness ratio or
incremental cost-effectiveness ratio |
Cost
year |
|---|---|---|---|---|---|---|
| Schistosomiasis alone | ||||||
| Hotez
et al.
(DCP2) 20 |
Hypothetical setting
(Schistosomiasis) |
Not clearly stated | Annual mass
school-based treatment |
Not stated | US$336–692 per DALY averted
(note that this is incorrectly quoted as US$3.36–6.92 within the report) |
Unclear |
| GiveWell 21 | Hypothetical setting
(Schistosomiasis) |
1 year | Annual mass
school-based treatment |
US$0.27-0.47
per treatment (including drug costs) |
US$30–$80 per DALY averted | Unclear |
| Lo et al. 57 | Hypothetical setting
( S. mansoni) |
5 years | Annual mass
school-based treatment |
US$0.71 per
treatment (including drug costs) |
5% prevalence in SAC: US$1,050
per DALY averted, 15% prevalence in SAC: US$449 per DALY averted, 30% prevalence in SAC: US$160 per DALY averted |
2015
prices |
| Annual mass
community- wide treatment |
US$1.71 per
treatment (including drug costs) |
15% prevalence in SAC: US$1,031
per incremental DALY averted, 30% prevalence in SAC: US$443 per incremental DALY averted |
||||
| King et al. 55 | Kenya
(S. mansoni and S. haematobium) |
Liftime | Annual mass
school-based treatment |
Delivery cost:
US$0.811 per treatment (including drug costs) |
S. mansoni: US$17.76 per QALY
gained S. haematobium: US$17.18 per QALYgained |
Unclear |
| Annual mass
school-based treatment (double treatment) |
Delivery cost:
US$0.811 per treatment (including drug costs) |
S. mansoni: US$152.95 per
incremental QALY gained S. haematobium: US$210.83 per incremental QALY gained |
||||
| Annual mass
community- wide treatment |
Delivery cost:
US$0.811 per treatment (drug costs were also included) |
S. mansoni: US$47.90 per QALY
gained S. haematobium: US$45.58 per QALY gained |
||||
| Annual mass
community-wide treatment (double treatment) |
Delivery cost:
US$0.811 per treatment (drug costs were also included) |
S. mansoni: US$291.07 per incremental
QALY gained S. haematobium: US$432.76 per incremental QALY gained |
||||
| Ndeffo
Mbah et al. 27 |
Zimbabwe
(S. haematobium) |
Lifetime | Provision of
clean water, sanitation, and health education with annual administration of praziquantel to school-aged children |
US$0.41 per
treatment (including drug costs) Costs of clean water, sanitation, and health education were varied. |
- | Unclear |
| Schistosomiasis and soil-transmitted helminths (STH): | ||||||
| Lo et al. 56 | Four communities
in Côte d'Ivoire (Schistosomiasis) |
15 years | Annual mass
school-based treatment |
US$0.71 per
treatment (including drug costs) |
US$118 (US$87–141) per DALY
averted (92% of the disability resulted from Schistosoma infections) |
2014
prices |
| Annual mass
community-wide treatment for schistosomiasis (biannual for STH) |
US$1.71 per
treatment (including drug costs) |
US$167 (US$101 to 463) per incremental
DALY averted |
||||
| Miguel and
Kremer 53 |
Kenya
( S. mansoni and STH) |
1 year | Annual mass
school-based treatment |
US$0.49 per pupil per
year (including drug costs) |
US$5 per DALY averted (99% of
the benefit was due to averted schistosomiasis) |
Unclear |
| Warren
et al. 50 |
Hypothetical setting
(Schistosomiasis and STH) |
10 years | Annual
mass school treatment with a mobile team |
US$0.8–1.80 per
child per year (including drug costs) |
US$6–33 per DALY averted | Unclear |
DALY: Disability-adjusted life year, QALY: Quality-adjusted life year, SAC: School-aged children
STH: Soil-transmitted helminths.
The cost-effectiveness analyses
We identified 41 cost-effectiveness analyses of schistosomiasis interventions ( Table 1). The majority investigated preventive chemotherapy, with few looking at the cost-effectiveness of water, sanitation and hygiene (WASH) and behavioural change ( Table 1). A range of effectiveness metrics were used by the different studies, including specific types of morbidity averted (such as anaemia), cases prevented, heavy infections averted, decreases in the infection rate (in humans, animal hosts and snails) and DALYs averted.
One of the key areas of analysis was whether selective treatment should be used i.e. where only those that are tested positive for infection (or suspected to be infected) are treated. This strategy uses less praziquantel relative to mass preventive chemotherapy. Some earlier studies found that selective treatment could be more cost-effective than mass treatment 24, 48. However, as the price/value of praziquantel and delivery declined over time, mass school-based or community-wide treatment became more cost-effective than using screening/selective based approaches, particularly in high prevalence settings 19, 31, 48. By adopting cheaper methods of performing selective treatment, such as water contact surveys rather than testing for infection with diagnostics, selective treatment may become more cost-effective in certain settings 30, 68.
The estimated cost per DALY averted for annual mass school-based preventive chemotherapy for schistosomiasis (or both STH and schistosomiasis) ranged widely between US$5-692 in moderate and high prevalence settings ( Table 2– cost year variable) ( Box 1). In many settings, annual school-based preventive chemotherapy would be classed as cost-effective (depending on the cost-effectiveness threshold ( Box 2)). Lo et al. 57 demonstrated that the cost-effectiveness of annual school-based preventive chemotherapy was highly influenced by the setting’s prevalence of schistosome infections (5% prevalence in SAC: US$1,050, 15% prevalence in SAC: US$449, and 30% prevalence in SAC: US$160 (2015 prices)). This shows that in moderate to high prevalence settings ( Box 1), annual preventive chemotherapy is generally cost-effective supporting the WHO recommendation of less frequent treatment in low prevalence settings. The range in these estimates was also influenced by the method used for calculating the number of DALYs averted, particularly the choice of disability weight. Community-wide preventive chemotherapy tended not to be more cost-effective than school-based treatment for schistosomiasis control 55– 57, i.e. it did not have a lower cost per DALYs averted. However, it could be classed as cost-effective depending on the cost-effectiveness threshold ( Box 2). De Neve et al. 58 highlighted that the overall cost-effectiveness of preventive chemotherapy depends on which other infections are co-endemic.
Box 2. Cost-effectiveness thresholds.
To determine whether an intervention is cost-effective using a cost-utility analysis, the cost per DALY averted is compared to a cost-effectiveness threshold. An often misunderstood aspect of cost-utility analysis is that when comparing mutually exclusive interventions (such as school-based vs community-wide preventive chemotherapy), the goal is to find the most effective intervention which has an incremental cost-effectiveness ratio below an established cost-effectiveness threshold. It is not about finding the intervention/strategy with the lowest cost-effectiveness ratio (i.e. the strategy with the lowest cost per DALY averted). The findings of cost-utility analysis are often miscommunicated to policymakers and many refer to an intervention being the “most cost-effective”, when instead they mean it is the optimal intervention for the given cost-effectiveness threshold.
The most appropriate cost-effectiveness thresholds are under debate within the global health field 69– 71. Some studies used the cost-effectiveness threshold set by the WHO-CHOICE 72, namely a cost per DALY averted < 3 times the country’s GDP per capita. However, this is now considered to be too high and has been widely criticised 69– 71, 73, 74. Interestingly, recent analyses have indicated that a significantly lower cost-effectiveness threshold closer to < ½ the country’s per capita GDP would be more appropriate for low-income countries 73, 75. The Disease Control Priorities project (Third Edition) also used a more conservative threshold of US$200 per DALY averted to identify priority interventions for consideration in low-income countries 76. These different thresholds are shown in the Table below. It is vital that studies are interpreted in light of such changes and reduced thresholds – as conclusions regarding what interventions or strategies are cost-effective may no longer hold. This will be an important consideration when using cost-effectiveness analysis to inform new schistosomiasis treatment guidelines, particularly guidance on the use of community-wide treatment.
Cost-effectiveness thresholds recommended for low-income countries.
| Threshold source | Average cost per DALY averted
threshold (2017 prices) |
|---|---|
| Previous WHO threshold 72 | US$2,355 (highly cost effective: US$785) |
| Proposed update to WHO
threshold 73, 75 |
US$392.5 |
| World Bank 77 | US$235.50 * |
* The value has been adjusted to 2017 prices using GDP implicit price deflators relating to US$ 78.
DALY: disability-adjusted life year, WHO: World Health Organization, GDP: Gross Domestic Product.
