Abstract
The plantar aponeurosis in the human foot has been extensively studied and thoroughly described, in part, because of the incidence of plantar fasciitis in humans. It is commonly assumed that the human plantar aponeurosis is a unique adaptation to bipedalism that evolved in concert with the longitudinal arch. However, the comparative anatomy of the plantar aponeurosis is poorly known in most mammals, even among non‐human primates, hindering efforts to understand its function. Here, we review previous anatomical descriptions of 40 primate species and use phylogenetic comparative methods to reconstruct the evolution of the plantar aponeurosis and its relationship to the plantaris muscle in primates. Ancestral state reconstructions suggest that the overall organization of the human plantar aponeurosis is shared with chimpanzees and that a similar anatomical configuration evolved independently in different primate clades as an adaptation to terrestrial locomotion. The presence of a plantar aponeurosis with clearly developed lateral and central bands in the African apes suggests that this structure is not prohibitive to suspensory locomotion and that these species possess versatile feet adapted for both terrestrial and arboreal locomotion. This plantar aponeurosis configuration would have been advantageous in enhancing foot stiffness for bipedal locomotion in the earliest hominins, prior to the evolution of a longitudinal arch. Hominins may have subsequently evolved thicker and stiffer plantar aponeuroses alongside the arch to enable a windlass mechanism and elastic energy storage for bipedal walking and running, although this idea requires further testing.
Keywords: human evolution, locomotor behaviour, non‐human primates, plantar aponeurosis, plantaris muscle
The plantar aponeurosis is commonly assumed to be an adaptation to bipedal walking in humans that evolved in concert with the longitudinal arch. However, there is still considerable uncertainty about the comparative anatomy of plantar aponeurosis in non‐human primates, which has undermined attempts to test adaptive hypotheses about this structure. In this study, we review and score descriptions of plantar aponeurosis anatomy in 40 primate species and apply new comparative phylogenetic methods to test hypotheses about the evolution of the plantar aponeurosis in non‐human primates.
1. INTRODUCTION
The plantar aponeurosis is a connective tissue structure in the plantar region of the foot that has been most thoroughly characterized in humans, but is also present in numerous mammals. In humans, this structure, also called the plantar fascia, is a broad sheet of highly fibrous tissue in which collagen fibres are regularly orientated to span the entire plantar aspect of the foot from the heel to the toes (Standring and Gray, 2016). Its general anatomy in adults, illustrated in Figure 1, has been variously described (Sinnatamby and Last, 2011; Sarrafian, 2011; Abrahams, 2013; Moore et al. 2014; Netter, 2014; Standring and Gray, 2016), but embryonic development studies confirm that it usually consists of two distinct parts (Dylevský, 1991; Kalicharan et al. 2017). First, there is a prominent central band that attaches proximally to the medial tubercle of the calcaneus and fans out distally to attach to the subcutaneous tissue and joint capsules of the second through to the fifth metatarsophalangeal joints as well as the plantar bases of the corresponding proximal phalanges (Bojsen‐Moller and Flagstad, 1976). Second, a lateral band that attaches proximally to the lateral tubercle of the calcaneus and distally to the joint capsule at the fifth tarsometatarsal joint is also usually present (Stecco et al. 2013). A third, medial band has also been described, but it is much thinner than the other two parts (Stecco et al. 2013) and is likely to be part of the fascial sheath of the abductor hallucis muscle (Kalicharan et al. 2017). Outside of humans, the comparative anatomical organization of the plantar aponeurosis is not widely known in most mammal species, even among non‐human primates. This lack of comparative data hinders efforts to understand the functional purpose of the plantar aponeurosis in humans, despite its clinical and evolutionary importance.
Figure 1.
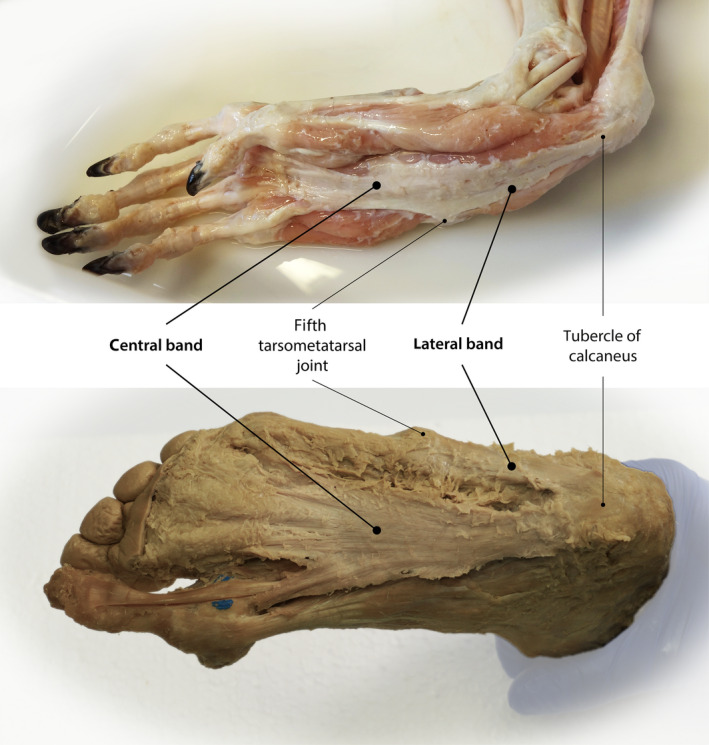
General anatomy of the plantar aponeurosis in baboons (Papio anubis) and humans. Both share a prominent central band and a lateral band that attaches proximally to the lateral tubercle of the calcaneus and distally to the joint capsule at the fifth tarsometatarsal joint. In baboons, the lateral band progresses further distal. The central band attaches proximally to the medial tubercle of the calcaneus and fans out distally to attach to the subcutaneous tissue and joint capsules of the first to the fifth metatarsophalangeal joints as well as the plantar bases of the corresponding proximal phalanges. The photos were provided with permission from Anthony Herrel (baboon) and Hanno Steinke (human).
Beyond being an anatomically interesting structure, the human plantar aponeurosis has received considerable clinical attention because of plantar fasciitis, a pathological inflammation of the connective tissue that can cause pain and immobility. Currently, about one in 10 Britons experiences plantar fasciitis at some point during his or her life (Garrow et al. 2004; Menz et al. 2010), and in the USA the condition accounts for an average of one million patient visits per year (Riddle and Schappert, 2004). Aetiologically, plantar fasciitis is recognized as an injury caused by excessive and repetitive loading of the foot's longitudinal arch (Wearing et al. 2006). The longitudinal arch is a structure unique to humans that is defined geometrically by the conformation of the tarsal and metatarsal bones and is maintained by soft tissue structures of the foot, including the plantar aponeurosis. Purported risk factors for plantar fasciitis include a tight Achilles tendon, weak intrinsic foot muscles, excessive foot pronation, overuse from too much running, obesity, prolonged standing and physical inactivity (Hill and Cutting, 1989; Kaya, 1996; Rome, 1997; Rome et al. 2001; Wearing et al. 2006). Despite the contradictory nature of some of these risk factors, all apparently interfere with what is thought to be the fundamental role of the plantar aponeurosis in humans: supporting the longitudinal arch during walking and running (Hicks, 1955).
The anatomical relationship between the plantar aponeurosis and the longitudinal arch has been used to define two biomechanical mechanisms for human foot function. First, in the windlass mechanism proposed by Hicks (1954), the plantar aponeurosis is described as wrapping around the metatarsal heads like a cable wrapping around a drum to insert onto the proximal phalanges of the toes (Figure 2A). Thus, dorsiflexion of the toes creates tension in the plantar aponeurosis that tends to pull the calcaneus towards the metatarsal heads. This motion creates an upward force in the longitudinal arch that effectively counters compressive forces from above and stiffens the foot. The windlass mechanism is believed to be activated during push‐off in walking and running when the toes are dorsiflexed following heel lift. The plantar aponeurosis has also been shown to behave like an energy‐storing spring during bipedal running (Ker et al. 1987; McDonald et al. 2016; Stearne et al. 2016; Wager and Challis, 2016; Figure 2B). When the foot is loaded in stance phase, the longitudinal arch is compressed, and the foot elongates, causing the plantar aponeurosis to stretch like a rubber band and store elastic energy. The plantar aponeurosis then recoils as it is unloaded, returning most of this elastic energy to the foot to aid in push‐off and reduce the metabolic energy required for running.
Figure 2.
