Abstract
The chemogenetic technology DREADD (designer receptors exclusively activated by designer drugs) is widely used for remote manipulation of neuronal activity in freely moving animals. DREADD technology posits the use of “designer receptors,” which are exclusively activated by the “designer drug” clozapine N-oxide (CNO). Nevertheless, the in vivo mechanism of action of CNO at DREADDs has never been confirmed. CNO does not enter the brain after systemic drug injections and shows low affinity for DREADDs. Clozapine, to which CNO rapidly converts in vivo, shows high DREADD affinity and potency. Upon systemic CNO injections, converted clozapine readily enters the brain and occupies central nervous system–expressed DREADDs, whereas systemic subthreshold clozapine injections induce preferential DREADD-mediated behaviors.
The chemogenetic technology designer receptors exclusively activated by designer drugs (DREADD) (1) is a powerful approach for remote and transient manipulation of cellular activity in laboratory animals, with potential for clinical therapeutics (2). DREADD technology has been applied to the investigation of various biological questions and disease mechanisms (3) by numerous laboratories around the world. In the 10 years since its inception, there have been more than 800 reports referring to DREADD, with the number of publications and laboratories using the technology growing exponentially (2).
DREADD technology posits the use of “designer receptors”—namely, mutated human muscarinic receptors (hM3Dq and hM4Di) that are not activated by acetylcholine or other endogenous neurotransmitters but exclusively via the “designer drug” clozapine N-oxide (CNO), an inert and inactive clozapine metabolite (1). However, the in vivo mechanism of action of CNO at DREADDs has never been confirmed.
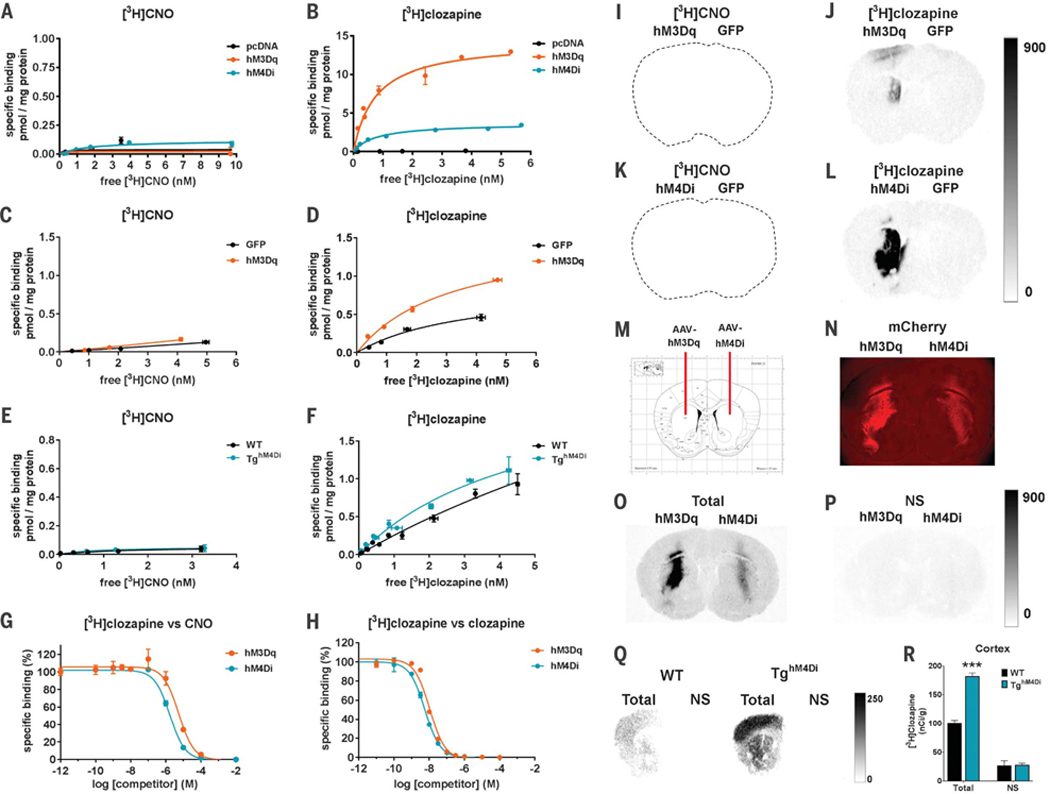
We first carried out saturation binding experiments in DREADD-expressing human embryonic kidney (HEK) 293 membranes and observed no detectable specific [3H]CNO binding (Fig. 1A). In contrast, we observed high specific binding of [3H]clozapine (Fig. 1B). Similar experiments were performed by using brain tissue from mice injected with hM3Dq or green fluorescent protein (GFP) adeno-associated virus (AAV) constructs and from hM4Di transgenic (hM4Di-Tg) and wild-type (WT) mice (4). Again, [3H]clozapine showed preferential binding to DREADDs over other endogenous sites, whereas [3H]CNO did not bind to either DREADD-expressing or naïve tissues (Fig. 1, C to F).
