Abstract
With the advent of immunotherapy as a realistic and promising option for cancer treatment, adoptive cellular therapies are gaining significant interest in the clinic. Whilst the recent successes of chimeric antigen receptor T-cell therapies for haematological malignancies are widely known, they have yet to show great success in solid cancers. However, immune cells transduced with T-cell receptors have been shown to traffic to and exert anti-cancer effects on solid tumour cells with some great successes. In this review, we explore the field of transgenic T-cell receptor immunotherapy, highlighting some of the key clinical trials which have paved the way for this type of cellular immunotherapy. Some trials have shown amazing clinical results, including long-term remissions and minimal toxicity, and can be looked at as an exemplar for this adoptive cell therapy. There have also been key trials where unexpected, fatal, off-tumour toxicity has occurred, and these trials have also been instrumental in shaping safer clinical trials, particularly regarding preclinical testing. In addition to previous trials, we analysed the current clinical trial space for T-cell receptor T-cell therapy, showing which trials are dominating in the clinic and which targets are being prioritised by researchers around the world. By looking at both past and current trials, we have been able to identify key drivers in developing transgenic T-cell receptor immunotherapy for the future.
Keywords: adoptive cell therapy, clinical trials, immunotherapy, T-cell receptor, TCR, transgenic
Introduction
Immunotherapy has recently begun to realise its promise within the field of cancer treatment. There are already some immunotherapies being used in the clinic including the use of cytokines such as IL-2, which help support endogenous T-cells directed against cancer, and checkpoint inhibitors such as nivolumab, an anti-PD-1 drug.1,2 However, these therapies are just scratching the surface of some potentially more effective and successful immunotherapies, which are currently being developed and trialled. One of the most exciting areas of these up-and-coming immunotherapies is adoptive cell therapy (ACT). This approach involves the isolation, expansion and in some cases, genetic engineering of T-cells before re-administration to the patient, either autologously or allogeneically. Tumour-infiltrating lymphocyte (TIL) therapy is a form of ACT where T-cells are extracted from the patient’s tumour, expanded in vitro and given back to the patient, along with pre-conditioning lymphodepletion and an IL-2 regime.3 The other two major types of adoptive T-cell immunotherapy, chimeric antigen receptor (CAR) and T-cell receptor (TCR) T-cell therapies, involve genetically manipulating patient T-cells with the introduction of a cancer-associated antigen receptor, and will be discussed below. With adoptive immune cell therapies beginning to enter the clinic, it is important to reflect on past successes and failures to best map out the future directions of cancer immunotherapy.
One of the biggest breakthroughs in the clinic, and a benchmark of a new class of cancer therapy, has been the advent of CAR T-cell therapy. Autologous lymphocytes are transduced with a CAR that recognises antigens on the surface of tumour cells directly, bypassing the requirement for the antigen to be processed and presented to TCRs by major histocompatibility complex (MHC) molecules. The CARs used thus far are of very high affinity and can detect even small amounts of tumour antigen on the surface of cells. However, there have been some safety concerns about this approach, such as the induction of cytokine release syndrome and neurological toxicity.4 Another drawback of this approach is that, to date, the major success of CARs has been restricted to haematological malignancies, with much poorer response rates in solid tumours; these range from 3/11 complete remission in neuroblastoma, to no clinical response in renal cell carcinoma.5,6
Another immunotherapeutic option for targeting solid cancers is TCR T-cell therapy, which involves transducing patient lymphocytes with a specific TCR that often recognises a known antigen expressed by cancer cells and presented in the context of MHC, which leads to induction of cytotoxic T-cell effector functions and the killing of targeted tumour cells. This approach requires that the patient tests positive for the target antigen and expresses the correct restricting MHC haplotype for the TCR. If these prerequisites are met, then transfer of the TCR can take place. TCR T-cell therapy carries certain benefits over CAR T-cell therapy. TCR T-cell therapy has been able to produce durable responses for patients with solid tumours, which is highlighted later in this review, and is something that has not been reflected in the CAR T-cell field. One factor that could contribute to this observation is that TCRs can target intracellular proteins, as the proteins are processed and presented to the TCR by MHC molecules, whereas CARs can only target extracellular proteins. Another benefit is that general cytokine storm for TCR T-cell therapy is considerably lower than that produced by CAR T-cell therapy, however there are reported instances of on-target, off-tumour responses, which are discussed further below.
Current state of the clinic
Factors to consider when designing a TCR for therapeutic use are: (a) choice of target antigen, (b) efficiency of antigen presentation, and (c) the relative levels of antigen expression on tumour cells compared with other healthy cells in the body. These factors can dictate how successful the TCR therapy is likely to be. An ideal antigen target would be expressed highly and specifically on all tumour cells. To date, several antigen targets have been identified for a variety of cancer types, and TCRs identified or engineered to target them. These can be broken down into broad categories, listed here according to definitions from the Cancer Antigen Peptide Database.7
Mutation antigens: unique antigens resulting from specific point mutations and expressed throughout the tumour, such as B-RAF mutation in melanoma.
Tumour-specific antigens: shared antigens expressed in tumours, while being absent from healthy tissue, such as NY-ESO-1 found in many tumour types.
Differentiation antigens: shared antigens expressed by tumours, as well as the tissue the tumour originated from, such as MART-1 in melanoma.
Overexpressed antigens: shared antigens which are expressed in different healthy tissues, but are overexpressed in tumours, such as p53.
There are advantages and disadvantages to targeting each of these antigen types. Often, the more tumour-specific antigens are not present as prevalently in tumours, or in as many patients. Differentiation and overexpressed antigens tend to be present in more patients, and at a higher amount in the tumours, but carry the increased risk of off-tumour toxicity due to presence in healthy tissues. This can induce mild side effects, such as in melanoma where corresponding healthy tissue is dispensable and corresponding healthy melanocytes express the same antigen that is being targeted on the tumour, leading to the development of vitiligo. However, off-tumour toxicity can have more serious consequences, which are discussed later in the review.
