Abstract
Almadasuchus figarii is a basal crocodylomorph recovered from the Upper Jurassic levels of the Cañadón Calcáreo Formation (Oxfordian–Tithonian) of Chubut, Argentina. This taxon is represented by cranial remains, which consist of partial snout and palatal remains; an excellently preserved posterior region of the skull; and isolated postcranial remains. The skull of the only specimen of the monotypic Almadasuchus was restudied using high‐resolution computed micro tomography. Almadasuchus has an apomorphic condition in its skull shared with the closest relatives of crocodyliforms (i.e. hallopodids) where the quadrates are sutured to the laterosphenoids and the otoccipital contacts the quadrate posterolaterally, reorganizing the exit of several cranial nerves (e.g. vagus foramen) and the entry of blood vessels (e.g. internal carotids) on the occipital surface of the skull. The endocast is tubular, as previously reported in thalattosuchians, but has a marked posterior step, and a strongly projected floccular recess as in other basal crocodylomorphs. Internally, the skull of Almadasuchus is heavily pneumatized, where different air cavities invade the bones of the suspensorium and braincase, both on its dorsal or ventral parts. Almadasuchus has a large basioccipital recess, which is formed by cavities that excavate the basioccipital and the posterior surface of the basisphenoid, and unlike other crocodylomorphs is connected with the basisphenoid pneumatizations. Ventral to the otic capsule, a pneumatic cavity surrounded by the otoccipital and basisphenoid is identified as the rhomboidal recess. The quadrate of Almadasuchus is highly pneumatized, being completely hollow, and the dorsal pneumatizations of the braincase are formed by the mastoid and facial antra, and a laterosphenoid cavity (trigeminal diverticulum). To better understand the origins of pneumatic features in living crocodylomorphs we studied cranial pneumaticity in the basal members of Crocodylomorpha and found that: (a) prootic pneumaticity may be a synapomorphy for the whole clade; (b) basisphenoid pneumaticity (pre‐, postcarotid and rostral recesses) is a derived feature among basal crocodylomorphs; (c) quadrate pneumatization is acquired later in the history of the group; and (d) the rhomboidal sinus is a shared derived trait of hallopodids and crocodyliforms. The marine thallatosuchians exhibit a reduction of the pneumaticity of the braincase and this reduction is evaluated considering the two phylogenetic positions proposed for the clade.
Keywords: anatomy, braincase, Crocodylomorpha, CT scan, evolution, pneumaticity

We describe the anatomy (external and internal) of a basal crocodylomorph, Almadasuchus figarii. Almadasuchus displays a high degree of pneumatization in its braincase, and its comparison with other taxa allowed us to study the evolution of this trait during the origins of Crocodyliformes. Our results indicate that derived crocodylomorphs have a well developed pneumaticity in the quadrates and dorsal and ventral elements of the braincase.
1. INTRODUCTION
Crocodylomorpha is the only group of pseudosuchian archosaurs that survive the Triassic‐Jurassic extinction that affected terrestrial environments (Nesbitt, 2011). Crocodylomorpha originated in the Late Triassic and, subsequently, in the Mesozoic and Cenozoic, diversified into a broad diversity of lineages including those of small terrestrial carnivores (e.g. Protosuchus richardsoni; Colbert & Mook, 1951), large terrestrial predators (e.g. Baurusuchus salgadoensis; Carvalho et al. 2005), small herbivores (e.g. Simosuchus clarki; Buckley et al. 2000) and even marine forms (e.g. Pelagosaurus typus; Pierce and Benton, 2006). The diversification of this clade was associated with the consolidation of an akinetic skull, which is one of the hallmarks of Crocodyliformes (Langston, 1973, as Crocodilia) and allowed the development of the strong biting forces of modern crocodylians (Erickson et al. 2012; Pol et al. 2013). Thus, this transition (i.e. the origin of Crocodyliformes) is a key event that influenced the evolutionary success of the clade for the rest of the Mesozoic and Cenozoic. In crocodylomorphs, unlike other non‐theropod reptiles, the skull is pneumatized by several cavities. This trait was described early in the literature (e.g. Owen, 1850), and several reviews have focused on it (e.g. Colbert, 1946a; Tarsitano, 1985; Dufeau and Witmer, 2015); however, the general anatomy of this region in non‐crocodyliform crocodylomorphs is poorly known, as these cavities are associated with the braincase and are difficult to observe. Furthermore, non‐crocodyliform crocodylomorphs have additional pneumatizations which are not homologous to the condition of crocodyliforms (Walker, 1990), which makes the identification and comparison of these cavities even more complicated.
The focus of this contribution is the crocodylomorph Almadasuchus figarii (Pol et al. 2013). The only known specimen of Almadasuchus is very well preserved but incomplete, missing most of the rostrum and palate, but including the posterior region of the braincase and temporal arches. Pol et al. (2013) briefly described this specimen and recognized the main changes associated with the establishment of an akinetic skull within Crocodylomorpha. Almadasuchus was recovered as a basal crocodylomorph in that contribution, taking a position as the sister group of Crocodyliformes. The matter was later tackled by Leardi et al. (2017) who, upon adding additional data, analyzed the general phylogenetic patterns of basal crocodylomorphs and found Almadasuchus allied to the Late Jurassic taxa Macelognathus and Hallopus, forming the clade Hallopodidae that was recovered as the sister group of Crocodyliformes.
In this contribution we describe the braincase of Almadasuchus in detail through the use of high‐resolution micro‐computed tomography (CT), which allows the reconstruction of internal structures such as the endocast, inner ear and pneumatic cavities. This study represents the first time these structures have been reconstructed in a basal crocodylomorph; therefore, we will discuss the pneumatic cavities of the skull of Almadasuchus and compare them with those in other crocodylomorph taxa in order to establish homologies with those of modern crocodylians. Finally, we discuss the significance of the pneumatic transformations of the crocodylomorph skull within a phylogenetic framework for the evolution of this clade.
2. MATERIALS AND METHODS
2.1. Specimen
The specimen analyzed in this contribution is the holotype of A. figarii (Pol et al. 2013; MPEF‐PV 3838) which is represented by the posterior half of a skull, isolated cranial material (left premaxilla, left palatine, left ectopterygoid) and the posterior end of the right mandibular ramus. The type specimen also includes isolated but well preserved appendicular material (left radiale and left femur). The specimen comes from a sedimentary sequence at the locality of Puesto Almada of the Cañadón Calcáreo Formation, from one of the sandstone layers located 30 m above the fish beds of the Almada fauna (López‐Arbarello et al. 2008; Pol et al. 2013) and 20 m below the lowest radioisotopic dating of the sequence (157.387 ± 0.045 Ma; Late Jurassic, Oxfordian; Cúneo et al. 2013).
2.2. CT analysis
The skull of A. figarii (MPEF‐PV 3838) was scanned at the Microscopy and Imaging Facility of the American Museum of Natural History, using a high‐resolution CT scanner (GE Phoenix v|tome|x s 240). The skull remains were scanned in the sagittal plane. Because of the size of the specimen, the skull was scanned in two steps, each scan starting from a different side, and then stitched into a single group of images using the software Fiji (Schindelin et al. 2012) . This process resulted in a total of 1038 slices, each with a slice thickness of 0.038 mm, with interslice spacing of 0.038, and a pixel resolution of 905 × 620. The matrix was eliminated and the skull was segmented into its individual bones using the Mimics software (V 16; Materialise). Cavities (endocranial, inner ear, and pneumatic) were recognized and filled manually for their posterior reconstruction.
2.3. Comparisons
In order to contrast adequately the range of morphologies of the taxa studied in this contribution, these are compared with a wide array of crocodylomorphs. The source of information for each individual taxon is supplied separately in Table 1. For the sake of comparisons in the text, thalattosuchians will be included in Crocodyliformes following recent contributions (Leardi et al. 2017; Ristevski et al. 2018); however, alternative placements of the group as the sister group of Crocodyliformes (Wilberg, 2015) will be discussed later (see Discussion).
Table 1.
List of taxa used for comparisons in the text
| Taxon | Source |
|---|---|
| Stagonolepis robertsoni | Walker (1961); Gower and Walker (2002) |
| Postosuchus kirkpatricki | Chatterjee (1985); Weinbaum (2011) |
| Pseudhesperosuchus jachaleri | PVL 3830; Bonaparte (1972) |
| Trialestes romeri | PVL 2561, 3889; Reig (1963); Lecuona et al. (2016) |
| Saltoposuchus conectens | Sereno and Wild (1992) |
| Terrestrisuchus gracilis | Crush (1984); Allen (2010) |
| Litargosuchus leptorhynchus | BP/1/5237; Clark and Sues (2002) |
| Hesperosuchus agilis | AMNH FR 6758; CM 29894; YPM 41198; Colbert (1952); Clark et al. (2000) |
| Kayentasuchus walkeri | UCMP 131830; Clark and Sues (2002) |
| Dromicosuchus grallator | Sues et al. (2003) |
| Sphenosuchus acutus | SAM‐PK 3014; Walker (1990) |
| Dibothrosuchus elaphros | IVPP V 7907; Wu and Chatterjee (1993) |
| Junggarsuchus sloani | IVPP V 14010; Clark et al. (2004) |
| Macelognathus vagans | LACM 5572/150148; Leardi et al. (2017) |
| Orthosuchus stormbergi | SAM‐PK 409; Nash (1975) |
| Protosuchus richardsoni | AMNH 3024; MCZ 6727; Clark (1986) |
| Protosuchus haughtoni | BP/1/4746, 4770, 4946, 5290; SAM‐PK 8026; Busbey and Gow (1984); Gow (2000) |
| Sichuanosuchus shushanensis | IVPP V 10594; Wu et al. (1997) |
| Notosuchus terrestris | MLP‐64‐IV‐16‐5; MACN‐PV RN 22, 1022, 1037; Barrios et al. (2018) |
| Simosuchus clarki | UA 8679; Kley et al. (2010) |
| Sebecus icaeorhinus | AMNH 3160; Colbert (1946b) |
| Pelagosaurus typus | BSGP 1890; Walker (1990); Pierce and Benton (2006); Pierce et al. (2017) |
| Stenosaurus bollensis | Herrera et al. (2018) |
| Cricosaurus araucanensis | MLP 72‐IV‐7‐1, 72‐IV‐7‐3, 72‐IV‐7‐4, 86‐XI‐5‐7; Herrera et al. (2018) |
| Caiman yacare | MACN 15145, 30531, 30522 |
| Alligator mississipiensis | Dufeau and Witmer (2015) |
| Crocodylus johnstoni | Witmer et al. (2008) |
With regard to the description of the pneumatic diverticula of the skull of Almadasuchus, we will use the terminology of Dufeau and Witmer (2015); however, we will also reference the classic names of these pneumatizations (e.g. Colbert, 1946a; Walker, 1990) as they have a long history in the study of this peculiar region of the crocodyliform skull. The new diverticular terminology of Dufeau and Witmer (2015) fails to describe the complexity of the structure in particular areas, such as the basioccipital‐basisphenoid pneumaticity.
2.4. Institutional abbreviations
AMNH, American Museum of Natural History (Fossil Reptiles), New York, United States; BSGP, Bayerische Staatssammlung fur Palaontologie und Geologie, Munich, Germany; BP, Evolutionary Studies Institute (formerly Bernard Price Institute for Palaeontological Research), University of the Witwatersrand, Johannesburg, South Africa; CM, Carnegie Museum of Natural History, Pittsburg, United States; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; LACM, Natural History Museum of Los Angeles County, Los Angeles, CA, USA; MACN, Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina; MCZ, Museum of Comparative Zoology, Cambridge, MA, USA; MLP, Museo de La Plata, La Plata, Argentina; MPEF, Museo Paleontológico Egidio Feruglio, Trelew, Argentina; PVL, Museo Miguel Lillo, San Miguel de Tucumán, Argentina; SAM, Iziko South African Museum, Cape Town, South Africa; UA, University of Antananarivo, Antananarivo, Madagascar; UCMP, University of California Museum of Paleontology, Berkeley, United States; YPM, Yale Peabody Museum, New Haven, United States.
3. RESULTS
3.1. General features of the skull
The braincase of Almadasuchus was found in an isolated block, separated from the rest of the skeletal elements of the holotype (MPEF‐PV 3838). The remains of the snout (premaxilla), palatal (palatine) and mandibular (articular) elements were associated with the postcranial remains of the specimen. No supernumerary elements were recovered, which, together with the similar preservational features and size, implies that all the bones are from a single individual.
The posterior part of the skull of MPEF‐PV 3838 is preserved from the posterior half of the frontals to the occipital condyle, and anteroventrally up to the dorsal half of the descending process of the postorbital (Figure 1A–E). Posterior to the descending process of the postorbital, the ventral region of the posterior part of the skull of Almadasuchus is well preserved, with most of its elements completely preserved. The exception is the pterygoids which have not been preserved (Figure 1B). The braincase of Almadasuchus measures 95 mm from the anterior part of the preserved frontals to the occipital condyle and 124 mm from the lateral condyle of the left quadrate to the lateral condyle of the right quadrate. This places Almadasuchus as one the largest taxa among the non‐crocodyliform crocodylomorphs. The dermal elements of the skull of Almadasuchus have well‐developed ornamentation, consisting of irregular ridges and furrows, which supports the hypothesis that this specimen represents an adult individual (Figure 1A).
Figure 1.

Digital reconstruction of the segmented elements of the posterior region of the skull of Almadasuchus figarii (MPEF‐PV 3838) in A, dorsal, B, ventral, C, right lateral, D, posterior and E, anterior views. ‘bor’, basioccipital recess (‘sensu lato’); cqp, cranioquadrate passage; endoc, endocranial cavity: fic, foramen for the internal carotids; fov, foramen ovale; ftoa, temporoorbital foramen; ic, internal carotids; itf, infratemporal fenestra; nc, nuchal crest; ota, otic aperture; ptf, post‐temporal fenestra; qf, quadrate fenestra; sc, sagittal crest; stf, supratemporal fenestra; st, sella turcica; stfo, supratemporal fossa; trc, triangular concavity of the squamosal; vg, vagus foramen; XII, exit for cranial nerve XII
Only the posterodorsal region of the orbit is preserved, as most of the anteroventral elements of the skull are not preserved. The dorsal margins of the orbits are not significantly raised (Figure 2C), unlike the condition of extant crocodylians (e.g. Caiman yacare, Gavialis) and pholidosaurs (e.g. Sarcosuchus, Rhabdognathus) where these borders are dorsally projected, delimiting a deep groove on the frontal between the orbits. Unfortunately, the precise orientation of the orbits cannot be established, as it greatly depends on its ventral border formed by the jugal and lacrimal, which are not preserved in MPEF‐PV 3838.
Figure 2.

