Abstract
We examined the morphology of the lungs of five species of high‐altitude resident ducks from Lake Titicaca in the Peruvian Andes (yellow‐billed pintail [Anas georgica], cinnamon teal [Anas cyanoptera orinomus], puna teal [Anas puna], speckled teal [Anas flavirostris oxyptera], and ruddy duck [Oxyura jamaicensis ferruginea]) and compared them with those of the high‐altitude migratory bar‐headed goose (Anser indicus) and the low‐altitude migratory barnacle goose (Branta leucopsis). We then determined the relationship between mass‐specific lung volume, the volume densities of the component parts of the lung, and previously reported hypoxia‐induced increases in pulmonary O2 extraction. We found that the mass‐specific lung volumes and the mass‐specific volume of the exchange tissue were larger in the lungs of high‐altitude resident birds. The bar‐headed goose had a mass‐specific lung volume that fell between those of the low‐altitude species and the high‐altitude residents, but a mass‐specific volume of exchange tissue that was not significantly different than that of the high‐altitude residents. The data suggest that the mass‐specific volume of the lung may increase with evolutionary time spent at altitude. We found an inverse relationship between the percentage increase in pulmonary O2 extraction and the percentage increase in ventilation across species that was independent of the volume density of the exchange tissue, at least for the resident Andean birds.
Keywords: altitude, birds, hypoxia, lungs, morphometry, pulmonary exchange tissue, pulmonary O2 extraction
We found that the mass‐specific lung volumes and the mass‐specific volume of exchange tissue were larger in the lungs of high‐altitude resident waterfowl than in those of low‐altitude resident and high‐altitude migratory waterfowl. We also found evidence of an inverse relationship between the percentage increase in pulmonary O2 extraction and the percentage increase in ventilation across species that is independent of the volume density of the exchange tissue.

1. INTRODUCTION
In a previous study, it was reported that some high‐altitude resident waterfowl, such as the Andean goose (Chloephaga melanoptera), have evolved fundamentally different mechanisms for maintaining oxygen (O2) supply in hypoxic (low O2) environments at altitude compared with the bar‐headed goose (Anser indicus). The bar‐headed goose, which migrates at high altitude to cross the Himalayas, responds to hypoxia by increasing ventilation and heart rate, whereas the Andean goose, which resides year‐round at altitude, increases lung O2 extraction and cardiac stroke volume (Lague et al., 2017). It was hypothesized that transient high‐altitude performance favoured the evolution of enhanced convective O2 uptake in hypoxia, whereas lifelong high‐altitude residency favoured the evolution of morphological enhancements to the lungs that increase lung‐O2 diffusion capacity (Lague et al., 2017). In a subsequent study, it was shown that the Andean goose does indeed possess pulmonary structural specializations that produce a mass‐specific total pulmonary O2 diffusing capacity of the lung that is among the highest values reported in birds (Maina et al., 2017). More recently, a study undertaken on multiple species residing at the same high elevations in the Andes of Peru found that distinct physiological strategies for coping with hypoxia exist across different high‐altitude lineages of ducks (Ivy et al., 2019). In this study, six populations of ducks that independently colonized the Peruvian Andes were compared with six closely related low‐altitude populations or species. Individuals from all populations responded to hypoxia with large increases in total ventilation combined with significant increases in pulmonary O2 extraction, regardless of whether they were taken from high‐ or low‐altitude populations. Two species, the yellow‐billed pintail (Anas georgica) and the torrent duck (Merganetta armata), had significantly greater increases in total ventilation on exposure to severe hypoxia compared with their low‐altitude counterparts. Although there was a trend for all high‐altitude birds to increase pulmonary O2 extraction more than their low‐altitude counterparts, this was significant only for the yellow‐billed pintail (Ivy et al., 2019).
Air entering the bird lung flows from the primary bronchus into multiple secondary bronchi that give rise to the parabronchi. These parabronchi give rise to the atria and infundibula at the entrance to the air capillaries. These air capillaries are closely connected to blood capillaries and are the site of gas exchange (Brown et al., 1997).
For the present study we obtained the lungs from five of the same species of high‐altitude ducks studied previously by Ivy et al. (2019). We also obtained the lungs of the high‐altitude migratory bar‐headed goose as well as the low‐altitude migratory barnacle goose. We examined the morphology of these lungs to determine the relationship between previously reported hypoxia‐induced increases in pulmonary O2 extraction and the mass‐specific lung volumes, and the volume densities of the component parts of the lung (primary bronchus, secondary bronchus including parabronchi, atria and infundibula, blood vessels larger than capillaries and exchange tissue). We hypothesized that the morphological features of the high‐altitude resident species would be more similar to the Andean goose than other low‐altitude species. We also hypothesized that there would be a positive correlation between the mass‐specific volumes of exchange tissue and blood vessels in the high‐altitude resident species and their reported values for pulmonary O2 extraction, and an inverse relationship with the magnitude of their ventilatory responses.
