Abstract
Objectives
Caregiver burden in neurologic Wilson disease (NWD) has received little attention. We investigated predictors of caregiver burden in Chinese NWD patients.
Methods
Participants in this retrospective study were NWD patients admitted to The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine from 1 August to 31 December 2019. Sociodemographic information was recorded for caregivers and NWD patients. Caregiver burden was evaluated using the Caregiver Burden Inventory (CBI). Cognitive impairment, functional problems, depression and anxiety were evaluated by professional interviewers. Path analysis was used to evaluate predictors of CBI scores.
Results
Sixty NWD patients were enrolled (mean age: 21.35 ± 4.89 years; mean NWD duration: 7.85 ± 3.11 years). The mean CBI score was 52.00 ± 17.16. Care duration had a significant direct effect on CBI score after controlling for confounders (r = 0.493). Cognitive impairment (r = −0.426), functional problems (r = 0.581), depression (r = 0.349) and anxiety (r = 0.317) had significant indirect effects on CBI score.
Conclusion
Caregivers of NWD patients may experience a medium level of caregiver burden. NWD duration, cognitive impairment, functional problems, depression and anxiety in NWD patients may be useful predictors of caregiver burden.
Keywords: Caregiver burden, neurologic Wilson disease, predictors, cognitive impairment, depression, anxiety, functional problems
Introduction
Wilson disease (WD) is an inherited copper metabolism disorder.1 The prevalence rate of WD is estimated to be 1/10,000 to 1/30,000 worldwide.2–4 One Chinese study reported a prevalence rate of 5.87/100,000.5 A lack of copper excretion causes excessive copper deposits in the liver, brain and other tissues.6 Patients with untreated WD usually present with hepatic or neurologic disorders, and death may occur in severe cases.7 Neurological symptoms and cognitive–behavioural and affective disturbances are caused by copper deposition and subsequent brain degeneration, which is termed neurologic Wilson disease (NWD).8,9 Although understanding of the characteristics and aetiology of NWD has increased, data are still inconclusive. Almost all NWD patients are cared for at home by a coresident family member. It is therefore important to recognize that NWD affects not only patients but also families, particularly caregivers.
Caring for a person with physical or mental disability can be a chronically stressful experience that increases informal caregivers’ risk of reduced well-being and enhanced morbidity and mortality.10,11 Previous studies indicate that caregiver burden is a unique aspect of the caregiving experience, and can be affected by patient characteristics, disease-related factors, patient and caregiver sociodemographical factors, and caregiving-related factors.12,13 To date, the caregiver burden of caring for NWD patients has received little attention. In the present study, we aimed to investigate the predictors of caregiver burden in NWD patients.
Materials and methods
Study design and population
This was a retrospective study of NWD patients admitted to The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine from 1 August to 31 December 2019. Medical records and questionnaire results were obtained from newly diagnosed NWD patients. Patients were excluded from the study if they had malignant, severe organic disease or psychiatric diseases that made it impossible to complete the study questionnaire, or if they did not provide informed consent. Each patient was asked to identify his or her primary caregiver. We recruited those caregivers who met the definition of a family caregiver established by a previous study.14 The exclusion criteria for caregivers were the same as for patients. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the human ethics committee of The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine. Written informed consent was obtained from patients and caregivers.
The primary outcome was caregiver burden. This was defined as the physical, psychological or emotional, social and financial problems that may be experienced by family members or friends who care for impaired adults.15 Caregivers were asked to provide the sociodemographic information of age and care duration, and were also asked to complete a Caregiver Burden Inventory (CBI) to evaluate their caregiving burden. The American College of Hepatology Guidelines define NWD as a neurodegenerative disorder characterized by a combination of neurologic symptoms.16 All patients in the present study, including those with discharge diagnoses, referrals, and admission diagnoses, were re-evaluated according to the American Collage of Hepatology Guidelines. The following demographic and clinicopathologic data for NWD patients were obtained from their medical records and from questionnaires: age, sex, NWD duration, marital status, educational level, residential area and annual income. All eligible NWD patients were also asked to complete the Montreal Cognitive Assessment (MoCA), the Activities of Daily Living (ADL) Scale, the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS) to evaluate their cognitive impairment, functional problems, depression and anxiety. These tools (CBI, MoCA, HDRS, HARS) have been validated and are widely used in the diagnosis and management of Chinese NWD patients and caregiver burden, based on the American College of Hepatology Guidelines.16
Quality control procedures
Quality control procedures involved checking the accuracy of responses recorded by experienced interviewers. All patients received a questionnaire regardless of their inclusion in the study. Participants were individually interviewed by qualified interviewers who had received comprehensive training in computer-assisted personal interviewing techniques. Participants’ data were recorded in the interviews. All suspected NWD cases were reviewed by a panel of two experienced investigators who evaluated all pertinent medical records. Data quality control procedures comprised a computerized logic check, sequential recording and phone-call checks by the quality controllers, and re-interview checks by the experienced investigators.
