Abstract
Objective
The growing prevalence of chronic conditions requiring changes in lifestyle and at-home self-management has increased interest in and need for supplementing clinic visits with data generated by patients outside the clinic. Patient-generated health data (PGHD) support the ability to diagnose and manage chronic conditions, to improve health outcomes, and have the potential to facilitate more “connected health” between patients and their care teams; however, health systems have been slow to adopt PGHD use in clinical care.
Materials and Methods
We surveyed current and potential users of PGHD to catalog how PGHD is integrated into clinical care at an academic health center. The survey included questions about data type, method of collection, and clinical uses of PGHD. Current users were asked to provide detailed case studies of PGHD use in research and care delivery.
Results
Thirty-one respondents completed the survey. Seventeen individuals contributed detailed case studies of PGHD use across diverse areas of care, including behavioral health, metabolic and gastrointestinal conditions, musculoskeletal/progressive functional conditions, cognitive symptoms, and pain management. Sensor devices and mobile technologies were the most commonly reported platforms for collection. Clinicians and researchers involved in PGHD use cited the potential for PGHD to enhance care delivery and outcomes, but also indicated substantial barriers to more widespread PGHD adoption across healthcare systems.
Conclusion
The results of our survey illustrate how PGHD is used in targeted areas of one healthcare system and provide meaningful insights that can guide health systems in supporting the widespread use of PGHD in care delivery.
Keywords: patient health data, digital health, data integration, electronic health data
INTRODUCTION
With the growing prevalence of chronic conditions requiring changes in lifestyle and at-home self-management, there is increased interest in and need for supplementing clinic visits with data generated by patients outside the clinic. Integrating patient-generated health data (PGHD) into clinical care holds potential for the successful diagnosis and improved management of these health conditions.1,2 PGHD are defined as “data created, recorded, and gathered by and from patients” often through the use of technology such as smartphones and wearable devices.3,4 Wearable devices, mobile health apps, and geolocation technologies place the ability to track, monitor, and report data into individuals’ hands and provide a novel way to capture information about health and well-being, monitor activity levels, improve self-awareness of health, and leverage tools to better manage health conditions.5–7 The surge in the consumer market for healthcare-related technology-driven solutions continues at an exponential rate that is anticipated to correlate with growth in PGHD generation.1,2,8
Within the healthcare system, efforts to improve access to and use of health information include web-based patient portals to view lab results and communicate with healthcare providers, the contribution of patient-reported information to medical records, and access to provider documentation through OpenNotes.9,10 “Connected health,” facilitated by patients collecting and reporting data outside of clinical care, has great potential to impact delivery of and access to healthcare through remote monitoring, increased access to longitudinal data about individual health, and improved engagement and communication with providers and healthcare teams.5,6,11–13 While PGHD offers an opportunity to provide a more robust view of an individual’s health and wellness, healthcare systems have been slow to formally integrate PGHD into clinical workflows and leverage PGHD for care transformation.6 Supporting the scale and spread of PGHD use requires better understanding of current PGHD utilization, including the diversity of data collected, intentions for data use, impact on health information technology systems, and effect on healthcare delivery, in order to prioritize next steps.1,14,15
To explore experiences with PGHD (primarily collected through use of technology) in clinical care, we sought to identify stakeholders across diverse clinical settings to understand the context of PGHD use (eg, prevention and wellness, condition or symptom-specific management, etc.), types of technology used for data collection (eg, wearable devices), measures collected (eg, sleep, movement), data analytics used, and experiences related to the integration of data into clinical care.
MATERIALS AND METHODS
The setting of our study was the University of Washington (UW) Medicine system, a large healthcare system in the Pacific Northwest. Ethics approval was obtained from UW Institutional Review Board, and an exempt determination was granted on May 31, 2017. In response to a changing healthcare and technology environment, UW Medicine established the Committee on Digital and Connected Health (CDCH) in 2016 to guide the role of digital health in healthcare delivery and care transformation. The CDCH is composed of 50 members, representing diverse experiences in software engineering and development, patient care, health system administration, patient-centered outcomes research, patient engagement, user-centered design, and health IT administration. The objective of this study was to engage with the CDCH members and broader stakeholders across UW Medicine to understand the current landscape of PGHD use within our health system.