A comprehensive search of the National Institutes of Health (NIH) clinical trials database revealed 104 clinical trials which use TCR T-cell therapy to treat a range of cancer types, the details of which can be found in Tables 1 and 2. Melanoma still dominates in the clinic, but this is likely a reflection on the immunogenicity of the tumour and having well-validated antigens to target. In fact, melanoma was the only cancer type being targeted by TCRs to differentiation antigens, such as melanoma-restricted antigens gp100 and MART-1, shown in Figure 1. The lack of similar antigens for other cancer types is possibly one factor restricting TCR T-cell therapy in the broader sense. Following melanoma, the next most frequently targeted cancers are head and neck cancers, including oropharyngeal, and gynaecological cancers. Although not harbouring the high number of mutations of melanoma, these cancers are relatively high in mutational load and/or present viral antigens, and are therefore more immunogenic.8 It is likely that, with increased research efforts into these tumours, new suitable targets can be identified, broadening the scope of TCR T-cell therapy.
Table 1.
List of completed and recruiting clinical trials using T-cell receptor engineered T-cells, data correct as of 6 April 2020.
| Target | Condition | Phase of trial | Country | ClinicalTrials.gov identifier (status) |
|---|---|---|---|---|
| AFP | Hepatocellular cancer | Phase I | Spain, United Kingdom, United States | NCT03132792 (recruiting) |
| AFP | Hepatocellular carcinoma | Phase I | China | NCT03971747 (not yet recruiting) |
| CMV | Haematological malignancies | Phase I | United Kingdom | NCT02988258 (suspended, protocol being re-written to allow inclusion of more patients) |
| EBV | Nasopharyngeal carcinoma | Phase I/II | China | NCT03648697 (not yet recruiting) |
| EBV | Head and neck squamous cell carcinoma | Phase I/II | China | NCT04139057 (recruiting) |
| EBV | Nasopharyngeal carcinoma | Phase I | China | NCT03925896 (recruiting) |
| gp100 | Melanoma | Phase I | United States | NCT00085462 (completed, no results posted) |
| gp100 | Melanoma | Phase I | United Kingdom, United States | NCT01211262 (completed, no results posted) |
| gp100 | Uveal melanoma | Phase I/II | Canada, Germany, Spain, United Kingdom, United States | NCT02570308 (active, not recruiting) |
| HA-1 | Recurrent acute leukaemia | Phase I | United States | NCT03326921 (recruiting) |
| HBV | Hepatocellular carcinoma | Phase I | China | NCT02686372 (recruiting) |
| HBV | Hepatocellular carcinoma | Phase I | China | NCT02719782 (recruiting) |
| HBV | Hepatocellular carcinoma | Phase I | China | NCT03899415 (recruiting) |
| HERV-E | Acute myeloid leukaemia | Phase I/II | United States | NCT02770820 (active, not recruiting) |
| HPV-16 E6 | Cervical cancer, head and neck squamous cell carcinoma | Phase I | China | NCT03578406 (recruiting) |
| HPV-16 E6 | Vulvar high grade squamous intraepithelial lesion | Phase I | United States | NCT03197025 (completed) – 1/1 non-CR/non-PD |
| HPV-16 E6 | Vaginal cancer, cervical cancer, anal cancer, penile cancer, oropharyngeal cancer | Phase I/II | United States | NCT02280811 (completed) – 2/12 PR |
| HPV-16 E7 | Cervical cancer, vulvar neoplasms | Phase I/II | United States | NCT02858310 (recruiting) |
| HPV-16 E7 | Oropharyngeal cancer | Phase II | United States | NCT04015336 (suspended pending approval of a new IND) |
| HPV-16 E7 | Oropharyngeal cancer | Phase II | United States | NCT04044950 (not yet recruiting) |
| HPV-16 E7 | HPV-16+ve cancers | Phase I | United States | NCT03912831 (recruiting) |
| HPV-16 E7 | Vulvar high-grade squamous intraepithelial lesion | Phase II | United States | NCT03937791 (recruiting) |
| KRAS G12D | Gastrointestinal cancer, pancreatic cancer, colon cancer, rectal cancer | Phase I/II | United States | NCT03745326 (recruiting) |
| KRAS G12V | Pancreatic cancer, gastrointestinal cancer, colon cancer, rectal cancer | Phase I/II | United States | NCT03190941 (recruiting) |
| KRAS G12V | Pancreatic cancer | Phase I/II | China | NCT04146298 (recruiting) |
| MAGE-A10 | Non-small cell lung cancer | Phase I | Canada, Spain, United Kingdom, United States | NCT02592577 (active, not recruiting) |
| MAGE-A10 | Urothelial carcinoma, head and neck cancer, melanoma | Phase I | Canada, Spain, United States | NCT02989064 (active, not recruiting) |
| MAGE-A3/A6 (KITE-718) | Solid tumour | Phase I | United States | NCT03139370 (recruiting) |
| MAGE-A3-DP0401/0402 | Cervical cancer, renal cancer, urothelial cancer, melanoma, breast cancer | Phase I/II | United States | NCT02111850 (recruiting) |
| MAGE-A4 | Non-small cell lung carcinoma, malignant melanoma, oesophageal carcinoma, head and neck carcinoma | Phase I | China | NCT01694472 (unknown) |
| MAGE-A4 | Solid tumours | Phase I | Japan | NCT02096614 (unknown) |
| MAGE-A4 | Bladder cancer, melanoma, head and neck cancer, ovarian cancer, non-small cell lung cancer, oesophageal cancer, gastric cancer, synovial sarcoma, myxoid round cell, liposarcoma | Phase I | Canada, United States | NCT03132922 (recruiting) |
| MART-1 | Melanoma | Phase I | United States | NCT00091104 (completed, no results posted) |
| MART-1 | Melanoma | Phase I/II | Netherlands | NCT02654821 (active, not recruiting) |
| MART-1 and gp100 | Melanoma | Phase II | United States | NCT00923195 (completed) – 4/4 PD |
| MART-1 F5 | Metastatic melanoma | Phase II | United States | NCT00509288 (completed) – 6/21 PR, 15/21 PD |
| MART-1 F5 | Metastatic melanoma | Phase II | United States | NCT00910650 (completed, no results posted) |
| MCPyV | Merkel cell cancer | Phase I/II | United States | NCT03747484 (suspended due to COVID19) |
