Abstract
Nickel (Ni) oral hyposensitization treatment (NiOHT) is an effective management approach for Ni allergy. No health-related quality of life (HRQoL) data exist for the pre- and post-treatment with NiOHT in systemic nickel allergy syndrome (SNAS). The aims of this study were (a) to explore HRQoL in SNAS patients, (b) to assess changes of HRQoL after 1 year of NiOHT; (c) to evaluate psychological status of patients. SNAS patients completed the Short-Form 36-Item Health Survey and Psychological General Well-Being Index before and 1 week after the end of NiOHT. Moreover, psychological state was assessed with the Minnesota Multiphasic Personality Inventory (MMPI-2). A total of 52 patients self-reported pre- and post-treatment questionnaires. HRQoL was poor at baseline. After 1 year of NiOHT, all outcome measure scores improved by about 20% with respect to baseline data (P < 0.01 for all indices, except depressed mood). Finally, 33 patients performed the MMPI-2. High rates for hypochondriasis and depression were noted. Furthermore, most of the patients had high scores for anxiety, depression, and health concerns. This is the first study showing that NiOHT improves HRQoL of SNAS patients, which can be considered a “personalized medicine” approach.
Keywords: food allergy, immunotherapy and tolerance induction, personalized medicine, quality of life, systemic nickel allergy syndrome
Introduction
Nickel (Ni) is widely distributed in the environment, and it may be nutritionally essential. Moreover, it has been reported to be one of the most common causes of allergic contact dermatitis (ACD), affecting nearly 15%–20% of the general population.1 Ni-hypersensitivity can induce less frequently respiratory allergies (RAs). In approximately 20% of Ni-systemic contact dermatitis (SCD) patients, the metal causes a more complex condition called systemic nickel allergy syndrome (SNAS).2,3 SNAS is characterized by a combination of cutaneous symptoms, in regions of the skin without direct nickel contact, and extra-cutaneous gastrointestinal symptoms, after the ingestion of Ni-rich foods, especially vegetables.4 A low-Ni diet, following positive patch tests, represents an effective diagnostic and therapeutic tool controlling the systemic manifestation of the syndrome, determining significant clinical improvements.2,5 However, low-Ni diet can be a difficult treatment choice for several reasons. First, the Mediterranean diet contains considerable amounts of Ni, representing a nutritional problem, especially for vegetarians and vegans. In addition, a low-Ni diet is relatively fibre poor, increasing constipation risks.6 Moreover, moderate to severe stress was reported in patients regarding the calculation of exact, daily oral-Ni-intake, due to the fact that the content of this metal varies greatly in soil and water, consequently also in vegetables.7 Finally, this restrictive, unbalanced diet is difficult to follow over long periods and is potentially social discriminating. All those facts negative impact social, physical, and emotional well-being of SNAS patients.8
Nickel oral hyposensitization treatment (NiOHT) is an effective Ni allergy management approach, especially in a subset of SNAS patients, inducing immunological and clinical tolerance to the metal at the normal diet intake dose.9 A phase III study conducted in 2014 as a multicenter prospective double-blind placebo-controlled trial validated this therapy allowing patients to consume Ni-rich foods in the absence of substantial side effects, after 1 year of treatment.3
Although a large number of clinical trials focused on health-related quality of life (HRQoL) in allergic diseases,10–14 data on the expectations, needs, and psychosocial characteristics of patients affected by SNAS are limited5 and pre- and post-treatment data specifically for NiOHT is unavailable. Given the high safety profile and beneficial effects of immunotherapy on HRQoL of patients with allergic rhinitis, we hypothesized similar positive results even after oral Ni desensitization.
Objectives
The aims of this study are (a) to explore HRQoL, (b) to assess changes between baseline HRQoL scores compared to 1 year after Ni hyposensitization treatment (primary outcomes), and (c) to evaluate the psychological status of SNAS patients (secondary outcome).
Materials and methods
Study design
This single-centre prospective observational cohort study investigated HRQoL of SNAS patients before and after 12 months of NiOHT.
Setting
The study was approved by the local Ethical Committee of the Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome (approval number: 4133/15), and registered in the ClinicalTrial.gov (NCT03731494). Written informed consent was obtained from all subjects before the study.
Participants
We adopted inclusion and exclusion criteria to select SNAS patients in order to reduce confounding effects related to medical conditions or drugs.
We enrolled patients with (a) history of SNAS (coexistence of typical cutaneous and gastrointestinal symptoms); (b) positive Ni-patch test; (c) clinical improvement of at least 70% from baseline after 4 weeks of low-Ni diet; (d) positivity of a double-blind placebo-controlled oral Ni challenge (DBPCO).
Exclusion criteria included (a) age < 18 years and >65 years; (b) other organic gastrointestinal diseases, such as peptic ulcer, inflammatory bowel diseases, celiac disease, gastrointestinal infections, and small intestinal bacterial overgrowth; (c) diabetes mellitus; (d) hepatic, renal or cardiac dysfunction; (e) thyroid disease or tumour; (f) concomitant treatment with steroids and/or antihistamines in the previous 4 weeks; (g) pregnancy and lactation; and (h) smoking, abuse of alcohol, coffee, tea, and cola intake.