Previous work has examined the use of PGHD for monitoring specific health conditions, its potential role in improving patient engagement in care, its use in clinical decision making, and the technological implications of its integration in clinical care.2,4,7,10,13,16 To our knowledge, no previous studies have cataloged the diversity of PGHD uses within a health system. Therefore, in collaboration with the CDCH we developed a cataloging survey to capture various aspects of respondents’ experiences using PGHD. We piloted the survey to ensure clarity of survey text, user friendliness, and ease of completion. The survey included both closed- and open-ended questions. The detailed characteristics of PGHD use addressed in the survey were guided by the relevant domains of the Sittig-Singh sociotechnical model for health information technology (Table 1), which organizes factors of technology design around hardware/software; clinical content; human–computer interface; people, workflow, and communication; organizational policies and procedures; external rules and regulations; and system measurement and monitoring.17 The survey addressed the individual’s interest in and use of PGHD. Respondents were also asked to indicate perceived barriers and evidence gaps for PGHD in the context of broader scale use in healthcare delivery. The subset of respondents who identified as current users of PGHD were asked to identify one specific type of patient-generated data used in their work (eg, physical activity, blood pressure, etc.), and were asked a series of detailed questions on these “case studies” of PGHD in actual use. By focusing on these case studies, we sought to better understand the workflow for how PGHD is collected and integrated into healthcare decision-making. Survey questions are detailed in Supplementary Appendix A.
Table 1.
Application of Sittig-Singh sociotechnical model to cataloging survey data elements17
| Sociotechnical domain | Brief description | Cataloging survey data elements |
|---|---|---|
| Hardware/software | Physical devices (hardware) and the applications (software) required keeping these devices running. |
|
| Clinical content | Data or information (structured or unstructured, images, scanned resources, etc.) that is stored within the system to facilitate clinical knowledge. |
|
| Human computer interface | Any interaction (see, touch, hear) between users and the system. |
|
| People | The human aspect (staff, patients, developers) of developing, learning, and using the system, and their reactions to it. |
|
| Workflow and communication | The process of completing tasks with the system (or alongside other systems) in order to accomplish care delivery. |
|
| Organizational policies and procedures | The internal policies, procedures, and guidelines that may influence an organization’s implementation or use of the system. |
|
| External rules, regulations, and pressures | External constraints that impact the implementation and use of the system (payment, federal laws, etc.). |
|
| System measurement and monitoring | The process for measuring system success, including system availability and use by clinical team members, impact on patient care, and any unintended consequences resulting from system use. | None included. |
CDCH committee members and other stakeholders (including clinicians, researchers, and developers) known for their involvement with PGHD at UW Medicine were invited to respond to the survey. The survey was distributed via several internal listservs, and directly to UW study teams with PGHD-related projects cited on clinicaltrials.gov. Snowball sampling was also used as survey recipients identified additional stakeholders involved in PGHD work at UW, and the team regularly reviewed survey responses to ensure diverse case studies were represented and identify potentially missing respondents.18 Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Washington.19 REDCap is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. All survey responses were reviewed and cleaned to ensure duplicate case studies were not included. Using the Sittig-Singh model as a framework, the team descriptively analyzed cataloging survey responses to better understand the breadth of sociotechnical considerations involved in PGHD use and identify potential patterns of commonality across case studies.17 Learnings from the cataloging survey were shared with CDCH stakeholders to inform discussions around how our health system can better support digital health advancement.
