Abstract
The infrapatellar fat pad (IFP) is an extrasynovial, intracapsular, adipose body occupying the space in the knee joint between the inferior border of the patella, the femoral condyles, tibial plateau and patellar tendon. Little is known about the anatomy and normal function of the IFP, but it has been suggested to play a role in the aetiology of Anterior knee pain syndrome, including that associated with osteoarthritis. Forty‐three knees from 11 male and 15 female embalmed cadavers (mean age 84 years; range 55–97 years) were investigated. The cadavers were donated and the study performed in compliance with the provisions of the UK Human Tissue Act (2004). The quadriceps tendon and the medial and lateral patellar retinacula were dissected from the patella, which was then reflected antero‐distally. The IFP was carefully excised and details of its morphology and attachments to components of the knee joint were recorded, together with the presence of articular surface pathology on the patella and femoral condyles. The principal novel findings of the current study were that 81% of IFPs were attached to the superior border of the patella by supero‐medial extensions and 65% were attached by supero‐lateral extensions; the supero‐medial extensions were larger than the supero‐lateral extensions. The superior extensions of the IFP were always attached anteriorly to the patellar retinacula and in four individuals the extensions formed a full loop around the superior border of the patella. The volume of IFPs with attachments to the superior border of the patella was significantly greater (p = .007) than those without, and the IFP was attached to the medial meniscus in significantly (p = .009) more knees with IFP attachment to the superior border of the patella than those without. All IFPs were attached to the medial anterior horn of the meniscus and the medial Kaplan’s ligament. Ninety‐seven per cent were attached to the lateral anterior horn of the meniscus and 97% to the lateral Kaplan’s ligament. The length of IFP attachment to the lateral meniscus was significantly longer (p = .004) than that to the medial meniscus. Ninety‐seven per cent of IFPs were attached to the superior portion of the patellar tendon with the mean tendon attachment being 60%. Ninety‐one per cent of IFPs were attached to the inferior border of the patella. Significantly fewer knees with patellar (p = .001) and femoral (p = .002) articular surface osteophytes exhibited superior IFP extensions and these extensions were significantly shorter in knees with patellar (p = .000) and femoral (p = .006) osteophytes, compared with those without. The IFP was attached to the medial meniscus in significantly fewer knees with femoral (p = .050) and patellar (p = .023) osteophytes than those without. All IFPs not attached to the anterior horn of the lateral menisci, medial Kaplan’s ligament, superior patella or inferior border of the patella, were in knees with articular surface osteophytes. This relationship between IFP morphology and knee joint pathology suggests a functional role for the IFP that requires further investigation.
Keywords: anterior knee pain, infrapatellar fat pad, osteoarthritis
A posterior view of an isolated left infrapatellar fat pad, showing its principal morphological features. Note particularly the superior lateral and superior medial extensions that encircle the patella (removed). (1) Superior lateral extension, (2) lateral extension, (3) central body, (4) ligamentum mucosum, (5) vertical cleft, (6) superior tag, (7) medial extension, (8) superior medial extension.

1. INTRODUCTION
The infrapatellar fat pad (IFP), also known as Hoffa’s fat pad (Hoffa, 1904), is adipose tissue that has been described as occupying the space in the knee joint bounded by the inferior border of the patella, patellar tendon, tibial plateau and femoral condyles (Gallagher et al., 2005). It is an intracapsular, but extrasynovial structure (Duri et al., 1996; Biedert and Sanchis‐Alfonso, 2002; Gallagher et al., 2005). The exact size, shape and volume of the fat pad have been reported to be highly variable (Metheny and Mayor, 1988; Duri et al., 1996; Biedert and Sanchis‐Alfonso, 2002). Previous anatomical studies have been performed on a small number of cadavers (Hoffa, 1904; Metheny and Mayor, 1988; Duri et al., 1996; Gallagher et al., 2005), on magnetic resonance imaging (MRI; Saddik et al., 2004; Ozkur et al., 2005; Abreu et al., 2008) and at arthroscopy (Brooker et al., 2009). The attachment of the IFP to surrounding structures was not investigated specifically in any of these studies.
A number of authors have reported that the IFP is innervated by the posterior articular branch of the tibial nerve (Kennedy et al., 1982; Bennell et al., 2004; Clements et al., 2004). However, probably the most detailed study of the innervation of the knee (Gardner, 1948) described numerous branches supplying different regions of the IFP. The anteromedial section of the IFP was reported to be supplied by the nerve to vastus medialis, the anterolateral portion by the nerve to vastus lateralis and branches from the saphenous, tibial, recurrent peroneal and common peroneal nerves were also reported to supply the IFP. All of these nerves were reported to travel with blood vessels into the IFP, but little is known about the types of nerve endings in the IFP or their function. However, immunohistochemistry of the IFP has revealed free nerve fibres and nerves surrounded by multilaminated connective tissue capsules, with the presence of type VIa free nerve endings that may mediate pain, pressure and temperature information (Biedert and Sanchis‐Alfonso, 2002; Macchi et al., 2016).
The blood supply to the IFP has been reported to be derived from arteries running vertically on either side of it; the medial and lateral genicular arteries. These arteries first supply the patella before passing into the medial and lateral segments of the IFP respectively and continuing inferiorly to exit the fat pad medial and lateral to the tibial tuberosity respectively, before anastomosing with the inferior medial artery of the knee and the recurrent artery of the tibia. These vertical arteries, descending in the IFP immediately behind the patellar tendon, anastomose via two to three arteries running horizontally between them (Davies and Edwards, 1948; Kohn et al., 1995; McDougall et al., 1998).
The function of the IFP has long been debated and there is not a consensus in the literature. Historically, Havers (1691) attributed the release of synovial fluid into the joints to synovial fat pads, such as the IFP. More recently, MacConaill (1950) suggested that such fat pads were simply fillers of dead space within joints, providing lubrication of the joint and ensuring that the space was maintained. However, it has been suggested more recently that the IFP may have a more specific function, because of its rich arterial blood supply (Kohn et al., 1995; Nemschak and Pretterklieber, 2012) and its sensory innervation (Lehner et al., 2008). Interestingly the IFP is only metabolised in severe malnutrition (Davies and White, 1961; Apostolaki et al., 1999), suggesting a role other than simply an energy store. Histological investigation of the IFP (Macchi et al., 2016) has led to the suggestion that it acts as a pressure absorber during knee articulation and that the relatively high density of nerves within the IFP may indicate a mechanoreceptor/proprioceptor role for the IFP. Furthermore, Geraghty and Spear (2017) have suggested that together with the plica of the knee, the IFP provides internal support for the patella, mirroring the support given by the external retinacula.