Variables
We chose two primary outcomes (Short-Form 36-Item Health Survey (SF-36v2) and Psychological General Well-Being Index (PGWBI)) to evaluate HRQoL and degree of well-being. Moreover, we used the Minnesota Multiphasic Personality Inventory (MMPI-2) as a tool to evaluate psychological state (secondary outcome).
Primary outcome measures were evaluated at baseline and 1 week after the end of the treatment (NiOHT); while the secondary outcome measure was obtained only at baseline.
Measurement
Patch test
All patients underwent skin-patch test with 5% Ni sulphate (NiSO4) in petrolatum (Hermal, Hamburg, and Germany) according to International Contact Dermatitis Research Group Guidelines.15
Patch tests were evaluated 48 and 72 h after their application and were considered positive if an eczematous-vesicular reaction occurred at the contact site with the allergen. The intensity was assessed with the following criteria: (a) ±, faint and non-palpable erythema; (b) +, palpable erythema; (c) ++, strong infiltrate, numerous papules, vesicles present, and strong reaction; and (d) +++, coalescing vesicles, bullae, or ulceration extreme reaction erythema.15
Low Nickel diet
The absolute removal of Ni from the diet is impractical because of its ubiquitous presence in almost all foods; therefore, we excluded all foods with a high content of Ni (Ni 100 μg/kg–Ni > 500 μg/kg) following BraMa-Ni diet4 as a guide for 12 weeks (Table 1). The diet therapy approach based on low-Ni foods was validated in the randomized, double-blind, placebo-controlled trial (EUDRACT No. 2009-013923-43).3 Furthermore, patients were asked to avoid the use of stainless-steel utensils to reduce Ni contamination during cooking.
Table 1.
Nickel-rich foods.
| Ni = 100 μg/kg | Ni = 200 μg/kg | Ni = 500 μg/kg | Ni > 500 μg/kg |
|---|---|---|---|
| Carrots | Apricots | Artichoke | Almonds |
| Figs | Broccoli | Asparagus | Chichpeas |
| Lettuce | Corn | Beans | Cocoa |
| Green salad | Lobster | Cabbage | Concentrated tomato |
| Licorice | Onions | Cauliflower | Lentils |
| Mushrooms | Pears | Green beans | Oats |
| Plaice and cod | Raisins | Integral flour | Peanuts |
| Rhubarb | Yeast | Walnuts | |
| Tea | Margarine | ||
| Mussels | |||
| Oysters | |||
| Potatoes | |||
| Peas | |||
| Plums | |||
| Spinach | |||
| Tomatoes |
Oral Ni challenge
Ni oral challenge was carried out during an asymptomatic period, administering increasingly Ni doses, from 1.25, 2.5, 3.75 to 4.5 mg, (capsules made by Lofarma SpA, Milan, Italy) until appearance of SNAS symptoms. Each dose was administered in weekly intervals. All subjects received specific instructions to avoid any possible sources of allergic contact and to continue their diet, as described above.9
Outcome measurements (questionnaires)
SF-36v2 (Italian version) is a self-reported questionnaire comprising 36-items measuring eight dimensions of general HRQoL: physical functioning (10 items), role limitation due to physical health problems (4 items), bodily pain (2 items), general health perceptions (5 items), vitality (4 items), social functioning (2 items), role limitations due to emotional problems (3 items), and general mental health (5 items). In addition to individual dimension scores, two summary scores assessing physical and mental dimensions of health and well-being can be calculated: Physical Component Summary (PCS) score and the Mental Component Summary (MCS) score, respectively. Each question’s score was coded, summed up, and transformed to a scale of 0 (worst possible health state measured by the questionnaire) to 100 (best possible health state) (see supplemental material).16
The PGWBI is a 22-item, self-report rating inventory that allows six possible responses for each item. Its score is proportional to the positivity of “well being” reported during the last 4 weeks, with scores between “0” (the worst condition) and “110” (the best condition). The results are grouped according to well-being levels as: positive well-being (score ⩾ 96), no distress (⩾73 to ⩽95), moderate distress (⩾60 to ⩽72), and severe distress (⩽60). In addition, the scale consists of six domains or dimensions: anxiety (five items; range 0–25), depression (three items; range 0–15), well-being (four items; range 0–20), self-control (three items; range 0–15), general health (three items; range 0–15), and vitality (four items; range 0–20). The response format is graded 1–6 (i.e. total range 22–132), with the highest value corresponding to optimal well-being (see supplemental material).17