Survey instruments
The CBI is a 24-item multidimensional questionnaire that measures caregiver burden on five subscales: physical, social, emotional, time dependence and developmental burden.17 Item scores are summed; higher scores indicate greater burden. The Montreal Cognitive Assessment (MoCA) provides a total score ranging from 0 to 30; lower scores indicate more substantial cognitive impairment.18 The Activities of Daily Living (ADL) Scale measures two central domains of functioning in NWD patients and comprises a Physical Self-Maintenance Scale, which assesses routine self-care ability, and an Instrumental Activities of Daily Living Scale, which assesses a more complex set of behaviours.19,20 Item scores range from 1 to 4; higher scores indicate more functional problems. The Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS) are the most widely used measures of patient depression and anxiety, respectively.21,22 A score of 0 to 7 was considered normal, a score of >7 was considered to indicate depression or anxiety, and higher scores indicated greater severity of depression or anxiety.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) and percentages. The internal consistency of the scales was evaluated using Cronbach’s alpha coefficient; alpha coefficients ≥0.70 were considered satisfactory using reliability analysis. Pearson and Spearman correlation coefficient tests were used to assess correlations between CBI scores and the other variables. Cognitive function, functional problems, depression, anxiety and NWD duration were treated as original primary stressors. The primary appraisal variable was care duration. The mediator variable was patient annual income. Path analysis was used to examine interrelationships among caregiver burden and patient/caregiver factors. All data analyses were performed using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA) and SPSS AMOS, version 17.0 (IBM Corp., Armonk, NY, USA).
Results
Patient and caregiver sociodemographic characteristics and measures
The average survey completion time was 60 minutes (range: 40–90 minutes). Of the 108 NWD patients screened in this study, 13 were unable to complete the questionnaires and 35 WD patients were diagnosed with other types of WD that involved liver (n = 16), kidney (n = 11) or other organ (n = 8) damage. Thus, 60 WD patients and their respective caregivers were recruited. The sociodemographic characteristics of NWD patients and their caregivers are shown in Table 1. Of 60 patients, 67.7% were men and the mean age was 21.35 ± 4.89 years. The NWD duration was 7.85 ± 3.11 years. The mean age of the 60 caregivers was 42.15 ± 5.27 years; the care duration was 7.07 ± 3.75 years.
Table 1.
Sociodemographic characteristics of patients and caregivers.
| Variables | |
|---|---|
| NWD patients | |
| Age (years) | 21.35 ± 4.89 |
| Male sex (%) | 40 (67.7%) |
| NWD duration (years) | 7.85 ± 3.11 |
| Unmarried (%) | 56 (93.3%) |
| Junior high school education (%) | 55 (91.7%) |
| City (%) | 20 (33.3%) |
| Annual income*(%) | |
| <50 | 21 (35.0%) |
| 50–100 | 24 (40.0%) |
| >100 | 15 (25.0%) |
| Caregivers | |
| Age (years) | 42.15 ± 5.27 |
| Caring duration (years) | 7.07 ± 3.75 |
*1000 CNY/year. NWD: neurologic Wilson disease; CNY: Chinese yuan.
The means, SDs, Cronbach’s alphas and possible scale score ranges for the measures are shown in Table 2. For NWD patients, the mean MoCA score was 25.90 ± 1.43, the mean ADL score was 63.25 ± 17.94, the mean HDRS score was 18.40 ± 4.26 and the mean HARS score was 19.92 ± 7.60. All Cronbach’s alpha values for the measures were >0.70.
Table 2.
