Abstract
Aryl sulfinates are precursors to a diverse number of sulfonyl-derived arenes, which are common motifs in pharmaceuticals and agrochemicals. Here, we report a site-selective two-step C–H sulfination sequence via aryl sulfonium salts to access aryl sulfonamides. Combined with site-selective aromatic thianthrenation, an operationally simple one-pot palladium-catalyzed protocol introduces the sulfonyl group using sodium hydroxymethylsulfinate (Rongalite) as a source of SO22–. The hydroxymethyl sulfone intermediate generated from the catalytic process can be employed as a synthetic handle to deliver a variety of sulfonyl-containing compounds.
Sulfur occurs in several different oxidation states; the most stable hexavalent organosulfur compounds such as sulfonamides, sulfones, and sulfonyl fluorides are abundant motifs in pharmaceuticals and agrochemicals.1 Commonly, sulfonyl functionality is introduced into arenes by electrophilic aromatic substitution with reagents such as chlorosulfuric acid.2 The limitations of such transformations are the formation of constitutional isomers and the low functional group tolerance.3 Synthetically valuable sulfonyl-containing molecules can be obtained by using aryl sulfinates as versatile intermediates, which can be formed from prefunctionalized arenes such as aryl halides,4 aryl boronic acids,5,6 aryl Grignard reagents,7,8 or aryl iodonium salts9 in the presence of sulfur dioxide surrogates.10 Additionally, primary11,12 and secondary13 sulfonamides can generate sulfinates in situ acting as terminal functional groups. However, the site selectivity of the synthesis of the starting materials remains a challenge for many of those substrates.14−16 Here, we present a two-step C–H sulfination sequence of site-selectively formed aryl thianthrenium salts under the action of a palladium catalyst and the inexpensive industrial reagent sodium hydroxymethanesulfinate (Rongalite) to synthetically access the sulfinate salt precursor, hydroxymethylsulfone. Subsequent electrophilic trapping of the sulfinate can be useful for functional group diversification (Scheme 1).
Scheme 1. Synthetic Approaches to Aryl Sulfonyl Groups.
Given that sulfinates can often be difficult to purify, strategies for accessing sulfonamides among other valuable sulfonyl-containing groups entail a two-step one-pot procedure by using the aryl sulfinate in a subsequent transformation.17−23 Aryl sulfinates can be obtained from the reaction of aryl nucleophiles with SO2, which is a toxic gas and is therefore frequently replaced with solid SO2 surrogates such as the adduct of SO2 with DABCO, called DABSO.24 Suitable aryl nucleophiles are Grignard reagents,7 aryl-zinc compounds,8 and arylboronic acids,5,6 all of which react well with DABSO. The generation of arylsulfinates from aryl electrophiles such as aryl halides4 and SO2 (surrogates) is also possible when using an additional reducing agent.19 An alternative route for generating sulfinates from aryl electrophiles, without the need for an exogenous reducing reagent, would be the reaction with a source of SO22–, such as Rongalite (sodium hydroxymethylsulfinate), an industrial bleaching and reducing reagent.25 While alkyl halides have been converted to alkyl sulfinates by reaction with Rongalite,26 methods for achieving aromatic sulfinylation with Rongalite remain unexplored. Although previous methods for making aryl sulfinates are practical when synthetic handles already exist, selective installation of halo or boryl substituents at a late stage can be challenging.14−16 The combination of site-selective thianthrenation and the first example of a palladium-catalyzed C–S bond forming reaction using Rongalite grants access to aryl hydroxymethyl sulfones, masked sulfinates that undergo a base-mediated fragmentation to release aryl sulfinates.