The MMPI-2 questionnaire is one of the most applied standardized psychological instruments to assess the main structural features of personality and emotional disorders. It consists of 567 items, which can be answered with true, false, or cannot say.18 MMPI-2 includes eight validity scales, which assess whether the patient has completed the questionnaire with sincerity and accuracy. Moreover, it includes 10 clinical scales that are designed to evaluate the most significant features of the patient’s personality; 16 additional scales that deepen the themes of clinical scales, and 15 content scales that deepen different personality variables. Each raw score obtained must be converted to a standardized score on scale T. Point T expresses the position of a subject close to the reference population. The average T-score for the MMPI (T ± SD = 50 ± 10) is commonly considered as normal values, while MMPI-2 with a value of >65 T indicate an elevated score and distinct psychological problems or pathology. In this study, the Italian version of MMPI-2 was used (see supplemental material).18
NiOHT
Nickel oral hyposensitization (NiOH) was performed with hard gelatine capsules containing Ni sulphate (NiSO4) at different dosages (0.1 ng, 1 ng, 10 ng, 0.1 μg, and 0.5 μg) and microcrystalline cellulose as excipient (TIO Nickel, Lofarma SpA, Milan, Italy). Treatment was given three times a week increasing progressively the dose from 0.1 ng to 3 μg in 10 weeks with a maintenance phase of 1.5 μg a week over a period of 12 months.3
After 6 months of treatment with maintenance dose, patients were instructed to gradually re-introduce food with maximum 100 μg/kg nickel content during the seventh month. Foods with maximum 200 μg/kg nickel content were re-introduced during the eighth month of treatment. In the following 2 months (9th and 10th), there was the prescription to re-introduce foods with maximum 500 μg/kg nickel content. Finally, all other Ni-rich foods were introduced from 11th month. During all before-mentioned phases, patients were educated to re-insert one food at time and in small quantities and fill a clinical diary in order to support their treatment compliance. In the last month (12th), Ni dose was progressively reduced by 0.5 μg per week until discontinuation. Throughout the treatment period, information on side effects, more severe adverse reactions and anti-allergic drug needs (corticosteroids and anti-histamine drugs) were collected.
Study size
SNAS patients showed a statistically significant improvement of PCS index of SF-36 after a 3-month period of low nickel diet (average pre-diet: 46.8, average post-diet: 51.9; P = 0.018).5 Therefore, setting the type I error at 1% (α = 0.01) in a one-tailed test of significance and a type II error of 5%, which corresponds to a study power of 95%, the calculated sample size indicated 31 patients. Considering a dropout rate of 30%, we planned to enrol at least 40 patients.
Quantitative variables
The socio-demographic and clinical characteristics of the studied population were reported as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables.
Statistical methods
The Wilcoxon signed-rank test was used to compare the values of SF-36 and PGWBI before and after NiOHT, while MMPI-2 indices were evaluated only at baseline. P value < 0.01 was considered significant. Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) version 16.0.
Results
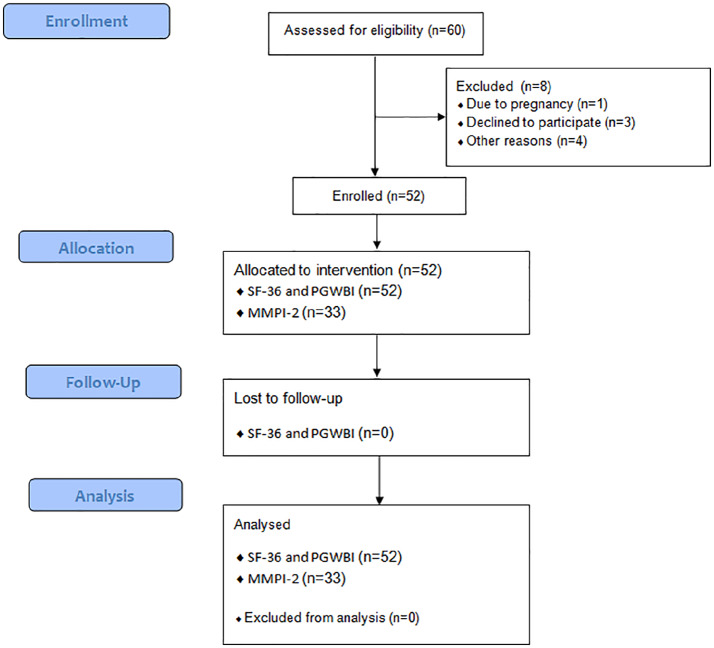
During the study period, 60 patients were considered eligible. Among them, one patient was excluded from the study due to pregnancy; three did not sign written informed consent; four patients dropped out because the immunotherapy programme was too extensive or bothersome. Therefore, 52 patients were enrolled and completed pre- and post-treatment questionnaires (Figure 1). All patients reached the maintenance dose and were able to re-introduce the highest category of nickel-rich foods without adverse reactions.
Figure 1.
Flow diagram of the study.
Table 2 displays the descriptive characteristics of SNAS patients at baseline. Our study sample consisted mainly of females (92% of sample). The mean age was 41 ± 11 years, ranging from 24 to 65 years. The body mass index (BMI) of the majority of the study population was normal. Concerning socio-demographics factors, participants were predominantly married, occupationally active and with a medium-high education level.
Table 2.