Digital reconstruction of the bones of the skull roof of Almadasuchus figarii. Frontals in A, dorsal and B, ventral views. Parietal in C, dorsal and D, ventral views. cc, crista cranii; f. fr, facet for the frontals; f. lsf, facet for the laterosphenoid; f. par, facet for the parietals; f. po, facet for the postorbital; f. pro, facet for the prootic; f. soc, facet for the supraoccipital; nc, nuchal crest; sc, sagittal crest; stf, supratemporal fenestra; stfo, supratemporal fossa; su, interfrontal suture
The supratemporal fenestrae of Almadasuchus are very large, occupying two‐thirds of the preserved dorsal surface of the skull roof, and are triangular in dorsal view (Figure 1A). The lateral edges of the supratemporal fenestrae are oblique, while the medial ones are almost straight and very close to each other, separated by a prominent sagittal crest. The supratemporal fossae are strongly asymmetrical, as they are very wide on the medial and posterior margins, but they are not developed on the lateral edges of the supratemporal fenestrae. The infratemporal fenestra is only preserved on its dorsal half as the elements that form its ventral half are not preserved in MPEF‐PV 3838 (Figure 1C). The dorsal part of the infratemporal fenestra of Almadasuchus is anteroposteriorly narrow, a condition shared with other Jurassic non‐crocodyliform crocodylomorphs (Junggarsuchus, Dibothrosuchus, Sphenosuchus) and basal crocodyliforms (Hemiprotosuchus, P. richardsoni), but contrasting with the wider and more triangular shaped dorsal region of the infratemporal fenestrae of Pseudhesperosuchus, Saltoposuchus, Terrestrisuchus and Hesperosuchus. Almadasuchus bears an autapomorphic condition on its infratemporal fenestra, in that the narrow dorsal region of this fenestra is obliquely oriented with its main axis oriented posterodorsally. In contrast, in other Jurassic non‐crocodyliform crocodylomorphs (Junggarsuchus, Dibothrosuchus and Sphenosuchus) and basal crocodyliforms (e.g. P. richardsoni) that have the infratemporal fenestra dorsally projected, its main axis is oriented anterodorsally.
Finally, the otic aperture is a large subtriangular opening on the lateral surface of the skull of Almadasuchus (Figure 1C). This feature can be observed better in ventrolateral view, as it is partially hidden by an anteroventral flange of the squamosal (see below) in lateral view. This feature is further modified by the posterior closure of the otic notch, a synapomorphic feature of hallopodids, shartegosuchids and mesoeucrocodylians (Clark, 2011; Pol et al. 2013; Leardi et al. 2017).
3.2. Description of the skull
The posterior halves of both frontals were preserved in MPEF‐PV 3838 (Figure 2A,B). As in most archosaurs, and in particular, non‐mesoeucrocodylian crocodylomorphs (e.g. Pseudhesperosuchus, Hesperosuchus, Sphenosuchus, P. richardsoni) with the exception of shartegosuchids (e.g. Fruitachampsa), the frontals are paired elements, contrasting with the fused nature in the later. The suture between them is straight. Like the rest of the dorsal dermal bones of the skull roof of Almadasuchus, the frontals are sculpted with low ridges and furrows, a condition shared with crocodyliforms (e.g. P. richardsoni, Sichuanosuchus shuhanensis), but absent in other basal crocodylomorphs (e.g. Hesperosuchus, Sphenosuchus, Junggarsuchus). The dorsal surface of the frontals of MPEF‐PV 3838 lacks the presence of an elevated central region, limited by strong lateral crests as in Litargosuchus, Dibothrosuchus and Sphenosuchus. Even in taxa where this elevated region is absent (Dromicosuchus, Junggarsuchus and Hesperosuchus) a central blunt crest is present along the contact between the frontals, but such a structure is also absent in Terrestrisuchus, Saltoposuchus, Almadasuchus and in most crocodyliforms. Anteriorly, no remains of the articular surfaces with the prefrontals were preserved. Posterolaterally, the frontals bear a short laterally directed process for the articulation with the postorbitals, where an interdigitated suture can be seen. The frontals participate on the anterior border of the supratemporal fenestra, particularly on the anteromedial angle of it. Posteroventrally, the part of the supratemporal fossae on the frontals are very deep and narrow. Posteriorly, the frontals overlap the parietal on its dorsolateral region.
On the ventral surface, the frontals bear a deep olfactory groove, limited by two very dorsoventrally high cristae cranii (Figure 2B). The cristae cranii exceed the dorsoventral development of the rest of the frontals in lateral view, a similar height was already noted in other non‐crocodyliform crocodylomorphs (Leardi et al. 2017; J. M. Clark, pers. obs.). The crests end abruptly posteriorly, just anterior to the rough area that represents the articular surface of the laterosphenoids.
The parietal is an unpaired bone, a condition shared with Sphenosuchus, Dibothrosuchus, Junggarsuchus, Macelognathus and crocodyliforms (e.g. P. richardsoni). The parietal is ‘T’‐shaped in dorsal view because of the presence of two conspicuous posterolateral processes which articulate with the squamosal on their lateral ends (Figure 2C). The marked lateral projection of these processes is also present in Junggarsuchus, Dibothrosuchus and Sphenosuchus, where these attain a lateral length nearly half of the anteroposterior development of the parietal in dorsal view. Hesperosuschus agilis (CM 29894) and Dromicosuchus also have parietals with laterally projected posterior processes, but to a minor extent (30% of the parietals length). In contrast, these posterolateral processes are very short in lateral development in Litargosuchus and Pseudhesperosuchus, where the parietals lose the ‘T’ shape in dorsal view. In crocodyliforms (e.g. P. richardsoni, Notosuchus, Caiman yacare), the squamosals overlie the parietal giving the latter a subrectangular shape in dorsal view. The dorsal surface of the parietal has a sharp sagittal crest all along its anteroposterior axis (Figure 2C). Sharp sagittal crests are present among most non‐crocodyliform crocodylomorphs with the exception of Litargosuchus and some thalattosuchians (e.g. Peipehsuchus, Stenosaurus bollensis). Terrestrisuchus has been rescontructed by Crush (1984) as lacking a sagittal crest, however, after a review of the specimens of this taxon Allen (2010) concluded that the dorsal surface of the parietals is not preserved well enough to make such a claim. In Almadasuchus, the sagittal crest is connected to a sharp nuchal crest present on the posterior margin of the parietal and on the posteromedial margin of the squamosals. The nuchal crest meets the sagittal crest at almost a right angle, contrasting with the condition in Pseudhesperosuchus, Dromicosuchus, Hesperosuchus, Saltoposuchus (inferred from the morphology of the squamosal), and Litargosuchus where the nuchal crests meet the sagittal crest in oblique fashion (Clark et al. 2000).
The ventral surface of the parietal of Almadasuchus is strongly concave along its main axis (Figure 2D). The ventral margin of the parietal, within the supratemporal fossa, is sutured to the laterosphenoids and prootics. The posterolateral processes of the parietal bears two different facets, separated by an oblique crest which divides these processes in two almost equal halves. The anterior facet corresponds to the articular surface for the prootics while the posterior one represents the articular area of the supraoccipital. The central region of the posterior end of the parietal has a deep triangular depression (Figure 2D), which also represents an articular surface for the supraoccipital, in particular, its dorsal process (see below).
The parietal is posteriorly exposed in Almadasuchus, forming a sub‐rectangular surface (Figure 1D). In its broadest part the parietal attains a height that it is comparable to the height of the supraoccipital in occipital view. This morphology is also present in other basal crocodylomorphs (Sphenosuchus, Dibothrosuchus), but not in crocodyliforms (e.g. P. richardsoni, Sichuanosuchus, Simosuchus, Pelagosaurus, Caiman yacare). This region is strongly concave, a condition further highlighted by the large posterior projection of the nuchal crests. The lateral margins of the occipital surface of the parietal are formed by the suture with the squamosal that runs ventromedially down to the triple contact between the squamosal, parietal and supraoccipital. This point is located dorsomedially from the post‐temporal fenestra. The ventral margin of the occipital surface of the parietal is delimited by the contact with the supraoccipital that runs dorsomedially up to the lateromedial midpoint of the occiput.
The postorbital has a flat dorsal surface that forms the anterolateral region of the skull roof ( Figures 1A–E and 3A,B). This surface is ornamented with pits and grooves that disappear gradually towards the postorbital‐squamosal contact (Figure 3A). The anterior region of the postorbital is lateromedially broad and separates the supratemporal fossa from the orbit. The posterodorsal ramus of the postorbital is anteroposteriorly elongated. This process forms the majority of the dorsal border of the infratemporal fenestra and more than 75% of the lateral margin of the supratemporal fenestra (Figure 1A). An elongated posterodorsal process of the postorbital is also present in Sphenosuchus, Junggarsuchus, Pseudhesperosuchus and Hesperosuchus, contrasting with the anteroposteriorly shorter posterodorsal ramus of the postorbital of crocodyliforms, Terrestrisuchus, Saltoposuchus, Dromicosuchus, Litargosuchus and Dibothrosuchus. Along this region, the dorsal surface of the postorbital has raised medial and lateral margins (Figure 3A), being dorsally concave in cross‐section. The rounded posterior end of the postorbital overlaps dorsally the anterior flange of the squamosal.
Figure 3.

Digital reconstruction of the bones of the left temporal region of Almadasuchus figarii. Postorbital in A, dorsal and B, lateral views. Squamosal in C, dorsal, D, lateral and E, posterior view. dcon, dorsal concavity of the postorbital; df po, descending flange of the postorbital; df sq, distal flange of the squamosal; f. fr, facet for the frontals; f. oto, facet for the otoccipital; f. par, facet for the parietal; f. po., facet for the postorbital; f. sq, facet for the squamosal; jp, jugal process of the postorbital; hp, hooked process of the squamosal; oc c, occipital crest of the squamosal; pfq, process for the articulation with the quadrate; ptf, post‐temporal fenestra; trc, triangular concavity of the squamosal
The jugal process of the postorbital is located at the anterolateral corner of the postorbital and projects ventrolaterally as a flattened laminar process (Figure 3B). The ventral margin of this process is directed posterodorsally forming the anterodorsal border of the infratemporal fenestra. The postorbital has a descending flange exposed along the lateral margin of its posterodorsal process. The lateral surface of the descending flange of the postorbital is slightly smooth and concave in lateral view, and extends between the infratemporal fenestra and the lateral margin of the skull roof. The posterior end of the descending flange is sutured posteriorly to an equivalent descending flange of the squamosal. The development of a descending flange on the posterodorsal process of the postorbital seems to be an autapomorphy of Almadasuchus, although this region is not known in Macelognathus and Hallopus. Pseudhesperosuchus has a medially exposed surface of the postorbital, but this exposure is caused by a medial twisting of the posterodorsal process of the postorbital and no descending flange is present on the lateral surface of this process. Junggarsuchus also has a laterally exposed posterodorsal process of the postorbital, but it does not form a proper descending flange as in Almadasuchus. Instead, the postorbital of Junggarsuchus is convex in dorsal view, and that rounded dorsal surface is more ventrally projected than the medial border of this process. The ventral surface of the postorbital bears a rough depression at its anteromedial corner, which receives the capitate process of the laterosphenoid.
The squamosal is a complex bone that forms part of the skull roof, supratemporal fossa, occipital surface and the margin of the external otic recess of the skull (Figure 1A–D). The squamosal forms the posterolateral region of the skull table, which is ornamented with irregular pits and grooves (Figure 3C,D). The anterodorsal process of the squamosal is remarkably short and contacts the posterodorsal process of the postorbital in the posterior part of the supratemporal fossa (Figure 3C). This process limits the posterolateral margin of the supratemporal fossa. The posterodorsal region of the squamosal is projected laterally as a broad hook‐shaped process, forming a deep and extensive dorsal roof over the otic recess. This lateral region of the skull roof is slightly deflected, so that the otic recess is partially hidden in lateral view (Figures 1C and 3D). The squamosal contribution to the roof of the otic recess is lateromedially broader than in other crocodylomorphs, being almost as long as broad. Additionally, in dorsal view, the lateral margin of the skull roof is markedly concave, forming a deep notch separating the descending process of the postorbital and the posterolateral projection of the squamosal. This morphology of the dorsolateral surface of the squamosal is unusual among crocodylomorphs, as most taxa have a sub‐quadrangular skull roof in dorsal view (e.g. Dromicosuchus, Sphenosuchus, Dibothrosuchus, P. richardsoni). A similar morphology is present in Junggarsuchus, where a marked triangular posterolateral flange of the squamosal is present. In these taxa, the posterolateral flange of the squamosal is clearly separated from the rest of the bone in dorsal view; however, no marked anterior notch is present in Junggarsuchus, unlike the condition of Almadasuchus. In Almadasuchus, a posteromedial process of the squamosal extends into the supratemporal fossa and meets the parietal at the posterior surface of this opening. The lateral region of this process is sutured to the primary head of the quadrate, while the medial one contacts the prootic. The squamosal wedges between the prootic and the parietal inside the supratemporal fossa and forms the dorsolateral border of the temporoorbital foramen. Thus, the posteromedial process of the squamosal precludes the quadrate‐parietal contact on the posterior region of the supratemporal fossa (Figure 1A). This condition is also observed in other ‘sphenosuchians’ (Litargosuchus, Dibothrosuchus and Sphenosuchus), but not in crocodyliforms (e.g. Protosuchus haughtoni). The squamosal also forms the posterolateral margin of the supratemporal fossa, which is delimited by an elevated crest that continues posteriorly in the parietal (i.e. the nuchal crests) and in medial border with the posterodorsal process of the postorbital.
The squamosal also contributes to the occipital surface of the skull, occupying the dorsolateral region of this surface (Figures 1D and 3E). The dorsomedial margin is sutured to the parietal and the squamosal meets the supraoccipital dorsomedial to the post‐temporal fenestra. The squamosal‐supraoccipital suture runs ventromedial to this point, passing just medial to the post‐temporal fenestra where this suture meets the otoccipital. Thus, the post‐temporal fenestra is completely enclosed between the squamosal and the otoccipital, with the squamosal forming the dorsal, lateral and ventrolateral margins of this opening. The ventral half of the occipital surface of the squamosal forms a dorsally concave suture with the dorsal margin of the paroccipital process. This contact continues ventrally up to the level of the ventral margin of the foramen magnum and medially up to the level of the medial border of the post‐temporal fenestra; therefore, the squamosal covers the entire dorsal surface of the paroccipital process. The occipital exposure of the squamosal of Almadasuchus is dorsal to the level of the foramen magnum. The occipital surface of the squamosal is limited laterally by a vertical crest, which borders the lateral otic groove posterior to the otic aperture (see below).
The squamosal has a deep concave surface located posteriorly on its lateral surface, posterior to the shelf that overhangs the otic recess and more medially than the lateral surface of the hooked lateral process of the squamosal (Figure 3C–E). This concavity is sub‐triangular as it broadens gradually ventrally, having its apex pointed dorsally. This triangular depression is limited anteriorly by the posterior surface of the hooked process of the squamosal and posteriorly by the vertical crest of the lateral end of the occipital surface of the squamosal. This peculiar concave surface of the squamosal is only known in Junggarsuchus among crocodylomorphs, although in Junggarsuchus this concavity is less developed. This posterior depression on the lateral surface of the quadrate is difficult to associate with any given structure of modern crocodylians, due to its position and size. This concave surface could be the origin of the M. depressor mandibulae, as in complete specimens (i.e. Junggarsuchus), this structure is aligned with the retroarticular process of the mandible (J. M. Clark, pers. obs). However, this interpretation would imply an anterolateral displacement of the origin of the muscle mass when compared with extant reptiles, as this muscle originates on the lateral side of occipital surface of the skull in these forms (Iordansky, 2010; Holliday et al. 2013). An alternative interpretation is that the depression corresponds to the musculature of the ear flap. The ear flap is a unique adaptation present in living crocodyliforms, and it has been identified in the basal crocodylomorphs Kayentasuchus and fossil crocodyliforms such as Protosuchus due to the presence of a dorsal groove on the squamosal (Clark and Sues, 2002). The triangular depression is in a similar position to the origin of the M. levator auriculae superior (Shute and Bellairs, 1955; Montefeltro et al. 2016). Nevertheless, this muscle does not leave a distinct scar or depression on the ventrolateral aspect of the squamosal of modern crocodylians or any fossil crocodyliform (Montefeltro et al. 2016), and there is no other indication of an ear flap muscle in Almadasuchus. Regardless, this remains a unique feature of Almadasuchus and Junggarsuchus which could be correlated with the large size of their otic apertures.
