Abstract
There is increasing evidence that the ∼20 routinely monitored perfluoroalkyl and polyfluoroalkyl substances (PFASs) account for only a fraction of extractable organofluorine (EOF) occurring in the environment. To assess whether PFAS exposure is being underestimated in marine mammals from the Northern Hemisphere, we performed a fluorine mass balance on liver tissues from 11 different species using a combination of targeted PFAS analysis, EOF and total fluorine determination, and suspect screening. Samples were obtained from the east coast United States (US), west and east coast of Greenland, Iceland, and Sweden from 2000 to 2017. Of the 36 target PFASs, perfluorooctane sulfonate (PFOS) dominated in all but one Icelandic and three US samples, where the 7:3 fluorotelomer carboxylic acid (7:3 FTCA) was prevalent. This is the first report of 7:3 FTCA in polar bears (∼1000 ng/g, ww) and cetaceans (<6–190 ng/g, ww). In 18 out of 25 samples, EOF was not significantly greater than fluorine concentrations derived from sum target PFASs. For the remaining 7 samples (mostly from the US east coast), 30–75% of the EOF was unidentified. Suspect screening revealed an additional 37 PFASs (not included in the targeted analysis) bringing the total to 63 detected PFASs from 12 different classes. Overall, these results highlight the importance of a multiplatform approach for accurately characterizing PFAS exposure in marine mammals.
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are a diverse class of chemicals used throughout society.1,2 Perfluoroalkyl chains possess a wide range of unique properties, including extreme stability and combined oil/water repellency. These attributes have led to the use of PFASs in a broad range of products, including fire-fighting foams, textiles, nonstick cookware, food wrapping paper, paints, and cosmetics, in addition to many other industrial applications.3,4 The most well-studied PFASs are the perfluoroalkyl acids (PFAAs), in particular the perfluoroalkyl carboxylic acids (PFCAs), such as perfluorooctanoic acid (PFOA), and the perfluoroalkyl sulfonic acids (PFSAs), such as perfluorooctanoic sulfonic acid (PFOS). PFSAs and PFCAs are suggested to be the final breakdown products of most PFASs.5
The bioaccumulation potential of PFASs is strongly correlated with perfluoroalkyl chain length; structures containing ≥8 fluorinated carbons for PFCAs and ≥6 fluorinated carbons for PFSAs are considered bioaccumulative.6−8 PFAAs are present in the blood of humans and wildlife globally, including remote polar regions.9−11 Unlike classical persistent organic pollutants (e.g., polychlorinated biphenyls), PFASs accumulate primarily in protein-rich tissues such as liver and blood.12 PFASs have been linked to various toxicological effects, for example, reproductive deficits,13,14 immunotoxicity,15,16 thyroid hormone disruption,17−19 and disturbance of lipid metabolism.20 Because of their persistent, bioaccumulative, and toxic properties as well as their widespread distribution, PFASs have received global attention over the last few decades leading to several regulatory initiatives.21−23 However, development and manufacturing of alternative PFASs (which are largely uncharacterized in terms of risks) remains ongoing, despite an increasing number of examples of their environmental occurrence,24,25 and therefore, they are hard to detect or often overseen in analyses of environmental samples and wildlife tissue samples.
Recent research by the Organization for Economic Co-operation and Development (OECD) identified 4730 CAS numbers related to PFASs.2 However, considering that only a small fraction (<20) of these substances are routinely monitored, PFAS exposure may be underestimated. Indeed, the large quantities of unidentified extractable organofluorine (EOF) in environmental samples (56–100%),26−29 cosmetics (68–100%),30 aqueous film forming foam (AFFF; ∼50%),31 human blood (15–67%),32 and wildlife (68–90%)33,34 are a cause for considerable concern. Moreover, recent investigations using nontarget and suspect-screening analytical workflows have uncovered an unprecedented number of novel PFAS structures, some of which may account for this unidentified organofluorine.25,35−39 However, because standards are unavailable for most of these compounds, the importance of their contribution to overall PFAS exposure remains unclear.
As top predators, marine mammals are particularly susceptible to exposure to persistent and bioaccumulative substances, including PFASs. Consumption of marine mammals in northern communities may therefore represent a major source of exposure to PFASs.40 Recent investigations in polar bear serum identified 35 additional PFASs that were not included in targeted analyses.41 This included cyclic or unsaturated PFSAs, ether PFSAs, unsaturated ether-, cyclic ether- or carbonyl PFSAs, and x:2 chlorinated perfluoroalkyl ether sulfonates. The present study builds upon the work of Liu et al.41 by combining suspect screening with organofluorine mass balance to comprehensively assess PFAS exposure in 11 different marine mammal species from different locations within the Northern Hemisphere (Table S1). To the best of our knowledge, this is the first time organofluorine mass balance combined with suspect screening has been conducted in marine mammals.
Materials and Methods
Sample Collection
Marine mammal liver samples included in this study originated from five different locations within the Northern Hemisphere. A full list of samples, including information on species (including Latin names), year, age, sex, sampling location, weight, and length are provided in Table S1. A brief overview is provided here. Species from the US Atlantic coast included grey seal, harbor seal, harbor porpoise, and pygmy sperm whale; samples were obtained between the years 2000 and 2012 from stranded animals. Samples from Sweden were collected between 2011 and 2016 from by-caught animals (seals), animals shot during domestic hunting (seals), or from stranded animals (harbor porpoise). Grey and harbor seals as well as harbor porpoise were collected from the south, while ringed seals were collected from the northern Baltic Sea. Samples from Greenland included harp and ringed seals, harbor porpoise, white beaked dolphin, killer whale, humpback whale, minke whale (fetus), and polar bear (including a mother and cub) were collected with help from local Inuit subsistence hunters from 2000 to 2016. The ringed seal (2012), polar bear (2012), and killer whale (2013) samples from East Greenland were analyzed previously for target PFAS by Gebbink et al.42 These samples were re-analyzed for target PFASs in the present work along with new measurements of TF/EOF and suspect screening. Icelandic seal samples were derived from animals that were by-caught in 2009 and 2010 and included grey, harbor, and harp seals. CITES numbers for export and import permissions are provided in the Supporting Information (Table S2). Liver tissues were shipped in individual polypropylene (PP) tubes on dry ice, after which they were stored at −20 °C until analysis. Under these conditions, PFAA-precursor degradation is not expected to occur.43 The present study was originally designed so that every sample would include a pool of liver tissue from multiple animals, with mixed sexes and ages. However, this was not possible for some species due to low sample availability, and therefore some samples consist of liver tissues from only one animal, while pooled samples consisted of liver tissues from 2 to 10 animals.
Chemicals and Reagents
Native and isotopically labelled PFAS standards included in the targeted analysis were purchased from Wellington Labs (Guelph, Canada). Structures and abbreviations of individual PFASs are provided in Table S3. A total of 36 PFASs were targeted in the present work, including 14 PFCAs (C4–16, C18), 8 PFSAs (C4–11), perfluorooctane sulfonamide (FOSA), 3 perfluoroalkane sulfonamidoacetic acids (FOSAA, MeFOSAA, EtFOSAA), 2 chlorinated polyfluorinated ether sulfonates (Cl-PFESAs; 9Cl-PF3ONS, 11Cl-PF3OUdS), ADONA, HFPO-DA (GenX), 3 fluorotelomer sulfonates (4:2, 6:2, and 8:2 FTSAs), and 3 fluorotelomer carboxylic acids (3:3, 5:3, and 7:3 FTCAs). Linear (L) and branched (br) isomers were determined separately for some substances (see Table S4). For some target analytes, an analogous internal standard (IS) was lacking, and these were therefore semi-quantified (see Table S4).
Overview of Fluorine Mass Balance Approach
The experimental approach for assessing fluorine mass balance is depicted in Figure S1 and was performed as follows. Three portions of tissues were removed from homogenates of a single liver or pooled sample. The first portion was fortified with an IS mix, extracted as described in the next paragraph, and analyzed using both UPLC-MS/MS (targeted analysis) and UPLC-Orbitrap-MS (suspect screening). The second portion was extracted using the same methods but without addition of the IS, and the resulting extract was analyzed for EOF by combustion ion chromatography (CIC). For comparability to targeted PFAS concentrations, EOF concentrations were recovery-corrected based on the results of a spike-recovery experiment (see QC section in the Supporting Information). The third portion of tissues was combusted directly on the CIC for determination of total fluorine (TF). Approximately 25% of the samples were run in triplicate. Assuming that all liver tissues display similar instrumental variation, the highest relative standard deviation (RSD) for each analyte was used to estimate standard deviations for all other samples (i.e., those not run as replicates).
Sample Preparation
Liver samples were stored in 13 mL PP tubes at −20 °C prior to analysis. Subsampling was done using a stainless-steel knife of which the blades were precleaned with methanol. For targeted analysis, approximately 0.5 g of liver homogenate was thawed at room temperature, and IS solution was added prior to extraction using the procedure described by Powley et al.44 (detailed description is provided in the Supporting Information). The final extract was fortified with recovery standards (RSs; 13C8-PFOA and 13C8-PFOS) and 500 μL of 4 mM NH4OAc (aq) and then stored at −20 °C until analysis. The extraction procedure for EOF analysis was the same as that for target PFAS analysis, with the exception that standards and buffer were not included, and the final extracts were concentrated to ∼200 μL under a stream of nitrogen. For TF analysis, 100 mg of neat liver was analyzed directly, with no fortification of standards.
