Abstract
Coordinated Oral health Promotion (CO-OP) Chicago is a two-arm cluster-randomized trial with a wait-list control. The primary aim is to evaluate the efficacy of an oral health community health worker (CHW) intervention to improve oral health behaviors in low-income, urban children under the age of three years. Exploratory aims will determine cost-effectiveness, and if any CHW intervention impact on child tooth brushing behaviors varies when CHWs are based out of a medical clinic compared to a community setting. This paper describes progress toward achieving these aims. Participating families were recruited from community social service centers and pediatric primary care medical clinics in Cook County, Illinois. Sites were cluster-randomized to CHW intervention or usual services (a wait-list control). The intervention is oral health support from CHWs delivered in four visits to individual families over one year. The trial sample consists of 420 child/caregiver dyads enrolled at the 20 participating sites over 11 months. Participant demographics varied across the sites, but primary outcomes values at baseline did not. Data on brushing frequency, plaque, and other oral health behaviors are collected at three timepoints: baseline, 6-, and 12-months. The primary analysis will assess differences in caregiver-reported child brushing frequency and observed plaque score between the two arms at 12-months. The trial is currently in the active intervention phase. The trial’s cluster-randomized controlled design takes a real-world approach by integrating into existing health and social service agencies and collecting data in participant homes. Results will address an important child health disparity. ClinicalTrials.gov identifier: NCT03397589.
Clinical trial registration
University of Illinois at Chicago Protocol Record 2017-1090
National Institutes of Dental & Craniofacial Research of the National Institutes of Health (NIDCR) Protocol Number: 17-074-E
Keywords: Pediatric dentistry, child, tooth brushing, oral health, community health workers, public health
1. Introduction
Dental caries, the most common chronic disease of childhood, has been documented in nearly half of United States (US) children two to eleven years old [1]. Fifteen percent of children aged six to eleven have had untreated caries [1]. The prevalence of both caries and untreated caries in elementary school children in Illinois and Chicago has surpassed national figures [2]. Caries and its associated morbidities have been shown to disproportionately affect low-income and minority children [3–6]. Unhealthy diets, inadequate exposure to fluoride, and poor oral hygiene all contribute to caries [7–9]. Effective multilevel interventions to improve oral health and reduce health disparities in low-income children are needed.
Community health workers (CHWs) have contributed to disease prevention and health promotion efforts in US communities for decades[10] and could potentially address oral health disparities. A growing number of studies support the work of CHWs to improve a range of health outcomes [11–17]. CHWs are non-clinicians that provide health education, social support, care coordination, navigation, and advocacy services to expand individual and community capacity for health [10, 18, 19]. CHWs are thought to be effective because they reside in the same communities (have social proximity) and have faced many of the same challenges as the target population; thus, they have a comparable knowledge base and understanding [10, 19]. Previous studies have demonstrated that oral health education provided to parents had a positive effect on knowledge, intended behavioral change (including snacking and sugar intake), and caries prevalence for children [20–22]. However, limited data exist that support CHW intervention efficacy in changing oral health outcomes in children or associated family behaviors [20, 23–26]. The goal of the COordinated Oral health Promotion (CO-OP) Chicago trial is to expand these data. The trial design and baseline characteristics of participants are described in this paper.
1.1. Trial Objectives
The CO-OP Chicago Trial primary objective is to evaluate the efficacy of an oral health CHW intervention to improve oral health behaviors in low-income, urban children under the age of three years. We hypothesize that participants receiving the oral health CHW intervention, compared to usual care (no direct study intervention), will have improved oral health behaviors. The difference will be measured using caregiver-reported brushing frequency and observed plaque score at 12-months. Exploratory aims will determine cost-effectiveness, and if any CHW intervention impact on child tooth brushing behaviors varies when CHWs are based out of a medical clinic compared to a community setting. The results of this trial will inform future programs that include oral health specific CHWs.
2. Methods
2.1. Design
Formative work informed the CO-OP Chicago final design [27] of a two-arm, cluster-randomized controlled trial with repeated measurements. Child/caregiver dyads recruited from ten Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) centers and ten pediatric medical clinics were randomized into two arms: CHW intervention and usual care. Matched randomization of clusters was employed, site race/ethnicity characteristics (Black, Hispanic, or White), site size (large, medium, small), and setting (WIC or clinic) as cluster-level covariates. Research assistants (RAs) collect outcome measures at baseline, six months, and 12 months.
2.2. Population
CO-OP Chicago targeted healthy, low-income, predominantly minority children under the age of three years, and their families in Cook County, Illinois. Eligibility criteria included children ages 6–36 months, with a minimum of two fully erupted central maxillary incisors, who were active patients/clients in the clinics/WIC centers from where they were recruited. Eligibility criteria also required that caregivers be age 18 or older, speak English or Spanish, and be the children’s primary caregivers. We defined “primary caregiver” as the person (or one of the people) consistently responsible for the child’s daily routines and who is a legal guardian. For children residing in multiple households, the primary caregiver had to live with the child at least five days out of the week to qualify. Child/caregiver dyads were excluded from the trial if the child had a medical condition that would limit his or her ability to conduct the study activities, such as severe developmental or cognitive delay, ventilator or oxygen dependence, oral aversion, or severe facial deformities.
2.3. Interventions
2.3.1. CHW training and hiring
Because of limited national and local formal certification or licensing programs for CHWs [28], training curriculums are typically developed for individual programs and are not standardized for any disease area. CHWs also require training in building relationships, self-advocacy, teamwork and conflict resolution, crisis and emergency management, and home visitation before going into the field [19, 29, 30], CO-OP Chicago built and tested a comprehensive oral health CHW training curriculum [29], available at https://go.uic.edu/COOPChicago. Applicants for the CO-OP Chicago CHW positions were recruited through existing UIC programs, community list-serves, participating medical clinics and WIC sites, and word of mouth. Candidates who had already worked as CHWs and had experience with the participating clinics or WIC centers were given preference.
CHW selection began with a pre-hire oral health specific training session for 23 individuals who were interested in the position. Pre-hire training served several purposes: 1) it built community capacity and skills around oral health, 2) it allowed investigators to observe candidates in group settings and role-plays, and 3) it provided a pool of back-up candidates for the future. This unpaid five-hour training was conducted by the principal investigator (a general pediatrician), with support from a dental hygienist. The training included standardized role-plays to evaluate skill attainment and content delivery. After training completion, the CHW positions were posted, and the trainees were invited to apply. Three CHWs with social proximity to the target population were hired based on their performance in the training, special skills (e.g., Spanish fluency), and subsequent interviews. After the CHWs were hired, they underwent additional training (Table 1) for which their time was compensated.
Table 1:
CHW Training Components
| CHW Training | Hours | Training Leader |
|---|---|---|
| Oral health training | 5 hours | PI (general pediatrician) |
| Human Subjects | 3 hours | CIRT* |
| Good Clinical Practices | 3-4 hours | CITI Program** |
| Information Privacy & Security | 2-4 hours | CITI Program** |
| Mental Health First Aid | 8 hours | Community social service agency |
| Motivational Interviewing Training | 2 hours | Co-I (health psychologist) |
| Home Visit Training | 2 hours | PI (general pediatrician) and Project Manager |
| Paper Case Report Forms Training | 1 hour | Project Manager |
| REDCap Database Training | 2-3 hours | Coordinating Center and Study Data Manager |
| Protocol Training | 2-3 hours | Project Manager |
| Manual of Procedures Training | 1-2 hours | Project Manager |
| CHW Core Curriculum Manual Training | 3 hours | Dental Hygienist |
| Dental Clinic clinician shadowing | 3-5 hours | Co-I (pediatric dentist) |
| Pediatric Clinic clinician shadowing | 3-5 hours | PI (general pediatrician) |
CHW = community health worker, Co-I = co-investigator, PI = principal investigator
Anderson EE. CIRTification: Community Involvement in Research Training. Facilitator Manual. Center for Clinical and Translational Science. University of Illinois at Chicago. 2011.
CITI Program Human Subjects Research, and Information Privacy & Security. www.citiprogram.org
As part of field training, the first few CHW home visits were conducted with the CHW supervisor. The CHW supervisor scored each visit using the CHW Fidelity Assessment Form, which was modified from Project MATCH: Training for a Promotora Intervention [31]. CHWs were assessed for the accuracy and clarity of the content, personalization, openness to questions, action planning, and interpersonal skills. Each item was scored from one to five (1=needs review and additional practice, 2=demonstrates basic understanding, 3=demonstrates full understanding but needs more practice, 4=ready to enter the field, 5=ready to be a role model for others). After achieving a score of at least four in each category on three visits, each CHW was cleared to visit participants alone. Continual training includes monthly reviews of topics of interest to the CHW and of oral health topics as needed.
2.3.2. CHW supervision
CHWs report directly to the CHW supervisor (the CO-OP Chicago project manager). The supervisor is responsible for day-to-day support and oversight. Additionally, every two weeks, CHWs meet with the CHW supervisor and available investigators (usually the principal investigator) to discuss self-management skills, clinical issues, and continuing education. In these meetings, a team approach is used to facilitate self-discovery and group learning. Every two months, or more frequently, if needed, a clinical psychologist meets with the CHWs to discuss their mental health in relation to the CHW job and trial participants. Strategies to resolve mental health concerns are generated during the meetings. This support is important for CHWs due to the stressful nature of the job. CHWs experience challenges with difficult participants, hectic households, unclean environments, poverty issues, and, sometimes, personal safety issues. Many CHWs also struggle or have struggled with these same issues themselves. Therefore, the supervision and support provided to CHWs, as outlined above, is crucial.