RESULTS
Thirty-one individuals completed the survey; 10 identified themselves as clinician researchers, 12 exclusively as clinicians, 8 exclusively as researchers (2 as developers or system engineers), and 1 as involved in digital health commercialization. Of the 31 total respondents, 23 individuals identified as current users of PGHD in research or clinical care, while 8 identified as interested in using PGHD in the future. Of the 23 survey respondents who identified as current users of PGHD, 17 respondents identified a case study from their experience with PGHD exemplifying use of a single data type and answered detailed questions pertaining to the case study (the remaining 6 current PGHD users did not identify a case study). Among the 22 clinicians, 6 (27%) indicated that patients currently bring PGHD unprompted by the clinical team, 3 (13%) indicated that their clinical setting collects PGHD as part of care delivery, and 7 (32%) indicated a combination of approaches for collecting PGHD from patients. Four (18%) indicated that their clinical setting does not collect PGHD at all, and 1 care provider did not respond to the question. The results of the survey, including the 17 case studies, are detailed below, organized around the following themes: (1) PGHD types and goals for clinical care, (2) technological infrastructure, (3) workflow for data collection and review, and (4) barriers to scale and spread.
PGHD types and goals for clinical care
The survey asked respondents to indicate their primary interest in PGHD use. Among current users of PGHD, clinical areas were heterogeneous. Examples included behavioral health conditions (eg, mood, depression, substance use), metabolic conditions (eg, blood pressure, diabetes), musculoskeletal or progressive functional conditions (eg, multiple sclerosis, osteoarthritis, cerebral palsy), cognitive conditions, pain, gastrointestinal conditions, and perioperative care, with an overwhelming focus on chronic versus acute conditions. Goals for incorporating PGHD (summarized in Table 2) focused on enhancing provider abilities to monitor and respond to patient experiences with disease and leverage new data to further align care with clinical practice guidelines.
Table 2.
Current goals for PGHD use reported by users of PGHD
| Goal | No. of times citeda |
|---|---|
| Improving symptom monitoring and assessment between visits | 8 |
| Personalizing interventions and monitoring plans | 4 |
| Assess clinical outcomes | 3 |
| Self-management and behavior change support | 2 |
| Prevention | 1 |
| Improving care delivery and quality assurance | 1 |
Number of times cited within the 17 case studies; the question allowed for multiple responses and two respondents indicated more than one goal for PGHD use.
PGHD users reported 71 distinct types of PGHD they currently use for research and/or clinical practice. Table 3 shows the spectrum of data types, as reported by survey respondents. To account for the wide range of settings within which PGHD is captured and utilized, the survey item addressing PGHD types solicited free-text responses. We did not solicit responses from a pre-determined set of data types and some types (eg, data relating to social determinants of health) may be underreported. On average, PGHD users reported using 4 unique types of data, with a range of 1 to 8 types. The most commonly used clinical category of PGHD was “physical activity”, followed by “mood-related symptoms” and “sleep.”
Table 3.
PGHD types
| Data types | Frequency reported by users of PGHD |
|---|---|
| Physical activity | 11 |
| Mood-related symptoms | 8 |
| Sleep | 6 |
| Medication use | 5 |
| Pain level (pain interference, mobility) | 4 |
| Blood pressure | 4 |
| GPS/location data | 4 |
| Accelerometer data | 3 |
| Paralinguistic (vocal data, spoken language) | 3 |
| Food diaries or logs | 3 |
| Weight | 3 |
| Blood sugar | 3 |
| Substance (alcohol, marijuana) use | 2 |
| Photo of wounds / stretches | 2 |
| Texting/device/technology use | 2 |
| Headache (frequency, characteristics) | 2 |
| Temperature | 1 |
| Social media use | 1 |
| Cognitive symptoms (memory, concentration) | 1 |
| Photo of drug metabolite test strips | 1 |
| Heart rate | 1 |
| Fatigue | 1 |
Seventeen survey respondents provided in-depth case studies detailing use of a single type of PGHD, including information about platforms used for capturing PGHD. Within the 17 case studies, 8 (47%) utilized sensor device or mobile functionality (eg, GPS, camera, smartphone) to collect PGHD, and 7 (41%) utilized wearable devices (eg, Fitbit). Additional platforms cited for PGHD collection included medical devices (3), Interactive Voice Response (IVR) (1), and direct patient report (electronic or paper based) (5). Five (29%) case studies utilized multiple platforms for PGHD collection. Of the 17 case studies, 16 (94%) required use of an affiliated app, web portal, or specialty software. Table 4 highlights the platforms used for capturing PGHD by type of data collected.