2. MATERIALS AND METHODS
Five species of ducks were captured at high altitude (3,812 m) at the Lake Titicaca National Reserve (near Puno, Peru): yellow‐billed pintails (A. georgica; .53 ± .02 kg, n = 3) cinnamon teal (Anas cyanoptera orinomus; .41 ± .10 kg, n = 3), puna teal (Anas puna; .41 ± .05 kg, n = 3), speckled teal (Anas flavirostris oxyptera; .31 ± .01, n = 2), and Andean ruddy ducks (Oxyura jamaicensis ferruginea; .63 ± .05 kg, n = 3). Bar‐headed geese (A. indicus; 2.8 ± .14 kg, n = 3) and barnacle geese (Branta leucopsis; 2.4 ± .16 kg, n = 3) were obtained from the Sylvan Heights Bird Park, NC, USA. Waterfowl from Lake Titicaca were collected in accordance with permits issued by the Servicio Nacional de Áreas Naturales Protegidas (001‐2017‐SERNANP‐DGANP‐RNT/J). All procedures were conducted according to guidelines approved by the Animal Care Committee at the University of Miami (17‐107) and University of British Columbia (A16‐0019) in accordance with the Canadian Council on Animal Care.
2.1. Fixation of the lungs
The birds were weighed and then killed by intravenous injection of propofol into the tibiotarsal vein (>20 mg/kg). With the body in a supine position, the lungs and the air sacs were fixed by intratracheal instillation with 2% glutaraldehyde buffered with either sodium cacodylate or phosphate (osmolarity 350 mOsm and pH 7.4) at a pressure head of 3,000 Pa (1 cm H2O = 100 Pa). The top of the funnel was held 30 cm above the sternum of the supine bird. When it stopped flowing by gravity, the body wall was gently massaged repeatedly to expel the air in the lung and the air sacs and allow better penetration of the fixative. The trachea was ligated and the fixative left in the respiratory system for approximately 4 hr at room temperature. After 4 hr, the lungs were removed, immersed in fixative, and stored at 4°C for 48–72 hr. They were then stored in buffer without glutaraldehyde at 4°C until sampling.
2.2. Determination of the lung volume (VL)
The extrapulmonary primary bronchus was trimmed close to the hilum, and all adhering fat and connective tissue were removed. Volume of the fixed lung (VL) was then determined by the weight displacement method of Scherle (1970). Due to the narrowing of the lungs at the sulci, the volume may be a slight underestimate; however, our estimates are comparable to those previously reported by Maina et al. (2017).
2.3. Sampling and analysis of the structural components of the lung
Details on lung sampling, tissue processing for microscopy, and morphometric analyses are outlined in Maina (2002) and are described briefly below.
The left lung of each bird was cut into five slices along the costal sulci and the slices in turn cut into halves dorsal to the primary bronchus. The 10 half‐slices were processed and embedded in paraffin wax with the cranial face of the lung facing anteriorly. Sections were cut at 8 µm thickness and stained with haematoxylin and eosin. The first five suitable slices off each lung section were used for analysis (Maina, 2002). Slices were not taken randomly and a vertical section (VUR) method was used. Images of the slides were taken field‐by‐field using a Zeiss Axioplan Fluorescent Microscope. The images were analysed using imagej software. The volume densities of the lumina of the intrapulmonary primary bronchus, the secondary bronchi and parabronchi (including the atria and infundibulae) (Pb), the exchange tissue (air and blood capillaries), and the blood vessels larger than capillaries were determined field‐by‐field by point‐counting using a 192‐point grid lattice at a final magnification of ×100 (Figure 1). The volume densities were calculated by averaging the percentage component of each slice. The absolute volumes of the structural parameters were calculated by multiplying the VL with the percentage volume density.
Figure 1.