Variable means, SDs, Cronbach’s alphas and scale score ranges.
| Variables | Measure | Mean | SD | Cronbach’s alpha | Possible scale score ranges |
|---|---|---|---|---|---|
| Cognitive impairment | MoCA | 25.90 | 1.43 | 0.763 | Lower: more cognitive impairment (0–30) |
| Functional problems | ADL | 63.25 | 17.94 | 0.702 | Higher: more functional problems (35–95) |
| Depression | HDRS | 18.40 | 4.26 | 0.729 | A score of 0–7 was considered normal |
| Anxiety | HARS | 19.92 | 7.60 | 0.746 | A score of 0–7 was considered normal |
| Caregiver burden | CBI | 52.00 | 17.16 | 0.828 | Higher: greater caregiver burden (0–96) |
SD: standard deviation; MoCA: Montreal Cognitive Assessment; ADL: Activities of Daily Living; HDRS: Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale; CBI: Caregiver Burden Inventory.
Correlations between CBI and other variables
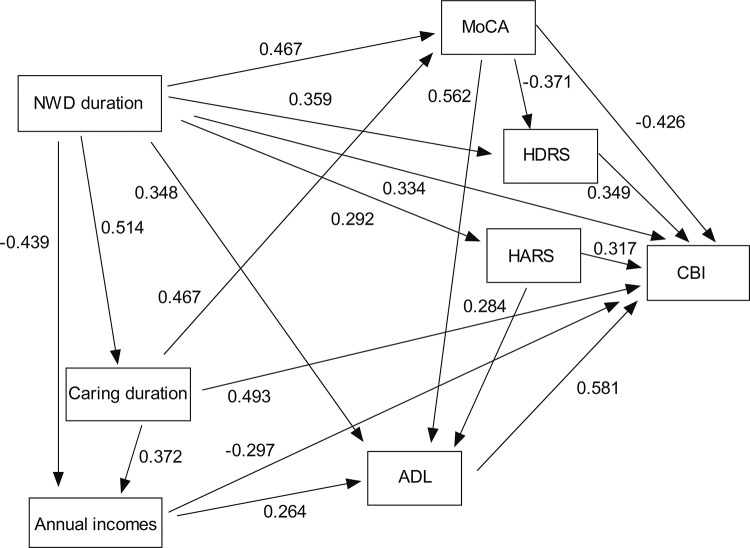
We found that the mean CBI score was 52.00 ± 17.16 (a medium level of burden), and Cronbach’s alpha for CBI score was 0.828. The correlations between variables and CBI scores are shown in Table 3. Caregiver CBI score was associated with NWD patient MoCA (r = −0.448, P < 0.001), ADL (r = −0.682, P < 0.001), HDRS (r = 0.362, P = 0.004) and HARS scores (r = 0.325, P = 0.011). Standard regression coefficients generated by the path model are shown in Figure 1. For the primary stressors, NWD duration had a statistically significant direct effect on CBI score after controlling for confounders. NWD duration was positively associated with CBI score (r = 0.334, P < 0.001), with longer patient NWD duration leading to higher CBI score. Care duration also had a statistically significant indirect effect on CBI score in the model. Care duration was positively associated with CBI score (r = 0.493, P < 0.001), with greater care duration leading to higher CBI score. Annual income had a statistically significant indirect effect on CBI score in the model. Annual income was negatively associated with CBI score (r = −0.297, P = 0.015), with lower income leading to a higher CBI score in caregivers. Of the primary stressors, cognitive impairment, functional problems, depression and anxiety were predicted by NWD duration, with longer NWD duration leading to higher scores on the MoCA (r = 0.467, P < 0.001), HDRS (r = 0.359, P < 0.001), HARS (r = 0.292, P < 0.001) and greater ADL dependency (r = 0.348, P < 0.001). Cognitive function, functional problems, depression and anxiety were not directly associated with caregiver burden. MoCA score was negatively associated with CBI score (r = −0.426, P < 0.001), with lower patient MoCA score leading to higher caregiver CBI score in caregivers. Functional problems were positively associated with CBI score (r = 0.581, P < 0.001), with higher patient ADL score leading to higher caregiver CBI score. Depression was positively associated with CBI score (r = 0.349, P < 0.001), with higher patient HDRS score leading to higher caregiver CBI score. Anxiety was positively associated with CBI score (r = 0.317, P < 0.001), with higher patient HARS score leading to higher caregiver CBI score.
Table 3.
