Abstract
Objective
We evaluated the extent to which studies that tested short message service (SMS)– and application (app)-based interventions for diabetes self-management education and support (DSMES) report on factors that inform both internal and external validity as measured by the RE-AIM (Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance) framework.
Materials and Methods
We systematically searched PubMed, Embase, Web of Science, CINAHL (Cumulative Index of Nursing and Allied Health Literature), and IEEE Xplore Digital Library for articles from January 1, 2009, to February 28, 2019. We carried out a multistage screening process followed by email communications with study authors for missing or discrepant information. Two independent coders coded eligible articles using a 23-item validated data extraction tool based on the RE-AIM framework.
Results
Twenty studies (21 articles) were included in the analysis. The comprehensiveness of reporting on the RE-AIM criteria across the SMS- and app-based DSMES studies was low. With respect to internal validity, most interventions were well described and primary clinical or behavioral outcomes were measured and reported. However, gaps exist in areas of attrition, measures of potential negative outcomes, the extent to which the protocol was delivered as intended, and description on delivery agents. Likewise, we found limited information on external validity indicators across adoption, implementation, and maintenance domains.
Conclusions
Reporting gaps were found in internal validity but more so in external validity in the current SMS- and app-based DSMES literature. Because most studies in this review were efficacy studies, the generalizability of these interventions cannot be determined. Future research should adopt the RE-AIM dimensions to improve the quality of reporting and enhance the likelihood of translating research to practice.
Keywords: mobile phone–based intervention, diabetes self-management education and support, SMS, app, RE-AIM
INTRODUCTION
Diabetes self-management education and support (DSMES) is a necessary component in diabetes care. DSMES plays an important role in glycemic management and preventing or delaying diabetes-related complications when administered alongside medical care and management.1,2 However, many barriers such as competing priorities, transportation barriers, underperceived seriousness of diabetes, and lack of accessible services discourage patients from obtaining traditional institution-based self-management education or training.3–6 There is a need for developing alternative DMSES interventions that are accessible and low cost for populations with diabetes.
Mobile phone technology can serve as an effective platform to deliver DSMES because of its ubiquitous availability and adoption by all populations.7,8 The 2 most common tools used in mobile phone–based interventions are short message service (SMS) and smartphone applications (apps). SMS and app-based interventions have been applied in multiple behavioral interventions, including promoting physical activity, tracking healthy eating, monitoring blood glucose, taking medication, monitoring complications, and problem solving, and show promising results.9–17 One recent meta-analysis and systematic review focused on multiple strategies of health technology interventions in diabetes management identified a larger effect size in mobile phone–based interventions compared with other forms of technological interventions, such as computer- or Internet-based interventions.18 However, despite the increased popularity and demonstrated efficacy of mobile phone–based interventions in diabetes management, it remains largely unknown whether these interventions can be translated beyond the research setting and be broadly adopted in clinical and other settings. Additionally, review studies in this area have concentrated on reporting issues through the lens of internal validity.10,13 To date, no systematic review has evaluated domains of external validity and identified gaps that could inform the generalizability and translatability of mobile phone–based interventions in the specific area of DSMES.
To bridge efficacy and effectiveness, or research and practice, Glasgow et al developed the RE-AIM (Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance) framework.19,20 The RE-AIM framework consists of 5 dimensions: reach into the target population and representativeness of the study sample; efficacy or effectiveness of the intervention on the primary outcome tested under either optimal or real-world conditions, effect on quality of life, and avoidance of unintended or negative consequences; adoption by target setting, organizations, and staff; implementation on consistency and cost of delivery; and maintenance of intervention effect over time.19,20 The RE-AIM framework has been utilized in reporting internal and external validity across numerous behavioral intervention studies, including physical activity, weight loss maintenance, and health literacy.21–27 These findings provide recommendations and future directions to improve the quality of reporting and to enhance the likelihood of translating research to practice. The goal of this review is to evaluate the extent to which studies, both randomized controlled trials (RCTs) and non-RCTs, testing SMS and app-based interventions to facilitate DSMES report on factors that inform both internal and external validity of the intervention. In addition to data extracted from literature databases, we also included data directly collected from study authors via emails. Recommendations to improve the quality of reporting and the likelihood of broad dissemination of effective SMS- and app-based interventions are provided based on 2 sources of data and other relevant evaluations of behaviors interventions.
MATERIALS AND METHODS
Database search and study inclusion
We adopted the validated PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) approach to conduct the literature review. PRISMA is an evidence-based minimum set of items for reporting in systematic reviews.28 We systematically searched PubMed for eligible articles from January 1, 2009 to February 28, 2019, using combinations of the following MeSH (Medical Subject Headings) (M) and text word (TW) search terms: (1) Diabetes Mellitus Type 2 (M), non insulin dependent diabetes (T), T2DM (T); (2) self-management (M), self care (M); and (3) mHealth (TW), mobile health (TW), cell phone (M), cell phone (TW), mobile phone (TW), short message service (TW), and text messaging (M). Similar searches were conducted in Embase, Web of Science, CINAHL (Cumulative Index of Nursing and Allied Health Literature), and the IEEE Xplore Digital Library. We also performed supplementary searches using the reference lists of eligible articles and relevant systematic review and other review articles. We decided to search from 2009, as 2009 marked a shift in technology from basic mobile phones to smartphones, and therefore the use of apps.29
Eligibility criteria
Studies were included if they were peer-reviewed studies testing the effect of SMS- or app-based interventions on DSMES among patients with type 2 diabetes. We excluded studies that did not use SMS or an app as the primary intervention (ie, used only phone calls or wearables devices for remote monitoring). In addition, we excluded qualitative research (ie, exclusive evaluations on patients’ attitudes or perceptions on interventions), studies of cost-effectiveness analyses, and protocol articles.