Most of the studies related to S. japonicum in China evaluated programmes using a combination of interventions together (such as chemotherapy, snail control and health education). These studies often looked at one specific setting whilst evaluating the currently used interventions, though some evaluated more comprehensive interventions 36 or alternative strategies 30, 31. Many of these studies investigated the cost per reduction in the infection rate, often considering humans, animal hosts and snails ( Table 1). For example, Zhou et al. 35, performed a retrospective economic evaluation of the national schistosomiasis control programme in China from 1992 to 2000. Based on data derived from the six study counties, they estimated that the average cost for case detection was 12.48 Chinese yuan per person (cost year varied). The average costs to reduce the human infection rate of S. japonicum per 100 persons by 1% was 7732.42 Chinese yuan (cost year varied) and the bovine S. japonicum infection rate per 100 cattle by 1% was 162891.10 Chinese yuan (cost year varied). Reducing the snail-infested areas by 1000m 2 by mollusciciding costed 3573.18 Chinese yuan (cost year varied). Due to the contrasting effectiveness metrics, it is difficult to make comparisons to the studies on other settings or diseases.
Most studies evaluating snail control were in China and looked at several types of control method, including environmental modification and molluscicide use. These were typically part of a programme and not a standalone intervention. It is critical not to overgeneralise studies regarding the impact and cost-effectiveness of snail control. For example, the benefit of snail control will likely be higher for zoonotic species such as S. japonicum. Only Lo et al. 28 investigated the cost-effectiveness of snail control for controlling S. haematobium using DALYs as the effectiveness metric. Their results supported the use of snail control within schistosomiasis control strategies, particularly in high prevalence settings, transmission hotspots, and settings with high noncompliance to preventive chemotherapy 28.
King et al. 55 evaluated the likely cost-effectiveness of giving repeated (or double) treatment, whereby two rounds of treatment are provided 2 to 8 weeks apart to enhance impact. Using Markov modelling of potential lifetime gains, they found that although schedules for repeated treatment with praziquantel require greater inputs in terms of direct costs and community participation, there are incremental benefits to this approach at an estimated incremental cost of US$153 ( S. mansoni) – US$211 ( S. haematobium) per QALY gained when repeated treatment is employed within school-based programmes.
Ndeffo Mbah et al. 26 performed a cost-effectiveness analysis of annual administration of praziquantel to SAC as a potential measure to reduce the burden of HIV in sub-Saharan Africa. Epidemiological data have shown that genital infection with S. haematobium – known as female genital schistosomiasis (FGS) - increases the risk of HIV infection in young women 79, 80. They found that praziquantel administration could potentially be a cost-effective and even cost saving way of reducing HIV infections in sub-Saharan Africa, particularly where S. haematobium and HIV are highly prevalent. Programme costs per case of HIV averted were similar to, and under some conditions better than, other interventions that are currently implemented in Africa to reduce HIV transmission. These cost-savings occurred due to the low cost and high efficacy of praziquantel, along with the increased HIV risk faced by women infected with FGS. In addition to FGS, schistosome infections have also been associated with increased transmission of HIV in males 81.
A further study by Ndeffo Mbah et al. 27 evaluated the cost-effectiveness of a community-based intervention for averting S. haematobium infections and resultant HIV. The intervention integrated the provision of clean water, sanitation, and health education (WSHE) for the entire community with annual praziquantel treatment of SAC. The cost-effectiveness of the community-based intervention was found to vary with the cost of WSHE, the efficacy of WSHE for reducing S. haematobium transmission and the duration of the intervention. The intervention remained cost-effective for a range of WSHE efficacies and became more cost-effective over time. Overall, the results indicated that this integrated community-based approach towards schistosomiasis control could effectively reduce the health and economic burden associated with S. haematobium and HIV infections in sub-Saharan Africa.
The cost-benefit analyses and estimates of economic benefits
Schistosomiasis can be debilitating and can negatively impact productivity 2, 67, 82– 91. A recent systematic review and meta-analysis found that Schistosoma infection/non-treatment was significantly associated with educational, learning and memory deficits in SAC 92.
A number of studies have estimated the economic benefits or performed cost-benefit analyses of schistosomiasis interventions ( Table 1). For example,
Redekop et al. 61 estimated US$17.4 billion in economic benefit would be generated (between 2011–2013) if the WHO 2020 roadmap goals for schistosomiasis were achieved (2010 prices). The majority of this benefit was due to prevented anaemia.
De Neve et al. 58 estimated the health, financial, and education gains of investing in preventive chemotherapy for schistosomiasis, STH, and lymphatic filariasis in Madagascar. They found that preventive chemotherapy could avert a notable amount of school absenteeism and reduce patients’ out-of-pocket expenditure.
Zhou et al. 35 investigated the cost-benefit of the national schistosomiasis control programme in China (1992–2000). The net benefit-cost ratio was 6.20.
Miguel and Kremer 53 found that deworming for STH and schistosomiasis was likely to increase the net present value of wages by over US$30 per treated child based on the estimated rate of return to education in Kenya. Baird et al. 67 also subsequently estimated that mass deworming may generate more in future government revenue than it costs in subsidies.
These studies indicate that schistosomiasis interventions may generate notable economic benefits. These types of analyses were less common compared to cost-effectiveness analyses. This is likely due to the methodological challenges of placing a monetary value on the benefits of schistosomiasis interventions. There was notable variation regarding the methodology of these identified studies and what was used to justify the assumptions made. It should be highlighted that the results of such studies can be highly sensitive to the methodology used. Further standardisation is urgently needed in this area (not just for studies on schistosomiasis).
Discussion
We identified a wide range of economic evaluations of schistosomiasis interventions. Due to the variation in methodology and epidemiological settings, it was not possible to make definitive policy recommendations based on the identified studies. However, the results of the review indicate that annual schistosomiasis treatment interventions are generally estimated to be cost-effective in moderate and high prevalence settings ( Box 1) and can generate notable economic benefits. In low prevalence settings aiming for morbidity control or elimination as a public health problem ( Box 1), annual mass preventive chemotherapy may not be cost-effective, supporting the current WHO recommendation of less frequent treatment in such settings 10. There are also a growing number of studies evaluating alternative strategies to those currently recommended, such as snail control and WASH.
Most of the studies related to S. haematobium and S. mansoni evaluated mass preventive chemotherapy. Contrastingly, most of the studies relating to S. japonicum evaluated comprehensive control programmes with multiple components (such as snail control, preventive chemotherapy, health education). These studies were difficult to compare with others as they tended to use metrics based on reductions in infection rates (humans, animal hosts and snails) rather than DALYs or cases/morbidity averted.
There was notable variation in methodology across the different studies (which likely lead to the wide range in the estimated costs per DALY averted ( Table 2)). Key sources of variation included 1) the assumed costs, 2) the epidemiolocal setting, and 3) the methods used to quantify the effectiveness of an intervention.
Variation in the assumed cost of interventions
The average delivery cost of annual preventive chemotherapy using praziquantel was typically assumed (or in some cases estimated) to be between US$0.20-0.50 per treatment 93. This is consistent with a recent systematic review by Salari et al. 94, which found that the average delivery cost of preventive chemotherapy for schistosomiasis (with or without an educational component) was US$0.30 per treatment. However, the methods used to parameterise the costs of different interventions within these economic evaluations varied widely making it difficult to understand the relative costs of different interventions and how costs may vary across countries/regions. This variation also made it difficult to directly compare the different studies. Salari et al. 94 also found that the degree of transparency for most of the costing studies of schistosomiasis interventions they identified was limited. An important consideration is whether financial or economic costs are being used ( Box 1). Economic costs are considered the gold standard within economic evaluations as they better reflect the sustainability and replicability of interventions. Many studies did not formally state if they were using financial or economic costs. There was also variation regarding whether the cost of donated praziquantel had been included within the analysis as an economic cost ( Table 2).
When interpreting and comparing the studies, it is vital to consider that integrating drug distribution through the school system rather than using mobile teams notably reduced the delivery costs associated with preventive chemotherapy. However, many of the earlier health economic studies identified (pre-2000) related to the use of mobile teams to deliver treatments, which is more costly than the currently used school-based platform. Additionally, in the past, the cost of praziquantel was substantially higher 47, 95 with many of the studies having praziquantel costing US$0.60 per treatment. However, several forms of generic praziquantel tablets are now available, currently costing around US$0.08 per tablet 96, 97 and some countries (such as China 95, 98) produce their own. Merck KGaA have also committed to donate 250 million tablets of praziquantel annually (primarily for SAC in Africa) 10. Consequently, the findings of many of the earlier studies cannot be directly generalised to current control programmes.
Variation in the epidemiological setting
Pre-control endemicity is a notable driver in the estimated cost-effectiveness of schistosomiasis interventions. As pre-control endemicity increases, the intervention generally becomes more cost-effective. This needs to be considered when comparing studies and using them to inform policy.
