Abstract
Objectives
This study aimed to investigate the factors affecting the quantity of DNA and RNA extractable from human formalin-fixed paraffin-embedded (FFPE) tissues stored for different lengths of time.
Methods
We randomly selected 20 FFPE specimens harvested from hysteromyoma patients with uterine fibroids during 2010, 2015, and 2017 at the Department of Pathology, Jiading District Central Hospital Affiliated Shanghai University of Medicine and Health Sciences. DNA and RNA extractions were performed using a DNA/RNA FFPE kit. DNA and RNA concentrations and their OD260/OD280 ratios were determined by a NanoDrop 2000 spectrophotometer. The human β-globin gene and aldehyde dehydrogenase-2 (ALDH2) gene were amplified from nucleic acids using a LightCycler 480 Real-Time PCR System, and PCR amplification products were electrophoresed on 1% agarose gels.
Results
Specimens that were stored for longer showed more degradation and a reduced concentration of DNA and RNA after nucleic acid extraction. However, there was no significant difference in DNA or RNA purity. β-globin and ALDH2 genes could be amplified from more than 99% of specimens.
Conclusion
We found that FFPE tissues stored for longer had a reduced quantity of extractable DNA and RNA. However, these tissues could be used for the analysis of some small target genes.
Keywords: FFPE tissues, storage time, internal reference gene amplification, DNA and RNA quantity, preservation, nucleic acid extraction
Introduction
Formalin-fixed paraffin-embedded (FFPE) tissues are a highly valuable source of genetic material for molecular analyses both in research and clinical diagnostics. In recent years, the use of FFPE tissues for molecular pathological detection, such as tumor-related gene mutations1 and the molecular detection of targeted drugs,2,3 has rapidly developed from scientific research to in vitro pathological diagnosis. Indeed, retrospective gene research is increasingly used in tumor molecular biology. However, because the tissue samples collected during scientific research are mostly FFPE, the quantity of DNA and RNA that they yield will directly affect the outcome of molecular experiments.4,5
Through a combination of long-term experimental exploration and published studies,6–8 optimal DNA and RNA extraction methods have been determined. Technical optimization includes avoiding the use of wax blocks with bleeding, necrosis, or autolysis; selecting areas with a high cell density; favoring a thickness of 8 to 10 μm; using 20- to 30-mg slices for each extraction; choosing xylene dewaxing; and using a digestive enzyme at a concentration of 20 g/L for 3 to 5 hours.
Whether DNA and RNA extracted from long-term preserved FFPE tissues meet the requirements of molecular biology experiments has become the focus of many studies.9 Here, we compared the extent of degradation, concentration, purity, and capacity for amplification of DNA and RNA extracted from FFPE tissues stored for several years under standard conditions. The aim of our study was to determine the effects of storage time on the quantity of DNA and RNA extracted from FFPE tissues.
Materials and methods
Ethical approval
This protocol was approved by the Ethics Review Board of Jiading District Central Hospital (Ethics Committee reference number 2020-GZR-16-330282198803169204). Written consent for study participation was received from the patients or their guardians.
Reagents
Formalin, ethanol, xylene, and isoamyl alcohol were purchased from the National Pharmaceutical Corporation (Shanghai, China). DNA/RNA extraction kits were purchased from Qiagen Inc. (Valencia, CA, USA). Primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The 5× All-In-One MasterMix was purchased from abm (Zhenjiang, China). Premix Ex Taq™ II was bought from Takara Bio Inc. (Shiga, Japan).
Sample selection
Sixty FFPE specimens from hysteromyoma patients diagnosed with uterine fibroids and collected during 2010, 2015, and 2017 (20 per year) were selected for this study. The samples had been removed during surgery, fixed in formalin for 12 hours, then a 1-cm × 1-cm × 0.2-cm area of tissue had been cut and embedded in paraffin (Solarbio, Beijing, China) which had a melting point of 56°C. Paraffin samples had been preserved in the dark at 25°C with 50% humidity in the archives of the Department of Pathology, Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences. Paraffin blocks were selected to carry out complete serial sections, according to HE staining sections of specimens made from previous hospital treatments. The selected specimens were preserved completely and found under a microscope to have no tissue autolysis, necrosis, or massive hemorrhage. All articles were sterilized and six FFPE sections of 10-μm thickness were placed in the 1.5-mL centrifugal tube for backup.