Fig. 1. CNO binds to DREADDs with low affinity, whereas clozapine binds to DREADDs with high affinity.
(A and B) [3H]CNO- or [3H]clozapine-specific binding to HEK-293 membranes expressing DREADDs or pcDNA (control). (C to F) Mouse striatum transduced expressing [(C) and (D)] hM3Dq or GFP (n = 2 mice), or [(E) and (F)] hM4Di-Tg and WT mouse whole-brain tissue (n = 2 mice). (G and H) Competition curves for [3H]clozapine (2.5 nM) against (G) CNO or (H) clozapine in HEK-293 membranes expressing DREADDs. All data points (means ± SEM) are from a representative experiment performed in triplicate. (I to L) [3H]CNO (1 nM) and [3H]clozapine (1 nM) autoradiograms from mice expressing [(I) and (J)] hM3Dq-mCherry or [(K) and (L)] hM4Di-mCherry in the left striatum and GFP in the right striatum (n = 4 mice). (M) AAV injection schematic. (N) Immunofluorescence image showing high DREADD-mCherry expression in dorsal striatum. (O and P) [3H]clozapine (3.5 nM) autoradio-grams from a rat injected with DREADDs as described above showing total and nonspecific (NS) binding. (Q) [3H]clozapine (3.5 nM) autoradiograms showing total and NS binding in hM4Di-Tg and WT mice (n = 2 mice). (R) Densitometric quantification of binding in the cortex of hM4Di-Tg and WT mice. Eight sections from each mouse were mounted onto two slides and assessed for total binding; four sections were mounted onto separate slides and assessed for NS binding. Statistical significance calculated by use of two-way analysis of variance (ANOVA) with genotype and binding condition as factors and Tukey’s post hoc test comparing WT versus hM4Di-Tg mice (***P < 0.001). All values represent mean ± SEM.
We also performed competition binding assays using HEK-293 membranes with [3H]clozapine and increasing concentrations of nonradioactive CNO (Fig. 1G) or clozapine (Fig. 1H). CNO showed weak micromolar-level inhibition of [3H]clozapine binding (hM3DqKi = 3.8 ± 0.3 mM and hM4DiKi = 15 ± 0.5 mM, where Ki is the inhibition constant), whereas clozapine showed low nanomolar-level inhibition of [3H]clozapine binding (hM3DqKi = 7± 2 nM and hM4DiK = 3.5 ± 0.7 nM).
To visualize the specificity of [3H]clozapine binding to DREADDs in intact brain tissue sections, we performed [3H]clozapine and [3H]CNO autoradiography using brains from mice injected with DREADD or GFP-expressing AAVs. We observed no [3H]CNO-specific binding (Fig. 1,1 and K). In contrast, we observed high specific binding of [3H]clozapine at the AAV-DREADD injection sites but not at the AAV-GFP injection sites nor at other brain areas containing endogenous clozapine binding sites (Fig. 1, J and L) (5). Similar experiments were carried out in rat sections (Fig. 1M), where DREADD-mCherry immune-fluorescence (Fig. 1N) colocalized with specific binding of [3H]clozapine to DREADDs (Fig. 1, O and P). Last, we observed significantly higher [3H]clozapine-specific binding in the hM4Di-Tg mouse brain (cortex) as compared with that of wild type (Fig. 1, Q and R) and an overall hM4Di distribution as previously reported by using immunohistochemistry (4).