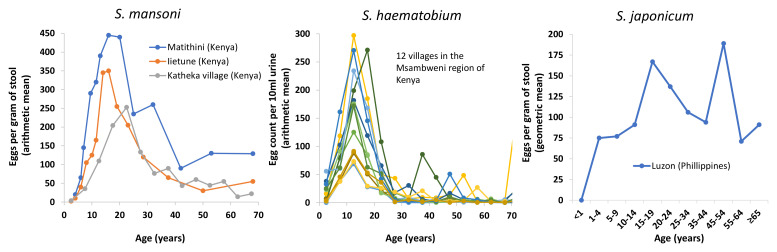
In contrast, the importance of the age profile of infection is more subtle and often ignored. Although the assumed age profile of infection would have a relativity small impact on the estimated cost-effectiveness of the recommended school-based treatment, it can have notable implications regarding the relative benefit of alternative interventions, particularly regarding the benefit of targeting additional age groups with preventive chemotherapy. Consequently, it needs to be considered in studies investigating the cost-effectiveness of alternative schistosomiasis interventions to school-based treatment ( Table 3) and has substantial consequences regarding the generalisability of economic evaluations and their conclusions. Figure 3 illustrates some of the available age profile data. It should be noted that the shape of the typical age profile (and variation around them) differs for each species. The importance of these age profiles of infection and their variation is often not accounted for in modelling studies and policy recommendations 99. There is a real danger that overgeneralising in this area could lead to highly inefficient recommendations. Caution is needed when parameterising this aspect of models when performing economic evaluations. For example, if the data relating to adults are not representative, such as being from high-risk adults only, the assumed average burden of adult infection and cost-effectiveness of switching to community-wide treatment will be overestimated 100.
Table 3. Age profiles of infection assumed in previous modelling studies on Schistosoma mansoni.
| Study | Measure | Mean burden in
Pre-SAC |
Mean burden in
SAC |
Mean burden in
adults |
Sources |
|---|---|---|---|---|---|
| Chan
et al. and
Guyatt et al. 47, 101– 103 |
Peak age of water contact
(mean EPG at peak) |
15 years old (EPG:
300 and 500) |
47, 101 | ||
| Lo et al. 56 | EPG – four different communities
were modelled |
37.1
6.1 5.1 0 |
94.2
229.5 30.1 1.1 |
138.9
446.9 89.6 4.8 |
104
105, 106 107 108 |
| Lo et al. 57 | Relative prevalence by age group | 0.625 | 1 | 0.8333 | 109 |
| Turner et al. 99 | EPG for three scenarios with low,
moderate and high relative adult burdens * |
Higher transmission
setting: 3 12.3 3.1 Lower transmission setting: 1.2 4.8 1.2 |
Higher transmission
setting: 308.7 267.3 215.4 Lower transmission setting: 157.7 133.3 101.5 |
Higher transmission
setting: 149.2 184.4 232.1 Lower transmission setting: 71.6 88.8 114.4 |
110, 111 |
Pre-SAC: pre-school-aged children (2–4 years old), SAC: school-aged children (5–14 years old), adults: 15+ years old, EPG: eggs per gram.
Note that these show the mean infection intensity for the entire age group (i.e. including non-infected individuals). * The values from Turner et al. 99 were converted from worm burden to EPG.
Figure 3. The observed cross-sectional host-age and mean infection intensity profiles for schistosome infections.
The data are from the following sources: Iietune village, Kenya 110, Matithini village, Kenya 110, Katheka village, Kenya 111, 12 villages in the Msambweni region of Coastal Kenya 112, and Luzon, Philippines 113. Note that these show the mean infection intensity for the entire sampled population (i.e. including both infected and non-infected individuals).
A further factor is whether other species are included within the economic evaluation ( Table 1 and Table 2). Based on the available studies, we cannot make inferences on the relative cost-effectiveness for different species. Generally, accounting for other species would increase the cost-effectiveness or cost-benefit of preventive chemotherapy. In addition, Lo et al. highlighted that the optimal strategy for schistosomiasis can depend on if it is co-endemic with STH 57.
Variation in the methods used to quantify the effectiveness of interventions
A key area of uncertainty and variation is how the health gains/effectiveness of interventions were quantified. This is important as the chosen method impacts the outcome of an economic evaluation, potentially leading to different policy recommendations. For example, when basing effectiveness on the number of worm years or heavy cases years averted (both metrics related to infection intensity), models find that the total effectiveness tends to increase with the transmission setting 99. In contrast, when the effectiveness is based on reductions in the prevalence of infection, the estimated effectiveness can be greater in lower transmission settings 99. This would imply that when modelling reductions in morbidity are based on reductions in prevalence, the results could find that it is more cost-effective to treat in lower transmission settings – which is likely to be misleading in terms of morbidity control. The effectiveness metric also has a particularly notable influence on the relative benefit of annual community-wide to school-based treatment: metrics based on reduction in infection intensity tend to estimate a lower benefit relative to those based on infection prevalence 99.
Typically, the ideal metric for evaluating different control strategies in low- and middle-income countries is the number of DALYs averted ( Box 1). As this metric is used for a wide range of diseases, the cost-effectiveness estimates can be directly compared to other healthcare interventions. This makes it possible to have standardised thresholds to class whether an intervention is cost-effective ( Box 2) which is rarely possible for disease-specific metrics. However, the DALY burden of schistosomiasis is often calculated by simply applying a disability weight (representing the disability of an `average' prevalent case of schistosomiasis) to the prevalence of infection. Although this approach may be a suitable method for approximating the disease burden of schistosomiasis at a given point in time, we would argue that it is misleading to apply this framework to evaluate the effectiveness of schistosomiasis interventions 99. This is because the relationship between schistosome infection and morbidity is complex and often not merely due to the presence or absence of infection 99, 114. Only quantifying health gains based on reductions in prevalence would implicitly assume that curing a very light infection has a health benefit whereas reducing a heavy infection to a light infection has none.
The DALY disability weights have also been controversial for schistosomiasis (defined in terms of 0 being perfect health and 1 being death ( Box 1)) 2, 115, 116. The Global Burden of Disease (GBD) 1990 study gave schistosomiasis a disability weight of 0.005 117, implying a very minimal level of disability for “average” schistosomiasis. In contrast, a higher weight of 0.02 was proposed by King et al. 2, 118. The GBD now has an average weight of 0.006 for mild infection, with higher disability weights for specific forms of morbidity (such as anaemia, bladder pathology, and haematemesis) 119. The notable variation in the methods used to estimate DALYs related to schistosomiasis makes it difficult to directly compare the results of different studies reporting a cost per DALY averted for schistosomiasis interventions ( Table 2). It is vital that this is recognised when interpreting these studies.
King et al. 55 estimated quality-adjusted life year (QALY) disability weights for schistosomiasis (defined in terms of 1 being perfect health and 0 being death) ( Box 1). These were estimated to be 0.9 for moderate-heavy infection and 0.986 for light infection. Despite their similarities, disability weights for QALYs and DALYs are not interchangeable, i.e. one minus a QALY weight does not equal a DALY weight (although some do use this adjustment method) 120.
The method used to quantify the effectiveness of interventions and the associated uncertainty needs greater consideration when using schistosomiasis related economic evaluations for informing policy. The choice of metrics will need to reflect the goal and will therefore likely need to be changed as settings move from aiming for morbidity control to interruption of transmission. In most African settings, this will likely mean that the metrics used are currently related to the level of morbidity, whereas in China the metrics will need to reflect the level transmission.
A further source of variation is the time horizon of the economic evaluation ( Box 1 and Table 2). This determines the duration over which the outcomes and costs are calculated and can generate notable differences across studies. Studies with short time horizons may underestimate cost-effectiveness of an intervention.
Limitations of this analysis
A potential source of bias of the search strategy is that it did not capture economic evaluations published outside of the searched electronic databases, such as grey literature, policy documents/reports, and non-English language publications. In addition, texts without available abstracts or those not clearly identifiable as an economic evaluation may be missed. Efforts were made to minimise this bias by searching the bibliographies of selected studies. There could also be a degree of publication bias, with economic evaluations with negative or less favourable results being less likely to be published. A number of the studies did not have the full text published in English. These were included within the results based on information from their abstracts ( Table 1) but further analysis was limited.
A further limitation was that it was not possible to adjust the results of the studies to a single reference year 121. This makes it more difficult to compare the different studies (due to inflation).
Research needs for future health economic analyses
There are important inconsistencies and research gaps that need to be addressed as we move towards the post-2020 WHO goals. In the following subsections we outline several key research needs and considerations for future economic evaluations ( Box 3 and Box 4).
Box 3. Key data needs and questions that require additional economic evaluations.
Key data needs
Further cost data on the currently used preventive chemotherapy strategies.