DNA and RNA extraction
Six sections of 1 cm × 1 cm × 10 µm of FFPE were used for DNA and RNA extractions. Dewaxing was achieved by adding 1 mL xylene to FFPE tissues, placing them in a 55°C water bath for 10 minutes and centrifuging at 7800 × g for 5 minutes. This was repeated three times. Sections were then washed twice by adding 1 mL anhydrous ethanol, shaking to mix, centrifuging at 7800 × g for 5 minutes, and drying at 60°C for preparation. DNA and RNA extractions were performed simultaneously using an All Prep® DNA/RNA FFPE Kit (Qiagen) according to the manufacturer’s instructions. For RNA extraction, 10 µL protease K was added and incubated at 56°C for 15 minutes, then protein cross-linking was removed by incubating at 80°C for 15 minutes. For DNA extraction, 40 µL of protease K was added and incubated at 56°C for 1 hour, then protein cross-linking was removed by incubating at 90°C for 2 hours. RNA was removed from DNA samples by adding 4 µL RNase A (100 mg/ml), and DNA was removed from RNA samples by adding 80 µL DNase I. The final elution volume for each sample was 30 µL. All extractions were performed in the Pathology Department of Jiading District Central Hospital, and samples were stored at –20°C.
DNA and RNA evaluation
The yield and quantity (260/280 optical density [OD] ratios) of DNA and RNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA) from 2-μL aliquots of each sample. When OD260/OD280 was 1.8 ± 0.1 the total DNA extracted was eligible, and when OD260/OD280 was 2.0 ± 0.1 the total RNA extracted was eligible. If the concentration of DNA or RNA was more than 10 ng/μL, the total DNA or RNA extracted was eligible.
The DNA and RNA integrity was checked by electrophoresis on 1% agarose gels stained with ethidium bromide. Degradation of DNA and RNA and the amplification of internal reference genes were observed using a BTS-20.M automatic digital imaging system and LUV-260D ultraviolet projector (both Tanon, Shanghai, China).
Amplification of internal reference genes
Primers to amplify internal reference genes (the human β-globin gene and ALDH2) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Primer sequences are listed in Table 1. A total of 5 µL RNA from each specimen was first transcribed into cDNA using the AccuRT Genomic DNA Removal Kit (abm) and 5× All-In-One MasterMix (abm) with oligo dT primers. SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) was used to perform fluorescence quantitative (q)PCR amplification with 2 µL cDNA samples. PCR conditions were as follows: denaturation at 95°C for 30 s, then 30 cycles of 95°C for 5 s and 60°C for 20 s. qPCR was analyzed by the PCR amplification curve and Ct value. PCR-amplified products (5 µL) were electrophoresed on a 1% agarose gel containing ethidium bromide and including a 100-bp DNA ladder marker.
Table 1.
Primer sequences used to amplify human β-globin and ALDH2 genes.
| Primer name | Sequence (5′–3′) | Amplification product size (bp) |
|---|---|---|
| Human β-globin forward primer | GAAGAGCCAAGGACAGGTAC | 268 |
| Human β-globin reverse primer | CAACTTCATCCACGTTCACC | |
| ALDH2 forward primer | CAAATTACAGGGTCAACTGCT | 135 |
| ALDH2 reverse primer | CCACACTCACAGTTTTCACTT |
Data analysis
Data are shown as means ± standard deviation of the means. Because the data are nonparametric, statistical significance was analyzed by Wilcoxon and Kruskal–Wallis tests using GraphPad v5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 was considered indicative of a statistically significant difference.