Table 4.
Data type and platform for collection of PGHD
| Type of data collected | Platform for data collection |
|||
|---|---|---|---|---|
| Wearable | Sensor/mobile | Medical device | Patient report | |
| Mood-related symptoms | x | x | ||
| Physical activity | x | x | ||
| Blood pressure | x | x | x | |
| Wound tracking (photos) | x | |||
| Food diaries or logs | x | x | x | x |
| Pain level and mobility | x | x | ||
| Accelerometer data | x | |||
| Paralinguistic (vocal data, spoken language) | x | |||
| Social media use | x | |||
| Medication use | x | |||
| Heart rate | x | x | ||
Data collection and review (training, timing, and clinical decision support)
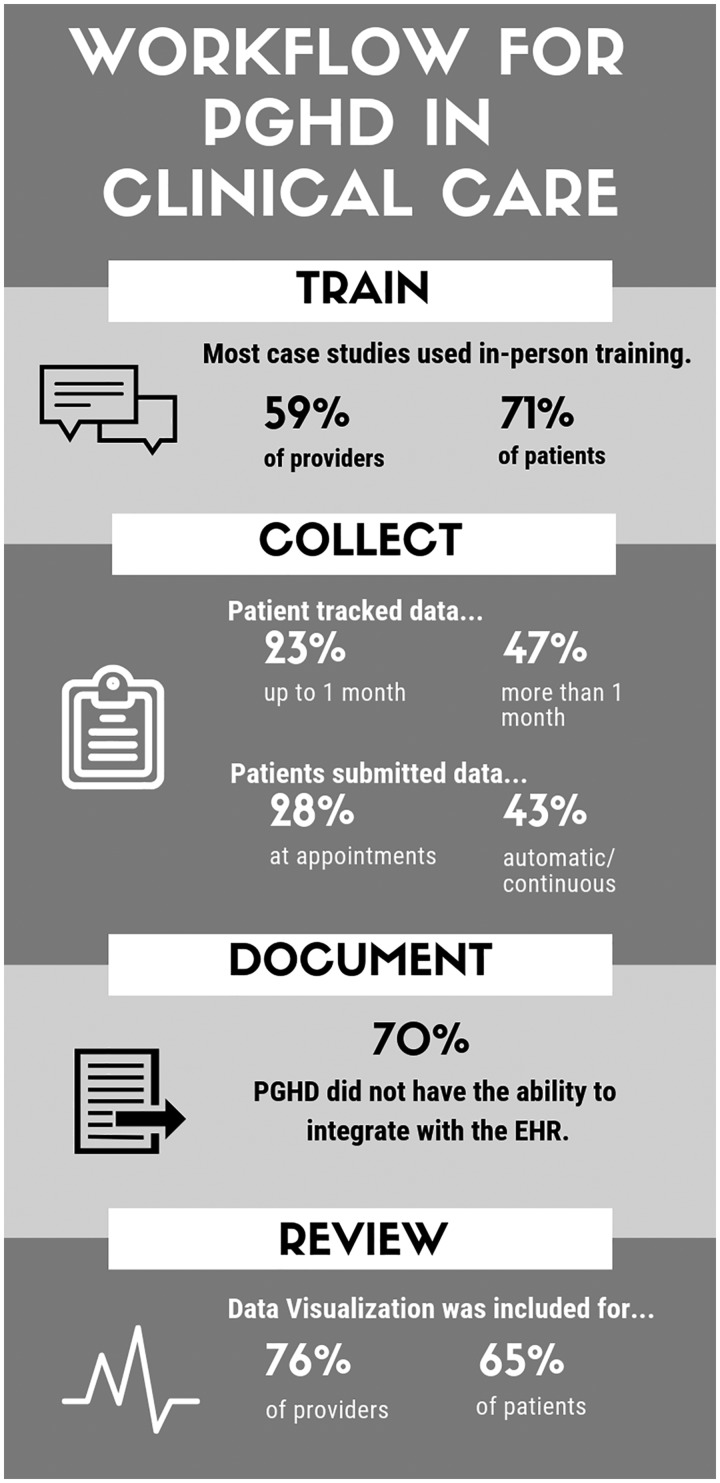
The survey examined several aspects of PGHD capture and reporting, and related communication in clinical practice. Figure 1 provides an overview of salient aspects of data collection and review reported in the 17 case studies provided by current users of PGHD.