Within the otic recess, the squamosal is extensively sutured with the quadrate, covering the dorsolateral region of the quadrate primary head. This suture is located deep within the otic recess and is hidden by the ventral deflection of the squamosal hooked process. The suture deflects ventrally down to the posterodorsal margin of the otic recess. The squamosal has a descending flange that meets the quadrate at the posteroventral corner of the otic recess, closing completely the external otic recess (Figures 1C and 3D). This morphology resembles the derived condition of mesoeucrocodylians. Other basal crocodylomorphs (e.g. Sphenosuchus and Dibothrosuchus) and basal crocodyliforms (e.g. P. richardsoni and P. haughtoni) lack a posterior closure of the otic notch due to a squamosal‐quadrate contact (Pol et al. 2013).
The quadrates lack the condylar region, but otherwise, both elements are almost complete (Figures 1A–E and 4A–D). The main body of the quadrate is straight or slightly curved, while the distal portion of the quadrate is lateromedially broad and anteroposteriorly flattened (Figure 4A). The posterior border of the quadrate, as in most crocodylomorphs, is curved and forms the anterior, dorsal and ventral borders of the otic aperture. As in Macelognathus, the otic aperture of Almadasuchus is very large, occupying almost half of the lateral height of the quadrate, having a smoothly curved and dorsoventrally high anterior margin. The low curvature and dorsoventral height of the anterior margin of the otic aperture resembles the condition of the quadrate otic notch of other basal crocodylomorphs but contrasts with the condition in crocodyliforms, where the otic aperture has a strongly concave anterior margin (e.g. P. richardsoni). Anterior to the otic aperture, the lateral surface of the quadrate is smooth and bears a shallow periotic fossa that is delimited anteriorly by a slightly elevated ridge that extends from the anterodorsal region of the squamosal‐quadrate suture down to the anteroventral margin of the quadrate. The quadrate is sutured to the ventral margin of the paroccipital processes of the otoccipitals through a broad posterodorsally projected posteroventral process (Figure 4A,C), a condition also present in Junggarsuchus, hallopodids and crocodyliforms (Pol et al. 2013; Leardi et al. 2017). Unlike other non‐crocodyliform crocodylomorphs (e.g. Sphenosuchus, Dibothrosuchus, Pseudhesperosuchus), the quadrate contacts with the squamosal on the posteroventral border of the otic aperture. This condition resembles the posterior closure of the otic aperture present in mesoeucrocodylians and it has been recognized as a synapomorphy of hallopodids (Leardi et al. 2017).
Figure 4.

Digital reconstruction of the right quadrate of Almadasuchus figarii in A, lateral, B, anterior, C, medial and D, posterior views. c ‘B’, crest B; f. lsf, facet for the laterosphenoid; f. oto, facet for the otoccipital; f. pro, facet for the prootic; f. qj, facet for the quadratojugal; f. sq, facet for the squamosal; ota, otic aperture; pdp, posterodorsal process of the quadrate; pneu 1‐3, pneumatic foramen 1‐3; ptp, pterygoid process of the quadrate; qf, quadrate fenestra; qh, quadrate head
Anterior to the otic aperture, on the anteroventral region of the lateral surface the quadrate bears a remarkably large and dorsoventrally elongated quadrate fenestra (Figure 4A). This opening is much broader than in other basal crocodylomorphs (e.g. Dromicosuchus, Junggarsuchus, Sphenosuchus, Dibothrosuchus), but resembles the condition described for Terrestrisuchus (Crush, 1984) and Hesperosuchus agilis (CM 29894). Also in A. figarii the fenestra is completely bounded by the quadrate as in Sphenosuchus, Dibothrosuchus and Junggarsuchus; while in Terrestrisuchus (Crush, 1984), Hesperosuchus and Dromicosuchus there is participation of the quadratojugal on at least its anteromedial margin (Leardi et al. 2017). The quadrate fenestra in Almadasuchus is clearly of pneumatic nature as it opens internally into the quadrate. The quadrate of Almadasuchus is filled with air cavities, evidencing a complex internal structure of the infundibular diverticulum (sensu Dufeau and Witmer, 2015). The quadrate fenestra opens into a large internal cavity, which invades the main body of the quadrate and opens into the middle ear cavity just anteroventral to the posteromedial process of the quadrate that contacts the otoccipital. This large pneumatic cavity extends ventrally, branching into no more than three smaller diverticula that reach up to the level of the condyles, and dorsally forming an isolated, much smaller diverticulum. The latter opens independently into the middle ear cavity through a small foramen. This foramen is the smallest and the ventral‐most of three dorsal medial pneumatic foramina that open into the middle ear cavity (Figure 4C). The two more dorsal ones correspond to the exit of two additional pneumatic cavities which invade the dorsal part of the quadrate. However, neither of these reach up to the level of the quadrate primary head. Quadrate pneumatization was described for Dibothrosuchus, Macelognathus and crocodyliforms (Leardi et al. 2017), with the condition of Almadasuchus (i.e. several internal pneumatic chambers) closely resembling that of Macelognathus.
Dorsally, the quadrate contacts the squamosal through its primary head or otic process (Figure 4A,C,D). The quadrate primary head of Almadasuchus is sickle‐shaped in lateral view, due to the marked hooked process developed posteriorly. In dorsal view, the quadrate otic process is differentiated in two regions: an anterior articular surface which is lateromedially narrow and articulates with the anterodorsal process of the squamosal; and, a posterior one that is lateromedially expanded, strongly convex and that articulates with the occipital process of the squamosal. Ventrally, within the adductor chamber, the quadrate is convex anteriorly (Figure 4B). Dorsally the quadrate extends into the supratemporal fossa articulating with both the prootic and the squamosal via its orbital process. The dorsal part of the orbital process articulates with the prootic, forming an oblique suture, while the ventral part of the orbital process is sutured to the laterosphenoid along a poorly developed and rugose vertical crest. The quadrate‐prootic suture runs dorsolaterally from this crest and passes lateral to the temporoorbital foramen. The quadrate‐laterosphenoid contact is absent in other basal crocodylomorphs (Litargosuchus, Sphenosuchus, Dibothrosuchus, Kayentasuchus and Junggarsuchus and thalattosuchians (Clark, 1986; Herrera et al. 2018), while it is present in crocodyliforms (e.g. P. richardsoni, P. haughtoni). More ventrally on the adductor chamber, the anteroventral surface of the quadrate bears an elevated crest B (Iordansky, 1973). This crest is broad and obliquely oriented, almost reaching the medial border of the orbital process of the quadrate (Figure 4B).
The ventral surface of the quadrate bears a short and blunt pterygoid process that is directed anteromedially (Figures 1B and 4B,C). This process bears posterior elongated facet that is interpreted as the articular surface of the pterygoids, which were not preserved in Almadasuchus. Anterior to the quadrate condyles, a groove passes anterodorsally between the crest ‘B’ and the base of the pterygoid process of the quadrate. This groove can be interpreted as an osteological correlate of the maxillomandibular artery and vein (Porter et al. 2016).
The prootic of Almadasuchus is extensively exposed within the supratemporal fossa and extends ventrally within the adductor chamber, wedging between the quadrate, parietal and laterosphenoid (Figures 1A and 5). The extensive exposure of the prootic within the supratemporal fossa is also present in other basal crocodylomorphs (Litargosuchus, Kayentasuchus, Sphenosuchus, Dibothrosuchus and Junggarsuchus) and thalattosuchians (e.g. S. bollensis, Pelagosaurus typus, Cricosaurus araucanensis) but is absent in Crocodyliformes (e.g. P. richardsoni, Notosuchus, Caiman yacare), where it is covered by the laterosphenoid. Posteriorly, within the supratemporal fossa, the prootic forms the ventral, medial and lateral borders of the temporoorbital foramen, while the dorsal border of this foramen is limited by the squamosal (Figures 1A and 6C). The prootic contacts the laterosphenoid anteriorly via an almost straight suture in lateral view and, laterally, it articulates with the dorsal region of the quadrate through an oblique suture in dorsal view. The posterior surface of the prootic contacts three elements: dorsomedially with the supraoccipital; ventrally, and occupying most of the posterior surface, with the otoccipital; and, dorsolaterally with the posteromedial border of the squamosal within the supratemporal fossa (Figures 1A and 5).
Figure 5.

(A) Digital reconstruction of the braincase in lateral view of Almadasuchus figarii. (B) Occipital region of the braincase in posteroventral view. (C) Detail of the otic capsule. bsp pl, basisphenoid plate; bsp r, basisphenoid rostrum; bt, basal tubera of the basioccipital; cap, capitate process of the laterosphenoid; cif, crista interfenestralis; cmet, crista metotica; cpq, cranioquadrate passage; fic, foramen for the internal carotids; fm, foramen magnum; fmet, metotic foramen; fov, fenestra ovalis; fro, fenestra rotunda; orb a, exit of the orbital artery; otspc, otosphenoidal crest; paro, paroccipital recess; rhom, rhomboidal sinus; scb, subcapsular buttress; vg, vagus foramen; V, foramen ovale; VII, groove for the VII cranial nerve; XII, exit of the XII cranial nerve
Figure 6.

Digital reconstruction of the bones of the posterodorsal region of the braincase of Almadasuchus figarii. Right prootic in A, dorsal, B, lateral and C, anteroventrolateral views. Supraoccipital in D, dorsal, E, anterior and F, posterior views. apyp, anterior pyramidal projection; dp soc, dorsal process of the supraoccipital; fa, facial antrum; fm, foramen magnum; f. lsf, facet for the laterosphenoid; f. q + lsf, facet for the quadrate + laterosphenoid; f. soc, facet for the supraoccipital; fov, fenestra ovale; ftoa, temporoorbital foramen; lf soc, lateral flange of the supraoccipital; ma, mastoid antrum; og, oblique groove of the supraoccipital; stfo, supratemporal fossa; VII‐VIII, exit for cranial nerves VII‐VIII
The prootic is a complex bone, as it is heavily pneumatized in its dorsal region (prootic diverticulum sensu Dufeau and Witmer, 2015). Pneumatization in the prootic is a feature widely present in crocodyliforms (e.g. P. richardsoni, Alligator mississipiensis) and has been reported in few early non‐crocodyliform Crocodylomorpha (Hesperosuchus, Kayentasuchus, Dibothrosuchus). However, this condition is as yet unknown in the most basal members of the clade (Pseudhesperosuchus, Trialestes, Saltoposuchus, Terrestrisuchus, Litargosuchus) or any large‐bodied crocodylomorphs (i.e. Carnufex, Redondavenator, CM 73372). The prootic pneumatic cavity in Almadasuchus is restricted to the dorsal region of the element and connects with the middle ear cavity through two closely placed lateral foramina (Figure 6B,C). Internally, it is subdivided into two main cavities: a simple anterior one that communicates externally through the anterior foramen (the facial antrum), and, a posterior cavity that is subdivided into two posterior pneumatizations that open externally via the posterior foramen (the mastoid antrum).
On the lateral surface, near the ventral margin and at the same level as the foramen of the mastoid antrum, the prootic forms along with the otoccipital the otosphenoidal crest (Figures 5 and 6B,C). The prootic forms the medial part of the crest and, unlike the condition of Sphenosuchus (SAM‐PK 3014) and Macelognathus (Leardi et al. 2017), the otosphenoidal crest is not projected dorsally. On the anterior surface of this crest, a deep groove is present, which is inferred to be the exit of the palatine branch of the facial nerve (VII; Figure 6C). The area of articulation between the prootic and the otoccipital in the otosphenoidal crest bears a small recess corresponding to the internal pathway of the facial nerve. Medially the prootic of Almadasuchus is strongly concave, bearing a dorsal excavation which corresponds to the auricular (floccular) recess of the cerebellum. Posterior to this medial excavation the prootic bears a ventral bulge that projects medially. The medial bulge of the prootic delimits the ventral extension of the articular surface of the supraoccipital with the prootic.
The supraoccipital is an unpaired element and it is exposed posteriorly where it forms the dorsal border of the foramen magnum, as in most non‐crocodyliform crocodylomorphs with the possible exception of Junggarsuchus (Figures 1D and 6F). In posterior view, the supraoccipital is lateromedially wider than dorsoventrally tall, a condition shared with Litargosuchus, Junggarsuchus, Macelognathus, and crocodyliforms (Leardi et al. 2017). The morphology of the supraoccipital in posterior view is unique among non‐crocodyliform crocodylomorphs, as the supraoccipital of Almadasuchus bears a central dorsal process and two well‐developed lateral flanges (Figure 6F), while in most ‘sphenosuchians’ (e.g. Dibothrosuchus, Sphenosuchus, Macelognathus) the lateral flanges are poorly developed. The central dorsal process of the supraoccipital is triangular in dorsal view and it fits into a posteroventral socket of the parietal (Figure 6E). Anterolateral to this process there are two wide, lateral oblique grooves, which are limited anterolaterally by strong crests (Figure 6D). Ventrolaterally the supraoccipital has two ventral pyramidal projections, one on each side, on its anterior half (Figure 6E). This structure has a crest that separates the articular surfaces of the anterior articular surface for the prootic of the posterior articular surface for the otoccipital. As in other non‐crocodyliform crocodylomorphs (e.g. Macelognathus, Sphenosuchus, Junggarsuchus) and thalattosuchians (C. araucanensis, Stenesaurus) the supraoccipital does not bear any pneumatization connecting both mastoid antra (i.e. intertympanic diverticulum, here used excluding the mastoid antrum unlike Dufeau and Witmer, 2015).