2.3.3. CHW intervention pedagogy
CHWs use social cognitive theory to help families shape and maintain behaviors [32], The CO-OP Chicago Oral Health CHW intervention applies formal self-management skills, including decision-making, problem-solving, patient/doctor partnership, resource utilization, and taking action [33–36], The CHWs introduce a topic after exploring participants’ needs. When education alone is not sufficient to resolve a deficit or fix a behavior, the CHWs incorporate relevant self-management skills and create goals. For example, a CHW may suspect the family is not brushing the child’s teeth regularly, but rather than confront the family with this suspicion, the CHW has the family prospectively track how often the child brushes over one week. The family then “self-discovers” that they do not brush as often as recommended, and the CHW continues intervention by having the family brainstorm possible reasons for why this is happening (bathroom organization, parent work schedules, behavioral issues, etc.). Once they have listed the problems, the family creates an action plan which is a self-identified goal for which the family feels a high level of efficacy or motivation. Action plans goals are small goals for a specific change, to be completed over a designated period. The action plan for this example might be to buy containers at the dollar store that can be used to organize the bathroom better. On subsequent CHW visits and follow-up telephone calls, CHWs revisit past action plans, revise them, and create new ones.
2.3.4. CHW intervention administration
Intervention-arm families are offered four in-person visits and four phone call follow-ups over 12 months. The expectation is that two visits occur in the first six months, and two in the second six months. Phone call follow-ups occur after the in-person visits, usually one to two weeks following the home visit. Visits can take place at any location of the family’s choosing, such as the home, clinic, WIC, or anywhere they feel comfortable. All scheduling and contact efforts with families begin on the telephone. CHWs follow a detailed contact protocol that requires multiple attempts at different times of the day using telephone, followed by text and email and letters that are hand delivered to the home address. Then site partners are engaged. Finally, certified letters are sent. CHWs do not stop trying to reach families until their intervention windows close.
At in-person visits, which can be held any day of the week (including weekends), CHWs spend one to two hours with the family. The first several minutes are spent in social engagement (getting to know the people in the family and their interests, as well as the family getting to know the CHW, or catching up socially if families are already known). CHWs try to engage all family members in the visits, if possible. At the first visit, usually, the CHWs administer the non-clinical portion of the Caries-risk Assessment Tool [37] for all family members present. CHWs follow this with tailored education based upon the risk assessment results using a variety of live demonstrations, pictorial, video, and written resources. Oral health topics are drawn from the core curriculum [27, 29], All topics are intended to be addressed by the CHWs over the one-year intervention, but not all topics are covered at each individual visit. Visits include 15 minutes of action planning with the families. At the start of subsequent visits, previous Action Plans are reviewed and discussed. During the follow-up call or text, the CHW reviews actions and reinforces concepts from the previous visit.
CHWs apply popular education methods [38], meaning they rarely use written materials. Instead, they engage families in discussion. These are the same techniques and methods used in the CHW training. For equipment, CHWs have computers with Wi-Fi access (via hotspots) to view videos, and models for demonstrating tooth brushing, which they practice with families. CHWs have access to online community resources and facilitate family-wide dental care access by identifying accepting providers, supplying families with provider contact information, and assisting in making appointments as needed. CHWs document topics covered and materials used immediately after each visit in the trial’s database, Research Electronic Data Capture (REDCap, version 9.1.3, Nashville, TN), electronic data capture tools hosted at the University of California, San Francisco [39,40], REDCap is secure, web-based software supporting clinical research data capture which includes 1) a validated data capture interface; 2) audit trails for tracking data edits and exports; 3) automated export for download to popular statistical packages; and 4) data integration and external source interoperability tools.).
CHWs also visit the clinics and WIC sites where recruitment occurred to provide education to non-study participants. Families at the five clinics and five WIC centers that were randomized to the CHW intervention receive these visits over the year after randomization. Families at the sites in the usual care arm are offered CHW services after the final 12-month data collection is complete. During site visits, CHWs educate patients/clients at the clinics/WIC offices on oral health and help make oral health referrals. This service is provided once a week for three to four hours for nine to 11 months. When talking with families, the CHWs provide a handout called “A Healthy Mouth for Your Baby” [41] for the caregiver to read and discuss with the CHW if they choose. The CHWs can also discuss a variety of other topics, such as how to obtain medical insurance, and provide many other health-related handouts that go beyond just oral health. During or immediately after the encounters, the CHWs record non-identifiable basic demographics for the individuals with which they engaged in REDCap.
2.3.5. CHW fidelity monitoring
CHWs manage caseloads that change as participants are randomized and as the intervention is completed; CHWs can have a caseload of up to 73 participants. CHW visit documentation is reviewed weekly by the CHW supervisor and investigators to ensure consistency and fidelity of the intervention content, and to inform the trial team about areas of focus and challenge. Reports from these data indicate core curriculum topics covered and pending per participant, resources used, and overall trends in topics [29]. While the goal is to cover each core curriculum topic throughout the intervention with each family, CHWs may deviate due to family needs and resources [29]. Deviations are regularly reviewed; if they are due to CHW issues (lack of comfort with a topic or poor organization), additional training is provided either to the specific CHW or to the group as needed. The CHW supervisor accompanies each CHW on a minimum of one visit out of every 30 (8% of total visits). The CHW supervisor assesses the CHW using the CHW Fidelity Assessment Form. If a CHW scores less than four on any category, additional training and support are provided. Depending on the specific issue, training/support could include extra sessions with the investigators, additional shadowing of more experienced CHWs, and time in the dental clinic. Visit supervision and fidelity scoring is implemented until the CHW consistently scores fours on all categories.
2.4. Measures
Measures (Table 2) are collected via caregiver self-report, clinical assessment, and observation of tooth brushing behaviors and equipment at baseline, six months, and 12 months. To reduce travel and childcare burdens, and maximize participant comfort, data are collected in participant homes or other agreed-upon locations. RAs verbally ask questions (using prompt cards when appropriate) about demographics, brushing, general health, social support, access to care, and health behaviors. RAs communicate in English, Spanish, or a mixture depending on the participants’ preferences. Children are then laid on their backs, their teeth exposed, and a disclosing solution applied to the teeth that temporarily stains dental plaque red. The RAs photograph the teeth before and after application of the disclosing solution. Images are later scored by calibrated clinicians at UIC, resulting in an Oral Hygiene Index - Maxillary Incisor Simplified (OHI-MIS) plaque score [42–44], Photographs are also screened for severe caries that may pose an acute risk to the child. If the clinician deems there to be an acute risk to the child, the RA will contact the family to inform them to seek dental care using the method described in the study protocol. The process of plaque disclosing, clinician calibration, and image scoring will be described in detail in other publications.
Table 2:
Measures Collected at Baseline, 6-months, and 12-months
| Domain | Question | Collection method | Details, Citations |
|---|---|---|---|
| Demographics | Child and caregiver: Age, sex, race, ethnicity. Caregiver relationship to child, highest degree, relationships status. Household income, size, number and ages of children. | Caregiver report | |
| Child and caregiver health insurance | Caregiver report | ||
| Child weight | Measured | ||
| Child medical history | Caregiver report | ||
| Child medications | Caregiver report | ||
| Child brushing behavior | Child brushing frequency* | Caregiver report | [45,46] |
| Amount of plaque * | Observed | Oral Hygiene Index-Maxillary Incisor Simplified Scale [42–44] | |
| Child age started brushing | Caregiver report | NHANES [47] | |
| Caregiver assistance with brushing | Caregiver report and observed | [45, 46] | |
| Child duration of brushing | Caregiver report and observed | ||
| Child age started using toothpaste | Caregiver report | ||
| Child type of toothpaste | Caregiver report and observed | ||
| Child amount of toothpaste | Caregiver report and observed | NHANES [47] | |
| Type of toothbrush | Observed | ||
| Mouth rinsed | Observed | ||
| Parenting behaviors during brushing | Observed | Toothbrushing Observation System [48] | |
| Child oral health other behaviors | Child dental care utilization | Caregiver report | NHANES [47] |
| Child barriers to dental care | Caregiver report | NHANES [47] | |
| Child type of drinking water | Caregiver report | [45, 46] | |
| Sugar sweetened beverages | Caregiver report | [45, 46] | |
| Child type of cup/bottle | Caregiver report | [45, 46] | |
| Child cup/bottle at night | Caregiver report | [45, 46] | |
| Child caries history | Child caries history | Caregiver report | |
| Child history of general anesthesia for dental | Caregiver report | ||
| Child oral health quality of life | Child oral health quality of life | Caregiver report | ECOHIS [49] |
| Caregiver factors related to child oral health behaviors | Caregiver knowledge and attitudes on oral health | Caregiver report | [45, 46] |
| Caregiver self-efficacy | Caregiver report | [45, 46] | |
| Caregiver sources of support for brushing | Caregiver report | ||
| Caregiver barriers to brushing | Caregiver report | ||
| Caregiver/family oral health | Caregiver general oral health assessment | Caregiver report | NHIS [50] |
| Caregiver oral health quality of life | Caregiver report | Oral Health Impact Profile [51, 52, 53] | |
| Caregiver brushing frequency | Caregiver report | [45, 46] | |
| Caregiver dental care utilization | Caregiver report | NHANES [47] | |
| Caregiver reason for last dental visit | Caregiver report | NHANES [47] | |
| Caregiver barriers to dental care | Caregiver report | NHANES [47] | |
| Sibling history of general anesthesia for dental | Caregiver report | ||
| Caregiver physical and psychosocial health | Caregiver general health | Caregiver report | NHIS [50] |
| Caregiver social functioning | Caregiver report | PROMIS Ability to Participate in Social Roles and Activities [54–57] | |
| Caregiver depression | Caregiver report | PROMIS Depression [54–57] | |
| Caregiver anxiety | Caregiver report | PROMIS Anxiety[54–5 7] | |
| Caregiver emotional, instrumental, informational social support | Caregiver report | PROMIS Social Support [54–57] | |
| Family functioning | Caregiver report | Confusion, Hubbub and Order Scale [58–61] | |
| Cost effectiveness | CHW intervention delivery costs | Study process data |
Primary outcome
When the data collection occurs in the home, families are asked to demonstrate how the child’s teeth are brushed. Parent involvement in the process, the technique and equipment used, and the length of the activity are documented. Due to the visual, auditory, and hands-on demands of collecting observational and timed data, RAs perform data collection in pairs. All data are entered directly into REDCap. If technology is not working, data are documented on paper forms for later entry.