CNO is considered inert (1); however, it has not been evaluated, to our knowledge, for its interactions with endogenous receptors at the high (micromolar) concentrations that we observed were necessary for CNO to bind to DREADDs. Therefore, it is unknown whether its inert profile would be maintained at such high concentrations. Accordingly, we screened CNO for its ability to competitively inhibit binding to multiple endogenous receptors and enzymes. CNO did not show any substantial interaction at 100 nM concentration (fig. S1). However, at 10 μM, CNO competitively inhibited binding at several receptors, including histamine H1 (~90%), 5-HT2A (~53%), muscarinic M1 (~58%), M3 (~43%), M4 (~58%), and dopamine D1 (~24%) and D2 (~23%) (fig. S1).
Despite the substantial number of publications reporting successful use of DREADDs, the degree to which CNO enters the brain to occupy DREADDs has not been thoroughly investigated. On the basis of our findings, we argue that this is a critical component of the DREADD system. That is because (i) CNO converts to clozapine in vivo (6–12), (ii) clozapine shows high brain permeability (4,13), and (iii) clozapine binds with very high affinity to DREADDs. Therefore, a small amount of converted clozapine from systemic CNO delivery would presumably occupy CNS-expressed DREADDs in vivo.
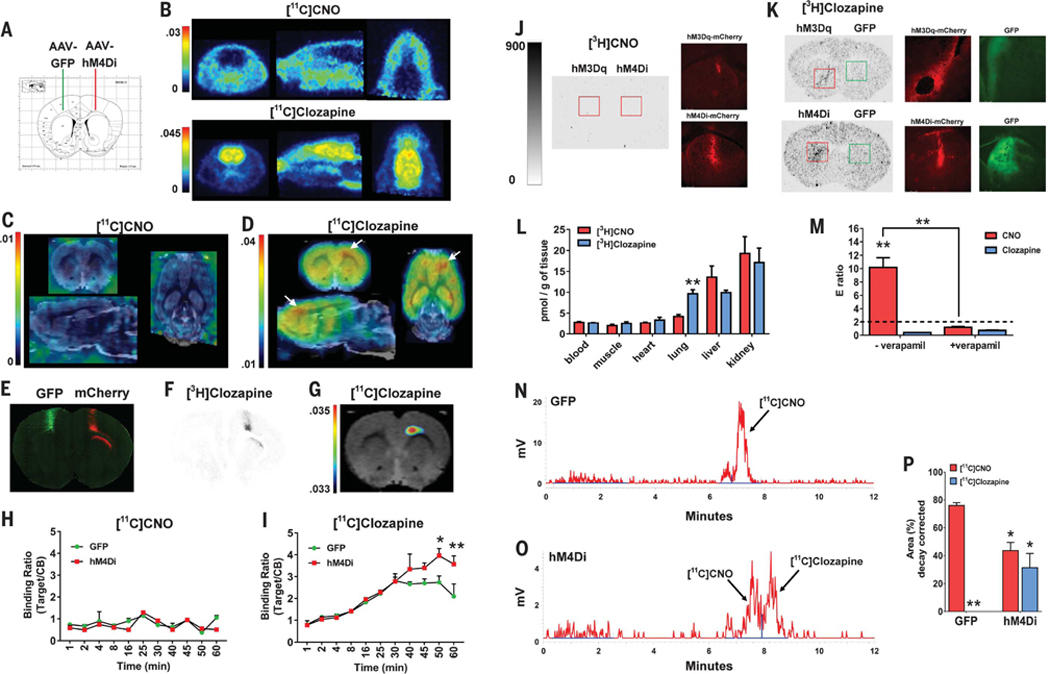
On the basis of the above considerations, we investigated the hypotheses that (i) CNO does not readily cross the blood-brain barrier (BBB) and (ii) the hM4Di-specific [11C]CNO binding signal observed previously (4) could be due to converted [11C]clozapine. We injected into the cortex of rats hM4Di (right hemisphere) or GFP (left hemisphere) AAVs (Fig. 2A). Rats were then scanned by using positron emission tomography (PET) after a bolus intravenous dose of [11C]CNO or [11C]clozapine (~0.3 to 0.5 nmol/kg each). [11C]CNO showed no observable brain uptake, whereas [11C]clozapine showed substantial brain uptake (Fig. 2B). PET images were then coregistered (14) to a rat magnetic resonance imaging (MRI) template so as to localize radioligand uptake in putative hM4Di expression sites. No [11C]CNO signal was found (Fig. 2C). In contrast, highly localized [11C]clozapine binding was observed at the putative injection site of the AAV-hM4Di (Fig. 2D), which colocalized (Fig. 2, E to G) with both hM4Di-mCherry expression and the [3H]clozapine autoradiography signal. Dynamic [11C]CNO and [11C]clozapine occupancy curves showed no in vivo binding or hM4Di occupancy by [11C]CNO (ratio < 1) (Fig. 2H) but high overall in vivo binding and specific hM4Di occupancy 45 min after [11C]clozapine injection (Fig. 2I).