As in all crocodylomorphs, the opistotic and the exoccipital are fused, forming the otoccipital (Clark, 1986). The otoccipital forms most of the lateral region of the occipital surface of Almadasuchus and it also participates in the posterior region of the floor of the otic capsule (Figures 1D and 5). The posteroventral participation in the otic capsule is given by the anteroventral contact with the quadrate, where it closes posteriorly the external otic meatus (Figure 1C). This contact has been previously recognized as a derived feature of Junggarsuchus, hallopodids and crocodyliforms (Pol et al. 2013; Leardi et al. 2017). In posterior view, the otoccipitals form the lateral margins of the foramen magnum but do not contact each other, unlike the condition of Junggarsuchus and crocodyliforms (Clark et al. 2004; Figure 7C). In occipital view, the otoccipital articulates with the basioccipital along its ventromedial border, having a rounded dorsal process in the region of the occipital condyle, while more ventrally the suture is simple and obliquely oriented (Figures 1D and 7C). The paroccipital process of the otoccipital is very wide in Almadasuchus as it expands greatly ventrally, while in other taxa (e.g. Dibothrosuchus, Kayentasuchus) this ventral expansion is more restricted. The posterior surface of the otoccipital, lateral to the foramen magnum, is pierced by several foramina (Figure 7C,D). The medial‐most one is located at the ventral level of the occipital condyle and it has participation of both the otoccipital, on its lateral margin, and the basioccipital, on its ventral margin. This foramen was identified by Pol et al. (2013) as the entry of the internal carotid artery (sensu Porter et al. 2016), this being a shared feature between Junggarsuchus (Clark et al. 2004), Almadasuchus and crocodyliforms. More laterally and entirely on the otoccipital, the paired exits for cranial nerve XII are present: one placed dorsomedial to the internal carotid foramen, and the other lateral to that foramen. Finally, on the lateral half of the paroccipital processes two large and ventrally facing foramina can be observed. The smallest and more laterally placed is identified as the vagus foramen (exit for cranial nerves IX‐XI) and the large lateral one corresponds to the cranioquadrate passage. The presence of a vagus foramen is also shared with crocodyliforms (e.g. P. richardsoni, Gobiosuchus, Orthosuchus, C. araucanensis, Steneosaurus, Pelagosaurus). On the other hand, the cranioquadrate passage is a feature present in many crocodyliforms, including those closely related to Mesoeucrocodylia (e.g. Gobiosuchus, Sichuanosuchus), thalattosuchians (e.g. C. araucanensis, Steneosaurus, Pelagosaurus) and Macelognathus (Leardi et al. 2017). However, it is worth mentioning the peculiar condition present in Almadasuchus, where the cranioquadrate passage is only formed by the otoccipital (Figure 7C,D), while in Macelognathus and crocodyliforms with this feature (e.g. Gobiosuchus, Sichuanosuchus) the quadrate forms the ventral border of this passage. However, the identity of this foramen is beyond dispute in Almadasuchus, as it connects with the middle ear cavity as it does in modern crocodylians (Iordansky, 1973).
Figure 7.

Digital reconstruction of the right otoccipital of Almadasuchus figarii in A, anterior, B, lateral, C, posterior and D, ventral views. cif, crista interfenestralis; cqp, cranioquadrate passage; fm, foramen magnum; f. met, metotic foramen; f. boc, facet for the basioccipital; f. pro, facet for the prootic; fov, foramen ovale; f. q, facet for the quadrate; f. sq, facet for the squamosal; otspc, otosphenoidal crest; paroc, paroccipital process; PTR, posterior tympanic recess; rhom, rhomboidal recess; scb, subcapsular bulge; vg, vagus foramen; VII, groove for cranial nerve VII; XII, exit for cranial nerve XII
Within the middle ear cavity the anterior surface of the paroccipital process of the otoccipital is excavated in Almadasuchus (Figure 7A,B). This excavation forms a deep furrow that originates medially, at the lateral border of the subcapsular buttress, and expands laterally towards the lateral border of the paroccipital process. This furrow is interpreted as the posterior tympanic recess (PTR; sensu Wu and Chatterjee, 1993; otoccipital diverticulum sensu Dufeau and Witmer, 2015). The condition of Almadasuchus contrasts with the ones reported for Dibothrosuchus and Macelognathus, where this recess forms an isolated internal chamber in the otoccipital. Sphenosuchus has a slight groove on the posterior region of the otic capsule and was identified by Walker (1990) as an ‘incipient PTR’. This depression is located on the anterior surface of the paroccipital process, resembling the condition present in Almadasuchus. Medial to this depression the metotic foramen is present, however, no further details about it can be given as medially the otoccipital is affected by several fractures (Figure 7B). Anteroventrally, the subcapsular buttress contacts the anterior surface of the paroccipital process within the middle ear cavity through a laterally directed flange. This flange forms the dorsal roof of an isolated cavity within the otoccipital that has slight participation of the basisphenoid, as this element enters it along its suture with the otoccipital (Figures 5 and 7A,B). Due to its position, this cavity is identified as the rhomboidal sinus. Isolated rhomboidal sinuses have also been identified in Macelognathus (Leardi et al. 2017), Eopneumatosuchus (Crompton and Smith, 1980) and crocodyliforms (e.g. P. haughtoni; Busbey and Gow, 1984). Dorsal to the rhomboidal sinus, the otoccipital contacts the prootic along its anterodorsal surface.
The basioccipital of Almadasuchus is triangular in posterior aspect (Figure 1D). This element forms the vast majority of the occipital condyle and the posterior‐most floor of the endocranial cavity. Anteriorly the basioccipital contacts the basisphenoid (Figures 5 and 8E). The lateral contact with both otoccipitals is through a linear, oblique suture along its ventrolateral flange. The occipital condyle is fairly well projected from the posterior surface of the basioccipital, bearing a distinct neck. The basal tubera of the basioccipital are not markedly projected and, as in most crocodylomorphs, these are connected by a transverse and thick crest (Nesbitt, 2011; Figure 8F). This transverse crest, together with the ventral borders of the ventrolateral flanges of the basioccipital, forms a posteroventral wall that separates the posterior from the ventral surfaces of the basioccipital.
Figure 8.

Digital reconstruction of the bones of the lateral region and floor of the braincase of Almadasuchus figarii. Left laterosphenoid in A, lateral and B, posterior view. Basisphenoid in C, lateral and D, ventral views. Basioccipital in E, lateral and F, ventral views. bor, basioccipital recess (‘sensu lato’); bsp p, basisphenoid process of the laterosphenoid; bsp r, basisphenoid rostrum; bt, basal tubera; cap, capitate process; cr cot, cotylar crest; ds, dorsum sellae; for pn, foramen for the exit of the pneumatic cavities of the basisphenoid; f. boc, facet for the basioccipital; f. bsf, facet for the basisphenoid; f. oto, facet for the otoccipital; f. pro, facet for the prootic; f.q., facet for the quadrate; occ, occipital condyle; tgr, trigeminal recess; tub cr, intertuberal crests; vlf boc, ventrolateral facet of the basioccipital; Vg, foramen ovale; vpl, ventral plate of the basisphenoid; IV, exit for cranial nerve IV
In ventral view, the basioccipital of Almadasuchus bears a well‐developed basioccipital recess (Figure 8F). This structure is widely present among non‐crocodyliform crocodylomorphs (e.g. Terrestrisuchus, Hesperosuchus, Dromicosuchus, Sphenosuchus, Macelognathus), but it has not been reported in crocodyliforms (e.g. P. richardsoni, Orthosuchus) or non‐crocodylomorph pseudosuchians (Nesbitt, 2011). Nesbitt (2011) mentioned the lack of this recess in the type specimen of Hesperosuchus agilis (AMNH FR 6576); however, there are no remains of the basioccipital and basisphenoid anterior to the basal tubera of the former bone in that specimen. The homology of this structure will be discussed below (see Discussion – Cranial pneumaticity). In Almadasuchus, as in other crocodylomorphs where this structure is well preserved (e.g. Sphenosuchus, Dibothrosuchus, Macelognathus), this recess is a complex structure as it divides into two blind tubes that excavate the posterior region of the basioccipital. The only exception to this is Junggarsuchus, where this recess is absent.
The basisphenoid is a rather short and robust bone (Figure 8C,D). Dorsally along most of its length the basisphenoid contacts the ventral surface of the laterosphenoid, while the prootic articulates only at the posterodorsal border. The anterodorsal region of the basisphenoid is free of articulation and has a rounded notch that is limited anteriorly by a narrow bony strut. This notch and bony bridge represent the ventral and anterior borders of the foramen ovale (V), which is capped dorsally by the laterosphenoid (Figure 5; see below). The basisphenoid articulates with the basioccipital posteriorly through its posterodorsal surface. Externally and in ventral view, the basisphenoid has a large and triangular ventral plate (Figure 8D), which, as in other non‐crocodyliform crocodylomorphs (e.g. Junggarsuchus, Dibothrosuchus, Sphenosuchus), does not contact the anterolateral border of the basioccipital. However, as in crocodyliforms (e.g. P. richardsoni, Orthosuchus), the basisphenoid of Almadasuchus lacks basipterygoid processes. Anterior to the ventral plate, the basisphenoid bears a short and high basisphenoid rostrum, as it has the same dorsoventral development as the region of the basisphenoid that is located ventral to the hypophyseal fossa (Figures 1C, 5 and 8C). This condition is similar to the one observed in crocodyliforms (Clark, 1994), but contrasts with the one observed in the basal crocodylomorphs Sphenosuchus and Macelognathus where an elongate cultriform process is present. This trait is mostly unknown in other non‐crocodyliform crocodylomorphs as it is either broken, absent or cannot be observed. However, it is important to mention the morphology observed in Dibothrosuchus (IVPP V 7907). In this specimen, the rostrum of the basisphenoid is not preserved, but a crest ventral to the hypophyseal fossa that can be inferred as the base of this process is. This crest is developed from the base of the hypophyseal fossa to the base of the anterior surface of the basisphenoid, thus it would imply a tall cultriform, at least at its base.
Internally, the basisphenoid is highly pneumatic, housing a large hollow cavity located ventral to the hypophyseal fossa, and extends anteriorly into the cultriform process (Figure 9B–E). A similar ventral pneumatic cavity is present in Sphenosuchus and Dibothrosuchus. This pneumatic cavity was named the rostral recess by Walker (1990) and with no clear homologue in extant crocodylians (Walker, 1990, p. 80). This diverticulum seems to communicate with the rest of the pharyngotympanic system towards the posterior region of the basisphenoid, on its lateral surface, although these dorsolateral communications cannot be discarded as caused by damage in the specimen. The basisphenoid of Junggarsuchus is highly pneumatized (J. M. Clark, pers. obs.), although the position of this cavity with respect to the hypophyseal fossa, and thus its homology, could not be specified. In Almadasuchus, two large, paired cavities can be observed both dorsal and ventral to the hypophyseal fossa and are also identified as pneumatic cavities (pre‐ and post‐carotid recesses; see Discussion, Cranial pneumaticity).
Figure 9.

Serial coronal slices through the skull of Almadasuchus figarii (MPEF‐PV 3838). The position of each slice (A–H) is indicated on a digital reconstruction above. bor ss, basioccipital recess ‘sensu stricto’; bsp r, basisphenoid rostrum; cif, crista interfenestralis; cp, carotid pillar; cpq, cranioquadrate passage; dcon, dorsal concavity of the postorbital; d tgr, dorsal trigeminal recess; endo, endocranial cavity; fa, facial antrum; fov, fenestra ovalis; hyfo, hypophyseal fossa; hp, hooked process of the squamosal; ifd, infundibular diverticulum; jp, jugal process of the postorbital; ma, mastoid antrum; occ, occipital condyle; ota, otic aperture; pneu, internal pneumatic connection of the quadrate pneumatizations; pocr, postcarotid recess; prcr, precarotid recess; ptf, post‐temporal fenestra; q, quadrate; qd, quadrate diverticulum; qf, quadrate fenestra; rhom, rhomboidal recess; ros, rostral recess; sbsr, subasisphenoid recess; sc, sagittal crest; st, sella turcica; vf, vagus foramen; v tgr, ventral trigeminal recess; vpl, ventral plate of the basisphenoid
Both laterosphenoids are well preserved on Almadasuchus, with only the anterior most part missing, and they form the anterior part of the floor of the braincase (Figures 1E and 5). CT data on the laterosphenoid do not reveal any evidence of sutures, preventing us from identifying any remains of an epipterygoid (Holliday and Witmer, 2009). Anterolaterally, the laterosphenoid bears an elongate capitate process, which almost reaches the lateral development of the posterolateral flange (see below; Figure 8A). Ventral to the capitate process a slight oblique cotylar crest is present, which is the osteological correlate of the origin of the anterior belly of the M. pseudotemporalis superficialis (Holliday and Witmer, 2009). Anteroventrally both laterosphenoids contact each other, marking the anterior extension of the hypophyseal fossa. Lateral to the exit of the hypophysis there is a small foramen for the exit of the trochlear nerve (IV). At the same dorsoventral level as this foramen, but more posteriorly placed, a larger dorsoventrally directed foramen is present. This foramen forms a slight fossa around it and connects to the internal cavity of the braincase, and it is identified as the foramen ovale (V) (Figures 5A and 8A). No additional grooves are observed that can be associated with any of the rami of the trigeminal nerve. The foramen ovale is limited anteriorly by a thin ventrally directed basisphenoid process of the laterosphenoid.
Posteriorly, the laterosphenoid of Almadasuchus bears a lateral flange that contacts the quadrate (see quadrate above for further details on this contact; Figure 8B). The anteroventral border of this flange, together with the medial border of the quadrates, forms a rounded notch (Figure 5). This notch is in a position similar to the one from which the orbital artery exits the braincase in modern crocodylians (Porter et al. 2016). Medial to the posterolateral flange, the laterosphenoid contacts the prootic through the medial region of its posterior surface. Between these two articular surfaces, a large foramen excavates the laterosphenoids posteriorly, forming a blind cavity that reaches the mid length of the element (Figure 8B). This cavity is not connected with the internal braincase cavity, which suggests it is not related to the trigeminal ganglion; thus, it probably represents an anterior extension of the pharyngotympanic sinus, a derived trait among crocodylomorphs (see Cranial pneumaticity for more details). An additional cavity is also present, ventrally and more anteriorly extended than the one previously described. This cavity connects to the adductor chamber by a small foramen. A similarly placed pneumatic cavity in the laterosphenoid has been identified in Kayentasuchus, Dibothrosuchus and Macelognathus, and it has been named as trigeminal recess (Wu and Chatterjee, 1993).
3.3. Endocast, inner ear, and cranial pneumaticity
In the following section, the main soft organs (brain and inner ear) of Almadasuchus will be described as they are represented by the main cavities within the skull (Figure 9A–H). It is always worth remembering that in fossils the encephalic morphology is represented by the endocast, which is a rough estimator of that organ as it is the internal mold of the endocranial cavity which houses the brain, its associated membranes (i.e. meninges) and vascular elements (e.g. venous sinus; Hopson, 1979). Besides the brain and the inner ear, we will focus on the cranial pneumaticity of Almadasuchus which is not a soft tissue per se, but its development and the identification of these cavities is strongly dependent on their position relative to internal structures such as the inner ear (Colbert, 1946a; Walker, 1990; Dufeau and Witmer, 2015; Herrera et al. 2018; Figure 10A–E).
Figure 10.

Digital reconstruction of the skull of Almadasuchus figarii rendered transparent displaying the endocast (light blue), inner year (yellow) and the pneumatic diverticula (green) reconstructed in this contribution in A, dorsal, B, ventral, C, left lateral, D, anterior and E, posterior views
3.3.1. Brain endocast
The endocast of the neurocranium of Almadasuchus could be completely reconstructed with the exception of the anteriormost end of the olfactory bulbs, as the only available specimen lacks the rostrum up to the anterior third of the frontals (see above; Figures 19A–E and 11A,B). Unlike the brain of some non‐avian dinosaurs and birds (e.g. Witmer et al. ), the brain endocast is cylindrical as in other pseudosuchian archosaurs (Pierce et al. 2017; Figure 11B). In pseudosuchians (e.g. Almadasuchus) the dorsoventral development of the brain and the cerebellum are comparable, as the latter is slightly higher in lateral view. By contrast, in theropods (Witmer et al. ), the brain expands markedly posteriorly, with the cerebellum being much higher than the brain. The dorsal margin of the endocast of Almadasuchus is convex, where it describes a series of waves, being similar to the morphology reconstructed for Sebecus icaeorhinus. However this contrasts with the markedly convex dorsal border present in extant crocodylians (e.g. Gavialis gangeticus, Alligator mississippiensis, Crocodylus johnstoni) and Simosuchus; and with the condition noted in thalattosuchian crocodylomorphs where the dorsal border of the brain is almost straight (e.g. Steneosaurus cf. gracilirostris, S. bollensis, C. araucanensis). However, the relative angle between different regions of the endocast of Almadasuchus has similar values (cephalic flexure = 161.28º; pontine flexure = 170.27º) to the ones described in thalattosuchians, which have high values of their cephalic and pontine flexures (>160º; Pierce et al. 2017). Almadasuchus has a relatively straight olfactory tract when compared to other crocodyliforms (e.g. Simosuchus, Sebecus, Gavialis gangeticus, Alligator mississippiensis, Crocodylus johnstoni), and the rest of the regions of the encephalon are almost aligned among them. However, the generally straight shape of the endocast of Almadasuchus is heavily modified at the posterior‐most end, as it forms an abrupt step at this region, just at the transition between the cerebellum and the medulla oblongata (Figure 11B). A similar condition is present in Sphenosuchus.