| Mutated neoantigens | Glioblastoma, non-small cell lung cancer, ovarian cancer, breast cancer, gastrointestinal cancer, genitourinary cancer | Phase II | United States | NCT03412877 (recruiting) |
| Mutated neoantigens | Melanoma, urothelial carcinoma, ovarian cancer, colorectal cancer, breast cancer (HR+), or prostate cancer | Phase I | United States | NCT03970382 (recruiting) |
| NY-ESO-1 | Metastatic malignant neoplasm in the brain | N/A | United States | NCT02774291 (recruiting) |
| NY-ESO-1 | Advanced malignant solid tumours | Not applicable | China | NCT03047811 (unknown) |
| NY-ESO-1 | Adult and child solid metastatic neoplasm | Phase I | United States | NCT02775292 (completed, no results posted) |
| NY-ESO-1 | Bladder carcinoma, breast cancer, oesophageal carcinoma, lung cancer, melanoma, multiple myeloma, neuroblastoma, ovarian cancer, synovial sarcoma, other metastatic solid cancers | Phase I | China | NCT02457650 (unknown) |
| NY-ESO-1 | Bone sarcoma, soft tissue sarcoma | Phase I | China | NCT03462316 (recruiting) |
| NY-ESO-1 | Liver cancer, gastric cancer, oesophageal cancer, bone and soft tissue tumours, breast cancer, bladder carcinoma, prostate carcinoma, thyroid cancer, ovarian cancer, solid tumour | Phase I | China | NCT03159585 (completed, no results posted) |
| NY-ESO-1 | Locally advanced malignant neoplasm | Phase I | United States | NCT03240861 (recruiting) |
| NY-ESO-1 | Multiple myeloma, melanoma, synovial sarcoma, myxoid/round cell, liposarcoma | Phase I | United States | NCT03399448 (active, not recruiting) |
| NY-ESO-1 | Neoplasms | Phase I | United States | NCT02588612 (active, not recruiting) |
| NY-ESO-1 | Neoplasms | Phase I | United States | NCT03391778 (recruiting) |
| NY-ESO-1 | Fallopian tube carcinoma, ovarian carcinoma, primary peritoneal carcinoma | Phase I | United States | NCT03691376 (recruiting) |
| NY-ESO-1 | Fallopian tube carcinoma, ovarian carcinoma, primary peritoneal carcinoma | Phase I | United States | NCT03017131 (recruiting) |
| NY-ESO-1 | Solid tumours | Phase I | Japan | NCT02366546 (active, not recruiting) |
| NY-ESO-1 | Synovial sarcoma | Phase I | United States | NCT01343043 (completed, no results posted) |
| NY-ESO-1 | Synovial sarcoma, melanoma, oesophageal cancer, ovarian cancer, lung cancer, bladder cancer, liver cancer | Phase I | Canada | NCT02869217 (recruiting) |
| NY-ESO-1 | Adult solid neoplasm | Phase I/II | United States | NCT02650986 (recruiting) |
| NY-ESO-1 | Advanced non-small cell lung cancer | Phase I/II | China | NCT03029273 (recruiting) |
| NY-ESO-1 | Neoplasms | Phase II | United States, Canada, United Kingdom | NCT03967223 (recruiting) |
| NY-ESO-1 | Soft tissue sarcoma | Phase I | China | NCT04318964 (not yet recruiting) |
| NY-ESO-1 | Multiple myeloma | Phase I/II | United States | NCT01352286 (completed) – 3/25 CR, 18/25 PR |
| NY-ESO-1 | Synovial sarcoma | Phase I/II | Japan | NCT03250325 (active, not recruiting) |
| NY-ESO-1 | Malignant neoplasm | Phase II | United States | NCT01697527 (active, not recruiting) |
| NY-ESO-1 | Melanoma, meningioma, breast cancer, non-small cell lung cancer, hepatocellular cancer | Phase II | United States | NCT01967823 (recruiting) |
| NY-ESO-1 | Neoplasms | Phase II | United States | NCT02992743 (recruiting) |
| NY-ESO-1 | Neoplasms | Phase II | Canada, Spain, United States | NCT03709706 (recruiting) |
| NY-ESO-1 | Neoplasms | Phase II | United States | NCT03168438 (recruiting) |
| NY-ESO-1 or MAGE-A3 | Ovarian cancer | Phase I/II | United States | NCT01567891 (completed) – 0/6 |
| P53 | Metastatic melanoma, other malignancies | Phase II | United States |
NCT00393029 (completed) – 1/9 clinical tumour regression |
| PRAME | Acute myeloid leukaemia, myelodysplastic syndrome, uveal melanoma | Phase I/II | United States | NCT02743611 (active, not recruiting) |
| PRAME | High-risk myeloid and lymphoid neoplasms | Phase I/II | Germany | NCT03503968 (recruiting) |
| PRAME/COL6A3 | Platinum-resistant ovarian cancer | Phase I | United States | NCT03318900 (recruiting) |
| Tyrosinase | Melanoma | Phase I | United States | NCT02870244 (recruiting) |
| Tyrosinase | Melanoma | Phase I | United States | NCT01586403 (active, not recruiting) |
| Unknown – IMA101 | Solid tumours | Phase I | United States | NCT02876510 (active, not recruiting) |
| Unknown – IMA201 product | Head and neck squamous cell carcinoma, non-small cell lung cancer | Phase I | United States | NCT03247309 (recruiting) |
| Unknown – IMA202 product | Hepatocellular carcinoma, hepatocellular cancer, non-small cell lung cancer, liver cancer, lung cancer | Phase I | United States | NCT03441100 (recruiting) |
| Unknown - IMA203-101 product | Refractory cancer, recurrent cancer, solid cancer | Phase I | United States | NCT03686124 (recruiting) |
| Unknown (tumour-specific antigen) | Non-small cell lung cancer | Phase I | China | NCT03778814 (recruiting) |
| Unknown (tumour-specific antigen) | Advanced solid cancers | Phase I | China | NCT03891706 (recruiting) |
| WT-1 | Chronic myeloid leukaemia, acute myeloid leukaemia | Not stated | United Kingdom | ISRCTN11622375 (completed, no results posted) |
| WT-1 | Acute myeloid leukaemia, chronic myeloid leukaemia | Phase I/II | United Kingdom | NCT01621724 (Completed, no results posted) |
| WT-1 | Acute myeloid leukaemia | Phase I/II | United States | NCT01640301 (active, not recruiting) |
| WT-1 | Acute myeloid leukaemia | Phase I/II | United States | NCT02770820 (active, not recruiting) |
| WT-1 | Malignant mesothelioma, non-small cell lung carcinoma | Phase I/II | United States | NCT02408016 (active, not recruiting) |
| WT-1 | Myelodysplastic syndromes, acute myeloid leukaemia | Phase I/II | Belgium, Germany, United Kingdom | NCT02550535 (completed, no results posted) |
Table 2.