Instrumental Analysis and Quality Control
Targeted Analysis
Targeted analysis was carried out on an Acquity UPLC (Waters) coupled to a triple quadrupole mass spectrometer (Xevo TQS, Waters), equipped with an ethylene bridge hybrid (BEH) C18 column (1.7 μm, 50 × 2.1 mm, Waters), based on a previously described method.45 The gradient program is specified in Table S5. MS source conditions are provided in the Supporting Information. Quantification was performed using MassLynx 4.1 (Waters), via a 9-point calibration curve ranging from 0.008 to 150 ng/mL (linear, 1/x weighting). Precursor and product ions are presented in Table S4. Analytes lacking an analogous labeled standard were quantified using the IS with the closest retention time. While most targets lacking an exactly matched IS displayed reasonable percent recoveries (see further discussion below), some uncertainty remains for these targets given that the interspecies variability in liver matrix composition which was not captured by the spike/recovery experiment. Consequently, data for substances lacking an exactly matched IS are considered semi-quantitative (semiQ). Targets without authentic standards were quantified using the calibration curve of a structurally similar homologue (Table S3). Branched isomers were quantified using the calibration curve of the linear isomer. Limits of quantification (LOQs) are presented in Table S6.
To determine method accuracy and precision, spike/recovery experiments were performed using homogenized seal liver. Seal liver samples (0.5 g) spiked with 10 ng of native standard mix showed very good recoveries for most compounds (73–130%; Figure S2). The exceptions were PFHxDA, PFOcDA, 4:2 FTSA, and 8:2 FTSA, which showed very high recoveries (278, 397, 212, and 227%, respectively), while HFPO-DA, 3:3 FTCA, 5:3 FTCA, and 7:3 FTCA showed very low recoveries (22, 34, 55, and 53%, respectively). These deviating recoveries are likely due to matrix effects, which were not accounted for because of the absence of an exactly matching isotopically labelled IS (see detailed discussion in the Supporting Information and Figure S2). NIST certified reference material 1957 (CRM 1957) was used for external method validation, and results were generally in good agreement with certified values (see Table S7). Finally, each batch of samples was processed together with three method blanks and control seal liver tissue (spiked and unspiked), and between every 8–10 instrumental injections, a standard was included to monitor instrumental drift. Procedural blank concentrations were negligible for targeted PFAS analysis, and therefore no blank subtraction was performed.
Total- and EOF Analysis
Measurements of TF and EOF were carried out using CIC (Thermo-Mitsubishi) using previously described methods.30,46 A detailed description is provided in the Supporting Information, and the IC gradient program is provided in Table S8. Quantification was performed using a standard calibration curve prepared at 0.05 to 100 μg F/mL (R2 > 0.98). For EOF measurements, the mean fluoride concentration in samples was subtracted by the average method blank concentration for their respective batch. Method blanks ranged from 41 to 56 ng/g for batch 1 (n = 3) and from 77 to 125 ng/g for batch 2 (n = 3). For TF analysis, instrumental (boat) blanks were adopted as method blanks, but because fluoride concentrations were low (<3% of sample concentrations), the TF measurements were not blank-corrected. The method quantification limit (LOQ) was defined as the mean concentration plus three times the standard deviation of the method blanks.
Spike/recovery experiments with NaF and PFOS over a range of concentrations revealed that inorganic fluorine was removed efficiently by the extraction procedure, as intended, even at the highest fortification level of 2000 ng F (Figure S3). In contrast, fluorine concentrations increased linearly (R2 > 0.99) with increasing fortification of PFOS. A comparison of the measured concentration of PFOS using CIC to the amount fortified revealed an average recovery of 69 ± 2% (±standard deviation), which is reasonable considering that no internal standard is used for this procedure. This value was used for recovery-correction of all EOF concentrations.
For comparison of sum PFAS concentrations to EOF and TF, concentrations of target PFASs were converted to their corresponding concentration in fluorine equivalents (CF_PFAS) according to eqn
| 1 |
where CPFAS is the concentration of the target compound, nF is the number of fluorine atoms in the target compound, AF is the atomic weight of fluorine (g/mol), and MWPFAS is the molecular weight of the target compound. The sum of known extractable fluorine concentration (ΣCF_PFAS) was calculated by summing the fluorine concentrations from all individual PFASs. Values <LOQ were set to 0 for calculating ΣCF_PFAS. EOF concentrations (CF_EOF) were corrected using the average PFOS recovery, obtained from spike/recovery experiments. Correction for analyte-specific recoveries would presumably give more accurate results, but this is impossible for unknown PFASs or PFASs lacking standards which contribute to the EOF. Another option is to extract the samples without using ISs, split the final extract, and analyze this in both target and total fluorine analysis, adding IS to the fraction for targeted analysis only.47 Although this approach leads to inaccuracies in the targeted data (because these data would be uncorrected for procedural losses), an additional extraction for targeted analysis with ISs could be included, assuming sufficient sample availability. Overall, correcting the EOF data using PFOS recoveries is reasonable in this case given that (a) PFOS is the predominant PFAS in most samples, (b) PFOS recoveries are generally representative of recoveries for most PFAAs, and (c) targeted results were not compromised using this approach.
Statistical comparisons of ΣCF_PFAS and CF_EOF were done with 1-tailed T-tests with unequal variances, assuming that ΣCF_PFAS can only be less than or equal to the CF_EOF concentrations. In cases where the CF_EOF appeared to be lower than ΣCF_PFAS, the fluorine balance was considered closed.
Suspect Screening
Suspect screening was carried out using a Dionex Ultimate 3000 liquid chromatograph coupled to a Q Exactive HF Orbitrap (Thermo Scientific), based on a previously described method.48 Instrumental parameters are provided in the Supporting Information. The instrument was run in negative ion, full scan (200–1200 m/z) data-dependent acquisition MS/MS mode based on an inclusion list derived from a combination of online databases (abbreviated here as EPA,49 KemI,50 OECD,51 and Trier52), literature,38,41,53−56 and features identified from PFAS homologue series mining (details below) during prescreening experiments. The resolution was set to 120 000 (15 000 for MS/MS), and the automatic gain control was set to 3 × 106. Other instrumental parameters are presented in Table S9. Data processing was carried out using Xcalibur 3.1 and Compound Discoverer 3.1 (Thermo Scientific). The workflow included peak retention time alignment, peak integration (using a mass tolerance of 5 ppm, a minimum signal to noise (S/N) ratio of 30, and a minimum peak intensity of 1 × 106), grouping, and gap-filling (peak integration at S/N = 10 for peaks detected at S/N = 30 in at least one sample). Blank subtraction was carried out by removing all peaks with areas less than 3 times the average peak area in the method blank.
A total of 17 973 features remained following data preprocessing. These features were subjected to homologue series mining using the R-package “nontarget”57 which was used to screen exact masses for homologue series differing by −CF2– (49.9 Da) and −C2F4– (99.9 Da) fragments, which are characteristic for PFASs. Each homologue series was then checked manually in the extracted ion chromatogram (EIC) for good peak shapes and an increasing retention time with mass-to-charge. At this point, in-source fragments were removed by comparing retention times, exact mass, and MS/MS spectra (if available). The resulting list of exact masses and their MS/MS spectra were annotated through comparison to databases and/or literature. In one case, MS/MS spectra were predicted using the in silico fragmentation predictor MetFrag.58 Confidence levels (CLs) were assigned according to Schymanski et al.59 (see Supporting Information for details).