Figure 1.
Workflow for PGHD in clinical care.
Training
To start, the survey asked current users of PGHD about approaches to training around PGHD and collection tools, and found in-person training for providers (10 case studies) and patients (12 case studies) was the most common format. Other approaches included training via email, paper, and website or online tutorial. Several case studies reported no training for providers (4 case studies) or patients (2 case studies). PGHD collection involving sensor devices or mobile technology platforms had the highest number of training formats when compared to other data collection platforms.
Collection
Next the survey asked current users about collecting PGHD. Of the 17 case studies, 47% required that patients track their data for multiple months. In 23%, patients tracked data for 1 week to 1 month; in 18% patients engaged in open-ended tracking until the index problem was resolved; in 12% patients tracked data for the duration of their care. Timing of data submission varied across case studies; 43% had patients submit their data automatically and/or continuously, 28% had patients submit data at the time of the appointment, and 14% had patients submit data on a routine basis not related to appointments (eg, weekly). Among case studies that required the use of an app or specialty software program (94%), 50% required both the patient and provider to use the software to access data or other tools. The remaining case studies were split between requiring only the patient (25%) or only the provider (25%) to use it. One case study cited the need for patients to call the provider to alert them when data had been uploaded for review.
Documentation
Seventy percent of the case studies had no integration capabilities with the electronic health record (EHR). Eighteen percent reported some level of EHR integration (ie, ability to manually import PGHD into the EHR), and only 12% reported full EHR integration. The most common approach for receiving PGHD collected by the patient was uploading data from the collection device to an associated web portal. Once PGHD was received, users were likely to store PGHD in multiple locations, including research portals and manual uploads into the EHR.
Reviewing and sharing data
Of the 17 case studies, 76% included provider-facing data visualization tools to support PGHD review and clinical decision-making and 65% included a visualization tool for patients. For providers, the most frequently cited data visualization functionality was the ability to show total scores for individual patients (35% of case studies), followed by the ability to show aggregate level scores for patient populations (29% of case studies). Only 12% of case studies reported the capability to compare trends or provide statistical insight into population level data. In 12% of case studies, patients were able to view their data at the point of collection and in 29% of case studies patients could access aggregate or historical reports of their data. Twelve percent of case studies had interactive dashboards where patients could manipulate visualizations of their data.
Finally, current PGHD users indicated that PGHD reports were consistently shared with patients (cited by 10 case studies), research teams (10 case studies), and providers within the clinic or specialty (9 case studies). Two current users stated that PGHD reports were shared with administrators, and one indicated that data were shared with providers outside the clinic or specialty where it was collected. No current users shared PGHD with payers.
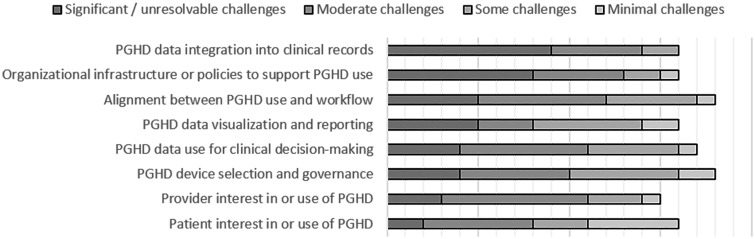
Barriers to scale and spread
Figure 2 shows how current PGHD users who identified case studies rated the barriers they experienced in their development and use of PGHD. The most significant barriers were (1) PGHD integration into clinical records and (2) organizational infrastructure or policies to support PGHD use. The area of PGHD use that had the least significant barriers was patient interest in or use of PGHD.