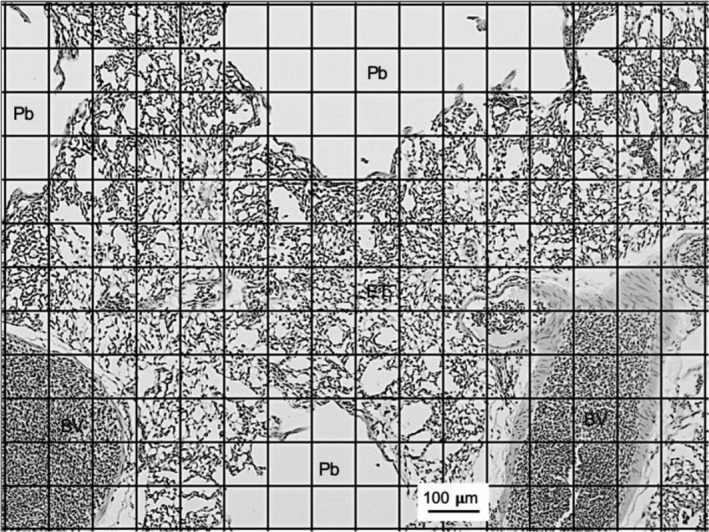
Sample haematoxylin and eosin‐stained section of a bird lung with a 192‐point grid lattice superimposed. Secondary bronchi and parabraonchi, blood vessels larger than capillaries, and exchange tissue are indicated. Scale bar: 100 µm
Standard errors were calculated to determine consistency within species and an ANOVA with a Tukey–Kramer post hoc test was performed in GraphPad prism and was used to test for statistically significant differences between species.
In Figures 2, 4, and 5, BG is low‐altitude, BHG is high‐altitude migratory, and all other species are ducks that reside at high altitude.
Figure 2.

Mass‐specific lung volumes (cm3 kg–1) of the species in this study. Barnacle goose, bar‐headed goose, yellow‐billed pintail, cinnamon teal, ruddy duck, puna teal, speckled teal, and Andean goose. Values for the Andean goose taken from Maina et al. (2017) are presented for comparison. Error bars represent standard error. * Indicates a significant difference between the groups indicated by the bar, † indicates a significant difference with all but the species indicated by the bar and ns, ‡ indicates a significant difference with all other species
Figure 4.

Mass‐specific volumes (cm3 kg–1) of the different components of the lung for each species. Barnacle goose, bar‐headed goose, yellow‐billed pintail, cinnamon teal, ruddy duck, puna teal, speckled teal, and Andean goose. Values for the Andean goose taken from Maina et al. (2017) are presented for comparison. Error bars represent standard error. † indicates a significant difference with all but the species indicated by the bar and ns, ‡ indicates a significant difference with all other species
Figure 5.

Volume densities (% of total volume) occupied by the different components of the lung for each species. Barnacle goose, bar‐headed goose, yellow‐billed pintail, cinnamon teal, ruddy duck, puna teal, speckled teal, and Andean goose. Values for the Andean goose taken from Maina et al. (2017) are presented for comparison. Error bars represent standard error. * indicates a significant difference between the groups indicated by the bar, and ‡ indicates a significant difference with all other species
3. RESULTS
The mean body mass and lung volumes (VL) of all species are given in Table 1 and the mass‐specific lung volumes are illustrated in Figure 2. In absolute terms, VL was largest for the ruddy duck and smallest for the speckled teal among these five species (Table 1). In proportion to bodyweight, the lungs of the barnacle goose were significantly smaller than those of all the high‐altitude species (F (7,10) = 51.9, p < .0137) except the bar‐headed goose (F (7,10) = 51.9, p = .0534). The bar‐headed goose lungs, in turn, were significantly smaller than those of the yellow‐billed pintail (F (7,10) = 51.9, p = .0063) and the speckled teal (F (7,10) = 51.9, p = .0068). For the Peruvian ducks from this study the only significant difference was between the ruddy duck and the speckled teal (F (7,10) = 51.9, p = .0431), with the speckled teal having the largest mass‐specific VL and the ruddy duck the smallest (Figure 2). Relative to body mass, the lungs of the Andean goose (values taken from Maina et al., 2017) were significantly larger than those of all birds from this study (F (7,10) = 51.9, p < .0099], including the bar‐headed goose by more than a factor of two.
Table 1.