Results and Discussion
Overview of PFAS Concentrations in Marine Mammals
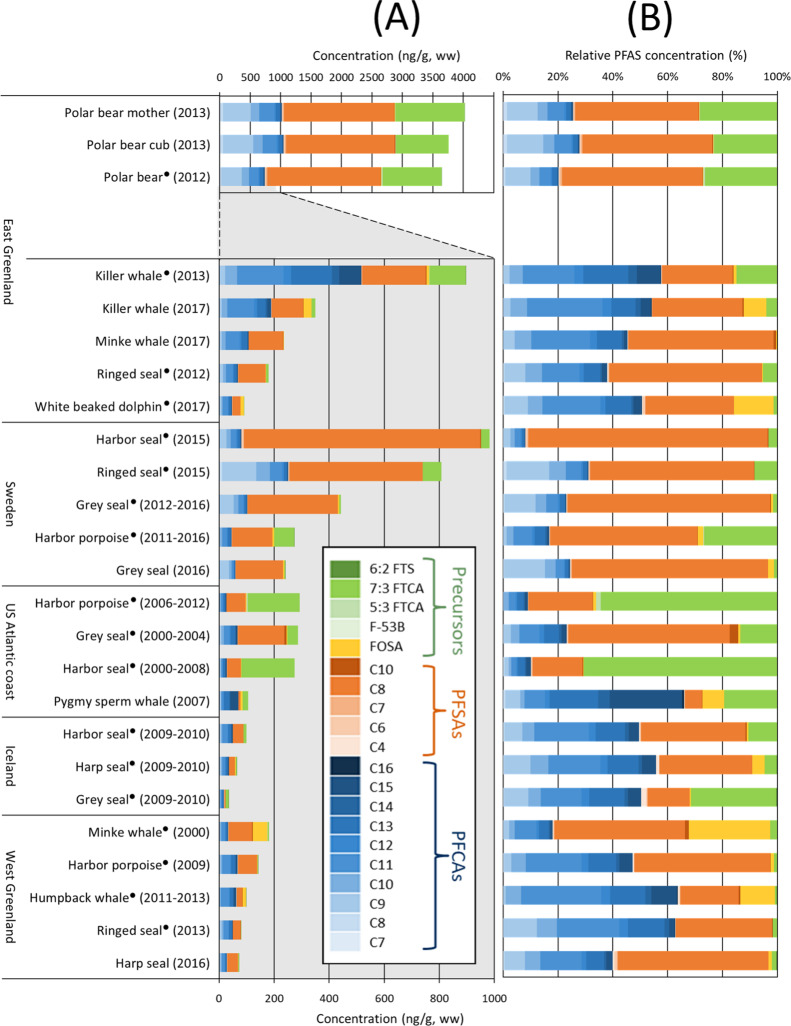
Of the 36 target PFASs analyzed in the present work, 20 were quantifiable in one or more samples: PFHpA, PFOA (L), PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFPeDA, PFHxDA, PFBS, PFHxS (L + Br), PFHpS, PFOS (L + Br), PFDS (L + Br), FOSA (L + Br), 9Cl-PF3OUdS, 5:3 FTCA, 7:3 FTCA, and 6:2 FTSA (Table S10). Peaks were also observable for FOSAA (L), MeFOSAA (L), EtFOSAA (L), and 11Cl-PF3OUdS, but concentrations were always <LOQ. PFBA, PFPeA, PFHxA, PFOA (Br), PFOcDA, PFPeS, PFNS, PFUnDS, FOSAA (Br), MeFOSAA (Br), EtFOSAA (Br), ADONA, HFPO-DA, 3:3 FTCA, 4:2 FTSA, and 8:2 FTSA were all below quantification limits in all samples. Both concentrations and PFAS profiles varied widely among species, sampling location, and sampling year (Figure 1). The highest sum PFAS concentrations (i.e., Σ36PFAS) among all species were observed in polar bears (3600–4000 ng/g), which were an order of magnitude higher than most other marine mammals (Figure 1). As apex predators, polar bears are among the most chemically contaminated species on the planet.60 The three most predominant compounds in polar bears were PFOS, 7:3 FTCA, and PFNA, which made up 45–51, 23–28, and 9–13% of the Σ36PFAS, respectively. 7:3 FTCA has not been reported in polar bears before, and it is therefore particularly surprising that this compound makes up such a large fraction of the total PFAS concentration. Σ36PFAS profiles were very similar between all polar bears and only slightly higher for the female polar bear compared to her cub, which is concerning due to health risks associated with chemical exposure at this early developmental stage.
Figure 1.
(A) Sum of targeted PFASs (note the separate concentration axis for polar bears) and (B) normalized concentrations for marine mammals sorted according to their sampling location. • = pooled samples (n = 2–10). Detailed sample information is available in Table S1.
In cetacean liver samples, the highest Σ36PFAS concentrations were observed in killer whales from East Greenland (614 ± 49 ng/g, ww), while in seals, the highest Σ36PFAS concentrations were detected in harbor seals (640 ± 51 ng/g, ww) and ringed seals (536 ± 43 ng/g, ww) from Sweden. PFOS dominated the Σ36PFAS fraction in samples from all locations, except for samples from the US Atlantic coast, where 7:3 FTCA was dominant. For harbor seal and harbor porpoise from the US Atlantic coast, 7:3 FTCA accounted for up to 64 and 71% of Σ36PFAS concentrations, respectively, which may indicate that these animals were located in closer proximity to an emission source of 7:3 FTCA and/or fluorotelomer alcohol (FTOH)-based substances. Seals from Iceland contained low Σ36PFAS levels compared to the other samples, that is, 23, 43, and 67 ng/g for grey seal, harp seal, and harbor seal, respectively.
Interspecies and Geographical Differences in PFCA Distribution
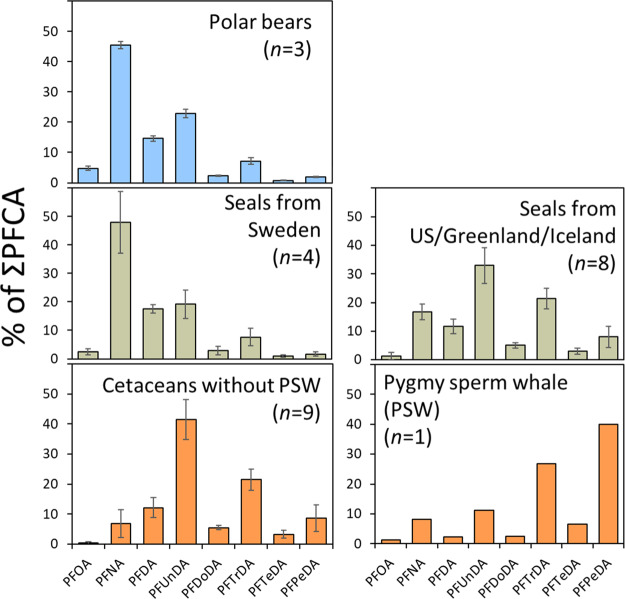
The distribution of PFCA homologues is shown in Figure 2. Among all samples, a characteristic odd/even chain length pattern was observed, wherein the concentration of a given odd chain-length PFCA in most cases exceeds the concentration of its adjacent even chain-length homologues (i.e., PFNA exceeds PFOA and PFDA, PFUnDA exceeds PFDA and PFDoDA, etc). This pattern has been widely reported in marine biota42,61−63 and is suggested to occur from atmospheric oxidation of FTOHs to corresponding even- and odd-chain length PFCAs, followed by preferential bioaccumulation of the odd (i.e., longer) chain-length homologue.11,64 Similar patterns have been observed in ice caps from the high Arctic, where the major even-odd pairs—PFHxA/PFHpA, PFOA/PFNA, and PFDA/PFUnDA—are thought to from 6:2, 8:2, and 10:2 FTOHs, respectively.65 Despite this consistent pattern, the overall distribution of PFCA homologues was remarkably different among species. Species-specific metabolism may explain these differences.66 For example, the dominant PFCA in polar bears from East Greenland was PFNA (C9), while PFUnDA (C11) was dominant in cetaceans (except for the pygmy sperm whale) from Greenland, the US, and Sweden. In comparison, the dominant PFCA in pygmy sperm whale was PFPeDA (C15; 28.0 ng/g, ww). The unique profile in pygmy sperm whale (n = 1) was not explainable by differences in sampling year amongst cetaceans. While C15 has not been quantified in pygmy sperm whales before, long-chain PFCAs (specifically PFTrDA (C13)) were previously reported to make up a large fraction of the total PFAS concentration in pygmy sperm whales.67,68 Diet may partly explain this unique pattern because pygmy sperm whales were one of the few species investigated here (in addition to white-beaked dolphin) that feed offshore on small fish, squid, octopus, and other invertebrates.69 However, we cannot be sure that the pattern is representative for the species because the liver of only one pygmy sperm whale was analyzed. For seals, the PFCA distribution varied among sampling locations, suggesting geographical differences in exposure source (Figure 2). In seals from Sweden (both Baltic Sea and west-coast Skagerrak/Kattegat straits), the most prevalent PFCA homologue was PFNA (C9), whereas for seals from the Atlantic Ocean (i.e., US, Greenland, Iceland), PFUnDA (C11) represented the highest fraction. These differences (which were not explainable by differences in sampling year) point to a common source of exposure in seals from the US, Greenland, and Iceland that is unique relative to that of the Baltic Sea and Skagerrak/Kattegat straits.
Figure 2.
Average percent contribution of PFCAs (C8–C15) to ΣPFCA concentrations (error bars represent standard deviation) in polar bears, seals (grouped by locations with similar patterns), and cetaceans (pygmy sperm whale and other cetaceans from Sweden/US/Greenland).
Interspecies Differences in FOSA Concentrations
FOSA/PFOS ratios were generally much higher for cetaceans (0.01–1.28; average 0.33), compared to other marine mammals (0–0.14; average 0.02). The exception was for harbor porpoises, which contained consistently lower FOSA/PFOS ratios (0.02–0.04; average 0.03). Previous studies have observed similar results, with Galatius et al.70 hypothesizing that smaller cetacean species (e.g. harbor porpoises) might have a higher capacity for transformation. FOSA is the most commonly observed PFOS precursor in wildlife. While FOSA usually occurs at lower concentrations than PFOS, a review of the current literature (see Figure S4) revealed that FOSA/PFOS ratios are mainly higher in cetaceans (0.2–1.0) compared to other marine mammals (ratio < 0.005; Figure S4).71−74 A phylogenetic difference in the ability of certain cetacean species to transform FOSA to PFOS was suggested by Galatius et al.70 and was later confirmed through in vitro hepatic microsome experiments.66 Other factors such as habitats and dietary structures may also play a role in the high FOSA/PFOS ratios in cetaceans.