List of terminated T-cell receptor T-cell therapy trials, data correct as of 6 April 2020.
| Target | Condition | Phase of trial | Country | ClinicalTrials.gov identfier | Reason for termination |
|---|---|---|---|---|---|
| CEA | Metastatic cancer | Phase I | United States | NCT00923806 | Poor accrual (3 participants) |
| gp100 | Melanoma, skin cancer | Phase II | United States | NCT00509496 | Low accrual (21 participants) |
| gp100 | Metastatic melanoma, skin cancer | Phase II | United States | NCT00610311 | Low accrual (3 participants) |
| hTG | Metastatic thyroid cancer | Phase I/II | United States | NCT02390739 | Withdrawn (0 participants) |
| MAGE-A3 | Breast cancer, cervical cancer, renal cancer, melanoma, bladder cancer | Phase I/II | United States | NCT02153905 | Not stated (3 patients) |
| MAGE-A3/12 | Metastatic cancer, metastatic renal cancer, metastatic melanoma | Phase I/II | United States | NCT01273181 | Not stated (9 participants) |
| MART-1 F5 | Melanoma | Phase II | United States | NCT00706992 | <11 subjects were enrolled to each arm (50 participants) |
| MART-1 F5 | Metastatic melanoma, skin cancer | Phase II | United States | NCT00612222 | Low accrual (4 participants) |
| NY-ESO-1 | Unspecified adult solid tumour | Phase I | United States | NCT02070406 | Low accrual (4 participants) |
| NY-ESO-1 | Multiple myeloma | Phase I | United States | NCT03506802 | Withdrawn (no participants enrolled) |
| NY-ESO-1 | Melanoma | Phase I/II | United States | NCT01350401 | Lack of enrolment (4 participants) |
| NY-ESO-1 | Metastatic cancer, metastatic renal cancer, metastatic melanoma | Phase I/II | United States | NCT01457131 | Not stated (2 participants) |
| NY-ESO-1 | Metastatic cancer, metastatic melanoma | Phase II | United States | NCT02062359 | Poor accrual (2 participants) |
| NY-ESO-1 | Metastatic cancer, metastatic renal cancer, metastatic melanoma | Phase II | United States | NCT00670748 | More highly selected protocol opened for patients with melanoma (45 participants) |
| NY-ESO-1 | Neoplasms | Phase II | No information provided | NCT03697824 | Withdrawn – internal decision, study to be replaced with a larger monotherapy trial (0 participants) |
| NY-ESO-1 | Multiple myeloma | Phase I/II | United States | NCT01892293 | Sponsor decision (6 participants) |
| P53 | Kidney cancer, melanoma, unspecified adult solid tumour | Phase II | United States | NCT00704938 | Withdrawal of support from collaborator (3 participants) |
| TGFβII | Metastatic colorectal cancer | Phase I/II | Norway | NCT03431311 | Terminated (Sponsor decision) |
| TRAIL bound to DR4 | Renal cancer, kidney cancer | Phase I | United States | NCT00923390 | Not stated (5 participants) |
Figure 1.
Clinical trials heatmap. The type of cancer being targeted by completed and active clinical trials is plotted against the type of antigen the T-cell receptor is directed against, as defined by the Cancer Antigenic Peptide Database.7 NSCLC, non-small cell lung cancer.
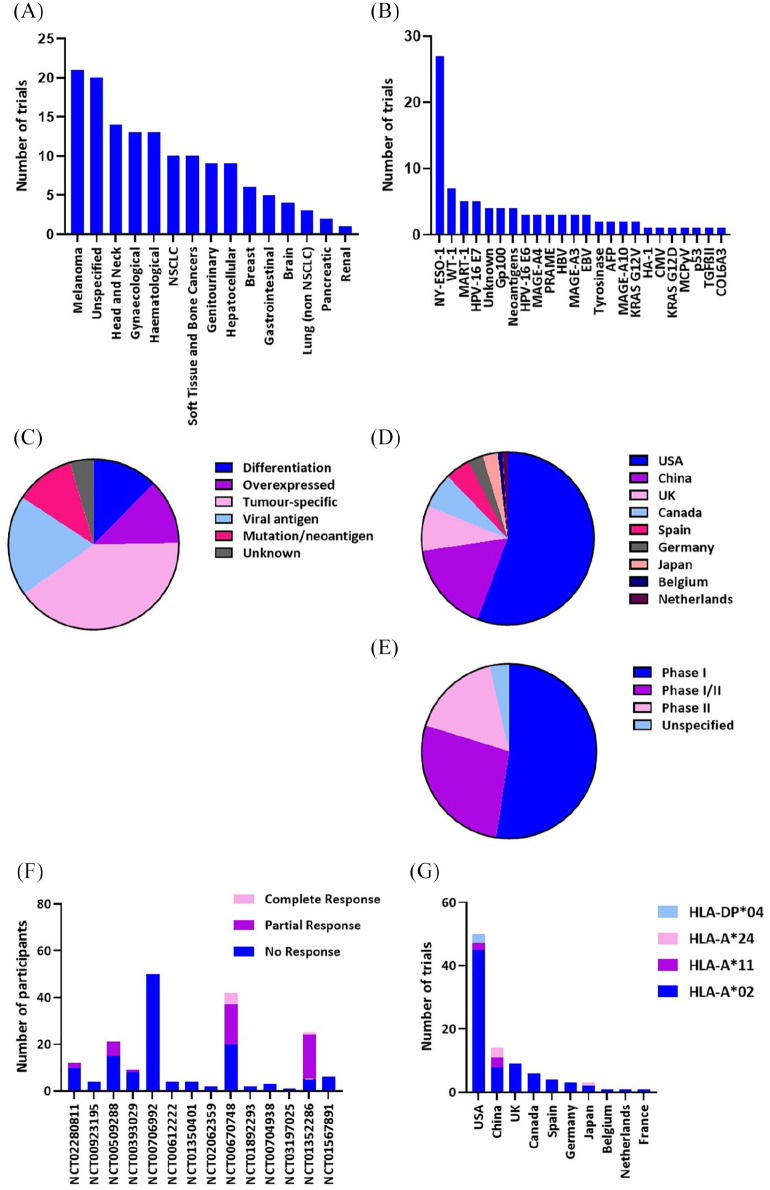
The 45 actively-recruiting trials evaluating TCR therapy target 19 different antigens collectively. Of these trials, 22 utilise TCRs directed towards NY-ESO-1 epitopes. This tumour-specific antigen can also be described as a cancer-testis (CT) restricted antigen, meaning that it is only found in tumour cells and early germline cells. It is a popular choice for targeting due to the highly specific nature of its expression in multiple types of cancer, whilst being absent in healthy tissues.9 The percentage of tumours that express NY-ESO-1 varies between different cancer types but has been reported to be as high as 80% of synovial sarcoma cases, and 45% of metastatic melanoma samples.10,11 The dominance of active or completed trials targeting this antigen can be visualised in Figure 2B and 2C.