In our previous work,27 we capitalized on the exquisite selectivity of aromatic C–H thianthrenation for subsequent site-selective functionalization in a two-step process to access various functional groups via palladium or photoredox catalysis.28−31 In this study, we envisioned a synthetic strategy for installing a masked sulfinate via a cross-coupling between aryl sulfonium salts and Rongalite. In contrast to our previous sulfone synthesis,27 the sulfinate precursor can be used in situ for further derivatization.32
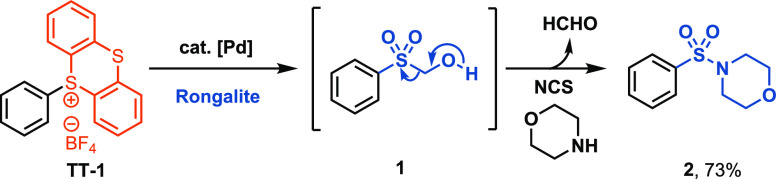
We developed reaction conditions to synthesize aryl hydroxymethyl sulfones [1 (Scheme 2)] from aryl thianthrenium salts via a palladium-catalyzed C–S bond formation by employing Pd(dppf)Cl2 as the catalyst and Rongalite as the coupling partner in iPrOH at 60 °C. The structure of 1 was confirmed by NMR spectroscopy and high-resolution mass spectrometry (see the Supporting Information). In the presence of a base, intermediate 1 loses formaldehyde, and the aryl sulfinate is generated in situ. The oxidative amination of the resulting aryl sulfinate with Et3N (2.0 equiv), morpholine (2.0 equiv), and N-chlorosuccinimide (NCS) (2.0 equiv) at 25 °C for 1 h resulted in sulfonamide 2 in 73% yield from the corresponding aryl sulfonium salt TT-1 (Scheme 2).
Scheme 2. Formation of Phenyl Hydroxymethyl Sulfone en Route to a Sulfonamide.
Two-step yield of the sulfonylation. Reaction conditions: (i) sulfonium salt (0.2 mmol), Pd(dppf)Cl2 (5 mol %), Rongalite (1.5 equiv), i-PrOH (0.2 M), 60 °C, 12 h; (ii) Et3N (2.0 equiv), morpholine (2.0 equiv), NCS (2.0 equiv), 25 °C, 1 h.
The optimal reaction conditions proved to be effective for generating a variety of structurally diverse sulfonamides with respect to the sulfonium salts, using morpholine as a representative amine component (Scheme 3A). Alkyl-substituted aryl sulfonamides (3–5) were obtained in 45–64% yields. A range of electron-rich arenes reacted under our conditions, providing aryl sulfonamides (6–9) in 51–65% yields. ortho-Substituted sulfonamides 10 and 11 were obtained in 50% and 77% yields, respectively. The hydrodefunctionalized compound was identified as the major side product for these substrates. Under our coupling conditions, the reactivity of sulfonium salts exceeds the reactivity of standard palladium cross-coupling partners, bromo and triflate groups, and compounds 12 and 13 were obtained in 74% and 60% yields, respectively. As a further demonstration of the utility of this methodology, late-stage functionalization of several active pharmaceuticals and agrochemicals was performed. For these more complex sulfonium salts (15–20), the solubility in isopropanol was low and proved to be an obstacle to achieving full conversion. However, when using the polar aprotic acetonitrile as a co-solvent, conversion improved and the morpholino sulfonyl compounds were obtained in 20–67% yields.
Scheme 3. Evaluation of Thianthrenium Salts for the Palladium-Catalyzed Coupling with Rongalite toward the Synthesis of Sulfonamides.
Reaction conditions: (1) sulfonium salt (0.1–0.2 mmol), Pd(dppf)Cl2 (5 mol %), Rongalite (1.5 equiv), i-PrOH (0.2 M), 60 °C, 12 h; (2) Et3N (2.0 equiv), R1R2NH (2.0 equiv), NCS (2.0 equiv), 25 °C, 1 h.
Yield of thianthrenation.
Two-step yield of sulfonylation.
Yield of the thianthrenation from ref (28).
Yield of the thianthrenation from ref (27).
Yield of the thianthrenation from ref (29).
MeCN was used as a co-solvent.
Two-step yield of the sulfonylation with (2) hydroxylamine-O-sulfonic acid (4.0 equiv) and sodium acetate (7.0 equiv), at 25 °C for 1 h, instead of Et3N and NCS.
We tested our two-step one-pot procedure with a set of primary (22–25), benzylic (26 and 27), and secondary (28–37) amines (Scheme 3B), resulting in 45–91% yields. In addition, the ammonia-derived sulfonamide 21 could be obtained in 62% yield with hydroxylamine-O-sulfonic acid in the presence of sodium acetate.