Hoffa (1904) was the first to describe IFP pathology, reporting that impingement of the IFP between the posterior patella and the femur caused anterior knee pain. However, there is a paucity of literature about IFP pathology, with most reports of primary pathology being case studies (Bulmer, 1966; Palumbo et al., 1994; Sakai et al., 1999; Choi, 2000; Aynaci et al., 2001; González, 2001). Some authors (Duri et al., 1997; Clockaerts et al., 2010) have suggested that most IFP pathology is secondary to other joint disease, particularly patello‐femoral osteoarthritis. Research has shown that in osteoarthritis, white adipose tissue such as the IFP can be infiltrated by immune cells and secrete inflammatory mediators and microscopic changes can occur to the IFP, such as thickening of the interlobular septa and an increase in vascularisation (Gardner, 1948; Caulfield et al., 1988; Maculé et al., 2005; Favero et al., 2017; Macchi et al., 2017). Moreover, the IFP has also been reported to contain large numbers of substance P‐positive nerve fibres and that their number increases in patients with rheumatoid arthritis (Bohnsack et al., 2005; Lehner et al., 2008). In view of the fact that the IFP largely comprises adipose tissue, it may also have an endocrine function and secrete leptin and adiponectin (Dumond et al., 2003; Fain, 2006), both of which have been reported to be pro‐inflammatory mediators in joint disease (Lago et al., 2008; Gomez et al., 2009). The IFP is in a position to secrete such inflammatory mediators directly into the knee joint because it is an intra‐articular structure. Therefore, obesity may affect the progression of osteoarthritis, beyond increasing mechanical pressure on weight‐bearing joints.
Anterior knee pain, also known as patello‐femoral pain syndrome (PFPS), is pain that occurs in the anterior knee, typically presenting on climbing stairs and squatting; it is one of the conditions described as ‘runner’s knee’. Patello‐femoral pain syndrome accounts for approximately 25% of knee disease (Collado and Fredericson, 2010) and occurs in 15%–33% of adults and 21%–45% of adolescents (Lindberg et al., 1986). However, its pathophysiology is poorly understood (Sanchis‐Alfonso, 2010). There appear to be a number of causes of PFPS, which can be broadly fit into three categories: malalignment of the patello‐femoral joint (Sanchis‐Alfonso et al., 1999; Heintjes et al., 2004), spasticity of the leg muscles (Heintjes et al., 2004; Tucker and Hodges, 2010) and overuse of or stress on the knee joint (Heintjes et al., 2004; Collado and Fredericson, 2010). Some definitive causes of PFPS have been identified including Hoffa’s disease (Hoffa, 1904), in which the IFP is impinged between the femoral condyles and the posterior patella, causing oedema of the IFP and maltracking of the patella (Subhawong et al., 2010).
Radiological diagnosis has revealed that patello‐femoral arthritis is a common disease affecting 36.1% of females and 32.7% of males over the age of 60 years (Davies et al., 2002), with 30% of females and 18.5% of males older than 55 years being symptomatic (McAlindon et al., 1992). Furthermore, 79% of cadavers with a mean age of 65 years (range 20–89 years) have been reported to have patello‐femoral arthritis (Noble and Hamblen, 1975), suggesting an increased incidence of PFPS with age. In the young, PFPS largely occurs as a result of sport injury (Sanchis‐Alfonso et al., 1999; Sanchis‐Alfonso, 2010) and there is increasing evidence that early life PFPS leads to the development of patello‐femoral arthritis in later life (Utting et al., 2005; Thomas et al., 2010; Antony et al., 2016). MRI studies have shown that individuals with incident osteoarthitis of the knee coupled with distinct structural changes are likely to have accelerated loss of cartilage and to be more symptomatic than those with common osteoarthritis (Roemer et al., 2009; Driban et al., 2016; Davis et al., 2018; Foreman et al., 2019). Furthermore, recent prospective quantitative MRI studies have revealed that baseline high IFP signal intensity was associated with subsequent structural knee abnormalities and that the rate of cartilage loss can be a predictor for total knee replacement (Han et al., 2018; Wang et al, 2018).
Therefore, the aim of this study is to provide a detailed description of the gross anatomy of the IFP, documenting its attachments, size and shape in a much larger population than has been investigated previously, and to examine a possible link between the morphology of the IFP and the presence of patello‐femoral arthritis.
2. METHODS
Twenty‐six embalmed cadavers were investigated in this study. Nine knees were excluded from the study because they had received total knee replacements. The remaining 43 knees were derived from soft‐embalmed (3 male [5 knees] and 6 female [10 knees]) and formalin‐embalmed (8 male [15 knees] and 9 female [13 knees]) cadavers (mean age 84 years, range 55–97 years). The cadavers were donated and the study performed in compliance with the provisions of the UK Human Tissue Act (2004). The cadavers were placed in a supine position, with their knees in full extension. For each knee, a semi‐circular incision was made through the skin and superficial fascia approximately 2 cm below the level of the tibial tuberosity and a second semi‐circular incision was made approximately 10 cm above the border of the superior border of the patella. A midline incision was then made over the rectus tendon, patella and patellar tendon between the two semi‐circular incisions. The iliotibial band was elevated from its insertion on the lateral tubercle of the tibia. The vastus medialis muscle was separated from the quadriceps tendon and femur on the medial side. The body of rectus femoris was transected perpendicular to its long axis and elevated to allow its tendon to be dissected from the three adjacent vasti muscles, until the superior part of the patella was visualised. Then the IFP, if present, was dissected off the superior border of the patella. This resection was continued around the medial and lateral borders of the patella and the inferior IFP was then dissected from the medial and lateral patellar retinacula until the inferior border of the patella was reached. At this point, the medial and lateral patellar retinacula were transected and the patella was reflected distally, facilitating dissection of the IFP from the inferior border of the patella and the deep surface of the patellar tendon up to its insertion into the tibial tuberosity.
The knee was then flexed and the IFP attachments (if present) to Kaplan’s (patello‐meniscal) ligaments medially and Kaplan’s fibres of the iliotibial band laterally, were cut. The IFP was then separated from the tibia, dissecting proximally until the anterior horns of the menisci were reached. The menisci were elevated from the tibial plateau medially and laterally until past their point of attachment to the IFP, to retain their attachment to it. The ligamentum mucosum was then cut if it extended into the femoral intercondylar notch and the menisci were cut to allow removal of the fat pad and its attachments to the menisci from the knee joint. Once the IFP‐meniscal complex was excised, the extent of the IFP attachment to the menisci was recorded on diagrammatic templates of the menisci. The excised parts of the menisci were then dissected off the IFP.