Data on the cost and effectiveness of alternative strategies (such as WASH, behaviour change, paediatric treatment, snail control and alternative preventive chemotherapy delivery platforms). It will be important that these data are from a range of settings – as the setting may influence the effectiveness of an alternative strategy.
Data on the link between schistosome infections and morbidity and mortality.
Further epidemiological data to parametrise the models used for schistosomiasis economic evaluations (such as prevalence and intensity of infection data across all ages, treatment coverage and adherence data, and WASH data).
Data from hotspot areas.
Key questions that require additional economic evaluations
What is the optimal strategy for achieving and maintaining elimination as a public health problem? This will include investigating whether different preventive chemotherapy strategies and/or complementary interventions (such as snail control) are cost-effective.
What is the optimal strategy for identifying and controlling hotspots?
When do alternative treatment strategies (such as test-and-treat) become more cost-effective than currently used preventive chemotherapy strategies?
Is it more cost-effective to maintain elimination as a public health problem or move towards interruption of transmission?
What is the cost-effectiveness of different surveillance strategies?
Box 4. Guidance for future economic evaluations of schistosomiasis interventions.
Follow standardised guidelines for the reporting of economic evaluations (such as CHEERS 122). In particular, clearly state the studies perspective, time horizon and cost year.
Clearly state how morbidity is modelled and investigate the impact of this on the study’s conclusions. If DALY calculations are used, any changes to the approach used by the Global Burden of Disease study needs to be clearly reported and justified.
Use economic costs rather than only financial costs (see definitions in Box 1).
Clearly state the source of the cost data and whether/how the value of donated drugs is included.
Clearly state the epidemiological setting under investigation (e.g. the pre-control endemicity, schistosome species and age profile of infection). Consider how generalizable the results are to other epidemiological settings.
Preventive chemotherapy cost data
The costs of preventive chemotherapy vary across different settings 16– 18, both within and between countries. A driver is the size of the targeted population 123– 125. This is because the delivery costs of preventive chemotherapy can have economies of scale, such that as the number of people treated increases, the cost per treatment tends to decrease 126, 127. Another key driver for the costs is the targeted age group. There is very little primary data on the relative cost of school vs community-based preventive chemotherapy 17. Many studies in this area make assumptions on the relative cost based on little data. This is an important research gap that needs to be filled to allow further analysis to inform whether and when to switch to community-wide preventive chemotherapy.
It should be noted that preventive chemotherapy delivery costs are not constant and will likely increase over time as countries develop and as expectations regarding the quality of distribution increase. Crucially, the cost per treatment of programmes will also likely increase considerably as they approach their “last mile”, particularly with an end goal of interruption of transmission. This is due to the increase in costs associated with expanding programmes to include harder-to-reach areas and groups 126, 128. Hence, a greater understanding of the variation in the costs of preventive chemotherapy across settings and quantifying its impact within subsequent economic evaluations is an important research gap for future studies 126.
A notable research gap is the lack of understanding of the costs of integrated NTD control 129, 130 and how integration may influence the costs and cost-effectiveness of implementing different control strategies 126.
Evaluation of alternative interventions and strategies
As more data on the costs and effectiveness of interventions become available, economic evaluations of alternative control strategies to preventive chemotherapy need to be conducted, particularly expanding to African settings. This includes WASH 131, vaccines 132, behaviour change, paediatric treatment, snail control and alternative preventive chemotherapy delivery platforms. Further studies are also needed to quantify the costs and effectiveness of alternative delivery strategies, beyond school-based and community-wide preventive chemotherapy. In particular, the coverage and adherence of different age groups when using various treatment delivery platforms needs to be assessed 133. For example, the number of high-risk adults treated through a school-based delivery system and the number of non-enrolled children missed needs to be quantified. It will also be important to investigate the cost-effectiveness of targeting high-risk adults within the community in comparison to targeting the whole community. If a sufficient coverage of high-risk adults could be achieved in addition to school-based treatment, it could be a more cost-effective alternative to mass community-wide preventive chemotherapy. This option is often missed in economics evaluations (due to the lack of data), with most studies only evaluating mass school-based treatment and community-wide preventive chemotherapy.
It is important to consider that as schistosomiasis programmes become more integrated with other NTD programmes or health/education interventions, the costs will likely go down (due to economies of scope 126), but the quality of the programme and treatment coverage may also decline.
Importantly, on-going mass treatment may no longer be optimal in low transmission settings and other strategies (such as selective treatment) may need to be considered. Furthermore, the level of treatment coverage that can be maintained with passive treatment at public health facilities needs to be investigated before stopping mass treatment.
Quantifying the health benefits of interventions
Currently, we would argue that it is difficult to accurately capture the impact of treatment on the morbidity related to schistosomiasis 99. There is an urgent need for the development of frameworks that can accurately estimate the number of DALYs averted by schistosomiasis interventions. An ideal framework will need to account for:
-
i.
The differences in how pathogenic different levels of infection are in different age groups (similar to the approach used for STH 134). A crucial area of uncertainty for this is the relative burden of light infections 99 and how this varies for different species.
-
ii.
Which forms of morbidity are permanent vs reversible with treatment 99. This would need to include what morbidity is present in individuals without a current infection 135.
Without a better framework, results regarding the benefit of expanding treatment beyond SAC will be highly dependent on assumptions from limited empirical evidence. In particular, overestimating the relative burden of light infections could overestimate the benefit and cost-effectiveness of community-wide treatment (and vice versa) 99. Further work is needed to accurately quantify the excess mortality related to schistosomiasis and to reassess the death estimates due to schistosomiasis.
Epidemiological data, one health and hotspots
Better epidemiological data are needed to parametrise models used for schistosomiasis economic evaluations. This includes prevalence and intensity of infection data across all ages to inform pre-control age profiles of infection, treatment coverage and adherence data, and WASH data. By collecting this data, economic evaluations can be more accurate and informative.
Animal populations have also been shown to be infected with schistosomiasis. The impact of zoonotic transmission to human infection differs by species and this influences the impact of different interventions. Some studies investigating S. japonicum included targeting the zoonotic reservoir ( Table 1) but further work is needed to investigate the costs and benefits of applying a one health approach across a range of settings 136.
Despite treatment, there are hotspots where infection remains at persistently high levels 14, 137– 139. Hotspots can be caused by various programmatic factors such as poor treatment coverage/adherence, movement of infected individuals and intense water contact. Additionally, it is possible that these may be caused by declining drug efficacy as some individuals remain infected following multiple treatment rounds 140, 141. These factors pose threats to the effectiveness of treatment programmes and need to be considered in future economic evaluations. Ignoring these could lead to the long-term cost-effectiveness of interventions being overestimated. Economic evaluations also have a role in informing the optimal strategies for identifying and controlling these hotspots.
The impact of the goal of the intervention
The goal of a programme is an important consideration when interpreting economic evaluations 16, 18. Some areas, particularly in Asia, are aiming to move beyond morbidity control and elimination as a public health problem to interruption of transmission 142 ( Box 1). Modelling studies have indicated that breaking transmission may theoretically be possible with mass preventive chemotherapy alone at high coverage and compliance 99, 143. Field studies and trials are currently underway to confirm if this is feasible in practice 144, 145. Moving towards elimination will likely require more intensive strategies, including treatment in low prevalence settings 14. It should be noted that an increase in programmatic costs would be required– at least in the short term. More intensive and expensive interventions may not be cost-effective in the context of morbidity control but could be when breaking transmission. For example, in some settings, annual school-based preventive chemotherapy may be the optimal strategy in terms of controlling schistosomiasis-related morbidity, but other more intensive strategies may be more cost-effective when the goal is the interruption of transmission.
Conclusions
A wide range of economic evaluations of schistosomiasis interventions have been conducted. Based on the identified studies, annual preventive chemotherapy has generally been found to be cost-effective in moderate to high prevalence settings, thereby supporting the WHO recommendation of less frequent treatment in low prevalence settings. However, the cost-effectiveness of mass preventive chemotherapy varies depending on the setting and it is difficult to generalise across species and regions/countries. There are also a growing number of studies evaluating alternative strategies to school-based preventive chemotherapy. Due to the variation in methodology and epidemiological settings, it was not possible to make definitive policy recommendations based on the identified studies. There are several important research gaps that need to be addressed as we move towards the post-2020 WHO goals ( Box 3). In particular, evaluations of interventions other than mass preventive chemotherapy are needed (especially in low transmission settings). Further work is also needed to develop frameworks that can more accurately quantify the health benefits of different strategies. It is also important that future health economics evaluations accurately account for the underlying epidemiology (such as the variation in the age profile of infection) and for the factors which may be driving persistent hotspots.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: Supporting Information - Economic evaluations of human schistosomiasis interventions: a systematic review and identification of associated research needs. https://doi.org/10.6084/m9.figshare.11961342.v1 15
This project contains the following extended data:
-
-
Supporting Infomation - Economic evaluations of human schistosomiasis interventions a systematic review and identification of associated research needs.docx (Word file with study search strategy and PRISMA checklist)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Reporting guidelines
PRISMA checklist for ‘Economic evaluations of human schistosomiasis interventions: a systematic review and identification of associated research needs’. https://doi.org/10.6084/m9.figshare.11961342.v1 15
Acknowledgements
We would like to thank Pauline N M Mwinzi, Aya Yajima, T. Deirdre Hollingsworth for valuable comments on the manuscript.