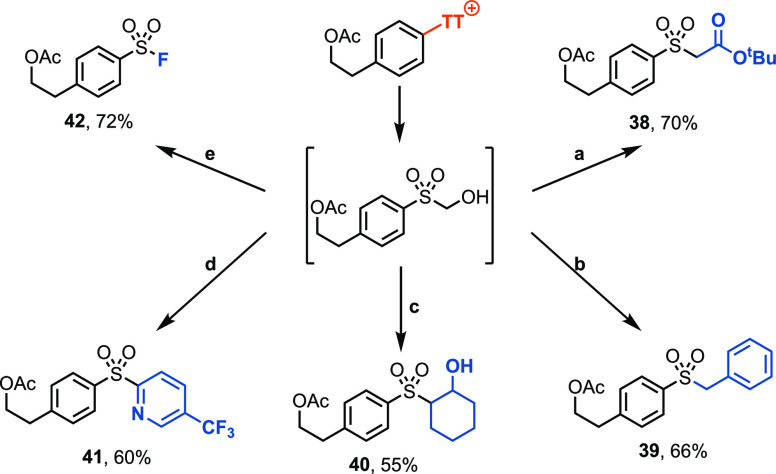
Finally, we evaluated several common electrophiles in the synthesis of sulfone derivatives via the hydroxymethyl sulfone intermediate (Scheme 4). The reaction with alkyl electrophiles, such as alkyl bromides or epoxides, afforded the alkylaryl sulfones (38–40) in 55–70% yields. Trapping with a heteroaryl electrophile in a nucleophilic aromatic substitution reaction yielded an aryl-heteroaryl sulfone (41) in 60% yield. Sulfonyl fluoride (42) can be obtained in 72% yield by reaction with the electrophilic fluorinating reagent N-fluorobenzenesulfonimide.33
Scheme 4. Functional Group Diversification via an Aryl Hydroxymethyl Sulfone.
For detailed experimental procedures, see the Supporting Information. The corresponding electrophiles were reacted: (a) α-bromo tert-butyl acetate, (b) benzyl bromide, (c) cyclohexene oxide, (d) 2-chloro-5-(trifluoromethyl)pyridine, and (e) N-fluorobenzenesulfonimide.
In conclusion, we have identified the readily available and inexpensive SO22– source, Rongalite, as a coupling partner in the palladium-catalyzed sulfination of aryl sulfonium salts. Besides a highly selective C–H functionalization, the two-step sequence grants access to valuable sulfinate precursors that can subsequently be unmasked and afford sulfonamides, which are important functional motifs in pharmaceuticals and agrochemicals among sulfones and sulfonyl fluorides.
Acknowledgments
The authors thank the MPI für Kohlenforschung for funding. The authors thank C. Farés and M. Kochius (MPI KOFO) for assistance with NMR structural assignments and S. Marcus and D. Kampen (MPI KOFO) for mass spectroscopy analysis. The authors thank P. S. Engl, A. Häring, and F. Ye (MPI KOFO) for providing aryl sulfonium salts. The cover was designed/conceived by Dr. Florian Berger and created by Dr. Florian Berger and Georg Berger with assistance by Luz Marily Ortiz Mendieta.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c00982.
Detailed experimental procedures and spectroscopic characterization (PDF)
Author Contributions
E.M.A. and M.B.P. developed the sulfination reaction. E.M.A. optimized follow-up transformations. All authors wrote the manuscript. T.R. directed the project.
The authors declare the following competing financial interest(s): A patent application (EP18204755.5, Germany) dealing with the use of thianthrene and its derivatives for CH functionalization has been filed, and F.B. and T.R. may benefit from royalty payments.