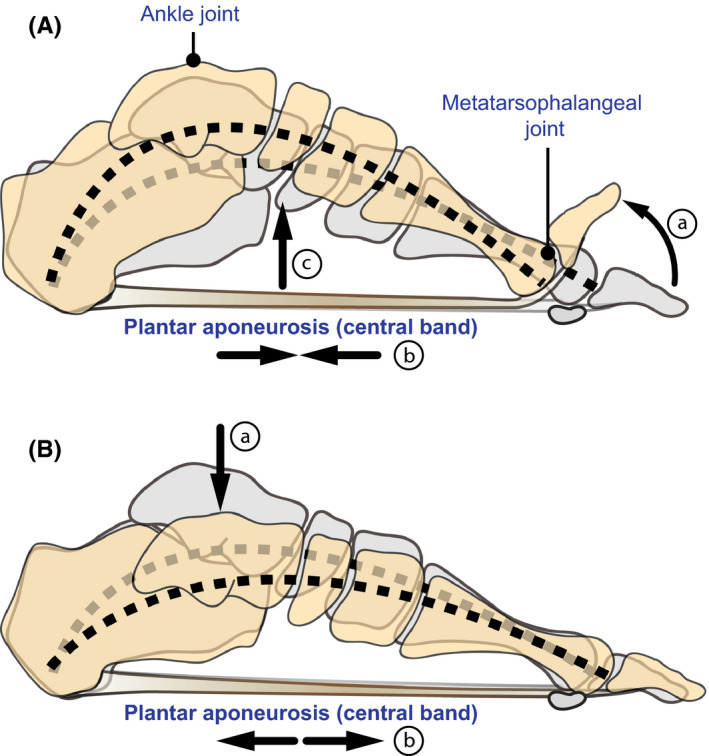
Biomechanics of the human foot involving the plantar aponeurosis. (A) In the windlass mechanism, the plantar aponeurosis is described as wrapping around the metatarsal heads like a cable wrapping around a drum to insert onto the proximal phalanges of the toes (a). Dorsiflexion of the toes creates tension in the plantar aponeurosis (b) that exerts a linear force that pulls the calcaneus forward and effectively raises the longitudinal arch (c). This makes the foot a stiff lever for effective power transmission from the ankle joint. (B) When the foot is loaded in stance phase (a), the longitudinal arch is compressed and the foot elongates, causing the plantar aponeurosis to stretch like a rubber band and store elastic energy (b). The plantar aponeurosis then recoils as it is unloaded, returning most of this elastic energy to the foot to aid in push‐off and reduce the metabolic energy required for running
Because of these special functions, it is commonly assumed that the human form of the plantar aponeurosis is an adaptation to bipedalism unique to humans that evolved in concert with the longitudinal arch (e.g. Aiello and Dean, 1990; Griffin et al. 2015). However, numerous studies have reported and/or described plantar aponeuroses or similar structures in the feet of other mammals, including some non‐human primates such as our closest living relatives, the great apes (Lewis, 1962; Swindler and Wood, 1982; Bennett et al. 1989; Vereecke et al. 2005; Wareing, 2016). While these reports demonstrate that the plantar aponeurosis is not a uniquely human structure, the degree to which it differs in humans from those of other animals, and particularly other primates, remains unclear. Lovejoy et al. (2009) argued that the human plantar aponeurosis more closely resembles those of distantly related cercopithecoid monkey species than those of closely related great apes. They suggested that the ‘thick and dense’ plantar aponeurosis in cercopithecoids is related to adaptations for propulsive behaviours such as above‐branch running and leaping, whereas the ‘minimal’ plantar aponeurosis in great apes allows midtarsal laxity for suspensory postures such as pedal grasping and vertical climbing. They argued further that humans inherited a thick plantar aponeurosis from their last common ancestor (LCA) with chimpanzees, and that extant great ape species independently experienced evolutionary reduction in their plantar aponeuroses as adaptations to suspensory locomotion. Based on this argument, one might predict that primates with adaptations for ‘propulsive’ foot postures possess more human‐like plantar aponeuroses, whereas those with adaptations for more ‘suspensory’ foot postures do not. However, although all great apes sometimes use their feet in suspensory postures, most locomotion in the African apes (Pan and Gorilla) is terrestrial (Doran, 1997), which benefits from a relatively stiff foot for enhanced propulsion (Holowka et al. 2017). Thus, an alternative hypothesis would be that more terrestrial primates, including the African apes, have human‐like plantar aponeurosis organization, whereas more arboreal primates do not.
Lovejoy et al. (2009) raised this issue in their description of the remains of Ardipithecus ramidus, a 4.4‐million‐year‐old fossil hominin from Ethiopia for which much of the postcranial skeleton has been recovered. Based on these remains, as well as variations in plantar soft tissue anatomy among extant catarrhines, Lovejoy et al. (2009) argued that the LCA of humans and chimpanzees had feet more like those of Old World monkeys in some respects than those of great apes and that this hominin would, therefore, have used different locomotor behaviours than great apes. Subsequent studies (e.g. Bates et al. 2013; White et al. 2015; Aerts et al. 2018; Simpson et al. 2019) have echoed this sentiment, and hence the notion of a non‐great ape‐like foot in the earliest hominins has gained some acceptance. However, the characterization of plantar aponeurosis anatomy in primates by Lovejoy et al. (2009) is based on a single source (Hartman et al., 1933) that includes only a vague, passing mention of the structure in rhesus macaques (Macaca mulatta). Thus, uncertainty about comparative plantar aponeurosis anatomy among primates persists and has major ramifications for our understanding of the evolution of human bipedalism.
To shed light on these issues, this study reviews previous anatomical descriptions and uses phylogenetic comparative methods to reconstruct the evolution of the plantar aponeurosis in primates. We focus on the comprehensive analysis by Loth (1908), who dissected and illustrated 126 primate feet, but whose descriptions have been generally overlooked, probably because they were published in German over 100 years ago. Importantly, Loth recognized the considerable variation among primates in specific aspects of plantar aponeurosis attachments and their relationship to the plantaris muscle, a plantarflexor of the ankle that is often categorized as belonging to the triceps surae muscles (Daseler and Anson, 1943; Langdon, 1990; Vereecke et al. 2005; Hanna and Schmitt, 2011). In various mammalian taxa, the plantaris muscle is primitively continuous with the plantar aponeurosis (Lewis, 1962). It usually originates from the femur just above the lateral condyle under cover of the lateral head of the gastrocnemius muscle and gives rise to a tendon which emerges medial to the Achilles tendon. In its primitive mammalian form, its distal portion presents a fibrous cap as it passes smoothly over the posterior end of the calcaneal bone to enter the plantar sole and blend with the plantar aponeurosis (Daseler and Anson, 1943; Lewis, 1962; Figure 3). Based on that primitive insertion, Lovejoy et al. (2009) argued that plantaris presence is developmentally related to the presence of other soft tissue structures in the foot, including the plantar aponeurosis.
Figure 3.
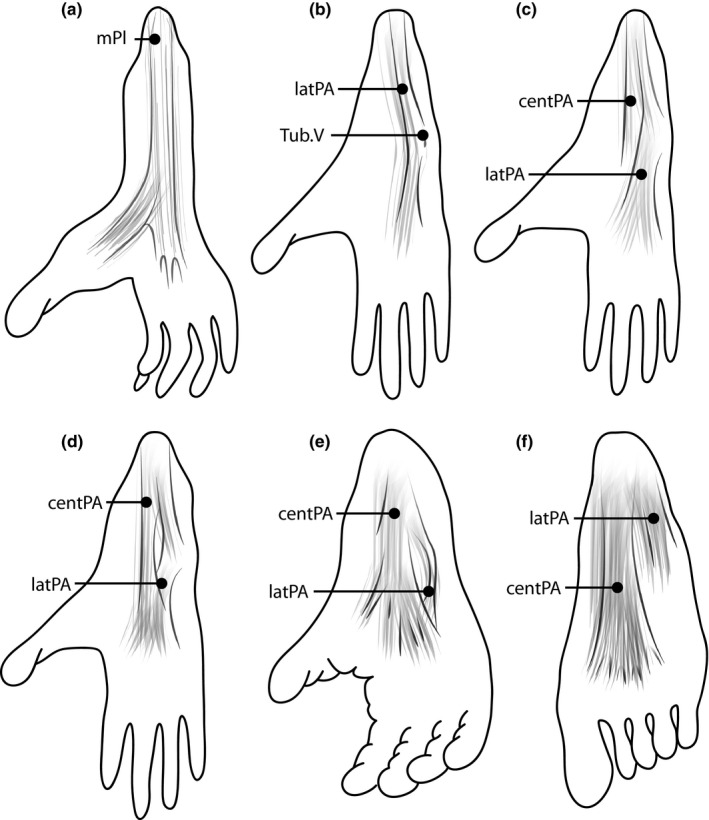
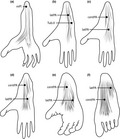
Illustrations of the anatomical variation of the plantar aponeurosis among primates. (a) The plantar aponeurosis is primitively continuous with the plantaris muscle (mPl). This form is described, for example, in black‐and‐white ruffed lemurs (Varecia variegate). (b) The plantar aponeurosis forms a lateral band (latPA) that attaches proximally to the lateral tubercle of the calcaneus and distally to the joint capsule at the fifth tarsometatarsal joint Tub.V), as described, for example, in Venezuelan red howlers (Alouatta seniculus). (c–f) Various formations of the plantar aponeurosis, with a lateral and a central band (centPA). While the lateral band appears prominent in olive baboons [Papio anubis (c)] and guinea baboons [Papio papio (d)], the central band is more prominent in chimpanzees [Pan troglodytes (e)] and humans [Homo sapiens (f)].
In light of the uncertainty about comparative plantar aponeurosis anatomy, and its relation to the plantaris muscle, the aim of the present study was to clarify variation and evolution of the plantar aponeurosis among primates and especially in humans by synthesizing previous anatomical descriptions and applying comparative phylogenetic methods to test four evolutionary hypotheses. First, we test the hypothesis that primates with a plantaris muscle also possess a plantar aponeurosis, whereas those that do not will lack both structures. Additionally, we test the hypothesis, following Lovejoy et al. (2009), that primates that use their feet in ‘propulsive’ postures (including frequent running and/or leaping) possess plantar aponeuroses with more human‐like configurations, including clear lateral and central bands. Conversely, primates that use their feet mainly in ‘suspensory’ postures (pedal grasping and vertical climbing) will lack features of the plantar aponeurosis found in humans. We also test an alternative hypothesis, that primates that engage in substantial terrestrial locomotion possess human‐like plantar aponeuroses, whereas those that are primarily arboreal do not. Finally, we test the more general hypothesis that humans possess a unique form of the plantar aponeurosis. By this, we refer to the qualitative presence or absence in other primates of the lateral and central bands comprising the human plantar aponeurosis, although we lack the data to test whether human plantar aponeuroses are thicker, stiffer or tougher than those of other primates.
2. METHODS
2.1. Included primate species
To compare the variation of plantar aponeurosis form among humans and other primates and clarify its relation to the plantaris muscle, we reviewed the existing literature on primate foot descriptions. As noted above, our data are primarily drawn from Loth (1908), but we also reviewed more recent reports of primate foot dissections to expand this dataset and test Loth's descriptions (Murie and Mivart, 1869; Keith, 1894; Straus, 1930; Hartman et al. 1933; Raven, 1936; Stilwell, 1957; Swindler and Wood, 1982; Langdon, 1990; Sarmiento, 1994; Aerts, 1998; Thorpe et al. 1999; Gebo, 2005; Vereecke et al. 2005; Carlson, 2006; Payne et al. 2006; Channon et al. 2009; Hirasaki and Kumakura, 2010; Diogo et al. 2013a; Sefczek and Dunham, 2014; Wareing, 2016; Table 1). We restricted our analysis to studies that contained detailed information in the form of text on the presence or absence of three prominent key characters: (1) a plantaris muscle; (2) a central band of the plantar aponeurosis; and (3) a lateral band of the plantar aponeurosis.
Table 1.