Funding Statement
This study was supported by the Wellcome Trust through a core grant to the Viet Nam Major Overseas Programme which supports HCT [106680]. This study was also supported by joint funding by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union [MR/R015600/1 supporting HCT]. JT gratefully acknowledges funding of the NTD Modelling Consortium by the Bill and Melinda Gates Foundation [OPP1184344].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. World Health Organization: Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2018. Weekly epidemiological record. 2019;50(94):601–12. Reference Source [Google Scholar]
- 2. King CH, Dickman K, Tisch DJ: Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–9. 10.1016/S0140-6736(05)66457-4 [DOI] [PubMed] [Google Scholar]
- 3. Gabrielli AF, Montresor A, Chitsulo L, et al. : Preventive chemotherapy in human helminthiasis: theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011;105(12):683–93. 10.1016/j.trstmh.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization: Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: WHO,2006. Reference Source [Google Scholar]
- 5. Utzinger J, Zhou XN, Chen MG, et al. : Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96(2–3):69–96. 10.1016/j.actatropica.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 6. Ross AG, Olveda RM, Acosta L, et al. : Road to the elimination of schistosomiasis from Asia: the journey is far from over. Microbes Infect. 2013;15(13):858–65. 10.1016/j.micinf.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarvel AK, Oliveira AA, Silva AR, et al. : Evaluation of a 25-year-program for the control of schistosomiasis mansoni in an endemic area in Brazil. PLoS Negl Trop Dis. 2011;5(3):e990. 10.1371/journal.pntd.0000990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergquist R, Tanner M: Controlling schistosomiasis in Southeast Asia: a tale of two countries. Adv Parasitol. 2010;72:109–44. 10.1016/S0065-308X(10)72005-4 [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization: New agreement expands access to schistosomiasis treatment for millions. Reference Source [Google Scholar]
- 10. World Health Organization: Schistosomiasis: progress report 2001-2011, strategic plan 2012-2020. Reference Source [Google Scholar]
- 11. World Health Assembly: Elimination of schistosomiasis: WHA Resolution 6521. Reference Source [Google Scholar]
- 12. World Health Organization: Accelerating work to overcome the global impact of neglected tropical diseases–A roadmap for implementation. Reference Source [Google Scholar]
- 13. World Health Organization: Schistosomiasis: Strategy. Reference Source [Google Scholar]
- 14. Secor WE, Colley DG: When Should the Emphasis on Schistosomiasis Control Move to Elimination? Trop Med Infect Dis. 2018;3(3): pii: E85. 10.3390/tropicalmed3030085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner H: “Supporting Information - Economic evaluations of human schistosomiasis interventions: a systematic review and identification of associated research needs”. Figshare V1.2020. 10.6084/m9.figshare.11961342.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gedge LM, Bettis AA, Bradley MH, et al. : Economic evaluations of lymphatic filariasis interventions: a systematic review and research needs. Parasit Vectors. 2018;11(1):75. 10.1186/s13071-018-2616-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turner HC, Truscott JE, Hollingsworth TD, et al. : Cost and cost-effectiveness of soil-transmitted helminth treatment programmes: systematic review and research needs. Parasit Vectors. 2015;8(355):355. 10.1186/s13071-015-0885-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner HC, Walker M, Pion SDS, et al. : Economic evaluations of onchocerciasis interventions: a systematic review and research needs. Trop Med Int Health. 2019;24(7):788–816. 10.1111/tmi.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prescott NM: The economics of schistosomiasis chemotherapy. Parasitol Today. 1987;3(1):21–4. 10.1016/0169-4758(87)90093-7 [DOI] [PubMed] [Google Scholar]
- 20. Hotez PJ, Bundy DAP, Beegle K, et al. : Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. In:, Jamison DT, Breman JG, et al. Disease Control Priorities in Developing Countries.Second Edition. Washington (DC): World Bank,2006;467–82. [Google Scholar]
- 21. GiveWell: Cost-Effectiveness in $/DALY for Deworming Interventions. Reference Source [Google Scholar]
- 22. Rosenfield PL, Smith RA, Wolman MG: Development and verification of a schistosomiasis transmission model. Am J Trop Med Hyg. 1977;26(3):505–16. 10.4269/ajtmh.1977.26.505 [DOI] [PubMed] [Google Scholar]
- 23. Korte R, Schmidt-Ehry B, Kielmann AA, et al. : Cost and effectiveness of different approaches to schistosomiasis control in Africa. Trop Med Parasitol. 1986;37(2):149–52. [PubMed] [Google Scholar]
- 24. Guyatt H, Evans D, Lengeler C, et al. : Controlling schistosomiasis: the cost-effectiveness of alternative delivery strategies. Health Policy Plan. 1994;9(4):385–95. 10.1093/heapol/9.4.385 [DOI] [PubMed] [Google Scholar]
- 25. Leslie J, Garba A, Oliva EB, et al. : Schistosomiasis and soil-transmitted helminth control in Niger: cost effectiveness of school based and community distributed mass drug administration [corrected]. PLoS Negl Trop Dis. 2011;5(10):e1326. 10.1371/journal.pntd.0001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ndeffo Mbah ML, Poolman EM, Atkins KE, et al. : Potential cost-effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa--the case of Zimbabwean women. PLoS Negl Trop Dis. 2013;7(8):e2346. 10.1371/journal.pntd.0002346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ndeffo Mbah ML, Kjetland EF, Atkins KE, et al. : Cost-effectiveness of a community-based intervention for reducing the transmission of Schistosoma haematobium and HIV in Africa. Proc Natl Acad Sci U S A. 2013;110(19):7952–7. 10.1073/pnas.1221396110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lo NC, Gurarie D, Yoon N, et al. : Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc Natl Acad Sci U S A. 2018;115(4):E584–E91. 10.1073/pnas.1708729114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang XY, Hong XL, Yan JL, et al. : Study on cost-effectiveness of strategy of examination and therapy with IHA after mass questionnaire in middle-endemic areas of schistosomiasis. Chin J Schisto Cont. 2000;12(5):277–9. Reference Source [Google Scholar]
- 30. Tang DL, Yu DB, Li YY, et al. : Analysis of cost-effectiveness on two chemotherapy schemes to schistosomiasis in hyperendemic villages of Hunan province. Prac Prev Med. 2001;8:165–8. Reference Source [Google Scholar]
- 31. Yu D, Sarol JN, Jr, Hutton G, et al. : Cost-effectiveness analysis of the impacts on infection and morbidity attributable to three chemotherapy schemes against Schistosoma japonicum in hyperendemic areas of the Dongting Lake region, China. Southeast Asian J Trop Med Public Health. 2002;33(3):441–57. [PubMed] [Google Scholar]
- 32. Jiang SD, Gao B, Huang XH, et al. : Cost effectiveness analysis on the project of schistosomiasis control in marshland and regions. Chin J Schisto Cont. 2002;14:385–7. [Google Scholar]
- 33. Zhao ZY, Wu ZW, Peng XP, et al. : Rapid control strategy in new hilly endemic areas of schistosomiasis in Taoyuan County - cost-effectiveness analysis of intervention measures. Chin J Schisto Cont. 2003;15(5):354–7. Reference Source [Google Scholar]
- 34. Zhang HZ, Wang WL, Yu BG, et al. : Cost effectiveness and benefit analysis of World Bank Loan on schistosomiasis control in Qiangjiang City. Chin J Schisto Cont. 2003:204–6. [Google Scholar]
- 35. Zhou XN, Wang LY, Chen MG, et al. : An economic evaluation of the national schistosomiasis control programme in China from 1992 to 2000. Acta Trop. 2005;96(2–3):255–65. 10.1016/j.actatropica.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 36. Lin DD, Zeng XJ, Chen HG, et al. : [Cost-effectiveness and cost-benefit analysis on the integrated schistosomiasis control strategies with emphasis on infection source in Poyang Lake region]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27(4):297–302. [PubMed] [Google Scholar]
- 37. Li SM, Chen SJ, Wu XJ, et al. : [Evaluation on cost-effectiveness of snail control project by environmental modification in hilly regions]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2011;23(1):85–8. [PubMed] [Google Scholar]