Results
Comparison of purity and concentration of DNA and RNA in FFPE tissues stored for different lengths of time
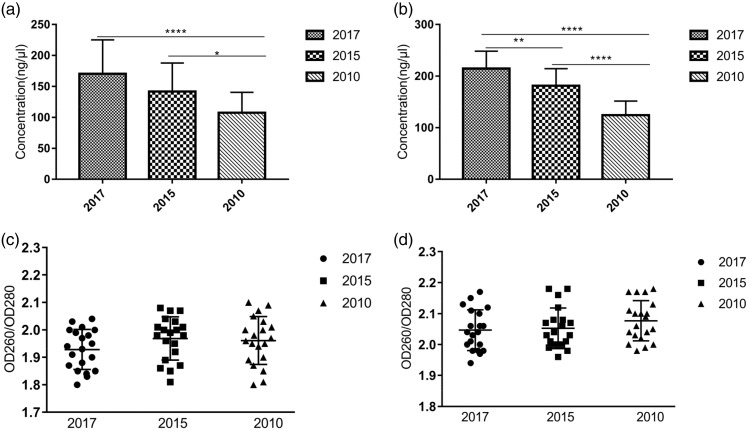
DNA and RNA were extracted from FFPE tissues stored for 1, 3, and 8 years. Typically, the concentration of DNA and RNA extracted was significantly lower from FFPE tissues that had been stored for longer than those stored for less time. The concentrations of DNA and RNA extracted from tissues stored for 1 and 3 years were significantly higher than those stored for 8 years but there was no significant difference between those stored for 1 and 3 years (Figure 1a, b). The OD260/OD280 of DNA and RNA extracted from FFPE tissues were between 1.8 to 2.1 and 1.9 to 2.2, respectively, indicating high levels of purity (Figure 1c, d). There was no significant difference between the purity of DNA or RNA.
Figure 1.
Concentration and purity of DNA and RNA extracted from FFPE tissues stored for different lengths of time. (a) Concentration of DNA. (b) Concentration of RNA (n = 20). (c) Purity of DNA. (d) Purity of RNA (n = 20). *P < 0.05, **P < 0.01, and ***P < 0.0001.
Comparison of DNA and RNA degradation in FFPE tissues stored for different lengths of time
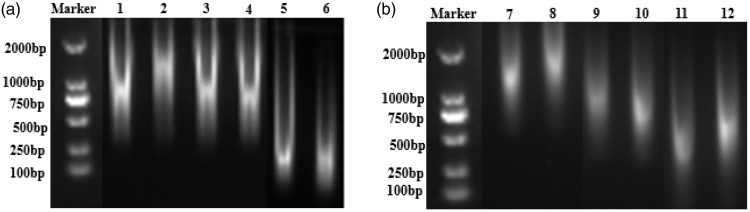
Gel electrophoresis revealed DNA and RNA molecular sizes of 100 to 2000 bp. DNA and RNA extracted from all samples were degraded, with increasing levels of degradation seen for samples stored for longer (Figure 2a, b). However, the difference was not significant. As the gel electrophoresis was only a qualitative analysis, fluorescent qPCR was used as an alternative method of quantification.
Figure 2.
Degradation of DNA and RNA extracted from FFPE tissues stored for different lengths of time. (a) Agarose gel electrophoresis of DNA. Lanes 1 and 2: FFPE tissues prepared in 2017; lanes 3 and 4: FFPE tissues prepared in 2015; lanes 5 and 6: FFPE tissues prepared in 2010. (b) Agarose gel electrophoresis of RNA. Lanes 7 and 8: FFPE tissues prepared in 2017; lanes 9 and 10: FFPE tissues prepared in 2015; lanes 11 and 12: FFPE tissues prepared in 2010.