The baseline socio-demographics and clinical characteristics of the participants.
| Variables | Patients (n = 52) |
||
|---|---|---|---|
| N | % | ||
| Socio-demographic factors | Female gender | 48 | 92 |
| Age (mean ± SD (range)) | 41 ± 11 (24–65) | ||
| BMI category, (kg/m2) | |||
| Underweight: <18.5 | 1 | 2 | |
| Normal weight: 18.5 – <25 | 43 | 83 | |
| Overweight: 25 – <30 | 8 | 15 | |
| Obese: ⩾30 | 0 | 0 | |
| Social class | |||
| Employed | 28 | 54 | |
| Unemployed | 7 | 13 | |
| Householder chores | 13 | 25 | |
| Student | 4 | 8 | |
| Education | |||
| Less than high school diploma | 9 | 17 | |
| High school diploma | 27 | 52 | |
| University degree | 16 | 31 | |
| Marital status | |||
| Married | 38 | 73 | |
| Single | 14 | 27 | |
| Clinical characteristics | Parental history of allergies | 8 | 15 |
| Lactose intolerance | 24 | 46 | |
| Concomitant others allergies | |||
| Known allergies (All) | 37 | 71 | |
| Allergic rhinoconjunctivitis and bronchial asthma | 27 | 52 | |
| Food allergies | 3 | 6 | |
| Adverse drug reaction | 7 | 13 | |
| Concomitant positivity to other haptens (palladium chloride, cobalt chloride, and potassium dichromate) | 12 | 23 | |
| Patch-grade nickela | |||
| Grade 1 (±) | 3 | 6 | |
| Grade 2 (+) | 16 | 31 | |
| Grade 3 (++) | 24 | 46 | |
| Grade 4 (+++) | 9 | 17 | |
SD: standard deviation; BMI: body mass index.
(1) ±, faint and non-palpable erythema; (2) +, palpable erythema; (3) ++, strong infiltrate, numerous papules, vesicles present, and strong reaction; and (4) +++, coalescing vesicles, bullae, or ulceration extreme reaction erythema.
Parental history of allergy and concomitant allergic conditions were reported by 15% and 71% of patients, respectively. Specifically, allergic rhinoconjunctivitis and bronchial asthma were the most common comorbidities (52%), followed by adverse drug reactions (13%) and food allergies (6%). Lactose intolerance occurred in about half of all patients enrolled. Regarding the Ni reactivity level, “+” grade occurred in 16 subjects, “++” grade in 24 and “+++” grade in nine participants. In some patients (n = 12, 23% of sample), a concomitant positivity to other metals, especially cobalt chloride (six patients), was noted.
Table 3 shows means and standard deviation of eight dimensions; two summary scores of SF-36, with six domains; and a total scale of PGWBI before and after the nickel oral hyposensitization, respectively.
Table 3.
Results of the QoL questionnaires before and after NiOHT.
| QoL questionnaries | Range | pre NiOHT |
post NiOHT |
Change |
P valuea | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| SF-36 | ||||||||
| Physical functioning (PF) | 0–100 | 47 | 10.7 | 53 | 5.5 | 6 | 3.5 | 0.0002 |
| Role physical (RP) | 0–100 | 46 | 11.2 | 53 | 5.7 | 8 | 3.5 | 0.0000 |
| Bodily pain (BP) | 0–100 | 46 | 11.6 | 54 | 8.4 | 8 | 7.0 | 0.0000 |
| General health (GH) | 0–100 | 40 | 10.0 | 48 | 9.6 | 7 | 6.5 | 0.0001 |
| Vitality or energy level (VT) | 0–100 | 45 | 11.5 | 55 | 10.8 | 10 | 10.5 | 0.0000 |
| Social functioning (SF) | 0–100 | 42 | 12.2 | 49 | 9.8 | 7 | 5.0 | 0.0042 |
| Role emotional (RE) | 0–100 | 44 | 12.2 | 52 | 8.8 | 9 | 8.0 | 0.0002 |
| Mental health (MH) | 0–100 | 43 | 13.7 | 50 | 10.7 | 7 | 5.0 | 0.0011 |
| Physical component summary (PCS) | 0–100 | 42 | 11.0 | 51 | 7.0 | 9 | 8.5 | 0.0000 |
| Mental component summary (MCS) | 0–100 | 42 | 11.1 | 50 | 7.9 | 8 | 9.0 | 0.0001 |
| PGWBI | ||||||||
| Anxiety | 0–25 | 16 | 5.0 | 20 | 4.2 | 4 | 5.0 | 0.0000 |
| Depressed mood | 0–15 | 11 | 3.0 | 12 | 2.6 | 1 | 3.2 | 0.0203 |
| General health | 0–20 | 10 | 2.8 | 12 | 2.6 | 2 | 3.3 | 0.0001 |
| Vitality | 0–15 | 12 | 3.8 | 15 | 3.0 | 3 | 4.2 | 0.0000 |
| Positive well-being | 0–15 | 11 | 3.7 | 14 | 3.2 | 3 | 4.4 | 0.0001 |
| Self-control | 0–20 | 10 | 3.5 | 12 | 2.7 | 2 | 3.8 | 0.0048 |
| PGWBI total score | 0–110 | 70 | 18.2 | 84 | 15.3 | 15 | 19.8 | 0.0000 |
QoL: quality of life; NiOHT: nickel (Ni) oral hyposensitization treatment; SF-36: Short-Form 36-Item Health Survey; PGWBI: Psychological General Well-Being Index.