- 38. Yu Q, Zhao GM, Hong XL, et al. : Impact and cost-effectiveness of a comprehensive Schistosomiasis japonica control program in the Poyang Lake region of China. Int J Environ Res Public Health. 2013;10(12):6409–21. 10.3390/ijerph10126409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu JY, Wang CX, Wang CF, et al. : [Cost-effectiveness of schistosomiasis comprehensive control in Lushan County from 2007 to 2012]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(5):513–5. [PubMed] [Google Scholar]
- 40. Zhang HM, Yu Q, Zhang X, et al. : [Cost-effectiveness evaluation on comprehensive control measures carrying out in schistosomiasis endemic areas with regard to different layers of administrative villages stratified by infection situation of human and domestic animals. I. Cost-effectiveness study in inner embankment of marshland and lake regions from 2006 to 2010]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2014;26(3):254–9. [PubMed] [Google Scholar]
- 41. Ya Y, Jian-Bing L, Hao L, et al. : [Cost-effectiveness of comprehensive schistosomiasis control strategy with focus on cattle and sheep removal in Junshan District, Yueyang City]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2017;30(1):14–7. 10.16250/j.32.1374.2017136 [DOI] [PubMed] [Google Scholar]
- 42. Hong-Chu W, Yu-Cai M, Zheng-Qiu Z, et al. : [Effect and cost-effectiveness of three commonly used molluscicides in largescale field application]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(6):619–24. 10.16250/j.32.1374.2018222 [DOI] [PubMed] [Google Scholar]
- 43. Jordan P: Schistosomiasis--research to control. Am J Trop Med Hyg. 1977;26(5 Pt 1):877–86. 10.4269/ajtmh.1977.26.877 [DOI] [PubMed] [Google Scholar]
- 44. Polderman AM: Cost-effectiveness of different ways of controlling intestinal schistosomiasis: a case study. Soc Sci Med. 1984;19(10):1073–80. 10.1016/0277-9536(84)90311-3 [DOI] [PubMed] [Google Scholar]
- 45. Guyatt HL, Evans D: Desirable characteristics of a schistosomiasis vaccine: some implications of a cost-effectiveness analysis. Acta Trop. 1995;59(3):197–209. 10.1016/0001-706x(95)91938-5 [DOI] [PubMed] [Google Scholar]
- 46. Evans DB, Guyatt HL: Human behaviour, cost-effectiveness analysis and research and development priorities: the case of a schistosomiasis vaccine. Trop Med Int Health. 1997;2(11):A47–54. [PubMed] [Google Scholar]
- 47. Guyatt HL, Chan MS: An investigation into the interaction between drug efficacy and drug price of praziquantel in determining the cost-effectiveness of school-targeted treatment for Schistosoma mansoni using a population dynamic model. Trop Med Int Health. 1998;3(6):425–35. 10.1046/j.1365-3156.1998.00248.x [DOI] [PubMed] [Google Scholar]
- 48. Carabin H, Guyatt H, Engels D: A comparative analysis of the cost-effectiveness of treatment based on parasitological and symptomatic screening for Schistosoma mansoni in Burundi. Trop Med Int Health. 2000;5(3):192–202. 10.1046/j.1365-3156.2000.00530.x [DOI] [PubMed] [Google Scholar]
- 49. Croce D, Porazzi E, Foglia E, et al. : Cost-effectiveness of a successful schistosomiasis control programme in Cambodia (1995-2006). Acta Trop. 2010;113(3):279–84. 10.1016/j.actatropica.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Warren KS, Bundy DAP, Anderson RM, et al. : Helminth infections.In: Disease Control Priorities in Developing Countries.Oxford: Oxford University Press.1993;131–60. [Google Scholar]
- 51. Carabin H, Chan MS, Guyatt HL: A population dynamic approach to evaluating the impact of school attendance on the unit cost and effectiveness of school-based schistosomiasis chemotherapy programmes. Parasitology. 2000;121(Pt 2):171–83. 10.1017/s0031182099006101 [DOI] [PubMed] [Google Scholar]
- 52. Guyatt HL, Brooker S, Kihamia CM, et al. : Evaluation of efficacy of school-based anthelmintic treatments against anaemia in children in the United Republic of Tanzania. Bull World Health Organ. 2001;79(8):695–703. [PMC free article] [PubMed] [Google Scholar]
- 53. Miguel E, Kremer M: Worms: Identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72(1):159–217. 10.1111/j.1468-0262.2004.00481.x [DOI] [Google Scholar]
- 54. Brooker S, Kabatereine NB, Fleming F, et al. : Cost and cost-effectiveness of nationwide school-based helminth control in Uganda: intra-country variation and effects of scaling-up. Health Policy Plan. 2008;23(1):24–35. 10.1093/heapol/czm041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. King CH, Olbrych SK, Soon M, et al. : Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5(9):e1321. 10.1371/journal.pntd.0001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lo NC, Bogoch II, Blackburn BG, et al. : Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Glob Health. 2015;3(10):e629–38. 10.1016/S2214-109X(15)00047-9 [DOI] [PubMed] [Google Scholar]
- 57. Lo NC, Lai YS, Karagiannis-Voules DA, et al. : Assessment of global guidelines for preventive chemotherapy against schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Infect Dis. 2016;16(9):1065–75. 10.1016/S1473-3099(16)30073-1 [DOI] [PubMed] [Google Scholar]
- 58. De Neve JW, Andriantavison RL, Croke K, et al. : Health, financial, and education gains of investing in preventive chemotherapy for schistosomiasis, soil-transmitted helminthiases, and lymphatic filariasis in Madagascar: A modeling study. PLoS Negl Trop Dis. 2018;12(12):e0007002. 10.1371/journal.pntd.0007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.[No authors listed]: How cost-effective would a vaccine be against schistosomiasis? CVI forum. 1996; (12):14. [PubMed] [Google Scholar]
- 60. Kirigia JM, Sambo LG, Kainyu LH: A cost-benefit analysis of preventive schistosomiasis interventions in Kenya. Afr J Health Sci. 2000;7(3–4):5–11. [PubMed] [Google Scholar]
- 61. Redekop WK, Lenk EJ, Luyendijk M, et al. : The Socioeconomic Benefit to Individuals of Achieving the 2020 Targets for Five Preventive Chemotherapy Neglected Tropical Diseases. PLoS Negl Trop Dis. 2017;11(1):e0005289. 10.1371/journal.pntd.0005289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen JE: Some potential economic benefits of eliminating mortality attributed to schistosomiasis in Zanzibar. Soc Sci Med. 1974;8(7):383–98. 10.1016/0037-7856(74)90124-3 [DOI] [PubMed] [Google Scholar]
- 63. Wiemer C: Optimal Disease-Control through Combined Use of Preventive and Curative Measures. J Dev Econ. 1987;25(2):301–19. 10.1016/0304-3878(87)90087-3 [DOI] [Google Scholar]
- 64. Liu GF, Li K, Zhang CH: [Snail control effect and eco-economical benefit of forest for snail control and schistosomiasis prevention in mountainous regions]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2011;23(4):386–9. [PubMed] [Google Scholar]
- 65. Tao HY, Xia A, Zhao YM, et al. : [Effect and cost-benefit of Oncomelania snail control by plowing and planting in Jiaobei Beach of Zhenjiang City]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2012;24(5):576–8. [PubMed] [Google Scholar]
- 66. Fenwick A: The costs and a cost-benefit analysis of an S. mansoni control programme on an irrigated sugar estate in northern Tanzania. Bull World Health Organ. 1972;47(5):573–8. [PMC free article] [PubMed] [Google Scholar]
- 67. Baird S, Hicks JH, Kremer M, et al. : Worms at Work: Long-run Impacts of a Child Health Investment. Q J Econ. 2016;131(4):1637–80. 10.1093/qje/qjw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li YS, Zhao ZY, Ellis M, et al. : Applications and outcomes of periodic epidemiological surveys for schistosomiasis and related economic evaluation in the People's Republic of China. Acta Trop. 2005;96(2–3):266–75. 10.1016/j.actatropica.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 69. Newall AT, Jit M, Hutubessy R: Are current cost-effectiveness thresholds for low- and middle-income countries useful? Examples from the world of vaccines. Pharmacoeconomics. 2014;32(6):525–31. 10.1007/s40273-014-0162-x [DOI] [PubMed] [Google Scholar]
- 70. Marseille E, Larson B, Kazi DS, et al. : Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–24. 10.2471/BLT.14.138206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leech AA, Kim DD, Cohen JT, et al. : Use and Misuse of Cost-Effectiveness Analysis Thresholds in Low- and Middle-Income Countries: Trends in Cost-per-DALY Studies. Value Health. 2018;21(7):759–61. 10.1016/j.jval.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hutubessy R, Chisholm D, Edejer TT: Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. 10.1186/1478-7547-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Woods B, Revill P, Sculpher M, et al. : Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value Health. 2016;19(8):929–35. 10.1016/j.jval.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shillcutt SD, Walker DG, Goodman CA, et al. : Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics. 2009;27(11):903–17. 10.2165/10899580-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ochalek J, Lomas J, Claxton K: Cost per DALY averted thresholds for low- and middle-income countries: evidence from cross country data.University of York, Centre for Health Economics, Working Paper 122.2015. Reference Source [Google Scholar]