Study screening
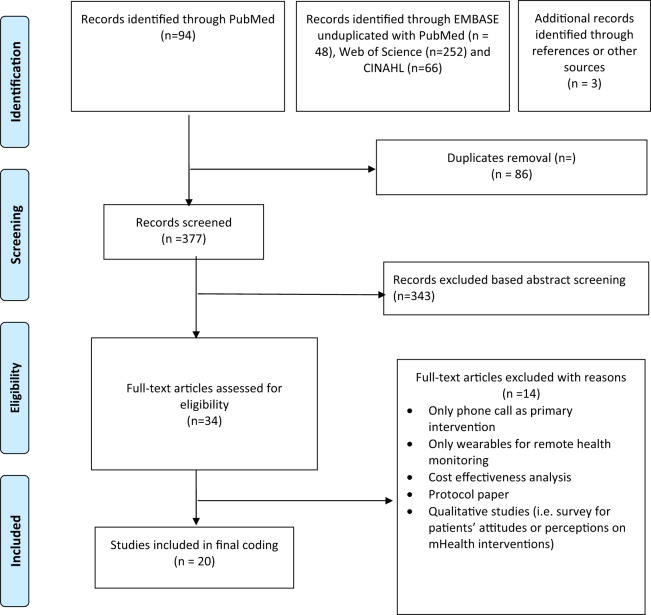
We used a multistage screening process in which search results were first pooled and transferred to EndNote software (Clarivate Analytics, Philadelphia, PA) for duplicate removal. Next, 2 reviewers (Y.Y. and S.J.P.) independently screened article titles and abstracts for relevance. Studies with “type 2 diabetes” or “non–insulin dependent diabetes” in the titles and abstracts and included a mobile phone–based intervention for DSMES were selected for full text review. Finally, the 2 reviewers conducted full text reviews of selected articles to confirm eligibility. Articles extracted from reference lists underwent an identical process (Figure 1).
Figure 1.
Article screening process (PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] 2018 flow diagram). CINAHL: Cumulative Index of Nursing and Allied Health Literature.
RE-AIM coding and scoring
A 23-item validated data extraction tool based on the RE-AIM framework was used to assess the extent of reporting study elements related to internal and external validity. Table 1 presents details on components that assess the RE-AIM dimensions. “Yes” or “no” were used to code the presence or absence of the RE-AIM components outlined in Supplementary Table 2. Each article was coded by 2 researchers independently; disagreement in coding was discussed until consensus was reached (Y.Y. and S.J.P.).
Table 1.
Evaluation dimensions and measures of the RE-AIM framework
| Dimension | Componenta | Internal or external validity indicator | Description22 |
|---|---|---|---|
| Reach | |||
| The number, proportion, and representativeness of individuals who are willing to participate in a given intervention. | Methods to identify target population | Internal | Description of the process by which the target population was identified in the intervention |
| Inclusion criteria | Internal | Description of characteristics of the target population that were used to determine if a potential participant was eligible in the intervention | |
| Exclusion criteria | Internal | Description of characteristics of the target population that prevent a potential participant from being eligible to participate | |
| Sample size | Internal | The number of people who agree to participate | |
| Participation rate | Internal | Sample size divided by the target population denominator | |
| Representativeness | Internal, external | Comparison of characteristics of the study participants in comparison to the target population | |
| Efficacy/effectiveness | |||
| The impact of an intervention on important outcomes, including potential negative effects. | Measures/results at least 1 follow-up | Internal | The study outcomes are measured at a time point after baseline |
| Intent-to-treat analysis utilized | Internal | Analyzing participants in trials in the groups to which they were randomized, regardless of whether they received or adhered to the allocated intervention | |
| Satisfactionb or potential negative outcomes | Internal | Measuring acceptability and usability of the intervention in participants; evaluate unintended consequences that may result from the intervention | |
| Attrition | Internal | The proportion that was lost of follow-up or dropped out of the intervention | |
| Adoption | |||
| The number, proportion, and characteristics of adopting settings and interventions agents | Description of intervention locationc | Internal, external | Description of characteristics of the location of the intervention |
| Description of staff who delivered the programc | Internal, external | Description of characteristics of staff who delivered the intervention | |
| Method to identify staff who delivered the intervention | External | Description of the process by which the staff was identified for participation in the study | |
| Level of expertise of delivery agent | External | Training and educational background among those who delivered the intervention | |
| Inclusion/exclusion criteria of delivery agent or setting | External | Description of the eligibility criteria of the setting/agent | |
| Adoption rate of delivery agent or setting | External | The number of participating delivery settings or agents divided by the number of eligible and approached delivery settings or agents | |
| Implementation | |||
| The extent to which the intervention is delivered as intended (eg, information on duration and frequency of intervention, fidelity to the intervention protocol, and cost including time and money) | Intervention duration and frequency | Internal | Length of the intervention (ie, days, weeks, months, and length of each intervention contact) and number of contacts with participants |
| Extent protocol delivered as intended (%) | Internal | Description of the fidelity to the intervention protocol | |
| Measures of cost of implementation | Internal, external | The costs including both money and time of delivery across all levels of implementation | |
| Maintenance | |||
| The extent to which a participant maintains the change due to intervention and an intervention becomes institutionalized or part of the routine organization practices | Assessed outcomes ≥6 mo postintervention | External | Description of follow-up outcome measures of individuals at some duration after intervention was terminated |
| Indicators of program level maintenance | External | Description of program continuation after completion of the research study | |
| Measures of cost of maintenance | External | The ongoing cost of maintaining delivery across all levels of the intervention | |
| Program adopted in other setting/populations | External | Description of the intervention being adopted beyond the original setting and population | |
RE-AIM: Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance.
Components were included to ensure relevancy with the mobile health interventions.
Components were informed by other RE-AIM framework reviews of health behavior interventions.21,48
Missing data inquiry from study authors
After the article screening and data extraction, we contacted corresponding authors by email when interventions included in the final review had missing or discrepant information. We introduced the purpose of our study and provided inquiries about missing or discrepant data according to RE-AIM criteria as well as additional questions regarding study implementation and generalizability. If there was no response to our initial email, reminders were sent at 7, 14, and 30 days.