Figure 2.
Clinical trials landscape. Number of clinical trials evaluating TCR T-cell therapy illustrating (A) cancer type and (B) target antigen. (C) Pie chart of number of clinical trials for each antigen type. (D) Pie chart of number of clinical trials being conducted by country. (E) Pie chart of clinical trials by phase. (F) Results of clinical trials comparing number of participants for each outcome. (G) Number of trials by human leukocyte antigen (HLA) type of participants by country. NSCLC, non-small cell lung cancer.
The majority of TCR T-cell therapy trials are being conducted in the USA (~57%), with the next highest being China (~12%) and the UK (~9%). The majority of trials are still in phase I, which focuses on safety and adverse events caused by the introduced TCR, shown in Figure 2E. Any trial that confirms the safety of using a specific TCR can progress to phase II clinical trials, which look at the comparative effectiveness of the TCR therapy. There are no trials being conducted that are in phase III, where much larger patient groups are used to compare efficacy against standard treatment. This is not surprising given the variation in trial results until this point; both successful and unsuccessful trials will be discussed later.
Unfortunately, out of the 104 trials found in this search, there have only been nine completed and only four of these have study results, potentially a reflection of the novelty of this field, or long-term follow-up status seen in some trials. A further seven trials which have been terminated have also posted study results, and a number of these trials are important examples of lack of clinical responses in TCR T-cell therapy trials. The most widely accepted reported outcome, particularly in phase II clinical trials, is clinical response, which is commonly defined by the Response Evaluation Criteria in Solid Tumours (RECIST) criteria.12 The RECIST criteria define a set of guidelines for categorising clinical response by reduction in tumour burden in the patient: complete response (CR), eradication of all target lesions; partial response (PR), 30% or greater reduction in sum of diameters of the target lesion; progressive disease (PD), 20% or greater increase in sum of diameters of target lesion (or appearance of new lesions); and stable disease (SD), increases or decreases that do not fit PR or PD criteria.13 Some clinical trials report the results as objective overall response, which is the CR and PR results taken together. The results of the completed TCR T-cell trials with regards to clinical response, as defined by RECIST criteria, are shown in Figure 2F and can demonstrate that even successful trials, such as the Chemotherapy Followed by ESO-1 Lymphocytes and Aldesleukin to Treat Metastatic Cancer Trial [ClinicalTrials.gov identifier: NCT00670748] that had a CR rate of just under 12%, can also be terminated for a variety of reasons.
One factor limiting the widespread application of TCR T-cell therapy is that the vast majority of laboratory and clinically validated TCRs are human leukocyte antigen (HLA)-restricted, meaning they recognise their cognate antigen when presented on a specific MHC molecule. HLA status varies between individuals, based on a genetic inheritance component, and some HLA types are much more prevalent in the population than others. The HLA restriction for TCRs stipulates the requirement for the patient to match the HLA restriction of the therapeutic TCR being used for the trial. This is a factor that has hindered the development of TCR T-cell therapy for large numbers of patients. A vast majority of trials use TCRs restricted to HLA-A*02, particularly in the USA, where this is statistically one of the highest represented HLA alleles in the population.14 This HLA restriction, while necessary, can result in immediate exclusion of some ethnic minority patients from TCR T-cell therapy clinical trials. Using advancing techniques such as single cell TCR sequencing, it could be possible to develop tumour-reactive TCRs for a much wider range of HLA haplotypes or find novel non-HLA-restricted TCRs, which would broaden the range of patients able to enrol on these clinical trials.15,16
Key successes in TCR T-cell therapy
Prior to the remarkable successes seen in CAR T-cell trials targeting CD19, there was great promise in TCR T-cell therapy, with a publication from the NIH demonstrating two PRs in patients using TCRs directed to MART-1.17 Since this report, NY-ESO-1 has been the favoured target of choice and, as such, the successes in the field have been demonstrated in this context with marked response rates in synovial sarcoma, melanoma and multiple myeloma patients.18,19
In the first reported trial of an NY-ESO-1 TCR T-cell therapy [ClinicalTrials.gov identifier: NCT00670748], patients positive for HLA-A*0201 and NY-ESO-1 tumour expression were enrolled onto two arms, one for melanoma and one for synovial sarcoma. Patients were infused with at least 1 × 108 autologous peripheral blood mononuclear cell derived T-cells transduced with a high-affinity NY-ESO-1-reactive TCR, after a lymphodepleting regime of cyclophosphamide (60 mg/kg/day for 2 days) and fludarabine (25 mg/m2/day for 5 days). Following T-cell administration, patients received up to 15 intravenous doses of exogenous IL-2 (Aldesleukin 720,000 IU/kg) to aid the expansion of the adoptively transferred T-cells in vivo.17,20 The median number of cells transferred to the patients was 5 × 1010 T-cells, and for all but one of these patients, the composition of the T-cells was > 68% CD8 positive. The median transduction rate, determined by staining of T-cells with antibodies directed to the Vβ13.1 domain within the introduced TCR β chain, was 92%. Response rates were reported as 45% (5/11) in the melanoma arm, with 18% (2/11) CRs, and 67% (4/6) showed PR in the sarcoma arm. Importantly, no severe off-target toxicity was observed, with the only side-effects being associated with the preconditioning regime and IL-2 supportive therapy, which both subsided with treatment.