Body mass (BM), absolute volume of the lung (VL), intrapulmonary primary bronchi (IPB), secondary bronchi and parabronchi (Pb), blood vessels larger than capillaries (BV) and exchange tissue (ET). Values are given as ± standard deviation
| Species | n | BM (kg) | VL (cm3) | IPB (cm3) | Pb (cm3) | BV (cm3) | ET (cm3) |
|---|---|---|---|---|---|---|---|
| Barnacle goose | 3 | 2.38 ± 0.16 | 26.0 ± 3.5 | a | 4.4 ± 3.2 | 6.0 ± 4.5 | 15.6 ± 3.6 |
| Yellow‐billed pintail | 3 | 0.53 ± 0.02 | 13.1 ± 0.1 | 0.5 ± 0.2 | 4.0 ± 1.0 | 1.4 ± 0.2 | 7.3 ± 0.7 |
| Cinnamon teal | 3 | 0.41 ± 0.10 | 9.8 ± 0.1 | 0.3 ± 0.1 | 2.5 ± 0.5 | 0.9 ± 0.1 | 6.1 ± 0.5 |
| Ruddy duck | 3 | 0.63 ± 0.05 | 13.3 ± 0.1 | 0.6 ± 0.5 | 3.5 ± 0.8 | 1.3 ± 0.3 | 7.9 ± 1.5 |
| Puna teal | 3 | 0.41 ± 0.05 | 11.2 ± 0.1 | 0.5 ± 0.1 | 3.6 ± 0.5 | 1.0 ± 0.2 | 6.1 ± 1.2 |
| Speckled teal | 2 | 0.31 ± 0.01 | 9.5 ± 0.1 | 0.3 ± 0.1 | 2.6 ± 0.1 | 0.8 ± 0.2 | 5.7 ± 0.3 |
| Andean goose | 3 | 2.67 ± 0.07 | 112.6 ± 5.4 | a | 32.4 ± 6.5 | 10.7 ± 3.3 | 69.5 ± 10.2 |
| Bar‐headed goose | 3 | 2.77 ± 0.14 | 46.9 ± 3.2 | a | 4.5 ± 2.9 | 8.8 ± 8.5 | 33.6 ± 8.9 |
Species for which the volume of the IPB was not measured, as the IPB was removed before sampling.
In Figure 3, an example of a low‐power histological micrograph of the lung from each species is shown illustrating the parabronchi leading into the exchange tissue. Intra‐parabronchial septa can be seen in some, but not all sections. This was true for the lungs of all species.
Figure 3.

Sample haematoxylin and eosin‐stained section from each species in this study. (a) yellow‐billed pintail, (b) cinnamon teal, (c) ruddy duck, (d) puna teal, (e) speckled teal, (f) barnacle goose, (g) bar‐headed goose. Scale bars: 100 µm except for the bar‐headed goose, which is 50 µm
The volumes of the main structural components of the lungs are given in Table 1, and the mass‐specific volumes of the structural components are illustrated in Figure 4. There were no differences among species in the mass‐specific volumes of intrapulmonary primary bronchi (F (4,9) = .3397, p > .7895) and blood vessels larger than capillaries (F (7,14) = .4744, p > .7895). The mass‐specific volume occupied by secondary bronchi and parabronchi was significantly smaller in barnacle and bar‐headed geese than in the Peruvian species (F (7,15) = 21.51, p < .0341) but there were no significant differences among the five species of high‐altitude ducks from this study (F (7,15) = 21.51, p > .0509); however, the mass‐specific volume occupied by secondary bronchi and parabronchi was larger in the Andean geese than in all other species (F (7,15) = 21.51, p < .0109) except the puna teal (F (7,15) = 21.51, p = .0945) and speckled teal (F (7,15) = 21.51, p = .1012). The mass‐specific volume of the exchange tissue was significantly smaller in the barnacle geese than in all other species (F (7,15) = 17.33, p < .0079) except the bar‐headed geese (F (7,15) = 17.33, p = .1271) and the ruddy duck (F (7,15) = 17.33, p = .0825), whereas that of the Andean goose was greater than in all other species (F (7,15) = 17.33, p < .0352).
These measurements expressed as volume densities (percentage of the lung volume occupied by each component) are listed in Table 2 and illustrated in Figure 5. When expressed this way, the volume density of blood vessels larger than capillaries was significantly greater in the bar‐headed geese (F (7,15) = 10.78, p < .0302) and barnacle geese (F (7,15) = 10.78, p < .0014) than in all other species, whereas the volume density of the secondary bronchi and parabronchi was smaller in the bar‐headed geese than in all other species (F (7,15) = 7.438, p < .0264). Notably, the volume density of the exchange tissue was similar across all species.
Table 2.
Volume densities of intrapulmonary primary bronchi (IPB), secondary bronchi and parabronchi (Pb), blood vessels larger than capillaries (BV), and exchange tissue (ET). Values are given as ± standard deviation
| Species | n | IPB (%) | Pb (%) | BV (%) | ET (%) |
|---|---|---|---|---|---|
| Barnacle goose | 3 | a | 17.0 ± 2.30 | 23.2 ± 3.45 | 59.8 ± 2.65 |
| Yellow‐billed pintail | 3 | 3.73 ± 1.89 | 30.53 ± 8.00 | 10.34 ± 1.80 | 55.40 ± 5.12 |
| Cinnamon teal | 3 | 3.07 ± 1.09 | 25.80 ± 4.80 | 8.82 ± 0.96 | 62.31 ± 4.66 |
| Ruddy duck | 3 | 4.74 ± 3.63 | 26.41 ± 6.09 | 9.43 ± 2.00 | 59.39 ± 11.58 |
| Puna teal | 3 | 4.16 ± 0.59 | 32.12 ± 4.73 | 8.87 ± 1.64 | 54.85 ± 6.69 |
| Speckled teal | 2 | 2.92 ± 0.57 | 27.89 ± 0.05 | 8.79 ± 2.06 | 60.4 ± 2.70 |
| Andean goose | 3 | a | 28.8 ± 4.45 | 9.5 ± 1.43 | 61.7 ± 7.39 |
| Bar‐headed goose | 3 | a | 9.60 ± 1.69 | 18.8 ± 6.05 | 71.6 ± 6.36 |
Species for which the volume of the IPB was not measured, as the IPB was removed before sampling.
