Abstract
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening diseases characterized by detachment of the epidermis and mucous membrane. SJS/TEN are considered to be on the same spectrum of diseases with different severities. They are classified by the percentage of skin detachment area. SJS/TEN can also cause several complications in the liver, kidneys, and respiratory tract. The pathogenesis of SJS/TEN is still unclear. Although it is difficult to diagnose early stage SJS/TEN, biomarkers for diagnosis or severity prediction have not been well established. Furthermore, optimal therapeutic options for SJS/TEN are still controversial.
Several drugs, such as carbamazepine and allopurinol, are reported to have a strong relationship with a specific human leukocyte antigen (HLA) type. This relationship differs between different ethnicities. Recently, the usefulness of HLA screening before administering specific drugs to decrease the incidence of SJS/TEN has been investigated.
Skin detachment in SJS/TEN skin lesions is caused by extensive epidermal cell death, which has been considered to be apoptosis via the Fas-FasL pathway or perforin/granzyme pathway. We reported that necroptosis, i.e. programmed necrosis, also contributes to epidermal cell death. Annexin A1, released from monocytes, and its interaction with the formyl peptide receptor 1 induce necroptosis. Several diagnostic or prognostic biomarkers for SJS/TEN have been reported, such as CCL-27, IL-15, galectin-7, and RIP3.
Supportive care is recommended for the treatment of SJS/TEN. However, optimal therapeutic options such as systemic corticosteroids, intravenous immunoglobulin, cyclosporine, and TNF-α antagonists are still controversial. Recently, the beneficial effects of cyclosporine and TNF-α antagonists have been explored. In this review, we discuss recent advances in the pathophysiology and management of SJS/TEN.
Keywords: Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, drug reaction, necroptosis
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe and life-threatening mucocutaneous reactions characterized by blisters and skin detachment. Drugs and infection, such as by Mycoplasma or the herpes simplex virus, are the main causes 1.
SJS/TEN are considered to be on the same spectrum of diseases with different severities. They are classified by the percentage of skin detachment area ( Table 1) 2. Although a study in the USA indicated that the incidence rate is 1.58 to 2.26 cases/million people, the overall incidence of SJS/TEN remains unclear. Contrary to its low incidence rate, the mortality rate is high (SJS: 4.8%, TEN: 14.8%) 3. Furthermore, even after recovery, sequelae such as blindness remain in some cases 1. Thus, patients with SJS/TEN should be accurately diagnosed, and appropriate treatment should commence as soon as possible. Therefore, a biomarker for early diagnosis and severity prediction is necessary. Further issues include the lack of evidence regarding the adequate management of SJS/TEN.
Table 1. Classification of SJS/TEN.
| Diagnosis | Skin detachment area (%) |
|---|---|
| SJS | <10 |
| SJS/TEN overlap | 10–30 |
| TEN | >30 |
SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis
In this review, we describe recent advances in the research and management of SJS/TEN.
Clinical features
The cutaneous symptoms for SJS/TEN are a painful erythematous rash, bullae, and erosion appearing on the face and trunk and spreading to the extremities. The early skin lesions appear as round lesions with only two nonpalpable zones with an indistinct border and are called “atypical targets”. Skin lesions typically test positive for the Nikolsky sign, which manifests with skin erosion upon gentle pressure 4. Malaise, fever, and upper respiratory tract symptoms often precede the onset of the skin rash by a few days. Almost all patients with SJS/TEN develop mucosal involvement of the eyes, mouth, and genitalia 5. The involvement of the eyes often relates to sequelae such as dry eyes, visual acuity, conjunctivitis, corneal erosions, and trichiasis. In severe cases, ocular sequelae can reach as far as blindness.
The severity-of-illness score for TEN (SCORTEN) is widely used to predict mortality for SJS/TEN 6. SCORTEN should be assessed within the first 24 hours after admission and again on day 3. SCORTEN is based on seven independent risk factors ( Table 2). The more risk factors that are present, the higher the mortality rate ( Table 3).
Table 2. Risk factors for SCORTEN.
| Age over 40 years |
| Heart rate >120 beats per minute |
| Presence of cancer or hematologic malignancy |
| Epidermal detachment area involving body surface area >10% |
| Blood urea nitrogen >28 mg/dL (10 mmol/L) |
| Blood glucose >252 mg/dL (14 mmol/L) |
| Bicarbonate <20 mEq/L |
SCORTEN, Score of Toxic Epidermal Necrosis
Table 3. Mortality rate in SCORTEN.
| Number of risk factors | Mortality rate (%) |
|---|---|
| 0–1 | 3.2 |
| 2 | 12.1 |
| 3 | 35.3 |
| 4 | 58.3 |
| ≥5 | 90 |
SCORTEN, Score of Toxic Epidermal Necrosis
SJS/TEN are mainly drug-induced diseases. The most frequently causative drugs include antibiotics, allopurinol, non-steroidal anti-inflammatory drugs, and antiepileptic drugs ( Table 4) 7.
Table 4. Medications associated with high risk of SJS/TEN.
| Nevirapine |
| Lamotrigine |
| Carbamazepine |
| Phenytoin |
| Cotrimoxazole and other anti-infective sulfonamides |
| Sulfasalazine |
| Allopurinol |
| Oxicam/NSAIDs |
NSAID, non-steroidal anti-inflammatory drug; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis
Genetic factors
There is increasing evidence of a genetic contribution to the incidence of cutaneous adverse reactions. In 2004, Chung et al. reported on a strong relationship between human leukocyte antigen (HLA)-B*15:02 and carbamazepine (CBZ)-induced SJS/TEN in a Han Chinese population 8. HLA alleles are divided into class I and class II, and they are specialized to present antigenic peptides to T cells, resulting in the activation of the immune response. In this study, 44 patients with CBZ-induced SJS/TEN were included, and all patients had the HLA-B*15:02 allele (100%). Following this, similar studies reported the relationship between CBZ-induced SJS/TEN and the HLA-B*15:02 allele in Asian populations including those in China, Thailand, Malaysia, and India 9– 20.
The relationship between SJS/TEN and HLA-B*15:02 has also been demonstrated in aromatic antiepileptic drugs other than CBZ. Although the incidence was lower than that seen with CBZ, HLA-*15:02 showed a strong association with phenytoin-, lamotrigine-, and oxcarbazepine-induced SJS/TEN 11, 21– 25. Conversely, there was no association between CBZ-induced SJS/TEN and HLA-B*15:02 in Japanese, Korean, and European populations 26– 32.
Ozeki et al. discovered that HLA-A*31:01 is also associated with CBZ-induced SJS/TEN 33. HLA-A*31:01 revealed a relationship with CBZ-induced SJS/TEN not only in Japanese but also in Korean and European populations 14, 32, 34, 35. Although the majority of CBZ-induced SJS/TEN is associated with HLA-B*15:02 in Asian populations, the association with HLA-A*31:01 is shown in multiethnic populations. Thus, the HLA association in SJS/TEN is different among different ethnicities.
In 2008, the US Food and Drug Administration released a recommendation to perform HLA-B*15:02 genotyping before administering CBZ 36. In Taiwan, it is reported that HLA-B*15:02 screening is strongly associated with a decrease in the incidence of CBZ-induced SJS/TEN 37.
As well as antiepileptic drugs, several other drugs, such as allopurinol and abacavir, have been reported to have HLA associations. Allopurinol is an anti-hyperuricemia drug which is a major cause of SJS/TEN. The relationship between HLA-B*58:01 and allopurinol-induced SJS/TEN has been reported in many ethnicities, including in Taiwanese, Japanese, Korean, Thai, and European individuals 26, 28, 30, 38– 45. Therefore, these data suggested that HLA-B*58:01 genotyping may be useful to prevent allopurinol-induced SJS/TEN.
Cost-effectiveness analysis of HLA-B*58:01 screening in Taiwan suggested a cost-saving effect in preventing allopurinol-induced SJS/TEN 46. In a US study, it was suggested that testing for HLA-B*5801 prior to allopurinol initiation is cost effective for Asians and African Americans but not for Caucasians or Hispanics 47.