Elevated Concentrations of 7:3 FTCA
The 7:3 FTCA was the second most prevalent PFAS (next to PFOS) and is reported here for the first time in cetaceans and polar bears. Because an isotopically labeled internal standard was unavailable for 7:3 FTCA, there is some degree of uncertainty with the reported concentrations; however, spike/recovery experiments revealed a recovery of 53% (Figure S2), and so actual concentration may be underreported. FTCAs are not used in consumer products or industrial applications75 but are major stable end-products from both aerobic and anaerobic biodegradation of fluorotelomer alcohols.76,77 7:3 FTCA has been observed previously in biological samples such as birds (16.2 ng/g, ww in water birds and 0.01–0.84 ng/g, dw in eagle-owl feathers),78,79 fish (0.07–0.21 ng/g, ww),79 human whole blood (from technicians working with ski wax; 3.9 ng/mL)80 and breast milk (<42 pg/mL),45 and seals (0.5–2.5 ng/g, ww).81 However, concentrations are typically much lower than those observed in the present study (e.g. polar bear mother: 1131 ng/g, ww and harbor seal: 192 ng/g, ww). Suspect screening also revealed the presence of other X:3 FTCA homologues (see section on Suspect Screening). Future investigations into the source of FTCAs should include lower trophic level organisms consumed as food by marine mammals but also potential emission sources such as wastewater treatment plants which may discharge FTCAs or FTCA precursors (e.g. FTOHs) into the marine environment.
Fluorine Mass Balance
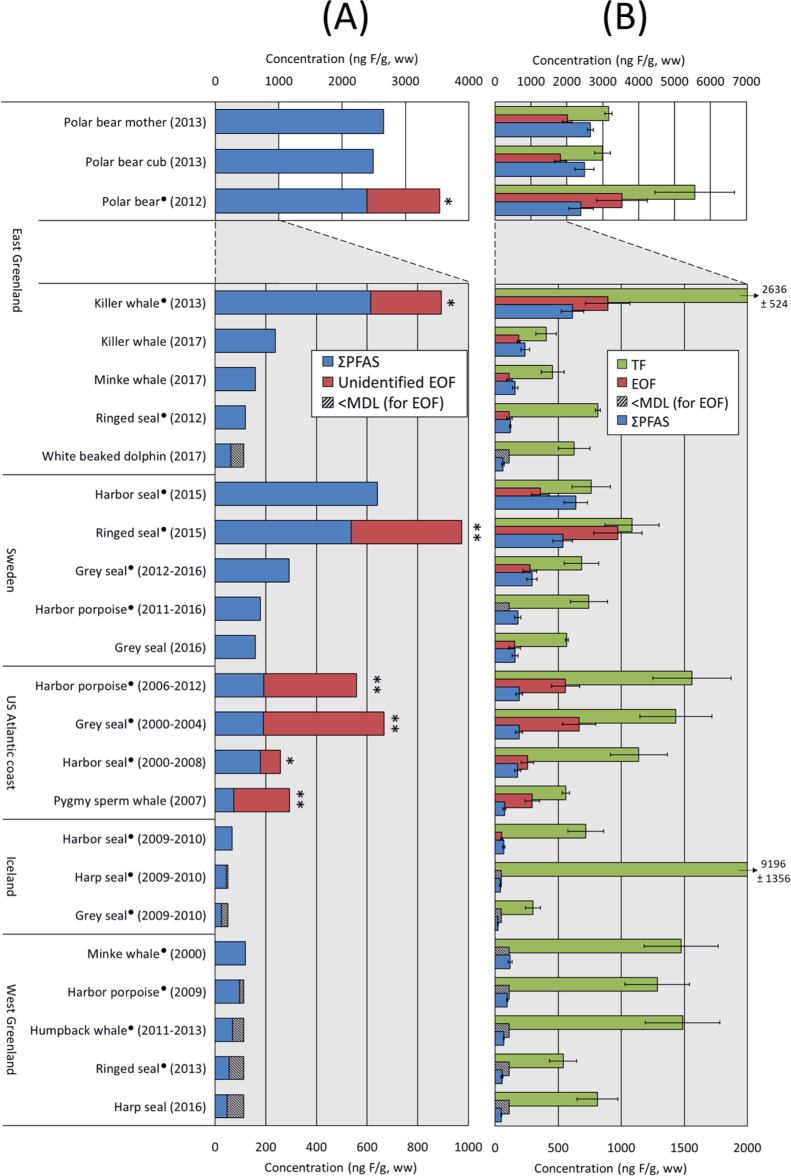
An overview of the fluorine mass balance including the sum target PFAS (ΣCF_PFAS), EOF (CF_EOF), and TF (CF_TF) concentrations is presented in Figure 3 and Table S10. A total of seven out of 25 samples displayed significantly (i.e., p < 0.05 or p < 0.1) higher CF_EOF compared to ΣCF_PFAS concentrations (Figure 3A). This included the pooled polar bear sample from East Greenland from 2012 (32% unidentified EOF); pooled East Greenland killer whale from 2013 (35% unidentified EOF); pooled ringed seal from Sweden from 2015 (45% unidentified EOF); and finally, the pooled harbor porpoise, pooled grey seal, pooled harbor seal, and the pygmy sperm whale (all sampled 2000–2012) from the US Atlantic coast (30–75% unidentified EOF). These results show that exposure of these species to organofluorine is indeed underestimated in some cases. Animals sampled from the US Atlantic coast contained the largest fraction of unidentified EOF, which may indicate that these animals are closer to the source(s) of unidentified organofluorine. Notable, however, is the fact that the US samples also tended to be older than those sampled at other sites. CF_EOF and ΣCF_PFAS concentrations were not significantly different in 9 of the samples, indicating a closed EOF mass balance. Another 9 samples displayed slightly lower CF_EOF than their respective ΣCF_PFAS concentrations, likely caused by under-reporting of CF_EOF due to recovery-correction using PFOS (see Materials and Methods section). While we considered the EOF mass balance to be closed for these samples, the source of this under-reporting requires further investigation. TF concentrations were consistently higher than EOF and target PFASs for all samples, which may be attributed to the presence of inorganic and/or nonextractable organic fluorine in the tissues. Overall, the percentage of unknown TF ranged from 10 to 93% (average 58%).
Figure 3.
(A) Sum target PFAS and unidentified EOF concentrations in ng F/g, ww. Significantly higher EOF concentrations are denoted by asterisks (*p < 0.1; **p < 0.05, 1-sided T-test, unequal variance). (B) Concentrations of target PFASs, EOF, and total fluorine (TF) in ng F/g, ww. Error bars indicate the standard deviation. Note the separate concentration axis for polar bears. • = pooled samples (n = 2–10). Detailed sample information is available in Table S1.
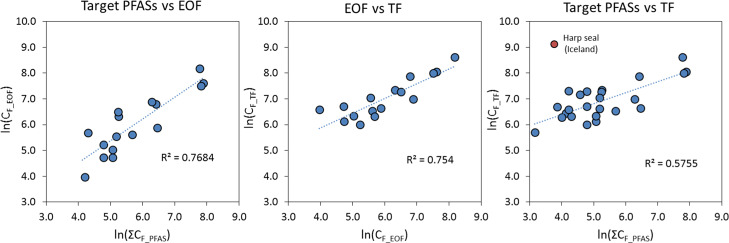
Sum target PFAS concentrations, EOF, and TF were natural log (ln)-linearly correlated with one another (Figure 4; p < 0.001; R2 0.58–0.77), which can be expected because the organofluorine mass balance was closed or nearly closed in most samples. The unidentified fraction of the EOF could consist of novel PFASs, metabolites, and/or transformation products of PFASs. Fluorinated pharmaceuticals and/or pesticides may also accumulate in marine mammals,82 but given their low percentage of fluorine (i.e., these substances typically only contain a few fluorine atoms83), they are not expected to make a significant contribution to EOF or TF concentrations unless they are present in very high abundance. Trifluoroacetic acid (TFA) was also considered because it occurs naturally in sea water at high concentrations (up to 17–190 ng/L in the Northern Atlantic84) and is ubiquitous throughout the entire aquatic environment.84 However, this was ultimately ruled out because TFA is nonbioaccumulative and therefore not expected to occur at high concentrations in marine mammals.85
Figure 4.
Natural log (ln)-linear correlations between sum target PFAS, EOF, and TF concentrations. Data <LOQ were excluded. P-values were <0.001 in all cases.
PFAS Suspect Screening
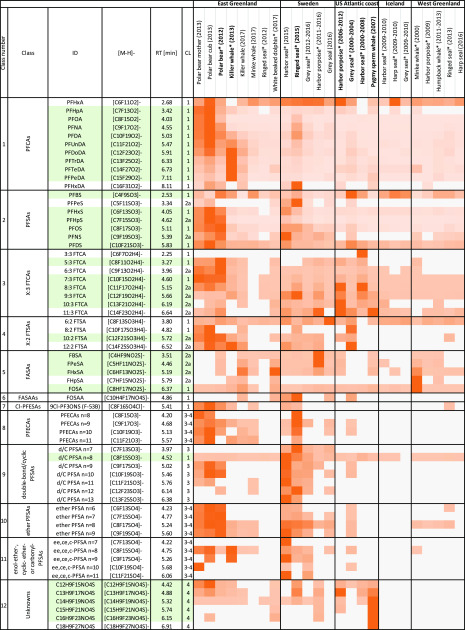
Figure 5 summarizes the PFASs that were identified via suspect screening along with the relative abundance of each suspect in individual samples. Classes 1–7 (PFCAs, PFSAs, FTCAs, FTSAs, FASAs, FASAAs, an Cl-PFESAs) were present in our target list, but additional homologues from some of these classes were identified through homologue series mining. Classes 8–11 (PFECAs, d/c PFSAs, ether PFSAs, and enol-ether/cyclic ether or carbonyl PFSAs) were identified by matching exact masses (and MS/MS fragments when available) to those in the literature. Finally, class 12 was flagged through homologue series mining; thereafter we attempted structural elucidation through database matching and comparison of MS/MS spectra to in silico fragmentation predictions.
