Abstract
The term “artificial intelligence” (AI) refers to the idea of machines being capable of performing human tasks. A subdomain of AI is machine learning (ML), which “learns” intrinsic statistical patterns in data to eventually cast predictions on unseen data. Deep learning is a ML technique using multi-layer mathematical operations for learning and inferring on complex data like imagery. This succinct narrative review describes the application, limitations and possible future of AI-based dental diagnostics, treatment planning, and conduct, for example, image analysis, prediction making, record keeping, as well as dental research and discovery. AI-based applications will streamline care, relieving the dental workforce from laborious routine tasks, increasing health at lower costs for a broader population, and eventually facilitate personalized, predictive, preventive, and participatory dentistry. However, AI solutions have not by large entered routine dental practice, mainly due to 1) limited data availability, accessibility, structure, and comprehensiveness, 2) lacking methodological rigor and standards in their development, 3) and practical questions around the value and usefulness of these solutions, but also ethics and responsibility. Any AI application in dentistry should demonstrate tangible value by, for example, improving access to and quality of care, increasing efficiency and safety of services, empowering and enabling patients, supporting medical research, or increasing sustainability. Individual privacy, rights, and autonomy need to be put front and center; a shift from centralized to distributed/federated learning may address this while improving scalability and robustness. Lastly, trustworthiness into, and generalizability of, dental AI solutions need to be guaranteed; the implementation of continuous human oversight and standards grounded in evidence-based dentistry should be expected. Methods to visualize, interpret, and explain the logic behind AI solutions will contribute (“explainable AI”). Dental education will need to accompany the introduction of clinical AI solutions by fostering digital literacy in the future dental workforce.
Keywords: decision-making, diagnostic systems, informatics, dental, deep learning, machine learning
Introduction
The term “artificial intelligence” (AI) was coined in the 1950s and refers to the idea of building machines that are capable of performing tasks that are normally performed by humans. Machine learning (ML) is a subfield of AI, in which algorithms are applied to learn the intrinsic statistical patterns and structures in data, which allows for predictions of unseen data (Fig. 1). A popular type of ML model are neural networks (NNs), which outperform more classical ML algorithms in particular on complex data structures such as imagery or language.
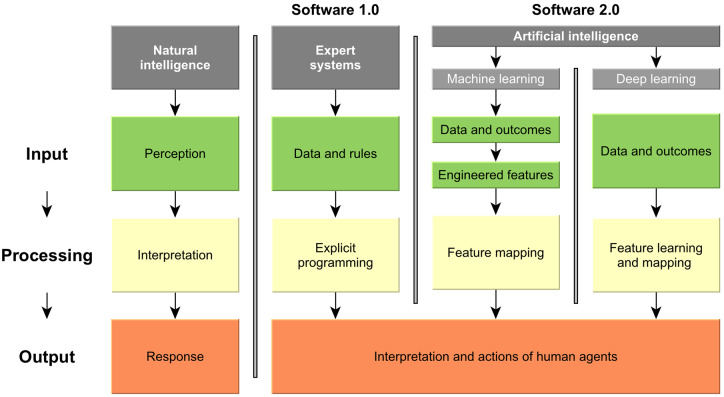
Figure 1.
Natural and computer intelligence. Natural intelligence is characterized by perception, interpretation and biological response. In contrast, computer intelligence does so far not replace human responses, but largely supports human interpretation and action. Traditional software (1.0) as one pillar of computer intelligence is supported by rules-based expert systems; they take data and explicitly programmed logical rules to generate narrow, specialized outcomes, thereby outperforming humans in these tasks. Software 2.0 instead uses data and outcomes to infer the rules: In classical machine learning, the features are first engineered by human experts and then learned (e.g., regression modeling). In deep learning, relevant features are learned and mapped in one step, without human feature engineering; this allows to leverage even complex data structures like imagery or language. Modified after Kolossváry et al. (2019).
The main constituent of any NN is the artificial neuron, which is a mathematical non-linear model that was inspired by the human neuron. By stacking and concatenating artificial neurons and connecting those layers using mathematical operations, a network is engineered that aims to solve a specific task like image classification (e.g., radiographic image showing a decayed tooth: yes or no).