Abacavir, a nucleoside reverse transcriptase inhibitor used to treat HIV infection, is reported to induce SJS/TEN in patients carrying HLA-B*57:01 48– 52. In 2008, HLA-B*57:01 screening was added to clinical care guidelines to reduce the risk of hypersensitivity reaction from abacavir 53. The frequency of HLA-B*57:01 screening then increased steadily, and the incidence of abacavir-induced SJS/TEN was decreased 54. However, many patients have not undergone HLA-B*57:01 screening. The expansion of HLA*B-57:01 screening is expected to reduce the incidence of abacavir-induced SJS/TEN.
Cytochrome P (CYP) is also an important genetic factor. CYPs are involved in drug metabolism. CYP450 genes have 57 variants, and each variant shows functional differences. Patients whose drug metabolism is slow because of CYP450 variants have a high risk of developing adverse drug reactions 55. Chung et al. discovered specific genetic factors associated with phenytoin-induced SJS/TEN 56. In this study, 16 significant single nucleotide polymorphisms in CYP2C9 were identified. Patients with phenytoin-induced SJS/TEN who had CYP2C9*3 showed a delayed clearance of phenytoin, resulting in increased disease severity.
Pathogenesis and diagnostic biomarkers
Immunopathogenesis
SJS/TEN is traditionally thought to be a T-cell-mediated disorder. T cells are activated by binding of drugs to T cell receptors (TCRs) from antigen-presenting cells (APCs). There are currently three hypotheses on T cell activation 57– 59 ( Figure 1): (1) the hapten/pro-hapten model, (2) the pharmacological interaction (p-i) concept, and (3) the altered peptide model. The majority of drugs and their metabolites are pro-haptens and do not act as haptens themselves. They acquire the immunogenicity by covalently binding to carrier proteins (hapten antigen). Hapten antigens form a complex with HLA in APCs and are recognized by TCRs. This stimulation triggers the drug-specific T cell activation. In this model, antigenic drugs are covalently bound to peptides presented by HLA molecules to TCRs 60– 63. However, some drugs can non-covalently bind directly to HLA and/or TCRs. This type of binding is termed the p-i concept. CBZ, lamotrigine, sulfamethoxazole, and celecoxib are known to fit this model 64– 68. In general, HLA polymorphisms are dependent on the antigen-binding cleft. It has been reported that unmodified abacavir binds to the antigen-binding cleft lying in the bottom of HLA-B*57:01 and changes the shape and chemistry of the antigen-binding cleft, altering the repertoire of endogenous peptides that can bind HLA-B*57:01 (altered peptide) 69, 70. The TCR profile is also associated with the development of SJS/TEN. Ko et al. identified the VB-11-ISGSY clonotype in 84% of patients with CBZ-associated SJS/TEN 71, 72. This clonotype was not present in CBZ-tolerant patients. The clonotype specificity is also reported in oxypurinol-induced SJS/TEN 73. Recently, Pan et al. investigated the TCR repertoire through next-generation sequencing and identified a public αβTCR from the cytotoxic T cells of patients with CBZ-induced SJS/TEN. This public αβTCR can bind with CBZ and mediate an immune response 74.
Figure 1. Models of T cell activation in Stevens-Johnson syndrome/toxic epidermal necrolysis.
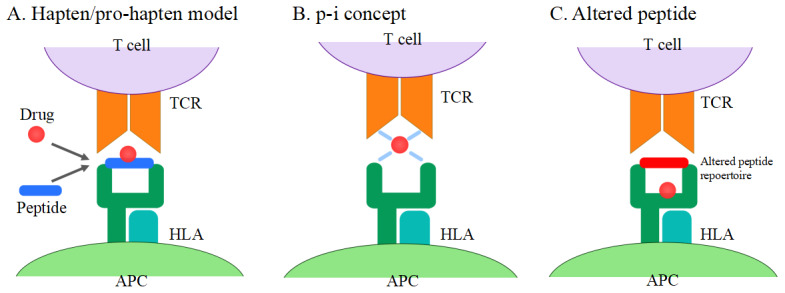
( A) Hapten/pro-hapten model: drugs or drug metabolites form a complex with carrier proteins and are presented as haptenated peptides in the peptide-binding groove of the HLA molecules. ( B) p-i concept: drugs directly bind to HLA and TCR non-covalently. ( C) Altered peptide model: drugs bind to the peptide-binding groove of HLA, resulting in the alteration of the HLA-binding peptide repertoire. APC, antigen-presenting cell; HLA, human leukocyte antigen; TCR, T cell receptor.
In the early stages of the disease, cytotoxic CD8 + T cells mainly infiltrate blister fluid and the epidermis, and CD4 + T cells mostly infiltrate the dermis 75, 76. Monocytes are present in the epidermis of TEN patients. In the later stages, lymphocytes are decreased and an increased number of monocytes is observed. Tohyama et al. reported that monocytes play an important role in epidermal damage, probably by enhancing the cytotoxicity of CD8 + T cells 77. In the serum and blister fluid of SJS/TEN patients, increased levels of soluble IL-2 receptors were observed 78. Soluble IL-2 receptors are a marker for activated T cells, indicating the importance of activated cytotoxic CD8 + T cells in the pathogenesis of SJS/TEN.
Keratinocyte death
The epidermal damage in the skin lesions of SJS/TEN patients is considered to be of apoptotic origin 79. Apoptosis is induced by cytotoxic CD8 + T cells through the Fas-Fas ligand (FasL) pathway or the perforin/granzyme pathway 80.
Cytotoxic CD8 + T cells and natural killer (NK) cells produce FasL, which binds Fas on target cells. Recognition of FasL causes activation of the caspase cascade and the resulting cells undergo apoptosis 80. Under normal conditions, Fas is present on the surface of keratinocytes and FasL is expressed intracellularly. FasL is transported to the cell surface when the cell needs to self-destruct 81. Viard et al. demonstrated that the cell surface of keratinocytes of TEN patients has FasL on it but not the keratinocytes of patients with maculopapular drug reactions 82. In addition, high levels of soluble FasL (sFasL) were found in the serum of TEN patients. sFasL also has the potential to mediate apoptosis 83.
We showed that FasL serum levels increased in patients with TEN 84, 85. This study revealed that sFasL was produced by peripheral blood mononuclear cells (PBMCs) when the causative drugs were added. sFasL released from PBMCs binds to Fas expressed on keratinocytes to cause apoptosis. This study suggested that elevated levels of serum sFasL may be a useful diagnostic marker for SJS/TEN. However, a correlation between sFasL levels and disease severity has not been established 84, 86.
Nassif et al. emphasized the importance of the perforin/granzyme pathway 87, 88. Upon recognition of a target cell, the cytotoxic CD8 + T cell releases perforin and granzyme B 80. This study revealed that mononuclear cells in the blister fluid of TEN patients have cytotoxic effects in the presence of a causative drug. This cytotoxicity is blocked by the perforin/granzyme pathway inhibitor. These findings suggest that the perforin/granzyme pathway causes epidermal damage in the skin lesions of SJS/TEN 87, 88.
In 2008, Chung et al. demonstrated the cytotoxic effect of granulysin in SJS/TEN 89. Granulysin is a pro-apoptotic protein which permits cell-mediated cytotoxicity without direct cell-to-cell contact. In SJS/TEN blisters, high levels of granulysin are detected. Granulysin is released from blister cells in skin lesions of SJS/TEN including cytotoxic CD8 + T cell and NK cells. The severity of the cutaneous lesions correlated with serum granulysin levels. We also reported granulysin as an early diagnostic marker 90. However, serum granulysin levels are also elevated in patients with drug-induced hypersensitivity syndrome/drug reactions with eosinophilia and systemic symptoms, which are other types of severe cutaneous adverse drug reaction characterized by a viral infection 91. Therefore, it is difficult to use granulysin as an SJS/TEN-specific biomarker.