- 76. Horton S: Cost-Effectiveness Analysis in Disease Control Priorities, Third Edition.In: rd, Jamison DT, Gelband H, et al. Disease Control Priorities: Improving Health and Reducing Poverty.Washington (DC): World Bank,2017. 10.1596/978-1-4648-0527-1_ch7 [DOI] [PubMed] [Google Scholar]
- 77. World Bank. World development report 1993: investing in health.New York: Oxford University Press.1993. Reference Source [Google Scholar]
- 78. The World Bank: GDP deflator (base year varies by country). Reference Source [Google Scholar]
- 79. Kjetland EF, Ndhlovu PD, Gomo E, et al. : Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593–600. 10.1097/01.aids.0000210614.45212.0a [DOI] [PubMed] [Google Scholar]
- 80. Kjetland EF, Ndhlovu PD, Mduluza T, et al. : Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72(3):311–9. [PubMed] [Google Scholar]
- 81. Wall KM, Kilembe W, Vwalika B, et al. : Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl Trop Dis. 2018;12(12):e0006902. 10.1371/journal.pntd.0006902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lenk EJ, Redekop WK, Luyendijk M, et al. : Productivity Loss Related to Neglected Tropical Diseases Eligible for Preventive Chemotherapy: A Systematic Literature Review. PLoS Negl Trop Dis. 2016;10(2):e0004397. 10.1371/journal.pntd.0004397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blas BL, Lipayon IL, Tormis LC, et al. : An attempt to study the economic loss arising from Schistosoma japonicum infection and the benefits derived from treatment. Southeast Asian J Trop Med Public Health. 2006;37(1):26–32. [PubMed] [Google Scholar]
- 84. Barbosa FS, Pereira Da Costa DP: Incapacitating effects of schistosomiasis mansoni on the productivity of sugar-cane cutters in northeastern Brazil. Am J Epidemiol. 1981;114(1):102–11. 10.1093/oxfordjournals.aje.a113156 [DOI] [PubMed] [Google Scholar]
- 85. Olveda RM, Tiu E, Fevidal P, Jr, et al. : Relationship of prevalence and intensity of infection to morbidity in schistosomiasis japonica: a study of three communities in Leyte, Philippines. Am J Trop Med Hyg. 1983;32(6):1312–21. 10.4269/ajtmh.1983.32.1312 [DOI] [PubMed] [Google Scholar]
- 86. Parker M: Re-assessing disability: the impact of schistosomal infection on daily activities among women in Gezira Province, Sudan. Soc Sci Med. 1992;35(7):877–90. 10.1016/0277-9536(92)90102-v [DOI] [PubMed] [Google Scholar]
- 87. Farooq M: A Possible Approach to the Evaluation of the Economic Burden Imposed on a Community by Schistosomiasis. Ann Trop Med Parasitol. 1963;57(3):323–31. 10.1080/00034983.1963.11686185 [DOI] [PubMed] [Google Scholar]
- 88. Fenwick A, Figenschou BH: The effect of Schistosoma mansoni infection of the productivity of cane cutters on a sugar estate in Tanzania. Bull World Health Organ. 1972;47(5):567–72. [PMC free article] [PubMed] [Google Scholar]
- 89. Ndamba J, Makaza N, Munjoma M, et al. : The physical fitness and work performance of agricultural workers infected with Schistosoma mansoni in Zimbabwe. Ann Trop Med Parasitol. 1993;87(6):553–61. 10.1080/00034983.1993.11812810 [DOI] [PubMed] [Google Scholar]
- 90. Foster R: Schistosomiasis on an irrigated estate in East Africa. III. Effects of asymptomatic infection on health and industrial efficiency. J Trop Med Hyg. 1967;70(8):185–95. Reference Source [Google Scholar]
- 91. Jia TW, Zhou XN, Wang XH, et al. : Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull World Health Organ. 2007;85(6):458–65. 10.2471/blt.06.033035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ezeamama AE, Bustinduy AL, Nkwata AK, et al. : Cognitive deficits and educational loss in children with schistosome infection-A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1):e0005524. 10.1371/journal.pntd.0005524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fitzpatrick C, Fleming FM, Madin-Warburton M, et al. : Benchmarking the Cost per Person of Mass Treatment for Selected Neglected Tropical Diseases: An Approach Based on Literature Review and Meta-regression with Web-Based Software Application. PLoS Negl Trop Dis. 2016;10(12):e0005037. 10.1371/journal.pntd.0005037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Salari P, Fürst T, Knopp S, et al. : Cost of interventions to control schistosomiasis: A systematic review of the literature. PLoS Negl Trop Dis. 2020;14(3):e0008098. 10.1371/journal.pntd.0008098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. World Health Organization: International Strategies for Tropical Disease Treatments - Experiences with Praziquantel - EDM Research Series No. 026,1998. Reference Source [Google Scholar]
- 96. Leslie J, Garba A, Boubacar K, et al. : Neglected tropical diseases: comparison of the costs of integrated and vertical preventive chemotherapy treatment in Niger. Int Health. 2013;5(1):78–84. 10.1093/inthealth/ihs010 [DOI] [PubMed] [Google Scholar]
- 97. Guyatt H: The cost of delivering and sustaining a control programme for schistosomiasis and soil-transmitted helminthiasis. Acta Trop. 2003;86(2–3):267–74. 10.1016/s0001-706x(03)00047-0 [DOI] [PubMed] [Google Scholar]
- 98. Wang XY, He J, Juma S, et al. : Efficacy of China-made praziquantel for treatment of Schistosomiasis haematobium in Africa: A randomized controlled trial. PLoS Negl Trop Dis. 2019;13(4):e0007238. 10.1371/journal.pntd.0007238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Turner HC, Truscott JE, Bettis AA, et al. : Evaluating the variation in the projected benefit of community-wide mass treatment for schistosomiasis: Implications for future economic evaluations. Parasit Vectors. 2017;10(1):213. 10.1186/s13071-017-2141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Toor J, Turner HC, Truscott JE, et al. : The design of schistosomiasis monitoring and evaluation programmes: The importance of collecting adult data to inform treatment strategies for Schistosoma mansoni. PLoS Negl Trop Dis. 2018;12(10):e0006717. 10.1371/journal.pntd.0006717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chan MS, Guyatt HL, Bundy DA, et al. : The development of an age structured model for schistosomiasis transmission dynamics and control and its validation for Schistosoma mansoni. Epidemiol Infect. 1995;115(2):325–44. 10.1017/s0950268800058453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chan MS, Anderson RM, Medley GF, et al. : Dynamic aspects of morbidity and acquired immunity in schistosomiasis control. Acta Trop. 1996;62(2):105–17. 10.1016/s0001-706x(96)00039-3 [DOI] [PubMed] [Google Scholar]
- 103. Guyatt H: Different approaches to modelling the cost-effectiveness of schistosomiasis control. Mem Inst Oswaldo Cruz. 1998;93(Suppl 1):75–84. 10.1590/s0074-02761998000700010 [DOI] [PubMed] [Google Scholar]
- 104. Coulibaly JT, N'Gbesso YK, Knopp S, et al. : Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6(12):e1917. 10.1371/journal.pntd.0001917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Keiser J, N'Goran EK, Traore M, et al. : Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Cote d'Ivoire. J Parasitol. 2002;88(3):461–6. 10.1645/0022-3395(2002)088[0461:PWSMGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 106. Utzinger J, N'Goran EK, Esse Aya CM, et al. : Schistosoma mansoni, intestinal parasites and perceived morbidity indicators in schoolchildren in a rural endemic area of western Cote d'Ivoire. Trop Med Int Health. 1998;3(9):711–20. 10.1046/j.1365-3156.1998.00292.x [DOI] [PubMed] [Google Scholar]
- 107. Raso G, Luginbuhl A, Adjoua CA, et al. : Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Cote d'Ivoire. Int J Epidemiol. 2004;33(5):1092–102. 10.1093/ije/dyh241 [DOI] [PubMed] [Google Scholar]
- 108. Becker SL, Sieto B, Silue KD, et al. : Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a Co-endemic setting. PLoS Negl Trop Dis. 2011;5(8):e1292. 10.1371/journal.pntd.0001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pullan RL, Smith JL, Jasrasaria R, et al. : Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. 10.1186/1756-3305-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fulford AJ, Butterworth AE, Ouma JH, et al. : A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitology. 1995;110( Pt 3):307–16. 10.1017/s0031182000080896 [DOI] [PubMed] [Google Scholar]