Range of score for each scale was reported.
Wilcoxon signed-rank test.
At baseline, both scores depicted a significant impairment of QoL in the total sample. In fact, all enrolled patients reported lower SF-36 scores in all subscales compared with those observed in a healthy general Italian population.16 Moreover, the domains of PGWBI confirmed a significant negative impact of SNAS on QoL.
In fact, we noticed a moderate to severe psychological distress in anxiety, depressed mood, general health, and self-control domains. Expressing the mean values of each domain as percentage of the upper limit of the reported ranges, we observed mean values between 61% and 72% of the corresponding upper limit, and remaining domains with mean values below 60%.17
After 1 year of NiOHT, all the selected outcome measure scores (SF-36 and PGWBI) improved by 20% with respect to baseline (P < 0.01 for all indices except depressed mood). Moreover, all subscales of PGWBI, except positive well-being, reached the minimum value observed in the general population, expressed as percentage (i.e. 73%).17
We evaluated the frequency distribution of changes of all dimensions and summary scores of SF-36 with domains and total scale of PGWBI after NiOHT. We identified three subgroups: the majority of scores improved after oral treatment, while some scores showed no change and others worsen. These response differences were statistically relevant, demonstrated with Kruskal–Wallis test setting up the significance level at P < 0.05 (Table 4).
Table 4.
Frequency distribution of changes of SF-36 and PGWBI after NiOHT.
| QoL questionnaries | Worsening |
No change |
Improvement |
P value* | ||
|---|---|---|---|---|---|---|
| No. of pts (%a) | Deltab | No. of pts (%a) | No. of pts (%a) | Deltab | ||
| SF-36 | ||||||
| Physical functioning | 8 | −7 | 12 | 32 | 11 | 0.0000 |
| Role physical | 7 | −5 | 9 | 36 | 12 | 0.0000 |
| Bodily pain | 9 | −6 | 9 | 34 | 14 | 0.0000 |
| General health | 12 | −8 | 1 | 39 | 12 | 0.0000 |
| Vitality | 8 | −10 | 2 | 42 | 14 | 0.0000 |
| Social functioning | 12 | −13 | 8 | 32 | 16 | 0.0000 |
| Role emotional | 5 | −16 | 18 | 29 | 19 | 0.0000 |
| Mental health | 11 | −14 | 2 | 39 | 13 | 0.0000 |
| Physical component summary | 6 | −6 | 4 | 42 | 12 | 0.0000 |
| Mental component summary | 9 | −12 | 3 | 40 | 13 | 0.0000 |
| PGWBI | ||||||
| Anxiety | 9 | −13 | 4 | 39 | 27 | 0.0000 |
| Depressed mood | 15 | −19 | 4 | 33 | 20 | 0.0000 |
| General health | 9 | −21 | 5 | 38 | 23 | 0.0000 |
| Vitality | 9 | −19 | 5 | 38 | 25 | 0.0000 |
| Positive well-being | 12 | −15 | 4 | 36 | 26 | 0.0000 |
| Self-control | 12 | −23 | 8 | 32 | 25 | 0.0000 |
| Total scale | 12 | −14 | 0 | 40 | 23 | 0.0000 |
SF-36: Short-Form 36-Item Health Survey; PGWBI: Psychological General Well-Being Index; NiOHT: nickel (ni) oral hyposensitization treatment; QoL: quality of life.
Percentage of total sample.
Mean value of changes.
Significance was tested by the Kruskal–Wallis test with a significance level of P < 0.05.
In order to explore potential influence factors of improvement or worsening of QoL after oral treatment, we performed a multivariable logistic regression. No significant association among socio-demographic, clinical characteristics (gender, age, BMI, social class, educational level, marital status, atopy, lactose intolerance, concomitant allergies, concomitant positivity to other haptens, and patch grade Nickel) and tested scores were found (data not shown).
Regarding secondary outcome, 33/52 performed the MMPI-2. We observed no pathological mean value (T > 65) among both clinical and content scales, analysing the MMPI-2. However, we found high rate for hypochondriasis and depression (27% and 24% of total sample, respectively). Considering the content scales, most of the patients had high score for anxiety, depression, health concerns, anger, low self-esteem, and negative treatment indicators (Table 5). Since only about 60% of the study participants took the MMPI, we performed Mann–Whitney U test for independent samples taking into account all socio-demographic and clinical characteristics of study population as well as all scores collected in order to investigate if there were any meaningful differences between those who completed the questionnaire and those who did not. The only clinically significant difference was the mean value of MCS of SF-36 questionnaire, at baseline, which was higher in those who completed the questionnaire (P = 0.0392).
Table 5.