In 2014, we reported that necroptosis induced by annexin A1–formyl peptide receptor 1 (FPR1) interaction contributes to keratinocyte death in SJS/TEN 92. Necroptosis is a type of programmed cell death which reveals morphological necrosis. Necroptotic cells release damage-associated molecular patterns (DAMPs), including a range of pro-inflammatory cytokines, resulting in inflammation, unlike apoptosis. Apoptotic cells are quickly phagocytosed by macrophages and degraded within phagolysosomes. No inflammatory reaction occurs with the process of apoptosis or with the removal of apoptotic cells 93. In general, necroptosis occurs through the stimulation of TNF-α under conditions in which apoptosis is blocked. In TNF-α stimulation, receptor interacting kinase 1 (RIP1) and receptor interacting kinase 3 (RIP3) are phosphorylated and form a “necrosome” complex. Furthermore, the mixed lineage kinase domain-like (MLKL) pseudokinase is recruited to the necrosome and phosphorylated by RIP3. The phosphorylated MLKL (pMLKL) is localized to the plasma membrane and induces cell death 93. Supernatant from PBMCs, which are exposed to the causative drug in SJS/TEN patients, induces the death of SJS/TEN keratinocytes. This cytotoxicity is blocked by necrostatin-1, a specific inhibitor of RIP1. In SJS/TEN skin lesions, keratinocytes express abundant FPR1 and monocytes secrete annexin A1. The interaction of annexin A1 and FPR1 induces necrosome formation ( Figure 2). Inhibition of necroptosis completely prevents SJS/TEN-like responses in a mouse model of SJS/TEN 92, 94. Therefore, these results suggest that necroptosis plays an important role in the pathogenesis of SJS/TEN.
Figure 2. Necroptosis pathway in Stevens-Johnson syndrome/toxic epidermal necrolysis.
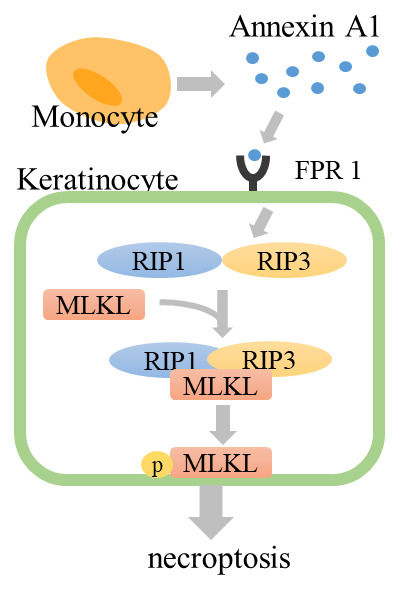
Drug-stimulated monocytes secrete annexin A1. Annexin A1 binds to FPR1, RIP1 and RIP3 form the necrosome, and MLKL is phosphorylated by RIP3. Phosphorylated MLKL translocates to the plasma membrane and induces cell death. FPR1, formyl peptide receptor 1; MLKL, mixed lineage kinase domain-like; RIP1, receptor interacting kinase 1.
Diagnostic biomarkers
Although SJS/TEN is a severe disease, clinical manifestations of early stage SJS/TEN are occasionally undistinguishable from those of maculopapular exanthema and erythema multiforme. However, useful biomarkers for the diagnosis or the prediction of severity have not been well established. Recently, some researchers discovered useful diagnostic or prognostic biomarkers for SJS/TEN. These biomarkers are now in the research phase and have not been used in the clinic yet.
Wang et al. revealed an increased concentration of CCL-27 in the serum of SJS/TEN patients, which correlated with disease activity 95, 96. CCL-27 is reported to be associated with cutaneous inflammatory diseases by regulating the trafficking of T cells to the skin 97. Tapia et al. found CCL-27 was highly expressed in the skin lesions of SJS/TEN patients 98. Wang et al. hypothesized that CCL-27 is produced by keratinocytes in the skin lesions found in SJS/TEN and released into the circulation.
Su et al. reported that interleukin-15 (IL-15) is associated with mortality and severity in SJS/TEN by measuring 28 serological factors using multiplex immunoassay or ELISA 99. They also revealed that IL-15 contributes to TEN severity by enhancing NK- and T-cell-mediated responses. IL-15 is known to induce the production of TNF-α and downstream cytokines/chemokines 100. The elevation of many cytokines/chemokines in SJS/TEN might be a secondary effect derived from IL-15.
We identified galectin-7 as a diagnostic biomarker using proteomics analysis 101. We hypothesized that certain soluble factors could be secreted only by drug-specific lymphocytes in SJS/TEN patients and not in those with a non-severe cutaneous adverse drug reaction. Hence, these soluble factors could be biomarkers for SJS/TEN. PBMCs from patients with SJS/TEN were cultured with the causative drugs and supernatant was collected. The elevated proteins in the supernatant underwent proteomic analysis 102. Hama, Nishimura, and colleagues concluded that this method allowed for the identification of new SJS/TEN-specific biomarkers that are not known to be associated with the pathogenesis of this condition.
Very recently, we focused on the mechanisms of epidermal necroptosis and identified serum RIP3 as a key mediator of necroptosis and as a diagnostic and severity marker 103. It is reported that the expression of RIP3 increased in cells undergoing necroptosis 104. We revealed that the expression of RIP3 increased in necroptotic keratinocytes as well, and the levels of serum RIP3 were high in the acute phase of patients with SJS/TEN. We also indicated that serum RIP3 levels may correlate with disease activity.
Management
In SJS/TEN patients, the epidermal and mucosal membranes are predominantly affected. However, SJS/TEN can also cause complications in several organs, such as the liver, kidneys, and respiratory tract. Thus, multidisciplinary assessment and early management in a specialized hospital environment are key for improving mortality.
Immediate discontinuation of suspected causative drugs is crucial in the initial management of SJS/TEN. In addition, supportive care including fluid replacement 105, nutritional assessment 106, pain relief 107, and supplemental oxygen is necessary. Since infection from skin detachment is a common complication in SJS/TEN patients and it is associated with the impairment of re-epithelialization and may lead to sepsis, daily skin care should be performed. Antibiotic treatment should be given when cutaneous infection is clinically suspected 108.
The optimal therapeutic strategy in SJS/TEN is still controversial 109. Although there have been some reports of benefits with the use of systemic corticosteroids, intravenous immunoglobulins (IVIGs), cyclosporine, TNF-α antagonists (infliximab and etanercept), and plasmapheresis (PP) 110, 111, evidence for systemic treatment is still insufficient. UK guidelines for the management of SJS/TEN, published in 2016, concluded that withdrawal of the culprit drug and multidisciplinary supportive care are prioritized over systemic treatment because of the lack of evidence to demonstrate the benefits of the latter 112. However, in Japanese guidelines for SJS/TEN, published in 2016, systemic treatment is prioritized over supportive care alone. This guideline recommended early initiation of systemic corticosteroid therapy as a first-line treatment. A combination of IVIG or PP therapy is added to systemic corticosteroid therapy if the clinical symptoms are severe or the disease is refractory to systemic corticosteroid alone.
The efficacy of systemic therapy may depend on the disease phase. For example, in the acute phase, immunosuppressive treatments are considered to be suitable since a strong inflammation-like “cytokine storm” occurs in the patient. However, at the peak period during which wide skin detachment develops, strong immunosuppressive treatment may avoid re-epithelization and increase the risk of infection. Previous studies have not considered this point and included all phase results, leading to discrepant results. We introduce each treatment below.
Systemic corticosteroids
Previous studies revealed that treatment with corticosteroids in SJS/TEN patients increased the risk of infection and overall complications, including higher mortality 113– 115. Analyses and systematic reviews have not revealed a survival advantage of systemic corticosteroids 116– 118.