Figure 11.

Digital reconstruction of the brain endocast of Almadasuchus figarii in A, dorsal and B, left lateral views. ce, cerebrum; ceh, cerebral hemispheres; crb, cerebellum; floc, floccular recess; ie, notch for the inner ear; mob, medulla oblongata; ob, olfactory bulbs; ot, olfactory tract; pas, posterior abrupt step
The forebrain of Almadasuchus is incomplete, as the olfactory bulbs are not entirely preserved (Figure 11A). Unlike most mesoeucrocodylians (e.g. Simosuchus, Sebecus icaeorhinus, C. araucanensis, Steneosaurus cf. gracilirostris, Alligator mississippiensis) but similar to the Gavialis gangeticus and Pelagosaurus, the olfactory tract is very wide, presenting a very slight narrowing when compared with the width of the cerebral hemispheres. These expand anteriorly to form the olfactory bulbs, which are undivided as in most crocodylomorphs with the exception of Pelagosaurus typus (Pierce et al. 2017). The cerebrum is moderately expanded at its hemispheres, being anteroposteriorly symmetrical in dorsal view. This contrasts with the more expanded cerebral hemispheres of other crocodylomorphs (e.g. Gavialis gangeticus, Pelagosaurus, Steneosaurus, Simosuchus, Alligator mississippiensis, Gavialis gangeticus) which are heart‐shaped in dorsal view. As in most crocodylomorphs, but unlike Pelagosaurus, the division between both cerebral hemispheres is not marked by a clear groove because the dorsal margin of the cerebrum of Almadasuchus is strongly convex and raised. This morphology has been associated with the development of a dorsal dural venous sinus (e.g. Hopson, 1979; Kley et al. 2010). Ventral to the cerebrum the pituitary gland has a marked posterior projection (Figure 11B), being anteroposteriorly longer than it is lateromedially wide. This condition is similar to the one observed in thalattosuchians and contrasts with the anteroposteriorly shorter pituitary gland of other crocodylomorphs (Pierce et al. 2017; Herrera et al. 2018).
The endocast of Almadasuchus is expanded at the level of the cerebellum, being dorsoventrally taller than the cerebrum (Figure 11B). A very marked floccular recess can be identified, limited posteriorly by the depression where the inner ear is housed in the skull, which forms a distinct dorsolateral depression in the endocast. The posterior border of the cerebellum is very steep, forming an abrupt posterior step, which is only present in Sphenosuchus. The medulla oblongata has a rounded section in posterior view.
3.3.2. Inner ear
Both inner ears of Almadasuchus are partially preserved (Figures 10, 12A–E and 10, 12); however, the quality is far from optimal, as a system of cracks affects the specimen on the posterior and medial borders of the otic capsule. Furthermore, the lateral margins of the endosseous labyrinth cannot be well observed in either of the sides, either due to poor preservation or the lack of difference between the densities of the rock matrix and the bone. As a result, the models of the endosseous labyrinths have some associated uncertainties, but some major features can be compared with other crocodylomorphs (Figure 12A–C).
Figure 12.

Digital reconstruction of the right inner ear of Almadasuchus figarii in A, lateral; B, posterior and C, dorsal views. asc, anterior semicircular canal: crc, crux communis; fov, fenestra ovalis; lag, lagena; lsc, lateral semicircular canal; posterior semicircular canal
As do other crocodylomorphs [e.g. Pelagosaurus, Steneosaurus, extant crocodylians (Brusatte et al. 2016; Figure 8)], the anterior semicircular canal of Almadasuchus has a greater anteroposterior development than the posterior one (Figure 12A,C). Although the anterior semicircular canal is incomplete, and judging by the anterodorsal border of the crus communis, both anterior and posterior semicircular canals have the same height (Figure 12A). This contrasts with the condition of crocodyliforms (e.g. Simosuchus, Gavialis gangeticus, Crocodylus johnstoni, Pelagosaurus, Steneosaurus, Cricosaurus) where the anterior semicircular canal attains a higher dorsal development than the posterior one. The crux communis is very wide in lateral view, being more than twice the width of either of the preserved canals (Figure 12A,B). The lateral semicircular canal is very badly preserved and could only be partially reconstructed on the right labyrinth. The lagena of Almadasuchus is very short, contrasting with the longer lagenae of extant crocodylians (see Figure 8 Brusatte et al. 2016) and with the very elongated condition present in thalattosuchians (Pierce et al. 2017; Figure 12A,B).
3.3.3. Cranial pneumaticity
Almadasuchus has well‐developed paratympanic pneumaticity which invades the quadrates, otoccipitals, basioccipital, basisphenoid, prootics and laterosphenoids (Figures 9A–H and 10A–E). Such an extension of these pneumatic cavities in the skull is a trait that has been reported in some crocodyliforms and closely related taxa (Busbey and Gow, 1984; Wu and Chatterjee, 1993; Leardi et al. 2017). In particular, the pneumatic cavities of Almadasuchus will be described in three parts: the quadrate inflations (infundibular diverticula, sensu Dufeau and Witmer, 2015), the sinuses on the dorsal region (mastoid and facial antra, and the trigeminal diverticula) and the ventral region (basioccipital, rostral recess and rhomboidal sinus) of the braincase.
One of the more extreme pneumatic features of Almadasuchus is the large cavity within its quadrates (Figures 9D–F, 10A–E and 13A–C). In this taxon, the quadrate is almost completely pneumatized, being internally hollow. This diverticulum even reaches the distal body of the quadrate and communicates with the middle ear cavity through several foramina placed ventromedial to the quadrate's primary head (Figure 4C). A heavily pneumatized condition of the quadrate is known in Macelognathus, where these pneumatic inflations also occupy the entire element along its dorsoventral length (Leardi et al. 2017); however, the pneumatic inflations of the quadrate of Macelognathus differ from those of Almadasuchus, as in the former taxon there are several (five) intercommunicated chambers within this element (Leardi et al. 2017). This contrasts with the more uniform internal cavity of the quadrate present in Almadasuchus (Figures 9E and 12B). However, this could be attributed to differences in their ontogenetic state as the specimen of Macelognathus where the internal structure of the skull is known (LACM 5572/150148) has been regarded as a juvenile due to its strongly convex skull roof (Leardi et al. 2017). More expanded internal air cavities of the ventral part of the braincase are observed in the juveniles of extant crocodiles, although the opposite is seen in the infundibular diverticulum in these taxa (see Figure 13 Dufeau and Witmer, 2015). Thus, ontogenetic causes for this difference in morphology should not be ruled out until more data are available on this sinus in other ontogenetic stages. Heavily pneumatized quadrates are common in Crocodyliformes, especially in their most basal members (P. richardsoni, MCZ 6727; Edentosuchus tianshanensis, IVPP V 3236). Among non‐crocodyliform crocodylomorphs, a hollow quadrate is observed in Dibothrosuchus. In the latter taxon (IVPP V 7907) both quadrates are broken at the level of their dorsal third, and a triangular cavity can be seen in ventral view in both the right and the left elements. Among other early crocodylomorphs the data about quadrate pneumaticity are scarcer, as it is observable from the outside only when the lateral walls of the quadrates are damaged and when the pneumatization occupies large parts of the quadrate. In Junggarsuchus only the left quadrate is well preserved, but it is not exposed internally as it is articulated with the rest of the skull, however, two pneumatic foramina are present in its lateral surface. Walker (1990, p. 80) homologized the posterolateral foramen on the quadrate of Sphenosuchus with the larger one of other crocodylomorphs (Protosuchus). However, the degree of pneumatization of Sphenosuchus within the quadrate seems to be different than the one present in Dibothrosuchus, hallopodids, and crocodyliforms. The right quadrate of Sphenosuchus (SAM‐PK‐K 3014) is preserved separated and it has its proximal end broken, with the primary head missing. In this region part of the internal structure is exposed which is not distinct from other spongy bone of other laminar bones, thus allowing us to infer that the quadrate of Sphenosuchus is not as pneumatized as in the above‐mentioned taxa (at least in its dorsal end of the bone). A similar observation is also applicable to the type specimen of Hesperosuchus (AMNH FR 6758), as the dorsal part of the right quadrate is exposed and lacks an internal cavity.
Figure 13.

Digital reconstruction of the pneumatic diverticula of the skull of Almadasuchus figarii in A, dorsal, B, anterior, and C, left lateral views. D, Detail of the medial pneumatizations of the skull in left lateral view. bor, basioccipital recess (‘sensu lato’); bor ss, basioccipital recess (‘sensu stricto’); con, connection between the basisphenoid pneumatizations and the sub‐basisphenoid recess; d tgr, dorsal trigeminal recess; fa, facial antrum; ifd, infundibular diverticulum; ma, mastoid antrum; rhom, rhomboidal recess; pocr, postcarotid recess; prcr, precarotid recess; ros, rostral recess; sbr, sub‐basisphenoid recess; tgr, trigeminal recess; v tgr, ventral trigeminal recess. Pneumatic diverticula from the different regions of the braincase have been colored differently: blue, dorsal; green, ventral; and, magenta, quadrate diverticula
Medial to the quadrate, the dorsal pneumatization of the skull of Almadasuchus forms isolated diverticula that invade some elements of the braincase (Figures 9D–F and 13A–D). The more posterior and dorsal of these is the mastoid antrum (prootic diverticulum sensu Dufeau and Witmer, 2015; Figure 13A,B,D). The mastoid antrum is one of the more widely distributed pneumatizations of the braincase among crocodylomorphs and is formed by an air cavity that invades, with different degrees of development, the prootic. This structure is present in every crocodylomorph where the prootic is adequately known and exposed (Hesperosuchus, Kayentasuchus, Sphenosuchus, Dibothrosuchus, Junggarsuchus, Almadasuchus, Macelognathus and crocodyliforms such as P. haughtoni), with the exception of thalattosuchians (Herrera et al. 2018). However, and considering the phylogenetic hypothesis of Leardi et al. (2017), it is unclear if this feature represents a synapomorphy of Crocodylomorpha or if it was acquired in a group of crocodylomorphs more closely related to Hesperosuchus than to Terrestrisuchus. This is due to the lack of well‐preserved prootics in the basal most crocodylomorphs, as some taxa have this element partially preserved (e.g. Terrestrisuchus), but its internal anatomy is not exposed. One taxon that might help solve this issue is Pseudhesperosuchus jachaleri, as the only known specimen (PVL 3810) has the braincase partially exposed on its right side. In that specimen, the lateral most part of the otic capsule is damaged, but part of the internal structure of the braincase is exposed. Anterior to the otic capsule, in a position consistent with the prootic, narrow bony struts separated by empty spaces (i.e. air filled cavities) are observed. This can be interpreted as a pneumatized prootic (i.e. presence of a mastoid antrum), making this trait at least a common derived feature of most crocodylomorphs in a classic point of view (small‐bodied crocodylomorphs sensu Zanno et al. 2015). However, the poor preservation of the only known specimen of Pseudhesperosuchus calls for additional caution when considering this feature, and additional information in other basal crocodylomorphs will help clarify this problem.
Anteroventral to the mastoid antrum, Almadasuchus has an additional pneumatization in its prootic (Figures 9E and 13A,D). Although it is seldom mentioned in any contribution dealing with crocodylomorph cranial pneumaticity (e.g. Dufeau and Witmer, 2015), this pneumatization is identified as the facial antrum due to its dorsoventral position with respect to the facial foramen and groove on the prootic (Walker, 1990; Figure 7B,C). This anterior diverticulum to the mastoid antrum has been identified in extant crocodylians and in Sphenosuchus (Walker, 1990). A similar feature could be present in Dibothrosuchus, as the fenestra identified as the exit of the mastoid antrum is divided internally, which could represent the division between the mastoid and the facial antra. A complex pneumatic structure of the prootic is also present in Kayentasuchus, as the mastoid antrum opens to the middle ear cavity through three foramina and the anterior most could represent this isolated anterior pneumatization. However, this claim requires additional internal data (CT data) to confirm this identity. In basal crocodyliforms (e.g. P. haughtoni; Busbey and Gow, 1984) pneumatizations are more anteriorly expanded in the prootic, thus the facial antrum could be present, but more internal data are needed to evaluate if an isolated diverticulum is formed. As with other prootic pneumatizations, the facial antrum is absent in thalattosuchians (Herrera et al. 2018).
Anterior to both prootic diverticula, Almadasuchus bears additional dorsal pneumatizations on its laterosphenoids. These excavations on the laterosphenoid are located dorsal to the foramen ovale and are represented by two different blind cavities (Figures 9C,D and 12A–D). The most dorsal one is larger and more complex, as its anterior end bifurcates attaining a T‐shape due to a dorsal projection (Figure 13D). This dorsal cavity opens to the middle ear space. On the other hand, the ventral pneumatization is rounded, only one third of the size of the dorsal one, and it opens into the adductor chamber. Comparing this condition with other crocodylomorphs is very difficult as the internal structure of the laterosphenoid is known in a very small number of specimens. This feature (trigeminal recess) was considered an autapomorphy of Dibothrosuchus (Wu and Chatterjee, 1993), but other crocodylomorph taxa besides Almadasuchus also display at least some degree of laterosphenoid pneumaticity, such as Kayentasuchus and Macelognathus. The trigeminal recess has not been found in thalattosuchians (Herrera et al. 2018). Dufeau and Witmer (2015) noted that the mastoid antrum (their prootic diverticulum) extended anteriorly in larger specimens of A. mississippiensis invading the laterosphenoid. Later in ontogeny, this connection can be lost and the recess in the laterosphenoid can even be obliterated in large individuals. Considering this, we infer that the trigeminal diverticula represent an anterior expansion of the mastoid antra.