Means, SD, and percentage of elevations in MMPI-2 scales (T > 65) in 33 patients suffering from SNAS.
| MMPI Scale | Mean ± SD | % Elevated |
|---|---|---|
| Clinical scales | ||
| Hypochondriasis (Hs) | 57 ± 13 | 27 |
| Depression (D) | 59 ± 11 | 24 |
| Hysteria (Hy) | 55 ± 11 | 12 |
| Psychopathic Deviate (Pd) | 53 ± 14 | 18 |
| Masculinity (Mfm)/femininity (Mff) | 50 ± 11 | 0 |
| Paranoia (Pa) | 55 ± 22 | 18 |
| Psychasthenia (Pt) | 54 ± 12 | 15 |
| Schizophrenia (Sc) | 54 ± 22 | 15 |
| Hypomania (Ma) | 52 ± 15 | 18 |
| Social Intravision (Si) | 53 ± 14 | 15 |
| Content scales | ||
| Anxiety (ANX) | 55 ± 13 | 27 |
| Fears (FRS) | 52 ± 12 | 12 |
| Obsessiveness (OBS) | 52 ± 13 | 15 |
| Depression (DEP) | 57 ± 13 | 27 |
| Health concerns (HEA) | 61 ± 15 | 37 |
| Bizarre mentation (BIZ) | 53 ± 17 | 18 |
| Anger (ANG) | 54 ± 13 | 21 |
| Cynicism (CYN) | 53 ± 11 | 15 |
| Antisocial practices (ASP) | 49 ± 10 | 6 |
| Type A (TPA) | 49 ± 12 | 15 |
| Low self-esteem (LSE) | 56 ± 15 | 30 |
| Social discomfort (SOD) | 52 ± 11 | 9 |
| Family problems (FAM) | 54 ± 14 | 15 |
| Work interference (WRK) | 56 ± 15 | 18 |
| Negative treatment indicators (TRT) | 54 ± 15 | 24 |
| MMPI mean T-score | 54 ± 2 | |
MMPI: Minnesota Multiphasic Personality Inventory; SNAS: systemic nickel allergy syndrome.
Discussion
HRQoL is a multidimensional concept focusing on the individuals’ perception on their physical, psychological, and social functioning. The assessment of the QoL is a topic of great relevance in clinical research.
To our knowledge, this is the first study that evaluates the oral nickel desensitizing therapy effects on SNAS patient’s QoL.
Three main observations derived from our study.
First, SNAS significantly affects patients’ QoL, influencing negatively patient’s perception on the syndrome.
Second, after 1 year of oral nickel desensitizing therapy, a very significant improvement was observed for all outcome measures (SF-36 and PGWBI).
Finally, the psychological evaluation by the MMPI-2 questionnaire depicts a specific profile of patients affected by SNAS, characterized by the prevalence of hypochondriasis, anxiety, depression, and health concerns.
Different studies explored HRQoL in allergic diseases.10–14,19–22
In 2001, Sicherer et al.20 measured parental perceptions on physical and psychosocial functioning with the Children’s Health Questionnaire (CHQ-PF50) and with an additional allergy-related questionnaire in a group of food-allergic children, demonstrating that childhood food allergies deeply influenced general health perception, limited family activities and had emotional impact on parents.
Recently, Meyer et al. investigated QoL of families with children suffering from food protein–induced non-IgE-mediated gastrointestinal allergies. The researchers adopted the Family Impact Module (FIM) of the Paediatric Quality of Life (PedsQL™) and noted particularly low daily activity scores.21
More recently, Stensgaard et al. compared self-reported and parent-reported HRQoL in different age groups of patients with a food allergy. Their multivariate models showed no significant differences in patient-reported HRQoL by age.22
A previous study by our group5 investigated the main structural features of personality and emotional disorders of a group of patients suffering from SNAS and irritable bowel syndrome. We demonstrated a high prevalence of psychiatric symptoms and a wide heterogeneity of clinical personality traits with anxiety profile prevalence.5
It is known that nickel hyposensitization is a disease-modifying treatment possibility, able to modulate inflammatory parameters that reduce symptoms and drug consumption in SNAS.9 SNAS-specific QoL has rarely been used as a primary end-point in the assessment of the effect of NiOHT on SNAS, investigating only the efficacy and safety of this treatment.