The term “deep learning” is a reference to deep (multi-layered) NN architectures. These are particularly useful for complex data structures, such as imagery, as they are capable of representing an image and its hierarchical features such as edges, corners, shapes, and macroscopic patterns. Deep NNs are considered universal approximation machines (Hornik 1991). Given a set of mathematical constraints, NNs are able to approximate any function and map any input (such as a radiographic image of a decayed tooth) to a given output (such as “decayed tooth”). If a sufficiently large amount of data and computational resources are available, such NNs can be trained to represent the intrinsic statistical patterns of the provided data. During the training process, data points and corresponding labels (classification task) or numerical results (regression task) are repetitively passed through the NN. Thereby, the connections between the neurons, also referred to as model weights, are iteratively optimized with respect to minimizing the prediction error (the difference between true and predicted outcome). A trained NN can predict the outcome of unseen data by passing the new data point through the network.
In the last 70 y, AI applications were perceived as both chance and menace (Fig. 2). During that period, numerous setbacks occurred, often referred to as “AI-winters,” where the expectations in this technology where not met by the actual outcomes. Today, the optimism is greater than ever before; the last decade was marked by extraordinary achievements in the field of ML and in a broader sense, AI. For instance, the textual output of state-of-the-art natural language models became so convincing that readers cannot distinguish between human written or artificially generated texts. Face recognition became so proficient that the technology’s potential to affect civil liberties caused activists, watchdog groups, and lawmakers to act upon it. It appears that finally AI technologies shifted from fantasy to reality; conversation about its impact on society, economics, healthcare, and politics are taking place in many different fields and disciplines. Dentistry should be among them.
Figure 2.
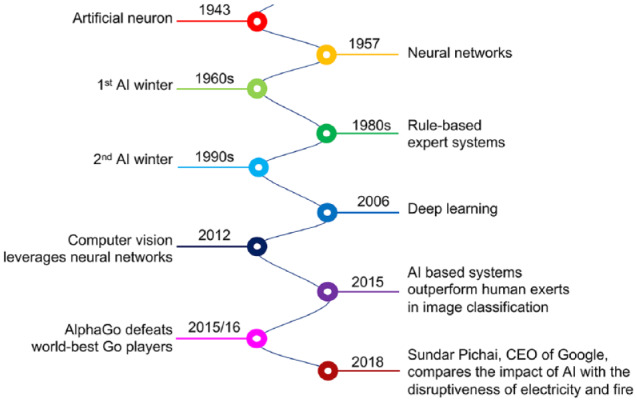
Milestones in the development of artificial intelligence (AI). AI refers to machines that are capable of performing tasks that are normally performed by humans. Machine learning (ML) involves the representation (learning) of intrinsic statistical patterns and structures in data, which allows for predictions for unseen data. “Deep Learning” is a form of machine learning in which multi-layered (deep) neural networks (NNs) are trained to learn features of complex data structures (e.g., image data or language). The history of AI is characterized by ups and downs; after numerous setbacks, optimism is greater today than ever before.