2.5. Recruitment, randomization, and retention
2.5.1. Recruitment
CO-OP Chicago aimed to recruit 18-23 dyads from each of the 20 sites (clinic or WIC center) for a total of 420 caregiver/child dyads. Sites were organized by geography, with three to four sites active for recruitment at a time. Because of anticipated differences in recruitment and enrollment across various sites, the most challenging sites were scheduled in the middle of the recruitment period. Sites took a median of 8 weeks to complete recruitment; the fastest site finished in 2 weeks, the slowest in 19 weeks. To identify participants, RAs approached caregivers of children who seemed to be under the age of three in the sites’ waiting areas. RAs described the trial verbally and using a short video, assessed interest, and screened the caregiver for eligibility if interested. If the family met the inclusion criteria, the RA obtained the caregiver’s contact information and scheduled an enrollment appointment. From January 20, 2018, to February 23, 2019, 2,225 families were approached: 653 refused the screening, while 1,572 agreed to be screened. Of those screened, 725 were eligible, 422 consented, and 420 were randomized after obtaining baseline data (Figure 1). Baseline data collection lasted an average of 36 minutes per visit, while overall recruitment required 2,026 RA hours, about 4.8 hours per randomized participant.
Figure 1:
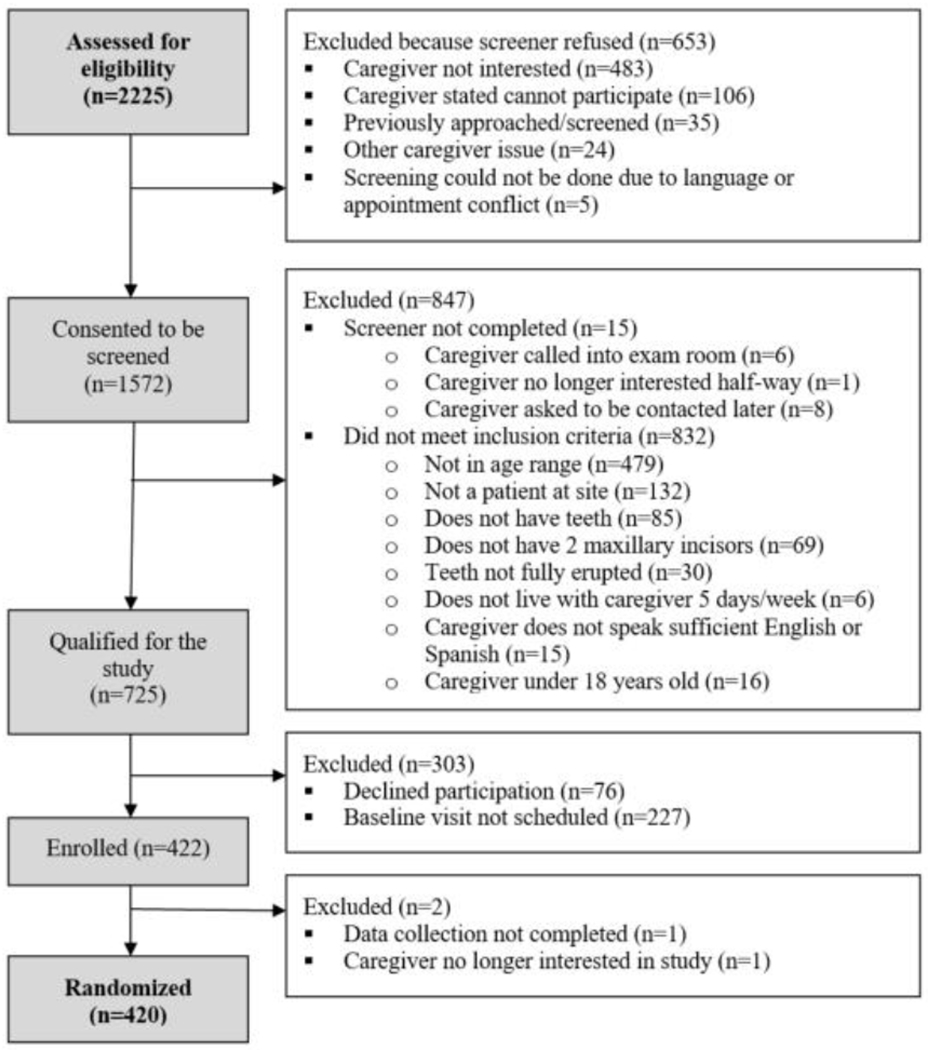
CO-OP Chicago Recruitment Diagram
2.5.2. Randomization
Sites were randomized once they reached 90% of their recruitment goal (18-23 families, depending on site size). Matched randomization of clusters was employed, using methods described by Ivers et al [62]. Cluster-level covariates regarding race/ethnicity characteristics of the site population (Black, Hispanic, or White), site size (large, medium, small), and setting (WIC or clinic) were used as cluster-level covariates. Cluster-level randomization was done by the NIDCR-appointed external University of California, San Francisco Dental Coordinating Center (CC) prior to recruitment with the randomization schedule stored in REDCap for concealment. Once a site was ready to be randomized, REDCap revealed the randomization arm to the project manager. She then informed the site leadership of the site assignment. For sites randomized to the intervention arm, the UIC project manager assigned a CHW to the site and the individual site participants. Usually, this was the same CHW, but some exceptions were made to accommodate CHW caseloads and language preferences. The project manager then informed the CHW of her participants and provided access to their contact information. UIC sent letters to participants informing them of their site’s assignment and their CHW’s information if applicable.
To reduce possible bias, RAs are blinded to the study arm, and communications with partners emphasize equipoise. Additionally, participants are reminded at every contact not to disclose any intervention they are or are not receiving. The project manager, investigators and staff working directly with the CHWs, and the CC are unblinded to treatment arm.
2.5.3. Retention
The retention plan was informed by formative work prior to starting the trial [27] Various types of contact information, such as phone numbers, e-mail addresses, home addresses, and alternative contact information, were collected for participants. At each time point, RAs obtain updated contact information, the caregivers’ preferred contact method, and when to best reach them. There was also careful vetting of all research materials through a community advisory board to ensure that participants feel connected to the trial, strong partnerships are maintained with clinics/WIC centers, and hired field staff are engaging, respectful, and non threatening. Additionally, participants receive remuneration for data collection. At the completion of the enrollment visit, participants were given $40 and a flyer describing basic oral health tips. After enrollment, participants are contacted again by RAs at six months and 12 months for subsequent data collection. Payment is the same at those visits ($40). In total, families that complete all data collections will have received up to $120.
2.6. Human subjects and study monitoring
The trial was approved by Institutional Review Boards at the University of Illinois at Chicago [2017-1090], the University of California San Francisco [16-19920], and the Chicago Department of Public Health [16–6], Informed consent was waived for screening. Caregivers provided written informed consent at the start of the enrollment visit. Enrolled children were too young to provide assent.
Trial oversight is provided by a Data Safety Monitoring Board (DSMB), an external monitor selected by the funder, and a Community Advisory Board (CAB). The DSMB is composed of members with appropriate clinical, statistical, scientific, and ethical expertise. Members were appointed by the National Institute of Dental and Craniofacial Research (NIDCR). The DSMB is coordinated by the CC. The DSMB met twice a year during the first two years of the trial and continues annually to assess safety and efficacy data (if applicable), trial progress, and data integrity. More frequent meetings may be held in the future if concerns arise. The external monitor, NIDCR’s Clinical Research Operations and Management Services contractor, visited the trial prior to launch, annually during the trial, and will visit again at closeout to review the regulatory binder, all informed consent forms, and a subset of all trial data. The CAB was established during the formative phase of the trial and includes a CHW, caregivers, and representatives from health, advocacy, and policy organizations. The CAB meets every three to six months to provide guidance on trial operations and trial relevance to community stakeholders. They give input regarding the development, recruitment and retention issues, intervention implementation, and outcomes of the clinical trial.
The complete approved protocol is available upon request from the authors.
2.7. Analyses
2.7.1. Sample size and power
Daily frequency of brushing as an ordinal variable and plaque score as a continuous variable were the two primary outcomes used in power analyses. We powered on detecting an effect size of 0.40 for plaque score and an odds ratio (OR) of 2.0 for the ordinal frequency of brushing. These effect sizes were based on results from our pilot work [27]. Estimates of OHI-MIS scores from our pilot study (mean=1.2, SD=0.78) suggested an effect size of 0.40 corresponded to a change of 0.31 units between arms. For brushing frequency, the percentages of responses in the pilot study was 16% brushed fewer than once a day, 26% reported brushing once a day, and 58% brushed twice or more times a day. This effect size (OR=2) translated to the following percentage of frequency of brushing in the intervention arm: 9% fewer than once a day, 18% once a day, and 73% twice or more times a day. The analysis accounted for repeated observations and 15% attrition at each follow-up. To achieve a power of 80%, 21 participants were estimated to be needed in each of the 20 clusters (420 total participants). The sample size calculation controlled for the multiplicity of having two outcome measures by Bonferroni adjustment (Type 1 error = 0.025). The proposed sample size was a conservative estimate as the simulation does not take into account stratification due to clinics and WICs. The analysis will control for outcome imbalance between clinics and WICs via covariate adjustment and hence increase the precision of the estimates.