Fig. 2. CNO does not cross the BBB while converted clozapine engages DREADDs in vivo.
(A) AAV injection sites. (B) Whole-head PET images (averaged from 1 to 60 min) after intravenous injection of [11C]CNO (n = 2 rats) or [11C]clozapine (n = 2 rats). (C) [11C]CNO or (D) [11C]clozapine PET brain images (averaged from 45 to 60 min) coregistered to a MRI rat template (white arrows indicate hM4Di expression site). (E) Immunohistochemical detection of GFP and hM4Di-mCherry from the rat shown above. (F) [3H]clozapine autoradiogram from an adjacent section to (E). (G) Thresholded [11C]clozapine PET-MRI coregistered image corresponding to (E) and (F). (H) [11C]CNO (n = 2 rats) or (I) [11C]clozapine (n = 2 rats) uptake quantified as target-to-reference (cerebellum) binding ratio, two-way repeated measures ANOVA with time versus group (GFP versus hM4Di) and Sidak’s post hoc test (50 min, P < 0.05; 60 min, P < 0.01). (J and K) Autoradiographic and fluorescent images from mice transduced with hM3Dq-mCherry, hM4Di-mCherry, or GFP in striatum. (L) Organ distribution of [3H]CNO or [3H]clozapine (unpaired Student’s t test). (M) Efflux (E) ratios of CNO and clozapine in the P-gp assay, two-way ANOVA, with drug and condition as factors and Tukey’s post hoc test. (N and O) Representative chromatograms of brain extracts from rats injected with [11C]CNO (n = 4 rats). (P) Percentage of [11C]CNO and converted [11C]clozapine, two-way ANOVA with drug and hemisphere as factors and Tukey’s post hoc test comparing GFP-injected [11C]CNO versus [11C]clozapine (P < 0.01), GFP-[11C]CNO versus hM4Di-[11C]CNO (P < 0.05), and GFP-[11C]clozapine versus hM4Di-[11C]clozapine (P < 0.05, one-tailed). *P < 0.05, **P < 0.01. All values represent mean ± SEM.
We next injected naïve mice with DREADD or GFP AAVs into the striatum and then injected them intraperitoneally with [3H]CNO or [3H]clozapine (~5 μg/kg dose). At 30 min later, we euthanized them and harvested blood, brain, and peripheral organs to examine uptake of each radioligand. No uptake was observed in brains from DREADD-expressing mice after systemic [3H]CNO injections (Fig. 2J). In contrast, we found high [3H]clozapine brain uptake and DREADD-specific binding (colocalized with DREADD-mCherry but not GFP expression) (Fig. 2K). The overall distribution profile for [3H]clozapine and [3H]CNO in peripheral organs was similar, with the exception of the lungs (Fig. 2L).
We hypothesized that CNO may be a substrate for the P-glycoprotein (P-gp) efflux pump, which limits permeability of molecules from crossing the BBB (15). We tested CNO and clozapine using a P-gp permeability assay. CNO exhibited a high efflux (E) ratio, which was reversed after P-gp inhibition, indicating that CNO is a P-gp substrate (Fig. 2M), as recently reported (12). exhibited a low E ratio (E < 2), indicating that it is not a P-gp substrate, as reported previously (15). How does one then reconcile the numerous publications documenting DREADD-specific CNOmediated effects on behavior?
CNO can convert to clozapine in vivo (6–12). Therefore, to demonstrate that systemic CNO injections lead to (i) clozapine conversion and (ii) DREADD occupancy via converted clozapine, we injected rats expressing hM4Di and GFP in the right and left motor cortex, respectively, with intravenous [11C]CNO (~2 to 4 nmol/kg) and harvested their blood and brain ~35 to 40 min later. Plasma [11C]CNO and [11C]clozapine accounted for ~40 and ~2%, respectively, of all radioactivity detected (other polar and unidentified metabolites accounted for ~58%) (fig. S2). In extracts from brain hemispheres receiving AAV-GFP, [11C]CNO accounted for ~76% of radioactivity detected (other metabolites accounted for ~24%), and no [11C]clozapine could be detected (Fig. 2, N and P). In extracts from brain hemispheres receiving AAV-hM4Di, [nC]CNO accounted for ~44%, whereas [11C]clozapine accounted for ~31% of total radioactivity detected (other metabolites accounted for ~25%) (Fig. 2, O and P).