Supplementary Material
References
- Feng M.; Tang B.; Liang S. H.; Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss B. S.; Hannaford A. J.; Smith P. W. G.; Tatchell A. R. In Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific and Technical, 1989; p 877. [Google Scholar]
- Bassin J. P.; Cremlyn R. J.; Swinbourne F. J. Review. Chlorosulfonation of Aromatic and Hetero-Aromatic Systems. Phosphorus, Sulfur Silicon Relat. Elem. 1991, 56, 245–275. 10.1080/10426509108038091. [DOI] [Google Scholar]
- Deeming A. S.; Russell C. J.; Willis M. C. Palladium(II)-Catalyzed Synthesis of Sulfinates from Boronic Acids and DABSO: A Redox-Neutral, Phosphine-Free Transformation. Angew. Chem., Int. Ed. 2016, 55, 747–750. 10.1002/anie.201508370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett E. J.; Hayter B. R.; Willis M. C. Palladium-Catalyzed Synthesis of Ammonium Sulfinates from Aryl Halides and a Sulfur Dioxide Surrogate: A Gas- and Reductant-Free Process. Angew. Chem., Int. Ed. 2014, 53, 10204–10208. 10.1002/anie.201404527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBergh J. R.; Niljianskul N.; Buchwald S. L. Synthesis of Aryl Sulfonamides via Palladium-Catalyzed Chlorosulfonylation of Aryl Boronic Acids. J. Am. Chem. Soc. 2013, 135, 10638–10641. 10.1021/ja405949a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeming A. S.; Russell C. J.; Hennessy A. J.; Willis M. C. DABSO-Based, Three Component, One-Pot Sulfone Synthesis. Org. Lett. 2014, 16, 150–153. 10.1021/ol403122a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke B. N.; Bahnck K. B.; Herr M.; Lavergne S.; Mascitti V.; Perreault C.; Polivkova J.; Shavnya A. Synthesis of Sulfones from Organozinc Reagents, DABSO, and Alkyl Halides. Org. Lett. 2014, 16, 154–157. 10.1021/ol4031233. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Luo M. Iron-catalyzed synthesis of arylsulfinates through radical coupling reaction. Chem. Commun. 2016, 52, 2980–2983. 10.1039/C5CC09830K. [DOI] [PubMed] [Google Scholar]
- Emmett E. J.; Willis M. C. The Development and Application of Sulfur Dioxide Surrogates in Synthetic Organic Chemistry. Asian J. Org. Chem. 2015, 4, 602–611. 10.1002/ajoc.201500103. [DOI] [Google Scholar]
- Fier P. S.; Maloney K. M. NHC-Catalyzed Deamination of Primary Sulfonamides: A Platform for Late-Stage functionalization. J. Am. Chem. Soc. 2019, 141, 1441–1445. 10.1021/jacs.8b11800. [DOI] [PubMed] [Google Scholar]
- Gómez-Palomino A.; Cornella J. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4. Angew. Chem., Int. Ed. 2019, 58, 18235–18239. 10.1002/anie.201910895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fier P. S.; Kim S.; Maloney K. M. Reductive Cleavage of Secondary Sulfonamides: Converting Terminal Functional Groups into Versatile Synthetic Handles. J. Am. Chem. Soc. 2019, 141, 18416–18420. 10.1021/jacs.9b10985. [DOI] [PubMed] [Google Scholar]
- Ishiyama T.; Takagi J.; Ishida K.; Miyaura N.; Anastasi N. R.; Hartwig J. F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- Saito Y.; Segawa Y.; Itami K. para-C-H Borylation of Benzene Derivatives by a Bulky Iridium Catalysts. J. Am. Chem. Soc. 2015, 137, 5193–5198. 10.1021/jacs.5b02052. [DOI] [PubMed] [Google Scholar]
- Tang R.-J.; Milcent T.; Crousse B. Regioselective Halogenation of Arenes and Heterocycles in Hexafluoroisopropanol. J. Org. Chem. 2018, 83, 930–938. 10.1021/acs.joc.7b02920. [DOI] [PubMed] [Google Scholar]
- Deeming A. S.; Emmett E. J.; Richards-Taylor C. S.; Willis M. C. Rediscovering the Chemistry of Sulfur Dioxide: New developments in Synthesis and Catalysis. Synthesis 2014, 46, 2701–2710. 10.1055/s-0034-1379042. [DOI] [Google Scholar]