The Chances of AI in Medicine and Dentistry
There has been a significant uptake of these technologies in medicine, too, so far mainly in the field of computer vision. A number of drivers for this uptake have been identified (Naylor 2018): 1) Diagnostic imaging is central in many healthcare fields, with AI being especially suitable to overcome the variability in subjective individual examination and to increase the effectiveness of care while lowering costs by eliminating routine tasks. 2) Digital health data are ubiquitously collected, and while so far these data are rather heterogenous, organizations are increasingly striving to provide cleaned, curated, and structured data. 3) AI allows to integrate different and heterogenous data domains, for example, medical/dental history, socio-demographic and clinical data, imagery data, biomolecular data, social network data, etc., thereby making the best use of these multi-level data and allowing to grasp their interaction. 4) AI facilitates research and discovery, by adding in silico experimentation options to conventional research hierarchies, complementing other research levels and existing modeling strategies. 5) As discussed, AI may streamline routine work and increase the face-to-face time doctors/dentists and their patients have (“humanizing care”). This may not only come via diagnostic assistance systems, but voice, speech, and text recognition and translation, enabling doctors/dentists to reduce time for record keeping (Israni and Verghese 2019). 6) AI also promises to make healthcare more participatory, especially if patients provide their data actively, for example using wearables, etc. Patients will be empowered by self-monitoring and self-management. 7) Using these continuously collected data may also overcome the disadvantages of “on-off-medicine” (Topol 2019), where patients are seen only for a few minutes, while most health conditions are usually acquired over years, and come and go in (oftentimes escalating) intervals (e.g., periodontal disease). Continuous non-invasive monitoring of health and behavior will enable a much deeper, individual understanding of the drivers and processes underlying health and disease. 8) Diagnostic and treatment costs may be decreased, thereby relieving healthcare systems burdened by an ageing society with an increasingly high numbers of complex, chronically ill cases. AI may also help to address shortages in workforce, as observed and expected to continue in many parts of the globe, thereby supporting to reach the World Health Organization (WHO)’s Sustainable Development Goals (https://www.who.int/sdg/en/).
The Challenges and Ways Forward
Despite all the potential, AI solutions have not by large entered routine medical practice. In dentistry, for example, convolutional NNs have only been adopted in research settings from 2015 onwards, mainly on dental radiographs, and the first applications involving these technologies are now entering the clinical arena (Schwendicke et al. 2019). This is all the more surprising when acknowledging that dentistry is especially suited to apply AI tasks: 1) In dentistry, imagery plays an important role and is at the cornerstone of most patients’ dental voyage, from screening to treatment planning and conduct. 2) Dentistry regularly uses different imagery materials from the same anatomical region of the same individual, regularly accompanied by non-imagery data like clinical records and general and dental history data, including systemic conditions, and medications. Moreover, data are often collected over multiple time points. AI is suited to integrate and cross-link these data effectively and improve diagnostics, prediction, and decision-making. 3) Many dental conditions (caries, apical lesions, periodontal bone loss) are relatively prevalent. Building up datasets with a high number of “affected” cases can be managed with limited efforts.
We see three main reasons why dentistry has not yet fully adopted AI technologies. Tackling these reasons will help to make dental AI technologies better and facilitate their uptake in clinical care.
First, medical and dental data are not as available and accessible as other data, due to data protection concerns and organizational hurdles. Data are often locked within segregated, individualized, and limitedly interoperable systems. Datasets lack structure and are often relatively small, at least when compared with other datasets in the AI realm. Data on each patient are complex, multi-dimensional, and sensitive, with limited options for triangulating or validating them. Medical and dental data, for example from electronic medical records, show low variable completeness, with data often missing systematically and not at random. Sampling often leads to selection bias, with either overly sick (e.g., hospital data), overly healthy (e.g., data collected by wearable devices), or overly affluent (e.g., data from those who afford dental care in countries lacking universal healthcare coverage) individuals being over-represented. AI applications developed on such data will be inherently biased (Gianfrancesco et al. 2018).
Second, processing data, and measuring and validating results is oftentimes insufficiently replicable and robust in dental AI research (Schwendicke et al. 2019). It remains unclear how datasets were selected, curated, and preprocessed. Data is oftentimes used for both training and testing, leading to “data snooping bias” (Gianfrancesco et al. 2018; England and Cheng 2019). It is usually not possible to define a “hard” gold standard and there is no agreement on how many experts are required to label a data point and how to merge different labels of such “fuzzy” gold standards (Walsh 2018).
Third, the outcomes of AI in dentistry are often not readily applicable: The single information provided by most of today’s dental AI applications will only partially inform the required and complex decision-making in clinical care (Maddox et al. 2019). Moreover, questions toward responsibilities and transparency remain.
The Table summarizes the limitations in existing AI approaches in dentistry and provides our assumptions how the field will tackle these in the future. Overall, and more generally, AI in medicine and dentistry needs to (Academy of Medical Sciences 2018; European Commission 2019):
Table.