We next examined the putative agonistic properties of clozapine at hM3Dq and hM4Di.
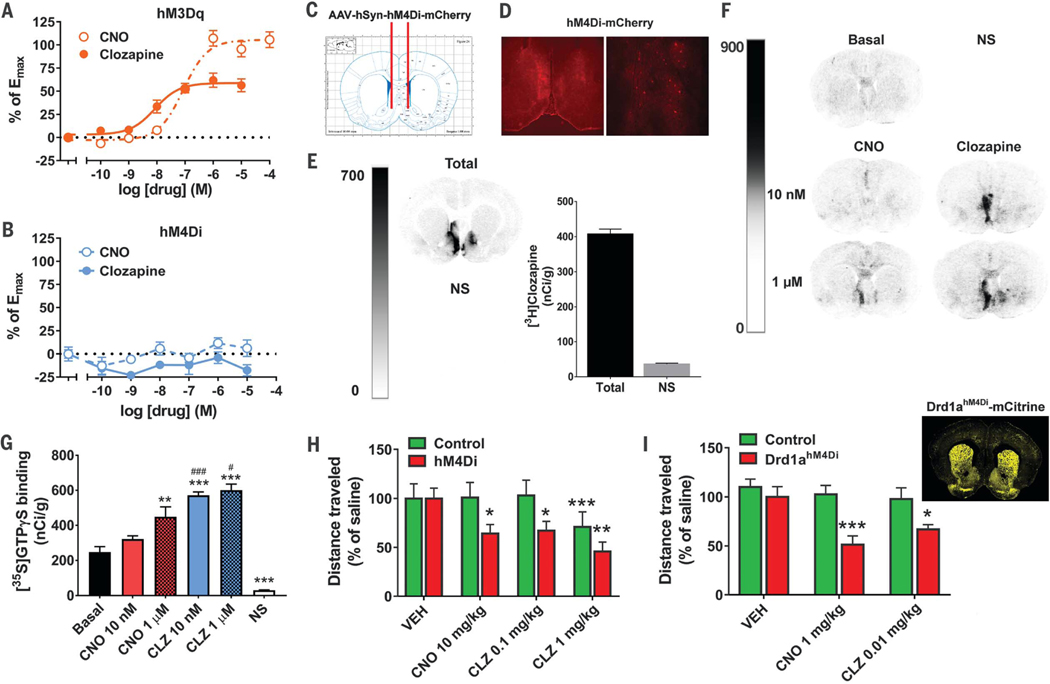
Clozapine showed greater potency [median effective concentration (EC50) = 5.4 ± 3.1 nM] at inducing increases in Ca2+ in hM3Dq-expressing HEK-293 cells compared with CNO (EC50 = 85 ± 17 nM) (Fig. 3A). CNO showed greater intrinsic efficacy, which, however, was only achieved at higher concentrations. As expected, hM4Di-expressing cells showed no Ca2+ response to either clozapine or CNO (Fig. 3B). Both clozapine and CNO induced significant increases in [35S]GTPgS binding to hM4Di, but clozapine did so with significantly greater potency at nanomolar concentrations (Fig. 3, C to G). In contrast, CNO-stimulated [35S]GTPγS binding to hM4Di required micromolar concentrations (Fig. 3, C to G). Similar findings were observed in WT and hM4Di-Tg mice (fig. S3).
Fig. 3. Clozapine potently activates DREADDs and leads to DREADD-specific behavioral responses.