The length of attachment of the IFP to the patellar tendon, the length of the patellar tendon and the lengths (± .5 mm) of its superior medial and superior lateral extensions were recorded using a rule. If a complete loop was formed by the extensions of the IFP around the patella, the lengths were measured to the midpoint of the loop. The maximal width (± .5 mm) of the IFP was recorded using callipers, the volume (± 2.5 ml) of the IFP was recorded using the displacement of water in a 1,000‐ml measuring cylinder and the height (± .5 mm) of the cadavers was measured using a tape measure.
The articular surfaces of the femoral condyles and posterior patella were photographed to record the presence or absence of osteophytes. Photographs were also taken of the IFPs to record their morphology (Figure 2).
Figure 2.
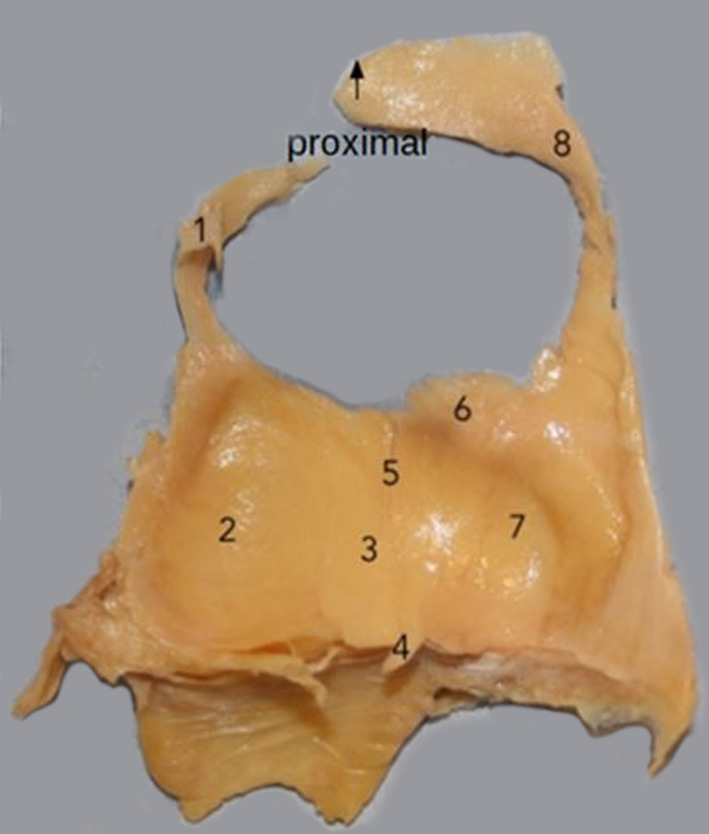
Posterior view of a left infrapatellar fat pad showing: (1) superior lateral extension; (2) lateral extension; (3) central body; (4) ligamentum mucosum; (5) vertical cleft; (6) superior tag; (7) medial extension; (8) superior medial extension
To investigate the attachments of the IFP statistically, a value of 1 was assigned to IFPs where an attachment was present and zero assigned to those where an attachment was not present. Thus, the means used were the percentages of IFPs with a given attachment. Six different variables were then independently assessed using Student's t tests to compare IFP datasets. These variables were: sex, embalming technique, presence of patello‐femoral disease, presence of horizontal cleft, presence of vertical cleft and superior border of the patellar attachment. The differences between the means were judged to be significant when p < .05.
The relationships between IFP volume, the thickness of the IFP, the height of the cadaver, the percentage of the length of the patellar tendon attached to the IFP and the length of the supero‐medial and supero‐lateral extensions of the IFP were investigated using Spearman’s rank correlation coefficient. The critical value of Spearman’s rank correlation coefficient was calculated to be r = ± .331 for p < .05, using a critical value table (Zar, 1972).
3. RESULTS
The IFP was present in all 43 knees investigated. Soft‐embalmed subjects exhibited a greater percentage of the ligamentum mucosum contiguous with the anterior cruciate ligament (100% vs. 86%), a greater percentage of IFP attachment at the inferior border of the patella (100% vs. 86%), a greater percentage presence of a vertical cleft in the IFP (93% vs. 43%) and a lower percentage of IFPs attached to the lateral meniscus (34% vs. 46%), compared with formalin‐embalmed subjects. These differences were statistically significant (p < .05) but relatively small and none of the other parameters investigated differed between embalming techniques. Moreover, embalming technique is unlike to have affected a binary parameter such as the presence or absence of an attachment. Therefore, soft‐ and formalin‐embalmed cadavers were included in the same dataset.
The ligamentum mucosum was present in IFPs from all knees investigated and it extended into the femoral intercondylar notch in 79% (Figure 1) and was contiguous with the anterior cruciate ligament in 91% of knees. All IFPs were attached to the medial anterior horns of the menisci and the medial Kaplan’s ligaments. Ninety‐seven per cent were attached to the lateral anterior horns of the menisci and 97% to the lateral Kaplan’s ligaments. However, the length of the IFP attachment to meniscus was significantly longer (p = .004) on the lateral (41.6% of lateral meniscus), compared to the medial side (32.1% of medial meniscus). Ninety‐seven per cent of IFPs were attached to the superior portion of the patellar tendon with the mean proportion of the tendon attachment being 60%. Ninety‐one per cent of IFPs were attached to the inferior border of the patella. All IFPs that were not attached to the anterior horn of the lateral menisci, medial Kaplan’s ligament, superior patella or inferior border of the patella were in knees with articular surface osteophytes.
Figure 1.

Anterior view of the ligamentum mucosum (1) extending from a left infrapatellar fat pad (2) into the femoral intercondylar notch
Eighty‐one per cent of IFPs had supero‐medial extensions (mean length 44 mm) and 65% had supero‐lateral extensions (mean length 20 mm). Furthermore, in 70% of IFPs these superior extensions were long enough to attach to the superior border of the patella (Figure 2). These superior extensions were attached anteriorly to the medial and lateral patellar retinacula in all knees in which they were present. The supero‐medial extensions were typically thicker and longer than the supero‐lateral extensions, with the exception of the four knees in which the extensions formed full loops around the patella.
All IFPs had a central body with a medial and typically larger, lateral extension. On the deep surface, 86% of IFPs had a superior tag, 46% had a horizontal cleft and 60% had a vertical cleft (Table 1; Figure 2). The mean volume of the IFPs was 27 ml ± 1.039 and the mean maximal thickness was 20 mm ± .501 (Table 2). Breaking down the data by sex, the mean volume (31.5 ml) and thickness (22.5 mm) of the male IFPs were significantly larger (p = .000 for both comparisons) than the mean volume (22.8 ml) and thickness (18.6 mm) of female IFPs.