Figure 5.
Heatmap showing relative abundance of PFASs identified by suspect screening across all samples. Data are normalized row-wise based on the maximum response observed across all samples for a given substance. Darker orange indicates high abundance, and lighter orange indicates low abundance. White indicated non-detects. Green shading denotes suspects identified by CompoundDiscoverer in contrast to manual inspection of the data. Bold font indicates samples where a significant gap in EOF was identified. CL = confidence level, * = pooled samples (n = 2–10).
Among the FTCAs, 5 additional homologues were detected that were not present in our target list (i.e., 6:3 and 8:3–11:3 FTCAs; <2 ppm mass error). These substances displayed a similar fragmentation pattern to target FTCAs; thus a high degree of confidence (CL = 2a) is ascribed to their assignment, despite an absence of standards (Figure S5). 5:3, 6:3, and 7:3 FTCAs showed highest abundancies in polar bears, while 8:3–11:3 FTCAs showed highest abundancy in harbor porpoise and ringed seals from the US, when comparing peak areas to other samples. All three samples from the US contained significant quantities of unidentified EOF. We posit that quantification of the full suite of FTCA homologues may account for a large portion of the missing EOF in these samples.
10:2 and 12:2 FTSAs (class 4) and C4–C7 FASAs (class 5) were not included in our target list and were identified through a combination of homologue series mining and by comparing their MS/MS fragments to those homologues for which standards were available (i.e., 6:2 and 8:2 FTSAs and FOSA; see Figures S6–S7). Notably, the peak area of 10:2 FTSA was elevated in all polar bear samples and the US harbor seal sample compared to other samples, suggesting that this target may contribute to the missing EOF observed in these samples. Among FASA homologues, perfluorobutane sulfonamide (FBSA) is particularly notable as this substance is a degradation product of a wide range of substances derived from perfluorobutanesulfonyl fluoride, which replaced PFOS precursors in the early 2000s.86 FBSA was present mainly in cetaceans and in all animals from Sweden. FBSA has previously been reported in several fish species in Canada and The Netherlands,87 and one study even reported FBSA in polar bear liver at concentrations of 0.4 ng/g ww.88
Perfluoroalkyl ether carboxylates (PFECAs; class 8, C8–11) were identified by matching the exact mass of multiple homologues to those reported previously in water89,90 and particulate matter.54 While C3–C889 and C10–C1554 PFECAs have been reported previously, to the best of our knowledge, this is the first report of C9 PFECA homologue in the environment. Similarly, a homologue series of double bond or cyclic PFSAs (d/c PFSAs; class 9, C8–C10) were identified by first matching the parent mass and MS/MS spectrum for perfluoroethylcyclohexanesulfonate (PFECHS; C8; Figure S10) to those reported previously in polar bear serum.41 After purchasing a 4-PFECHS standard, its retention time and MS/MS spectrum could be confirmed (Figure S10) and CL = 1 could be assigned. PFECHS was first reported in 2011 in top predator fish and water from the Great Lakes91 and has recently been detected in fish, water, and sediment from China;92,93 water, sediment, char, benthic invertebrates, and snow from the High Arctic;94,95 and in Baltic Sea water samples.96 Notably, in the present study, PFECHS was prevalent in both ringed seals and harbor seals from Sweden relative to other samples, and the former (i.e., ringed seals) had a significant quantity of missing EOF.
MS/MS data was not available for either C6–C9 ether-PFSAs (class 10) or C7–C9 enol-ether/cyclic-ether/carbonyl PFSAs (class 11) due to low peak intensities. Therefore, tentative identification (i.e., CL = 3–4) was carried out by matching the exact mass of the precursor ions to those reported previously in polar bear serum.41 For class 11, peaks for the C10 homologue eluted both at retention time 5.03 and 5.55, suggesting a mixture of structures (e.g., both an enol ether and a cyclic ether).
Finally, one of the compounds of the “unknown” class (class 12; CnF2n+1H10–C5SO4N) was originally matched with a methyl ester structure listed in both the OECD and KemI lists (CAS# 87988-69-0; mass error = 0.456 ppm). However, methyl esters are generally nondetectable by ESI-MS, so this structure was ruled out.72 Alternatively, this substance may be an isomer or in-source fragment of a neutral compound. This feature displayed the highest peak areas in the harbor porpoise and pygmy sperm whale from the US (which had a large fraction of unidentified EOF). Ultimately, confirming the identity of this substance and quantifying it is necessary to assess how much it contributed to the unidentified EOF fraction.
Overall, an additional 37 PFASs were identified through our suspect screening workflow, which were not included in the targeted analysis, bringing the total number of substances detected at a CL of 1–4 to 63 substances from 12 different PFAS classes (not including isomers). We note that the highest peak areas for suspects were not always in samples containing significant quantities of unknown EOF. This should not be surprising, considering that EOF measurements are based on fluorine equivalents, rather than molecular weight-based concentrations, and because the contribution to EOF from a few dominant substances (e.g., PFOS) may dwarf that of some important novel PFAS. Thus, while EOF remains an important tool for prioritizing samples for closer scrutiny; suspect screening (and ultimately quantification) of novel PFASs is clearly needed to obtain a complete picture of PFAS exposure in wildlife. Further development of MS databases to include newly developed commercial PFAS, but also substances formed through for example advanced treatment (UV, peroxide, persulfate, etc.) of industrial and urban wastewater, will only serve to improve the performance of suspect- and nontarget workflows. Finally, it is prudent to note that hazard data are currently unavailable for most of the novel PFAS detected in the present work. This is concerning, not only due to the unknown health risks posed to marine mammals but also due to risks posed to human populations that consume marine mammals as part of their diet.
Acknowledgments
The Danish Cooperation for Environment in the Arctic (DANCEA) is acknowledged for financial support and local subsistence hunters are for collection of East Greenland samples, respectively. BONUS BALTHEALTH that has received funding from BONUS (Art. 185), funded jointly by the EU, Innovation Fund Denmark (grants 6180-00001B and 6180-00002B), Forschungszentrum Jülich GmbH, German Federal Ministry of Education and Research (grant FKZ 03F0767A), Academy of Finland (grant 311966) and Swedish Foundation for Strategic Environmental Research (MISTRA) is acknowledged for support for the Baltic sampling and support for the work of BONUS BALTHEALTH collaborators engaged in the present study. A preprint version of this work is available on ChemRxiv.97
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b06773.
Further information on chemicals and reagents, sample preparation, instrumental analysis, along with results of spike/recovery experiments (targeted and CIC analysis), literature review of FOSA/PFOS ratios, EICs for suspects, detailed sampling information, CITES permits, target PFASs, LC mobile phase gradient, MS and RTs for target PFASs, LOQs, NIST results, eluent programs for CIC analysis, HRMS parameters, and concentrations for PFAS, EOF, and TF (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manage. 2011, 7, 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs). Ser. Risk Manag. 2018, 39, 1–24. [Google Scholar]
- Kissa E.Fluorinated Surfactants and Repellents, 2nd ed.; CRC Press: New York (NY), 2001; Vol. 97. [Google Scholar]
- Kemikalieinspektionen . Kemikalier i Textilier, 2014.