Summary of descriptions for the characterization of the plantaris muscle and plantar aponeurosis as well as classification of locomotor behaviour among primates.
| Species | Character state | Plantar aponeurosis general descriptionsc | Locomotor behaviourd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plantaris musclea | Lateral partb | Central partb | |||||||||
| n | 0 | 1 | 2 | 0 | 1 | 0 | 1 | S‐P | T‐A | ||
| Hominidae | |||||||||||
| Gorilla gorilla gorilla e | 6 | Loth (1908), Straus (1930), Diogo, (2011), Wareing (2016), Payne et al. (2006) | Loth (1908), Wareing (2016), Straus (1930) | Loth (1908), Wareing (2016), Straus (1930) | Raven (1936), Sarmiento (1994) | Suspensory | Terrestrial | ||||
| Fleagle (2013) | |||||||||||
| Homo sapiens e | 50 | Loth (1908), Langdon (1990) | Loth (1908), Stecco et al. (2013) | Loth (1908), Stecco et al. (2013) | Propulsive | Terrestrial | |||||
| Fleagle (2013) | |||||||||||
| Pan troglodytes troglodytes e | 11 | Loth (1908)f, Wareing (2016), Diogo (2013b), Thorpe et al. (1999), Carlson (2006) | Loth (1908)f, Wareing (2016), Thorpe et al. (1999), Carlson (2006) | Loth (1908), Wareing (2016) | Loth (1908), Wareing (2016) | Raven (1936) | Suspensory | Terrestrial | |||
| Gosselin‐Ildari (2013), Fleagle (2013) | |||||||||||
| Pan paniscus | 4 | Wareing (2016), Payne et al. (2006), Vereecke et al. (2005) | Wareing (2016), Vereecke et al. (2005) | Wareing (2016), Vereecke et al. (2005) | Suspensory | Terrestrial | |||||
| Gosselin‐Ildari (2013), Fleagle (2013) | |||||||||||
| Pongo pygmaeus e | 4 | Loth (1908)g, Payne et al. (2006) | Loth (1908)g, Diogo et al. (2013a), Langdon (1990) | Loth (1908), Wareing (2016), Sarmiento (1994) | Loth (1908), Wareing (2016), Sarmiento (1994) | Suspensory | Arboreal | ||||
| Gosselin‐Ildari (2013), Fleagle (2013) | |||||||||||
| Hylobatidae | |||||||||||
| Hylobates muelleri | 3 | Loth (1908) | Loth (1908) | Loth (1908) | Hirasaki and Kumakura (2010) | Suspensory | Arboreal | ||||
| Fleagle (2013) | |||||||||||
| Hylobates lar | 2 | Vereecke et al. (2005), Payne et al. (2006) | Vereecke et al. (2005) | Vereecke et al. (2005) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
| Symphalangus syndactylus | 11 | Loth (1908), Langdon (1990), Channon et al. (2009) | Loth (1908) | Loth (1908) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
| Nomascus leucogenys | 1 | Vereecke et al. (2005) | Vereecke et al. (2005) | Vereecke et al. (2005) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
| Cercopithecidae | |||||||||||
| Cercocebus torquatus atys | 3 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Chlorocebus pygerythrus | 3 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Chlorocebus sabaeus e | 4 | Loth (1908), Keith (1894) | Loth (1908), Keith (1894) | Loth (1908), Keith (1894) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Colobus vellerosus | 1 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Erythrocebus patas | 4 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca arctoides | 1 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca fascicularis | 6 | Loth (1908), Langdon (1990), Sefczek and Dunham (2014) | Loth (1908) | Loth (1908) | Sefczek and Dunham (2014) | Propulsive | Arboreal | ||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca maura | 3 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca mulatta | 4 | Loth (1908) | Loth (1908), Stilwell (1957), Herrel (2019)p.c. | Loth (1908), Stilwell (1957), Herrel 2019p.c. | Hartman et al. (1933) | Propulsive | Terrestrial | ||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca nemestrina | 6 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Macaca sinica | 4 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Mandrillus sphinx | 4 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Papio anubis | 5 | Loth (1908) | Loth (1908), Herrel (2019)p.c., Berillon (2019)p.c. | Loth (1908), Herrel (2019)p.c., Berillon (2019)p.c. | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Papio cynocephalus | 2 | Loth (1908) | Loth (1908) | Loth (1908) | Swindler & Wood (1973) | Propulsive | Terrestrial | ||||
| Gosselin‐Ildari (2013) | |||||||||||
| Papio hamadryas | 5 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Rowe et al. (1999) | |||||||||||
| Papio papio | 7 | Loth (1908) | Loth (1908), Herrel (2019)p.c. | Loth (1908), Herrel (2019)p.c. | Propulsive | Terrestrial | |||||
| Fleagle (2013) | |||||||||||
| Presbytis melalophos | 3 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Semnopithecus entellus | 4 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Terrestrial | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Trachypithecus cristatus | 2 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Cebidae | |||||||||||
| Cebus albifrons | 2 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Rowe et al. (1999) | |||||||||||
| Sapajus apella | 1 | Loth (1908), Langdon (1990) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Gosselin‐Ildari (2013) | |||||||||||
| Cebus capucinus | 1 | Loth (1908) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | |||||
| Rowe et al. (1999) | |||||||||||
| Callithrix jacchus | 7 | Loth (1908) | Langdon (1990) | Loth (1908) | Loth (1908) | Propulsive | Arboreal | ||||
| Fleagle (2013) | |||||||||||
| Atelidae | |||||||||||
| Alouatta seniculus | 1 | Loth (1908), Langdon (1990), Sarmiento (1983) | Loth (1908) | Loth (1908) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
| Ateles paniscus | 2 | Loth (1908), Langdon (1990) | Loth (1908) | Loth (1908) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
| Lemuridae | |||||||||||
| Eulemur fulvus fulvus | 1 | Loth (1908), Murie and Mivart (1869) | Loth (1908) | Loth (1908) | Gebo (2005) | Propulsive | Arboreal | ||||
| Rowe et al. (1999) | |||||||||||
| Eulemur macaco macaco | 1 | Loth (1908), Murie and Mivart (1869) | Loth (1908) | Loth (1908) | Gebo (2005) | Suspensory | Arboreal | ||||
| Rowe et al. (1999) | |||||||||||
| Eulemur mongoz | 1 | Loth (1908), Murie and Mivart (1869) | Loth (1908) | Loth (1908) | Gebo (2005) | Propulsive | Arboreal | ||||
| Rowe et al. (1999) | |||||||||||
| Varecia variegata variegata | 2 | Loth (1908) | Loth (1908) | Loth (1908) | Suspensory | Arboreal | |||||
| Rowe et al. (1999) | |||||||||||
| Galagidae | |||||||||||
| Galago gallarum | 2 | Loth (1908) | Loth (1908) | Loth (1908) | Aerts (1998) | Propulsive | Arboreal | ||||
| Fleagle (2013) | |||||||||||
| Lorisidae | |||||||||||
| Loris tardigradus | 5 | Loth (1908) | Loth (1908) | Loth (1908) | Suspensory | Arboreal | |||||
| Fleagle (2013) | |||||||||||
In total, 40 primate species of eight primate families were included into the analysis.
The plantaris muscle was coded into three states: plantaris muscle absent (0), plantaris muscle present, smoothly passing beneath the calcaneal bone to blend with the plantar aponeurosis (1) and plantaris muscle present, being bound to or inserting into the calcaneal bone with little to no further connection to the plantar aponeurosis (2).
The lateral and central band of the plantar aponeurosis were coded into two states: well‐defined, parallel‐oriented fibres absent (0) and present (1).
Character state reported, but details as provided by Loth (1908) are not given.
Locomotor behaviour was characterized by the two categories ‘suspensory’ or ‘propulsive’ (S‐P) and ‘terrestrial’ or ‘arboreal’.
Complete confirmation (all characters) of descriptions provided by Loth (1908).
Presence of the plantaris muscle in chimpanzee is quite variable: 54.3% present (Loth, 1908).
Presence of the plantaris muscle in orangutan is quite variable: 3.7% present (Loth, 1908).
, personal communication.
In total, we were able to include 40 primate species and one outgroup species in the analysis (Table 1). Of these, all three characters of five species were described in sufficient detail in other studies to compare with Loth's description: Gorilla gorilla (Straus, 1930; Diogo, 2011; Wareing, 2016), Homo sapiens (Daseler and Anson, 1943; Stecco et al. 2013), Pan troglodytes (Diogo, 2013b; Wareing, 2016), Pongo pygmaeus (Langdon, 1990; Diogo et al. 2013a; Wareing, 2016), and Chlorocebus sabaeus (Keith, 1894). Further, single characters of 10 species were described in sufficient detail in other studies to compare with Loth's description (Table 1). In addition, personal communications confirmed the plantar aponeurosis anatomy described by Loth (1908) in Papio anubis (G. Berillon, pers. comm.; A. Herrel, pers. comm.), Papio papio and Macaca mulatta (A. Herrel, pers. comm.).
2.2. Coding of anatomical plantar aponeurosis descriptions
Descriptions of plantar aponeurosis anatomy were considered for code assignment when they indicated presence or absence of regular, parallel‐oriented fibres, and descriptions of plantaris anatomy had to state whether the tendon passed smoothly over the calcaneus or adhered to it (Figure 3). To permit quantitative comparisons and reconstruct ancestral states, all character descriptions were coded into discrete character states. The plantaris muscle was coded into three states: 0 = plantaris muscle absent; 1 = plantaris muscle present, smoothly passing beneath the calcaneal bone to blend with the plantar aponeurosis (primitive insertion); 2 = plantaris muscle present, but with little or no control of tension in the plantar aponeurosis. State 2 covers various descriptions. At one extreme, it includes a plantaris muscle with a tendon that passes beneath the calcaneal bone to blend with the plantar aponeurosis but, in contrast to state 1, transverse ligamentous fibres bind its tendon strongly to the surrounding periosteal tissue. At the other extreme, it includes a plantaris muscle that inserts into the calcaneal bone with no further connection to the plantar aponeurosis. All descriptions have in common that the plantaris muscle cannot produce tension in the plantar aponeurosis. The central and lateral bands of the plantar aponeurosis were coded into two states: 0 = well‐defined, parallel‐oriented fibres absent; 1 = well‐defined, parallel‐oriented fibres present. For state 1, these structures were described as discreetly differentiated from other soft tissue structures in the foot, and consisting of fibres predominantly aligned with the long axis of the foot. Unfortunately, this coding scheme did not allow us to consider reported within‐species variation or conflicting descriptions of plantaris or plantar aponeurosis anatomy, so in select cases where such inconsistencies existed, we ran the analyses using alternate coding schemes.