RESULTS
Study characteristics
Our search yielded 20 studies (Supplementary Table 2). Of these, 14 were RCTs and 2 were pragmatic trials. Four were quasi-experimental studies, among which 1 was an intention-to-treat trial. Nine studies centered on app-based interventions, and 11 focused on SMS-based or the combination of SMS and phone call–based interventions. Eight studies were conducted in urban settings and 1 included both urban and rural settings. Five studies were conducted in the United States, 3 were conducted in Europe (Italy, Spain, and England), 4 were conducted in East Asia, 2 were conducted in India, 2 were conducted in Iran, 1 had locations in both Australia and Taiwan, 1 were conducted in Canada, 1 were conducted in New Zealand, and 1 were conducted in Bahrain. A total of 12 authors from 12 studies responded to our email inquiries, with 9 addressing our inquires (Supplementary Table 2).
RE-AIM analysis
Reach
All 20 studies reported specific inclusion and exclusion criteria and methods to identify target population. All 20 studies provided sample size (median sample size = 140; range, 32-427). Five studies did not report on number of eligible participants30–35; therefore, participation rate could not be calculated. Among 16 studies that provided the participation rates, median participation rate was 83% (range, 5.7%-100%). No studies in this review explicitly described characteristics of both participants and nonparticipants; therefore, we were unable to make conclusions about representativeness of the samples (Table 2).
Table 2.
Proportion of mobile health intervention on DSMES reporting RE-AIM framework dimensions and components (n = 20)
| Dimension and componenta | Proportion (%) | Notes |
|---|---|---|
| Reach | ||
| Methods to identify target population | 100 | |
| Inclusion criteria | 100 | |
| Exclusion criteria | 94.4 | |
| Sample size | 100 | Median sample size = 140 (range, 32-427) |
| Participation rate | 70 | Median rate 83% (range, 5.7%-100%) |
| Representativeness | 0 | |
| Efficacy/effectiveness | ||
| Measures/results at least 1 follow-up | 100 | |
| Intention-to-treat analysis utilized | 45 | |
| Satisfactionb or potential negative outcomes | 25 | |
| Attrition | 14.8 | Median attrition rate 15% (range, 2%-35%) |
| Qualitive methods to measure efficacy/effectiveness | 50 | |
| Adoption | ||
| Description of intervention locationc | 90 | |
| Description of staff who delivered the programc | 0 | |
| Method to identify staff who delivered the intervention | 0 | |
| Level of expertise of delivery agent | 45 | |
| Inclusion/exclusion criteria of delivery agent or setting | 5.5 (setting); 0 (staff) | |
| Adoption rate of delivery agent or setting | 0 | |
| Implementation | ||
| Intervention duration and frequency | 100 | Median follow-up 4.5 months, (range, 3-22 months) |
| Extent protocol delivered as intended | 30 | |
| Measures of cost of implementation | 15 | 2 studies reported monetary cost, 1 study reported time cost |
| Maintenance | ||
| Assessed outcomes ≥6 mo postintervention | 5 | |
| Indicators of program level maintenance | 0 | |
| Measures of cost of maintenance | 0 | |
| Program adopted in other setting/populations | 20 | |
DSMES: diabetes self-management education and support; RE-AIM: Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance.
Components were included to ensure relevancy with the mobile health interventions.
Effectiveness
All 20 studies reported on findings for at least 1 follow-up measurement. Six (33.3%) studies had more than 1 follow-up.31,37–42 Nine (45%) studies reported on intention-to-treat analysis.32,38–47 Eight (40%) provided information on missing data or imputation measures.37,39–45,47 The median attrition rate was 15% (range, 2%-35%). Five (25%) studies reported satisfaction or potential negative outcomes.37,40–43,48 Ten (50%) studies reported including qualitative methods to measure efficacy or effectiveness of their studies. Among these, 7 used satisfaction surveys or patients’ feedback from real-time monitoring systems to understand participants’ satisfaction and acceptability of the intervention.32,33,37,38,40–45,49 One study reported intermittent technical challenges faced by few participants,37 and another study reported intervention burden or poor response to participant requests as reasons for dropout from the study.42 Information on adverse outcomes related to communication mode such as misinformation, confusion, or data errors were not reported in any studies (Table 2).
Adoption
Ten of 20 (50%) studies provided information on adoption items.30,37–39,43–46,48,50 Most study participants (n = 18; 90%) were recruited in primary or secondary care settings and utilized physician or self-referral. One study recruited participants online,44 and another identified potential eligible participants using automatic search from anonymous pharmacy data of 40 pharmacies.45 Four studies (20%) provided brief descriptions of characteristics of the recruitment settings.37,38,43,48
Regarding intervention delivery, 10 (50%) studies reported level of staff expertise. Three studies used diabetes educators,39,44,50 1 used nurses,48 1 used physicians,40 1 used a combination of clinicians and diabetes educators,30 1 used a combination of nurses and research staff,46 1 used a combination of nurse and nutritionist,41,42 and 2 used only research staff to facilitate intervention delivery.38,47 No study provided characteristics, method to identify, eligibility, or adoption rate of intervention staff. Even though most studies reached patients in a primary or secondary care setting, the intervention may be delivered in nonclinical settings (eg, home) remotely though app and text. Only one study39 reported on eligibility for setting selection, method to identify setting, and setting adoption rate. However, this setting was the recruitment setting rather the intervention setting39 (Table 2).