Figure 2.
Barriers to PGHD use.
Survey respondents who were interested in using PGHD in the future also cited challenges related to making PGHD tools available in the health record, and lack of billing support for provider time spent reviewing PGHD outside the clinical visit.
DISCUSSION
The results of our survey illustrate how PGHD is used in targeted areas of one healthcare system and provide insights into how health systems can conceptualize integrating PGHD across clinical care as use of PGHD increases. Understanding common goals and data of interest across care settings can facilitate prioritization of future work. Further, it presents an opportunity to identify barriers and challenges faced and addressed by early adopters to guide system-wide initiatives to integrate PGHD.
Of the 71 different types of PGHD reported, the three most common uses of PGHD (representing 35% of the total PGHD types reported) were tracking physical activity, mood, and sleep; over half of the PGHD types reported track activities or symptoms that occur on a daily basis. These data are not easily captured through traditional care delivery workflows. Integration of PGHD into clinical care can address this gap by providing additional data to support clinical decision-making. It is also important to note that these data points do not always correlate with traditional areas of care or common use cases. For example, while tracking physical activity (eg, through the use of Fitbits) is often associated with primary care or weight management, several use cases reported here indicate an expanded value of this data, such as in measurement of functional status decline or for monitoring the impact of gastrointestinal disease on daily activities. This is significant not only because PGHD can support a more patient-centered approach to assessments of symptom burden, but also because PGHD could enhance understanding of patient experience beyond traditional disease markers and contribute to clinical decision-making processes in new ways.19,20 Our analysis also found that sensor devices and mobile technologies are the most frequently used platforms for PGHD collection (63%), therefore driving PGHD use more than wearables or medical devices. This finding aligns with research that shows an emerging trend toward use of mobile technologies to monitor health, access health information, and interact with the health system.21 As medical sensing abilities improve and are further integrated into smartphones and mobile devices, it is anticipated that the use of such technology for managing personal health will continue to increase.22 Continued focus on the quality of apps and equitable access to technology for those who may benefit most is warranted.23,24
Survey results also provided insight into the technological infrastructure of PGHD collection, indicating that 94% of PGHD case studies required the use of an app or specialty software, and that half of those use cases required the patient and their provider to interact with that software together to facilitate PGHD use, highlighting a significant impact to clinical workflow. Integration of PGHD into the EHR or other existing health system IT tools was limited and cited as the most significant barrier among PGHD users. In 35% of case studies, PGHD was entered into the EHR, often through manual processes, despite the attendant workflow burdens implicit in such an ad hoc system. EHRs remain limited in the ability to import data from external data sources especially where data standards are lacking.22,25 The fact that clinicians and researchers continue to use PGHD despite the burden to clinical workflow implied by the above findings highlights the value derived from the addition of PGHD to clinical care and reinforces its emerging usefulness for supporting patient engagement and clinical decision-making. Efforts to better integrate PGHD remain an important area of focus and may be better suited through advancing interoperability between HIT platforms through middleware, block chain technology, and application program interface specifications.25
Barriers to PGHD use highlight important areas for future work. Policy and organizational support for PGHD remain important facilitators for capturing PGHD. At the policy level, recently proposed changes to the 21st Century Cures Act support a mandate for prioritizing interoperability of heath IT.26 These proposals aim to increase access and exchange of electronic health information between patients and their providers. Health systems will likewise need to consider how to align with policy such that clinical implementations of PGHD meet proposed standards for health IT.27
In addition to improved integration with EHRs, more work is needed to understand how PGHD supports clinical decision-making. Ensuring data collected translates to actionable information requires work not only to capture the data but also evidence for how to translate the data such that it supports clinical decision-making.4 Promising examples exist of how health systems can overcome these challenges to scale programs that integrate PGHD into clinical care. Ochsner Health System’s initiative to incorporate home blood pressure monitoring into a comprehensive program for remote monitoring and treatment of hypertension is one such example.28 Another is Sutter Health’s use of patient-centric decision support tools to improve patient self-management of disease.28 These EHR-integrated tools are accessible by both patients and providers. The examples highlighted here point toward promising areas for future examination of how PGHD can be translated for use in clinical decision-making and in support of improved care processes.