2.3. Classification of primate locomotor behaviour
To test the relationship between plantar aponeurosis form and locomotor behaviour among primates, we used two classificatory schemes to compare alternative hypotheses. First, to test the hypothesis that plantar aponeurosis anatomy is related to suspensory or propulsive foot postures, we categorized species as ‘suspensory’ or ‘propulsive’ based on analyses that describe them using their feet in 'suspensory’ postures in Rowe et al. (1999) and Fleagle (2013; Table 1; Figure 4). The suspensory category includes regular use of the foot to hang from branches or grasp branches with the hallux in opposition to the other digits during vertical climbing and clambering. Primates who do not use these behaviours were considered to have feet adapted primarily for ‘propulsive’ postures, such as running and leaping. To test the second hypothesis, that plantar aponeurosis anatomy is related to terrestrial locomotion, we categorized species as ‘terrestrial’ if they spent at least 16% of their locomotion time on the ground, and arboreal if they did not, following Gosselin‐Ildari (2013). If the necessary data for that categorization were not available, we based our categorizations on descriptions in Fleagle (2013) and Rowe et al. (1999).
Figure 4.
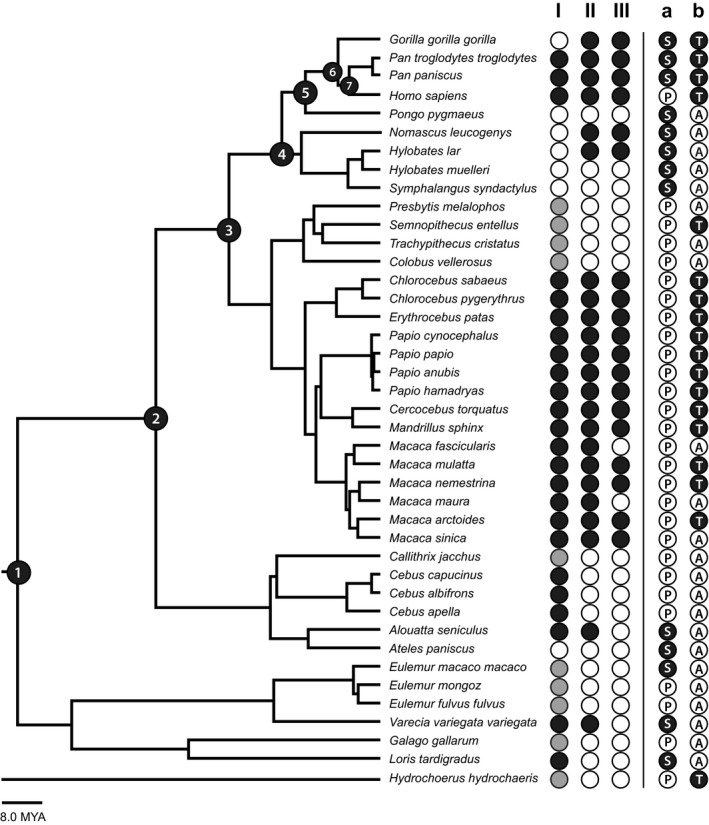
Documentation of the variation of plantar aponeurosis form among humans and other primates and classification of primate locomotor behaviour. The absence (white dot) or presence (black or grey dot) of three key characters was documented: plantaris muscle (I) as well as lateral (II) and central (III) band of a plantar aponeurosis. Two categories were used to classify primates’ locomotor behaviour. The first category indicates primates that have feet primarily adapted for suspensory (black dot with S), vs. propulsive (white dot with P). The second category indicates primates that spend significant amounts of time in terrestrial (black dot with T) vs. arboreal locomotion (white dot with A). To understand the evolutionary pattern that preceded humans the plantar aponeurosis form was analysed at each of the following major nodes (black numbered dots), representing last common ancestors of: all primates (1), all haplorhines (2), all catarrhines (3), all apes (4), great apes (5), African apes (6), and Pan/Homo (7).
2.4. Ancestral state reconstruction
To reconstruct the evolution of plantar aponeurosis form, phylogenetic data of all included primate species were downloaded from the 10k Tree website (Arnold et al. 2010). This phylogeny is based on genetic data, with branch lengths obtained from a Bayesian phylogenetic analysis (for more details see Arnold et al. 2010). The phylogeny was then imported into Mesquite reconstruction software (Mesquite version 3.40, www.mesquiteproject.org; Maddison and Maddison, 2006, 2018) to build a character matrix defining the state of the three documented key characters for each species (Figure 5). The capybara (Hydrochoerus hydrochaeris), the largest living rodent, which is comparable to primates in height and weight, was added to the character matrix as an outgroup species (García‐Esponda and Candela, 2016). To find the ancestral states for each of the three key characters, we used a likelihood reconstruction. For each ancestral node, this reconstruction finds the state assignment that maximizes the probability of arriving at the observed states in the terminal taxa. For our likelihood calculation, we applied the Mk1 model ('Markov k‐state 1 parameter model'). The single parameter in this model is the rate of change with any change, either a character gain or lost (from state 0 to 1 or state 1 to 0 for the central band, for example), being equally probable. Details on the statistics underlying Mk1 reconstructions are provided elsewhere (Pagel, 1994; Schluter et al. 1997). To reconstruct the evolutionary pattern that preceded humans, we analysed the plantar aponeurosis form at the nodes representing LCAs of all primates, all haplorhines, all catarrhines, all apes, great apes, African apes, and Pan/Homo. To test the two hypotheses about the relationship between plantar aponeurosis anatomy and locomotor behaviour, we mapped the above described behavioural categorizations onto the phylogeny and compared them to the distribution of coded anatomical features among the extant primate taxa.
Figure 5.
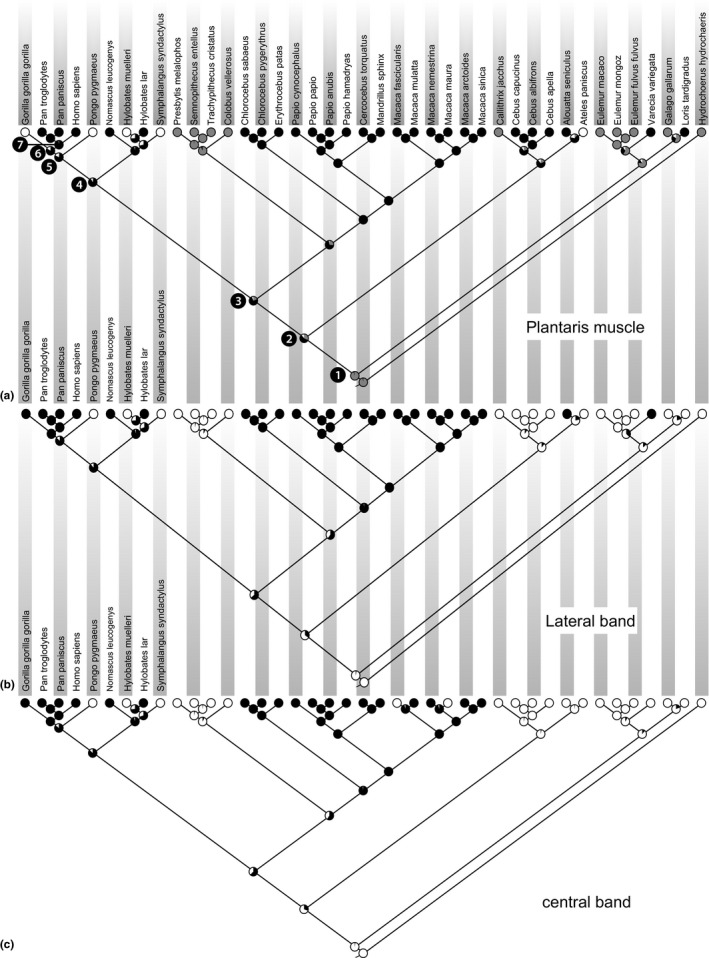
Ancestral state reconstruction to find the ancestral states for plantaris muscle (a), lateral band (b) and central band (c) of the planar aponeurosis. The absence (white dot) or presence (black or grey dot) of three key characters was documented for each taxon. For each ancestral node (pie chart) the reconstruction finds the state assignment that maximizes the probability of arriving at the observed states in the terminal taxa. Black numbered dots represent last common ancestors of: all primates (1), all haplorhines (2), all catarrhines (3), all apes (4), great apes (5), African apes (6), and Pan/Homo (7).
3. RESULTS
3.1. Plantaris muscle
As summarized in Figure 4, the plantaris muscle was absent in five species. In nine species the plantaris muscle was present in a primitive state, smoothly passing beneath the calcaneal bone to blend with the plantar aponeurosis. It was present but bound to or inserted into the calcaneal bone with limited or no further connection to the plantar aponeurosis in 26 species. Pan troglodytes was unusual in having an inconsistent presence of the plantaris muscle. In three of the nine described specimens (Loth, 1908; Wareing, 2016) it was absent or only unilaterally present (Diogo, 2013b; Wareing, 2016).
The presence or absence of the plantaris muscle was also analysed in relation to the presence or absence of a plantar aponeurosis (Figure 4). In nine species the plantaris was primitively continuous with the plantar aponeurosis. In 22 species the plantaris muscle and the plantar aponeurosis were both present, but not smoothly connected without plantaris adhesion or attachment to the calcaneus. In four species the plantaris muscle was present and inserted onto the calcaneus but the plantar aponeurosis was absent, and in one species the plantar aponeurosis was present while the plantaris muscle was absent. Both anatomical features were absent in four species.
The presence/absence of the plantaris muscle was further analysed with regards to the species locomotor behaviour. For the first hypothesis (propulsive vs. suspensory), it was absent in most suspensory species (state 0:5 of 13 species). In contrast, all propulsive species possess either a plantaris that is bound to or inserts into the calcaneus, with limited connection to the plantar aponeurosis (state 2, 19 of 27 species) or a primitive plantaris morphology with a tendon that smoothly blends into the plantar aponeurosis (state 1, eight of 27 species). For the second hypothesis, most arboreal species (52%) either lacked a plantaris muscle (state 0; three of 23 species) or possessed the primitive plantaris morphology (state 1; nine of 23 species). Conversely, almost all terrestrial species (88%) possessed plantaris muscles that are bound to or insert into the calcaneus (state 2). The only exceptions were Gorilla (state 0) and Semnopithecus (state 1).