Implementation
All 20 studies reported study duration (median length of follow-up = 4.5 months; range, 3-22 months). One study did not report intervention frequency or dosage.38 In terms of intervention fidelity, 6 (30%) studies reported that the intervention protocol was delivered as intended.31,37,40–43,48 One study author replied by email that their intervention was not carried out as intended.50 One study was an intention-to-treat trial,44 and 10 study authors did not respond to our inquiry on adoption components. Two studies measured monetary cost of the intervention,37,43 and 1 measured time cost.50 Two studies reported providing incentives to intervention staff,41,42,44 and 2 provided incentives to participants (Table 2).43,48
Maintenance
Only one study measured outcomes at 6-month follow-up after the study completed, according to study author’s response via email.37 No study reported indicators of program-level maintenance nor cost of maintenance. Authors from 4 studies informed us that their interventions have been adopted in other settings or patient populations41–43,45 or were being considered for a large scale implementation (Table 2).37
Applicability of studies conducted in the United States
Of the studies conducted in the United States, app-based intervention studies either provided mobile phones with unlimited service plans39 or required patients to own an iPhone,44 whereas SMS-based interventions required patients to own cellphones capable of receiving text messages.48,50 Most studies had predominantly White non-Hispanic participants, with one study including predominantly Hispanic participants,43 and another study had 39.3% African American participants.39 One study identified that patient messages and diabetes educator responses were in the electronic health records, where primary care physicians (PCPs) could access to all messages.50 In a study by Bauer et al,48 physicians received information on diagnosis and management of diabetic neuropathy as they exclusively recruited participants with peripheral diabetes neuropathy; however, change in treatment secondary to SMS or app was not tracked. Quinn et al39 compared the control group with 3 additional groups: (1) an app-based intervention group with no PCP involvement, (2) an app-based intervention group in which PCPs received unanalyzed raw data, and (3) an app-based intervention group in which PCPs received summarized report by fax or email with treatment recommendations. Findings indicate that addition of clinical decision support did not differ from an app-based intervention group with no physician involvement.39 Two studies had no physician involvement.43,44 Overall, mobile phone–based DSMES interventions in the United States did not provide actionable summarized data in the electronic health record for PCPs to provide clinical decision support.
Connecting mobile phone–based data to clinical management
Four studies conducted outside of the United States reported some form of provider management of mobile phone–based patient entered data. One study conducted in China provided patients with an action plan as previsit summaries for physician visits.33 Another Chinese study also provided guidance for blood glucose monitoring, diet, and exercise.35 Another study conducted in India had a web portal and smartphone app for physicians to communicate treatment titration or recommendations.38 One study from Bahrain gave patients the mobile number of the diabetes educators’ and physicians’ to allow for texting between visits.30 A study from Korea had medical staff from a hospital provide tailored recommendations based on patients’ self-monitoring data of glucose and blood pressure.49
DISCUSSION
Discussion notes for main results
This study used the RE-AIM framework to systematically review the state of research on SMS- and app-based interventions targeting DSMES from both internal and external validity perspectives. Methods to target populations and selection criteria were reported across studies. The participation rate (average at 70%) is similar to other behavioral interventions (average at 76%) that include physical activity promotion or smoking cessation at individual level.19 This participation rate is encouraging and suggests that SMS- and app-based DMSE programs are appealing to patients. However, it is important to note that most studies recruited participants at academic medical centers, hospitals systems, or other institutions focused on clinical care. Previous research has demonstrated that recruitment at clinical settings may offer increased access to patients who are ready to participate in trials because of better information provided by clinicians regarding the study.34,51–53 As a result, patients with no access to clinical care, compounded by low health literacy, mistrust of the system, or lack of information or awareness of research opportunities, are likely to be excluded.34 Additionally, no study in this review described the target population or indicated representativeness of the study sample to a larger population. Many mobile phone–based interventions in behavioral change fall short on describing the target population, raising concerns of generalizability of these interventions to varying sociodemographic groups.21,22,27,36,54 Similarly, the convenience sampling that interventions employed also challenges the understanding of whether the intervention is reaching subgroups of a population and those individuals that could benefit the most.22,34
Efficacy or effectiveness of SMS- and app-based interventions on diabetes disease or behavioral outcomes was reported in all studies, while information on maintenance of the changes was absent. This finding is consistent with interventions in other areas.36,55,56,21,24 Less than half of the studies performed an intention-to-treat analysis. The rest of the studies presented results of those who completed the follow-up. Whether the SMS- or app-based interventions produce lasting effects is questionable because most studies did not examine maintenance at least 6 months past an intervention. Researchers in a previous review focusing on promoting physical activity through mobile health technology suggested that mobile phone–based interventions are a relatively new area of research that the studies still emphasized to determine whether the interventions can initiate change.22 The researchers also indicated that mobile phone–based interventions may reduce the likelihood of maintaining disease or behavioral outcome changes, as fast advancements in technology could make current interventions obsolete.22 Moreover, the potential of technical problems may reduce motivation and discourage engagement.22 These reasons could in part explain the lack of description on maintenance in SMS- and app-based intervention studies in this review. Additionally, determinants of efficacy or effectiveness remain largely unexplained. Only 5 studies reported using qualitative methods to measure efficacy or effectiveness. The degree to which patients found mobile phone–based interventions to be acceptable, feasible, and sustainable to use are not documented, which hinders the understanding of the potential long-term effects of those interventions.
Organizational or delivery-level aspects of the RE-AIM framework have been historically underreported across behavioral interventions.57 This is also the case for SMS- and app-based DSMES research. We found very limited descriptions of the methods used to engage those who delivered the intervention or description of their characteristics, the extent to which the intervention was delivered as intended, and if any adaptions were made to the intervention during the study period. Furthermore, costs across the RE-AIM framework are also important to inform dissemination but are often missed in reporting.58 In mobile phone–based interventions specially, previous research has suggested other costs in addition to implementation costs need to be documented. This includes costs associated with recruitment, equipment, technology (eg, mobile phone, service plan, technical maintenance), and even future cost of continuing to use the service.22 Information on cost can help to determine the generatability or replicability of an intervention.