However, recent studies suggested a beneficial role for corticosteroid treatment. A European multicenter retrospective study and recent meta-analysis of observational studies showed the beneficial effects of corticosteroids 110, 119. An observational study reported that the short-term use of high-dose corticosteroids in the early stages of SJS/TEN reduced mortality without increasing the risk of infection 120. Since cutaneous infection is the most important point in the use of corticosteroids for SJS/TEN patients, short-term use of corticosteroids, improvement of infection control, and wound management are necessary to decrease the mortality rate.
IVIG
IVIG has been widely used for patients with SJS/TEN. However, the mechanisms of IVIG treatment remain unknown. While some case reports concluded that IVIG did not confer a beneficial effect in decreasing mortality 121– 123, there are some reports which revealed that IVIG had some beneficial effects for patients with SJS/TEN 124– 128. In the largest retrospective study in this field, the European Study of Severe Cutaneous Adverse Reactions (EuroSCAR), IVIG did not improve mortality compared with supportive care alone 119. However, recent meta-analyses have shown that high-dose IVIG (<2 g/kg) has a beneficial effect in decreasing the mortality of SJS/TEN 129. Thus, the use of IVIG for SJS/TEN patients is still controversial. Randomized controlled trials are required.
Cyclosporine
Cyclosporine, a calcineurin inhibitor, has been reported to have a therapeutic benefit in SJS/TEN. Cyclosporine affects T-lymphocyte-mediated cytotoxicity and inhibits FasL, nuclear factor-kB, and TNF-α 130. Some case reports and meta-analyses have shown that cyclosporine treatment improved mortality in SJS/TEN patients 131– 138. Gilbert and Scherrer reported that cyclosporine appears to have not only a mortality benefit in the treatment of SJS/TEN but also few side effects 139. These data support a potential role for cyclosporine in the treatment of SJS/TEN. However, the number of reported patients is small. Further studies are required to validate the efficacy of cyclosporine.
Plasmapheresis
Several case series have shown that PP is effective for the treatment of SJS/TEN 140– 145. The purpose of PP is to remove pathogenic factors such as a drug, drug metabolites, and disease-induced cytokines/chemokines from the patient’s blood. PP sessions are carried out every other day or daily. PP is a safe treatment and can be performed with few adverse side effects. Although one observational study has concluded PP treatment to be ineffective, the overall survival in this study was 87.5% 146. Narita et al. reported that PP was effective in TEN patients who were refractory to supportive therapy or systemic corticosteroid therapy and revealed that cytokine serum levels decreased after PP 147.
A study suggested a beneficial effect of combined PP and IVIG therapy 148. However, another study reported negative results for the combined therapy while treatment with PP alone revealed a good result 149. Randomized studies are needed to further define its usefulness.
Tumor necrosis factor inhibitors
Owing to the skin lesions and blister fluid in SJS/TEN containing high levels of TNF-α 150, 151, TNF-α inhibitors such as etanercept and infliximab have been used and, in some cases, beneficial effects have been suggested 152– 159. However, only a small number of cases have reported the use of TNF-α inhibitors for SJS/TEN. Additional studies are required to confirm the efficacy of these drugs in the treatment of SJS/TEN.
Future directions
Rapid withdrawal of the culprit drug and intensive supportive care are the basis of treatment for SJS/TEN. The use of systemic corticosteroids and IVIG is still controversial. However, recently, there has been an increasing number of studies suggesting the efficacy of cyclosporine or TNF-α inhibitors. Accumulating evidence of these treatments is desirable. In addition, the pathogenesis of SJS/TEN has been elucidated. It is hoped that this research will lead to the discovery of new therapeutic targets.
Conclusions
This review summarizes recent advances in the pathophysiology, diagnosis, and treatment of SJS/TEN. SJS/TEN is a severe disease which has a high mortality rate. However, its diagnostic method and treatment algorithm have not been well established. Further studies to elucidate the pathogenesis of SJS/TEN are needed.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Julia Spoendlin, Basel Pharmacoepidemiology Unit, Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland
Marc Vocanson, CIRI, International Center for Infectiology Research, Université de Lyon, Lyon, France; CIRI-INSERM U1111, Lyon, France
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, et al. : Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–76. 10.1007/s12016-017-8654-z [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 2. Bastuji-Garin S, Rzany B, Stern RS, et al. : Clinical Classification of Cases of Toxic Epidermal Necrolysis, Stevens-Johnson Syndrome, and Erythema Multiforme. Arch Dermatol. 1993;129(1):92–6. 10.1001/archderm.1993.01680220104023 [DOI] [PubMed] [Google Scholar]
- 3. Hsu DY, Brieva J, Silverberg NB, et al. : Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J Invest Dermatol. 2016;136(7):1387–97. 10.1016/j.jid.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 4. Griffiths C, Barker J, Bleiker T, et al. : Rook’s Textbook of Dermatology. 9th ed. Wiley.2016. 10.1002/9781118441213 [DOI] [Google Scholar]
- 5. Revuz J, Penso D, Roujeau JC, et al. : Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987;123(9):1160–5. 10.1001/archderm.1987.01660330071012 [DOI] [PubMed] [Google Scholar]
- 6. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. : SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–53. 10.1046/j.1523-1747.2000.00061.x [DOI] [PubMed] [Google Scholar]
- 7. Mockenhaupt M, Viboud C, Dunant A, et al. : Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study. J Invest Dermatol. 2008;128(1):35–44. 10.1038/sj.jid.5701033 [DOI] [PubMed] [Google Scholar]
- 8. Chung WH, Hung SI, Hong HS, et al. : Medical genetics: A marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. 10.1038/428486a [DOI] [PubMed] [Google Scholar]
- 9. Hung SI, Chung WH, Jee SH, et al. : Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. 10.1097/01.fpc.0000199500.46842.4a [DOI] [PubMed] [Google Scholar]
- 10. Man CBL, Kwan P, Baum L, et al. : Association between HLA-B*1502 Allele and Antiepileptic Drug-Induced Cutaneous Reactions in Han Chinese. Epilepsia. 2007;48(5):1015–8. 10.1111/j.1528-1167.2007.01022.x [DOI] [PubMed] [Google Scholar]
- 11. Locharernkul C, Loplumlert J, Limotai C, et al. : Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49(12):2087–91. 10.1111/j.1528-1167.2008.01719.x [DOI] [PubMed] [Google Scholar]
- 12. Mehta T, Prajapati L, Mittal B, et al. : Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol. 2009;75(6):579–82. 10.4103/0378-6323.57718 [DOI] [PubMed] [Google Scholar]
- 13. Ou GJ, Wang J, Ji X, et al. : A study of HLA-B*15: 02 in 9 different Chinese ethnics: Implications for carbamazepine related SJS/TEN. HLA. 2017;89(4):225–9. 10.1111/tan.12970 [DOI] [PubMed] [Google Scholar]
- 14. Khor AHP, Lim KS, Tan CT, et al. : HLA-A*31: 01 and HLA-B*15: 02 association with Stevens-Johnson syndrome and toxic epidermal necrolysis to carbamazepine in a multiethnic Malaysian population. Pharmacogenet Genomics. 2017;27(7):275–8. 10.1097/FPC.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Wang J, Zhao LM, et al. : Strong association between HLA-B*1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol. 2011;67(9):885–7. 10.1007/s00228-011-1009-4 [DOI] [PubMed] [Google Scholar]