The likelihood reconstruction (Figure 5a) for the plantaris muscle unambiguously predicts that the LCA of all primates most likely had a plantaris that blended with the plantar aponeurosis (state 1:94.0%). The likelihood of this anatomy dropped to 31.2% in the LCA of haplorhines, with the presence of a plantaris that was bound to or inserted into the calcaneal bone being more likely (56.9%). The plantaris was most likely to insert into the calcaneus (state 2) for the catarrhine LCA (75.3%), the LCA of all apes (88.3%), great apes (79.7%), and African apes (83.1%). Finally, the LCA of Pan and Homo probably had a plantaris that inserted into the calcaneus (state 2:98.0%). All probabilities described above use a state 2 coding for Pan troglodytes, but, as described earlier, the plantaris is not always present in Pan troglodytes specimens. Therefore, we performed ancestral state reconstructions coding the plantaris as absent in Pan. The results for most nodes showed no major change, and the LCA reconstruction of Pan and Homo decreased to a likelihood that the plantaris was present and inserted into the calcaneus of 86.0%.
3.2. Plantar aponeurosis: lateral band
As shown in Figure 4, a lateral band of the plantar aponeurosis with well‐defined, parallel fibres was absent in 17 species and present in 23 species. In the first locomotor behaviour hypothesis, the lateral band was present in 54% suspensory species and 60% of propulsive species. In the second locomotor behaviour hypothesis, the lateral band was present in all terrestrial species except Semnopithecus, and absent in most (70%) arboreal species.
Based on the ancestral state reconstructions (Figure 5b), the lateral band was most likely to have been absent in the LCA of all primates (97.0%), and haplorhines (65.3%). In contrast, the lateral band was likely to have been present in the LCA of catarrhines (64.4%), apes (87.6%), great apes (85.9%), African apes (99.0%) and the LCA of Pan and Homo (99.8%).
3.3. Plantar aponeurosis: central band
A central band of the plantar aponeurosis with well‐defined, parallel fibres was absent in 20 species and present in 20 species (Figure 4). There was some ambiguity in reports of this feature among two species. In the case of orangutans (P. pygmaeus), we found detailed descriptions from Loth (1908) and Wareing (2016). While Wareing (2016) characterizes orangutans as possessing a plantar aponeurosis, she describes the structure similarly to Loth (1908): a plantar sheet of fibrous tissue appears to be present, but it is very thin, mainly oblique or meshed, and with no discerning division into a lateral and central band. We therefore coded the plantar aponeurosis—the lateral and central band—as absent (state 0). In the case of chimpanzees (Pan troglodytes), the relative thickness of the central band varies between individuals, according to Loth (1908) and Wareing (2016), but both describe it as possessing clearly defined, parallel fibres regardless. Loth suggests that thicker plantar aponeuroses are related to the presence of a plantaris muscle, although his argument is based on the single specimen with a plantaris that he dissected.
In the first locomotor behaviour hypothesis, a central band was present in only 38% of suspensory species and was present in only 51% of propulsive species. In the second locomotor behaviour hypothesis, the central band was present in all terrestrial species except Semnopithecus entellus and was absent in all arboreal species except Nomascus leucogenys, Hylobates lar and Macaca sinica.
Based on the ancestral state reconstructions (Figure 5c), the central band was most likely to have been absent in the LCA of all primates (97.5%), and haplorhines (69.2%). In contrast, the central band was likely to have been present in the LCA of catarrhines (62.7%), apes (87.1%), great apes (85.5%), African apes (99.0%) and the LCA of Pan and Homo (99.8%).
4. DISCUSSION
This study reviewed published anatomical descriptions of the plantar aponeurosis among extant primates and used phylogenetic comparative methods to test evolutionary hypotheses. First, we tested the general hypothesis that humans possess a unique form of the plantar aponeurosis. Our results do not support this hypothesis as we found that 19 species had plantar aponeuroses with human‐like anatomy. Further, we tested two evolutionary hypotheses relating plantar aponeurosis anatomy to locomotor behaviour: first, that plantar aponeurosis anatomy reflects use of the foot in suspensory vs. propulsive locomotor postures (Lovejoy et al. 2009), or alternatively, that it reflects the proportion of time species engage in terrestrial locomotion. The latter hypothesis was strongly supported by our results, while the former was not. Finally, we tested the hypothesis that the plantaris muscle and plantar aponeurosis are integrated during development, and thus primates that possess a plantaris should also possess a plantar aponeurosis (Lovejoy et al. 2009). This hypothesis was rejected.
The findings of our study challenge the assumption that humans possess a unique form of the plantar aponeurosis that is primarily tied to its fundamental role in supporting the longitudinal arch during walking and running (Griffin et al. 2015). The human plantar aponeurosis is characterized as a broad sheet of highly fibrous tissue that can be separated into two distinct bands: a prominent central band and a smaller lateral band (Dylevský, 1991; Kalicharan et al. 2017). Surprisingly, a generally similar configuration of the plantar aponeurosis can be found in many primate taxa, including cercopithecines and our closest living relatives, the African apes. These findings are independently verified in multiple studies that report anatomical descriptions of the plantar aponeurosis in great apes, vervets, macaques and baboons (Keith, 1894; Loth, 1908; Straus, 1930; Swindler and Wood, 1982; Wareing, 2016; G. Berillon, pers. comm., A. Herrel, pers. comm.). Based on our ancestral state reconstruction, a human‐like plantar aponeurosis configuration was most likely to have been present in the LCA of apes, great apes and African apes, as well as in the LCA of Pan and Homo. It is therefore most probable that humans inherited the present form of the plantar aponeurosis from our LCA with chimpanzees. Alternatively, the present form could have evolved independently in extant hominids. While this possibility is considerably less parsimonious, there are other examples of homoplasy regarding the plantar aponeurosis within the animal kingdom. For instance, the plantar aponeurosis of the Tasmanian Devil (Sarcophilus harrisi), another predominantly terrestrial mammal (Owen and Pemberton, 2011), resembles that of primates in presenting clear lateral and central bands (Lewis, 1962).
The presence of a human‐like plantar aponeurosis configuration among non‐human primates and other mammals that lack longitudinal arches leads us to question inferences that these structures are evolutionarily related. Although it is unlikely that the appearance of the plantar aponeurosis corresponded with evolution of a longitudinal arch, humans may have re‐purposed the central band for arch‐related functions, namely enhancing the stiffness of the foot under loading. Limited data about the mechanical behaviour of the plantar aponeurosis in humans and African apes indicate that the stiffness of the central band in humans (Wright and Rennels, 1964; Guo et al. 2018) is about twice as high as in gorillas and chimpanzees (Wareing, 2016). This difference could be related to an assumed thickening of the plantar aponeurosis in humans (e.g. Susman, 1983), although we currently lack comparative quantitative data to verify this. Thus, future research should be directed at collecting more quantitative data on plantar aponeurosis material properties in a wide range of primates. We hypothesize that humans have evolved thicker, stiffer plantar aponeuroses relative to those of other primates to help facilitate the special adaptive functions of the human longitudinal arch (Holowka and Lieberman, 2018). Specifically, these changes may have enabled the human plantar aponeurosis to act as a more effective truss under loading (Lapidus, 1963), or helped facilitate the function of the windlass mechanism during push‐off in walking (Hicks, 1954; Caravaggi et al. 2009). However, it is likely that the plantar aponeuroses of other primates can effectively stiffen the midfoot to some extent, even in the absence of a longitudinal arch (Bennett et al. 1989; Wareing, 2016).
Another function of the plantar aponeurosis that might have been re‐purposed in humans with the evolution of a longitudinal arch is its spring‐like behaviour (Ker et al. 1987). During running, the longitudinal arch lengthens and lowers in early stance, which allows mechanical energy to be stored in the stretched plantar aponeurosis and consequently reduces the energetic cost of running through elastic recoil (Ker et al. 1987; McDonald et al. 2016; Stearne et al. 2016). Interestingly, Bennett et al. (1989) demonstrated a similar energy‐saving spring function of the plantar aponeurosis in cadaveric feet from pig‐tailed macaques (Macaca nemestrina) and vervet monkeys (Chlorocebus aethiops), which lack arches but possess human‐like plantar aponeuroses (Loth, 1908). Unlike the energy‐storing mechanism in humans, the feet of these monkeys bend to form a convex plantar surface when subjected to high loads, stretching the plantar aponeurosis and storing strain energy, which can be returned in the subsequent elastic recoil (Bennett et al. 1989). These findings indicate that the energy‐storing capacity of the plantar aponeurosis can be used by primates without longitudinal arches (Bennett et al. 1989). However, the estimated energy storage in human feet (17%, Ker et al. 1987) is greater than in monkey feet (12%, Bennett et al. 1989), potentially indicating an adaptation for improved endurance running performance in humans (Bramble and Lieberman, 2004).
Interestingly, Vereecke and Aerts (2008) discuss the possibility of a monkey‐like energy‐saving mechanism in the plantar aponeuroses and digital flexor tendons of gibbons during bipedalism, although they do not provide estimates of energy savings. In this regard, our analysis revealed potential variation among hylobatids in plantar aponeurosis anatomy. While Loth (1908) described two hylobatid species, Symphalangus syndactylus and Hylobates muelleri, as lacking a plantar aponeurosis with well‐defined, parallel‐oriented fibres, Vereecke et al. (2005) described the presence of a plantar aponeurosis consisting of a lateral and central band in two hylobatid species (Hylobates lar and Nomascus leucogenys). Future research should investigate the possibility of plantar aponeurosis variation among hylobatids, and its implications for the relationship between plantar aponeurosis anatomy and locomotor behaviour among primates.