The most obvious omission in reporting is maintenance at the organizational level. Few research projects have the resources to ensure that their interventions can be sustained at the organizational level. Moreover, readiness to adopt and implement mobile phone–based programs are also hindered by financial resources, policies, and workplace culture.59,60 Indeed, in email conversations with study authors, only one author informed us that their SMS-based diabetes self-management program is being considered for national implementation.37
Mobile phone–based programs have the potential to gather large amounts of health data that can be used to better inform interventions and clinical management plans.61 These data may also meet the interests of major government stakeholders looking for fiscal efficiencies in healthcare delivery.61 However, most mobile-based interventions fail to move beyond the pilot or efficacy trial phase, owing to barriers to implementation and sustainability. Previous research discussion indicates that compared with other industries, health care is relatively reluctant to adopt new technologies because of resistance to change within organizations, absence or inadequacy of policies, and funding issues.60,62–65 Ross et al63 reported that a frequent reason for unsuccessful implementation of a new technology is that it does not fit well with work practices or daily clinical work. They also suggested that unless a technology is adaptable to fit with roles, tasks, and workflows of clinicians and there is access to dedicated technical support staff, resistance to implementing a new technology will remain a challenge.63 More importantly, intervention researchers need to understand the standards and policies regarding data safety and privacy, professional liability, and potential pitfalls of data sharing between systems and organizations.64,65 Another major implementation barrier is the termination of funding support, as the additional costs of privacy and security testing, ongoing technology support and development, and software maintenance do not fit well with government supported funding cycles for research and development.60,66 In our review, termination of funding is also one of the most frequently reported implementation barriers by study authors.
Recommendations for future research
Table 3 provides recommendations for future research to improve the assessment and reporting on individual and organizational level factors that will support the internal and external validity of SMS- and app-based intervention in DSMES. We constructed this table using evidence from the current review as well as recommendations from other relevant evaluations on behavioral interventions.22,57,67–71 In addition to these recommendations, efforts from other stakeholders including funding organizations and practitioners should also be in place to promote translatability of mobile phone–based solutions in diabetes management. Such efforts include encouraging reporting of negative or unintended outcomes and positive outcomes across RE-AIM dimensions, so that the feasible and efficacious parts of an intervention can be replicated.23 Funding organizations may consider requiring researchers to develop a plan for sustainability throughout the research. Practitioners should also demand more information on external validity of an intervention with demonstrated efficacy. Information on adoption, implementation, and maintenance will help them clarify whether an efficacious intervention is a good fit for their organization.23
Table 3.
Recommendations based on gaps identified from the RE-AIM framework evaluation
| Dimension and component | Key issues | Overall and specific recommendations |
|---|---|---|
| Reach | Representativeness of study sample to target population | Document and understand access, awareness, and appropriateness of intervention to meet target population needs67 |
| Compare characteristics (eg, sociodemographic, economic, and behavioral) of participants with nonparticipants or the general local population to understand the representativeness of the sample22 | ||
| Include inclusion criteria (eg, health/disease conditions, such as hemoglobin A1c ≥8.0%, capable of using mobile phone) and exclusion to Provide explanation on why certain individuals were not eligible for participation22 | ||
| Include recruitment setting, methods, and recruitment adaptations22 | ||
| Efficacy/effectiveness | Suitability and credibility of study design, data collection, and evaluation | Document participants’ satisfaction, negative outcomes, and subgroup effects in addition to reporting primary outcome67 |
| Report study design (eg, randomized controlled trial) and whether a comparison group is included | ||
| Report use intention-to-treat analysis22 and missing data procedure68 | ||
| Report qualitative methods to acceptability and usability of the intervention; report potential negative outcomes of the intervention22 | ||
| Report subgroup effects (eg, sex, race, age, or health/disease condition that influence the intervention effect)22 | ||
| Adoption | Diffusion of intervention program at organizational and delivery level and factors influence the adoption | Document and understand contextual factors related to adoption and developing guides to help users enhance adoption67 |
| Report characteristics of intervention location and delivery agent and their selection criteria22; if applicable, describe adoption rate of intervention location and delivery agent69 | ||
| If applicable, describe the expertise of the delivery staff69 | ||
| If applicable, describe participation rate of delivery setting/agent69 | ||
| Provide information on the level of human involvement required for an SMS- and app-based intervention compared with the level of human involvement for a routine application70 | ||
| Provide information on the prompts/reminders required for SMS- and app-based intervention compared with the level of prompts/reminders for a routine application70 | ||
| Provide information on any interventions (including training sessions/support) that are implemented in addition to the targeted SMS- and app-based intervention70 | ||
| Implementation | Fidelity of intervention program, including intervention uptake, development, monitoring, and adaptation and factors influence the implementation | Document standardized measures for capturing implementation fidelity, multilevel assessment of cost (both monetary and time) and adaption made for implementation67 |
| Describe intervention content and use parameters (eg, frequency, optimal timing for use, heaviness of use)70, and frequency of inter-person and virtual sessions, if applicable. | ||
| Provide information intervention costs including price for mobile phone, mobile phone data plan, and incentives for program development/participation given to staff or participants68 | ||
| Record percent delivered as intended (eg, SMS sent/unsent/received/not received; any application functioning problems)22 | ||
| Describe adaptations made to the intervention during implementation (eg, fitting strategies and methods to user culture)68 | ||
| Report strategies to monitoring and gathering feedback (from both participants and staffs) during implementation69 | ||
| Report time cost to participants and staff in addition to time used for intervention implementation. | ||
| Maintenance | Sustainability of intervention program at both the individual and organizational levels | Document and understand dynamic, complex, and multilevel factors leading to sustainment67 |
| Include an assessment of maintenance of the intervention outcomes (clinical and/or behavioral, such as hemoglobin A1c reduction, healthy eating) 6 mo after the completion of the intervention71 | ||
| Report broader outcomes (eg, policy development associated with the intervention)57 | ||
| Provide a context in which to evaluate the long-term outcome (eg, ongoing institutional and policy support for program maintenance and ongoing cost)57 | ||
| Include a sustainability plan regarding how the intervention could be sustained at both the individual and organizational levels or, if applicable, provide data on the degree to which the intervention is sustained over time22,71 |
RE-AIM: Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance; SMS: short message service.