- Jahnke A.; Berger U. Trace Analysis of Per- and Polyfluorinated Alkyl Substances in Various Matrices-How Do Current Methods Perform?. J. Chromatogr. A 2009, 1216, 410–421. 10.1016/j.chroma.2008.08.098. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Mabury S. A.; Solomon K. R.; Muir D. C. G. Dietary Accumulation of Perfluorinated Acids in Juvenile Rainbow Trout (Oncorhynchus Mykiss). Environ. Toxicol. Chem. 2003, 22, 189–195. 10.1002/etc.5620220125. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Mabury S. A.; Solomon K. R.; Muir D. C. G. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. 10.1002/etc.5620220126. [DOI] [PubMed] [Google Scholar]
- Conder J. M.; Hoke R. A.; Wolf W. d.; Russell M. H.; Buck R. C. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- Giesy J. P.; Kannan K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Houde M.; Martin J. W.; Letcher R. J.; Solomon K. R.; Muir D. C. G. Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environ. Sci. Technol. 2006, 40, 3463–3473. 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Houde M.; De Silva A. O.; Muir D. C. G.; Letcher R. J. Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ. Sci. Technol. 2011, 45, 7962–7973. 10.1021/es104326w. [DOI] [PubMed] [Google Scholar]
- Jones P. D.; Hu W.; De Coen W.; Newsted J. L.; Giesy J. P. Binding of Perfluorinated Fatty Acids to Serum Proteins. Environ. Toxicol. Chem. 2003, 22, 2639–2649. 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Lau C.; Butenhoff J. L.; Rogers J. M. The Developmental Toxicity of Perfluoroalkyl Acids and Their Derivatives. Toxicol. Appl. Pharmacol. 2004, 198, 231–241. 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Apelberg B. J.; Goldman L. R.; Calafat A. M.; Herbstman J. B.; Kuklenyik Z.; Heidler J.; Needham L. L.; Halden R. U.; Witter F. R. Determinants of Fetal Exposure to Polyfluoroalkyl Compounds in Baltimore, Maryland. Environ. Sci. Technol. 2007, 41, 3891–3897. 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- Loveless S. E.; Hoban D.; Sykes G.; Frame S. R.; Everds N. E. Evaluation of the Immune System in Rats and Mice Administered Linear Ammonium Perfluorooctanoate. Toxicol. Sci. 2008, 105, 86–96. 10.1093/toxsci/kfn113. [DOI] [PubMed] [Google Scholar]
- Sunderland E. M.; Hu X. C.; Dassuncao C.; Tokranov A. K.; Wagner C. C.; Allen J. G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Exposure Sci. Environ. Epidemiol. 2019, 29, 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R.; Dewailly É.; Pereg D.; Dery S.; Ayotte P. Thyroid Function and Plasma Concentrations of Polyhalogenated Compounds in Inuit Adults. Environ. Health Perspect. 2009, 117, 1380–1386. 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. M.; Andersson P. L.; Lamoree M. H.; Leonards P. E. G.; Van Leeuwen S. P. J.; Hamers T. Competitive Binding of Poly- and Perfluorinated Compounds to the Thyroid Hormone Transport Protein Transthyretin. Toxicol. Sci. 2009, 109, 206–216. 10.1093/toxsci/kfp055. [DOI] [PubMed] [Google Scholar]
- Ji K.; Kim S.; Kho Y.; Paek D.; Sakong J.; Ha J.; Kim S.; Choi K. Serum Concentrations of Major Perfluorinated Compounds among the General Population in Korea: Dietary Sources and Potential Impact on Thyroid Hormones. Environ. Int. 2012, 45, 78–85. 10.1016/j.envint.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nelson J. W.; Hatch E. E.; Webster T. F. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population. Environ. Health Perspect. 2010, 118, 197–202. 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environment and Climate Change Canada - PFCAs and their precursors in perfluorinated products. https://www.ec.gc.ca/epe-epa/default.asp?lang=En&n=AE06B51E-1 (accessed Oct 1, 2019).
- United States Environmental Protection Agency . Fact Sheet: 2010/2015 PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program (accessed Oct 1, 2019).
- Stockholm Convention on Persistent Organic Pollutants. http://www.pops.int/TheConvention/Overview/tabid/3351/Default.aspx (accessed Oct 1, 2019).
- Gebbink W. A.; Van Asseldonk L.; Van Leeuwen S. P. J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S.; McMahen R.; Stoeckel J. A.; Chislock M.; Lindstrom A.; Strynar M. Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ. Sci. Technol. 2017, 51, 1544–1552. 10.1021/acs.est.6b05330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y.; Yamashita N.; Rostkowski P.; So M. K.; Taniyasu S.; Lam P. K. S.; Kannan K. Determination of Trace Levels of Total Fluorine in Water Using Combustion Ion Chromatography for Fluorine: A Mass Balance Approach to Determine Individual Perfluorinated Chemicals in Water. J. Chromatogr. A 2007, 1143, 98–104. 10.1016/j.chroma.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Wang P.; Wang T.; Giesy J. P.; Lu Y. Perfluorinated Compounds in Soils from Liaodong Bay with Concentrated Fluorine Industry Parks in China. Chemosphere 2013, 91, 751–757. 10.1016/j.chemosphere.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Yeung L. W. Y.; De Silva A. O.; Loi E. I. H.; Marvin C. H.; Taniyasu S.; Yamashita N.; Mabury S. A.; Muir D. C. G.; Lam P. K. S. Perfluoroalkyl Substances and Extractable Organic Fluorine in Surface Sediments and Cores from Lake Ontario. Environ. Int. 2013, 59, 389–397. 10.1016/j.envint.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Tan B.; Wang T.; Wang P.; Luo W.; Lu Y.; Romesh K. Y.; Giesy J. P. Perfluoroalkyl Substances in Soils around the Nepali Koshi River: Levels, Distribution, and Mass Balance. Environ. Sci. Pollut. Res. 2014, 21, 9201–9211. 10.1007/s11356-014-2835-6. [DOI] [PubMed] [Google Scholar]
- Schultes L.; Vestergren R.; Volkova K.; Westberg E.; Jacobson T.; Benskin J. P. Per- and Polyfluoroalkyl Substances and Fluorine Mass Balance in Cosmetic Products from the Swedish Market: Implications for Environmental Emissions and Human Exposure. Environ. Sci.: Processes Impacts 2018, 20, 1680–1690. 10.1039/c8em00368h. [DOI] [PubMed] [Google Scholar]
- Weiner B.; Yeung L. W. Y.; Marchington E. B.; D’Agostino L. A.; Mabury S. A. Organic Fluorine Content in Aqueous Film Forming Foams (AFFFs) and Biodegradation of the Foam Component 6:2 Fluorotelomermercaptoalkylamido Sulfonate (6:2 FTSAS). Environ. Chem. 2013, 10, 486–493. 10.1071/EN13128. [DOI] [Google Scholar]
- Yeung L. W. Y.; Miyake Y.; Taniyasu S.; Wang Y.; Yu H.; So M. K.; Jiang G.; Wu Y.; Li J.; Giesy J. P.; Yamashita N.; Lam P. K. S. Perfluorinated Compounds and Total and Extractable Organic Fluorine in Human Blood Samples from China. Environ. Sci. Technol. 2008, 42, 8140–8145. 10.1021/es800631n. [DOI] [PubMed] [Google Scholar]
- Yeung L. W. Y.; Yamashita N.; Taniyasu S.; Lam P. K. S.; Sinha R. K.; Borole D. V.; Kannan K. A Survey of Perfluorinated Compounds in Surface Water and Biota Including Dolphins from the Ganges River and in Other Waterbodies in India. Chemosphere 2009, 76, 55–62. 10.1016/j.chemosphere.2009.02.055. [DOI] [PubMed] [Google Scholar]
- Loi E. I. H.; Yeung L. W. Y.; Taniyasu S.; Lam P. K. S.; Kannan K.; Yamashita N. Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environ. Sci. Technol. 2011, 45, 5506–5513. 10.1021/es200432n. [DOI] [PubMed] [Google Scholar]
- Place B. J.; Field J. A. Identification of Novel Fluorochemicals in Aqueous Film-Forming Foams Used by the US Military. Environ. Sci. Technol. 2012, 46, 7120. 10.1021/es301465n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino L. A.; Mabury S. A. Identification of Novel Fluorinated Surfactants in Aqueous Film Forming Foams and Commercial Surfactant Concentrates. Environ. Sci. Technol. 2014, 48, 121–129. 10.1021/es403729e. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson K. A.; Roberts S. C.; Choyke S.; Oetjen K.; McAlees A.; Riddell N.; McCrindle R.; Ferguson P. L.; Higgins C. P.; Field J. A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yu N.; Zhu X.; Guo H.; Jiang J.; Wang X.; Shi W.; Wu J.; Yu H.; Wei S. Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park. Environ. Sci. Technol. 2018, 52, 11007–11016. 10.1021/acs.est.8b03030. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Pereira A. D. S.; Martin J. W. Discovery of C5-C17 Poly- and Perfluoroalkyl Substances in Water by in-Line Spe-HPLC-Orbitrap with in-Source Fragmentation Flagging. Anal. Chem. 2015, 87, 4260–4268. 10.1021/acs.analchem.5b00039. [DOI] [PubMed] [Google Scholar]
- Hu X. C.; Dassuncao C.; Zhang X.; Grandjean P.; Weihe P.; Webster G. M.; Nielsen F.; Sunderland E. M. Can Profiles of Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum Provide Information on Major Exposure Sources?. Environ. Health: Global Access Sci. Source 2018, 17, 1–15. 10.1186/s12940-018-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Richardson E. S.; Derocher A. E.; Lunn N. J.; Lehmler H.-J.; Li X.; Zhang Y.; Cui J. Y.; Cheng L.; Martin J. W. Hundreds of Unrecognized Halogenated Contaminants Discovered in Polar Bear Serum. Angew. Chem., Int. Ed. 2018, 57, 16401–16406. 10.1002/anie.201809906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink W. A.; Bossi R.; Rigét F. F.; Rosing-Asvid A.; Sonne C.; Dietz R. Observation of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in Greenland Marine Mammals. Chemosphere 2016, 144, 2384–2391. 10.1016/j.chemosphere.2015.10.116. [DOI] [PubMed] [Google Scholar]
- Woudneh M. B.; Chandramouli B.; Hamilton C.; Grace R. Effect of Sample Storage on the Quantitative Determination of 29 PFAS: Observation of Analyte Interconversions during Storage. Environ. Sci. Technol. 2019, 53, 12576–12585. 10.1021/acs.est.9b03859. [DOI] [PubMed] [Google Scholar]
- Powley C. R.; George S. W.; Ryan T. W.; Buck R. C. Matrix Effect-Free Analytical Methods for Determination of Perfluorinated Carboxylic Acids in Environmental Matrixes. Anal. Chem. 2005, 77, 6353–6358. 10.1021/ac0508090. [DOI] [PubMed] [Google Scholar]
- Nyberg E.; Awad R.; Bignert A.; Ek C.; Sallsten G.; Benskin J. P. Inter-Individual, Inter-City, and Temporal Trends of per- and Polyfluoroalkyl Substances in Human Milk from Swedish Mothers between 1972 and 2016. Environ. Sci.: Processes Impacts 2018, 20, 1136–1147. 10.1039/c8em00174j. [DOI] [PubMed] [Google Scholar]
- Schultes L.; Peaslee G. F.; Brockman J. D.; Majumdar A.; McGuinness S. R.; Wilkinson J. T.; Sandblom O.; Ngwenyama R. A.; Benskin J. P.; Benskin J. P. Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform?. Environ. Sci. Technol. Lett. 2019, 6, 73–78. 10.1021/acs.estlett.8b00700. [DOI] [Google Scholar]
- Kärrman A.; Wang T.; Kallenborn R.; Langseter A. M.; Grønhovd S. M.; Ræder E. M.; Lyche J. L.; Yeung L.; Chen F.; Eriksson U.; Aro R.; Fredriksson F.. PFASs in the Nordic Environment; Nordic Council of Ministers, 2019. [Google Scholar]
- Miaz L. T.; Plassmann M. M.; Gyllenhammer I.; Bignert A.; Sandblom O.; Lignell S.; Glynn A.; Benskin J. P. Temporal Trends of Suspect- and Target-per/Polyfluoroalkyl Substances (PFAS), Extractable Organic Fluorine (EOF) and Total Fluorine (TF) in Pooled Serum from First-Time Mothers in Uppsala, Sweden, 1996-2017. Environ. Sci.: Processes Impacts 2020, 10.1039/c9em00502a. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency . Chemistry Dashboard | PFAS: Cross-Agency Research List. https://comptox.epa.gov/dashboard/chemical_lists/EPAPFASRL (accessed Oct 1, 2019).