Table 1.
The number and percentage of infrapatellar fat pads (IFPs) with identified features in males, females and both sexes combined
| IFP feature | Superior tag | Medial extension | Lateral extension | Superior medial extension | Superior lateral extension | Ligamentum mucosum | Horizontal cleft | Vertical cleft | Synovial fringe |
|---|---|---|---|---|---|---|---|---|---|
| % | 86.05 | 100.00 | 100.00 | 81.40 | 65.12 | 100.00 | 46.51 | 60.47 | 51.16 |
| Number of IFPs (total = 43) | 37.00 | 43.00 | 43.00 | 35.00 | 28.00 | 43.00 | 20.00 | 26.00 | 22.00 |
| Male % | 80.00 | 100.00 | 100.00 | 75.00 | 75.00 | 100.00 | 55.00 | 60.00 | 50.00 |
| Male number of IFPs (total = 20) | 16.00 | 20.00 | 20.00 | 15.00 | 15.00 | 20.00 | 11.00 | 12.00 | 10.00 |
| Female % | 91.30 | 100.00 | 100.00 | 86.96 | 56.52 | 100.00 | 39.13 | 60.87 | 52.17 |
| Female number of IFPs (total = 23) | 21.00 | 23.00 | 23.00 | 20.00 | 13.00 | 23.00 | 9.00 | 14.00 | 12.00 |
Table 2.
The means and SD in males, females and both sexes combined of the volume, maximal thickness and lengths of the patellar tendon, the attachment of the infrapatellar fat pad (IFP) to the patellar tendon, the superior medial extension (SME) of the IFP and the superior lateral extension (SLE) of the IFP, the percentage of the medial and lateral menisci attached to the IFP and the percentage of the patellar tendon attached to the IFP
| Attachment length to patellar tendon (mm) | Patellar tendon length (mm) | % of patellar tendon attached | IFP volume (ml) | % of Medial menisci attached | % of Lateral menisci attached | IFP maximal thickness (mm) | SME length (mm) | SLE length (mm) | Age (year) | Height (cm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 32.02 | 53.60 | 59.81 | 26.86 | 32.09 | 41.63 | 20.37 | 43.58 | 19.74 | 83.93 | 165.22 |
| SD | 10.23 | 7.98 | 16.29 | 6.82 | 14.11 | 15.15 | 3.29 | 25.46 | 19.83 | 10.30 | 10.97 |
| Male mean | 37.00 | 57.10 | 64.70 | 31.50 | 31.00 | 43.50 | 22.45 | 45.55 | 25.20 | 85.55 | 174.05 |
| Male SD | 8.83 | 7.00 | 12.97 | 5.40 | 15.18 | 17.40 | 3.15 | 29.12 | 19.94 | 8.34 | 8.83 |
| Female mean | 27.70 | 50.57 | 55.55 | 22.83 | 33.04 | 40.00 | 18.57 | 41.87 | 15.00 | 82.52 | 157.54 |
| Female SD | 9.51 | 7.64 | 17.91 | 5.18 | 13.38 | 13.06 | 2.17 | 22.33 | 18.89 | 11.74 | 5.45 |
The mean and SD of subject age and height are also given.
The volume of IFPs with attachments to the superior border of the patella was significantly greater (p = .007) than in those without such an attachment. Significantly more IFPs with an attachment to the superior border of the patella had a superior medial and superior lateral extension than did those without an attachment to the superior border of the patella (p = .001 and p = .000, respectively). The lengths of the superior medial and superior lateral extensions of IFPs with attachment to the superior border of the patella were significantly greater than in those with such an attachment (p = .000) for both comparisons. The IFP was attached to the medial meniscus in significantly (p = .009) more knees with IFP attachment to the superior border of the patella than in those without such an attachment.
There was a significant positive correlation between IFP volume and subject height in both males (r = +1.0, p < .05) and females, (r = +.687, p < .05), between the IFP volume and thickness (r = +.714, p < .05), between IFP volume and superior lateral extension length (r = +.347, p < .05), between height and the percentage of the patellar tendon attached to the IFP (r = +.411, p < .05), between IFP height and maximum thickness (r = +.674, p < .05), between IFP maximum thickness and the superior lateral extension length (r = +.376, p < .05), between IFP maximum thickness and the percentage of the patellar tendon attached to it (r = +.345, p < .05), and between the lengths of the superior lateral and superior medial extensions of the IFP (r = +.470, p < .05).
Articular surface osteophytes were present on the femoral condyles of 16 knees. Significantly fewer knees with femoral osteophytes had superior medial extensions and superior lateral extensions of the IFP compared with those without femoral osteophytes (p = .002 and p = .000 respectively). The lengths of the superior medial and superior lateral extensions of the IFP in knees with femoral osteophytes were significantly smaller than in those knees without femoral osteophytes (p = .000 and p = .006), respectively. The IFP was attached to the medial meniscus in significantly fewer knees with femoral osteophytes than in those without (p = .050). Articular surface osteophytes were present on the posterior surface of the patella in 14 knees.
Significantly fewer knees with patellar osteophytes had superior medial extensions and superior lateral extensions of the IFP than did those without patellar osteophytes (p = .001 and p = .000, respectively). The lengths of the superior medial and superior lateral extensions of the IFP in knees with patellar osteophytes were significantly smaller than in those knees without patella osteophytes (p = .000 and p = .000, respectively). The IFP was attached to the medial meniscus in significantly fewer knees with patellar osteophytes than in those without (p = .023). Although not statistically significant (p = .10), the IFPs in knees with patello‐femoral osteophytes were numerically smaller than those in knees without. The mean volume of the IFP in knees with femoral osteophytes was 24.6 ml and in those knees without femoral osteophytes 28.1 ml. Furthermore, the mean volume of the IFP was 24.6 ml in knees with patellar osteophytes compared with 27.9 ml in knees without (p = .14).
4. DISCUSSION
The results of the current study provide a comprehensive description of the gross anatomy of the IFP and for the first time describe superior medial and superior lateral extensions of the IFP along the sides of the patella, and attachments of the IFP to the superior border of the patella and to both the medial and lateral patellar retinacula. Preliminary results of this study have been published in abstract form (Leese and Davies, 2017) and the extensions of the IFP have subsequently also been described following dissections of fresh‐frozen knees (Stephen et al., 2018).