- 111. King CH, Magak P, Salam EA, et al. : Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Int Health. 2003;8(2):109–17. 10.1046/j.1365-3156.2003.00994.x [DOI] [PubMed] [Google Scholar]
- 112. Gurarie D, King CH, Yoon N, et al. : Refined stratified-worm-burden models that incorporate specific biological features of human and snail hosts provide better estimates of Schistosoma diagnosis, transmission, and control. Parasit Vectors. 2016;9(1):428. 10.1186/s13071-016-1681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. WHO Workshop. Quantitative aspects of the epidemiology of Schistosoma japonicum infection in a rural community of Luzon, Philippines. WHO workshop. Bull World Health Organ. 1980;58(4):629–38. [PMC free article] [PubMed] [Google Scholar]
- 114. Andrade G, Bertsch DJ, Gazzinelli A, et al. : Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11(2):e0005372. 10.1371/journal.pntd.0005372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Goldberg EM, Pigott D, Shirude S, et al. : Underestimation of the global burden of schistosomiasis - Authors' reply. Lancet. 2018;391(10118):308. 10.1016/S0140-6736(18)30124-7 [DOI] [PubMed] [Google Scholar]
- 116. King CH, Galvani AP: Underestimation of the global burden of schistosomiasis. Lancet. 2018;391(10118):307–8. 10.1016/S0140-6736(18)30098-9 [DOI] [PubMed] [Google Scholar]
- 117. Murray CJL, Lopez AD: The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020.Boston: Harvard School of Public Health,1996. Reference Source [Google Scholar]
- 118. King CH: Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113(2):95–104. 10.1016/j.actatropica.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Global Burden of Disease Study: Global Burden of Disease Study 2016 (GBD 2016) Disability Weights. Reference Source [Google Scholar]
- 120. Maertens de Noordhout C, Devleesschauwer B, Gielens L, et al. : Mapping EQ-5D utilities to GBD 2010 and GBD 2013 disability weights: results of two pilot studies in Belgium. Arch Public Health. 2017;75(1):6. 10.1186/s13690-017-0174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Turner HC, Lauer JA, Tran BX, et al. : Adjusting for Inflation and Currency Changes Within Health Economic Studies. Value Health. 2019;22(9):1026–32. 10.1016/j.jval.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 122. Husereau D, Drummond M, Petrou S, et al. : Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]
- 123. Ndyomugyenyi R, Lakwo T, Habomugisha P, et al. : Progress towards the elimination of onchocerciasis as a public-health problem in Uganda: opportunities, challenges and the way forward. Ann Trop Med Parasitol. 2007;101(4):323–33. 10.1179/136485907X176355 [DOI] [PubMed] [Google Scholar]
- 124. Katabarwa M, Mutabazi D, Richards F, Jr: The community-directed, ivermectin-treatment programme for onchocerciasis control in Uganda--an evaluative study (1993-1997). Ann Trop Med Parasitol. 1999;93(7):727–35. 10.1080/00034989957989 [DOI] [PubMed] [Google Scholar]
- 125. Katabarwa MN, Habomugisha P, Richards FO, Jr: Implementing community-directed treatment with ivermectin for the control of onchocerciasis in Uganda (1997-2000): an evaluation. Ann Trop Med Parasitol. 2002;96(1):61–73. 10.1179/000349802125000529 [DOI] [PubMed] [Google Scholar]
- 126. Turner HC, Toor J, Hollingsworth TD, et al. : Economic Evaluations of Mass Drug Administration: The Importance of Economies of Scale and Scope. Clin Infect Dis. 2018;66(8):1298–303. 10.1093/cid/cix1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Conteh L, Engels T, Molyneux DH: Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375(9710):239–47. 10.1016/S0140-6736(09)61422-7 [DOI] [PubMed] [Google Scholar]
- 128. Turner HC, Truscott JE, Fleming FM, et al. : Cost-effectiveness of scaling up mass drug administration for the control of soil-transmitted helminths: a comparison of cost function and constant costs analyses. Lancet Infect Dis. 2016;16(7):838–46. 10.1016/S1473-3099(15)00268-6 [DOI] [PubMed] [Google Scholar]
- 129. Keating J, Yukich JO, Mollenkopf S, et al. : Lymphatic filariasis and onchocerciasis prevention, treatment, and control costs across diverse settings: a systematic review. Acta Trop. 2014;135(0):86–95. 10.1016/j.actatropica.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 130. Brady MA, Hooper PJ, Ottesen EA: Projected benefits from integrating NTD programs in sub-Saharan Africa. Trends Parasitol. 2006;22(7):285–91. 10.1016/j.pt.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 131. Grimes JE, Croll D, Harrison WE, et al. : The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8(1):156. 10.1186/s13071-015-0766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hsieh MH, Brotherton JM, Siddiqui AA: Hepatitis B Vaccines and HPV Vaccines Have Been Hailed as Major Public Health Achievements in Preventing Cancer--Could a Schistosomiasis Vaccine be the Third? PLoS Negl Trop Dis. 2015;9(5):e0003598. 10.1371/journal.pntd.0003598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shuford KV, Turner HC, Anderson RM: Compliance with anthelmintic treatment in the neglected tropical diseases control programmes: a systematic review. Parasit Vectors. 2016;9:29. 10.1186/s13071-016-1311-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chan MS, Medley GF, Jamison D, et al. : The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109(Pt 3):373–87. 10.1017/s0031182000078410 [DOI] [PubMed] [Google Scholar]
- 135. Bustinduy AL, King CH: Parasitic Helminths. In: Fratamico PM, Smith JL, Brogden KA. Post-Infectious Sequelae and Long-Term Consequences of Infectious Diseases.Washington: American Society for Microbiology Press.2009;291–329. 10.1128/9781555815486.ch17 [DOI] [Google Scholar]
- 136. Gower CM, Vince L, Webster JP: Should we be treating animal schistosomiasis in Africa? The need for a One Health economic evaluation of schistosomiasis control in people and their livestock. Trans R Soc Trop Med Hyg. 2017;111(6):244–7. 10.1093/trstmh/trx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pennance T, Person B, Muhsin MA, et al. : Urogenital schistosomiasis transmission on Unguja Island, Zanzibar: characterisation of persistent hot-spots. Parasit Vectors. 2016;9(1):646. 10.1186/s13071-016-1847-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wiegand RE, Mwinzi PNM, Montgomery SP, et al. : A Persistent Hotspot of Schistosoma mansoni Infection in a Five-Year Randomized Trial of Praziquantel Preventative Chemotherapy Strategies. J Infect Dis. 2017;216(11):1425–33. 10.1093/infdis/jix496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kittur N, King CH, Campbell CH, et al. : Persistent Hotspots in Schistosomiasis Consortium for Operational Research and Evaluation Studies for Gaining and Sustaining Control of Schistosomiasis after Four Years of Mass Drug Administration of Praziquantel. Am J Trop Med Hyg. 2019;101(3):617–27. 10.4269/ajtmh.19-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Crellen T, Walker M, Lamberton PH, et al. : Reduced Efficacy of Praziquantel Against Schistosoma mansoni Is Associated With Multiple Rounds of Mass Drug Administration. Clin Infect Dis. 2016;63(9):1151–9. 10.1093/cid/ciw506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lamberton PH, Hogan SC, Kabatereine NB, et al. : In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am J Trop Med Hyg. 2010;83(6):1340–7. 10.4269/ajtmh.2010.10-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tchuem Tchuenté LA, Rollinson D, Stothard JR, et al. : Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty. 2017;6(1):42. 10.1186/s40249-017-0256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Anderson RM, Turner HC, Farrell SH, et al. : Studies of the Transmission Dynamics, Mathematical Model Development and the Control of Schistosome Parasites by Mass Drug Administration in Human Communities. Adv Parasitol. 2016;94:199–246. 10.1016/bs.apar.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 144. Knopp S, Person B, Ame SM, et al. : Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: a cluster-randomised trial. Lancet Glob Health. 2019;7(8):e1118–e29. 10.1016/S2214-109X(19)30189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mekete K, Ower A, Dunn J, et al. : The Geshiyaro Project: a study protocol for developing a scalable model of interventions for moving towards the interruption of the transmission of soil-transmitted helminths and schistosome infections in the Wolaita zone of Ethiopia. Parasit Vectors. 2019;12(1):503. 10.1186/s13071-019-3757-4 [DOI] [PMC free article] [PubMed] [Google Scholar]