Our findings do not support the argument put forward by Lovejoy et al. (2009) that plantar aponeurosis anatomy reflects use of the foot in propulsive vs. suspensory locomotor postures, as highlighted by differences in plantar aponeurosis anatomy among extant non‐human great apes. All these species use their feet to grasp arboreal supports during suspensory behaviours (such as vertical climbing), yet the African apes have human‐like plantar aponeurosis organization while orangutans (and some hylobatids) do not. This difference is probably related to substrate use: whereas orangutans are primarily arboreal (Fleagle, 2013), a significant component of African ape locomotion occurs on the ground (Doran, 1993, 1997). In another revealing comparison, spider monkeys (Ateles paniscus) also lack a plantar aponeurosis (Loth, 1908; Langdon, 1990). Spider monkeys and other atelines have been argued to converge on non‐human apes in many respects related to their use of suspensory locomotor behaviours (Fleagle et al. 1981), but like the orangutans, they are mainly restricted to arboreal travel (Fleagle, 2013). Thus, the presence of a well‐defined plantar aponeurosis among the African apes appears to be closely related to frequent use of terrestrial substrates during locomotion.
The terrestrial underpinnings of a well‐defined plantar aponeurosis are highlighted by the nearly universal presence of both central and lateral bands of the plantar aponeurosis among primate species with a significant terrestrial component in their locomotor behaviour. This includes most of the cercopithecine taxa in our sample, which were mostly categorized as terrestrial. Of the three arboreal cercopithecines included in this study, two lacked a central band, further supporting the notion that plantar aponeurosis anatomy is strongly related to substrate use. Based on ancestral state reconstructions, it is likely that central and lateral bands of the plantar aponeurosis evolved in the earliest cercopithecines, and were maintained in all taxa that continued to engage in significant terrestrial locomotion. Bennett et al. (1989) found that the plantar aponeurosis confers considerable stiffness in the feet of two of the monkey species (Chlorocebus sp. and Macaca nemestrina) coded as terrestrial in our analysis. Thus, it is likely that this added stiffness is advantageous during locomotion on the ground, but that it is unimportant to locomotion on arboreal substrates.
The absence of a well‐defined plantar aponeurosis in orangutans, potentially gibbons, colobines, and platyrrhines, suggests that a human‐like organization of the plantar aponeurosis evolved convergently in cercopithecines and African apes, likely as an adaptation to terrestrial locomotion. This scenario supports the argument that the LCA of humans and chimpanzees frequently engaged in terrestrial quadrupedalism (Gebo, 1992; Richmond et al. 2001; Wrangham and Pilbeam, 2002; Pilbeam and Lieberman, 2017). Some researchers favour the alternative, less parsimonious inference that chimpanzees and gorillas could have evolved human‐like plantar aponeurosis anatomy along with other adaptations for terrestrial knuckle‐walking convergently (Kivell and Schmitt, 2009), while humans evolved their plantar aponeurosis anatomy independently for bipedal locomotion. Regardless of these different evolutionary scenarios, our results do not support the argument that the LCA of humans and chimpanzees had a foot that was poorly adapted for suspensory locomotion (Lovejoy et al. 2009). Even if this species possessed human‐like plantar aponeurosis anatomy, this should not have had a major effect on its suspensory locomotor capabilities. Similarly, our results run counter to the argument that the feet of African apes are adapted for extreme pliability to the detriment of their use as stiff levers during terrestrial locomotion (Lovejoy et al. 2009). Unfortunately, we were not able to assess the relative thickness or mechanical properties of the plantar aponeurosis across species but, based on previous descriptions, it is probable that the plantar aponeuroses of the African apes cannot confer the same stiffness to the foot as in humans (Susman, 1983; Wareing, 2016). Nevertheless, the retention or independent evolution of lateral and central bands in humans and African apes conforms to the notion that this structure was selected to provide some degree of foot stiffness (Bennett et al. 1989), and thus likely contributes to the versatility that makes the feet of chimpanzees and other African apes well adapted for both terrestrial and arboreal locomotor behaviours (Holowka et al. 2017). The ability to stiffen the foot might also be attributed to a change in the developmental relationship between the plantaris muscle and the plantar aponeurosis.
Our phylogenetic reconstruction shows that the LCA of all primates most likely had a plantaris muscle that was primitively continuous with the plantar aponeurosis. This primitive feature is shared by many mammals (Lewis, 1962; Anapol and Barry, 1996; Perry, 2004; McClearn, 22005; Warburton et al. 2012; García‐Esponda and Candela, 2016), including most of the strepsirrhines in our study. However, among most extant catarrhines, the tendon of the plantaris is strongly bound to the periosteal tissue of the calcaneus, and therefore the plantaris loses its ability to directly increase tension in the plantar aponeurosis. This transition may reflect a developmental de‐coupling of these structures, as our data indicate that within species the plantaris and plantar aponeurosis are not necessarily both present or absent. For example, in gorillas and in some chimpanzees and humans, a plantar aponeurosis is present while the plantaris is missing (Loth, 1908; Langdon, 1990; Diogo, 2011; 2013b; Wareing, 2016). In contrast, capuchin monkeys (Cebus capucinus, Cebus albifrons and Cebus apella) and the red slender loris (Loris tardigradus) have a plantaris that inserts into the calcaneal bone, but a plantar aponeurosis is absent (Loth, 1908). Another example that may reflect the gradual de‐coupling of the plantaris muscle and the plantar aponeurosis is the anatomical condition in chimpanzees. Both Loth (1908) and Wareing (2016) observed well‐defined fibre bundles forming lateral and central bands, but they varied somewhat in qualitative thickness between individuals. Loth (1908) relates this variation to the presence of a plantaris muscle, based on his observation that the plantar aponeurosis was thickest in the one specimen he dissected with a plantaris muscle present. Although this argument is based on only one specimen and therefore requires further investigation, it supports a primitive developmental link between these structures. Conversely, his observations also highlight the developmental de‐coupling, as we observe the presence of a central and lateral band independent of the presence or absence of a plantaris muscle. This condition contrasts with cercopithecines, where the central and lateral band are continuous with a regularly present plantaris muscle, although the muscle's tendon adheres strongly to the calcaneus (Keith, 1894; Loth, 1908). Therefore, the plantar aponeurosis and its relation to the plantaris muscle in African apes most closely resembles the condition described in humans (Loth, 1908). In humans, the plantar aponeurosis is always present while the plantaris is missing in about 10% of humans (Daseler and Anson, 1943; Langdon, 1990). We therefore reject the hypothesis that the plantaris has a strong developmental relationship to the plantar aponeurosis in all primates (Lovejoy et al. 2009). In its most primitive form, the plantar aponeurosis is essentially a continuation of the plantaris (Loth, 1908). Selection may have favoured the separation and de‐coupling of these structures in some primates to allow the plantar aponeurosis to stiffen the midfoot passively without the necessity of activating the plantaris, which is an ankle plantarflexor. This may have been advantageous in more terrestrial primates that use plantigrade or semi‐plantigrade foot postures (Schmitt and Larson, 1995), where greater foot stiffness could enhance propulsive power production during locomotion.
It is crucial to bear in mind that the functional implications of this study are restricted to anatomical descriptions of the plantar aponeurosis. Unfortunately, comparative data of the thickness and mechanical properties are missing on a large scale. While the human pattern of the plantar aponeurosis may not be unique among primates, it may well be derived in terms of thickness and material properties, highlighting the need to develop a full picture of the anatomical and mechanical variability of the plantar aponeurosis among primates and its relation to locomotor function. A further limitation of this study was that we had to rely on a few published descriptions of plantar aponeurosis anatomy in determining our classification scheme. Because a clear, thorough description including specific information about the central and lateral bands was necessary to code species for this study, we relied primarily on Loth (1908). While we were able to independently verify the descriptions in Loth for several of the species in this study, there were partly conflicting descriptions for plantar aponeurosis anatomy in orangutans (P. pygmaeus) in Wareing (2016). We coded the plantar aponeurosis as absent in this species because, without the description of consistent longitudinal organization or clearly defined central and lateral bands, the plantar aponeurosis cannot be clearly distinguished from the epimysium overlying the flexor digitorum brevis. Similarly, we coded the plantar aponeurosis as absent in Müeller's gibbon (Hylobates muelleri) and siamangs (Symphalangus syndactylus). Nevertheless, if these species do possess clearly defined plantar aponeuroses, several of our major findings would have to be adjusted. First, our argument that plantar aponeurosis presence is related to terrestrial locomotion would be weakened, but so would the argument that suspensory locomotion selected against a plantar aponeurosis (Lovejoy et al. 2009). Second, this finding would bolster the idea that hominins inherited a functional plantar aponeurosis from their LCA with chimpanzees, and re‐purposed this structure for bipedal locomotion. Further study of plantar aponeurosis anatomy in these and other primate species could help provide further clarity to these possibilities.
Finally, higher sample sizes and independent observations could shed more light on within‐species variation in plantaris and plantar aponeurosis anatomy. The variation described for species such as chimpanzees and orangutans (Loth, 1908; Wareing, 2016) is fascinating and begs further investigation. While our study used a simplistic coding scheme to categorize these structures, this approach may mask real species‐level variation that could be functionally relevant, as well as indicative of the directionality of selective forces. Besides inheritance and selection, variation is an essential ingredient of adaptive evolution. Some of the conflicting anatomical descriptions we encountered in our study may be attributable to heritable variation within species. Since our ancestral state reconstruction did not allow the consideration of within‐species variation, more complex evolutionary models that take such variation into account (e.g. Goolsby, 2017) could provide greater insight into the evolution of plantar aponeurosis and plantaris anatomy. Perhaps more importantly, future investigation of these structures using continuous morphological measurements will greatly enhance our understanding of their adaptive significance with regard to primate locomotion, including human bipedalism.