The results of this study also provide evidence for a link between the size and shape of the IFP and the presence of gross knee pathology in the form of osteophytes on the femoral condyles and posterior surface of the patella. They also expand on previous descriptions of the attachments of the IFP to surrounding tissues, the regional morphology of the IFP, its volume and width (Hoffa, 1904; Davies and White, 1961; Duri et al., 1996; Saddik et al., 2004; Gallagher et al., 2005; Swan, 2005; Brooker et al., 2009; Mace et al., 2016).
The age of the cadavers investigated (mean 84 years and all but one over the age of 69 years) provides a relatively homogeneous population with respect to age, effectively removing it as a variable from the data comparisons made. Furthermore, previous research has shown little variance in the weight of synovial fat pads from adolescence to old age (Davies and White, 1961), but little is known about the effect of age on the morphology of the IFP.
The dissection technique used on embalmed bodies in a previous study in which it was described (Gallagher et al., 2005), closely followed the medial and lateral borders of the patella, completely separating it from the patellar retinacula. The current study employed a more conservative dissection approach, as described in the Methods section above. This conservative dissection technique and the greater number of subjects investigated than in previous studies allowed the identification of the previously undescribed supero‐medial and supero‐lateral extensions of the IFP. Newell (1991) reported that the IFP and suprapatellar fat pad formed one continuous fat pad around the patella. However, in the current study, the superior extensions of the IFP were not found to have a connection with the suprapatellar fat pad.
The results of the current study revealed that significantly fewer knees with patello‐femoral osteophytes had superior medial and superior lateral extensions of the IFP compared with those without osteophytes. The IFP was attached to the medial meniscus in significantly fewer knees with patello‐femoral osteophytes than in those without. Furthermore, the IFP was numerically smaller in knees with patello‐femoral osteophytes than in those without. These results suggest that the IFP is involved in PFPS. This view is supported by the findings of Brushøj et al. (2008) who reported that 40% of their subjects with PFPS were tender to specific palpation of the IFP, and Culvenor et al. (2011) who reported a link between IFP volume on MRI and patellar tendonitis. Patients with patellar tendinopathy were shown to have larger IFPs compared with healthy controls, and Fontanella et al. (2019) reported a decrease in IFP volume on MRI in patients with osteoarthritis compared to subjects without. Further research is needed to investigate the link between IFP morphology and size and osteoarthritis and to understand the role that the IFP plays in PFPS.
There is a paucity of data about the histological structure of the IFP and presence of different types of nerve endings, such as pain, mechanoreceptor or substance P‐positive fibres in the IFP and whether these vary with the region of the IFP, age or pathology. The results of the current study provide a basis for selecting regions of the IFP for investigation. This study builds on the work of Gallagher et al. (2005) but is the first comprehensively to record the attachments of the IFP to its surrounding tissues. Gallagher et al (2005) studied only four cadavers and found that 75% had horizontal and vertical clefts; the results of this study showed that only 46.5% had horizontal clefts and 60.5% vertical clefts. It would be of interest to investigate the histology of the vertical and horizontal clefts of the IFP in comparison with the central body and superior medial and superior lateral extensions, because they were only present in a few knees and were associated with a slight difference in the presence of the horizontal cleft in taller men (males with horizontal clefts had a mean height of 178 cm ± 1.53, whereas males without a horizontal cleft had a mean height of 169 cm ± 2.05; p = .041) and with the presence of vertical clefts in women (88.9% of women with horizontal clefts had vertical clefts, whereas only 42.8% of women without horizontal clefts had vertical clefts [p = .0165]). A possible hypothesis for their presence could be as a result of stress on those regions of the IFP in which case they may be structurally different from other regions of the IFP and have a different innervation. This view is supported by Macchi et al. (2018), and indicates that the biomechanical properties of the IFP change during osteoarthritis, with a reduction of proper stress‐strain behaviour under mechanical loads.
In view of the fact that in the current study of aged cadavers, the superior extensions of the IFP were not found to be continuous around the superior border of the patella in all knees investigated, it would be of interest to investigate whether the IFP is continuous around the patella during earlier life and whether this continuity disappears with age and/or pathology, or if the absence of a continuity in some aged adults is congenital. If the latter is the case, then the correlation between the absence/reduced length of superior extensions of the IFP and pathology of the patello‐femoral articular surfaces found in the current study would suggest that the morphology of the superior extensions of the IFP could signpost a predisposition to patello‐femoral osteoarthritis.
In the past two decades, some surgeons have begun to resect the IFP in an attempt to treat PFPS (Ogilvie‐Harris and Giddens, 1994; Tanaka, et al., 2003; Doner and Noyes, 2014). However, varying results have been reported, with some suggesting that the resection of the IFP reduces anterior knee pain (Ogilvie‐Harris and Giddens, 1994; Tanaka et al., 2003; Doner and Noyes, 2014) and others that resection of the IFP is linked to an increase in anterior knee pain (Pinsornsak et al., 2014; White et al., 2015). None of the research regarding the resection of the IFP as a treatment for PFPS provides any evidence that the IFP is a cause of knee pain, beyond the known aetiology of Hoffa’s disease (Hoffa, 1904; Metheny and Mayor, 1988; Subhawong et al., 2010). It is therefore a concern that IFP resection is being carried out for any aetiologies of PFPS other than fat pad impingement, when there is evidence suggesting that anterior knee pain increases following IFP resection and that its removal may affect the biomechanics and kinematics of the knee joint as a whole (Bohnsack et al., 2004). Therefore, more research is needed into the function of the IFP and the effect of its resection to inform the surgical treatment of anterior knee pain.
A systematic review of the literature revealed that 15%–76% of adults with chronic knee pain had radiographic osteoarthritis and 15%–81% of those with radiographic knee osteoarthritis had knee pain (Bedson and Croft, 2008). These results suggest a correlation between osteoarthritis and knee pain. This correlation appears to be stronger in old age, because Porcheret et al. (2007) reported that most knee pain in the elderly is attributable to osteoarthritis and Duncan et al. (2006) reported that radiographic osteoarthritis is present in over 70% of adults over 50 years of age with knee pain. The link between IFP morphology and patello‐femoral pathology suggested by the results of the current study of knees from cadavers with a mean age of 84 years, may have a bearing on anterior knee pain in osteoarthritis and therefore requires further investigation, as does the possibility that anterior knee pain with other aetiologies may also be related to changes in IFP morphology. Such investigations would lay the foundation for understanding the role of the IFP in the aetiology of PFPS.