In 2014, Di Gioacchino et al.3 conducted the first randomized, double-blind, placebo-controlled trial (EUDRACT No. 2009-013923-43) evaluating NiOHT in SNAS patients. The researchers enrolled adults with positive Ni-patch test, who reported symptoms suggesting SNAS, which improved after Ni-poor diet and were positive to Ni-oral challenge. Patients were randomly assigned to three treatments (1.5 μg, 0.3 μg, or 30 ng Ni/week) or placebo for a year, with progressive re-introduction of Ni-rich foods beginning from the fifth month. The researchers observed that during Ni-rich food re-introduction, the 1.5 μg Ni/week group had a mean visual acuity score (VAS) significantly higher than placebo, with significant gastrointestinal improvements.3
Recently, Epstein-Rigbi et al.19 performed a prospective cohort study aimed to characterize changes in QoL of food-allergic patients during and after oral immunotherapy (OIT). They adopted the Food Allergy Quality of Life Questionnaire-Parental Form (FAQLQ-PF) and noted that scores significantly improved from OIT initiation to reaching full maintenance and partial maintenance, whereas no change were noted in control patients.19
We confirm this and, for the first time, add new findings in relation to the effects of oral nickel desensitizing therapy on patient’s QoL suffering from SNAS. In this study, all enrolled patients reported low scores in all subscales and scores of SF-36 before NiOHT. Moreover, the analysis of PGWBI revealed moderate to severe psychological distress. Interestingly, after 1 year of NiOHT, all the selected outcome measure scores (SF-36 and PGWBI) improved by about 20% with respect to baseline (P < 0.01 for all indices except depressed mood). Moreover, all subscales of PGWBI, except positive well-being, reached the minimum value as observed in general population, expressed as percentage (i.e. 73%).17
Finally, this study confirms previous findings by our group23 highlighting that the analysis of the MMPI-2 reveals no pathological mean value (T > 65) among both clinical and content scales, but SNAS patients seem to experience higher levels of hypochondriasis, depression, anxiety, and health concerns. The low level of QoL in patients with SNAS can be related to anxiety, depression, and low self-esteem. This condition represents the “chicken-and-egg” dilemma: does being depressed and/or anxious adversely affect the quality of life of SNAS patients? Or, is SNAS a breeding ground for a psycho-physical imbalance characterized by depressive-anxious tendencies? This requires further investigations.
This study has some limitations. The major criticism is the relatively limited number of enrolled patients, due to highly selective criteria. However, our results reached statistical significance. Moreover, the study sample derives from the same hospital unit and country, and this limits the generalizability of the study results. Another potential drawback is that we were unable to provide information about long-term treatment effects. It would be of added value to collect data to get insight on long-term QoL improvements after NiOHT, adding an adequate follow-up period. Finally, bias is possible because the HRQoL assessment was performed using a non disease-specific questionnaires, applicable to all health conditions.
Despite these limitations, our QoL data confirm that SNAS may be considered the emblem of how a non–life threatening illness, may deeply interfere on patients’ life.
Conclusion
OIT is a treatment milestone for food allergy in “personalized medicine” with systemic effects, able to not only allow Ni-rich food re-introduction but also to improve SNAS patients QoL.
Supplemental Material
Supplemental material, Attached_file__CONSORT-2010-Checklist-MS-Word for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, MMPI-2 for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, PGWBI for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, SF-36v2TM for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Acknowledgments
The authors thank Dr Franziska Michaela Lohmeyer, PhD(c), MSc, BcOT of Direzione Scientifica IRCCS of Fondazione Policlinico Universitario A. Gemelli IRCCS for editing the manuscript.
Footnotes
Authorship statement: Guarantor of article: Prof. Eleonora Nucera is the submission’s guarantor. She takes responsibility for the integrity of the work as a whole, from inception to published article.
Specific author contributions: E.N., A.R., A.D.R., and A.G. have made substantial contributions to conception and design, analysis, and interpretation of data. Moreover, they have been involved in drafting the manuscript and gave final approval of the version to be published. A.B., A.A., V.C., A.G.R., M.C., S.M., and L.R. have made substantial contributions to acquisition of data, interpretation of data and been involved in drafting the manuscript. D.S. has been involved in revising the manuscript critically for important intellectual content. R.I. has made substantial contributions to analysis and interpretation of data, been involved in revising the manuscript critically for important intellectual content. All authors approved the final version of the article, including the authorship list.
Availability of data and materials: The data sets generated during this study are available from the corresponding author on the reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosure statement: The project was conducted with no specific support.
Ethics approval: Ethical approval for this study was obtained from the local Ethical Committee of the Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome (approval number: 4133/15).
Informed consent: Written informed consent was obtained from all subjects before the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Trial registration: ClinicalTrials.gov; No. NCT03731494; URL: www.clinicaltrials.gov.
ORCID iDs: Angela Rizzi  https://orcid.org/0000-0002-6795-746X
https://orcid.org/0000-0002-6795-746X
Arianna Aruanno  https://orcid.org/0000-0002-6746-9044
https://orcid.org/0000-0002-6746-9044
Riccardo Inchingolo  https://orcid.org/0000-0003-2843-9966
https://orcid.org/0000-0003-2843-9966
Supplemental material: Supplemental material for this article is available online.