The volume densities of the different structural components did not differ significantly progressing from the first to the fifth slice cut along the costal sulci of the lungs (F (4,10) = .1190, p > .1449). There was a trend for the volume density of the blood vessels to decrease and of the exchange tissue to increase from anterior to posterior, but these trends were very modest and not significant (F (4,10) = .1190, p > .1449). These measurements were only taken for the five species from Lake Titicaca and given that there were no differences between species, are averaged together and shown in Figure 6.
Figure 6.

Mean volume densities of the secondary bronchi, primary bronchi, blood vessels larger than capillaries and exchange tissue for sections 1–5 of each lung of each of the five Andean duck species averaged together. The insert is a schematic of the lung showing the sulci where the lung interdigitates with the ribs and where the cuts were made to obtain the five sections. Error bars represent standard error
4. DISCUSSION
The high‐altitude speckled teal and puna teal diverged from their low‐altitude ancestors approximately 1 million years ago or more, as did the Andean goose (McCracken et al., 2009). The other three species have only been established at altitude for multiples of tens of thousands of years, as estimated by population divergence times calculated from genetic data (McCracken et al., 2009). Although not significant, the trends in the data from this study suggest that as time since colonization of high‐altitude increases, so does mass‐specific lung, secondary bronchi and parabronchi, and exchange tissue volume. The Andean goose, which likely has been established at high‐altitude the longest, has the largest lungs and highest mass‐specific exchange tissue volume (Maina et al., 2017), whereas the bar‐headed goose, which is not a resident but is transitory at high altitude, has much smaller lungs and a reduced mass‐specific volume of exchange tissue compared with the percentage of exchange tissue.
The lungs of the low‐altitude barnacle goose were significantly smaller than those of all the high‐altitude species except the bar‐headed goose. The bar‐headed goose lungs, in turn, were significantly smaller than those of the yellow‐billed pintail and the speckled teal. For the Peruvian ducks from this study, the speckled teal had relatively larger lungs than the ruddy duck, and the lungs of the Andean goose (values taken from Maina et al., 2017) were significantly larger than those of all other birds in this study. Figure 7 compares the lung volumes of these birds with those of other avian species for which data are available (Maina et al., 2017; Table 3) and demonstrates that the values measured in the present study are consistent with trends seen more broadly.
Figure 7.

Comparison of the bird lung volumes in the present study (circles in red) with those of the population of birds for which similar data are available (circles in cyan, taken from Maina et al., 2017: table S8)
Table 3.
Mass‐specific volume (cm3/kg) of intrapulmonary primary bronchi (IPB), secondary bronchi and parabronchi (Pb), large blood vessels (BV), and exchange tissue (ET) for various high‐ and low‐altitude species
| IPB | Pb | BV | ET | |
|---|---|---|---|---|
| Andean goose b | 1.27 | 10.96 | 4.04 | 26.22 |
| Speckled teal | 0.88 | 8.43 | 2.66 | 18.26 |
| Puna teal | 1.14 | 8.78 | 2.42 | 14.99 |
| Cinnamon teal | 0.73 | 6.14 | 2.10 | 14.84 |
| Yellow‐billed pintail | 0.92 | 7.52 | 2.55 | 13.64 |
| Ruddy duck | 1.01 | 5.60 | 2.00 | 12.60 |
| Bar‐headed goose | – | 1.63 | 3.18 | 12.13 |
| Passeriformes a | 0.50 | 9.19 | 1.84 | 12.41 |
| Mallard duck a | 0.70 | 14.85 | 1.98 | 11.95 |
| Greylag goose a | 0.43 | 12.66 | 1.72 | 10.02 |
| Charadriiformes a | 0.79 | 16.62 | 2.74 | 10.01 |
| Barnacle goose | – | 1.86 | 2.54 | 6.54 |
Table 3 extends this comparison to a variety of other species. The values we obtained for the low‐altitude barnacle goose in the present study compare well to those of other low‐altitude species such as the greylag goose, the mallard duck, and species from the orders Charadriiformes (shorebirds) and Passeriformes (perching birds). As previously reported (Maina et al., 2017), the Andean goose continues to stand out with an exceptional mass‐specific volume of exchange tissue, whereas the five species of Andean ducks in the present study have higher mass‐specific volumes of exchange tissue than the low‐altitude species or transient high‐altitude migrant. The higher mass‐specific lung volumes in the resident Peruvian ducks may reflect the reduced muscle mass of these short‐distance fliers compared with longer distance migratory birds.