Correlations between variables and CBI scores (Pearson and Spearman correlations).
|
CBI scores |
Physical burden |
Social burden |
Emotional burden |
Time dependence burden |
Developmental burden |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Patients | ||||||||||||
| NWD duration | 0.334 | 0.009 | 0.341 | 0.008 | – | – | – | – | 0.316 | 0.014 | – | – |
| MoCA | −0.448 | <0.001 | −0.370 | 0.004 | −0.415 | 0.001 | −0.275 | 0.033 | −0.451 | <0.001 | −0.288 | 0.026 |
| ADL | 0.682 | <0.001 | −0.569 | <0.001 | −0.578 | <0.001 | −0.542 | <0.001 | −0.509 | <0.001 | −0.450 | <0.001 |
| HDRS | 0.362 | 0.004 | – | – | 0.424 | 0.001 | – | – | −0.265 | 0.041 | 0.381 | 0.003 |
| HARS | 0.325 | 0.011 | 0.297 | 0.021 | 0.452 | <0.001 | – | – | – | – | 0.323 | 0.012 |
| Annual income | −0.321 | 0.013 | −0.347 | 0.007 | – | – | – | – | – | – | – | – |
| Caregivers | ||||||||||||
| Age | 0.535 | <0.001 | 0.513 | <0.001 | 0.310 | 0.016 | 0.300 | 0.020 | 0.505 | <0.001 | 0.529 | <0.001 |
| Care duration | 0.552 | <0.001 | 0.464 | <0.001 | 0.309 | 0.016 | 0.329 | 0.010 | 0.519 | <0.001 | 0.476 | <0.001 |
Only significant coefficients are shown. CBI: Caregiver Burden Inventory; NWD: neurologic Wilson disease; MoCA: Montreal Cognitive Assessment; ADL: Activities of Daily Living; HDRS: Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale.
Figure 1.
Path analysis plot with standardized coefficients. Only significant coefficients are shown. NWD: neurologic Wilson disease; MoCA: Montreal Cognitive Assessment; ADL: Activities of Daily Living; HDRS: Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale; CBI: Caregiver Burden Inventory.
Discussion
This study attempted to use a path model to evaluate the complexities involved in caregiving and to measure the effects of several variables in the model on caregiver burden. The exploration of modifiable, easily measured factors in NWD patients and caregivers could help to alleviate burden and improve both the care given to NWD patients and the caregiving experience. In the present study, we found that NWD duration had a significant positive direct effect on CBI score, even after controlling for other variables. Additionally, as a modifiable and easily measured factor, annual income had a significantly negative indirect effect on CBI score.
WD is characterized by a deleterious accumulation of copper in the liver and brain.23 The prevalence of WD is higher in China and other Asian countries than in Western countries.5 Neurological symptoms are the most frequent clinical symptoms of WD. Initial neurological presentation occurs in 18% to 68% of patients; the mean age at symptom onset is 20 to 30 years.24 The primary clinical spectrum of neurological symptoms includes different movement disorders with a wide variety of involuntary movements such as dysarthria, gait and posture disturbances, drooling and dysphagia. These disturbances may severely affect activities of daily living.25–27 Family members often act as caregivers out of love, respect, dedication and a sense of responsibility for the person concerned. Given the high demand and burden of care, caring for this population is a challenge.28 Caring for patients with mental disabilities can be a chronically stressful experience; as a result, informal caregivers may be at greater risk of reduced well-being and enhanced morbidity and mortality.10,11 The level of caregiver burden may be affected by the caregiver’s culture. In Chinese culture, caregivers often do not distinguish their caregiver burden role from other daily activities. They often consider caring for patients to be just another aspect of family life. Compared with caregivers of dementia patients in Western society, caregivers of dementia patients in China score higher on depression and caregiver burden.29
Studies on caregiver burden in patients with mental disorders have received much attention. One study from Turkey reported that the disease duration and disability of 30 patients with early stages of idiopathic Parkinson’s disease had a remarkable impact on caregiver burden.30 Another study using linear regression models showed that the main determinants of caregiver burden were positively associated with disease duration in 94 patients with Parkinson’s disease.31 It is worth noting that the authors of these two studies failed to present mean CBI scores. There are few studies on the correlation between NWD duration and caregiver burden in NWD patients. We found that NWD duration, as an unmodifiable factor, had a significant positive direct effect on caregiver burden. After controlling for other variables, NWD duration still had a positive effect on caregiver burden.