Further to this study, a long-term follow-up study was carried out by the same investigators to evaluate prolonged survival rates in patients who received NY-ESO-1 TCR therapy, and expanded the data with a further 12 synovial sarcoma patients added to the 6 from the original cohort, and a further 9 melanoma patients added to 11 from the original cohort.21 One month after TCR transfer, 61% (11/18) of patients with synovial sarcoma had an objective clinical response, while 55% (11/20) of metastatic melanoma patients experienced an objective clinical response. PRs for synovial sarcoma patients lasted between 3 and 18 months, 3-year survival rate was predicted to be 38%, and 5-year survival was estimated at 14%. PR for melanoma patients was better, lasting between 3 and 28 months, with both 3- and 5-year survival rates predicted to be 33%. As a comparison, Kim et al. carried out a study of patients treated through chemotherapy or combination chemotherapy, and their data shows the 5-year survival rate for metastatic melanoma to be 16%.22 As checkpoint blockade therapies are now commonly used to treat metastatic melanoma, they might be a better comparator for survival rates. The 1- and 2-year survival rates for nivolumab have been reported as 62% and 43%, respectively, and a more recent report of treatment with pembrolizumab or ipilimumab state the 2-year survival rates to be 55% and 43%, respectively.2,23 Whilst these appear to be more favourable survival rates in comparison with TCR therapy, it is noteworthy that most patients treated in the TCR T-cell trials have often already failed several therapies, including checkpoint blockade therapies.
In a phase II trial investigating TCR T-cell therapy against NY-ESO-1 in multiple myeloma patients [ClinicalTrials.gov identifier: NCT01352286], 14 of the 20 patients (70%) achieved near or full CRs. This was despite a mean transfusion of 8 × 109 cells, with mean transduction efficiency of 33%, considerably lower than the initial investigation carried out by Rapoport et al.19 Another factor that varied from the previous trial was that the proportion of CD8+ T-cells in the product was lower, 42% being the median, ranging from 18% to 75%. Despite these differences, the results were very promising, with 14 patients having near or full CR, 4 PR, 1 SD and just 1 patient had PD. These response rates were assessed at day 100 post-treatment with a further follow-up at a median of 21 months, which revealed 75% of the patients were alive, 50% alive and disease free and only five patients died due to disease progression.
Other antigens that have been targeted by TCR T-cell therapy include over-expressed antigens gp100 and MART-1, whose expression on healthy melanocytes is low but can be found at much higher expression on melanoma cells. There have been mixed results from trials targeting overexpressed melanoma antigens. A collection of three clinical trials [ClinicalTrials.gov identifiers: NCT00509288, NCT00923195 and NCT01273181] were performed by the same group to investigate TCR T-cell therapy.17,24,25 They used TCRs to target melanoma-associated antigens gp100, MART-1 and MAGE-A3. In the first, Morgan et al. report using an anti-MART-1 TCR (DMF4) isolated from a patient TIL clone from a melanoma tumour to treat 17 melanoma patients. Whilst only 12% (2/17) had a clinical response, as set out by the RECIST criteria, no severe off-target toxicity was observed. This landmark study was the first to provide strong evidence that autologous lymphocytes transduced with TCRs could invoke clinical responses in a cancer setting, sparking increased interest in the field.
Subsequently, a high-affinity variant of the anti-MART-1 TCR (DMF5) was developed, and used to treat 20 additional patients, of which 6 had a clinical response (30%).24 In the same study, an anti-gp100 TCR isolated from a transgenic mouse model was used to treat 16 patients, 3 of whom had a clinical response (~19%). However, a major drawback of this study was that approximately 80% (29/36) patients treated with either the high affinity MART-1 or gp100 TCR experienced severe off-tumour side effects, where normal melanocytes of the skin, eye and inner ear were also targeted by the transduced lymphocytes. For the MART-1 TCR, the targeting resulted in widespread erythema (14/20 patients), anterior uveitis (11/20 patients) and ototoxicity leading to temporary loss of hearing (10/20 patients); all symptoms improved with or without intervention. This contrasted with observations of the aforementioned study conducted by Morgan et al. utilising the DMF4 TCR, where none of these toxicities occurred.17 This likely represents a situation where increasing the affinity of the innate TCR, and therefore sensitivity to lower levels of the MART-1 target antigen, resulted in T-cell activation in normal, healthy tissues where MART-1 is present at low levels. This is a key example of where increasing the affinity of a TCR might not be beneficial to patient outcome.
Key failings in the field of TCR T-cell therapy
As well as key successes in the area of TCR T-cell therapy, there have also been some important failings. The first notable instance of death due to TCR T-cell therapy was reported in patients receiving a HLA-A*02-restricted TCR that could recognise epitopes of MAGE-A3/A9/A12. Out of the nine patients treated, five had durable clinical responses, including one patient who was classed as a complete responder over 15 months post-treatment. However, a couple of days following infusion of the TCR-transduced T-cells, three patients experienced changes to their mental state; two of these patients went into comas and experienced multiple seizures, and eventually passed away despite various efforts to improve their mental and physical states.26 Both patients’ autopsy reports showed necrotic brain tissue, with massive infiltrations of lymphocytes. Through further analysis by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), NanoString quantitation and deep sequencing, the presence of MAGE-A12 in the brains of the deceased patients was revealed, indicating that the infused T-cell product was likely responsible for the massive brain inflammation and, ultimately, the deaths of these patients. These events highlight the need for improved characterisation of antigen expression on healthy tissues. Moreover, stratification and pre-treatment testing for antigen expression may be a necessary measure to avoid severe off-target side effects, while still allowing treatment of patients most likely to gain significant clinical benefit.
Another notable failing from which we can learn comes from a trial that used a high-affinity HLA-A*01-restricted TCR targeting MAGE-A3. Having undergone extensive pre-clinical testing of the TCR, two patients were enrolled on trials to receive MAGE-A3 TCR T-cells to treat either melanoma or myeloma. Unfortunately, within 5 days of receiving the TCR-transduced T-cells, both patients passed away from serious adverse events.27 The first patient had a history of silent myocardial infarctions, so whilst clinical test results revealed cardiac tissue damage and cardiac arrest was recorded as the cause of death, these were not seen as related to the T-cell infusion received 4 days prior. Subsequently, the second patient, who did not have a history of cardiac complications, died following severe cardiac shock, causing the case of the first patient to be re-examined. Both patients had large immune infiltrates in their cardiac tissues, and cytokine analysis revealed the activated status of these T-cells. Further in vitro assays led to the discovery that the high-affinity MAGE-A3 TCR could recognise a cross-reactive epitope derived from titin, a protein expressed in striated muscle and in culture by beating cardiac myocytes derived from induced pluripotent stem cells (iPSCs). Mass spectroscopy confirmed MHC expression of titin peptides and quantitative polymerase chain reaction (qPCR) confirmed high levels of titin expression in cardiac autopsy samples from both patients. Together with the autopsy report and other in vitro results, it was concluded that the high-affinity MAGE-A3 TCR could recognise and target cells expressing an unrelated epitope of high similarity to MAGE-A3, derived from the titin protein.