2.7.2. Aims and Analysis plan
The primary aim is to evaluate the efficacy of a one-year oral health CHW intervention compared to usual care to improve oral health behaviors in low-income urban children under the age of three years. Oral health behaviors are assessed as the frequency of brushing and OHI-MIS plaque score. For brushing frequency, a random-effects ordinal regression model will be employed. The OHI-MIS score will be analyzed as a continuous variable using a linear random-effect model. For both outcomes, the between-subjects factors -usual care or CHW arm, within-subjects occasions of measurement - time, and their interaction will be in the model. The interaction term is the focus of the analyses. We will employ Bonferroni-Holm adjustments to control for multiplicity [63], Fixed effect indicators for clinic and WIC sites, as well as size and race/ethnicity variables used in restricted randomization, will be included in all models, using methods specific to cluster-randomized trials [64–66], Each site was a unit of randomization, hence any imbalance in site characteristics is due to random variation (‘random confounding’). Random confounding by covariates could influence the treatment effects. We will not test for imbalance of baseline covariates, as recommended by the CONSORT statement. Instead, analyses will include subject-level covariates that are determined a priori based on the current best evidence. The impact of clustering of participants in sites is larger variability of the estimated treatment (group) variances, which is reflected in the intraclass correlation coefficient (ICC). Covariate adjustment, when the covariate uncovers meaningful confounding related to the intervention effect, might increase or decrease the ICC. We will a priori choose covariates carefully with consideration of their influence on the ICC and the power of the trial.
The analyses will be carried out under intendon-to-treat principles [67–70], Missing data will be imputed using methods adapted for cluster-randomized controlled trials. We anticipate data missing at random, but will be vigilant for data not missing at random as well.
The first exploratory aim is to determine if the oral health CHW intervention impact on child tooth brushing behaviors varies when the CHWs are based out of a medical clinic compared to a community WIC center. The null hypothesis is that there will be no difference between clinic and WIC sites. This will extend models described above with a three-way interaction: time by group by type of site. The analyses will follow standard mixed model approaches described by Hedeker and Gibbons [71], We will estimate the intervention effect (average change in the outcomes) at the last follow-up conditional on site type. The 95% confidence interval (Cl) of site difference in the estimated means will be compared to a prespecified acceptance criterion (scientifically justified maximum difference of no clinical significance). If the 95% Cl is contained within the acceptance criteria, the equivalence between sites can be declared (based on two one-sided tests approach) [72].
The second exploratory aim is to determine the cost-effectiveness of the CHW intervention compared to usual care from the perspective of a service delivery (system-level) entity. Cost data will first be summed and presented on the system-level (program costs, separate for clinic and WIC sites). Cost-effectiveness will also be assessed by determining the mean total cost per participant versus the change in primary outcomes [73]. The incremental cost-effectiveness ratio (ICER) for the CHW home intervention compared to usual care is found using the equation ICERt = (C1 – C0) / (E1t – E0t), where C corresponds to the average cost and E corresponds to the average effectiveness. Subscript 1 signifies the CHW home intervention (treatment group), and subscript 0 signifies the comparison condition (control group). The time period (six months and 12 months) is denoted by subscript t. In order to assess the uncertainty in the results, 95% CIs will be calculated for the ICERs [74–76], To determine if the ICERs are sensitive to conceivable changes in the values of the key parameters, one-way and multi-way sensitivity analyses on the key parameters influencing the ICERs will be completed. Sensitivity analyses will define the strength of the comparisons and the key parameters affecting the ICERs [74].
2.8. Dissemination
Results will be disseminated through presentations at national scientific meetings and local grand rounds, faculty workshops, publications in peer-reviewed journals, local data releases via media, the study website, and advocacy outreach (handouts, videos, and presentations for partner sites and communities). The investigators, partners, and CAB have influence on many levels of local, state, and national policy. They will ensure that if indicated, trial results will be applied towards the development of new research studies, workforce development protocols, and reimbursement policies.
3. Results
3.1. Demographics (Table 3)
Table 3:
Trial Participant Demographics at Baseline
| Caregiver N=420 | Child N=420 | |
|---|---|---|
| Female (%) | 405 (96.4) | 213 (50.7) |
| Age, mean (SD) | 29.6 (6.6) years | 21.5 (6.9) months |
| Highest degree earned (%) | NA | |
| Less than high school | 68 (16.2) | |
| High school/GED | 132 (31.4) | |
| More than high school | 220 (52.4) | |
| Hispanic (%) | 219 (52.1) | 226 (53.8) |
| Mexican (% of Hispanic) | 184 (84.0) | 166 (73.1) |
| Race (%) | ||
| White | 57 (13.6) | 54 (12.9) |
| Black | 176 (41.9) | 176 (41.9) |
| Other | 187 (44.5) | 190 (45.2) |
| Household income in last year (%) | NA | |
| <30k | 98 (23.3) | |
| 30k-60k | 74 (17.6) | |
| >60k | 28 (6.7) | |
| Unknown | 219 (52.1) | |
| Refused | 1 (0.2) | |
| Caregiver relationship status (%) | NA | |
| Single | 142 (33.8) | |
| Living with partner/ spouse | 257 (61.2) | |
| Separated/ divorced/ widowed | 21 (5.0) | |
| Household size, mean (SD); [range] | 4.8 (1.7); [2–11] | NA |
| Children in household, mean (SD); [range] | 2.5 (1.3); [1–9] | NA |
| Child medical problems (%) | NA | |
| Ear infections | 135 (32.1) | |
| Nose/eye/mouth/throat problems | 88 (21.0) | |
| Diarrhea | 215 (51.2) | |
| Constipation | 191 (45.5) | |
| Skin problems | 124 (29.5) | |
| Sleeping problems | 31 (7.4) | |
| Breathing problems (including asthma) | 63 (15.0) | |
| Other | 52 (12.4) | |
| Taking prescription medicines (%) | NA | 57 (13.6) |
| Taking over-the-counter medicines (%) | NA | |
| None | 187 (44.5) | |
| Vitamins | 61 (14.5) | |
| Herbal medicine/Other | 172 (41.0) | |
| Has health insurance (%) | 319 (76.0) | 401 (95.5) |
| Medicaid (%, N=319/401) | 247 (77.4) | 358 (89.3) |
| Has dental insurance (%) | 276 (65.7) | 344 (81.9) |
| Medicaid (%, N=276/344) | 217 (78.6) | 311 (90.4) |
The mean (SD) child age was 21.5 months (6.9) at the time of baseline data collection, and 50.7% were female. Most caregivers (96.4%) were female. Ethnicity of caregivers and children were similar, with 53.8% of children reporting Hispanic ethnicity; the primary subethnicity was Mexican (73.1% of Hispanic participants). Many caregivers (mainly those who chose Hispanic ethnicity) reported “other” for their and their child’s race, and 41.9% of caregivers and children identified as Black. Families struggled to answer the household income question. Those that responded were mostly low-income. The majority of caregivers were married/living with a partner (61.2%). The mean (SD) household size was 4.8 (1.7) people, with a mean (SD) of 2.5 (1.3) children per household. Child medical issues were common for their age range, including diarrhea, constipation, and ear infections. Few children took prescription medications (13.6%), but many took vitamins, herbal remedies, or over the counter medicines (41%). Almost all children had health insurance (95.5%), mainly through Medicaid (89.3% of those with insurance), while 76% of caregivers had health insurance. Similarly, most children had dental insurance (81.9%), mainly through Medicaid (90.4% of those with insurance), while only 65.7% of caregivers had dental insurance.