- 16. Wu XT, Hu FY, An DM, et al. : Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy Behav. 2010;19(3):405–8. 10.1016/j.yebeh.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Zhou JQ, Zhou LM, et al. : Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure. 2011;20(6):446–8. 10.1016/j.seizure.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 18. Tassaneeyakul W, Tiamkao S, Jantararoungtong T, et al. : Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51(5):926–30. 10.1111/j.1528-1167.2010.02533.x [DOI] [PubMed] [Google Scholar]
- 19. Chang CC, Too CL, Murad S, et al. : Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol. 2011;50(2):221–4. 10.1111/j.1365-4632.2010.04745.x [DOI] [PubMed] [Google Scholar]
- 20. Then SM, Rani ZZM, Raymond AA, et al. : Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol. 2011;29(3):290–3. [PubMed] [Google Scholar]
- 21. Hung SI, Chung WH, Liu ZS, et al. : Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11(3):349–56. 10.2217/pgs.09.162 [DOI] [PubMed] [Google Scholar]
- 22. Chen CB, Hsiao YH, Wu T, et al. : Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. 2017;88(1):78–86. 10.1212/WNL.0000000000003453 [DOI] [PubMed] [Google Scholar]
- 23. Chang CC, Ng CC, Too CL, et al. : Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharmacogenomics J. 2017;17(2):170–3. 10.1038/tpj.2016.10 [DOI] [PubMed] [Google Scholar]
- 24. Shi YW, Min FL, Zhou D, et al. : HLA-A*24:02 as a common risk factor for antiepileptic drug–induced cutaneous adverse reactions. Neurology. 2017;88(23):2183–91. 10.1212/WNL.0000000000004008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung YK, Cheng SH, Chan EJM, et al. : HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54(7):1307–14. 10.1111/epi.12217 [DOI] [PubMed] [Google Scholar]
- 26. Kaniwa N, Saito Y, Aihara M, et al. : HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9(11):1617–22. 10.2217/14622416.9.11.1617 [DOI] [PubMed] [Google Scholar]
- 27. Kaniwa N, Saito Y, Aihara M, et al. : HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51(12):2461–5. 10.1111/j.1528-1167.2010.02766.x [DOI] [PubMed] [Google Scholar]
- 28. Park HJ, Kim YJ, Kim DH, et al. : HLA Allele Frequencies in 5802 Koreans: Varied Allele Types Associated with SJS/TEN According to Culprit Drugs. Yonsei Med J. 2016;57(1):118–26. 10.3349/ymj.2016.57.1.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alfirevic A, Jorgensen AL, Williamson PR, et al. : HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics. 2006;7(6):813–8. 10.2217/14622416.7.6.813 [DOI] [PubMed] [Google Scholar]
- 30. Lonjou C, Borot N, Sekula P, et al. : A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99–107. 10.1097/FPC.0b013e3282f3ef9c [DOI] [PubMed] [Google Scholar]
- 31. Ramírez E, Bellón T, Tong HY, et al. : Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. 2017;115:168–178. 10.1016/j.phrs.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Lee KW, Song WJ, et al. : Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97(1-2):190–7. 10.1016/j.eplepsyres.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 33. Ozeki T, Mushiroda T, Yowang A, et al. : Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20(5):1034–41. 10.1093/hmg/ddq537 [DOI] [PubMed] [Google Scholar]
- 34. McCormack M, Alfirevic A, Bourgeois S, et al. : HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N Engl J Med. 2011;364(12):1134–43. 10.1056/NEJMoa1013297 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35. Amstutz U, Shear NH, Rieder MJ, et al. : Recommendations for HLA-B*15: 02 and HLA-A*31: 01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55(4):496–506. 10.1111/epi.12564 [DOI] [PubMed] [Google Scholar]
- 36. Ferrell PB, McLeod HL: Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9(10):1543–6. 10.2217/14622416.9.10.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen P, Lin JJ, Lu CS, et al. : Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–33. 10.1056/NEJMoa1009717 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 38. Hung SI, Chung WH, Liou LB, et al. : HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–9. 10.1073/pnas.0409500102 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 39. Tassaneeyakul W, Jantararoungtong T, Chen P, et al. : Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19(9):704–9. 10.1097/FPC.0b013e328330a3b8 [DOI] [PubMed] [Google Scholar]
- 40. Niihara H, Kaneko S, Ito T, et al. : HLA-B*58: 01 strongly associates with allopurinol-induced adverse drug reactions in a Japanese sample population. J Dermatol Sci. 2013;71(12):150–2. 10.1016/j.jdermsci.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 41. Cheng L, Xiong Y, Qin CZ, et al. : HLA-B*58: 01 is strongly associated with allopurinol-induced severe cutaneous adverse reactions in Han Chinese patients: a multicentre retrospective case-control clinical study. Br J Dermatol. 2015;173(2):555–8. 10.1111/bjd.13688 [DOI] [PubMed] [Google Scholar]
- 42. Sukasem C, Jantararoungtong T, Kuntawong P, et al. : HLA-B*58: 01 for Allopurinol-Induced Cutaneous Adverse Drug Reactions: Implication for Clinical Interpretation in Thailand. Front Pharmacol. 2016;7:186. 10.3389/fphar.2016.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonçalo M, Coutinho I, Teixeira V, et al. : HLA-B*58: 01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013;169(3):660–5. 10.1111/bjd.12389 [DOI] [PubMed] [Google Scholar]
- 44. Yu KH, Yu CY, Fang YF: Diagnostic utility of HLA-B*5801 screening in severe allopurinol hypersensitivity syndrome: An updated systematic review and meta-analysis. Int J Rheum Dis. 2017;20(9):1057–71. 10.1111/1756-185X.13143 [DOI] [PubMed] [Google Scholar]
- 45. Wu R, Cheng YJ, Zhu LL, et al. : Impact of HLA-B*58: 01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies. Oncotarget. 2016;7(49):81870–9. 10.18632/oncotarget.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ke CH, Chung WH, Wen YH, et al. : Cost-effectiveness Analysis for Genotyping Before Allopurinol Treatment to Prevent Severe Cutaneous Adverse Drug Reactions. J Rheumatol. 2017;44(6):835–43. 10.3899/jrheum.151476 [DOI] [PubMed] [Google Scholar]
- 47. Jutkowitz E, Dubreuil M, Lu N, et al. : The Cost-Effectiveness of HLA-B*5801 Screening to Guide Initial Urate-Lowering Therapy for Gout in the United States. Semin Arthritis Rheum. 2017;46(5):594–600. 10.1016/j.semarthrit.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mallal S, Nolan D, Witt C, et al. : Association Between Presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and Hypersensitivity to HIV-1 Reverse-Transcriptase Inhibitor Abacavir. Lancet. 2002;359:727–32. 10.1016/s0140-6736(02)07873-x [DOI] [PubMed] [Google Scholar]
- 49. Hetherington S, Hughes AR, Mosteller M, et al. : Genetic Variations in HLA-B Region and Hypersensitivity Reactions to Abacavir. Lancet. 2002;359(9312):1121–2. 10.1016/S0140-6736(02)08158-8 [DOI] [PubMed] [Google Scholar]
- 50. Martin AM, Nolan D, Gaudieri S, et al. : Predisposition to Abacavir Hypersensitivity Conferred by HLA-B*5701 and a Haplotypic Hsp70-Hom Variant. Proc Natl Acad Sci U S A. 2004;101(12):4180–5. 10.1073/pnas.0307067101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hughes AR, Mosteller M, Bansal AT, et al. : Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004;5(2):203–11. 10.1517/phgs.5.2.203.27481 [DOI] [PubMed] [Google Scholar]
- 52. Saag M, Balu R, Phillips E, et al. : High Sensitivity of Human Leukocyte Antigen–B*5701 as a Marker for Immunologically Confirmed Abacavir Hypersensitivity in White and Black Patients. Clin Infect Dis.. 2008;46(7):1111–8. 10.1086/529382 [DOI] [PubMed] [Google Scholar]