Limitations
Several limitations concerning this review need to be considered. First, there was a small number of eligible studies available for this study. However, we used a validated PRISMA approach when we selected literature and extracted results from studies using RCTs or quasi-experimental designs. Authors’ comments were also incorporated into findings of the review. Small sample size and inadequate blinding of some studies in this review may still contribute to risk of bias. Larger and methodologically robust trails are very much needed. Second, it is possible that our search strategy could not identify all relevant articles. For example, we excluded articles that were published in non–English-language or trial registry data. However, we searched 3 main databases including PubMed, Embase, and Web of Science, in addition to 2 topic-specific databases, CINAHL Plus and the IEEE Xplore Digital Library. We used broad inclusion and exclusion criteria to increase the likelihood of capturing relevant studies to minimize publication bias. We also used a manual search of reference lists of eligible articles for relevant systematic reviews and narrative reviews. We addressed a focused area of mobile phone–based solutions, specifically interventions delivered by SMS and apps that are the most common in technology based behavioral interventions due to increased smartphone ownership. The exclusive review on SMS- and app-based interventions, rather than a broad area of mobile health interventions including various wearable devices, improves the transferability of our results to a prime area of smartphone-based interventions. Further, some authors did not report on specific adoption, implementation, and maintenance strategies that were in fact used in their studies, increasing the risk of reporting bias. To address this concern, we contacted the authors directly to inquire missing information regarding external validity.
CONCLUSION
SMS- and app-based interventions may have potential to promote diabetes self-management among patients with diabetes as access to smartphone increase; however, the population impact in the United States and other countries remains to be confirmed. Our systematic review based on the RE-AIM framework synthesized important findings from this emergent body of literature. This review is among the first to address issues in external validity of SMS- and app-based DSMES studies that is lacking from traditional reviews predominately focused on internal validity and intervention efficacy or effectiveness. Our review demonstrated that the comprehensiveness of reporting on RE-AIM criteria across the mobile phone–based DSMES studies was relatively low and with many gaps in internal validity reporting (ie, extent to which the protocol was delivered as intended) and, more so in external validity (across domains in adoption, implementation, and maintenance). Without this information, it is difficult to determine the internal validity and external validity of mobile phone–based interventions in DSMES. We encourage researchers to improve reporting around RE-AIM dimensions, specifically information on intention-to-treat analysis; mixed methods to understand acceptability, usability, and implementation of an intervention; costs; location, delivery agent selection; characteristics; and maintenance planning. Continued efforts in improving quality of research development and reporting will ensure mobile phone–based interventions to address diabetes will be broadly applicable across diverse settings and populations.
FUNDING
This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award numbers P30DK092950 and P30DK020579) and the Centers for Disease Control and Prevention (Cooperative Agreement number U48DP006395). SJP’s contribution was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002346. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
YY designed the study, undertook the literature search and data extraction, contacted individual study authors, and wrote the article. SJP refined the coding book, verified the coding results, and assessed clinical applicability of the included studies. RCB, SAB, MK, VAF, and EJS provided critical appraisals and edits to the article. RD, KW, DAG, AT, AR, and CM provided important information regarding their individual studies.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr Eirik Arsand from Norwegian Centre for E-health Research, University Hospital of North Norway, Tromsø, Norway, for his information on an app that was extended from the app included in an article under our review. We would like to thank Dr Maryam Peimani from Tehran University of Medical Sciences in Iran, Ms Marcia Vervloet from the Netherlands Institute for Health Services Research in the Netherlands, and Dr Sanjay Arora from Keck School of Medicine at University of Southern California in the United States for their responses to our inquires. We would also like to thank Ms Lauren Hartz from University of Tokyo to reply our inquires in English on behalf of KM. Finally, we would like to thank Ms Allison Phad from Center for Diabetes Translation Research Washington University in St. Louis for providing her edits to the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management Education and Support. Diabetes Educ 2017; 43 (5): 449–64. [DOI] [PubMed] [Google Scholar]
- 2. Haas L, Maryniuk M, Beck J, et al. National standards for diabetes self-management education and support. Diabetes Educ 2012; 38 (5): 619–29. [DOI] [PubMed] [Google Scholar]
- 3. Benoit SR, Ji M, Fleming R, Philis-Tsimikas A.. Predictors of dropouts from a San Diego diabetes program: a case control study. Prev Chronic Dis 2004; 1: A10. [PMC free article] [PubMed] [Google Scholar]
- 4. Gucciardi E, Demelo M, Offenheim A, Stewart DE.. Factors contributing to attrition behavior in diabetes self-management programs: a mixed method approach. BMC Health Serv Res 2008; 8 (1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peyrot M, Rubin RR.. Access to diabetes self-management education. Diabetes Educ 2008; 34 (1): 90–7. [DOI] [PubMed] [Google Scholar]
- 6. Li R, Shrestha SS, Lipman R, et al. Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes–United States, 2011-2012. MMWR Morb Mortal Wkly Rep 2014; 63 (46): 1045–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Pew Research Center. Mobile Technology Fact Sheet. Washington, DC: Pew Research Center; 2019. [Google Scholar]