Ventrally, the floor of the braincase of Almadasuchus is pneumatized by an interconnected system of diverticula that excavate the basioccipital and the basisphenoid (Figures 9F,G and 10A–E; 13A–D). The basioccipital bears a large foramen that communicates with the pharynx. This large pneumatic cavity is usually termed the basioccipital recess (e.g. Clark et al. 2000; Leardi et al. 2017). However, internal data reveal that this cavity is divided internally into two regions: an anterior simpler one, and a posterior one that is subdivided in two ascending but short blind tubes (Figure 13D). Such division in the basioccipital pneumatic cavity was noted by Walker (1990) who named the anterior pneumatization the sub‐basisphenoid recess, as it excavates the posteroventral surface of the basisphenoid, and the posterior divided subchamber the basioccipital recess in its strict sense (‘bor ss’), as it excavates only that bone. Following Walker’s (1990) ideas, there has been a general trend (e.g. Clark, 1986; Nesbitt, 2011; Pol et al. 2013) to homologize the whole ventral recess of the basioccipital to the median Eustachian tube, with the sub‐basisphenoid recess being equivalent to the anterior median branch or anterior communicating canal and the basioccipital recess being homologous to the posterior median one or posterior communicating canal. Furthermore, the posterior divisions of the basioccipital recess would represent the right and left branches of the posterior communicating canal, thus implying that non‐crocodyliform crocodylomorphs have a very short initial segment of this canal (Walker, 1990); however given the blind nature of these tubes (see above; Leardi et al. 2017; Figures 9G and 13D), it seems that in these basal forms the Eustachian system did not connect the pharynx with dorsal pneumatic cavities associated with the middle ear cavity. Based on the evidence provided by specimens of several crocodylomorphs with their internal anatomy exposed (Busbey and Gow, 1984; Clark, 1986) and CT data (Dufeau and Witmer, 2015; Brusatte et al. 2016; Pierce et al. 2017; Leardi et al. 2017; Herrera et al. 2018), the connection between the pharynx and the dorsal pneumatizations of the braincase is a common derived feature of crocodyliforms including thalattosuchians among crocodylomorphs.
Anteriorly, the basisphenoid of Almadasuchus is hollow, with this internal cavity reaching even the anterior region of the rostrum of this bone. Such inflations in the body and rostrum of the basisphenoid were previously noted in Sphenosuchus, Dibothrosuchus, Junggarsuchus, Eopneumatosuchus, and in basal crocodyliforms (i.e. P. haughtoni). These have been homologized with the ATR present in crocodiles (Walker, 1990; Wu and Chatterjee, 1993). This system is composed of four diverticula: three recesses on the basisphenoid (precarotid, postcarotid and rostral recesses), and, a prootic recess (Figure 13D). The latter, as its name reflects, is developed in the prootics and is equivalent to the facial antrum of modern crocodylians and other basal crocodylomorphs (see above). The precarotid and postcarotid recesses represent pneumatization of the floor of the braincase (Figure 9D,E), which can extend into the basipterygoid processes (if present), while the rostral recess is an anterior expansion of those diverticula into the rostrum of the basisphenoid (Walker, 1990; Figure 9B,C). In Almadasuchus these recesses can be clearly identified within the basisphenoid, sharing the same condition as Sphenosuchus and P. haughtoni. However, this contrasts with what was reported for Dibothrosuchus in its description by Wu and Chatterjee (1993), where it was proposed that the pre‐ and postcarotid recesses were fused. However, paired cavities dorsal to the hypophyseal fossa were identified as the carotid pillars in an illustrated cross‐section (see Figure 5 in Wu and Chatterjee, 1993). This identification is dubious, as it implies that the internal carotid arteries passed dorsal to the hypophyseal fossa, a pathway not reported in any archosaur (Herrera, pers. comm.; e.g. Lautenschlager and Butler, 2016; Herrera et al. 2018). Furthermore, these cavities strongly resemble the postcarotid recesses of Almadasuchus, and thus such identity is proposed here for these in Dibothrosuchus in this contribution. However, this identification is still tentative as the identification of the carotid passage on internal studies of Dibothrosuchus is still pending. In Almadasuchus these pneumatic spaces within the basisphenoid open laterally through foramina present on the lateral walls of the basisphenoid, as it is also observed in Dibothrosuchus (Figure 9E). Wu and Chatterjee (1993) also homologized these pneumatizations on the basisphenoid to the lateral branches of the anterior communicating canal of the median Eustachian tube based on the idea that ventral pneumatizations of the braincase in Dibothrosuchus opened into the anterior part of the sub‐basisphenoid recess. However, on personal examination (J. M. Leardi, pers. obs.), no connection between the ATR and the sub‐basisphenoid recess could be observed in Dibothrosuchus. Given the preservation of the specimen where these observations were made (IVPP V 7907) internal (CT) data are needed to back this claim.
The internal anatomy of Almadasuchus may help solve this identification, as in this taxon the basisphenoid inflations are connected to the sub‐basisphenoid recess by a common canal on the posteroventral region of the basisphenoid, supporting Wu and Chatterjee’s (1993) homology assignment (Figure 13D). Basisphenoid pneumaticity extending further anteriorly reaching the base of the basisphenoid rostrum, forming a rostral recess (Walker, 1990), is present in other Jurassic basal crocodylomorphs (i.e. Sphenosuchus, Macelognathus) besides Almadasuchus. In Almadasuchus the rostral recess is further extended into the basisphenoid rostrum itself, making this element hollow almost to its anterior end (Figure 9B). This recess has also been mentioned in Dibothrosuchus (Wu and Chatterjee, 1993), however, the base of the basisphenoid recess, which is broken (IVPP V 7907), does not display any significant air chamber. Thus, if such structure is present in Dibothrosuchus it does not reach the anterior portion of the cultriform process. Walker (1990) noted the lack of any pneumatizations in this region of the basisphenoid of extant crocodylians, and thus considered this structure a unique ‘sphenosuchian’ feature and not homologous with any cavity of crocodylians, a notion followed in this study also. It is also worth noting that, to date, there have not been any reports of basisphenoid pneumaticity extending to the base of the cultriform process (i.e. the presence of a rostral recess) in crocodyliforms.
Almadasuchus has a distinct sinus located at the posteroventral end of the tympanic cavity (Figures 9G and 13A–C). This diverticulum is limited by the anteroventral surface of the paroccipital processes of the otoccipital and a flange of bone between the anterolateral process and the subcapsular buttress (Figures 5, 7A and 10D,E). In this contribution, we have identified this sinus as the rhomboidal recess (see above), as it is consistent with its position and the elements limiting it. A rhomboidal sinus is present as a ventral expansion of the tympanic cavity in modern crocodylians (Owen, 1850; Colbert, 1946b) and has also been identified in the non‐crocodyliform crocodylomorph Macelognathus (Leardi et al. 2017), Eopneumatosuchus (Crompton and Smith, 1980), and Pelagosaurus (Walker, 1990; not reconstructed by Pierce et al. 2017, who also did not to reconstruct the lateral Eustachian tubes present in this taxon (Herrera et al. 2018). However, the presence of this feature in Eopneumatosuchus should be handled with care as several parts of the braincase were reconstructed in its original description (Clark, 1986). The recognition of a rhomboidal sinus in other basal crocodylomorphs is heavily biased as it depends on the access of internal morphology, which can be observed via CT data or fortuitous access due to breakage (e.g. Sphenosuchus). Thus, the presence of this recess is difficult to evaluate, but it seems to be a derived condition within Crocodylomorpha as no such structure has been identified in the well‐known taxon Sphenosuchus (Walker, 1990).
The final recess to discuss is the PTR. This recess is developed in the anterior surface of the paroccipital processes of the opistotics/otoccipitals and is present in birds, where it is connected with another recess (superior tympanic recess), but absent in extant crocodylians (Walker, 1990). As with other recesses, it is difficult to evaluate its presence in taxa where the internal anatomy is not known. The PTR is present in many crocodylomorphs (Sphenosuchus + more derived crocodylomorphs, sensu Leardi et al. 2017), including basal crocodyliforms such P. haughtoni (see Figure 8 in Busbey and Gow, 1984), although its shape and degree of development varies. Almadasuchus displays a concavity along the anterior surface of the paroccipital processes with its major axis oriented mediolaterally. The PTR in Almadasuchus also forms a slight depression on the lateral aspect of the otoccipital (Figure 7A,B), just posterior to the metotic foramen. A similar morphology is also observed in Sphenosuchus and might also be present in Junggarsuchus (J. M. Clark, pers. obs.). The condition present in these crocodylomorphs contrasts with the one observed in Dibothrosuchus, Macelognathus and the basal crocodyliform P. haughtoni, where the PTR penetrates into the otoccipital and forms a deep cavity bounded mostly by the otoccipital and limited anteriorly by the prootic (see Wu and Chatterjee, 1993; Leardi et al. 2017).
4. DISCUSSION
The excellent preservation of the type specimen of Almadasuchus and its phylogenetic position among basal crocodylomorphs allows us to evaluate the condition of several characters among these taxa. This is highlighted by the fact that Almadasuchus is the first non‐crocodyliform crocodylomorph where the internal anatomy is reconstructed using CT data. In particular, in recent years there has been a renewed discussion about the phylogenetic position of Thalattosuchia (e.g. Wilberg, 2015), with one of the topologies considered for this large group as the sister group of Protosuchidae and other crocodyliforms (i.e. the sister‐group of Crocodyliformes). Thus, knowing the character state in the taxa most closely related to crocodyliforms will facilitate an assessment of the changes that occurred in the origins of Crocodyliformes and to evaluate the condition of other taxa/lineages in that light. Some of the topics have been discussed in previous recent contributions; in particular those regarding the changes in cranial kinesis among non‐crocodyliform crocodylomorphs (Pol et al. 2013; Leardi et al. 2017). Thus, for further detail on that matter the reader is referred to that bibliographic material.
The following discussion will be mostly focused on the results of the cranial endocasts and the pneumatic cavities.
4.1. Brain endocast
The general structure of the brain of fossil pseudosuchians is fairly well documented as several brain endocasts have been published, either using manual techniques (e.g. Hopson, 1979) or CT data (e.g. Lautenschlager and Butler, 2016; Herrera et al. 2018). However, the condition among basal crocodylomorphs is not well represented, as the only reported endocast is a partial reconstruction of Sphenosuchus that only comprises the posterior part of it (Walker, 1990). This absence of data is also highlighted by the fact that currently no non‐crocodylomorph paracrocodylomorph endocast is known, not allowing assessing the basal condition for the brain of the clade.
As in most basal pseudosuchians, the brain has an elongated architecture (Hopson, 1979; Lautenschlager and Butler, 2016). However, crocodylomorphs have a dorsoventrally lower endocast in lateral view when compared with other basal pseudosuchians (Hopson, 1979; Lautenschlager and Butler, 2016). This is produced by the general ventral displacement of the cerebellum with respect to the cerebrum in basal pseudosuchians, resulting in considerably lower values for the cephalic flexure angle (i.e. Pierce et al. 2017). Thus, considering the data available at hand, the straight endocast is a derived feature of crocodylomorphs, while the thalattosuchians exacerbate this feature even more. Almadasuchus displays a rather short olfactory tract (see above), contrasting with the elongated tracts of phytosaurs (Lautenschlager and Butler, 2016), thalattosuchians (Herrera et al, 2018) and modern crocodylians (Witmer et al. ; Witmer et al. ; Pierce et al. 2017). Thus, at least in pseudosuchians, this feature in the olfactory tract is highly dependent on the elongation of the snout, as displayed by the short olfactory tracts of short‐snouted taxa (e.g. Simosuchus, Kley et al. 2010).
The cerebral hemispheres in phytosaurs (Lautenschlager and Butler, 2016) are almost anteroposteriorly symmetrical in dorsal view, a condition also present in Almadasuchus and thalattosuchians (Pierce et al. 2017; Herrera et al. 2018), but contrasting with the asymmetrical profile of the cerebral hemispheres of mesoeucrocodylians (e.g. Colbert, 1946b; Witmer et al. 2008; Kley et al. 2010). Unfortunately no endocast is known for any non‐mesoeucrocodylian crocodyliform, thus we are not able to propose when this change in the shape of the cerebral hemisphere might have happened in the phylogeny of Crocodylomorpha. The pituitary gland projects ventral to the cerebrum, and the general aspect in crocodylomorphs contrasts with the one in other pseudosuchians. In the latter, the pituitary is anteroposteriorly short but markedly projected ventrally, having a dorsoventral development comparable with one of the cerebral hemispheres (see Figure 9 in Hopson, 1979; see Figures 2 and 3 in Lautenschlager and Butler, 2016). In contrast, in crocodyliforms (S. icaeorhinus, Simosuchus, Alligator misssissippiensis, Gavialis gangeticus, Pelagosaurus, S. bollensis, C. araucanensis) the pituitary projects posteriorly. Thalattosuchians further modify this pattern in having an anteroposteriorly elongated pituitary gland (see Herrera et al. 2018), as it has a greater development in its anteroposterior axis than on its dorsoventral one. Almadasuchus has a pituitary gland that shares a similar morphology to the one described for thalattosuchians.
Finally, as mentioned above (see Endocast), Almadasuchus has a very abrupt posterior step between the cerebellum and the medulla oblongata. A similar morphology is present in Sphenosuchus (see Figure 46 in Walker, 1990) and could represent a feature more widely distributed among basal crocodylomorphs. This feature is attenuated in crocodyliforms and it is absent in thalattosuchians (e.g. S. bollensis, C. araucanensis), contributing to the marked tubular aspect of the endocast of this clade (Pierce et al. 2017; Herrera et al. 2018).
4.2. Cranial pneumaticity
In order to properly account for the changes in pneumaticity among crocodylomorphs, a brief comment on non‐crocodylomorph pseudosuchians is necessary. In a review of the general braincase anatomy of the clade, Gower (2002) concluded that crocodylomorphs are the only pseudosuchians in which the bony elements surrounding the middle ear cavity are pneumatized. The only elements which have evidence of braincase pneumaticity in non‐crocodylomorph pseudosuchians are those restricted to its posteroventral region, in particular the basioccipital and basisphenoid (Gower & Nesbitt, 2006; Nesbitt, 2007; Nesbitt, 2011; Figure 14). Several members of Pseudosuchia [Stagonolepis (Walker, 1964), Riojasuchus (PVL 3827), Effigia (AMNH FR 30587), Xilosuchus (IVPP V 6026)] have these two bones with a ventral excavation: the basioccipital has a recess on the anterior part of its ventral surface; and the basisphenoid has a relatively deep but blind pit located just anteriorly to the basioccipital tubera. These have been referred as the basioccipital and parabasisphenoid recess, respectively (Gower, 2000; Nesbitt, 2011). However, these ventral recesses have different relationships and placement than the ones observed in crocodylomorphs. The ‘basioccipital recess’ is present in basal suchians (aetosaurs like Stagonolepis and ornithosuchids like Riojasuchus) and poposauroids (Xilosuchus; Arizonasaurus [Gower and Nesbitt, 2006]), while it is absent in paracrocodylomorphs closer to crocodylomorphs [e.g. Saurosuchus gallei (Alcober, 2000); Postosuchus kirkpatricki (Weinbaum, 2011)]. When present, this recess is posterior to the basioccipital tubera and separated by a crest from the parabasisphenoid recess (Gower & Nesbitt, 2006; Nesbitt, 2007). Thus, it is probably not homologous with the basioccipital recess of crocodylomorphs (which is anterior to the basioccipital tubera) and the usage of the term should be restricted to the one in Crocodylomorpha. On the other hand, the parabasisphenoid recess of these pseudosuchians shares a similar relative position with the basioccipital and sub‐basisphenoid recesses of crocodylomorphs. Again, there is a difference between the condition observed in non‐loricatan pseudosuchians and loricatans, as in the latter the parabasisphenoid recess is significantly deeper (Nesbitt, 2011).
Figure 14.