3.2. Child oral health status (Table 4)
Table 4:
Oral Health Outcomes at Baseline
| Total Caregiver Report N=420 | Total Observed N=420 | |
|---|---|---|
| Child’s brushing frequency (%) | ||
| Never/has not started | 25 (6.0) | |
| Sometimes, but not everyday | 64 (15.2) | |
| Once a day | 142 (33.8) | |
| Twice a day | 168 (40.0) | |
| More than twice a day | 21 (5.0) | |
| OHI-MIS plaque score,1 mean (SD) n=419 | 1.9 (0.6) | |
| Age child started brushing teeth (months), mean (SD) n=394 | 9.6 (4.9) | |
| Do adults help with brushing (%)2 | ||
| No, child brushes alone | 11 (2.6) | |
| Sometimes | 71 (17.0) | |
| Most of the time | 80 (19.0) | |
| Always | 233 (55.5) | |
| Actual parent involvement (%)n=348 | ||
| Parent did all brushing | 226 (64.9) | |
| Both parent and child participated in brushing | 108 (31.0) | |
| Parent wiped, teeth not brushed | 12 (3.5) | |
| Child refused brushing | 2 (0.6) | |
| Length of brushing (%)2 | Caregiver reported | Observed, mean seconds (SD) n=347 |
| 0-59 seconds | 128 (30.5) | |
| 60-119 seconds | 137 (32.6) | |
| 120 or more seconds | 128 (30.5) | 83.6 (59.0) |
| Don’t know | 2 (0.5) | |
| Age started using toothpaste in months, mean (SD) n=348 | 12.7 (4.8) | |
| Toothpaste has fluoride (%) | Caregiver reported | Observed n=348 |
| Child does not brush/use toothpaste | 72 (17.1) | 54 (15.5) |
| No | 80 (19.1) | 99 (28.5) |
| Yes | 93 (22.2) | 195 (56.0) |
| Don’t know | 175 (41.7) | 0 (0.0) |
| Amount of toothpaste used (%) | Observed n=342 | |
| Does not brush/use toothpaste | 72 (17.1) | 54 (15.8) |
| Smear | 220 (52.4) | 134 (39.2) |
| Pea sized | 94 (22.4) | 99 (29.0) |
| Half load or covers half the brush | 24 (5.7) | 33 (9.7) |
| Full load or covers the full brush | 9 (2.1) | 22 (6.4) |
| Don’t know | 1 (0.2) | 0.0 |
| Length since child’s last dental visit (%) n=419 | ||
| Never has been | 250 (59.7) | |
| ≤ 6 months | 139 (33.2) | |
| > 6 months but < 1 year | 17 (4.1) | |
| > 1 year but < 2 years | 13 (3.1) | |
| Child needed dental care but could not get it in the past 12 months (%) | 31 (7.4) | |
| Reasons (N=31): | ||
| Cost/no insurance coverage | 14 (45.2) | |
| Caregiver too busy/no time | 6 (19.4) | |
| Another dentist recommended not to | 6 (19.4) | |
| Afraid/did not think anything was wrong | 5 (16.1) | |
| Child has had dental cavity or tooth decay (%) | 18 (4.3) | |
| Child put to sleep in clinic or hospital due to cavity or toothache (%) | 3 (0.7) | |
| Child oral health quality of life.3 median (IQR) | 2.0 (5.0) | |
For the first primary outcome of child brushing frequency, caregivers reported 5% brushed more than twice a day, 40% twice a day, 33.8% once a day, 15.2% sometimes but not every day, and 6% no brushing. The other primary outcome—OHI-MIS plaque score—is interpreted as 0-0.6=good, 0.7-1.8= fair, and 1.9-3.0=poor [77], Scores range from 0 to 3 with higher scores reflecting higher amounts of plaque. The mean (SD) OHI-MIS score of our sample was 1.9 (0.6). Thirty-one percent of caregivers reported brushing their child’s teeth for 0–59 seconds, 32.6% for 60-119 seconds, and 30.5% for 120 seconds or more. However, RAs measured the mean (SD) brushing time to be 83.6 (59.0) seconds. The presence of fluoride in the child’s toothpaste was unknown or underreported by many caregivers. Over 41% reported that they did not know if the child’s toothpaste had fluoride. While 22.2% of the caregivers reported that the child’s toothpaste had fluoride, 56.0% of all observed toothpaste had fluoride. Caregivers generally underestimated the quantity of toothpaste used as well. While 52.4% of the caregivers reported using a smear, only 39.2% of all brushing observations showed that caregivers actually used a smear. Sixteen percent of children did not use any toothpaste at all when demonstrating their regular brushing routine. Over half of children (59.7%) had never been to the dentist and if the sample is limited to children one year or older (N=401) to better align with standard recommendations, 55.5% had never been to the dentist. Child oral health quality of life was measured using the Early Childhood Oral Health Impact Scale (ECOHIS), which sums the frequency of occurrence for 13 child oral health-related problems [78], (Response choices are never=0, hardly ever=1, occasionally=2, often=3, and very often=4) [78], The median ECOHIS score was 2.0 (IQR 5.0).
3.3. Differences by site
Cook County is large, with well-defined geographic areas that differ greatly from each as a result of segregation. Clinic and WIC sites were located mostly throughout the west and south sides of Cook County. The specific twenty sites were chosen to facilitate the recruitment of low-income Hispanic and Black families into the trial. As shown in Table 5, the participants recruited were primarily Hispanic and Black, but race and ethnicity varied dramatically by site (p<0.001). For example, participants from one medical clinic were exclusively of Black race with none endorsing Hispanic ethnicity. At another, all claimed Hispanic ethnicity and none Black race. Caregiver highest degree served as a proxy for socioeconomic status due to the high amount of unknown/refused responses on household income. Here again, we saw tremendous variation by site (p=0.03), with some sites having a third of participants with less than a high school education while other sites had over 70% with some college/trade school or more. Child age was not consistent across sites (p=0.01). The primary outcomes—child brushing frequency and average OHI-MIS plaque scores—did not vary across sites.
Table 5:
Differences across 20 Participating Sites
| Medical Clinics | WIC Centers | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Data | Total N=420 | 1 N=22 | 2 N=21 | 3 N=18 | 4 N=19 | 5 N=19 | 6 N=21 | 7 N=23 | 8 N=18 | 9 N=22 | 10 N=19 | Ave N=202 | 1 N=23 | 2 N=23 | 3 N=19 | 4 N=23 | 5 N=23 | 6 N=23 | 7 N=20 | 8 N=21 | 9 N=21 | 10 N=22 | Ave N=218 |
| Caregiver highest degree earned (%) | |||||||||||||||||||||||
| Less than high school | 68 (16.2) | 1 (4.6) | 7 (33.3) | 2 (11.1) | 1 (5.3) | 4 (21.1) | 5 (23.8) | 4 (17.4) | 3 (16.7) | 6 (27.3) | 1 (5.3) | 34 (16.8) | 1 (4.4) | 3 (13.0) | 3 (15.8) | 8 (34.8) | 4 (17.4) | 2 (8.7) | 2 (10.0) | 5 (23.8) | 3 (14.3) | 3 (13.6) | 34 (15.6) |
| High school/GED | 132 (31.4) | 4 (18.2) | 7 (33.3) | 5 (27.8) | 3 (15.8) | 6 (31.6) | 5 (23.8) | 4 (17.4) | 5 (27.8) | 7 (31.8) | 8 (42.1) | 54 (26.7) | 6 (26.1) | 9 (39.1) | 9 (47.4) | 8 (34.8) | 4 (17.4) | 5 (21.7) | 8 (40.0) | 8 (38.1) | 13 (61.9) | 8 (36.4) | 78 (35.8) |
| More than high school | 220 (52.4) | 17 (77.3) | 7 (33.3) | 11 (61.1) | 15 (79.0) | 9 (47.4) | 11 (52.4) | 15 (65.2) | 10 (55.6) | 9 (40.9) | 10 (52.6) | 114 (56.4) | 16 (69.6) | 11 (47.8) | 7 (36.8) | 7 (30.4) | 15 (65.2) | 16 (69.6) | 10 (50.0) | 8 (38.1) | 5 (23.8) | 11 (50.0) | 106 (48.6) |
| Caregiver Hispanic (%) | 219 (52.1) | 7 (31.8) | 19 (90.5) | 10. (55.6) | 1 (5.3) | 0 (0.0) | 14 (66.7) | 23 (100) | 1 (5.6) | 9 (40.9) | 11 (57.9) | 95 (47.0) | 9 (39.1) | 12 (52.2) | 16 (84.2) | 20 (87.0) | 10 (43.5) | 3 (13.0) | 17 (85.0) | 20 (95.2) | 16 (76.2) | 1 (4.6) | 124 (56.9) |
| Caregiver race (%) | |||||||||||||||||||||||
| White | 57 (13.6) | 5 (22.7) | 3 (14.3) | 1 (5.6) | 1 (5.3) | 0 (0.0) | 1 (4.8) | 5 (21.7) | 2 (11.1) | 4 (18.2) | 3 (15.8) | 25 (12.4) | 3 (13.0) | 5 (21.7) | 5 (26.3) | 2 (8.7) | 2 (8.7) | 2 (8.7) | 8 (40.0) | 2 (9.5) | 3 (14.3) | 0 (0.0) | 32 (14.7) |
| Black | 176 (41.9) | 12 (54.6) | 4 (19.1) | 8 (44.4) | 17 (89.5) | 19 (100) | 8 (38.1) | 0 (0.0) | 15 (83.3) | 9 (40.9) | 8 (42.1) | 100 (49.5) | 12 (52.2) | 10 (43.5) | 0 (0.0) | 2 (8.7) | 11 (47.8) | 17 (73.9) | 0 (0.0) | 2 (9.5) | 3 (14.3) | 19 (86.4) | 76 (34.9) |
| Other | 187 (44.5) | 5 (22.7) | 14 (66.7) | 9 (50.0) | 1 (5.3) | 0 (0.0) | 12 (57.1) | 18 (78.3) | 1 (5.61 | 9 (40.9) | 8 (42.1) | 77 (38.1) | 8 (34.8) | 8 (34.8) | 14 (73.7) | 19 (82.6) | 10 (43.5) | 4 (17.4) | 12 (60.0) | 17 (81.0) | 15 (71.4) | 3 (13.6) | 110 (50.5) |
| Child’s age (mouths), mean(SD) | 21.5 (6.9) | 24.0 (6.9) | 26.4 (6.2) | 23.3 (6.1) | 23.4 (7.6) | 23.0 (7.7) | 20.3 (7.1) | 20.9 (7.4) | 20.7 (7.8) | 23.5 (6.5) | 21.2 (8.4) | 22.7 (7.3) | 19.1 (6.0) | 18.9 (6.5) | 19.8 (5.8) | 21.5 (6.4) | 21.6 (7.9) | 20.0 (6.6) | 22.8 (6.1) | 21.9 (5.3) | 17.8 (6.8) | 21.2 (5.7) | 20.4 (6.4) |
| Length since child’s last dental visit (%)n=419 | |||||||||||||||||||||||
| Never has been | 250 (59.7) | 13 (59.1) | 7 (33.3) | 11 (61.1) | 15 (79.0) | 12 (63.2) | 10 (50.0) | 14 (60.9) | 14 (77.8) | 14 (63.6) | 12 (63.2) | 122 (60.7) | 18 (78.3) | 16 (69.6) | 8 (42.1) | 9 (39.1) | 13 (56.5) | 16 (69.6) | 10 (50.0) | 13 (61.9) | 10 (47.6) | 15 (68.2) | 128 (58.7) |
| ≤ 6 months | 139 (33.2) | 8 (36.4) | 14 (66.7) | 6 (33.3) | 2 (10.5) | 4 (21.1) | 9 (45.0) | 8 (34.8) | 4 (22.2) | 7 (31.8) | 6 (31.6) | 68 (33.8) | 3 (13.0) | 4 (17.4) | 9 (47.4) | 12 (52.2) | 9 (39.1) | 6 (26.1) | 6 (30.0) | 5 (23.8) | 11 (52.4) | 6 (27.3) | 71 (32.6) |
| > 6 months, < 1 year | 17 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 2 (8.7) | 3 (13.0) | 1 (5.3) | 2 (8.7) | 1 (4.4) | 1 (4.4) | 2 (10.0) | 2 (9.5) | 0 (0.0) | 1 (4.6) | 15 (6.9) |
| >1 year, < 2 years | 13 (3.1) | 1 (4.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 3 (15.8) | 1 (5.0) | 1 (4.4) | 0 (0.0) | 1 (4.6) | 1 (5.3) | 9 (4.5) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.0) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 4 (1.8) |
| Caregiver oral health knowledge total score, mean (SD) | 4.2 (0.8) | 4.2 (0.8) | 4.0 (0.9) | 4.3 (0.8) | 4.4 (0.7) | 4.0 (0.8) | 4.3 (0.6) | 3.9 (1.1) | 4.4 (0.9) | 4.5 (0.8) | 4.4 (1.0) | 4.2 (0.9) | 4.2 (0.7) | 3.7 (0.0) | 3.6 (0.7) | 4.4 (0.8) | 4.1 (0.7) | 4.3 (0.8) | 4.1 (0.9) | 4.1 (0.8) | 4.1 (0.9) | 4.1 (0.8) | 4.1 (0.8) |
| Caregiver has health insurance (%) | 319 (76.0) | 20 (90.9) | 13 (61.9) | 17 (94.4) | 14 (73.7) | 19 (100) | 18 (85.7) | 15 (65.2) | 17 (94.4) | 16 (72.7) | 16 (84.2) | 165 (81.7) | 18 (78.3) | 18 (78.3) | 9 (47.4) | 12 (52.2) | 17 (73.9) | 23 (100) | 14 (70.0) | 12 (57.1) | 11 (52.4) | 20 (90.9) | 154 (70.6) |
P<0.05 for comparisons across all 20 sites.