- Fischer S.S14|KEMIPFAS|PFAS Highly Fluorinated Substances List: KEMI. 2017.
- Wang Z.S25/OECDPFAS/List of PFAS from the OECD. Zenodo, 2018.
- Trier X.; Lunderberg D.. S9|PFASTRIER|PFAS Suspect List: Fluorinated Substances. 2015.
- Liu Y.; D’Agostino L. A.; Qu G.; Jiang G.; Martin J. W. High-Resolution Mass Spectrometry (HRMS) Methods for Nontarget Discovery and Characterization of Poly- and per-Fluoroalkyl Substances (PFASs) in Environmental and Human Samples. Trends Anal. Chem. 2019, 121, 115420. 10.1016/j.trac.2019.02.021. [DOI] [Google Scholar]
- Yu N.; Guo H.; Yang J.; Jin L.; Wang X.; Shi W.; Zhang X.; Yu H.; Wei S. Non-Target and Suspect Screening of Per- and Polyfluoroalkyl Substances in Airborne Particulate Matter in China. Environ. Sci. Technol. 2018, 52, 8205–8214. 10.1021/acs.est.8b02492. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Qian M.; Ma X.; Zhu L.; Martin J. W. Nontarget Mass Spectrometry Reveals New Perfluoroalkyl Substances in Fish from the Yangtze River and Tangxun Lake, China. Environ. Sci. Technol. 2018, 52, 5830–5840. 10.1021/acs.est.8b00779. [DOI] [PubMed] [Google Scholar]
- Mejia-Avendaño S.; Munoz G.; Vo Duy S.; Desrosiers M.; Benoît P.; Sauvé S.; Liu J. Novel Fluoroalkylated Surfactants in Soils Following Firefighting Foam Deployment during the Lac-Mégantic Railway Accident. Environ. Sci. Technol. 2017, 51, 8313–8323. 10.1021/acs.est.7b02028. [DOI] [PubMed] [Google Scholar]
- Loos M.; Singer H. Nontargeted Homologue Series Extraction from Hyphenated High Resolution Mass Spectrometry Data. J. Cheminf. 2017, 9, 1–11. 10.1186/s13321-017-0197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttkies C.; Schymanski E. L.; Wolf S.; Hollender J.; Neumann S. MetFrag Relaunched: Incorporating Strategies beyond in Silico Fragmentation. J. Cheminf. 2016, 8, 1–16. 10.1186/s13321-016-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski E. L.; Jeon J.; Gulde R.; Fenner K.; Ruff M.; Singer H. P.; Hollender J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Routti H.; Atwood T. C.; Bechshoft T.; Boltunov A.; Ciesielski T. M.; Desforges J.-P.; Dietz R.; Gabrielsen G. W.; Jenssen B. M.; Letcher R. J.; McKinney M. A.; Morris A. D.; Rigét F. F.; Sonne C.; Styrishave B.; Tartu S. State of Knowledge on Current Exposure, Fate and Potential Health Effects of Contaminants in Polar Bears from the Circumpolar Arctic. Sci. Total Environ. 2019, 664, 1063–1083. 10.1016/j.scitotenv.2019.02.030. [DOI] [PubMed] [Google Scholar]
- Bossi R.; Dam M.; Rigét F. F. Perfluorinated Alkyl Substances (PFAS) in Terrestrial Environments in Greenland and Faroe Islands. Chemosphere 2015, 129, 164–169. 10.1016/j.chemosphere.2014.11.044. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Smithwick M. M.; Braune B. M.; Hoekstra P. F.; Muir D. C. G.; Mabury S. A. Identification of Long-Chain Perfluorinated Acids in Biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38, 373–380. 10.1021/es034727+. [DOI] [PubMed] [Google Scholar]
- Shaw S.; Berger M. L.; Brenner D.; Tao L.; Wu Q.; Kannan K. Specific Accumulation of Perfluorochemicals in Harbor Seals (Phoca Vitulina Concolor) from the Northwest Atlantic. Chemosphere 2009, 74, 1037–1043. 10.1016/j.chemosphere.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Ellis D. A.; Martin J. W.; De Silva A. O.; Mabury S. A.; Hurley M. D.; Sulbaek Andersen M. P.; Wallington T. J. Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2004, 38, 3316–3321. 10.1021/es049860w. [DOI] [PubMed] [Google Scholar]
- Pickard H. M.; Criscitiello A. S.; Spencer C.; Sharp M. J.; Muir D. C. G.; De Silva A. O.; Young C. J. Continuous Non-Marine Inputs of per- and Polyfluoroalkyl Substances to the High Arctic: A Multi-Decadal Temporal Record. Atmos. Chem. Phys. 2018, 18, 5045–5058. 10.5194/acp-18-5045-2018. [DOI] [Google Scholar]
- Letcher R. J.; Chu S.; McKinney M. A.; Tomy G. T.; Sonne C.; Dietz R. Comparative Hepatic in Vitro Depletion and Metabolite Formation of Major Perfluorooctane Sulfonate Precursors in Arctic Polar Bear, Beluga Whale, and Ringed Seal. Chemosphere 2014, 112, 225–231. 10.1016/j.chemosphere.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Reiner J. L.; O’Connell S. G.; Butt C. M.; Mabury S. A.; Small J. M.; De Silva A. O.; Muir D. C. G.; Delinsky A. D.; Strynar M. J.; Lindstrom A. B.; Reagen W. K.; Malinsky M.; Schäfer S.; Kwadijk C. J. A. F.; Schantz M. M.; Keller J. M.; Reiner J. L. Determination of Perfluorinated Alkyl Acid Concentrations in Biological Standard Reference Materials. Anal. Bioanal. Chem. 2012, 404, 2683–2692. 10.1007/s00216-012-5943-5. [DOI] [PubMed] [Google Scholar]
- Fujii Y.; Kato Y.; Kozai M.; Matsuishi T.; Harada K. H.; Koizumi A.; Kimura O.; Endo T.; Haraguchi K. Different Profiles of Naturally Produced and Anthropogenic Organohalogens in the Livers of Cetaceans from the Sea of Japan and the North Pacific Ocean. Mar. Pollut. Bull. 2018, 136, 230–242. 10.1016/j.marpolbul.2018.08.051. [DOI] [PubMed] [Google Scholar]
- Kannan K.; Koistinen J.; Beckmen K.; Evans T.; Gorzelany J. F.; Hansen K. J.; Jones P. D.; Helle E.; Nyman M.; Giesy J. P. Accumulation of Perfluorooctane Sulfonate in Marine Mammals. Environ. Sci. Technol. 2001, 35, 1593–1598. 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- Galatius A.; Bossi R.; Sonne C.; Rigét F. F.; Kinze C. C.; Lockyer C.; Teilmann J.; Dietz R. PFAS Profiles in Three North Sea Top Predators: Metabolic Differences among Species?. Environ. Sci. Pollut. Res. 2013, 20, 8013–8020. 10.1007/s11356-013-1633-x. [DOI] [PubMed] [Google Scholar]
- Benskin J. P.; Holt A.; Martin J. W. Isomer-Specific Biotransformation Rates of a Perfluorooctane Sulfonate ( PFOS ) -Precursor by Cytochrome P450 Isozymes and Human Liver Microsomes. Environ. Sci. Technol. 2009, 43, 8566–8572. 10.1021/es901915f. [DOI] [PubMed] [Google Scholar]
- D’eon J. C.; Mabury S. A. Exploring Indirect Sources of Human Exposure to Perfluoroalkyl Carboxylates (PFCAs): Evaluating Uptake, Elimination, and Biotransformation of Polyfluoroalkyl Phosphate Esters (PAPs) in the Rat. Environ. Health Perspect. 2011, 119, 344–350. 10.1289/ehp.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.; Zhang S.; Sun J.; Zhang Z.; Giesy J. P.; Hu J. Isomer-Specific Accumulation of Perfluorooctanesulfonate from (N -Ethyl Perfluorooctanesulfonamido)Ethanol-Based Phosphate Diester in Japanese Medaka (Oryzias Latipes). Environ. Sci. Technol. 2014, 48, 1058–1066. 10.1021/es404867w. [DOI] [PubMed] [Google Scholar]