Many studies have confirmed that patient cognitive impairment, functional problems, depression and anxiety are positively related to caregiver burden.32–35 A study from the United States reported that in 75 patients with mild cognitive impairment, reduced awareness of cognitive issues is associated with increased caregiver burden, independent of neuropsychiatric symptoms, functional abilities and cognition.32 Another study of real-world data confirmed an association between increased caregiver burden and severity of patients’ cognitive impairment by analysing a wide range of validated measures of caregiver burden.33 A study from China showed that depression, anxiety and sleep problems are the main challenges faced by family caregivers of patients with Alzheimer’s disease.35 To the best of our knowledge, the relationships between caregiver burden and cognitive impairment, functional problems, depression and anxiety in patients with NWD has received little attention. The present findings are consistent with those of the previous studies mentioned above, although the study populations differ.
Our study had several strengths. First, we used a path analysis model of the direct and indirect effects of caregiver and patient factors on caregiver burden. Second, both caregiver and patient factors were evaluated. Finally, all the Cronbach’s alpha values were >0.70, suggesting that the validated measures had relatively high internal consistency. There were several study limitations. First, this was a single-centre retrospective study with small sample sizes, so the data cannot be used to infer causality. Second, we did not collect data on other indicators such as social support and positive aspects of caregiving, which may affect caregiver burden. Third, our sample contained 13 patients with hyperkinetic movement disorders. Unfortunately, as this number was low, we were unable to perform a subgroup analysis of these patients. However, we plan to investigate caregiver burden with these types of patients in the near future. Finally, as all caregivers and NWD patients were Chinese, these findings may not apply to NWD patients of other ethnicities.
Caregivers of patients with NWD may have a medium caregiver burden, which is directly associated with NWD duration and indirectly associated with cognitive impairment, functional problems, depression, anxiety and annual income. Thus, early diagnosis and management of NWD patients may effectively ease caregiver burden.
Acknowledgements
We express our gratitude to all patients who participated in the study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of Anhui Province of China (1908085MH266) and the Natural Science Foundation of Anhui University of Chinese Medicine (2012qn007).
ORCID iD
Yuancheng Bao https://orcid.org/0000-0002-5629-0989
References
- 1.Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine (Baltimore) 1992; 71: 139–164. DOI: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Reilly M, Daly L, Hutchinson M. An epidemiological study of Wilson’s disease in the Republic of Ireland. J Neurol Neurosurg Psychiatry 1993; 56: 298–300. DOI: 10.1136/jnnp.56.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey AJ, Durkie M, Hague S, et al. A genetic study of Wilson’s disease in the United Kingdom. Brain 2013; 136: 1476–1487. DOI: 10.1093/brain/awt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gialluisi A, Incollu S, Pippucci T, et al. The homozygosity index (HI) approach reveals high allele frequency for Wilson disease in the Sardinian population. Eur J Hum Genet 2013; 21: 1308–1311. DOI: 10.1038/ejhg.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie JJ, Wu ZY. Wilson’s disease in China. Neurosci Bull 2017; 33: 323–330. DOI: 10.1007/s12264-017-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrukhin K, Fischer SG, Pirastu M, et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet 1993; 5: 338–343. DOI: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- 7.Socha P, Janczyk W, Dhawan A, et al. Wilson’s disease in children: a position paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018; 66: 334–344. DOI: 10.1097/MPG.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 8.Frota NA, Barbosa ER, Porto CS, et al. Cognitive impairment and magnetic resonance imaging correlations in Wilson’s disease. Acta Neurol Scand 2013; 127: 391–398. DOI: 10.1111/ane.12037. [DOI] [PubMed] [Google Scholar]
- 9.Hegde S, Sinha S, Rao SL, et al. Cognitive profile and structural findings in Wilson’s disease: a neuropsychological and MRI-based study. Neurol India 2010; 58: 708–713. DOI: 10.4103/0028-3886.72172. [DOI] [PubMed] [Google Scholar]