Also differences across sites noted for type of drinking water (p<0.01), caregiver brushing frequency (p=0.03). caregiver has dental insurance (p<0.01). and PROMIS instrumental support (p<0.01).
Not significant differences for child brushing frequency, plaque index score, caregiver age, child health insurance, child dental insurance, sugary foods consumption, sugary beverage consumption, caregiver oral health status, caregiver brushing frequency, child has caries, ECOHIS score, PROMIS anxiety score, PROMIS depression score, PROMIS functioning score, PROMIS emotional support score, PROMIS informational support score, household chaos.
4. Discussion
4.1. Study Design Discussion
CO-OP Chicago is a cluster-randomized controlled trial that aims to determine if receipt of an oral health CHW intervention improves tooth brushing behaviors for young children in low-income urban families. Our protocol resulted in successful recruitment, enrollment, and baseline data collection for 420 child/caregiver dyads from twenty community partner sites. Participants reflect the population we intended to reach: low-income, minority families with young children. Their oral health behaviors and quality of life are comparable to other low-income non-clinical cohorts [79], The study population was chosen to fill gaps in our understanding of oral health behaviors in young children and low-income minority families. While caries can be treated, the ultimate goal in pediatrics is primary prevention; restorative dental care (i.e., fillings, crowns, extractions) typically fails to control the disease trajectory. Targeting families with children under the age of three years old may help establish healthy behaviors for a lifetime [80], Additionally, low-income, predominantly minority families have higher documented rates of caries development than the general population [81, 82], The primary outcome measures show sufficient rigor and variability to allow for future determination of intervention efficacy in this high-risk population.
The trial’s design will also allow for the determination of implementation factors related to the CHW intervention. CHWs typically work out of either a clinical location or a community agency. Therefore, CO-OP Chicago places CHWs in both pediatric primary care clinics and WIC centers. Analyses will determine if intervention effects vary by type of site. The CHW intervention will be delivered over four in-person visits within one year; this “dosage” was chosen in order to align with well-child medical checks and WIC center appointments (typically every three months). Four visits per year have been shown in asthma to be acceptable to families and associated with cost savings [15]. The necessary amount of CHW visits to achieve changes in oral health behaviors will be explored in analyses. The cost to deliver the intervention will also be determined.
Some of the participant characteristics (but not the primary outcome measures) vary by site. This is a reflection of the segregation inherent to the Chicago area. We anticipate rates of change in the study measures and efficacy of the intervention will vary in relation to these site-level differences. Site differences also offer an opportunity to explore factors associated with better and worse intervention efficacy. Randomization was on the site level, making it unlikely that site variation is unequal in the two arms. To minimize any impact this could have on the treatment effect, analyses will adjust for covariates that are determined a priori based on the current best evidence.
4.2. Limitations
Because our trial focuses mainly on low-income urban minority families, the generalizability of the finding will be limited. Another limitation is that the trial targets behaviors and is not powered to detect changes in caries. This decision was made because the children were very young to develop caries and because we felt it more appropriate to first test the impact of the intervention on the proximal behavior of tooth brushing. Effective tooth brushing is known to reduce caries risk [83–85]. Future studies will follow families to assess caries incidence when children are five years old. A common challenge in cluster-randomized trials is intervention adherence [86]. This trial does not require participants to accept the intervention; we will carefully measure intervention adherence to determine its influence on treatment effects.
4.3. Conclusions
The strengths and unique features of CO-OP Chicago position it to address important health disparity issues in the US. The trial focuses on children under the age of three years. This age group is frequently excluded from oral health research, even though the process of caries prevention should start early [87], even before birth [88]. CHW interventions have demonstrated efficacy in many disease areas, but studies in oral health are limited [20, 23, 24, 89, 90]; this trial will address that gap. Finally, the cluster-randomized controlled design of the trial takes a real-world approach by integrating CHWs into existing health and social service agencies and collecting data in participant homes. Site-level influences on participant measures and intervention uptake will contribute to our understanding of intervention implementation.
Acknowledgments
We would like to thank the other members of the CO-OP Chicago Steering Committee that did not participate as authors, including Michael Berbaum, Jennifer Bereckis, Marcio da Fonseca, William Frese, Mark Minier, Jennie Pinkwater, Sheela Raja, Shojanny Salazar, and Rebecca Van Horn. A special thanks is offered to Anabelen Diaz, Nadia Ochoa, Nia O’Neal, Nusirat Williams who collected the data and our community health workers Melissa Hernandez Contreras, Monserrath Espinosa, Hope Opuada, and Mayra Pereddo. Our Community Advisory Board (https://co-opchicago.ihrp.uic.edu/) provided support and guidance. Finally, we thank the families, staff, providers, and administrators at our partner clinics and WIC centers: Aunt Martha’s Pediatric Health and Wellness Center, Aunt Martha’s South Holland Community Health Center, Aunt Martha’s Southeast Side Community Health Center, CDPH WIC Friend Family Health Center, CDPH WIC Greater Lawn Health Center, and CDPH WIC Westside Health Partnership, CEDA WIC Blue Island, CEDA WIC Diversey, CEDA WIC Harvey, CEDA WIC Irving Park, CEDA WIC Maywood, CEDA WIC Oak Park, CEDA WIC Summit, Mile Square Health Center Back of the Yards, Mile Square Health Center Cicero, Mile Square Health Center Englewood, Mile Square Health Center Main, Mile Square Health Center South Shore, UI Health Child and Youth Center, and Vida Pediatrics.
Funding source
This work was supported by the National Institutes of Dental & Craniofacial Research of the National Institutes of Health [Grant No. UH3DE025483, Principal Investigator: Molly A. Martin, and Coordinating Center Award No. U01DE025507, Principal Investigator: Stuart A. Gansky, University of California, San Francisco).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Financial disclosures
The authors have no conflicts of interest or financial disclosures.
References
- [1].Fleming E and Afful J, Prevalence of Total and Untreated Dental Caries Among Youth: United States, 2015–2016 NCHIS Data Brief, no 307. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- [2].Chicago Community Oral Health Forum. Healthy Smiles, Health Growth 2013-2014: Assessing the Oral Health Status and Body Mass Index of Third Grade Children in Illinois. Available from: https://www.heartlandalliance.org/wp-content/uploads/sites/3/2016/02/healthy-smiles-healthy-growth_final.pdf (accessed 12 Dec 2019).