(A and B) Ca2+ dose-response for CNO and clozapine in HEK-293 cells expressing (A) hM3Dq or (B) hM4Di (from four experiments performed in triplicate). (C) Schematic illustration of AAV-hM4Di bilateral injection. (D) Fluorescence microscopy of brain sections from (C). (E) [3H]clozapine autoradiography of total and NS binding in brain sections from the rat in (D). (F and G) [35S]GTPγS autoradiography with adjacent sections treated with vehicle (Basal), CNO, or clozapine, or nonradioactive GTP (NS) illustrated (F) as a panel and (G) after densitometric quantification (n = 6 sections per condition, performed in duplicate). Statistical significance was calculated by means of one-way ANOVA followed by a Sidak post hoc multiple comparison test (**P < 0.01, **P < 0.001 compared with Basal, and #P < 0.05, ###P < 0.001 compared with the respective dose of CNO). (H) Rats expressing hM4Di-mCherry (n = 10 rats) and controls (GFP or sham) (n = 9 rats) in the accumbens/basal forebrain were tested for locomotor activity after injection of vehicle, CNO, and clozapine. Statistical significance was calculated by using repeated measures two-way ANOVA with group (control versus hM4Di) and drug as factors and Tukey’s post hoc multiple comparison test (*P < 0.05, **P < 0.01, ***P < 0.001 compared with the respective vehicle). (I) Drd1ahM4Dl mice (n = 6 or 7) and controls (n = 6 to 9) were injected with vehicle, CNO, or clozapine, and their ambulatory activity was recorded. Statistical significance was calculated by using repeated measures two-way ANOVA with group (control versus Drd1ahM4Di) and drug as factors and Tukey’s post hoc multiple comparison test (*P < 0.05, ***P < 0.001 compared with the respective vehicle). All values represent mean ± SEM.
To investigate DREADD-specific behavioral responses, rats were injected bilaterally with hM4Di and GFP AAVs or received sham surgery into the accumbens/basal forebrain and were tested for locomotor activity after intraperitoneal injections of vehicle, CNO, or clozapine. A low, subthreshold 0.1 mg/kg dose of clozapine significantly decreased locomotor activity in hM4Di- expressing rats but not in controls (Fig. 3H). The magnitude of this effect was comparable with that induced by 10 mg/kg CNO [~100-fold higher dose, which is consistent with plasma levels of converted [11C]clozapine (~2%) after [11C]CNO injection]. At a higher clozapine dose (1 mg/kg), both hM4Di-expressing rats and controls showed decreases in locomotor activity, likely because of sedative effects of clozapine at endogenous sites. We also performed similar experiments in Drd1ahM4Di mice and controls. Drd1ahM4Di mice exhibited widespread hM4Di expression (Fig. 3I), and thus, locomotor activity was examined after lower doses of CNO and clozapine. We found that 1 mg/kg CNO and 0.01 mg/kg clozapine both significantly decreased locomotor activity (Fig. 3I) selectively in Drd1ahM4Di mice and not in controls.
It was recently reported that converted clozapine reaches maximal cerebrospinal fluid (CSF) concentrations at 2 to 3 hours after CNO injection (12). We hypothesized that any effects of CNO on locomotor activity would thus be more likely to occur at this time point. Indeed, we observed significant decreases in locomotor activity in non-DREADD control mice at 2 to 3 hours after 1 mg/kg CNO injection, whereas 0.01 mg/kg clozapine had no such effect (fig. S4). Accordingly, 1 mg/kg CNO strongly inhibited locomotor activity in Drd1ahM4Di mice at 2 to 3 hours after injection (fig. S4).
Our collective findings indicate that systemic CNO shows extremely low CNS presence and highly unfavorable in vitro and in vivo pharmacology for DREADD activation. The likely reason for which [11C]CNO was detected in extracts from both GFP- and hM4Di-injected brain tissue is that [11C]CNO is circulating in brain blood vessels or in CSF [but not within the brain (16)], as shown previously where CNO CSF/plasma ratios are extremely low (11,12). Moreover, the fact that [11C]CNO was detected in both AAV-GFP and AAV-hM4Di extracts indicates that [11C]CNO presence in these samples is not due to specific DREADD binding. Last, lack of [11C]clozapine detection in AAV-GFP extracts is in line with CNO being inert at low (as in the trace doses we administered here) but not at higher systemic doses (6). Therefore, its above efficacy profile would not be relevant for in vivo CNS DREADD applications that use systemic CNO delivery. Furthermore, for studies in which such concentrations would be relevant, such as after intracranial injections of micromolar CNO concentrations (17), any CNO agonistic effects could be confounded by off-target effects at endogenous receptors (if present in the targeted region) (fig. S1). Additionally, results may be further confounded by local CNO-to-clozapine conversion (18) and thus effects of converted clozapine on both DREADDs and, if converted clozapine reaches high enough concentrations, endogenous clozapine binding sites as well. Additionally, experimental time frames should be carefully considered because of late-onset nonspecific effects of CNO, especially with repeated administration. Furthermore, intrinsic differences in metabolism between experimental animals or future patients may introduce experimental variability and lead to poor reproducibility of findings. Last, impurities in CNO sourced commercially (such as low CNO purity and/or residual presence of clozapine) may further complicate interpretation of experimental results and may be responsible for inconsistencies observed across laboratories.