- Chen Y.; Willis M. C. Copper (I)-Catalyzed Sulfonylative Suzuki-Miyaura Cross-Coupling. Chem. Sci. 2017, 8, 3249–3253. 10.1039/C6SC05483H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. K. In Sulfonic Acids and Their Derivatives in Comprehensive Organic Chemistry; Barton D. H. R., Ollis W. D., Jones D. N., Eds.; Pergamon Press: Oxford, U.K., 1979; Vol. 3, pp 331– 350. [Google Scholar]
- Deeming A. S.; Russell C. J.; Willis M. C. Combining Organometallic Reagents, the Sulfur Dioxide Surrogate DABSO, and Amines: A One-Pot Preparation of Sulfonamides, Amenable to Array Synthesis. Angew. Chem., Int. Ed. 2015, 54, 1168–1171. 10.1002/anie.201409283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Shen Y.; Deng Q.; Huang C.; Tu T. One-Pot Bimetallic Pd/Cu-Catalyzed Synthesis of Sulfonamides from Boronic Acids, DABSO and O-Benzoyl Hydroxylamines. Chem. - Asian J. 2017, 12, 706–712. 10.1002/asia.201601732. [DOI] [PubMed] [Google Scholar]
- Flegeau E. F.; Harrison J. M.; Willis M. C. One-Pot Sulfonamide Synthesis Exploiting the Palladium Catalyzed Sulfination of Aryl Iodides. Synlett 2015, 27, 101–105. 10.1055/s-0035-1560578. [DOI] [Google Scholar]
- Lo P. K. T.; Chen Y.; Willis M. C. Nickel (II)-Catalyzed Synthesis of Sulfinates from Aryl and Heteroaryl Boronic Acids and the Sulfur Dioxide Surrogate DABSO. ACS Catal. 2019, 9, 10668–10673. 10.1021/acscatal.9b04363. [DOI] [Google Scholar]
- Woolven H.; González-Rodríguez C.; Marco I.; Thompson A. L.; Willis M. C. DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide preparation. Org. Lett. 2011, 13, 4876–4878. 10.1021/ol201957n. [DOI] [PubMed] [Google Scholar]
- Kotha S.; Khedkar P. Rongalite: A Useful Green Reagent in Organic Synthesis. Chem. Rev. 2012, 112, 1650–1680. 10.1021/cr100175t. [DOI] [PubMed] [Google Scholar]
- Shavnya A.; Coffey S. B.; Hesp K. D.; Ross S. C.; Tsai A. S. Reaction of Alkyl Halides with Rongalite: One-Pot and Telescoped Synthesis of Aliphatic Sulfonamides, Sulfonyl Fluorides and Unsymmetrical Sulfones. Org. Lett. 2016, 18, 5848–5851. 10.1021/acs.orglett.6b02894. [DOI] [PubMed] [Google Scholar]
- Berger F.; Plutschack M. B.; Riegger J.; Yu W.; Speicher S.; Ho M.; Frank N.; Ritter T. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 2019, 567, 223–228. 10.1038/s41586-019-0982-0. [DOI] [PubMed] [Google Scholar]
- Engl P. S.; Häring A. P.; Berger F.; Berger G.; Peréz-Bitrián A.; Ritter T. C-N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141, 13346–13351. 10.1021/jacs.9b07323. [DOI] [PubMed] [Google Scholar]
- Ye F.; Berger F.; Jia H.; Ford J.; Wortman A.; Borgel J.; Genicot C.; Ritter T. Aryl Sulfonium Salts for Site-Selective Late-Stage Trifluoromethylation. Angew. Chem., Int. Ed. 2019, 58, 14615–14619. 10.1002/anie.201906672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang R.; Korkis S.; Su W.; Ye F.; Engl P.; Berger F.; Ritter T. Site-Selective C-H Oxygenation via Aryl Sulfonium Salts. Angew. Chem., Int. Ed. 2019, 58, 16161–16166. 10.1002/anie.201908718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Chen J.; Sang R.; Ham W.-S.; Plutschack M. B.; Berger F.; Chabbra S.; Schnegg A.; Genicot C.; Ritter T. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 2020, 12, 56–62. 10.1038/s41557-019-0353-3. [DOI] [PubMed] [Google Scholar]
- Willis M. C. New catalytic reactions using sulfur dioxide. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 654–657. 10.1080/10426507.2019.1602623. [DOI] [Google Scholar]
- Davies A. T.; Curto J. M.; Bagley S. W.; Willis M. C. One-Pot Palladium-Catalyzed Synthesis of Sulfonyl Fluorides from Aryl Bromides. Chem. Sci. 2017, 8, 1233–1237. 10.1039/C6SC03924C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.