- [3].Schwendicke F, Dorer CE, Schlattmann P, Page LF, Thomson WM, and Paris S Socioeconomic Inequality and Caries: A Systematic Review and Meta-Analysis. 2015, SAGE Publications: Los Angeles, CA: p. 10–18. [DOI] [PubMed] [Google Scholar]
- [4].Como DH, Stein Duker LL, Polida JC, Cermak SA The Persistence of Oral Health Disparities for African American Children: A Scoping Review. Int J Environ Res Public Health, 2019. 16(5). 10.3390/ijerph16050710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mouradian WE, Wehr E, and Crall JJ Disparities in children’s oral health and access to dental care. Jama, 2000. 284(20): p. 2625–31. 10.1001/jama.284.20.2625. [DOI] [PubMed] [Google Scholar]
- [6].Flores G and Tomany-Korman SC Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics, 2008. 121(2): p. e286–98. https://doi.Org/10.1542/peds.2007-1243. [DOI] [PubMed] [Google Scholar]
- [7].Krol DM, et al. , Maintaining and Improving the Oral Health of Young Children. Pediatrics, 2014. 134(6): p. 1224–1229. 10.1542/peds.2014-2984. [DOI] [PubMed] [Google Scholar]
- [8].Casamassimo PS, Lee JY, Marazita ML:, Milgrom P, Chi DL, and Divaris K Improving children’s oral health: an interdisciplinary research framework. J Dent Res, 2014. 93(10): p. 938–42. 10.1177/0022034514547273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fisher-Owens SA, Gansky SA, Platt LJ, Wentraub JA, Soobader MJ, Bramlett MD, and Newacheck PW Influences on children’s oral health: a conceptual model. Pediatrics, 2007. 120(3): p. e510–20. 10.1542/peds.2006-3084. [DOI] [PubMed] [Google Scholar]
- [10].Swider SM Outcome effectiveness of community health workers: an integrative literature review. Public Health Nurs, 2002. 19(1): p. 11–20. 10.1046/j.1525-1446.2002.19003.x. [DOI] [PubMed] [Google Scholar]
- [11].Viswanathan M, Kraschnewski J, Nishikawa B, Morgan LC, Thieda P, Honeycutt A, Lohr KN, and Jonas D Outcomes of community health worker interventions. Evid Rep Technol Assess (Full Rep), 2009(181): p. 1–144, A1,–2, B1–14, passim. [PMC free article] [PubMed] [Google Scholar]
- [12].Viswanathan M, Kraschnewski J, Nishikawa B, Morgan LC, Honeycutt A, Thieda P, Lohr KN, and Jonas DE Outcomes and costs of community health worker interventions: a systematic review. Med Care, 2010. 48(9): p. 792–808. [DOI] [PubMed] [Google Scholar]
- [13].Lewin SA, Dick J, Pond P, Zwarenstein M, Aja G, Van Wyk B, Bosch-Capblanch X, and Patrick M Lay health workers in primary and community health care. Cochrane Database Syst Rev, 2005(1): p. Cd004015 10.1002/14651858.CD004015.pub2. [DOI] [PubMed] [Google Scholar]
- [14].Rhodes SD, Foley KL, Someta CS, Bloom FR Lay Health Advisor Interventions Among Hispanics/Latinos. A Qualitative Systematic Review. American Journal of Preventive Medicine, 2007. 33(5): p. 418–427. 10.1016/j.amepre.2007.07.023. [DOI] [PubMed] [Google Scholar]
- [15].Campbell JD, Brooks M, Hosokawa P, Robinson J, Song L, and Krieger J Community Health Worker Home Visits for Medicaid-Enrolled Children With Asthma: Effects on Asthma Outcomes and Costs. Am J Public Health, 2015. 105(11): p. 2366–72. 10.2105/AJPH.2015.302685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johnson SL and Gunn VL Community Health Workers as a Component of the Health Care Team. Pediatr Clin North Am, 2015. 62(5): p. 1313–28. 10.1016/j.pcl.2015.06.004. [DOI] [PubMed] [Google Scholar]
- [17].Rothschild SK, Martin MA, Swider SM, Tumialan Lynas CM, Janssen I, Avery EF, and Powell LH Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. Am J Public Health, 2014. 104(8): p. 1540–8. 10.2105/AJPH.2013.301439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].U.S. Health Resources and Services Administration, Health Resources and Services Administration, Bureau of Health Professions. Community Health Workers National Workforce Study. 2007, HRSA: Rockville, Md: https://bhw.hrsa.gov/sites/default/files/bhw/nchwa/projections/communityhealthworkforce.pdf (accessed 12 Dec 2019). [Google Scholar]
- [19].Rosenthal EL The final report of the national community health advisor study. 1998, Annie E. Casey Foundation: Baltimore, MD. [Google Scholar]
- [20].Feldens CA, Giugliani ERJ, Duncan BB, Drachler M, and Vitolo MR. Long-term effectiveness of a nutritional program in reducing early childhood caries: A randomized trial. Community Dentistry and Oral Epidemiology, 2010. 38(4): p. 324–332. 10.1111/j.1600-0528.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- [21].Strippel H Effectiveness of structured comprehensive paediatric oral health education for parents of children less than two years of age in Germany. Community Dental Health, 2010. 27(2): p. 74–80. [PubMed] [Google Scholar]
- [22].Rothe V, Kebriaei A, Pitner S, Balluff M, and Salama F Effectiveness of a presentation on infant oral health care for parents. International Journal of Paediatric Dentistry, 2010. 20(1): p. 37–42. 10.1111/j.1365-263X.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- [23].Kowash MB, Pinfield A, Smith J, and Curzon ME Effectiveness on oral health of a long-term health education programme for mothers with young children. British Dental Journal, 2000. 188(4): p. 201–205. 10.1038/sj.bdj.4800431. [DOI] [PubMed] [Google Scholar]
- [24].Plonka KA, Pukallus ML, Barnett A, Holcombe TF, Walsh LJ, and Seow WK A controlled, longitudinal study of home visits compared to telephone contacts to prevent early childhood caries. International Journal of Paediatric Dentistry, 2013. 23(1): p. 23–31. 10.1111/j.1365-263X.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- [25].Grover J, The community dental health coordinator. A valued new member of the dental team. Todays FDA, 2014. 26(2): p. 56–7. [PubMed] [Google Scholar]
- [26].A Statement from the American Dental Association. Breaking Down Barriers to Oral Health for All Americans: The Community Dental Health Coordinator. 2012. [cited 2017 6/26]; Available from: http://www.ada.org/~/media/ADA/Advocacy/Files/ADA_Breaking_Down_Barriers-Community_Dental_Health_Coordinator.ashx. (accessed 12 Dec 2019).
- [27].Martin MA, Lee H, Landa J, Minier M, Avenetti D, and Sandoval A, Formative Research Implications on Design of a Randomized Controlled Trial for Oral Health Promotion in Children. Pilot and Feasibility Studies, 2018. 4: p. 155 10.1186/s40814-018-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Community Health Workers: Critical Connections in Communities. updated 2011 May 20 [cited 2013 September 7]; Available from: https://www.cdc.gov/diabetes/projects/pdfs/comm.pdf. (accessed 12 December 2019).
- [29].Martin M, Freese W, Lumsden C, and Sandoval A, Building a pediatric oral health training curriculum for community health workers. J Public Health Manag Prac 2018. May/June 24(3):39–e18. 10.1097/PHH.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martin MA, Perry-Bell K, Minier M, Glasssgow AE, and,Van Voorhees BW A Real-World Community Health Worker Care Coordination Model for High-Risk Children. Health Promot Pract, 2019. 20(3): p. 409–418. 10.1177/1524839918764893. [DOI] [PubMed] [Google Scholar]
- [31].Swider SM, Martin M, Lynas C, and Rothschield S Project MATCH: training fora promotora intervention. Diabetes Educ, 2010. 36(1): p. 98–108. 10.1177/0145721709352381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bandura A, Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 1977. 84(2): p. 191–215. [DOI] [PubMed] [Google Scholar]
- [33].Lorig KR, Sobel DS, Stewart AL, Brown BW, Bandura A, Ritter P, Gonzalez VM, Laurent DD, Holman HR Evidence Suggesting That a Chronic Disease Self-Management Program Can Improve Health Status While Reducing Hospitalization: A Randomized Trial. Medical Care, 1999. 37(1): p. 5–14. 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- [34].Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, Bauman A, Hensley MJ, and Walters EH Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev, 2003(1): 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- [35].Von Korff M, Moore J, Lorig K, Cherkin D, Suanders K, Gonzalez V, Luarent D, Rutter C, and Comite F A randomized trial of a lay person-led self-management group intervention for back pain patients in primary care. Spine, 1998. 23(23): p. 2608–2615. 10.1097/00007632-199812010-00016. [DOI] [PubMed] [Google Scholar]
- [36].Lorig KR, Ritter PL, and Jacquez A Outcomes of Border Health Spanish/English Chronic Disease Self-management Programs. The Diabetes Educator, 2005. 31(3): p. 401–409. 10.1177/0145721705276574. [DOI] [PubMed] [Google Scholar]
- [37].Guideline on Caries-risk Assessment and Management for Infants, Children, and Adolescents. Pediatr Dent,, 2016. 38(6): p. 142–149. [PubMed] [Google Scholar]
- [38].Freire P, Pedagogy of the Oppressed. 2000, New York: Bloomsbury Academic. [Google Scholar]
- [39].Harris PA, Taylor R, Thielke R, Payne J, Nonzalez N, Conde JG Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. April;42(2):377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN REDCap Consortium, The REDCap consortium: Building an international community of software partners. J Biomed Inform. 2019. May 9 10.1016/jjbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].National Institute of Dental and Craniofacial Research. NIH Publication No. 17–2884 A Healthy Mouth for Your Baby. 2017. [Google Scholar]
- [42].Greene JG and Vermillion JR The Simplified Oral Hygiene Index. The Journal of the American Dental Association, 1964. 68(1): p. 7–13. 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- [43].Gansky SA, Jue B, Avenetti D, Cheng N, Lindau H, Ramos-Gomez F, Hyde S, Shiboski C, Dental plaque score reliability from Photographs in preschoolers: BEECON Trial International Association of Dental Research, Vancouver, Canada. [Google Scholar]
- [44].Martin M, Rosales G, Avenetti D, Van Horn R, Shiboski C, Jue B, and Hyde S Accurately Scoring Dental Plaque Photographs in Young Children: CO-OP Chicago. International Association of Dental Research, Vancouver, Canada. [Google Scholar]
- [45].Wilson A, Brega AG, Batliner TS, Henderson W, Campagna EJ, Fehringer K, Gallegos J, Daniels D, and Albino J Assessment of parental oral health knowledge and behaviors among American Indians of a Northern Plains tribe. J Public Health Dent. 2014. Spring;74(2): 159–67. 10.1111/jphd.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Albino J, et al. The basic research factors questionnaire for studying early childhood caries. BMC Oral Health, May 2017, 17(1):83 10.1186/s12903-017-0374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2013-2014. www.cdc.gov/nchs/nhanes. [Google Scholar]
- [48].Collett BR, Huebner CE, SeminarioA L, Wallace E, Gray KE, and Speltz ML Observed child and parent toothbrushing behaviors and child oral health. Int J Paediatr Dent. 2016. May;26(3): 184–92. 10.1111/ipd.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pahel BT, Rozier RG, and Slade GD Parental perception of children’s oral health: The Early Childhood Oral Health Impact Scale (ECOHIS). Health Qual of Life Outcomes 2007;5:6–17. 10.1186/1477-7525-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health Interview Survey (NHIS). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008. www.cdc.gov/nchs/nhis. [Google Scholar]
- [51].Slade GD, Nuttal N, Sanders AE, Steele JG, Allen PF, and Lahti S>. Impacts of oral disorders in the United Kingdom and Australia. Br Dent J. 2005. April 23;198(8):489–93. 10.1038/sj.bdj.4812252. [DOI] [PubMed] [Google Scholar]
- [52].Slade GD Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol 1997; 25:284–90. 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- [53].Slade GD, and Spencer AJ Development and evaluation of the Oral Health Impact Profile. Community Dent Health, 1994; 11:3–11. [PubMed] [Google Scholar]
- [54].Patient-Reported Outcomes Measurement Information System. http://www.nihpromis.org/measures/translations. (accessed 24 Sept 14).