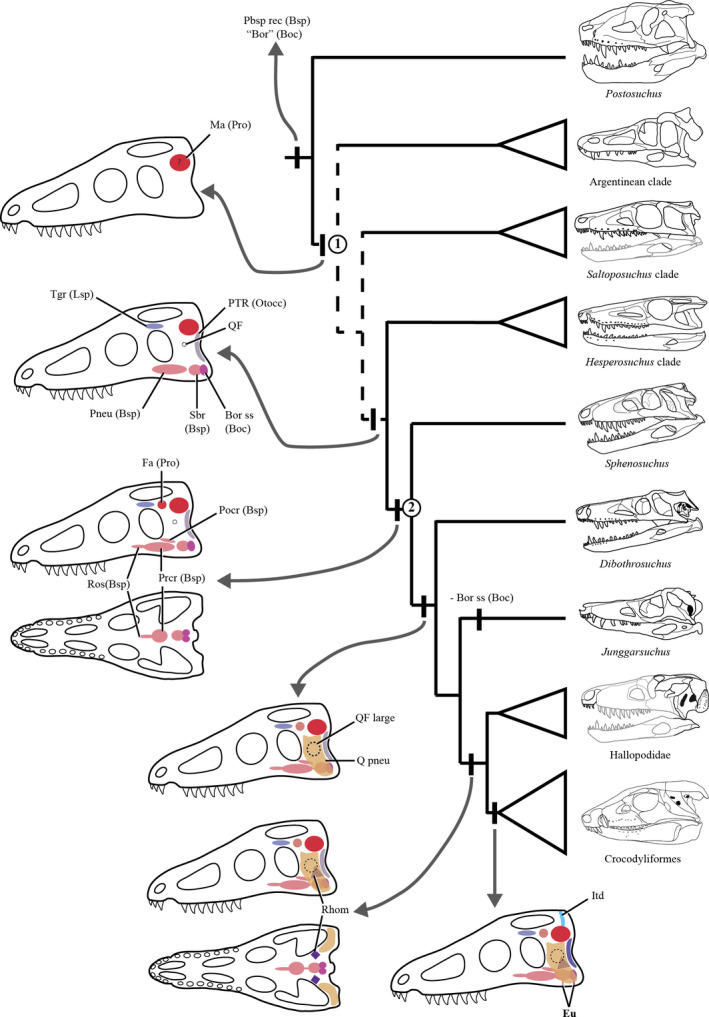
Cladogram based on the hypothesis of Leardi et al. (2017) of non‐crocodyliform crocodylomorphs illustrating the major transitions in the pneumatic diverticula during among basal members of Crocodylomorpha. Thalattosuchians are depicted in two phylogenetical positions: as the sister group of Crocodyliformes; or, as crocodyliforms. The captions indicate the pneumatic diverticulum and between parenthesis specifies the bone that is pneumatized. Dotted line represents the ambiguous optimization of the pneumatic features in basal forms due to the lack of information (see text). Nodes: (1) Crocodylomorpha; (2) ‘advanced crocodylomorphs’. Boc, basioccipital; ‘Bor’, basioccipital recess (sensu lato); Bor (ss), basioccipital recess (strict sense); Bsp, basisphenoid; Eu, eustachian tubes; Fa, facial antrum; Itd, intertympanic diverticulum; Ma, mastoid antrum; Pbsp rec, parabasisphenoid recess; Pneu, pneumatized condition; Otoc, otoccipital; Pcr, precarotid recess; pocr, postcarotid recess; Pro, protic; PTR, posterior tympanic recess; Q, quadrate; QF, quadrate foramen; Ros, rostral recess; Rhom, rhomboidal recess; sbr, sub‐basisphenoidal recess; Soc, supraoccipital; Tgr, trigeminal recess. Question mark (?) indicates the accelerated transformations (ACCTRAN) in the case of ambiguous optimizations due to missing data (see main text). Sample taxa modified from Chatterjee (1985); Clark (1986); Clark et al. (2000, 2004); Nesbitt (2011); Pol et al. (2013); Sereno and Wild (1992); Walker (1990); Wu and Chatterjee (1993)
Among the most basal crocodylomorphs, the detailed internal structure of the basioccipital recess is unknown, as it is either not preserved or covered by matrix. The sole exception is Hesperosuchus (CM 29894) where the recess is exposed partially and is very wide in ventral view, displaying two internal elliptical depressions suggesting an internal division, as in Jurassic crocodylomorphs (Sphenosuchus + more derived taxa, sensu Leardi et al. 2017). As was described above, in the basal crocodylomorphs Sphenosuchus, Dibothrosuchus, Macelognathus, and Almadasuchus the basioccipital recess is subdivided into the posterior basioccipital recess (in the strict sense) which divides in two blind cavities, and an anterior, single sub‐basisphenoid recess (Figure 14). As a whole, this excavation is homologous to the medial Eustachian foramen (Walker, 1990), with the basioccipital recess homologous to the posterior communicating canal and the sub‐basisphenoid recess homologous to the anterior communicating canal. Almadasuchus further illustrates this division, displaying a connection between the sub‐basisphenoid recess and the basisphenoid pneumatic diverticula (see above). However, in no ‘sphenosuchian’ is there direct evidence of a connection with the middle ear cavity, as these recesses are blind. On the other hand, Junggarsuchus (IVPP V 14010) displays an autapomorphic condition among basal crocodylomorphs, as the basioccipital recess is absent.
Basisphenoid pneumaticity can form a recess, which was discussed above, or else penetrate into the bone forming internal hollow cavities. The development of such internal cavities is present in crocodylomorphs such as Sphenosuchus, Dibothrosuchus, Macelognathus, Junggarsuchus, Almadasuchus, and crocodyliforms (Figure 14, node 2). In the rest of the crocodylomorphs this trait cannot be evaluated as it is not preserved or the internal anatomy cannot be accessed. A hollow internal cavity on the anteroventral region of the basisphenoid was reported and illustrated in Kayentasuchus (Clark and Sues, 2002), however, it was damaged and lost in the holotype specimen (UCMP 131830). Thus, the absence of basisphenoid pneumatizations in more basal forms is still unknown and more internal anatomy studies are necessary to evaluate if this is a common feature of Crocodylomorpha or a subgroup of this clade. It was previously considered that the basisphenoid body was expanded when it was invaded by pneumatizations (Clark et al. 2004), however, taxa that do not have the basisphenoid expanded (Sphenosuchus and Dibothrosuchus) can display extensive air cavities within the bone.
A similar lack of information pertains to the presence of a mastoid antrum in basal Crocodylomorpha, as it has been identified in several forms (e.g. Kayentasuchus, Dibothrosuchus, Junggarsuchus, Macelognathus), but in the basal most members of the lineage its presence is equivocal (Pseudhesperosuchus) or the prootic is not preserved (see above). The putative presence of this feature in basal members of the lineage implies that the presence of prootic pneumatizations could be a synapomorphy for all crocodylomorphs, at least in a traditional view (i.e. ‘small‐bodied crocodylomorphs’ sensu Zanno et al. 2015). A similar argument can be made for the presence of the facial antrum and the trigeminal recess. The former has been observed in Sphenosuchus and Almadasuchus, and could be present in other basal crocodylomorph [Dibothrosuchus, Kayentasuchus (see above)] and crocodyliform taxa (e.g. P. haughtoni). The trigeminal recess has been reported in Dibothrosuchus, hallopodids (Macelognathus and Almadasuchus) and in Kayentasuchus [reported as pneumatization within the laterosphenoids (Clark and Sues, 2002; Figure 14)]. The presence of both in modern crocodylians (Walker, 1990 for the facial antrum; Dufeau and Witmer, 2015 for the trigeminal diverticulum) could imply that this pneumatization also represents a crocodylomorph synapomorphy; however, better preserved braincases of basal members of the lineage will help solve this matter.
Unlike other ‘sphenosuchians’, hallopodids (Almadasuchus, Macelognathus) share a derived condition with crocodyliforms, the presence of a rhomboidal sinus (i.e. an expansion at the base of the middle ear cavity). This feature is absent in other crocodylomorphs close to crocodyliforms (e.g. Dibothrosuchus, Junggarsuchus). However, the rhomboidal sinus of non‐crocodyliform crocodylomorphs is not coupled with the extension of the pharyngotympanic system so that it opens into this sinus, which is restricted to crocodyliforms and thalattosuchians.
Finally, the quadrate pneumatizations are better represented in fossil taxa, as most of the taxa preserve at least partial remains of the quadrates, allowing better understanding of the changes in this structure. Unlike other pseudosuchians (e.g. Stagonolepis, Batrachotomus, Postosuchus), most crocodylomorphs have some evidence of quadrate pneumatization due to the presence of a pneumatic foramen that pierces the body of the quadrate. The exceptions to this are Pseudhesperosuchus (Clark et al. 2000), and Saltoposuchus (Clark et al. 2004), while in other basal forms the lateral surface of the quadrate is not preserved (Trialestes, Litargosuchus). However, most taxa that display this pneumatic fenestra within the quadrate do not have an internal expanded diverticulum, as breakage of this element shows no significant air cavity within it (e.g. Kayentasuchus, Hesperosuchus, Sphenosuchus). In contrast, Dibothrosuchus, Junggarsuchus, Macelognathus, Almadasuchus and crocodyliforms have a highly pneumatic quadrate, where the body of this bone is internally hollow (Figure 14). In these taxa, the increased pneumatic nature of the quadrate is coupled either with enlargement (e.g. Almadasuchus) or an increased number of quadrate fenestrae (e.g. Junggarsuchus, crocodyliforms; Leardi et al. 2017).
4.2.1. The thalattosuchian condition
The pneumatic features on the skull of thalattosuchians have been one of the most common features cited to support their non‐crocodyliform crocodylomorph affinities (e.g. Wilberg, 2015). The main trait mentioned is the lack of a communication between both middle ear cavities through the supraoccipital, a feature recognized even before the ubiquitous use of CT in studies of the group (Clark, 1986). However, the original observations mentioned the lack of communication between both mastoid antra, an affirmation that is now known to be equivocal. Recent studies (Herrera et al. 2018) have revealed that thalattosuchians lack prootic pneumatizations (i.e. facial and mastoid antra), a feature widely distributed among crocodylomorphs that may even be a synapomorphy for the whole clade (see above). Thalattosuchians also lack other derived crocodylomorph pneumatic features, as their quadrates are internally massive and do not display any quadrate pneumatic fenestra. Thus, the clade suffered a major transformation in the diverticular expansions that invaded the braincase and the quadrates, and when any transformation (in particular lack of any diverticula) is invoked as supporting any phylogenetic position, it should be based on a wide comparison with other crocodyliforms and crocodylomorphs.
The different phylogenetic positions proposed for Thalattosuchia imply different pneumatic transformations on their skulls. As has been mentioned before (e.g. Clark, 1986, 1994), the crocodyliform hypothesis for Thalattosuchia requires the loss of a pneumatic supraoccipital. However, the lack of communicating canal between both middle ears is not surprising when we consider the absence of other dorsal pneumatic diverticula in the dorsal region of the braincase. However, the hypothesis placing Thalattosuchia outside Crocodyliformes requires further pneumatic transformations on the braincase of thalattosuchians, as this clade also lacks the ventral diverticula within the basisphenoid (rostral, pre‐ and postcarotid recesses) which are now widely recorded among crocodyliforms and their closest relatives among the successive sister groups of Crocodyliformes (see above). If each of these diverticula is considered as independent characters, the non‐crocodyliform hypothesis for Thalattosuchia is in fact less parsimonious than the crocodyliform one. When thalattosuchians are positioned as the sister group of Crocodyliformes it implies the loss of three pneumatic diverticula observed in other non‐crocodyliform crocodylomorphs: pre‐ and postcarotid recesses, and the anteriormost extension of the pneumaticity on the basisphenoid (rostral recess). The former two (pre and postcarotid recesses) have been identified in basal crocodyliforms while the absence of the rostral recess could represent a shared feature with crocodyliforms, as it is absent in these taxa (see Cranial pneumaticty). On the other hand, the placement of Thalattosuchia within Crocodyliformes only implies the loss of the intertympanic diverticulum. However, this absence is not unexpected as this diverticulum communicates the mastoid antra of both sides of the skull, a feature also absent in thalattosuchians.
In conclusion, the present analysis of the braincase anatomy of A. figarii reveals a series of characters that have been observed in crocodylomorphs closely related to crocodyliforms (Pol et al, 2013; Leardi et al. 2017). Although some differences (e.g. the posterolateral sutures of the basioccipital with the otoccipitals) were noted from those originally described (Pol et al. 2013), the main novelty of this contribution is the data of the internal anatomy of Almadasuchus. The CT data allowed us to evaluate numerous osteological structures of the braincase of this taxon, and our study represents the first time the brain, inner ear and pneumatic cavities of a non‐crocodyliform crocodylomorph have been digitally reconstructed. Almadasuchus displays many traits that have been previously recognized in a group of Jurassic crocodylomorphs known as Hallopodidae, such as the presence of a cranioquadrate passage, a laterosphenoid‐quadrate contact, a vagus foramen, a posteriorly closed otic notch, and a wide supraoccipital in posterior view, among others (Leardi et al. 2017). The study of the anatomy of Almadasuchus also revealed features that were thought to be apomorphies of other taxa (e.g. the posterior groove on the squamosal shared with Junggarsuchus), expanding their distribution among basal crocodylomorphs.
The brain of Almadasuchus represents the first case where we can compare this structure with those of crocodyliforms. The endocast of Almadasuchus is straight in lateral view and has an elongated and posteriorly projected hypophysis, conditions thought to be restricted to thalattosuchians (Pierce et al. 2017; Herrera et al. 2018). However, unlike the later, Almadasuchus has a strongly projected floccular recess and a posterior abrupt step on the cerebellum, a feature also present in other non‐crocodyliform crocodylomorphs (i.e. Sphenosuchus acutus). These features could represent the basal condition for crocodyliforms, but the lack of knowledge of both non‐crocodyliform crocodylomorphs and basal crocodyliforms precludes this analysis.
The specimen of Almadasuchus provides a unique chance to study the pneumatic recesses of the skull of this taxon and to evaluate them within the context of basal crocodylomorphs. Almadasuchus has a highly pneumatic braincase, displaying several air‐filled chambers within the quadrates, prootics, laterosphenoids, otoccipitals, basisphenoid and basioccipital. The infundibular pneumatizations invade the quadrates, fully pneumatizing the element, which represents a derived feature within crocodylomorphs. The ventral pneumatizations of the braincase are well‐developed in Almadasuchus and, unlike other crocodylomorphs known to date, are interconnected. However, as in all non‐crocodyliform taxa, the Eustachian system is not composed of three discrete exits and does not connect with the middle ear cavity. However, unlike more basal taxa (e.g. Sphenosuchus), Almadasuchus shares with Macelognathus and crocodyliforms the presence of a rhomboidal sinus. Finally, the dorsal elements of the braincase are also pneumatic displaying facial, mastoid antra and a divided trigeminal recess.
Finally, this study is in line with other contributions in the past (Leardi et al. 2017), which aim to expand the knowledge of the internal structures in basal crocodylomorphs. Future studies in non‐crocodyliform crocodylomorphs and basal crocodyliforms (i.e. non‐mesoeucrocodylians) are still needed to fully understand the early cranial modifications of the lineage.
ACKNOWLEDGEMENTS
We would like to thank S. Goldberg, M. Hill and H. Towbin from the Microscopy and Imaging Facility of the American Museum of Natural History for their valuable help during the CT process and M. Norell for arranging for us to use it. We would like to thank an anonymous reviewer and the editor (E. Fenton), whose comments greatly improved the final quality of this manuscript. M. Carrizo (MACN) is thanked for his help in Figure 14. This research was made possible by the grants PICT 2013‐2725 and 2016‐0267 (both to JML) and NSF grant EAR 1636753 (to JMC). This is J.M.L.'s R296 contribution to the Instituto de Estudios Andinos Don Pablo Groeber.