References
- 1. Thyssen JP, Linneberg A, Menné T, et al. (2007) The epidemiology of contact allergy in the general population – Prevalence and main findings. Contact Dermatitis 57(5): 287–299. [DOI] [PubMed] [Google Scholar]
- 2. Ricciardi L, Arena A, Arena E, et al. (2014) Systemic nickel allergy syndrome: Epidemiological data from four Italian allergy units. International Journal of Immunopathology and Pharmacology 27: 131–136. [DOI] [PubMed] [Google Scholar]
- 3. Di Gioacchino M, Ricciardi L, De Pità O, et al. (2014) Nickel oral hyposensitization in patients with systemic nickel allergy syndrome. Annals of Medicine 46: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braga M, Quecchia C, Perotta C, et al. (2013) Systemic nickel allergy syndrome: Nosologic framework and usefulness of diet regimen for diagnosis. International Journal of Immunopathology and Pharmacology 26: 707–716. [DOI] [PubMed] [Google Scholar]
- 5. Rizzi A, Nucera E, Laterza L, et al. (2017) Irritable bowel syndrome and nickel allergy: What is the role of the low nickel diet? Journal of Neurogastroenterology and Motility 23: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lusi EA, Di Ciommo VM, Patrissi T, et al. (2015) High prevalence of nickel allergy in an overweight female population: A pilot observational analysis. PLoS ONE 10: e0123265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pizzutelli S. (2011) Systemic nickel hypersensitivity and diet: Myth or reality? European Annals of Allergy and Clinical Immunology 43: 5–18. [PubMed] [Google Scholar]
- 8. Perino A. (2005) Nickel and food. International Journal of Immunopathology and Pharmacology 18: 15–17. [PubMed] [Google Scholar]
- 9. Minelli M, Schiavino D, Musca F, et al. (2010) Oral hyposensitization to nickel induces clinical improvement and a decrease in TH1 and TH2 cytokines in patients with systemic nickel allergy syndrome. International Journal of Immunopathology and Pharmacology 23(1): 193–201. [DOI] [PubMed] [Google Scholar]
- 10. Baiardini I, Braido F, Brandi S, et al. (2006) Allergic diseases and their impact on quality of life. Annals of Allergy, Asthma & Immunology 97: 419–428; quiz 429–430, 476. [DOI] [PubMed] [Google Scholar]
- 11. Kansen HM, Le T-M, Meijer Y, et al. (2018) The impact of oral food challenges for food allergy on quality of life: A systematic review. Pediatric Allergy and Immunology 29(5): 527–537. [DOI] [PubMed] [Google Scholar]
- 12. Muraro A, Dubois AEJ, DunnGalvin A, et al. (2014) EAACI food allergy and anaphylaxis guidelines. Food allergy health-related quality of life measures. Allergy 69: 845–853. [DOI] [PubMed] [Google Scholar]
- 13. Rak S, Yang WH, Pedersen MR, et al. (2007) Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: A double-blind, randomised study. Quality of Life Research 16(2): 191–201. [DOI] [PubMed] [Google Scholar]
- 14. Novakova SM, Staevska MT, Novakova PI, et al. (2017) Quality of life improvement after a three-year course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis: Results from real-life. Health and Quality of Life Outcomes 15: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calnan CD, Fregert S, Magnusson B. (1976) The international contact dermatitis research group. Cutis 18: 708–710. [PubMed] [Google Scholar]
- 16. Apolone G, Mosconi P. (1998) The Italian SF-36 Health Survey: Translation, validation and norming. Journal of Clinical Epidemiology 51(11): 1025–1036. [DOI] [PubMed] [Google Scholar]
- 17. Grossi E, Groth N, Mosconi P, et al. (2006) Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S). Health and Quality of Life Outcomes 4: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai S, Molfino A, Mecarelli O, et al. (2018) Neurological and psychological changes in hemodialysis patients before and after the treatment. Therapeutic Apheresis and Dialysis 22(5): 530–538. [DOI] [PubMed] [Google Scholar]
- 19. Epstein-Rigbi N, Goldberg MR, Levy MB, et al. (2019) Quality of life of food-allergic patients before, during, and after oral immunotherapy. The Journal of Allergy and Clinical Immunology 7: 429–436.e2. [DOI] [PubMed] [Google Scholar]
- 20. Sicherer SH, Noone SA, Muñoz-Furlong A. (2001) The impact of childhood food allergy on quality of life. Annals of Allergy, Asthma & Immunology 87(6): 461–464. [DOI] [PubMed] [Google Scholar]
- 21. Meyer R, Godwin H, Dziubak R, et al. (2017) The impact on quality of life on families of children on an elimination diet for non-immunoglobulin E mediated gastrointestinal food allergies. World Allergy Organization Journal 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stensgaard A, Bindslev-Jensen C, Nielsen D, et al. (2017) Quality of life in childhood, adolescence and adult food allergy: Patient and parent perspectives. Clinical and Experimental Allergy 47(4): 530–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Attached_file__CONSORT-2010-Checklist-MS-Word for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, MMPI-2 for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, PGWBI for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology
Supplemental material, SF-36v2TM for Impact of nickel oral hyposensitization on quality of life in systemic nickel allergy syndrome by Angela Rizzi, Alessia Di Rienzo, Alessandro Buonomo, Arianna Aruanno, Valentina Carusi, Anna Giulia Ricci, Michele Centrone, Simona Mezzacappa, Lilli Romeo, Domenico Schiavino, Riccardo Inchingolo, Antonio Gasbarrini and Eleonora Nucera in International Journal of Immunopathology and Pharmacology