Leese J, Davies DC. An investigation of the anatomy of the infrapatellar fat pad and its possible involvement in anterior pain syndrome: a cadaveric study. J. Anat. 2020;237:20–28. 10.1111/joa.13177
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abreu, M.R. , Chung, C.B. , Trudell, D. and Resnick, D. (2008) Hoffa’s fat pad injuries and their relationship with anterior cruciate ligament tears: new observations based on MR imaging in patients and MR imaging and anatomic correlation in cadavers. Skeletal Radiology, 37, 301–306. [DOI] [PubMed] [Google Scholar]
- Antony, B. , Jones, G. , Jin, X. and Ding, C. (2016) Do early life factors affect the development of knee osteoarthritis in later life: a narrative review. Arthritis Research & Therapy, 18, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolaki, E. , Cassar‐Pullicino, V.N. , Tyrrell, P.N. and McCall, I.W. (1999) MRI appearances of the infrapatellar fat pad in occult traumatic patellar dislocation. Clinical Radiology, 54, 743–747. [DOI] [PubMed] [Google Scholar]
- Aynaci, O. , Ahmetoğlu, A. , Reis, A. and Turhan, A.U. (2001) Synovial hemangioma in Hoffa’s fat pad (case report). Knee Surgery, Sports Traumatology, Arthroscopy, 9, 355–357. [DOI] [PubMed] [Google Scholar]
- Bedson, J. and Croft, P.R. (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskeletal Disorders, 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennell, K. , Hodges, P. , Mellor, R. , Bexander, C. and Souvlis, T. (2004) The nature of anterior knee pain following injection of hypertonic saline into the infrapatellar fat pad. Journal of Orthopaedic Research, 22, 116–121. [DOI] [PubMed] [Google Scholar]
- Biedert, R.M. and Sanchis‐Alfonso, V. (2002) Sources of anterior knee pain. Clinics in Sports Medicine, 21, 335–347. [DOI] [PubMed] [Google Scholar]
- Brushøj, C. , Hölmich, P. , Nielsen, M.B. and Albrecht‐Beste, E. (2008) Acute patellofemoral pain: aggravating activities, clinical examination, MRI and ultrasound findings. British Journal of Sports Medicine, 42, 64–67. [DOI] [PubMed] [Google Scholar]
- Bohnsack, M. , Wilharm, A. , Hurschler, C. , Rühmann, O. , Stukenborg‐Colsman, C. and Wirth, C.J. (2004) Biomechanical and kinematic influences of a total infrapatellar fat pad resection on the knee. American Journal of Sports Medicine, 32, 1873–1880. [DOI] [PubMed] [Google Scholar]
- Bohnsack, M. , Meier, F. , Walter, G.F. , Hurschler, C. , Schmolke, S. , Wirth, C.J. , et al. (2005) Distribution of substance‐P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Archiv fur orthopadische und Unfall‐Chirurgie, 125, 592–597. [DOI] [PubMed] [Google Scholar]
- Brooker, B. , Morris, H. , Brukner, P. , Mazen, F. and Bunn, J. (2009) The macroscopic arthroscopic anatomy of the infrapatellar fat pad. Arthroscopy, 25, 839–845. [DOI] [PubMed] [Google Scholar]
- Bulmer, J.H. (1966) Torsion of the infrapatellar fat pad. British Medical Journal, 2(5514), 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield, J.P. , Hein, A. , Helfgott, S.M. , Brahn, E. , Dynesius‐Trentham, R.A. and Trentham, D.E. (1988) Intraarticular injection of arthritogenic factor causes mast cell degranulation, inflammation, fat necrosis, and synovial hyperplasia. Laboratory Investigation, 59, 82–95. [PubMed] [Google Scholar]
- Choi, N.H. (2000) Localized pigmented villonodular synovitis involving the fat pad of the knee. American Journal of Knee Surgery, 13, 117–119. [PubMed] [Google Scholar]
- Clements, K.M. , Burton‐Wurster, N. and Lust, G. (2004) The spread of cell death from impact damaged cartilage: lack of evidence for the role of nitric oxide and caspases. Osteoarthritis Cartilage, 12, 577–585. [DOI] [PubMed] [Google Scholar]
- Clockaerts, S. , Bastiaansen‐Jenniskens, Y.M. , Runhaar, J. , Van Osch, G.J. , Van Offel, J.F. , Verhaar, J.A. , et al. (2010) The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage, 18, 876–882. [DOI] [PubMed] [Google Scholar]
- Collado, H. and Fredericson, M. (2010) Patellofemoral pain syndrome. Clinics Sports Medicine (Auckland, N. Z.), 29, 379–398. [DOI] [PubMed] [Google Scholar]
- Culvenor, A.G. , Cook, J.L. , Warden, S.J. and Crossley, K.M. (2011) Infrapatellar fat pad size, but not patellar alignment, is associated with patellar tendinopathy. Scandinavian Journal of Medicine and Science in Sports, 21, e405–411. [DOI] [PubMed] [Google Scholar]
- Davies, A.P. , Vince, A.S. , Shepstone, L. , Donell, S.T. and Glasgow, M.M. (2002) The radiologic prevalence of patellofemoral osteoarthritis. Clinical Orthopaedics and Related Research, 402, 206–212. [DOI] [PubMed] [Google Scholar]
- Davies, D.V. and Edwards, D.A. (1948) The blood supply of the synovial membrane and intra‐articular structures. Annals of the Royal College of Surgeons of England, 2, 142–146. [PMC free article] [PubMed] [Google Scholar]
- Davies, D.V. and White, J.E. (1961) The structure and weight of synovial fat pads. Journal of Anatomy, 95, 30–37. [PMC free article] [PubMed] [Google Scholar]
- Davis, J.E. , Harkey, M.S. , Ward, R.J. , Mackay, J.W. , Lu, B. , Price, L.L. , et al. (2018) Characterizing the distinct structural changes associated with self‐reported knee injury among individuals with incident knee osteoarthritis: data from the Osteoarthritis Initiative. Clinical Anatomy, 31, 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doner, G. and Noyes, F.R. (2014) Arthroscopic resection of fat pad lesions and infrapatellar contractures. Arthroscopy Techniques, 3, e413–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban, J.B. , Price, L.L. , Eaton, C.B. , Lu, B. , Lo, G.H. , Lapane, K.L. , et al. (2016) Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clinical Rheumatology, 35, 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumond, H. , Presle, N. , Terlain, B. , Mainard, D. , Loeuille, D. , Netter, P. , et al. (2003) Evidence for a key role of leptin in osteoarthritis. Arthritis and Rheumatism, 48, 3118–3129. [DOI] [PubMed] [Google Scholar]