- 8. Greenwood DA, Gee PM, Fatkin KJ, Peeples M.. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol 2017; 11 (5): 1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arambepola C, Ricci-Cabello I, Manikavasagam P, Roberts N, French DP, Farmer A.. The impact of automated brief messages promoting lifestyle changes delivered via mobile devices to people with type 2 diabetes: a systematic literature review and meta-analysis of controlled trials. J Med Internet Res 2016; 18 (4): e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnhold M, Quade M, Kirch W.. Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res 2014; 16 (4): e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi J, Lee JH, Vittinghoff E, Fukuoka Y.. mHealth physical activity intervention: a randomized pilot study in physically inactive pregnant women. Matern Child Health J 2016; 20 (5): 1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coughlin SS. Mobile technology for self-monitoring of blood glucose among patients with type 2 diabetes mellitus. Mhealth 2017; 3: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haider R, Sudini L, Chow CK, Cheung NW.. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2019; 150: 27–37. [DOI] [PubMed] [Google Scholar]
- 14. Hall AK, Cole-Lewis H, Bernhardt JM.. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health 2015; 36 (1): 393–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kankanhalli A, Shin J, Oh H.. Mobile-based interventions for dietary behavior change and health outcomes: scoping review. JMIR Mhealth Uhealth 2019; 7 (1): e11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orr JA, King RJ.. Mobile phone SMS messages can enhance healthy behaviour: a meta-analysis of randomised controlled trials. Health Psychol Rev 2015; 9 (4): 397–416. [DOI] [PubMed] [Google Scholar]
- 17. Park JYE, Li J, Howren A, Tsao NW, De Vera M.. Mobile phone apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR Mhealth Uhealth 2019; 7 (1): e11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida Y, Boren SA, Soares J, Popescu M, Nielson SD, Simoes EJ.. Effect of health information technologies on glycemic control among patients with type 2 diabetes. Curr Diab Rep 2018; 18 (12): 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P.. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Ann Behav Med 2004; 27 (1): 3–12. [DOI] [PubMed] [Google Scholar]
- 20. Glasgow RE, Vogt TM, Boles SM.. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999; 89 (9): 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akers JD, Estabrooks PA, Davy BM.. Translational research: bridging the gap between long-term weight loss maintenance research and practice. J Am Diet Assoc 2010; 110 (10): 1511–22, 22 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blackman KC, Zoellner J, Berrey LM, et al. Assessing the internal and external validity of mobile health physical activity promotion interventions: a systematic literature review using the RE-AIM framework. J Med Internet Res 2013; 15 (10): e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bull SS, Gillette C, Glasgow RE, Estabrooks P.. Work site health promotion research: to what extent can we generalize the results and what is needed to translate research to practice? Health Educ Behav 2003; 30 (5): 537–49. [DOI] [PubMed] [Google Scholar]
- 24. Dzewaltowski DA, Estabrooks PA, Klesges LM, Bull S, Glasgow RE.. Behavior change intervention research in community settings: how generalizable are the results? Health Promot Int 2004; 19 (2): 235–45. [DOI] [PubMed] [Google Scholar]
- 25. Klesges LM, Dzewaltowski DA, Glasgow RE.. Review of external validity reporting in childhood obesity prevention research. Am J Prev Med 2008; 34 (3): 216–23. [DOI] [PubMed] [Google Scholar]
- 26. McGoey T, Root Z, Bruner MW, Law B.. Evaluation of physical activity interventions in children via the reach, efficacy/effectiveness, adoption, implementation, and maintenance (RE-AIM) framework: A systematic review of randomized and non-randomized trials. Prev Med 2016; 82: 8–19. [DOI] [PubMed] [Google Scholar]
- 27. White SM, McAuley E, Estabrooks PA, Courneya KS.. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Ann Behav Med 2009; 37 (1): 10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). PRISMA 2009 Checklist. Helsinki, Finland: PRISMA; 2009. [Google Scholar]
- 29. Sutter JD. The top 10 tech trends of 2009. CNN http://www.cnn.com/2009/TECH/12/22/top.tech.trends.2009/index.html Accessed •••.
- 30. Hussein WI, Hasan K, Jaradat AA.. Effectiveness of mobile phone short message service on diabetes mellitus management; the SMS-DM study. Diabetes Res Clin Pract 2011; 94 (1): e24–6. [DOI] [PubMed] [Google Scholar]
- 31. Shetty AS, Chamukuttan S, Nanditha A, Raj RK, Ramachandran A.. Reinforcement of adherence to prescription recommendations in Asian Indian diabetes patients using short message service (SMS)–a pilot study. J Assoc Physicians India 2011; 59: 711–4. [PubMed] [Google Scholar]
- 32. Waki K, Fujita H, Uchimura Y, et al. DialBetics: a novel smartphone-based self-management support system for type 2 diabetes patients. J Diabetes Sci Technol 2014; 8 (2): 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Chen M, Yuan J, Sun Y.. Welltang—A smart phone-based diabetes management application—Improves blood glucose control in Chinese people with diabetes. Diabetes Res Clin Pract 2016; 116: 105–10. [DOI] [PubMed] [Google Scholar]
- 34. Zimmerman LP, Goel S, Sathar S, et al. A novel patient recruitment strategy: patient selection directly from the community through linkage to clinical data. Appl Clin Inform 2018; 9 (1): 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun C, Sun L, Xi S, et al. Mobile phone–based telemedicine practice in older chinese patients with type 2 diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth 2019; 7 (1): e10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen K, Zoellner J, Motley M, Estabrooks PA.. Understanding the internal and external validity of health literacy interventions: a systematic literature review using the RE-AIM framework. J Health Commun 2011; 16 (sup3): 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dobson R, Whittaker R, Jiang Y, et al. Text message-based diabetes self-management support (SMS4BG): study protocol for a randomised controlled trial. Trials 2016; 17 (1): 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V.. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health 2017; 23 (9): 733–40. [DOI] [PubMed] [Google Scholar]