Our study reveals the precise in vivo pharmacological mechanism of action of CNO at hM3Dq and hM4Di DREADDs by demonstrating that metabolically derived clozapine, arising from systemic CNO administration, is the in vivo DREADD actuator. Our findings provide context for interpretation of past studies implementing DREADDs and lead to the conclusion that investigators using DREADD technology should use subthreshold doses of clozapine as the pharmacological agent instead of high doses of CNO, whose mechanism of action at DREADDs is mediated by converted clozapine.
DREADD technology is considered a promising neuromodulation therapeutic for clinical use. However, using CNO in humans has been a limiting factor for clinical use of DREADDs because of known conversion to clozapine in vivo in humans. Although the use of clozapine in humans as a DREADD actuator could potentially lead to undesirable side effects, clozapine is already approved by the U.S. Food and Drug Administration, it has been used clinically for decades, and its pharmacokinetic and pharmacodynamic properties and side effects are known. Because DREADDs require very low, subthreshold clozapine doses for their selective activation in vivo, our findings address this critical limitation and elevate the translational potential of DREADD technology as a clinical CNS therapeutic.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIDA Intramural Research Program (ZIA000069 and ZIA000526). We thank M. Higuchi, B. Ji, and T. Minamimoto for providing access to WT and hM4Di transgenic mouse tissue. We thank M. Sanchez-Soto and D. Howard for assisting with in vitro studies. Last, we thank G. Smith, H. Davis, N. Ator, D. Jacob, J. Engles, B. Liu, and M. Morales for research and administrative support and Y. Shaham, A. Newman, and C. Bradberry for their comments on the manuscript. M.M. is a cofounder and owns stock in Metis Laboratories. All other authors declare no conflicts of interest. All data are stored in our laboratory and freely available upon request. J.L.G., J.B., and M.M. designed experiments, performed experiments, and wrote the manuscript. W.L., P.S.-S., C.T.R., W.B.M., L.A.R., and R.J.E. performed experiments. B.K.H., R.F.D., M.G.P., and A.B. provided access to administrative/research support, reagents, and/or equipment. All coauthors reviewed the manuscript and provided comments.
Footnotes
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, Proc. Natl. Acad. Sci. U.S.A 104, 5163–5168 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.English JG, Roth BL, JAMA Neurol. 72, 1361–1366 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Urban DJ, Roth BL, Annu. Rev. Pharmacol. Toxicol 55, 399–417 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Ji B et al. , J. Neurosci 36, 11544–11558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schotte A et al. , Psychopharmacology (Berl.) 124, 57–73 (1996). [DOI] [PubMed] [Google Scholar]
- 6.MacLaren DAA et al. , eNeuro 3, e0219–16.2016 (2016). [Google Scholar]
- 7.Jann MW, Lam YW, Chang WH, Arch. Int. Pharmacodyn. Ther 328, 243–250 (1994). [PubMed] [Google Scholar]
- 8.Chang WH et al. , Prog. Neuropsychopharmacol. Biol. Psychiatry 22, 723–739 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Lin G, McKay G, Midha KK, J. Pharm. Biomed. Anal 14, 1561–1577 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Guettier JM et al. , Proc. Natl. Acad. Sci. U.S.A 106, 19197–19202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldridge MA et al. , Nat. Neurosci 19, 37–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raper J et al. , ACS Chem. Neurosci 8, 1570–1576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender D, Holschbach M, Stöcklin G, Nucl. Med. Biol 21, 921–925 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Michaelides M et al. , J. Clin. Invest 123, 5342–5350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schinkel AH, Wagenaar E, Mol CA, van Deemter L, J. Clin. Invest 97, 2517–2524 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardridge WM, Expert Opin. Drug Deliv 13, 963–975 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Mahler SV et al. , Nat. Neurosci 17, 577–585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang J, Eur. J. Drug Metab. Pharmacokinet 25,109–114 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.