- Xu L.; Krenitsky D. M.; Seacat A. M.; Butenhoff J. L.; Anders M. W. Biotransformation of N-Ethyl-N-(2-Hydroxyethyl)Perfluorooctanesulfonamide by Rat Liver Microsomes, Cytosol, and Slices and by Expressed Rat and Human Cytochromes P450. Chem. Res. Toxicol. 2004, 17, 767–775. 10.1021/tx034222x. [DOI] [PubMed] [Google Scholar]
- Eriksson U.; Haglund P.; Kärrman A. Contribution of Precursor Compounds to the Release of Per- and Polyfluoroalkyl Substances (PFASs) from Waste Water Treatment Plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. 10.1016/j.jes.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Wang N.; Szostek B.; Buck R. C.; Folsom P. W.; Sulecki L. M.; Gannon J. T. 8-2 Fluorotelomer Alcohol Aerobic Soil Biodegradation: Pathways, Metabolites, and Metabolite Yields. Chemosphere 2009, 75, 1089–1096. 10.1016/j.chemosphere.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Szostek B.; McCausland P. K.; Wolstenholme B. W.; Lu X.; Wang N.; Buck R. C. 6:2 and 8:2 Fluorotelomer Alcohol Anaerobic Biotransformation in Digester Sludge from a WWTP under Methanogenic Conditions. Environ. Sci. Technol. 2013, 47, 4227–4235. 10.1021/es4000824. [DOI] [PubMed] [Google Scholar]
- Dahlberg Persson M. J.Levels of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Feathers of Eurasian Eagle-Owls (Bubo Bubo) in Norway; Norwegian University of Science and Technology, 2017. [Google Scholar]
- Loi E. I. H.; Yeung L. W. Y.; Taniyasu S.; Lam P. K. S.; Kannan K.; Yamashita N. Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environ. Sci. Technol. 2011, 45, 5506–5513. 10.1021/es200432n. [DOI] [PubMed] [Google Scholar]
- Nilsson H.; Kärrman A.; Rotander A.; van Bavel B.; Lindström G.; Westberg H. Biotransformation of Fluorotelomer Compound to Perfluorocarboxylates in Humans. Environ. Int. 2013, 51, 8–12. 10.1016/j.envint.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Powley C. R.; George S. W.; Russell M. H.; Hoke R. A.; Buck R. C. Polyfluorinated Chemicals in a Spatially and Temporally Integrated Food Web in the Western Arctic. Chemosphere 2008, 70, 664–672. 10.1016/j.chemosphere.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Yeung L. W. Y.; Miyake Y.; Wang Y.; Taniyasu S.; Yamashita N.; Lam P. K. S. Total Fluorine, Extractable Organic Fluorine, Perfluorooctane Sulfonate and Other Related Fluorochemicals in Liver of Indo-Pacific Humpback Dolphins (Sousa Chinensis) and Finless Porpoises (Neophocaena Phocaenoides) from South China. Environ. Pollut. 2009, 157, 17–23. 10.1016/j.envpol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Jeschke P. Latest Generation of Halogen-Containing Pesticides. Pest Manage. Sci. 2017, 73, 1053–1066. 10.1002/ps.4540. [DOI] [PubMed] [Google Scholar]
- Scott B. F.; Macdonald R. W.; Kannan K.; Fisk A.; Witter A.; Yamashita N.; Durham L.; Spencer C.; Muir D. C. G. Trifluoroacetate Profiles in the Arctic, Atlantic, and Pacific Oceans. Environ. Sci. Technol. 2005, 39, 6555–6560. 10.1021/es047975u. [DOI] [PubMed] [Google Scholar]
- Voogt P. De.Reviews of Environmental Contamination and Toxicology Perfluorinated Alkylated Substances; Whitacre D. M., Ed.; Springer, 2010; Vol. 208. [PubMed] [Google Scholar]
- Chu S.; Letcher R. J. Vitro Metabolic Formation of Perfluoroalkyl Sulfonamides from Copolymer Surfactants of Pre- and Post-2002 Scotchgard Fabric Protector Products. Environ. Sci. Technol. 2014, 48, 6184–6191. 10.1021/es500169x. [DOI] [PubMed] [Google Scholar]
- Chu S.; Letcher R. J.; McGoldrick D. J.; Backus S. M. A New Fluorinated Surfactant Contaminant in Biota: Perfluorobutane Sulfonamide in Several Fish Species. Environ. Sci. Technol. 2016, 50, 669–675. 10.1021/acs.est.5b05058. [DOI] [PubMed] [Google Scholar]
- Boisvert G.; Sonne C.; Rigét F. F.; Dietz R.; Letcher R. J. Bioaccumulation and Biomagnification of Perfluoroalkyl Acids and Precursors in East Greenland Polar Bears and Their Ringed Seal Prey. Environ. Pollut. 2019, 252, 1335–1343. 10.1016/j.envpol.2019.06.035. [DOI] [PubMed] [Google Scholar]
- Strynar M.; Dagnino S.; McMahen R.; Liang S.; Lindstrom A.; Andersen E.; McMillan L.; Thurman M.; Ferrer I.; Ball C. Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol. 2015, 49, 11622–11630. 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sun M.; Arevalo E.; Strynar M.; Lindstrom A.; Richardson M.; Kearns B.; Pickett A.; Smith C.; Knappe D. R. U. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- De Silva A. O.; Spencer C.; Scott B. F.; Backus S.; Muir D. C. G. Detection of a Cyclic Perfluorinated Acid, Perfluoroethylcyclohexane Sulfonate, in the Great Lakes of North America. Environ. Sci. Technol. 2011, 45, 8060–8066. 10.1021/es200135c. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Vestergren R.; Shi Y.; Cao D.; Xu L.; Cai Y.; Zhao X.; Wu F. Identification, Tissue Distribution, and Bioaccumulation Potential of Cyclic Perfluorinated Sulfonic Acids Isomers in an Airport Impacted Ecosystem. Environ. Sci. Technol. 2016, 50, 10923–10932. 10.1021/acs.est.6b01980. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhang Y.; Li J.; Wu N.; Li W.; Niu Z. Distribution, Partitioning Behavior and Positive Matrix Factorization-Based Source Analysis of Legacy and Emerging Polyfluorinated Alkyl Substances in the Dissolved Phase, Surface Sediment and Suspended Particulate Matter around Coastal Areas of Bohai Bay. Environ. Pollut. 2019, 246, 34–44. 10.1016/j.envpol.2018.11.113. [DOI] [PubMed] [Google Scholar]
- Macinnis J. J.; French K.; Muir D. C. G.; Spencer C.; Criscitiello A.; De Silva A. O.; Young C. J. Emerging Investigator Series: A 14-Year Depositional Ice Record of Perfluoroalkyl Substances in the High Arctic. Environ. Sci.: Processes Impacts 2017, 19, 22–30. 10.1039/c6em00593d. [DOI] [PubMed] [Google Scholar]
- Lescord G. L.; Kidd K. A.; De Silva A. O.; Williamson M.; Spencer C.; Wang X.; Muir D. C. G. Perfluorinated and Polyfluorinated Compounds in Lake Food Webs from the Canadian High Arctic. Environ. Sci. Technol. 2015, 49, 2694–2702. 10.1021/es5048649. [DOI] [PubMed] [Google Scholar]
- Joerss H.; Apel C.; Ebinghaus R. Emerging Per- and Polyfluoroalkyl Substances (PFASs) in Surface Water and Sediment of the North and Baltic Seas. Sci. Total Environ. 2019, 686, 360–369. 10.1016/j.scitotenv.2019.05.363. [DOI] [PubMed] [Google Scholar]
- Spaan K.; van Noordenburg C.; Plassmann M.; Schultes L.; Shaw S. D.; Berger M.; Heide-Jørgensen M. P.; Rosing-Asvid A.; Granquist S.; Dietz R.; Sonne C.; Rigét F.; Roos A.; Benskin J.. Fluorine Mass Balance and Suspect Screening in Marine Mammals from the Northern Hemisphere. 2019, ChemRxiv:10128653. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.