- 10.Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs 2008; 108: 23–27. DOI: 10.1097/01.NAJ.0000336406.45248.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA 1999; 282: 2215–2219. DOI: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 12.Kasuya RT, Polgar-Bailey P, Takeuchi R. Caregiver burden and burnout. A guide for primary care physicians. Postgrad Med 2000; 108: 119–123. DOI: 10.3810/pgm.2000.12.1324. [DOI] [PubMed] [Google Scholar]
- 13.Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev 2015; 62: 340–350. DOI: 10.1111/inr.12194. [DOI] [PubMed] [Google Scholar]
- 14.Perlick DA, Hohenstein JM, Clarkin JF, et al. Use of mental health and primary care services by caregivers of patients with bipolar disorder: a preliminary study. Bipolar Disord 2005; 7: 126–135. DOI: 10.1111/j.1399-5618.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 15.Stull DE, Kosloski K, Kercher K. Caregiver burden and generic well-being: opposite sides of the same coin? Gerontologist 1994; 34: 88–94. DOI: 10.1093/geront/34.1.88. [DOI] [PubMed] [Google Scholar]
- 16.Czlonkowska A, Litwin T, Chabik G. Wilson disease: neurologic features. Handb Clin Neurol 2017; 142: 101–119. DOI: 10.1016/B978-0-444-63625-6.00010-0. [DOI] [PubMed] [Google Scholar]
- 17.Chou KR, Jiann-Chyun L, Chu H. The reliability and validity of the Chinese version of the Caregiver Burden Inventory. Nurs Res 2002; 51: 324–331. DOI: 10.1097/00006199-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 2011; 24: 184–190. DOI: 10.1177/0891988711422528. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–919. DOI: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–186. [PubMed] [Google Scholar]
- 21.Obeid S, Abi Elias Hallit C, Haddad C, et al. Validation of the Hamilton Depression Rating Scale (HDRS) and sociodemographic factors associated with Lebanese depressed patients. Encephale 2018; 44: 397–402. DOI: 10.1016/j.encep.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Chang B, Zhu W, Li S. Effects of depression and anxiety on microvascular decompression outcome for trigeminal neuralgia patients. World Neurosurg 2019; 128: e556–e561. DOI: 10.1016/j.wneu.2019.04.194. [DOI] [PubMed] [Google Scholar]
- 23.Poujois A, Woimant F. Wilson’s disease: a 2017 update. Clin Res Hepatol Gastroenterol 2018; 42: 512–520. DOI: 10.1016/j.clinre.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer RF. Wilson’s disease. Semin Neurol 2007; 27: 123–132. DOI: 10.1055/s-2007-971173. [DOI] [PubMed] [Google Scholar]
- 25.Lorincz MT. Neurologic Wilson’s disease. Ann N Y Acad Sci 2010; 1184: 173–187. DOI: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- 26.Litwin T, Dziezyc K, Karlinski M, et al. Psychiatric disturbances as a first clinical symptom of Wilson’s disease – case report. Psychiatr Pol 2016; 50: 337–344. DOI: 10.12740/PP/45218. [DOI] [PubMed] [Google Scholar]
- 27.Svetel M, Kozic D, Stefanova E, et al. Dystonia in Wilson’s disease. Mov Disord 2001; 16: 719–723. DOI: 10.1002/mds.1118. [DOI] [PubMed] [Google Scholar]
- 28.Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract 2008; 20: 423–428. DOI: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 29.Torti FM, Jr, Gwyther LP, Reed SD, et al. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Dis Assoc Disord 2004; 18: 99–109. DOI: 10.1097/01.wad.0000126902.37908.b2. [DOI] [PubMed] [Google Scholar]
- 30.Yuksel B, Ak PD, Sen A, et al. Caregiver burden and quality of life in early stages of idiopathic Parkinson’s disease. Ideggyogy Sz 2018; 71: 343–350. DOI: 10.18071/isz.71.0343. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, et al. Neuropsychiatric symptoms and caregiver’s burden in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 629–634. DOI: 10.1016/j.parkreldis.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Kelleher M, Tolea MI, Galvin JE. Anosognosia increases caregiver burden in mild cognitive impairment. Int J Geriatr Psychiatry 2016; 31: 799–808. DOI: 10.1002/gps.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black CM, Ritchie CW, Khandker RK, et al. Non-professional caregiver burden is associated with the severity of patients’ cognitive impairment. PLoS One 2018; 13: e0204110. DOI: 10.1371/journal.pone.0204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WT, He B, Wang YH, et al. The relationships among Muslim Uyghur and Kazakh disabled elders’ life satisfaction, activity of daily living, and informal family caregiver’s burden, depression, and life satisfaction in far western China: a structural equation model. Int J Nurs Pract 2017; 23: e12521. DOI: 10.1111/ijn.12521. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Li C, Shi Z, et al. Caregiver burden and prevalence of depression, anxiety and sleep disturbances in Alzheimer’s disease caregivers in China. J Clin Nurs 2017; 26: 1291–1300. DOI: 10.1111/jocn.13601. [DOI] [PubMed] [Google Scholar]