- [55].Patient-Reported Outcomes Measurement Information System. http://www.assessmentcenter.net/documents/PROMIS%20Scoring%20Manual-%20CATs,%20Profiles,%20Short%20Forms.pdf. (accessed 8 May 14).
- [56].Pilkonis PA, et al. ; PROMIS Cooperative Group. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011. September;18(3):263–83. 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hahn EA, et al. ; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014. May; 33(5):490–9. 10.1037/hea0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Matheny A, Wachs T, Ludwig J, Phillips K Bringing order out of chaos: psychometric characteristics of Confusion, Hubbub and Order Scale. J Appl Dev Psychol. 1995;16:429–444 [Google Scholar]
- [59].Haach LM, Gerdes AC, Schneider BW, Hurtado GD Advancing our knowledge of ADHD in Latino children: Psychometric and cultural properties of Spanish-versions of parental/family functioning measures. J Abnorm Child Psychol. 2011;391:33–43. 10.1016/0193-3973(95)90028-4. [DOI] [PubMed] [Google Scholar]
- [60].Coldwell J, Pike A, and Dunn J Household chaos-links with parenting and child behaviour. J Child Psychol Psychiatry. 2006. November;47(11):1116–22. 10.1111/j.1469-7610.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- [61].Deater-Deckard K, et al. Conduct problems, IQ, and household chaos: a longitudinal multi-informant study. J Child Psychol Psychiatry. 2009;50(10):1301–1308. 10.1111/j.1469-7610.2009.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ivers NM, Halperin IJ, Barnsley J, Grimshaw JM, Shah BR, Tu K, Upshur R, and Zwarenstein M Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials, 2012. 13(1): p. 120 10.1186/1745-6215-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hole S, A simple sequentially rejective multiple test procedure. Journal of Statistics, 1979. 6(2): p. 65–70. [Google Scholar]
- [64].Donner A and Klar N Pitfalls of and Controversies in Cluster Randomization Trials. American Journal of Public Health, 2004. 94(3): p. 416–422. 10.2105/ajph.94.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Peters TJ, Richards SH, Bankeahd CR, Ades AE, and Sterne JAC Comparison of methods for analysing cluster randomized trials: An example involving a factorial design. Int J Epidemiol, 2003. 32(5): p. 840–846. 10.1093/ije/dyg228. [DOI] [PubMed] [Google Scholar]
- [66].Piaggio G, et al. , Methodological considerations on the design and analysis of an equivalence stratified cluster randomization trial. Stat Med, 2001. 20(3): p. 401–416. . [DOI] [PubMed] [Google Scholar]
- [67].Little R and Yau L Intent-to-Treat Analysis for Longitudinal Studies with Drop-Outs. Biometrics, 1996. 52(4): p. 1324–1333. [PubMed] [Google Scholar]
- [68].Gomes M, Diaz-Ordaz K, Grieve R, and Kenward MG Multiple Imputation Methods for Handling Missing Data in Cost-effectiveness Analyses That Use Data from Hierarchical Studies: An Application to Cluster Randomized Trials. Med Decis Making, 2013. 33(8): p. 1051–1063. 10.1177/0272989X13492203. [DOI] [PubMed] [Google Scholar]
- [69].Ma J, et al. , Imputation strategies for missing binary outcomes in cluster randomized trials. BMC Med Res Methodol, 2011. 11(1): p. 18–18. 10.1186/1471-2288-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Taljaard M, Donner A, and Klar N Imputation strategies for missing continuous outcomes in cluster randomized trials. Biom J, 2008. 50(3): p. 329–345. https://doir.org/10.1002/bimj.200710423. [DOI] [PubMed] [Google Scholar]
- [71].Hedeker D GR, Longitudinal Data Analysis. 2006: John Wiley & Sons. [Google Scholar]
- [72].Borman PJ, Chatfield MJ, Damjanov I, and Jackson P Design and Analysis of Method Equivalence Studies. Anal Chem, 2009. 81(24): p. 9849–9857. 10.1021/ac901945f. [DOI] [PubMed] [Google Scholar]
- [73].Breitenstein SM, Schoney M, Risser H, and Johnson T A study protocol testing the implementation, efficacy, and cost effectiveness of the ezParent program in pediatric primary care. Contemp Clin Trials, 2016. 50: p. 229–37. 10.1016/j.cct.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Polsky D, Glick HA, Willke R, and Schulman K Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ, 1997. 6(3): p. 243–52. . [DOI] [PubMed] [Google Scholar]
- [75].Tambour M and Zethraeus N Bootstrap confidence intervals for cost-effectiveness ratios: some simulation results. Health Econ, 1998. 7(2): p. 143–7. . [DOI] [PubMed] [Google Scholar]
- [76].Briggs AH, Wonderling DE, and Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health economics, 1997. 6(4): p. 327–340. [DOI] [PubMed] [Google Scholar]
- [77].Wei SH and Lang NP Periodontal epidemiological indices for children and adolescents: II. Evaluation of oral hygiene; III. Clinical applications. Pediatr Dent, 1982. 4(1): p. 64–73. [PubMed] [Google Scholar]
- [78].Pahel BT, Rozier RG, and Slade GD Parental perceptions of children’s oral health: the Early Childhood Oral Health Impact Scale (ECOHIS). Health Qual Life Outcomes, 2007. 5: p. 6 10.1186/1477-7525-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Born CD, Divaris K, Zeldin LP, and Rozier RG Influences on preschool children’s oral health-related quality of life as reported by English and Spanish-speaking parents and caregivers. J Public Health Dent, 2016. 76(4): p. 276–286. 10.1111/jphd.12152. [DOI] [PubMed] [Google Scholar]
- [80].National Institute of Child and Human Development Early Child Care Research Network. Duration and Developmental Timing of Poverty and Children’s Cognitive and Social Development From Birth Through Third Grade. Child Dev, 2005. 76(4): p. 795–810. 10.1111/j.1467-8624.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- [81].Blakely C, et al. , The fidelity-adaptation debate: implications for the implementation of public sector social programs. Am J Com Psychol, 1987. 15. [Google Scholar]
- [82].Promis Social Support. Available from: https://www.assessmentcenter.net/documents/InstrumentLibrary.pdf. (accessed 12 Dec 2019).
- [83].Goldman A, Leal SC, de Amorim RG, and Frencken JE, Treating High-Caries Risk Occlusal Surfaces in First Permanent Molars through Sealants and Supervised Toothbrushing: A 3-Year Cost-Effective Analysis. Caries Res, 2017. 51(5): p. 489–499. 10.1159/000477822. [DOI] [PubMed] [Google Scholar]
- [84].Hilgert LA, Leal SC, Bronkhorst EM, and Frencken JE Long-term Effect of Supervised Toothbrushing on Levels of Plaque and Gingival Bleeding Among Schoolchildren. Oral Health Prev Dent, 2017. 15(6): p. 537–542. 10.3290/j.ohpd.a39593. [DOI] [PubMed] [Google Scholar]
- [85].Marinho VC, Higgins JP, Sheiham A, and Logan S Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev, 2003(1): p. CD002278 10.1002/14651858.CD002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Eldridge SM, et al. , Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials, 2004. 1(1): p. 80–90. 10.1191/1740774504cn006rr. [DOI] [PubMed] [Google Scholar]
- [87].American Academy of Pediatrics. A Pediatric Guide to Children’s Oral Health. 2009: Elk Grove Village, IL: https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Oral-Health/Documents/OralHealthFCpagesF2_2_1.pdf (accessed 12 December 2019). [Google Scholar]
- [88].Xiao J, et al. , Prenatal Oral Health Care and Early Childhood Caries Prevention: A Systematic Review and Meta-Analysis. Caries Res, 2019. 53(4): p. 411–421. 10.1159/000495187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Villalta J, et al. , Developing an Effective Community Oral Health Workers-”Promotoras” Model for Early Head Start. Front Public Health, 2019. 7: p. 175 10.3389/fpubh.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mathu-Muju KR, et al. , The Children’s Oral Health Initiative: An intervention to address the challenges of dental caries in early childhood in Canada’s First Nation and Inuit communities. Can J Public Health, 2016. 107(2): p. e188–93. 10.17269/cjph.107.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]