Leardi JM, Pol D, Clark JM. Braincase anatomy of Almadasuchus figarii (Archosauria, Crocodylomorpha) and a review of the cranial pneumaticity in the origins of Crocodylomorpha. J. Anat. 2020;237:48–73. 10.1111/joa.13171
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alcober, O. (2000) Redescription of the skull of Saurosuchus galilei (Archosauria: Rauisuchidae). Journal of Vertebrate Paleontology, 20, 302–316. [Google Scholar]
- Allen, R.C. (2010) The anatomy and systematic of Terrestrisuchus gracilis (Archosauria, Crocodylomorpha). PhD Thesis, Northern Illinois University, DeKalb. [Google Scholar]
- Barrios, F. , Bona, P. , Paulina‐Carabajal, A. and Gasparini, Z. (2018) Re‐description of the cranio‐mandibular anatomy of Notosuchus terrestris (Crocodyliformes, Mesoeucrocodylia) from the Upper Cretaceous of Patagonia. Cretaceous Research, 83, 3–39. [Google Scholar]
- Bonaparte, J.F. (1972) Los tetrápodos del sector superior de la Formación Los Colorados, La Rioja, Argentina (Triásico Superior). 1 Parte . Opera Lilloana, 22, 1–183. [Google Scholar]
- Brusatte, S.L. , Muir, A. , Young, M.T. , Walsh, S. , Steel, L. and Witmer, L.M. (2016) The braincase and neurosensory anatomy of an Early Jurassic marine crocodylomorph: implications for crocodilian sinus evolution and sensory transitions. The Anatomical Record, 299, 1511–1530. [DOI] [PubMed] [Google Scholar]
- Buckley, G.A. , Brochu, C.A. , Krause, D.W. and Pol, D. (2000) A pug‐nosed crocodyliform from the Late Cretaceous of Madagascar. Nature, 405, 941–944. [DOI] [PubMed] [Google Scholar]
- Busbey, A.B. III and Gow, C. (1984) A new protosuchian crocodile from the Upper Triassic Elliot Formation of South Africa. Palaeontologia Africana, 25, 127–149. [Google Scholar]
- Carvalho, I.S. , Campos, A.C.A. and Nobre, P.H. (2005) Baurusuchus salgadoensis, a new Crocodylomorpha from the Bauru Basin (Cretaceous), Brazil. Gondwana Research, 8, 11–30. [Google Scholar]
- Chatterjee, S. (1985) Postosuchus, a new Thecodontian reptile from the Triassic of Texas and the origin of Tyrannosaurs. Philosophical Transactions of the Royal Society of London B, 309, 395–460. [Google Scholar]
- Clark, J.M. (1986) Phylogenetic relationships of the crocodylomorph archosaurs. PhD Thesis, University of Chicago, Chicago. [Google Scholar]
- Clark, J.M. (1994) Patterns of evolution in Mesozoic Crocodyliformes In: Fraser N.C. and Sues H.‐D. (Eds.) In the Shadow of the Dinosaurs, Early Mesozoic Tetrapods. Cambridge: Cambridge University Press, pp. 84–97. [Google Scholar]
- Clark, J.M. (2011) A new shartegosuchid crocodyliform from the Upper Jurassic Morrison Formation of western Colorado. Zoological Journal of the Linnean Society, 163, S152–S172. [Google Scholar]
- Clark, J.M. , Sues, H.‐D. and Berman, D.S. (2000) A new specimen of Hesperosuchus agilis from the Upper Triassic of New Mexico and the interrelationships of basal crocodylomorph archosaurs. Journal of Vertebrate Paleontology, 20, 683–704. [Google Scholar]
- Clark, J.M. and Sues, H.‐D. (2002) Two new basal crocodylomorph archosaurs from the Lower Jurassic and the monophyly of the Sphenosuchia. Zoological Journal of the Linnean Society, 136, 77–95. [Google Scholar]
- Clark, J.M. , Xu, X. , Forster, C.A. , Wang, Y. (2004) A Middle Jurassic “sphenosuchian” from China and the origin of the crocodilian skull. Nature, 430, 1021–1024. [DOI] [PubMed] [Google Scholar]
- Colbert, E.H. (1946a) The Eustachian tubes in crocodiles. Copeia, 1946, 12–14. [Google Scholar]
- Colbert, E.H. (1946b) Sebecus, representative of a peculiar suborder of fossil Crocodylia from Patagonia. Bulletin of the American Museum of Natural History, 87, 2017–2270. [Google Scholar]
- Colbert, E.H. (1952) A pseudosuchian reptile from Arizona. Bulletin of the American Museum of Natural History, 99, 561–592. [Google Scholar]
- Colbert, E.H. and Mook, C.G. (1951) The ancestral crocodilian Protosuchus . Bulletin of the American Museum of Natural History, 97, 143–182. [Google Scholar]
- Crompton, A.W. and Smith, K.K. (1980) A new genus and species of crocodilian from the Kayenta Formation (Late Triassic?) of Northern Arizona In: Jacobs L.L. (Ed.) Aspects of Vertebrate History: Essays in Honor of Edwin Harris Colbert. Flagstaff, UK: Museum of Northern Arizona Press, pp. 193–217. [Google Scholar]
- Crush, P.J. (1984) A late Upper Triassic sphenosuchid crocodilian from Wales. Palaeontology, 27, 131–157. [Google Scholar]
- Cúneo, N.R. , Ramezani, J. , Scasso, R. , Pol, D. , Escapa, I. , Zavattieri, A.M. and Bowring, S.A. (2013) High‐precision U‐Pb geochronology and a new chronostratigraphy for the Cañadón Asfalto Basin, Chubut, Central Patagonia: implications for terrestrial faunal and floral evolution in the Jurassic. Gondwana Research, 24, 1267–1275. [Google Scholar]
- Dufeau, D.L. and Witmer, L.M. (2015) Ontogeny of the middle‐ear air‐sinus system in Alligator mississippiensis (Archosauria: Crocodylia). PLoS One, 10, e0137060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, G.M. , Gignac, P.M. , Steppan, S.J. , Lappin, A.K. , Vliet, K.A. , Brueggen, J.D. et al (2012) Insights into the ecology and evolutionary success of crocodilians revealed through bite‐force and tooth‐pressure experimentation. PLoS ONE, 7, e31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow, C.E. (2000) The skull of Protosuchus haughtoni, an Early Jurassic crocodyliform from Southern Africa. Journal of Vertebrate Paleontology, 20, 49–56. [Google Scholar]
- Gower, D.J. (2000) Rauisuchian archosaurs (Reptilia, Diapsida): An overview. Neues Jahrbuch für Geologie und Paläeontologie Abhandlungen, 218, 447–488. [Google Scholar]
- Gower, D.J. (2002) Braincase evolution in suchian archosaurs (Reptilia: Diapsida): evidence from the rauisuchian Batrachotomus kupferzellensis. Zoological Journal of the Linnean Society, 136, 49–76. [Google Scholar]
- Gower, D.J. and Nesbitt, S.J. (2006). The braincase of Arizonasaurus babbitti–further evidence for the non‐monophyly of "rauisuchian" archosaurs. Journal of Vertebrate Paleontology, 26, 79–87. [Google Scholar]
- Gower, D.J. and Walker, A.D. (2002) New data on the braincase of the aetosaurian archosaur (Reptilia: Diapsida) Stagonolepis robertsoni Agassiz. Zoological Journal of the Linnean Society, 136, 7–23. [Google Scholar]
- Herrera, Y. , Leardi, J.M. and Fernández, M.S. (2018) Braincase and endocranial anatomy of two thalattosuchian crocodylomorphs and their relevance in understanding their adaptations to the marine environment. PeerJ, 6, e5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday, C.M. and Witmer, L.M. (2009) The epipterygoid of crocodyliforms and its significance for the evolution of the orbitotemporal region of eusuchians. Journal of Vertebrate Paleontology, 29, 715–733. [Google Scholar]
- Holliday, C.M. , Tsai, H.P. , Skiljan, R.J. , George, I.D. and Pathan, S. (2013) A 3D interactive mode and atlas of the jaw musculature of Alligator mississippiensis . PLoS One, 8, e62808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopson, J.A. (1979) Paleoneurology In: Gans C., Northcutt R.C. and Ulinski P. (Eds.) Biology of the Reptilia. New York: Academic Press, pp. 39–146. [Google Scholar]
- Iordansky, N.N. (1973) The skull of Crocodylia In: Gans C. and Parsons T.S. (Eds.) Biology of Reptilia, vol 4, New York: Academic Press, pp. 201–264. [Google Scholar]
- Iordansky, N.N. (2010) Pterygoideus muscles and other jaw adductors in amphibians and reptiles. Biology Bulletin, 37, 905–914. [Google Scholar]
- Kley, N.J. , Sertich, J.W. , Turner, A.H. , Krause, D.W. , O'Connor, P.M. and Georgi, J.A. (2010) Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Society of Vertebrate Paleontology Memoir, 10, 13–98. [Google Scholar]
- Langston, W.J. (1973) The crocodylian skull in historical perspective In: Gans C. and Parsons T.S. (Eds.) Biology of Reptilia, Vol. 4, pp. 263–289. New York: Academic Press. [Google Scholar]
- Lautenschlager, S. and Butler, R.J. (2016) Neural and endocranial anatomy of Triassic phytosaurian reptiles and convergence with fossil and modern crocodylians. PeerJ, 4, e2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leardi, J.M. , Pol, D. and Clark, J.M. (2017) Detailed anatomy of the braincase of Macelognathus vagans Marsh, 1884 (Archosauria, Crocodylomorpha) using high resolution tomography and new insights on basal crocodylomorph phylogeny. PeerJ, 5, e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuona, A. , Ezcurra, M.D. and Irmis, R.B. (2016) Revision of the early crocodylomorph Trialestes romeri (Archosauria, Suchia) from the lower Upper Triassic of Ischigualasto Formation of Argentina: one of the oldest‐known crocodylomorphs. Papers in Palaeontology, 2, 585–622. [Google Scholar]
- López‐Arbarello, A. , Rauhut, O.W.M. and Moser, K. (2008) Jurassic fishes of Gondwana. Revista de la Asociación Geológica Argentina, 63, 586–612. [Google Scholar]
- Montefeltro, F.C. , Andrade, D.V. and Larsson, H.C. (2016) The evolution of the meatal chamber in crocodyliforms. Journal of Anatomy, 228, 838–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, D.S. (1975) The morphology and relationships of a crocodilian, Orthosuchus stormbergi, from the Upper Triassic of Lesotho. Annals of the South African Museum, 67, 227–329. [Google Scholar]
- Nesbitt, S.J. (2007) The anatomy of Effigia okeeffeae (Archosauria, Suchia), theropod‐like convergence, and the distribution of related taxa. Bulletin of the American Museum of Natural History, 302, 84. [Google Scholar]
- Nesbitt, S.J. (2011) The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History, 352, 1–292. [Google Scholar]
- Owen, R. (1850) On the communications between the cavity of the tympanum and the palate in the Crocodilia (gavials, alligators and Crocodiles). Philosphical Transactions of the Royal Society of London, 140, 521–527. [Google Scholar]
- Pierce, S.E. and Benton, M.J. (2006) Pelagosaurus typus Bronn, 1841 (Mesoeucrocodylia: Thalattosuchia) from the Upper Lias (Toarcian, Lower Jurassic) of Somerset, England. Journal of Vertebrate Paleontology, 26, 621–635. [Google Scholar]
- Pierce, S.E. , Williams, M. and Benson, R.B.J. (2017) Virtual reconstruction of the brain and sinuses of the early Jurassic marine crocodylomorph Pelagosaurus typus (Thalattosuchia). PeerJ, 5, e3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, D. , Rauhut, O.W.M. , Lecuona, A. , et al. (2013) A new fossil from the Jurassic of Patagonia reveals the early basicranial evolution and the origins of Crocodyliformes. Biological Reviews, 88, 862–872. [DOI] [PubMed] [Google Scholar]
- Porter, W.R. , Sedlmayr, J.C. and Witmer, L.M. (2016) Vascular patterns in the heads of crocodilians: blood vessels and sites of thermal exchange. Journal of Anatomy, 229, 800–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig, O.A. (1963) La presencia de dinosaurios saurisquios en los “estratos de Ischigualasto” (Mesotriásico Superior) de las provincias de San Juan y La Rioja (República Argentina). Ameghiniana, 3, 3–20. [Google Scholar]
- Ristevski, J. , Young, M.T. , Andrade, M.B. and Hastings, A.K. (2018) A new species of Antephthalmosuchus (Crocodylomorpha, Goniopholidae) from the Lower Cretaceous of the Isle of Wigh, United Kingdom, and a review of the genus. Cretaceous Research, 84, 340–383. [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. et al (2012) Fiji: an open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno, P.C. and Wild, R. (1992) Procompsognathus: theropod, “thecodont” or both? Journal of Vertebrate Paleontology, 12, 435–458. [Google Scholar]
- Shute, C.C.D. and Bellairs, A.d'A. (1955) The external ear in Crocodilia. Proceedings of the Zoological Society of London, 124, 741–749. [Google Scholar]
- Sues, H.‐D. , Olsen, P.E. , Carter, J.G. and Scott, D.M. (2003) A new crocodylomorph archosaur from the Upper Triassic of North Carolina. Journal of Vertebrate Paleontology, 23, 329–343. [Google Scholar]
- Tarsitano, S.F. (1985) Cranial metamorphosis and the origin of the Eusuchia. Neues Jahrbuch für Geologie und Paläeontologie Abhandlungen, 170, 27–44. [Google Scholar]
- Walker, A.D. (1961) Triassic reptiles from the Elgin area: Stagonolepis, Dasygnathus and their allies. Philosophical Transactions of the Royal Society of London B, 244, 103–204. [Google Scholar]
- Walker, A.D. (1990) A revision of Sphenosuchus acutus Haughton, a crocodylomorph reptile from the Elliot Formation (late Triassic or early Jurassic) of South Africa. Philosophical Transactions of the Royal Society of London B, 330, 1–120. [Google Scholar]
- Weinbaum, J.C. (2011) The skull of Postosuchus kirkpatricki (Archosauria: Paracrocodyliformes) from the Upper Triassic of the United States. PaleoBios, 30, 18–44. [Google Scholar]
- Wilberg, E.W. (2015) What’s in an outgroup? The impact of outgroup choice on the phylogenetic position of thalattosuchia (Crocodylomorpha) and the origin of crocodyliformes. Systematic Biology, 64, 621–637. [DOI] [PubMed] [Google Scholar]
- Wu, X.‐C. and Chatterjee, S. (1993) Dibothrosuchus elaphros, a crocodylomorph from the Lower Jurassic of China and the phylogeny of the Sphenosuchia. Journal of Vertebrate Paleontology, 13, 58–89. [Google Scholar]
- Wu, X.‐C. , Sues, H.‐D. and Dong, Z.‐M. (1997) Sichuanosuchu shuhanensis, a new ?Early Cretaceous Protosuchian (Archosauria: Crocodyliformes) from Sichuan (China), and the monophyly of Protosuchia. Journal of Vertebrate Paleontology, 17, 89–103. [Google Scholar]
- Zanno, L.E. , Drymala, S. , Nesbitt, S.J. and Schneider, V.P. (2015) Early crocodylomorph increases top tier predator diversity during rise of dinosaurs. Scientific Reports, 5, 9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