AI in Dentistry Now and in a Possible Future Scenario.
| Aspect | Now | Future |
|---|---|---|
| Sample size | Largely under 2,000 instances/images | Millions of multi-level connected instances |
| Data sources | Single hospitals, insurance claims data | Federated learning; data from multiple institutions |
| Focus | Detection of structures on imagery, association modelling | Multi-class detection of pathologies, predictive modelling, decision support |
| Training mode | Supervised learning | Unsupervised or semi-supervised learning |
| Testing mode | Cross-validation | Hold-out test set, independent datasets |
| Metrics | Measures of accuracy (accuracy, area-under-the-curve, F1-score, segment overlap, etc.) | Measures of value (impact on treatment decision, clinical and patient-reported outcomes, cost-effectiveness) and trustworthiness (explainable AI) |
| Study types | Diagnostic accuracy studies on retrospectively collected data | Randomized controlled trials or large cohort studies collecting data prospectively |
. . . demonstrate value by; improving access to and quality of care; increasing efficiency and safety of provided services; empowering and enabling patients to participate and steer their healthcare; supporting medical research and innovation; increasing healthcare sustainability and ecologic responsibility. The latter is coming into focus: Despite their excellent performance, one drawback of large deep learning AI models is their extreme training complexity. It was estimated that training highly complex models produces 1,438 CO2 (lbs) emissions, which is almost as much as a roundtrip flight between New York City and San Francisco (1,984 CO2 (lbs) per passenger), and can increase up to 625,155 (lbs), which is more than the average lifetime emissions of a US car including fuel (126,000 CO2 (lbs)) (Strubell et al. 2019). The usage of more efficient hardware, application of smaller models and integration of prior knowledge (“hybrid models”) will assist in making AI more sustainable (Bubba et al. 2019).
. . . respect and protect individual privacy, rights and autonomy by; respecting data confidentiality and governance; meeting ethical, regulatory and legal requirements; being transparent about data usage and allowing traceability and accessibility of data. We assume a major shift from centralized to distributed/federated AI training schemes. Data will no longer be centrally gathered and processed, but AI models will go to where the data reside; training will be performed locally, and only updates will be shared between the distributed AI models. The advantages of this distributed/federated learning are scalability and privacy. Various robust and communication-efficient training schemes for federated learning have been developed (Sattler et al. 2019).
. . . maintain trustworthiness and ensure robustness and generalizability by; implementing continuous human agency, oversight and validation; providing a mechanism for evaluation and regulation comparable to those accepted and established in evidence-based medicine and dentistry; reporting the development and validation of any AI solution along the TRIPOD criteria (Moons et al. 2015); guarantying equitability and non-discrimination; retaining the responsibility with the healthcare professional, who in return needs to be educated accordingly; building a workforce with the required skillsets and capacities (digital literacy or “matureness”) (The Topol Review 2019). Two aspects need to be highlighted here: To foster trust in AI, it is of utmost importance to understand and to be able to explain what the model is doing. Due to their complexity, AI systems have been often regarded as black boxes, which do not provide any feedback why and how they arrive at their predictions. In the last few years, there have been enormous developments in the field of explainable AI (XAI). Various methods have been developed to visualize, interpret and explain what AI systems are doing (Samek et al. 2019) (see Fig. 3). Further, medical AI research needs standards (National Institute of Standards and Technology 2019). Dental researchers are called to action to participate in the development of such standards, for example, relating to 1) concepts and terminology, 2) data principles (Wilkinson et al. 2016), 3) sample size estimation (El Naqa et al. 2018); 4) metrics; 5) performance testing and methodology, 6) risk management; and 7) value and trustworthiness. The International Telecommunications Unit and the WHO have recently launched a focus group informing standardization of AI applications in medicine. A topic group on “Dental diagnostics and dentistry” has just been founded (https://www.itu.int/en/ITU-T/focusgroups/ai4h/Pages/default.aspx).