- Duncan, R.C. , Hay, E.M. , Saklatvala, J. and Croft, P.R. (2006) Prevalence of radiographic osteoarthritis—it all depends on your point of view. Rheumatology, 45, 757–760. [DOI] [PubMed] [Google Scholar]
- Duri, Z.A.A. , Aichroth, P.M. , Dowd, G. and Ware, H. (1997) The fat pad and its relationship to anterior knee pain. The knee, 4, 227–236. [Google Scholar]
- Duri, Z.A. , Aichroth, P.M. and Dowd, G. (1996) The fat pad. Clinical observations. The American Journal of Knee Surgery, 9, 55–66. [PubMed] [Google Scholar]
- Fain, J.N. (2006) Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the non‐fat cells. Vitamins Hormones, 74, 443–477. [DOI] [PubMed] [Google Scholar]
- Favero, M. , El‐Hadi, H. , Belluzzi, E. , Granzotto, M. , Porzionato, A. , Sarasin, G. , et al. (2017) Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology, 56, 1784–1793. [DOI] [PubMed] [Google Scholar]
- Fontanella, C.G. , Belluzzi, E. , Rossato, M. , Olivotto, E. , Trisolino, G. , Ruggieri, P. , et al. (2019) Quantitative MRI analysis of infrapatellar and suprapatellar fat pads in normal controls, moderate and end‐stage osteoarthritis. Anatomischer Anzeiger, 221, 108–114. [DOI] [PubMed] [Google Scholar]
- Foreman, S.C. , Neumann, J. , Joseph, G.B. , Nevitt, M.C. , McCulloch, C.E. , Lane, N.E. , et al. (2019) Longitudinal MRI structural findings observed in accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Skeletal Radiology, 48, 1949–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, J. , Tierney, P. , Murray, P. and O’Brien, M. (2005) The infrapatellar fat pad: anatomy and clinical correlations. Knee Surgery, Sports Traumatology, Arthroscopy, 13, 268–272. [DOI] [PubMed] [Google Scholar]
- Gardner, E. (1948) The innervation of the knee joint. Anatomical Record, 101, 109–130. [DOI] [PubMed] [Google Scholar]
- Geraghty, R.M. and Spear, M. (2017) Evidence for plical support of the patella. Journal of Anatomy, 231, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, R. , Lago, F. , Gomez‐Reino, J. , Dieguez, C. and Gualillo, O. (2009) Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. Journal of Molecular Endocrinology, 43, 11–18. [DOI] [PubMed] [Google Scholar]
- González, A.C. (2001) Fat pad entrapment in suprapatellar pouch following previous arthroscopic resection of infrapatellar fat pad and medial plica: rare complication. Journal of Musculoskeletal Pain, 9, 95–98. [Google Scholar]
- Han, W. , Aitken, D. , Zheng, S. , Wluka, A.E. , Zhu, Z. , Blizzard, L. , et al. (2018) Association between quantitatively measured infrapatellar fat pad high signal‐intensity alteration and magnetic resonance imaging–assessed progression of knee osteoarthritis. Arthritis Care & Research, 71, 638–646. [DOI] [PubMed] [Google Scholar]
- Havers, C. (1691) Osteologia Nova, or, Some new Observations of the Bones: … with the Manner of their Accretion, and Nutrition, Communicated to the Royal Society in Several Discourses. Vol. 1, London: The Royal Society. [Google Scholar]
- Heintjes, E. , Berger, M.Y. , Bierma‐Zeinstra, S.M. , Bernsen, R.M. , Verhaar, J.A. and Koes, B.W. (2004) Pharmacotherapy for patellofemoral pain syndrome. Cochrane Database of Systematic Reviews, (3), CD003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffa, A. (1904) The influence of adipose tissue with regard to pathology of the knee joint. JAMA, 42, 795–796. [Google Scholar]
- Kennedy, J.C. , Alexander, I.J. and Hayes, K.C. (1982) Nerve supply of the human knee and its functional importance. American Journal of Sports Medicine, 10, 329–335. [DOI] [PubMed] [Google Scholar]
- Kohn, D. , Deiler, S. and Rudert, M. (1995) Arterial blood supply of the infrapatellar fat pad. Anatomy and clinical consequences. Archives of Orthopaedic and Trauma Surgery, 114, 72–75. [DOI] [PubMed] [Google Scholar]
- Lago, R. , Gomez, R. , Otero, M. , Lago, F. , Gallego, R. , Dieguez, C. , et al. (2008) A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro‐inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage, 16, 1101–1109. [DOI] [PubMed] [Google Scholar]
- Leese, J. and Davies, D.C. (2017) An investigation of the anatomy of the infrapatellar fat pad: a cadaveric study. Journal of Anatomy, 231, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner, B. , Koeck, F.X. , Capellino, S. , Schubert, T.E.O. , Hofbauer, R. and Straub, R.H. (2008) Preponderance of sensory versus sympathetic nerve fibers and increased cellularity in the infrapatellar fat pad in anterior knee pain patients after primary arthroplasty. Journal of Orthopaedic Research, 26, 342–350. [DOI] [PubMed] [Google Scholar]
- Lindberg, U. , Lysholm, J. and Gillquist, J. (1986) The correlation between arthroscopic findings and the patellofemoral pain syndrome. Arthroscopy, 2, 103–107. [DOI] [PubMed] [Google Scholar]
- Macchi, V. , Porzionato, A. and Sarasin, G. (2016) The infrapatellar adipose body: a histotopographic study. Cells Tissues Organs, 201, 220–231. [DOI] [PubMed] [Google Scholar]
- Macchi, V. , Porzionato, A. , Rossato, M. , and De Caro, R. (2017) Regional differences between perisynovial and infrapatellar adipose tissue depots and their response to class II and III obesity in patients with OA: comment on the article by Harasymowicz et al. Arthritis & Rheumatology, 70, 146–147. [DOI] [PubMed] [Google Scholar]
- Macchi, V. , Stecco, E. , Stecco, C. , Belluzzi, E. , Favero, M. , Porzionato, A. , et al. (2018) The infrapatellar fat pad and the synovial membrane: an anatomo‐functional unit. Journal of Anatomy, 233, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConaill, M.A. (1950) The movements of bones and joints; the synovial fluid and its assistants. The Journal of Bone and Joint Surgery. British Volume, 32‐B(2), 244–252. [DOI] [PubMed] [Google Scholar]
- Mace, J. , Bhatti, W. and Anand, S. (2016) Infrapatellar fat pad syndrome: a review of anatomy, function, treatment and dynamics. Acta Orthopaedica Belgica, 82, 94–101. [PubMed] [Google Scholar]