As previously mentioned, the Andean goose responds to hypoxia with small increases in ventilation and large increases in oxygen extraction, whereas the bar‐headed goose responds with large increases in ventilation but only extracts half as much O2. It is possible that the entire adaptive features of the bar‐headed goose can be explained by the fact that it is exercising at altitude, in which case the respiratory frequency may be habitually and ergonomically coupled to wing beat (Scott et al., 2015). The Andean goose, the high‐altitude resident, maintains a low ventilation rate, allowing the inspired air to remain in the lung for longer and more O2 to be extracted per breath (Maina et al., 2017).
The ruddy duck, which had the smallest lung volume, is the only diving species among these ducks. The vital capacity of the ruddy duck, reflecting the volume of the air sacs as well as the lungs, is also the smallest of these species (York et al., 2017). The lung morphology of this species may not be optimized for gas exchange, but rather in order to reduce buoyancy and to prevent the structural collapse of the lung while diving (Kooyman and Ponganis, 2002).
The morphological components of the lung, particularly the exchange tissue, determine the O2 diffusion capability of the respiratory structure. For the most part, the mass‐specific volumes of the different components of the lung in the different species reflect the mass‐specific differences in total lung volume (compare Figures 2 and 3).
When expressed as volume densities (the percentage of the lung occupied by each component) the volume density of the exchange tissue is relatively uniform across all species. The exchange tissue is composed of the air and blood capillaries. Also of note is the reduction of the volume of the lung occupied by secondary bronchi and parabronchi, and the increase in the volume density of larger blood vessels in the low‐altitude barnacle goose as well as in the bar‐headed goose. The significance of this is not clear.
Large standard deviations in the volume densities of the various lung components reflect the large variation in the structural composition of the lung between individuals within each species. Keeping this in mind, in Figure 8 we compare the volume densities of birds from different habitats (low and high altitude) and different locomotor behaviours (flightless, residents, migratory gliders, and migratory flappers). Again, there is large variability within groups, particularly among the low‐altitude birds. The large variability within the groups may reflect the phylogenetic history of the birds, as many of these species have been separated by millions of years of independent evolution (Prum et al., 2015). There appears to be a trend for the percentage of the lung occupied by blood vessels to increase in the high‐altitude species (resident and migratory) but two notable exceptions are the flightless emu and the barnacle goose from the present study. The percentage of the lung occupied by exchange tissue is relatively large in the high‐altitude species but is not unique. Several of the low‐altitude species, including the barnacle goose, have similar values for the percentage of the lung occupied by exchange tissue, as does the flightless ostrich. These data suggest that although life at altitude appears to influence lung structure, many other factors are also at play in determining lung tissue composition. Of note in this regard, the flightless birds are comprised of the ostrich (Struthio camelus) and the emu (Dromaius novaehollandiae), both of which have high O2 diffusion capability; however, they have achieved this in different ways. The ostrich has a very high‐volume density of exchange tissue, whereas the emu has a very high‐volume density of blood vessels. Thus, both increased perfusion and blood gas surface area can increase O2 diffusion capacity.
Figure 8.