- 53. Mallal S, Phillips E, Carosi G, et al. : HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–79. 10.1056/NEJMoa0706135 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. Mounzer K, Hsu R, Fusco JS, et al. : HLA-B*57: 01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study. AIDS Res Ther. 2019;16(1):1. 10.1186/s12981-019-0217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 55. Wilkinson GR: Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21):2211–21. 10.1056/NEJMra032424 [DOI] [PubMed] [Google Scholar]
- 56. Chung WH, Chang WC, Lee YS, et al. : Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312(5):525–34. 10.1001/jama.2014.7859 [DOI] [PubMed] [Google Scholar]
- 57. Pichler WJ: Modes of presentation of chemical neoantigens to the immune system. Toxicology. 2002;181-182:49–54. 10.1016/s0300-483x(02)00254-8 [DOI] [PubMed] [Google Scholar]
- 58. Adam J, Pichler WJ, Yerly D: Delayed drug hypersensitivity: Models of T-cell stimulation. Br J Clin Pharmacol. 2011;71(5):701–7. 10.1111/j.1365-2125.2010.03764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abe R: Immunological response in Stevens-Johnson syndrome and toxic epidermal necrolysis. J Dermatol. 2015;42(1):42–8. 10.1111/1346-8138.12674 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Callan HE, Jenkins RE, Maggs JL, et al. : Multiple adduction reactions of nitroso sulfamethoxazole with cysteinyl residues of peptides and proteins: Implications for hapten formation. Chem Res Toxicol. 2009;22(5):937–48. 10.1021/tx900034r [DOI] [PubMed] [Google Scholar]
- 61. Weltzien HU, Padovan E: Molecular Features of Penicillin Allergy. J Invest Dermatol. 1998;110(3):203–6. 10.1046/j.1523-1747.1998.00122.x [DOI] [PubMed] [Google Scholar]
- 62. Naisbitt DJ, Hough SJ, Gill HJ, et al. : Cellular disposition of sulphamethoxazole and its metabolites: Implications for hypersensitivity. Br J Pharmacol. 1999;126(6):1393–407. 10.1038/sj.bjp.0702453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friedmann PS, Lee MS, Friedmann AC, et al. : Mechanisms in cutaneous drug hypersensitivity reactions. Clin Exp Allergy. 2003;33(7):861–72. 10.1046/j.1365-2222.2003.01718.x [DOI] [PubMed] [Google Scholar]
- 64. Pichler WJ: Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr Opin Allergy Clin Immunol. 2002;2(4):301–5. 10.1097/00130832-200208000-00003 [DOI] [PubMed] [Google Scholar]
- 65. Pichler WJ: Delayed Drug Hypersensitivity Reactions. Ann Intern Med. 2003;139:683 10.7326/0003-4819-139-8-200310210-00012 [DOI] [PubMed] [Google Scholar]
- 66. Yun J, Marcaida MJ, Eriksson KK, et al. : Oxypurinol Directly and Immediately Activates the Drug-Specific T Cells via the Preferential Use of HLA-B*58: 01. J Immunol. 2014;192(7):2984–93. 10.4049/jimmunol.1302306 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. Pichler WJ, Beeler A, Keller M, et al. : Pharmacological Interaction of Drugs with Immune Receptors: The p-i Concept. Allergol Int. 2006;55(1):17–25. 10.2332/allergolint.55.17 [DOI] [PubMed] [Google Scholar]
- 68. Yang CWO, Hung SI, Juo CG, et al. : HLA-B*1502-bound peptides: Implications for the pathogenesis of carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2007;120(4):870–7. 10.1016/j.jaci.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 69. Illing PT, Vivian JP, Dudek NL, et al. : Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486(7404):554–8. 10.1038/nature11147 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 70. Ostrov DA, Grant BJ, Pompeu YA, et al. : Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109(25):9959–64. 10.1073/pnas.1207934109 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 71. Ko TM, Chung WH, Wei CY, et al. : Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128(6):1266–1276.e11. 10.1016/j.jaci.2011.08.013 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 72. Wei CY, Chung WH, Huang HW, et al. : Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129(6):1562-9.e5. 10.1016/j.jaci.2011.12.990 [DOI] [PubMed] [Google Scholar]
- 73. Chung WH, Pan RY, Chu MT, et al. : Oxypurinol-Specific T Cells Possess Preferential TCR Clonotypes and Express Granulysin in Allopurinol-Induced Severe Cutaneous Adverse Reactions. J Invest Dermatol. 2015;135(9):2237–48. 10.1038/jid.2015.165 [DOI] [PubMed] [Google Scholar]
- 74. Pan RY, Chu MT, Wang CW, et al. : Identification of drug-specific public TCR driving severe cutaneous adverse reactions. Nat Commun. 2019;10(1):3569. 10.1038/s41467-019-11396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 75. Correia O, Delgado L, Ramos JP, et al. : Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement. Arch Dermatol. 1993;129(4):466–8. [PubMed] [Google Scholar]
- 76. Le Cleach L, Delaire S, Boumsell L, et al. : Blister fluid T lymphocytes during toxic epidermal necrolysis are functional cytotoxic cells which express human natural killer (NK) inhibitory receptors. Clin Exp Immunol. 2000;119(1):225–30. 10.1046/j.1365-2249.2000.01119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tohyama M, Watanabe H, Murakami S, et al. : Possible involvement of CD14+ CD16+ monocyte lineage cells in the epidermal damage of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2012;166(2):322–30. 10.1111/j.1365-2133.2011.10649.x [DOI] [PubMed] [Google Scholar]
- 78. Correia O, Delgado L, Roujeau JC, et al. : Soluble interleukin 2 receptor and interleukin 1alpha in toxic epidermal necrolysis: A comparative analysis of serum and blister fluid samples. Arch Dermatol. 2002;138(1):29–32. 10.1001/archderm.138.1.29 [DOI] [PubMed] [Google Scholar]
- 79. Paul C, Wolkenstein P, Adle H, et al. : Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996;134(4):710–4. 10.1111/j.1365-2133.1996.tb06976.x [DOI] [PubMed] [Google Scholar]
- 80. Elmore S: Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 2007;35(4):495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Viard-Leveugle I, Bullani RR, Meda P, et al. : Intracellular localization of keratinocyte Fas ligand explains lack of cytolytic activity under physiological conditions. J Biol Chem. 2003;278(18):16183–8. 10.1074/jbc.M212188200 [DOI] [PubMed] [Google Scholar]
- 82. Viard I, Wehrli P, Bullani R, et al. : Inhibition of Toxic Epidermal Necrolysis by Blockade of CD95 with Human Intravenous Immunoglobulin. Science. 1998;282(5388):490–3. 10.1126/science.282.5388.490 [DOI] [PubMed] [Google Scholar]
- 83. Tanaka M, Suda T, Haze K, et al. : Fas ligand in human serum. Nat Med. 1996;2(3):317–22. 10.1038/nm0396-317 [DOI] [PubMed] [Google Scholar]
- 84. Abe R: Toxic epidermal necrolysis and Stevens-Johnson syndrome: Soluble Fas ligand involvement in the pathomechanisms of these diseases. J Dermatol Sci. 2008;52(3):151–9. 10.1016/j.jdermsci.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 85. Abe R, Shimizu T, Shibaki A, et al. : Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome Are Induced by Soluble Fas Ligand. Am J Pathol. 2003;162(5):1515–20. 10.1016/S0002-9440(10)64284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stur K, Karlhofer FM, Stingl G: Soluble FAS ligand: A discriminating feature between drug-induced skin eruptions and viral exanthemas. J Invest Dermatol. 2007;127(4):802–7. 10.1038/sj.jid.5700648 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 87. Nassif A, Bensussan A, Bachot N, et al. : Drug Specific Cytotoxic T-Cells in the Skin Lesions of a Patient with Toxic Epidermal Necrolysis. J Invest Dermatol. 2002;118(4):728–33. 10.1046/j.1523-1747.2002.01622.x [DOI] [PubMed] [Google Scholar]
- 88. Nassif A, Bensussan A, Boumsell L, et al. : Toxic epidermal necrolysis: Effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. 2004;114(5):1209–15. 10.1016/j.jaci.2004.07.047 [DOI] [PubMed] [Google Scholar]