CONCLUSION
The results of this PGHD cataloging survey provide a framework to understand how PGHD is currently used within healthcare systems. The potential benefit that digital health can contribute to healthcare lies in its ability to improve our understanding of disease courses, push us beyond our current disease paradigms, and support better connectedness between patients and care providers.14,16,27 Yet PGHD technologies and their associated functionality (software) to support integration into clinical care are not developing in a systematic fashion. Additionally, the rapid pace and volatility of technology clashes with the goals of health systems, who seek to invest in longevity, not trends, in the pursuit of improving efficiencies in care delivery and health outcomes. This presents significant challenges to health systems that have constraints on technological flexibility and responsiveness and look to adopt practices and technologies that demonstrate the ability to provide benefits to both patients and the health system. In consideration of PGHD’s potential role in improving care processes and treatment paradigms, health systems must be proactive in establishing policies that govern the selection and integration of technologies that facilitate PGHD use.29
The work presented here represents an effort to better understand these complexities in the context of one health system. Limitations of this study include the use of a convenience sample within one health system. As such it may not represent all potentially relevant applications of PGHD in clinical care. In the next phase of this project we will conduct in-depth interviews with patients, clinicians, and administrators involved in PGHD use to further explore and refine the findings reported here. Future work will aim to clarify the most effective approaches to evaluating the impact of PGHD from the perspectives of multiple stakeholders, in order to support efficient evidence translation, and inform decision-making regarding the scale and spread of PGHD use across healthcare systems.15,27,29
FUNDING
The project described was supported by a Patient-Centered Outcomes Research Institute (PCORI) Program Award (4322-UOW), and by Funding Opportunity Number CMS-331-44-501 from the U.S. Department of Health & Human Services, Centers for Medicare & Medicaid Services.
AUTHOR CONTRIBUTIONS
EA, DA, RB, SOL, DM, SM, and DCL conceived of and designed the project. EA, DA, SOL, and DCL collected the data. EA, JRL, and DCL analyzed the data, and all authors contributed to interpretation of the data. EA, JRL, and DCL drafted the article. All authors provided critical revision of the article and final approval of the version to be published.
Supplementary Material
ACKNOWLEDGMENTS
The content provided is solely the responsibility of the authors and does not necessarily represent the official views of PCORI, or HHS or any of its agencies.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
REFERENCES
- 1. Lai AM, Hsueh P-Y, Choi YK, Austin RR.. Present and future trends in consumer health informatics and patient-generated health data. Yearb Med Inform 2017; 26 (01): 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sands DZ, Wald JS.. Transforming health care delivery through consumer engagement, health data transparency, and patient-generated health information. Yearb Med Inform 2014; 9 (1): 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health & Human Services. Consumer eHealth: Patient-generated health data 2015. https://www.healthit.gov/policy-researchers-implementers/patient-generated-health-data. Accessed September 13, 2019.