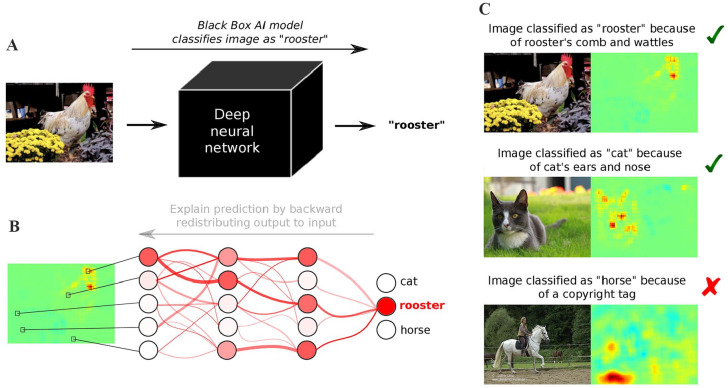
Figure 3.
More transparency through explainable AI (XAI). (A) Today’s AI models are often considered black boxes, because they take an input (e.g., an image) and provide a prediction (e.g., “rooster”) without saying how and why they arrived at it. (B) Recent XAI methods (Samek et al. 2019) redistribute the output back to input space and explain the prediction in terms of a “heatmap,” visualizing which input variables (e.g., pixels) were decisive for the prediction. (C) This allows to distinguish between meaningful and safe prediction strategies, for example, classifying rooster images by detecting the roster’s comb and wattles or classifying cat images by focusing on the cat’s ears and nose, and so-called Clever Hans predictors (Lapuschkin et al. 2019), for example, classifying horse images based on the presence of a copyright tag.
Notably, two further developments can be expected: AI systems will bring together different types of information (e.g., visual and textual) which are able to reason. For instance, recent Visual Question Answering systems (Osman and Samek 2019) are able to answer free text questions about a given image. The reasoning abilities of current AI models even go so far that they pass a medical licensing examination (Wu et al. 2018). Second, significant advances will be achieved in the field of embodied AI. These systems not only master the aspects of perception and reasoning but have some planning abilities to actively interact with the environment. In contrast to current narrow AI system which only solve specific tasks (playing Go, classifying images, detecting cancer, etc.), embodied AI aims to solve complex tasks similar to humans. There is progress in some components required to build embodied AI (e.g., continuous learning, multi-task learning, few-shot learning), however, a comprehensive general AI system is not in reach yet.
Conclusion
The next decade will prove if this time the expectations for tangible AI applications are met by actual outcomes or if once again an AI-winter buries hopes and excitement. In particular in healthcare, the stakes are high. There is reasonable concern about data protection and data security and about handing over critical medical decisions to computers. However, AI has the potential to revolutionize healthcare and, with it, dentistry; AI may assist in addressing the weaknesses harshly criticized in conventional dental care (Watt et al. 2019). Dentistry and, specifically, dental research, has a role to ensure that AI will make dental care better, at lower costs, to the benefit of patients, providers, and the wider society.
Author Contributions
F. Schwendicke, J. Krois, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; W. Samek, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: W. Samek  https://orcid.org/0000-0002-6283-3265
https://orcid.org/0000-0002-6283-3265
References
- Academy of Medical Sciences. 2018. Our data-driven future in healthcare [accessed 2020 Mar 9]. https://acmedsci.ac.uk/file-download/74634438.
- Bubba TA, Kutyniok G, Lassas M, März M, Samek W, Siltanen S, Srinivasan V. 2019. Learning the invisible: a hybrid deep learning-shearlet framework for limited angle computed tomography. Inverse Probl. 35(6):064002. doi: 10.1088/1361-6420/ab10ca. [DOI] [Google Scholar]
- El Naqa I, Ruan D, Valdes G, Dekker A, McNutt T, Ge Y, Wu QJ, Oh JH, Thor M, Smith W, et al. 2018. Machine learning and modeling: data, validation, communication challenges. Med Phys. 45(10):e834–e840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JR, Cheng PM. 2019. Artificial intelligence for medical image analysis: a guide for authors and reviewers. AJR Am J Roentgenol. 212(3):513–519. [DOI] [PubMed] [Google Scholar]
- European Commission. 2019. Ethics guidelines for trustworthy AI [accessed 2020 Mar 9]. https://ec.europa.eu/digital-single-market/en/news/ethics-guidelines-trustworthy-ai.
- Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. 2018. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern Med. 178(11):1544–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik K. 1991. Approximation capabilities of multilayer feedforward networks. Neural Netw. 4(2):251–257. [Google Scholar]
- Israni ST, Verghese A. 2019. Humanizing artificial intelligence. JAMA. 321(1):29–30. [DOI] [PubMed] [Google Scholar]
- Kolossváry M, De Cecco CN, Feuchtner G, Maurovich-Horvat P. 2019. Advanced atherosclerosis imaging by CT: radiomics, machine learning and deep learning. J Cardiovasc Comput Tomogr. 13(5):274–280. [DOI] [PubMed] [Google Scholar]
- Lapuschkin S, Wäldchen S, Binder A, Montavon G, Samek W, Müller K-R. 2019. Unmasking Clever Hans predictors and assessing what machines really learn. Nat Commun. 10(1):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox TM, Rumsfeld JS, Payne PRO. 2019. Questions for artificial intelligence in health care. JAMA. 321(1):31–32. [DOI] [PubMed] [Google Scholar]
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. 2015. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 162(1):W1–73. [DOI] [PubMed] [Google Scholar]
- Naylor CD. 2018. On the prospects for a (deep) learning health care system. JAMA. 320(11):1099–1100. [DOI] [PubMed] [Google Scholar]
- National Institute of Standards and Technology. 2019. U.S. leadership in AI: a plan for federal engagement in developing technical standards and related tools [accessed 2020 Mar 9]. https://www.nist.gov/system/files/documents/2019/08/10/ai_standards_fedengagement_plan_9aug2019.pdf.
- Osman A, Samek W. 2019. DRAU: dual recurrent attention units for visual question answering. Comput Vis Image Underst. 185:24–30. [Google Scholar]
- Samek W, Montavon G, Vedaldi A, Hansen L, Müller K. 2019. Explainable AI: interpreting, explaining and visualizing deep learning. Berlin: Springer. [Google Scholar]
- Sattler F, Wiedemann S, Müller K, Samek W. 2019. Robust and communication-efficient federated learning from non-i.i.d. data. IEEE Trans Neural Netw Learn Syst [epub ahead of print 01 Nov 2019] in press. https://ieeexplore.ieee.org/document/8889996. DOI: 10.1109/TNNLS.2019.2944481 [DOI] [PubMed]
- Schwendicke F, Golla T, Dreher M, Krois J. 2019. Convolutional neural networks for dental image diagnostics: a scoping review. J Dent. 91:103226. [DOI] [PubMed] [Google Scholar]
- Strubell E, Ganesh A, McCallum A. 2019. Energy and policy considerations for deep learning in NLP. Proceedings of the 57th Annual Meeting of the Association for Computational Linguistics. pp. 3645–3650. [Google Scholar]
- The Topol Review. 2019. The Topol review: preparing the healthcare workforce to deliver the digital future [accessed 2020 Mar 9]. https://topol.hee.nhs.uk/.
- Topol E. 2019. Deep medicine: how artificial intelligence can make healthcare human again. New York: Basic Books. [Google Scholar]
- Walsh T. 2018. Fuzzy gold standards: approaches to handling an imperfect reference standard. J Dent. 74 Suppl 1:S47–S49. [DOI] [PubMed] [Google Scholar]
- Watt RG, Daly B, Allison P, Macpherson LMD, Venturelli R, Listl S, Weyant RJ, Mathur MR, Guarnizo-Herreno CC, Celeste RK, et al. 2019. Ending the neglect of global oral health: time for radical action. Lancet. 394(10194):261–272. [DOI] [PubMed] [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, et al. 2016. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu X, Zhang X, He Z, Lv P. 2018. Master clinical medical knowledge at certificated-doctor-level with deep learning model. Nat Commun. 9(1):4352. [DOI] [PMC free article] [PubMed] [Google Scholar]