- 89. Chung WH, Hung SI, Yang JY, et al. : Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–50. 10.1038/nm.1884 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 90. Abe R, Yoshioka N, Murata J, et al. : Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann Intern Med. 2009;151(7):514–5. 10.7326/0003-4819-151-7-200910060-00016 [DOI] [PubMed] [Google Scholar]
- 91. Saito N, Abe R, Yoshioka N, et al. : Prolonged elevation of serum granulysin in drug-induced hypersensitivity syndrome. Br J Dermatol. 2012;167(2):452–3. 10.1111/j.1365-2133.2012.10921.x [DOI] [PubMed] [Google Scholar]
- 92. Saito N, Qiao H, Yanagi T, et al. : An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014;6(245):245ra95–245ra95. 10.1126/scitranslmed.3008227 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 93. Linkermann A, Green DR: Necroptosis. N Engl J Med. 2014;370(5):455–65. 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Saito N, Yoshioka N, Abe R, et al. : Stevens-Johnson syndrome/toxic epidermal necrolysis mouse model generated by using PBMCs and the skin of patients. J Allergy Clin Immunol. 2013;131(2):434–41.e1-9. 10.1016/j.jaci.2012.09.014 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 95. Wang F, He D, Tang X, et al. : Chemokine expression in diverse nonimmediate drug hypersensitivity reactions: Focus on thymus activation-regulated chemokine, cutaneous T-cell-attracting chemokine, and interleukin-10. Ann Allergy Asthma Immunol. 2014;113(2):204–8. 10.1016/j.anai.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 96. Wang F, Ye Y, Luo ZY, et al. : Diverse expression of TNF-α and CCL27 in serum and blister of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin Transl Allergy. 2018;8:12. 10.1186/s13601-018-0199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 97. McCully ML, Moser B: The Human Cutaneous Chemokine System. Front Immunol. 2011;2:33. 10.3389/fimmu.2011.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tapia B, Padial A, Sánchez-Sabaté E, et al. : Involvement of CCL27-CCR10 interactions in drug-induced cutaneous reactions. J Allergy Clin Immunol. 2004;114(2):335–40. 10.1016/j.jaci.2004.04.034 [DOI] [PubMed] [Google Scholar]
- 99. Su SC, Mockenhaupt M, Wolkenstein P, et al. : Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J Invest Dermatol. 2017;137(5):1065–73. 10.1016/j.jid.2016.11.034 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 100. McInnes IB, Leung BP, Sturrock RD, et al. : Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3(2):189–95. 10.1038/nm0297-189 [DOI] [PubMed] [Google Scholar]
- 101. Hama N, Nishimura K, Hasegawa A, et al. : Galectin-7 as a potential biomarker of Stevens-Johnson syndrome/toxic epidermal necrolysis: Identification by targeted proteomics using causative drug-exposed peripheral blood cells. J Allergy Clin Immunol Pract. 2019;7(8):2894–2897.e7. 10.1016/j.jaip.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 102. Vidova V, Spacil Z: A review on mass spectrometry-based quantitative proteomics: Targeted and data independent acquisition. Anal Chim Acta. 2017;964:7–23. 10.1016/j.aca.2017.01.059 [DOI] [PubMed] [Google Scholar]
- 103. Hasegawa A, Shinkuma S, Hayashi R, et al. : RIP3 as a diagnostic and severity marker for Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol Pract. 2020;8(5):1768–1771.e7. 10.1016/j.jaip.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 104. Qing DY, Conegliano D, Shashaty MGS, et al. : Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med. 2014;190(11):1243–54. 10.1164/rccm.201406-1095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shiga S, Cartotto R: What Are the Fluid Requirements in Toxic Epidermal Necrolysis? J Burn Care Res. 2010;31(1):100–4. 10.1097/BCR.0b013e3181cb8cb8 [DOI] [PubMed] [Google Scholar]
- 106. Coss-Bu JA, Jefferson LS, Levy ML, et al. : Nutrition requirements in patients with toxic epidermal necrolysis. Nutr Clin Pract. 1997;12(2):81–4. 10.1177/011542659701200281 [DOI] [PubMed] [Google Scholar]
- 107. Valeyrie-Allanore L, Ingen-Housz-Oro S, Chosidow O, et al. : French referral center management of Stevens–Johnson syndrome/toxic epidermal necrolysis. Dermatologica Sinica. 2013;31(4):191–5. 10.1016/j.dsi.2013.09.008 [DOI] [Google Scholar]
- 108. de Prost N, Ingen-Housz-Oro S, Duong Ta, et al. : Bacteremia in Stevens-Johnson syndrome and toxic epidermal necrolysis: Epidemiology, risk factors, and predictive value of skin cultures. Medicine (Baltimore). 2010;89(1):28–36. 10.1097/MD.0b013e3181ca4290 [DOI] [PubMed] [Google Scholar]
- 109. White KD, Abe R, Ardern-Jones M, et al. : SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation. J Allergy Clin Immunol Pract. 2018;6(1):38–69. 10.1016/j.jaip.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zimmermann S, Sekula P, Venhoff M, et al. : Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017;153(6):514–22. 10.1001/jamadermatol.2016.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 111. Schneider JA, Cohen PR: Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6):1235–44. 10.1007/s12325-017-0530-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Creamer D, Walsh SA, Dziewulski P, et al. : U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174(6):1194–227. 10.1111/bjd.14530 [DOI] [PubMed] [Google Scholar]
- 113. Ginsburg CM: Stevens-Johnson syndrome in children. Pediatr Infect Dis J. 1982;1:155–8. 10.1097/00006454-198205000-00005 [DOI] [PubMed] [Google Scholar]
- 114. Halebian PH, Corder VJ, Madden MR, et al. : Improved Burn Center Survival of Patients with Toxic Epidermal Necrolysis Managed without Corticosteroids. Ann Surg. 1986;204(5):503–12. 10.1097/00000658-198611000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kelemen JJ, 3rd, Cioffi WG, McManus WF, et al. : Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg. 1995;180(3):273–8. [PubMed] [Google Scholar]
- 116. Finkelstein Y, Soon GS, Acuna P, et al. : Recurrence and Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Children. Pediatrics. 2011;128(4):723–8. 10.1542/peds.2010-3322 [DOI] [PubMed] [Google Scholar]
- 117. Sekula P, Dunant A, Mockenhaupt M, et al. : Comprehensive Survival Analysis of a Cohort of Patients with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. J Invest Dermatol. 2013;133(5):1197–204. 10.1038/jid.2012.510 [DOI] [PubMed] [Google Scholar]
- 118. Roujeau JC, Bastuji-Garin S: Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug Saf. 2011;2(3):87–94. 10.1177/2042098611404094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schneck J, Fagot JP, Sekula P, et al. : Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33–40. 10.1016/j.jaad.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 120. Kardaun SH, Jonkman MF: Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Acta Derm Venereol. 2007;87(2):144–8. 10.2340/00015555-0214 [DOI] [PubMed] [Google Scholar]
- 121. Lee HY, Lim YL, Thirumoorthy T, et al. : The role of intravenous immunoglobulin in toxic epidermal necrolysis: A retrospective analysis of 64 patients managed in a specialized centre. Br J Dermatol. 2013;169(6):1304–9. 10.1111/bjd.12607 [DOI] [PubMed] [Google Scholar]
- 122. Bachot N, Revuz J, Roujeau JC: Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: A prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139(1):33–6. 10.1001/archderm.139.1.33 [DOI] [PubMed] [Google Scholar]
- 123. Brown KM, Silver GM, Halerz M, et al. : Toxic epidermal necrolysis: Does immunoglobulin make a difference? J Burn Care Rehabil. 2004;25(1):81–8. 10.1097/01.BCR.0000105096.93526.27 [DOI] [PubMed] [Google Scholar]
- 124. Huang YC, Li YC, Chen TJ: The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: A systematic review and meta-analysis. Br J Dermatol. 2012;167(2):424–32. 10.1111/j.1365-2133.2012.10965.x [DOI] [PubMed] [Google Scholar]