- Maculé, F. , Sastre, S. , Lasurt, S. , Sala, P. , Segur, J.M. and Mallofré, C. (2005) Hoffa’s fat pad resection in total knee arthroplasty. Acta Orthopaedica Belgica, 71, 714–717. [PubMed] [Google Scholar]
- McAlindon, T.E. , Snow, S. , Cooper, C. and Dieppe, P.A. (1992) Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Annals of the Rheumatic Diseases, 51, 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall, J.J. , Elenko, R.D. and Bray, R.C. (1998) Cholinergic vasoregulation in normal and adjuvant monoarthritic rat knee joints. Journal of the Autonomic Nervous System, 72, 55–60. [DOI] [PubMed] [Google Scholar]
- Metheny, J.A. and Mayor, M.B. (1988) Hoffa disease: chronic impingement of the infrapatellar fat pad. American Journal of Knee Surgery, 1, 134–139. [Google Scholar]
- Nemschak, G. and Pretterklieber, M.L. (2012) The patellar arterial supply via the infrapatellar at pad (of Hoffa): a combined anatomical and angiographical analysis. Anatomy Research International, 2012, 713838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell, R.L.M. (1991) A complete intra‐articular fat pad around the human patella. Journal of Anatomy, 179, 232. [Google Scholar]
- Noble, J. and Hamblen, D.L. (1975) The pathology of the degenerate meniscus lesion. Journal of Bone and Joint Surgery. British volume, 57, 180–186. [PubMed] [Google Scholar]
- Ogilvie‐Harris, D.J. and Giddens, J. (1994) Hoffa’s disease: arthroscopic resection of the infrapatellar fat pad. Arthroscopy, 10, 184–187. [DOI] [PubMed] [Google Scholar]
- Ozkur, A. , Adaletli, I. , Sirikci, A. , Kervancioglu, R. and Bayram, M. (2005) Hoffa’s recess in the infrapatellar fat pad of the knee on MR imaging. Surgical and Radiologic Anatomy, 27, 61–63. [DOI] [PubMed] [Google Scholar]
- Palumbo, R.C. , Matthews, L.S. and Reuben, J.M. (1994) Localized pigmented villonodular synovitis of the patellar fat pad: a report of two cases. Arthroscopy, 10, 400–403. [DOI] [PubMed] [Google Scholar]
- Pinsornsak, P. , Naratrikun, K. and Chumchuen, S. (2014) The effect of infrapatellar fat pad excision on complications after minimally invasive TKA: a randomized controlled trial. Clinical Orthopaedics and Related Research, 472, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheret, M. , Jordan, K. and Croft, P. (2007) Treatment of knee pain in older adults in primary care: development of an evidence‐based model of care. Rheumatology, 46, 638–648. [DOI] [PubMed] [Google Scholar]
- Roemer, F.W. , Zhang, Y. , Niu, J. , Lynch, J.A. , Crema, M.D. , Marra, M.D. , et al. (2009) Tibiofemoral joint osteoarthritis: risk factors for MR‐depicted fast cartilage loss over a 30‐month period in the multicenter osteoarthritis study. Radiology, 252, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddik, D. , McNally, E.G. and Richardson, M. (2004) MRI of Hoffa’s fat pad. Skeletal Radiology, 33, 433–444. [DOI] [PubMed] [Google Scholar]
- Sakai, H. , Tamai, K. , Iwamoto, A. and Saotome, K. (1999) Para‐articular chondroma and osteochondroma of the infrapatellar fat pad: a report of three cases. International Orthopaedics, 23, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Alfonso, V. (2010) Pathophysiology of Anterior Knee Pain In: Zaffagnini S., Dejour D. and Arendt E.A. (Eds.) Patellofemoral Pain, Instability, and Arthritis. Berlin: Springer, pp. 1–16. [Google Scholar]
- Sanchis‐Alfonso, V. , Rosello‐Sastre, E. and Martinez‐Sanjuan, V. (1999) Pathogenesis of anterior knee pain syndrome and functional patellofemoral instability in the active young. American Journal of Knee Surgery, 12, 29–40. [PubMed] [Google Scholar]
- Stephen, J.M. , Sopher, R. , Tullie, S. , Amis, A.A. , Ball, S. and Williams, A. (2018) The infrapatellar fat pad is a dynamic and mobile structure, which deforms during knee motion, and has proximal extensions which wrap around the patella. Knee Surgery, Sports Traumatology, Arthroscopy, 26, 3515–3524. [DOI] [PubMed] [Google Scholar]
- Subhawong, T.K. , Eng, J. , Carrino, J.A. and Chhabra, A. (2010) Superolateral Hoffa’s fat pad edema: association with patellofemoral maltracking and impingement. American Journal of Roentgenology, 195, 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan, A.M.S. (2005) Anatomy of the infrapatellar fat pad. New Zealand Journal of Physiotherapy, 33, 19–22. [Google Scholar]
- Tanaka, N. , Sakahashi, H. , Sato, E. , Hirose, K. and Isima, T. (2003) Influence of the infrapatellar fat pad resection in a synovectomy during total knee arthroplasty in patients with rheumatoid arthritis. Journal of Arthroplasty, 18, 897–902. [DOI] [PubMed] [Google Scholar]
- Thomas, M.J. , Wood, L. , Selfe, J. and Peat, G. (2010) Anterior knee pain in younger adults as a precursor to subsequent patellofemoral osteoarthritis: a systematic review. BMC Musculoskeletal Disorders, 11, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utting, M.R. , Davies, G. and Newman, J.H. (2005) Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? The Knee, 12, 362–365. [DOI] [PubMed] [Google Scholar]
- Tucker, K.J. and Hodges, P.W. (2010) Changes in motor unit recruitment strategy during pain alters force direction. European Journal of Pain, 14, 932–938. [DOI] [PubMed] [Google Scholar]
- Wang, K. , Ding, C. , Hannon, M.J. , Chen, Z. , Kwoh, C.K. , Lynch, J. , et al. (2018) Signal intensity alteration within infrapatellar fat pad predicts knee replacement within 5 years: data from the Osteoarthritis Initiative. Osteoarthritis and Cartilage, 26, 1345–1350. [DOI] [PubMed] [Google Scholar]
- White, L. , Hartnell, N. , Hennessy, M. and Mullan, J. (2015) The impact of an intact infrapatellar fat pad on outcomes after total knee arthroplasty. Advances in Orthopedic Surgery, 2015, 1–6. Article ID 817906. [Google Scholar]
- Zar, J.H. (1972) Significance testing of the Spearman rank correlation coefficient. Journal of American Statistical Association, 67, 578–580. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