In conclusion, the present review of plantar aponeurosis anatomy and variation in non‐human primates reveals that the overall anatomical configuration of this structure is not unique to humans. Furthermore, our ancestral state reconstructions suggest that humans probably inherited this configuration from their LCA with chimpanzees and that a similar anatomical configuration evolved independently in different primate clades as an adaptation to terrestrial locomotion. The presence of a well‐developed plantar aponeurosis with lateral and central bands in the African apes suggests that this structure is not prohibitive to suspensory locomotion and that these species possess versatile feet adapted for both terrestrial and arboreal locomotion. Whether independently acquired or not, this plantar aponeurosis anatomy would have been advantageous in enhancing foot stiffness for bipedal locomotion in the earliest hominins, prior to the evolution of a longitudinal arch. However, it is likely that hominins evolved thicker and stiffer plantar aponeuroses alongside the arch to enable a windlass mechanism and elastic energy storage for bipedal walking and running. In the future, more work is needed to determine the adaptive functions as well as within‐species variation of the plantar aponeurosis and plantaris muscle in non‐human primates and other mammals. Nevertheless, our findings shed light on the evolutionary origins of the human plantar aponeurosis and the specialized function of this structure in the human foot.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
The authors thank Jeroen B. Smaers (Department of Anthropology, Stony Brook University, Stony Brook, NY, USA) for his advices on the phylogenetic reconstruction. The authors also thank Hanno Steinke (Institute of Anatomy, University of Leipzig, Leipzig, Germany) for dissecting a human plantar aponeurosis. We would like to thank Gilles Berillon (Département Homme et Environnement, Muséum national d'Histoire naturelle, Paris) and Anthony Herrel (Bâtiment d'Anatomie Comparée, Muséum national d'Histoire naturelle, Paris) for sharing their anatomical observations with us. Special thanks also to Páll Þórsson.
Sichting F, Holowka NB, Ebrecht F, Lieberman DE. Evolutionary anatomy of the plantar aponeurosis in primates, including humans. J. Anat.. 2020;237:85–104. 10.1111/joa.13173
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- Abrahams, P.H. (2013) McMinn and Abrahams' Clinical Atlas of Human Anatomy. Edinburgh: Mosby. [Google Scholar]
- Aerts, P. (1998) Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philosophical Transactions of the Royal Society B: Biological Sciences, 353, 1607–1620. [Google Scholar]
- Aerts, P. , D'Août, K. , Thorpe, S. , Berillon, G. and Vereecke, E. (2018) The gibbon's Achilles tendon revisited. Consequences for the evolution of the great apes? Proceedings. Biological Sciences, 285(1880). pii: 20180859. 10.1098/rspb.2018.0859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, L. and Dean, C. (1990) An Introduction to Human Evolutionary Anatomy. London, San Diego: Academic Press. [Google Scholar]
- Anapol, F. and Barry, K. (1996) Fiber architecture of the extensors of the hindlimb in semiterrestrial and arboreal guenons. American Journal of Physical Anthropology, 99, 429–447. [DOI] [PubMed] [Google Scholar]
- Arnold, C. , Matthews, L.J. and Nunn, C.L. (2010) The 10kTrees website: a new online resource for primate phylogeny. Evolutionary Anthropology: Issues, News, and Reviews, 19, 114–118. [Google Scholar]
- Bates, K.T. , Collins, D. , Savage, R. , McClymont, J. , Webster, E. , Pataky, T.C. ,, et al. (2013) The evolution of compliance in the human lateral mid‐foot. Proceedings of the Royal Society B: Biological Sciences, 280, 20131818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M.B. , Ker, R.F. and Alexander, R.M. (1989) Elastic strain energy storage in the feet of running monkeys. Journal of Zoology, 217, 469–475. [Google Scholar]
- Bojsen‐Moller, F. and Flagstad, K.E. (1976) Plantar aponeurosis and internal architecture of the ball of the foot. Journal of Anatomy, 121, 599–611. [PMC free article] [PubMed] [Google Scholar]
- Bramble, D.M. and Lieberman, D.E. (2004) Endurance running and the evolution of Homo. Nature, 432, 345–352. [DOI] [PubMed] [Google Scholar]
- Caravaggi, P. , Pataky, T. , Goulermas, J.Y. , Savage, R. and Crompton, R.H. (2009) A dynamic model of the windlass mechanism of the foot: evidence for early stance phase preloading of the plantar aponeurosis. The Journal of Experimental Biology, 212, 2491–2499. [DOI] [PubMed] [Google Scholar]
- Carlson, K.J. (2006) Muscle architecture of the common chimpanzee (Pan troglodytes): perspectives for investigating chimpanzee behavior. Primates, 47, 218–229. [DOI] [PubMed] [Google Scholar]
- Channon, A.J. , Günther, M.M. , Crompton, R.H. and Vereecke, E.E. (2009) Mechanical constraints on the functional morphology of the gibbon hind limb. Journal of Anatomy, 215, 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daseler, E.H. and Anson, B.J. (1943) The plantaris muscle: an anatomical study of 750 specimens. JBJS, 25, 822. [Google Scholar]
- Diogo, R. (2011) Photographic and Descriptive Musculoskeletal Atlas of Gorilla. With Notes on the Attachments, Variations, Innervation, Synonymy, and Weight of the Muscles. Enfield, N.H: Science Publishers. [Google Scholar]
- Diogo, R. , Potau, J.M. , Pastor, J.F. , de Paz, F.J. , Barbosa, M. , Ferrero, E.M. ,, et al. (2013a) Photographic and Descriptive Musculoskeletal Atlas of Orangutans: With Notes on the Attachments, Variations, Innervations, Function and Synonymy and Weight of the Muscles. Boca Raton, FL: CRC Press, Taylor & Francis Group. [Google Scholar]
- Diogo, R. (2013b) Photographic and Descriptive Musculoskeletal Atlas of Chimpanzees. With Notes on the Attachments, Variations, Innervation, Function and Synonymy and Weight of the Muscles. Boca Raton, FL: CRC Press, Taylor & Francis Group. [Google Scholar]
- Doran, D.M. (1993) Sex differences in adult chimpanzee positional behavior: the influence of body size on locomotion and posture. American Journal of Physical Anthropology, 91, 99–115. [DOI] [PubMed] [Google Scholar]
- Doran, D.M. (1997) Ontogeny of locomotion in mountain gorillas and chimpanzees. Journal of Human Evolution, 32, 323–344. [DOI] [PubMed] [Google Scholar]
- Dylevský, I. (1991) Aponeurosis plantaris‐phylogenetic development. Sbornik Lekarsky, 93, 1–5. [PubMed] [Google Scholar]
- Fleagle, J. , Stern, J.T. , Jungers, W. , Susman, R.L. , Vangor, A.K. and Wells, J.P. (1981) Climbing: A biomechanical link with brachiation and with bipedalism. Symposia of the Zoological Society of London, 48, 359–375. [Google Scholar]
- Fleagle, J.G. (2013) Primate Adaptation and Evolution. Amsterdam, Boston: Academic Press. [Google Scholar]
- García‐Esponda, C.M. and Candela, A.M. (2016) Hindlimb musculature of the largest living rodent Hydrochoerus hydrochaeris (Caviomorpha): Adaptations to semiaquatic and terrestrial styles of life. Journal of Morphology, 277, 286–305. [DOI] [PubMed] [Google Scholar]
- Garrow, A.P. , Silman, A.J. and Macfarlane, G.J. (2004) The Cheshire Foot Pain and Disability Survey: a population survey assessing prevalence and associations. Pain, 110, 378–384. [DOI] [PubMed] [Google Scholar]
- Gebo, D.L. (1992) Plantigrady and foot adaptations in African apes: implications for hominid origins. American Journal of Physical Anthropology, 89, 29–58. [DOI] [PubMed] [Google Scholar]
- Gebo, D.L. (2005) The nature of the primate grasping foot. American Journal of Physical Anthropology, 67, 269–277. [Google Scholar]
- Gosselin‐Ildari, A.D. (2013) The Evolution of Cercopithecoid Locomotion: A Morphometric, Phylogenetic, and Character Mapping Approach: The Graduate School. Stony Brook, NY: Stony Brook University. [Google Scholar]
- Griffin, N.L. , Miller, C.E. , Schmitt, D. and D’Aoüt, K. (2015) Understanding the evolution of the windlass mechanism of the human foot from comparative anatomy: insights, obstacles, and future directions. American Journal of Physical Anthropology, 156, 1–10. [DOI] [PubMed] [Google Scholar]
- Goolsby, E.W. (2017) Rapid maximum likelihood ancestral state reconstruction of continuous characters: a rerooting‐free algorithm. Ecology and Evolution, 7, 2791–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. , Liu, X. , Ding, X. , Wang, L. and Fan, Y. (2018) Biomechanical and mechanical behavior of the plantar fascia in macro and micro structures. Journal of Biomechanics, 76, 160–166. [DOI] [PubMed] [Google Scholar]
- Hanna, J.B. and Schmitt, D. (2011) Comparative triceps surae morphology in primates: a review. Anatomy Research International, 2011, 191509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, C.G. , Straus, W.L. and Bast, T.H. (1933) The Anatomy of the Rhesus Monkey (Macaca mulatta). Baltimore: Williams & Wilkins. [Google Scholar]
- Hicks, J.H. (1954) The mechanics of the foot. Journal of Anatomy, 88, 25. [PMC free article] [PubMed] [Google Scholar]
- Hicks, J.H. (1955) The foot as a support. Cells Tissues Organs, 25, 34–45. [Google Scholar]
- Hill, J.J. and Cutting, P.J. (1989) Heel pain and body weight. Foot & Ankle, 9, 254–256. [DOI] [PubMed] [Google Scholar]
- Hirasaki, E. and Kumakura, H. (2010) Estimating the functional axis of the primate foot using the distribution of plantar muscles. International Journal of Primatology, 31, 239–261. [Google Scholar]
- Holowka, N.B. , O'Neill, M.C. , Thompson, N.E. and Demes, B. (2017) Chimpanzee ankle and foot joint kinematics: arboreal versus terrestrial locomotion. American Journal of Physical Anthropology, 164, 131–147. [DOI] [PubMed] [Google Scholar]
- Holowka, N.B. and Lieberman, D.E. (2018) Rethinking the evolution of the human foot: insights from experimental research. The Journal of Experimental Biology, 221(17). 10.1242/jeb.174425 [DOI] [PubMed] [Google Scholar]
- Kalicharan, A. , Pillay, P. , Rennie, C.O. , De, G. and Satyapal, K.S. (2017) The plantar aponeurosis in fetuses and adults: an aponeurosis or fascia? International Journal of Morphology, 35, 684–690. [Google Scholar]
- Kaya, B.K. (1996) Plantar fasciitis in athletes. Journal of Sport Rehabilitation, 5, 305–320. [Google Scholar]
- Keith, A. (1894) The ligaments of the Catarrhine Monkeys, with references to corresponding structures in man. Journal of Anatomy and Physiology, 28, 149–168. [PMC free article] [PubMed] [Google Scholar]
- Ker, R.F. , Bennett, M.B. , Bibby, S.R. , Kester, R.C. and Alexander, R.M. (1987) The spring in the arch of the human foot. Nature, 325, 147–149. [DOI] [PubMed] [Google Scholar]
- Kivell, T.L. and Schmitt, D. (2009) Independent evolution of knuckle‐walking in African apes shows that humans did not evolve from a knuckle‐walking ancestor. Proceedings of the National Academy of Sciences, 106, 14241–14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon, J.H. (1990) Variations in cruropedal musculature. International Journal of Primatology, 11, 575–606. [Google Scholar]
- Lapidus, P.W. (1963) Kinesiology and mechanical anatomy of the tarsal joints. Clinical Orthopaedics and Related Research, 30, 20–36. [PubMed] [Google Scholar]
- Lewis, O.J. (1962) The phylogeny of the crural and pedal flexor musculature. Journal of Zoology, 138, 77–109. [Google Scholar]
- Loth, E. (1908) Die Aponeurosis plantaris in der Primatenreihe. Mit spezieller Berücksichtigung des Menschen. Eine vergleichend‐morphologische und antropologische Untersuchung. Gegenbauers Morphologisches Jahrbuch, 38, 194–322. [Google Scholar]
- Lovejoy, C.O. , Latimer, B. , Suwa, G. , Asfaw, B. and White, T.D. (2009) Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science, 326, 72e1–72e8. [PubMed] [Google Scholar]
- Maddison, W.P. and Maddison, D.R. (2006) StochChar: A package of Mesquite modules for stochastic models of character evolution. Version 1.1. Available from: http://mesquiteproject.org
- Maddison, W.P. and Maddison, D.R. (2018) Mesquite: A modular system for evolutionary analysis. Available from: http://mesquiteproject.org.