Another example of TCR T-cell therapy where extensive side effects were reported was the use of anti-carcinoembryonic antigen (anti-CEA) TCR to target colorectal cancers.28 Three patients were treated with autologous T-cells transduced with an anti-CEA TCR that was raised in an immunised HLA-A*0201 transgenic mouse model. One patient had an initial PR with a reduction in tumour burden of ~50% but had PD by 6 months post-infusion. The other two patients did not respond or did not reach the RECIST criteria of reduction in tumour burden. The clinical trial was halted by the US Food and Drug Administration (FDA) regulations, owing to the side effects incurred by the TCR therapy; all patients experienced grade 2–3 diarrhoea, linked to the development of severe colitis, proven to be related to the infiltration of CD3+ T-cells in the colon.
Whilst unfortunate and unforeseen, the results of these trials have identified hurdles for successful therapy and triggered important investigations about the safety of high affinity, genetically engineered TCRs, and how we can shape pre-clinical safety testing for future trials. This has been particularly discussed with reference to TCRs recognising epitopes from the MAGE family of proteins; MAGE-A3 was a target in two of the trials mentioned above, however, it remains a promising target due to its high tumour expression. One suggestion for improving the safety testing of genetically modified TCRs in vitro is to carry out peptide scans of peptide homologs to gather a more complete cross-reactivity profile, such as that used to evidence the reactivity of MAGE-A3 TCR to titin.29 This would likely reduce the chance of unforeseen off-target recognition of peptides by TCRs. However, changes in expression profiles, such as that seen in the previously mentioned study where cardiac cells only expressed titin when differentiated into beating cardiomyocytes in vitro, prove to be pitfalls for this approach.
There are conflicting opinions in the field as to the value of high-affinity engineered TCRs. Whilst some evidence could indicate a clinical anti-cancer benefit from the high-affinity TCRs, as seen in the Johnson et al. trial discussed previously, there is a notable trade off with the safety of these adapted TCRs.24 Natural, unedited TCRs have undergone thymic selection so are very likely to be safe, whereas mutagenesis to achieve higher affinity for MHC:peptide complexes ultimately result in a new TCR that has not been through the same natural selection process and could, therefore, have an appreciable degree of reactivity to self-antigens. An ideal solution may be to identify naturally high-affinity TCRs from patient TIL or peripheral blood T-cells. Our current research aims to address this issue by using single-cell sequencing to identify the TCR repertoire of patient TIL and screening these TCRs against known melanoma antigens to identify tumour-reactive TCRs that have been thymically selected.30 These TCRs are likely to be very rare in the patient and it remains a challenge to identify such receptors quickly and efficiently; thus it has led to efforts to artificially engineer or generate high-affinity TCRs through in vivo models. New single-cell TCR sequencing techniques, and more routine screening of patient TIL for shared antigen TCRs, could combat these drawbacks and pave the way for a new wave of high-affinity, organic, tumour-reactive TCRs for TCR therapy. However, the MHC ligandome variation is different for each patient, which might result in knock-on effects of efficiency of presentation and possible cross-reactive targets.
Learning from previous trials to overcome challenges
A large aspect of current challenges in developing effective adoptive TCR T-cell therapy is target selection. Some trials have used TCRs directed to CT antigens such as NY-ESO-1 and MAGE-A3, with mixed success, as discussed previously. A key question in target validation for TCR therapy is: if the target antigen is expressed in healthy tissues, do the side effects resulting from on-target, off-tumour toxicity outweigh the clinical benefit of the TCR therapy?
The isolation and identification of effective, tumour-reactive TCRs has been a challenge. Some TCRs have been isolated from patient TIL, which have proven their potential to traffic to and exert an effect on tumour cells, such as the MART-1 TCR isolated by the Rosenberg group. Other TCRs have been isolated from a transgenic mouse model, such as the gp100154-162 TCR used in clinical trials, isolated by the same group. One clinical trial that is currently recruiting [ClinicalTrials.gov identifier: NCT03778814] is using patient-specific TCRs derived from patient TIL, which while effectively creating a bespoke patient-specific product, could prove to be the most-effective TCR therapy yet. By choosing the most effective cancer-targeting TCR on a patient-by-patient basis, it optimises the treatment to increase the likelihood of partial and full responses. However, one debate for TIL therapy over TCR therapy, such as this or more traditional examples, is the question of how homogenous the cancer is. Do all the metastases share the same expression profile of antigens being targeted? Does expression of the antigen change over time as the tumour grows and adapts? These questions can only be answered with further research, but it does explain why TIL therapy is one of the most effective treatment options for immunogenic cancers such as metastatic melanoma. It might be beneficial to look retrospectively at long-term survivors who have received TIL therapy and identify clones that are still present in the patient. These TCRs are not only likely to be highly relevant to the anti-cancer response but have also proven to be able to permit persistence of the associated T-cells in the body.
Another consideration for TCR T-cell and CAR T-cell therapy is the effect of cancer immunoediting that can result in relapse of antigen-negative tumours. It is a known phenomenon that tumours can undergo selection pressure and grow out resistant clones, an effect which has been shown in mouse models to be driven by IFN-γ-producing T-cells.31 It has also been demonstrated that relapsed tumours that are resistant to re-administration of T-cell therapy do not have to lose antigen to avoid targeting by T-cells, instead showing reduced numbers of CD8 T-cells and monocytes, which resulted in lower levels of chemoattractants and adhesion molecules to aid T-cell infiltration.32
As mentioned previously, a major hurdle to overcome in TCR T-cell therapy is MHC-restriction which limits the accessibility of this therapy to specific patients with the appropriate HLA type. If HLA-restriction was not a factor, patient recruitment, accessibility and total number of patients treatable would vastly increase. The identification of shared tumour antigens has allowed for the same TCR to treat multiple HLA-matched patients, however delivering such autologous therapies on a large scale remains an obstacle to widespread uptake of these forms of treatment. As such, efforts to generate allogeneic products which can be banked and used to treat multiple patents holds great promise.