- 125. Metry DW, Jung P, Levy ML: Use of intravenous immunoglobulin in children with stevens-johnson syndrome and toxic epidermal necrolysis: Seven cases and review of the literature. Pediatrics. 2003;112(6 Pt 1):1430–6. 10.1542/peds.112.6.1430 [DOI] [PubMed] [Google Scholar]
- 126. Prins C, Vittorio C, Padilla RS, et al. : Effect of High-Dose Intravenous Immunoglobulin Therapy in Stevens-Johnson Syndrome: A Retrospective, Multicenter Study. Dermatology. 2003;207(1):96–9. 10.1159/000070957 [DOI] [PubMed] [Google Scholar]
- 127. Faye O, Roujeau JC: Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IV Ig): Clinical experience to date. Drugs. 2005;65(15):2085–90. 10.2165/00003495-200565150-00002 [DOI] [PubMed] [Google Scholar]
- 128. Morici MV, Galen WK, Shetty AK, et al. : Intravenous immunoglobulin therapy for children with Stevens-Johnson syndrome. J Rheumatol. 2000;27(10):2494–7. [PubMed] [Google Scholar]
- 129. Barron SJ, Del Vecchio MT, Aronoff SC: Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: A meta-analysis with meta-regression of observational studies. Int J Dermatol. 2015;54(1):108–15. 10.1111/ijd.12423 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 130. Chung WH, Wang CW, Dao RL: Severe cutaneous adverse drug reactions. J Dermatol. 2016;43(7):758–66. 10.1111/1346-8138.13430 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131. Valeyrie-Allanore L, Wolkenstein P, Brochard L, et al. : Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163(4):847–53. 10.1111/j.1365-2133.2010.09863.x [DOI] [PubMed] [Google Scholar]
- 132. Kirchhof MG, Miliszewski MA, Sikora S, et al. : Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71(5):941–7. 10.1016/j.jaad.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 133. Kumar P, Das NK: Cyclosporine in toxic epidermal necrolysis: A brief review of the emerging therapeutic modality. Dermatol Online J. 2016;22(10): 13030/qt3m82s074. [PubMed] [Google Scholar]
- 134. Lee HY, Fook-Chong S, Koh HY, et al. : Cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis: Retrospective analysis of a cohort treated in a specialized referral center. J Am Acad Dermatol. 2017;76(1)106–113. 10.1016/j.jaad.2016.07.048 [DOI] [PubMed] [Google Scholar]
- 135. Ng QX, de Deyn MLZQ, Venkatanarayanan N, et al. : A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. 2018;11:135–142. 10.2147/JIR.S160964 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 136. González-Herrada C, Rodríguez-Martín S, Cachafeiro L, et al. : Cyclosporine Use in Epidermal Necrolysis Is Associated with an Important Mortality Reduction: Evidence from Three Different Approaches. J Invest Dermatol. 2017;137(10):2092–100. 10.1016/j.jid.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 137. Roujeau J-C, Mockenhaupt M, Guillaume J-C, et al. : New Evidence Supporting Cyclosporine Efficacy in Epidermal Necrolysis. J Invest Dermatol. 2017;137(10):2047–9. 10.1016/j.jid.2017.07.828 [DOI] [PubMed] [Google Scholar]
- 138. Shah R, Chen ST, Kroshinsky D: Use of Cyclosporine for the treatment of Steven Johnson Syndrome/ Toxic Epidermal Necrolysis. J Am Acad Dermatol. 2019;S0190–9622(19)30001-5. 10.1016/j.jaad.2018.09.063 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 139. Gilbert M, Scherrer LA: Efficacy and safety of cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol Ther. 2019;32(1):e12758. 10.1111/dth.12758 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 140. Kamanabroo D, Schmitz-Landgraf W, Czarnetzki BM: Plasmapheresis in Severe Drug-Induced Toxic Epidermal Necrolysis. Arch Dermatol. 1985;121(12):1548–1549. 10.1001/archderm.1985.01660120074023 [DOI] [PubMed] [Google Scholar]
- 141. Sakellariou G, Koukoudis P, Karpouzas J, et al. : Plasma Exchange (Pe) Treatment in Drug-Induced Toxic Epidermal Necrolysis (Ten). Int J Artif Organs. 1991;14(10):634–8. 10.1177/039139889101401006 [DOI] [PubMed] [Google Scholar]
- 142. Chaidemenos GC, Chrysomallis F, Sombolos K, et al. : Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol. 1997;36(3):218–21. 10.1046/j.1365-4362.1997.00192.x [DOI] [PubMed] [Google Scholar]
- 143. Egan CA, Grant WJ, Morris SE, et al. : Plasmapheresis as an adjunct treatment in toxic epidermal necrolysis. J Am Acad Dermatol. 1999;40(3):458–61. 10.1016/s0190-9622(99)70497-4 [DOI] [PubMed] [Google Scholar]
- 144. Bamichas G, Natse T, Christidou F, et al. : Plasma exchange in patients with toxic epidermal necrolysis. Ther Apher. 2002;6(3):225–8. 10.1046/j.1526-0968.2002.00409.x [DOI] [PubMed] [Google Scholar]
- 145. Yamada H, Takamori K, Yaguchi H, et al. : A Study of the Efficacy of Plasmapheresis for the Treatment of Drug Induced Toxic Epidermal Necrolysis. Ther Apher. 1998;2(2):153–6. 10.1111/j.1744-9987.1998.tb00094.x [DOI] [PubMed] [Google Scholar]
- 146. Furubacke A, Berlin G, Anderson C, et al. : Lack of significant treatment effect of plasma exchange in the treatment of drug-induced toxic epidermal necrolysis? Intensive Care Med. 1999;25(11):1307–10. 10.1007/s001340051063 [DOI] [PubMed] [Google Scholar]
- 147. Narita YM, Hirahara K, Mizukawa Y, et al. : Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: Is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol. 2011;38(3):236–45. 10.1111/j.1346-8138.2010.01154.x [DOI] [PubMed] [Google Scholar]
- 148. Lissia M, Figus A, Rubino C: Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: Preliminary report. Br J Plast Surg. 2005;58(4):504–10. 10.1016/j.bjps.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 149. Han F, Zhang J, Guo Q, et al. : Successful treatment of toxic epidermal necrolysis using plasmapheresis: A prospective observational study. J Crit Care. 2017;42:65–8. 10.1016/j.jcrc.2017.07.002 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 150. Paquet P, Nikkels A, Arrese JE, et al. : Macrophages and Tumor Necrosis Factor Alpha in Toxic Epidermal Necrolysis. Arch Dermatol. 1994;130(5):605–8. 10.1001/archderm.1994.01690050073012 [DOI] [PubMed] [Google Scholar]
- 151. Nassif A, Moslehi H, Le Gouvello S, et al. : Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123(5):850–5. 10.1111/j.0022-202X.2004.23439.x [DOI] [PubMed] [Google Scholar]
- 152. Wojtkiewicz A, Wysocki M, Fortuna J, et al. : Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88(4):420–1. [DOI] [PubMed] [Google Scholar]
- 153. Patmanidis K, Sidiras A, Dolianitis K, et al. : Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: Successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314. 10.1155/2012/915314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. : Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23(1):61–3. [PubMed] [Google Scholar]
- 155. Scott-Lang V, Tidman M, McKay D: Toxic Epidermal Necrolysis in a Child Successfully Treated with Infliximab. Pediatr Dermatol. 2014;31(4):532–4. 10.1111/pde.12029 [DOI] [PubMed] [Google Scholar]
- 156. Pham CH, Gillenwater TJ, Nagengast E, et al. : Combination therapy: Etanercept and intravenous immunoglobulin for the acute treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis. Burns. 2019;45(7):1634–1638. 10.1016/j.burns.2018.12.018 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 157. Paradisi A, Abeni D, Bergamo F, et al. : Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71(2):278–83. 10.1016/j.jaad.2014.04.044 [DOI] [PubMed] [Google Scholar]
- 158. Hunger RE, Hunziker T, Buettiker U, et al. : Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116(4):923–4. 10.1016/j.jaci.2005.06.029 [DOI] [PubMed] [Google Scholar]
- 159. Gubinelli E, Canzona F, Tonanzi T, et al. : Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36(3):150–3. 10.1111/j.1346-8138.2009.00616.x [DOI] [PubMed] [Google Scholar]