- 4. Cohen DJ, Keller SR, Hayes GR, et al. Integrating patient-generated health data into clinical care settings for clinical decision-making: lessons learned from Project HealthDesign. JMIR Hum Factors 2016; 3 (2): e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gollamudi SS, Topol EJ, Wineinger NE.. A framework for smartphone-enabled, patient-generated health data analysis. PeerJ 2016; 4: e2284.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hull S. Patient-generated health data foundation for personalized collaborative care. Comput Inform Nurs 2015; 33 (5): 177–80. [DOI] [PubMed] [Google Scholar]
- 7. Lv N, Xiao L, Simmons ML, Rosas LG, Chan A, Entwistle M.. Personalized hypertension management using patient-generated health data integrated with electronic health records (EMPOWER-H): six-month pre-post study. J Med Internet Res 2017; 19 (9): e311.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung AE, Jensen RE, Basch EM.. Leveraging emerging technologies and the “Internet of Things” to improve the quality of cancer care. J Oncol Pract 12 (10): 863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delbanco T, Walker J, Bell SK, et al. Inviting patients to read their doctors' notes: a quasi-experimental study and a look ahead. Ann Intern Med 2012; 157 (7): 461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arsoniadis EG, Rabindra T, Saif K, Cyrus J, Daren S, et al. Characterizing patient-generated clinical data and associated implications for electronic health records. Stud Health Technol Inform 2015; 216: 158–62. [PubMed] [Google Scholar]
- 11. Iglehart JK. Connected health: emerging disruptive technologies. Health Aff 2014; 33 (2): 190.. [DOI] [PubMed] [Google Scholar]
- 12. Mikk KA, Sleeper HA, Topol EJ.. The pathway to patient data ownership and better health. JAMA 2017; 318 (15): 1433.. [DOI] [PubMed] [Google Scholar]
- 13. Nundy A, Lu C-Y, Hogan P, et al. Using patient-generated health data from mobile technologies for diabetes self-management support: provider perspectives from an Academic Medical Center. J Diabetes Sci Technol 2014; 8 (1): 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reading MJ, Merrill JA.. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. JAMIA 2018; 25 (6): 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steward DA, Hofler RA, Thaldorf C, Milov DE.. A method for understanding some consequences of bringing patient-generated data into health care delivery. Med Decis Making 2010; 30 (4): E1–E13. [DOI] [PubMed] [Google Scholar]
- 16. Tiase VL. Navigating the patient-generated health data deluge. Nurs Manage 2017; 48 (12): 7–8. [DOI] [PubMed] [Google Scholar]
- 17. Sittig DF, Singh H.. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual Saf Health Care 2010; 19 (Suppl 3): i68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernard HR. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 5th ed. Lanham, MD: AltaMira Press; 2011. [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42 (2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purswani JM, Dicker AP, Champ CE, et al. Big data from small devices: the future of smartphones in oncology. Semin Radiat Oncol 2019; 29 (4): 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health apps and information survey. The Henry J Kaiser Family Foundation.http://files.kff.org/attachment/Topline-Health-Apps-and-Information-Survey-September-2019Accessed 13 September, 2019.
- 22. Singh K, Meyer SR, Westfall JM, et al. Consumer-facing data, information, and tools: Self-management of health in the digital age. Health Aff (Project Hope) 2019; 38 (3): 352–358. [DOI] [PubMed] [Google Scholar]
- 23. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff 2016; 35 (12): 2310–2318. [DOI] [PubMed] [Google Scholar]
- 24. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med 2016; 31 (12): 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kao CK, Liebovitz DM. Consumer mobile health apps: Current state, barriers, and future directions. PM&R 2017; 9 (5): S106–S115. [DOI] [PubMed] [Google Scholar]
- 26. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program. Federal Register 2019; 84 (42): 7424–7610.[CVOCROSSCVO] https://www.federalregister.gov/documents/2019/03/04/2019-02224/21st-century-cures-act-interoperability-information-blocking-and-the-onc-health-it-certification. Accessed September 13, 2019. [Google Scholar]
- 27. Petersen C, Adams SA, DeMuro PR.. mHealth: don’t forget all the stakeholders in the business case. Med 2015; 4 (2): e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tai-Seale M, Downing NL, Jones VG, et al. Technology-enabled consumer engagement: Promising practices at four health care delivery organizations. Health Aff 2019; 38 (3): 383–390. [DOI] [PubMed] [Google Scholar]
- 29. Hsueh P S, Dey S, Das S, Wetter T.. Making sense of patient-generated health data for interpretable patient-centered care: the transition from “More” to “Better.” Stud Health Technol Inform 2017; 245: 113–117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.