- 39. Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL.. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011; 34 (9): 1934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi MC, Nicolucci A, Di Bartolo P, et al. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care 2010; 33 (1): 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holmen H, Torbjornsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial renewing healTH. JMIR Mhealth Uhealth 2014; 2 (4): e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torbjornsen A, Jenum AK, Smastuen MC, et al. A low-intensity mobile health intervention with and without health counseling for persons with type 2 diabetes, part 1: baseline and short-term results from a randomized controlled trial in the Norwegian part of renewing health. JMIR Mhealth Uhealth 2014; 2 (4): e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arora S, Peters AL, Burner E, Lam CN, Menchine M.. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med 2014; 63 (6): 745–54.e6. [DOI] [PubMed] [Google Scholar]
- 44. Kumar S, Moseson H, Uppal J, Juusola JL.. A diabetes mobile app with in-app coaching from a certified diabetes educator reduces A1C for individuals with type 2 diabetes. Diabetes Educ 2018; 44 (3): 226–36. [DOI] [PubMed] [Google Scholar]
- 45. Vervloet M, van Dijk L, de Bakker DH, et al. Short- and long-term effects of real-time medication monitoring with short message service (SMS) reminders for missed doses on the refill adherence of people with Type 2 diabetes: evidence from a randomized controlled trial. Diabet Med 2014; 31 (7): 821–8. [DOI] [PubMed] [Google Scholar]
- 46. Wu CJ, Sung HC, Chang AM, Atherton J, Kostner K, McPhail SM.. Cardiac-diabetes self-management program for Australians and Taiwanese: a randomized blocked design study. Nurs Health Sci 2017; 19 (3): 307–15. [DOI] [PubMed] [Google Scholar]
- 47. Agarwal P, Mukerji G, Desveaux L, et al. Mobile app for improved self-management of type 2 diabetes: multicenter pragmatic randomized controlled trial. JMIR Mhealth Uhealth 2019; 7 (1): e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bauer V, Goodman N, Lapin B, et al. Text messaging to improve disease management in patients with painful diabetic peripheral neuropathy. Diabetes Educ 2018; 44 (3): 237–48. [DOI] [PubMed] [Google Scholar]
- 49. Kim HS, Choi W, Baek EK, et al. Efficacy of the smartphone-based glucose management application stratified by user satisfaction. Diabetes Metab J 2014; 38 (3): 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Greenwood DA, Hankins AI, Parise CA, Spier V, Olveda J, Buss KA.. A comparison of in-person, telephone, and secure messaging for type 2 diabetes self-management support. Diabetes Educ 2014; 40 (4): 516–25. [DOI] [PubMed] [Google Scholar]
- 51. Brandon DT, Isaac LA, LaVeist TA.. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc 2005; 97 (7): 951–6. [PMC free article] [PubMed] [Google Scholar]
- 52. Carpenter WR, Tyree S, Wu Y, et al. A surveillance system for monitoring, public reporting, and improving minority access to cancer clinical trials. Clin Trials 2012; 9 (4): 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greiner KA, Friedman DB, Adams SA, et al. Effective recruitment strategies and community-based participatory research: community networks program centers’ recruitment in cancer prevention studies. Cancer Epidemiol Biomarkers Prev 2014; 23 (3): 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glasgow RE, Bull SS, Gillette C, Klesges LM, Dzewaltowski DA.. Behavior change intervention research in healthcare settings: a review of recent reports with emphasis on external validity. Am J Prev Med 2002; 23 (1): 62–9. [DOI] [PubMed] [Google Scholar]
- 55. Antikainen I, Ellis R.. A RE-AIM evaluation of theory-based physical activity interventions. J Sport Exerc Psychol 2011; 33 (2): 198–214. [DOI] [PubMed] [Google Scholar]
- 56. Estabrooks P, Dzewaltowski DA, Glasgow RE, Klesges LM.. Reporting of validity from school health promotion studies published in 12 leading journals, 1996-2000. J Sch Health 2003; 73 (1): 21–8. [DOI] [PubMed] [Google Scholar]
- 57. Gaglio B, Shoup JA, Glasgow RE.. The RE-AIM framework: a systematic review of use over time. Am J Public Health 2013; 103 (6): e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Estabrooks PA, Allen KC.. Updating, employing, and adapting: a commentary on What does it mean to “employ” the RE-AIM model. Eval Health Prof 2013; 36 (1): 67–72. [DOI] [PubMed] [Google Scholar]
- 59. Joe J, Demiris G.. Older adults and mobile phones for health: a review. J Biomed Inform 2013; 46 (5): 947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. May CR, Finch TL, Cornford J, et al. Integrating telecare for chronic disease management in the community: what needs to be done? BMC Health Serv Res 2011; 11 (1): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nielsen JA, Mengiste SA.. Analysing the diffusion and adoption of mobile IT across social worlds. Health Informatics J 2014; 20 (2): 87–103. [DOI] [PubMed] [Google Scholar]
- 62. Hebert MA, Korabek B, Scott RE.. Moving research into practice: a decision framework for integrating home telehealth into chronic illness care. Int J Med Inform 2006; 75 (12): 786–94. [DOI] [PubMed] [Google Scholar]
- 63. Ross J, Stevenson F, Lau R, Murray E.. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implement Sci 2016; 11 (1): 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Benavides-Vaello S, Strode A, Sheeran BC.. Using technology in the delivery of mental health and substance abuse treatment in rural communities: a review. J Behav Health Serv Res 2013; 40 (1): 111–20. [DOI] [PubMed] [Google Scholar]
- 65. Jennett PA, Scott RE, Affleck Hall L, et al. Policy implications associated with the socioeconomic and health system impact of telehealth: a case study from Canada. Telemed J E Health 2004; 10 (1): 77–83. [DOI] [PubMed] [Google Scholar]
- 66. Matthew-Maich N, Harris L, Ploeg J, et al. Designing, implementing, and evaluating mobile health technologies for managing chronic conditions in older adults: a scoping review. JMIR Mhealth Uhealth 2016; 4 (2): e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health 2019; 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stellefson M, Chaney B, Barry AE, et al. Web 2.0 chronic disease self-management for older adults: a systematic review. J Med Internet Res 2013; 15 (2): e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Molleman GR, Ploeg MA, Hosman CM, Peters LH.. Preffi 2.0—a quality assessment tool. Promot Educ 2006; 13 (1): 9–14. [PubMed] [Google Scholar]
- 70. Eysenbach G, Group C.. E-CONSORT-E-HEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res 2011; 13 (4): e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kessler RS, Purcell EP, Glasgow RE, Klesges LM, Benkeser RM, Peek CJ.. What does it mean to “employ” the RE-AIM model? Eval Health Prof 2013; 36 (1): 44–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.