Comparisons of volume densities of primary bronchi, secondary bronchi and parabronchi (parabronchi), blood vessels, and exchange tissues for birds with different lifestyles. Lifestyles are flightless (FL), low‐altitude birds (LA), migratory glider (MG), high‐altitude resident (HAR), and high‐altitude migratory (HAM). FL: Ostrich (Struthio camelus), Emu (Dromaius novaehollandiae); LA: Barnacle goose (Branta leucopsis), Canada goose (Branta canadensis), Mute swan (Cygnus olor), Mallard duck (Anas platyrhynchos), Grosbeak weaver (Ambryispiza albifrons), White‐winged robin (Cercotrichas leukophrys), Yellow flycatcher (Chloropeta natalensis), Singing cisticola (Cisticola cantans), Robin chat (Cossypha cafra), Waxbill (Estrilda astrid), Yellow‐bellied waxbill (Estrilda melanotis), African rock martin (Hirundo fuligula), Red‐billed firefinch (Laganosticta senegala), Tropical boubou (Lanius aethiopicus), Fiscal shrike (Lanius collaris), Bronze mankin (Lonchura cucullate), Bronze sunbird (Nectarina kilimensis), Golden‐winged sunbird (Nectarina reichenowi), House sparrow (Passer domesticus), Baglafecht weaver (Ploceus baglafecht), Black‐headed weaver (Ploceus cucullatus), Spectacled weaver (Ploceus ocularis), Holub’s golden weaver (Ploceus xanthops), Tawny prinia (Prinia subflava), Canary (Serinus canaria), Yellow‐fronted canary (Serinus mozambicus), Common starling (Sturnus vulgaris), Redwing (Turdus iliacus), Olive thrush (Turdus olivaceus). MG: Razorbill (Alca torda), Spectacled guillemot (Cephus carbo), Herring gull (Larus argentatus), Common gull (Larus canus), Black‐headed gull (Larus ridibundus). HAR: Yellow‐billed pintail (Anas georgica), Cinnamon teal (Anas c. orinomus), Ruddy duck (Oxyura j. ferruginea), Speckled teal (Anas oxyptera), Puna teal (Anas puna), Andean goose (Chloephaga melanoptera). HAM: Bar‐headed goose (Anser indicus). Data are taken from Maina (2002) and Maina et al. (2017). Data from birds in the present study are shown in red. The ‘e’ and ‘o’ beside the data for the flightless birds indicate data from the emu and the ostrich
It was previously hypothesized that transient high‐altitude performance favoured the evolution of enhanced convective O2 uptake in hypoxia, whereas lifelong high‐altitude residency favoured the evolution of structural enhancements to the lungs that increase lung diffusion capacity (Lague et al., 2017; Maina et al., 2017). It was subsequently shown that the five species of Andean ducks used in the present study responded to hypoxia with large increases in total ventilation combined with significant increases in pulmonary O2 extraction. There was a trend for these species to increase pulmonary O2 extraction more than related low‐altitude populations or species do (Ivy et al., 2019). In Figure 9 we compare the volume density of exchange tissue with the increase in pulmonary O2 extraction vs. the increase in ventilation in various species of birds breathing a gas mixture containing only 5% O2. While we are unable to do statistics on these data, as they were single values derived from mean data from different individuals, there appears to be an inverse relationship between the percentage increase in pulmonary O2 extraction and the percentage increase in ventilation that are independent of the volume density of the exchange tissue for the Andean birds. This could reflect differences in the morphometrics of the exchange tissue (barrier thickness, pulmonary respiratory surface area of the blood gas barrier or capillary density) or physiological differences in perfusion of the lungs. This remains to be determined.
Figure 9.
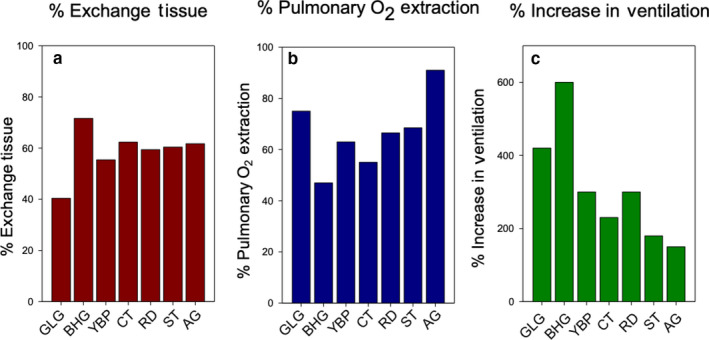
Comparison of percentage of exchange tissue, percentage of pulmonary O2 extraction, and percentage of ventilation increase. (a) Percentage of the lung composed of exchange tissue. (b) Percentage of O2 extracted from inspired air while breathing a hypoxic gas mixture (5% O2). (c) Percentage of increase in ventilation in birds breathing the same hypoxic gas mixture (100% is normal level). Data sources for (b) and (c) are Scott et al. (2015), Lague et al. (2017) and Ivy et al. (2019)
5. CONCLUSIONS
Our data indicate that the mass‐specific lung volumes and the mass‐specific volume of the exchange tissue are characteristically larger in the lungs of high‐altitude birds. The pulmonary specializations previously reported for the Andean goose, however, continue to be exceptional in this regard, even among high‐altitude residents (Maina et al., 2017). The bar‐headed goose that migrates at altitude twice each year has a mass‐specific lung volume that falls between those of low‐altitude species and the high‐altitude residents reported here, but a mass‐specific volume of exchange tissue that is not significantly lower than that of the high‐altitude residents. The data also suggest the mass‐specific volume of the lung may increase with time spent at altitude. The ruddy duck, the only true diving species among the high‐altitude ducks, had the smallest lungs and, based on previous reports, the smallest air sac volumes, suggesting a compromise between adaptations for life at altitude and adaptations for diving. There was an inverse relationship between the percentage increase in pulmonary O2 extraction and the percentage increase in ventilation that are independent of the volume density of the exchange tissue, at least for the Andean birds.