One concern that several groups have been keen to address is the potential ability of the introduced TCR to mis-pair with the endogenous TCR present in the patient’s T-cell population. In theory, mis-pairing of the TCR chains with endogenously expressed TCRs would result in a new TCR that has not been thymically selected and therefore could have significant off-target cytotoxicity to another antigen expressed elsewhere in the body, a concept explored in a mouse model by Bendle et al.33 Fortunately, there have been no clinical reports about such an event occurring, likely due to stringent preclinical investigation, and several groups have devised strategies of altering their TCRs to prevent mis-pairing. These include use of CD3ζ, single-chain TCRs and CD3ε linkage.34–36
There is a distinct lack of phase III trials for TCR T-cell therapy. This is likely a reflection on the quantity and inconsistency of phase II clinical trial data. Owing to the scale of these larger clinical trials, the initial data from phase II clinical trial needs to be very promising to warrant large-scale funding to pursue a TCR for therapy. This is one aspect where the HLA-restriction is a disadvantage; it would be more financially logical to fund a CAR T-cell or TIL therapy, where all patients can be treated, as opposed to a HLA-restricted TCR therapy, where only a proportion of patients could benefit, particularly in the UK where less than 30% of the population are HLA-A2*01 restricted.37 With more research being carried out in identifying and validating novel targets for TCR therapy, hopefully a more successful series of clinical trials could pave the way to phase III trial funding, and to licensed TCR T-cell therapy treatment. As single-cell sequencing techniques are becoming more mainstream in research, it is possible that in the future, patient tumour-reactive, or high-abundance T-cells could be sequenced and neoantigen targeting TCRs identified for personalised immunotherapy. Any possible mutations to high affinity TCRs could then be identified using peptide scanning approaches to avoid potential toxicity. This approach is currently possible but would need to be made more efficient to be a viable option, however feasibility and timescale could still be a limiting factor. Overall, while there has been a lot of progress in TCR T-cell therapy and promising potential for development, there are several other immunotherapies, such as TIL therapy, which offer attractive alternatives while bypassing some of the concerns with TCR T-cell therapy, like antigen heterogeneity and off-target toxicity.
Conclusion
In summary, the results of TCR T-cell therapy trials have been mixed, but there is clear scope for development of candidate TCRs for larger scale trials and potential medicinal products. There are several hurdles that need to be overcome including identification of optimal targets for specific tumours, and potentially a more defined approach to identify non-MHC restricted TCRs which overcome the limitations associated with MHC restricted TCRs, such as their variable affinity and restricted expression in different populations. However, we should not be left feeling discouraged as responses targeting solid cancers has been far more successful than equivalent CAR-based approaches.
Footnotes
Conflict of interest statement: REH and JSB are shareholders in Immetacyte Ltd. and InsTIL Bio.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Natasha Oppermans PhD Project is part of the EPSRC and MRC funded CDT in Regenerative Medicine programme at University of Manchester (grant number EP/L014904/1).
ORCID iDs: Natasha Oppermans  https://orcid.org/0000-0002-3399-9178
https://orcid.org/0000-0002-3399-9178
Gray Kueberuwa  https://orcid.org/0000-0001-9066-0181
https://orcid.org/0000-0001-9066-0181
John S. Bridgeman  https://orcid.org/0000-0002-2857-6031
https://orcid.org/0000-0002-2857-6031
Contributor Information
Natasha Oppermans, University of Manchester, Manchester, UK.
Gray Kueberuwa, Immetacyte Ltd., University of Manchester, Manchester, Greater Manchester, UK.
Robert E. Hawkins, Immetacyte Ltd, Manchester, UK
John S. Bridgeman, Immetacyte Ltd., University of Manchester, Manchester, Greater Manchester, UK
References
- 1. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994; 271: 907–913. [PubMed] [Google Scholar]
- 2. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamers CHJ, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006; 24: e20–e22. [DOI] [PubMed] [Google Scholar]
- 6. Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118: 6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Bruggen. Cancer antigenic peptide database. https://caped.icp.ucl.ac.be/about (n.d., accessed 20 May 2019).
- 8. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. (2013). Signatures of mutational processes in human cancer. Nature 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA 1997; 94: 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen ny-eso-1 but not mage-a1 or ct7. Int J Cancer 2001; 94: 252–256. [DOI] [PubMed] [Google Scholar]
- 11. Barrow C, Browning J, MacGregor D, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 2006; 12: 764–771. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer 2016; 62: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14. Cao K, Hollenbach J, Shi X, et al. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol 2001; 62: 1009–1030. [DOI] [PubMed] [Google Scholar]
- 15. De Simone M, Rossetti G, Pagani M. Single cell T cell receptor sequencing: techniques and future challenges. Front Immunol 2018; 9: 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowther MD, Dolton G, Legut M, et al. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol 2020; 21: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015; 21: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26: 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robbins PF, Kassim SH, Tran TLN, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015; 21: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim C, Lee CW, Kovacic L, et al. Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist 2010; 15: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 24. Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood, 2009; 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abate-Daga D, Hanada K, Davis JL, et al. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood 2013; 122: 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013; 36: 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011; 19: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cameron BJ, Gerry AB, Dukes J, et al. Identification of a Titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci Transl Med 2013; 5: 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spindler MJ, Nelson AL, Wagner EK, et al. Massively parallel interrogation and mining of natively paired human TCRαβ repertoires. Nat Biotechnol. Epub ahead of print 16 Mar 2020. DOI: 10.1038/s41587-020-0438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kmieciak M, Knutson KL, Dumur, et al. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol 2007; 37: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Straetemans T, Berrevoets C, Coccoris M, et al. Recurrence of melanoma following T cell treatment: continued antigen expression in a tumor that evades T cell recruitment. Mol Ther 2015; 23: 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 2010; 16: 565–570. [DOI] [PubMed] [Google Scholar]
- 34. Sebestyén Z, Schooten E, Sals T, et al. Human TCR that incorporate CD3ζ induce highly preferred pairing between TCR and Chains following gene transfer. J Immunol 2008; 180: 7736–7746 [DOI] [PubMed] [Google Scholar]
- 35. Aggen DH, Chervin AS, Schmitt TM, et al. Single-chain VαVβ T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther 2012; 19: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Govers C, Sebestyén Z, Roszik J, et al. TCRs genetically linked to CD28 and CD3ε do not mispair with endogenous TCR chains and mediate enhanced T cell persistence and anti-melanoma activity. J Immunol 2014; 193: 5315–5326. [DOI] [PubMed] [Google Scholar]
- 37. Neville MJ, Wanseon L, Humburg P, et al. High resolution HLA haplotyping by imputation for a British population bioresource. Hum Immunol 2017; 78: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]