- McClearn, D. (2005) Anatomy of raccoon (Procyon lotor) and coati (Nasua narica and N. nasua) forearm and leg muscles: relations between fiber length, moment‐arm length, and joint‐angle excursion. Journal of Morphology, 183, 87–115. [DOI] [PubMed] [Google Scholar]
- McDonald, K.A. , Stearne, S.M. , Alderson, J.A. , North, I. , Pires, N.J. and Rubenson, J. (2016) The role of arch compression and metatarsophalangeal joint dynamics in modulating plantar fascia strain in running. PLoS ONE, 11, e0152602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz, H.B. , Jordan, K.P. , Roddy, E. and Croft, P.R. (2010) Characteristics of primary care consultations for musculoskeletal foot and ankle problems in the UK. Rheumatology (Oxford, England), 49, 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K.L. , Dalley, A.F. and Agur, A.M.R. (2014) Clinically Oriented Anatomy. Philadelphia: Wolters Kluwer / Lippincott Williams & Wilkins. [Google Scholar]
- Murie, J. and Mivart, S.G. (1869) On the anatomy of the lemuroidea. The Transactions of the Zoological Society of London, 7, 1–113. [Google Scholar]
- Netter, F.H. (2014) Atlas of Human Anatomy. Munich: Elsevier, Urban & Fischer (Munich). [Google Scholar]
- Owen, D. and Pemberton, D. (2011) Tasmanian devil. A unique and threatened animal. Crows Nest, NSW: Allen & Unwin. [Google Scholar]
- Pagel, M. (1994) Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London, Series B: Biological Sciences, 255, 37–45. [Google Scholar]
- Payne, R.C. , Crompton, R.H. , Isler, K. , Savage, R. , Vereecke, E.E. , Günther, M.M. , et al. (2006) Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. Journal of Anatomy, 208, 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C.R. (2004) Phylogenetic analysis of the Australian genus Pseudophryne (Myobatrachidae) using morphological characters. United States ‐ Tennessee.
- Pilbeam, D.R. and Lieberman, D.E. (2017) Reconstructing the last common ancestor of chimpanzees and humans In: Muller M.N., Pilbeam D.R. and Wrangham R.W. (Eds.) Chimpanzees and Human Evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- Raven, H.C. (1936) Comparative anatomy of the sole of the foot. American Museum novitates, no. 871.
- Richmond, B.G. , Begun, D.R. and Strait, D.S. (2001) Origin of human bipedalism. The knuckle‐walking hypothesis revisited. American journal of physical anthropology, 116, 70–105. [DOI] [PubMed] [Google Scholar]
- Riddle, D.L. and Schappert, S.M. (2004) Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot & Ankle International, 25, 303–310. [DOI] [PubMed] [Google Scholar]
- Rome, K. (1997) Anthropometric and biomechanical risk factors in the development of plantar heel pain—a review of the literature. Physical Therapy Reviews, 2, 123–134. [Google Scholar]
- Rome, K. , Howe, T. and Haslock, I. (2001) Risk factors associated with the development of plantar heel pain in athletes. The Foot, 11, 119–125. [Google Scholar]
- Rowe, N. , Goodall, J. and Mittermeier, R.A. (1999) The Pictorial Guide to the Living Primates. Charlestown, RI: Pogonias Press. [Google Scholar]
- Sarmiento, E.E. (1983) The significance of the heel process in anthropoids. International Journal of Primatology, 4, 127–152. [Google Scholar]
- Sarmiento, E.E. (1994) Terrestrial traits in the hands and feet of gorillas. American Museum novitates; no. 3091.
- Sarrafian, S.K. (2011) Anatomy of the Foot and Ankle. Descriptive, Topographic, Functional. Philadelphia: J.B. Lippincott. [Google Scholar]
- Schluter, D. , Price, T. , Mooers, A.Ø. and Ludwig, D. (1997) Likelihood of ancestor states in adaptive radiation. Evolution, 51, 1699–1711. [DOI] [PubMed] [Google Scholar]
- Schmitt, D. and Larson, S.G. (1995) Heel contact as a function of substrate type and speed in primates. American Journal of Physical Anthropology, 96, 39–50. [DOI] [PubMed] [Google Scholar]
- Sefczek, T.M. and Dunham, N.T. (2014) Forelimb and Hindlimb Musculature of the Crab‐Eating Macaque (Macaca fascicularis). Available from: http://hdl.handle.net/1811/65221. [Google Scholar]
- Simpson, S.W. , Levin, N.E. , Quade, J. , Rogers, M.J. and Semaw, S. (2019) Ardipithecus ramidus postcrania from the Gona Project area, Afar Regional State, Ethiopia. Journal of human evolution, 129, 1–45. [DOI] [PubMed] [Google Scholar]
- Sinnatamby, C.S. and Last, R.J. (2011) Last's Anatomy. Regional and Applied. Edinburgh, New York: Churchill Livingstone/Elsevier. [Google Scholar]
- Standring, S. and Gray, H. (2016) Gray's Anatomy. The Anatomical Basis of Clinical Practice. Oxford: Elsevier. [Google Scholar]
- Stearne, S.M. , McDonald, K.A. , Alderson, J.A. , North, I. , Oxnard, C.E. and Rubenson, J. (2016) The foot’s arch and the energetics of human locomotion. Scientific Reports, 6, 19403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecco, C. , Corradin, M. , Macchi, V. , Morra, A. , Porzionato, A. , Biz, C. , et al. (2013) Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. Journal of Anatomy, 223, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilwell, D.L. (1957) The innervation of deep structures of the foot. American Journal of Anatomy, 101, 59–73. [DOI] [PubMed] [Google Scholar]
- Straus, W.L. (1930) The foot musculature of the highland Gorilla (Gorilla beringei). The Quarterly Review of Biology, 5, 261–317. [Google Scholar]
- Susman, R.L. (1983) Evolution of the human foot: evidence from Plio-Pleistocene hominids. Foot Ankle, 3, 365–376. [DOI] [PubMed] [Google Scholar]
- Swindler, D.R. and Wood, C.D. (1982) Atlas of Primate Gross Anatomy: Baboon, Chimpanzee, and Man. Malabar, FL: Krieger Publishing Company. [Google Scholar]
- Thorpe, S.K. , Crompton, R.H. , Günther, M.M. , Ker, R.F. and McNeill, A.R. (1999) Dimensions and moment arms of the hind‐ and forelimb muscles of common chimpanzees (Pan troglodytes). American Journal of Physical Anthropology, 110(2), 179–199. [DOI] [PubMed] [Google Scholar]
- Vereecke, E.E. , D’Août, K. , Payne, R. and Aerts, P. (2005) Functional analysis of the foot and ankle myology of gibbons and bonobos. Journal of Anatomy, 206, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke, E.E. and Aerts, P. (2008) The mechanics of the gibbon foot and its potential for elastic energy storage during bipedalism. The Journal of Experimental Biology, 211, 3661–3670. [DOI] [PubMed] [Google Scholar]
- Wager, J.C. and Challis, J.H. (2016) Elastic energy within the human plantar aponeurosis contributes to arch shortening during the push‐off phase of running. Journal of Biomechanics, 49, 704–709. [DOI] [PubMed] [Google Scholar]
- Warburton, N.M. , Yakovleff, M. and Malric, A. (2012) Anatomical adaptations of the hind limb musculature of tree‐kangaroos for arboreal locomotion (Marsupialia : Macropodinae). Australian Journal of Zoology, 60, 246–258. [Google Scholar]
- Wareing, K.A. (2016) Adaptation of the non‐human great ape lower limb in response to locomotor behaviour. Dissertation. Liverpool. Available from http://livrepository.liverpool.ac.uk/3001676/.
- Wearing, S.C. , Smeathers, J.E. , Urry, S.R. , Hennig, E.M. and Hills, A.P. (2006) The pathomechanics of plantar fasciitis. Sports Medicine, 36, 585–611. [DOI] [PubMed] [Google Scholar]
- White, T.D. , Lovejoy, C.O. , Asfaw, B. , Carlson, J.P. and Suwa, G. (2015) Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proceedings of the National Academy of Sciences of the United States of America, 112, 4877–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham, R. and Pilbeam, D.R. (2002) African Apes as Time Machines In Galdikas B.M.F., Briggs N.E., Sheeran L.K., Shapiro G.L. and Goodall J. (Eds.) All Apes Great and Small. Volume 1: African Apes. Boston, MA: Kluwer Academic Publishers. [Google Scholar]
- Wright, D.G. and Rennels, D.C. (1964) A study of the elastic properties of plantar fascia. The Journal of Bone & Joint Surgery, 46, 482–492. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