The comparisons between low‐ and high‐altitude birds in this study would be greatly strengthened by comparisons between the high‐altitude Andean species and their closely related low‐altitude conspecific or sister species or populations, as has been done in previous physiological studies (York et al., 2017; Ivy et al., 2019; Lague et al., 2020), but lungs for these birds are not yet available for study. These comparisons would also be strengthened by a more detailed analysis of the exchange tissue at the microscopic level.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
WKM and KGM designed the study. BC, JMY, WKM, and KGM acquired the samples, CB and ES analysed the samples and acquired the data. CB performed the statistical analysis and wrote the initial draft. CB, JMY, KGM, and WKM critically revised the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This research was funded by the NSERC of Canada (WKM) and a National Science Foundation grant (IOS‐0949439) and the James A. Kushlan Endowment for Waterbird Biology and Conservation from the University of Miami (K.G.M.).
Bakkeren C, Smith E, York JM, Chua B, McCracken KG, Milsom WK. A morphometric analysis of the lungs of high‐altitude ducks and geese. J. Anat. 2020;237:188–196. 10.1111/joa.13180
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Brown, R.E. , Brain, J.D. and Wang, N. (1997) The avian respiratory system: a unique model for studies of respiratory toxicosis and for monitoring air quality. Environmental Health Perspectives, 105(2), 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy, C.M. , Lague, S.L. , York, J.M. , Chua, B.A. , Alza, L. , Cheek, R et al (2019) Control of breathing and respiratory gas exchange in high‐altitude ducks native to the Andes. Journal of Experimental Biology, 222 (Pt 7), 10.1242/jeb.198622 [DOI] [PubMed] [Google Scholar]
- Kooyman, G.L. and Ponganis, P.J. (2002) The physiological basis of diving to depth: birds and Mammals. Annual Review of Physiology, 60, 19–32. [DOI] [PubMed] [Google Scholar]
- Lague, S.L. , Chua, B. , Alza, L. , Scott, G.R. , Frappell, P.B., Zhong, Y et al (2017) Divergent respiratory and cardiovascular responses to hypoxia in bar‐headed geese and Andean birds. Journal of Experimental Biology, 220(22), 4186–4194. [DOI] [PubMed] [Google Scholar]
- Lague, S.L. , Ivy, C.M. , York, J.M. , Chua, B.A. , Alza, L. , Cheek, R et al (2020) Cardiovascular responses to progressive hypoxia in ducks native to high altitude in the Andes. Journal of Experimental Biology, 10.1242/jeb.211250 [DOI] [PubMed] [Google Scholar]
- Maina, J.N. (2002) Some recent advances on the study and understanding of the functional design of the avian lung: morphological and morphometric perspectives. Biological Reviews of the Cambridge Philosophical Society, 77(1), 97–152. [DOI] [PubMed] [Google Scholar]
- Maina, J.N. , McCracken, K.G. , Chua, B. , York, J.M. and Milsom, W.K. (2017) Morphological and morphometric specializations of the lung of the Andean goose, Chloephaga melanoptera: A lifelong high‐altitude resident. PLoS One 12(3), e0174395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken, K.G. , Barger, C.P. , Bulgarella, M. , Johnson, K.P. , Kuhner, M.K. , Moore, A.V. et al (2009) Signatures of high‐altitude adaptation in the major hemoglobin of five species of Andean dabbling ducks. The American Naturalist, 174(5), 631–650. [DOI] [PubMed] [Google Scholar]
- Prum, R.O. , Berv, J.S. , Dornburg, A. , Field, D.J. , Townsend, J.P. , Lemmon, E.M. et al (2015) A comprehensive phylogeny of birds (Aves) using targeted next‐generation DNA sequencing. Nature, 526, 569–573. [DOI] [PubMed] [Google Scholar]
- Scherle, W. (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie, 26, 57–60. [PubMed] [Google Scholar]
- Scott, G.R., Hawkes, L.A. , Frappell, P.B. , Butler, P.J. , Bishop, C.M. and Milsom, W.K. (2015) How bar‐headed geese fly over the Himalayas. Physiology, 30(2), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York, J.M. , Chua, B.A. , Ivy, C.M. , Alza, L. , Cheek, R. , Scott, G.R et al (2017) Respiratory mechanics of eleven avian species resident at high and low altitude. Journal of Experimental Biology, 220(6), 1079–1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