Fluorescent qPCR analysis of human β-globin and ALDH2 gene amplification
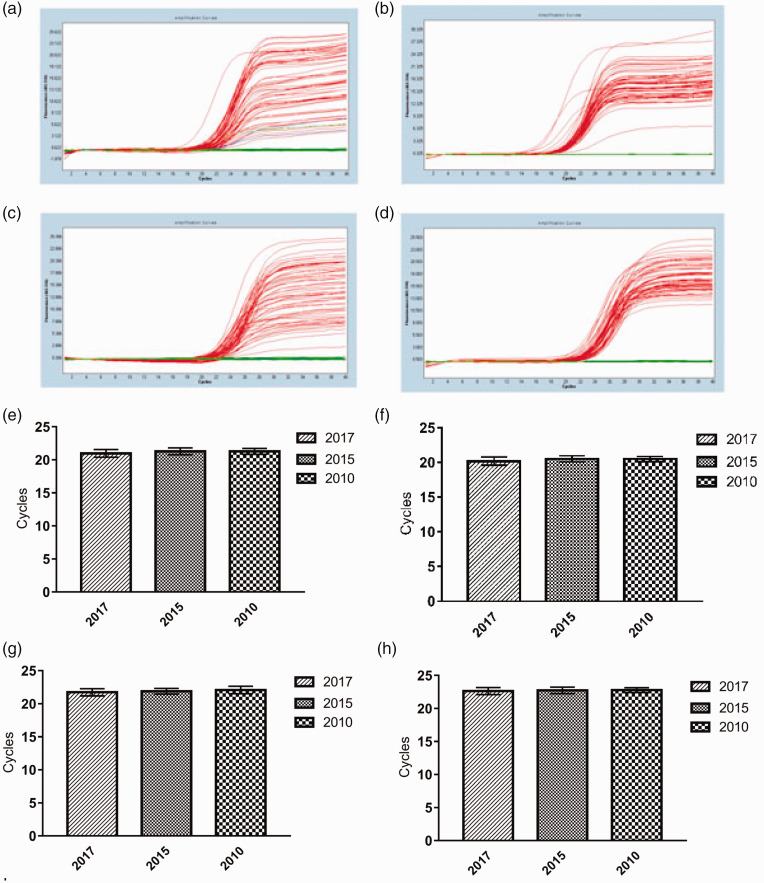
DNA and RNA extracted from FFPE tissues stored for 1, 3, and 8 years were used to amplify the human β-globin gene (268 bp) and ALDH2 gene (135 bp) by fluorescent qPCR. The kinetic curves (Figure 3a–d) showed that the exponential growth stage and the plateau stage were complete, indicating the reliability of the results. Ct values were between 17 and 25, with no significant differences (Figure 3e–h). Taken together, these results showed that FFPE tissues samples stored for as long as 8 years can still be used to analyze the expression of small target genes.
Figure 3.
Amplification of the human β-globin gene and ALDH2 gene. Amplification curves of human β-globin gene from DNA (a) and RNA (b). Amplification curves of ALDH2 gene from DNA (c) and RNA (d). Ct value of human β-globin gene from DNA (e) and RNA (f). Ct value of ALDH2 from DNA (g) and RNA (h). (n = 20).
Amplification results of internal reference bands
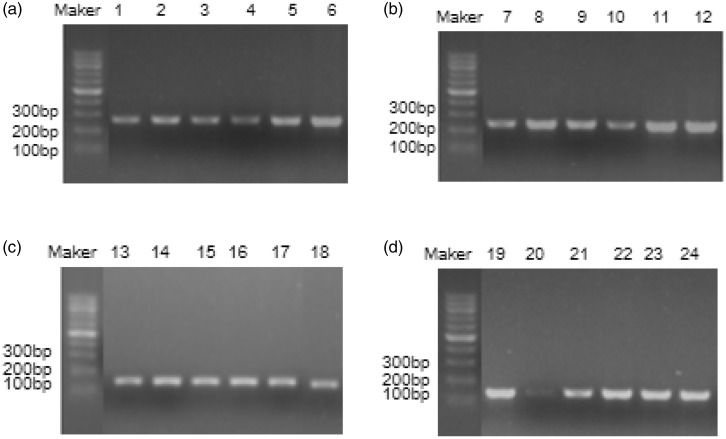
Fluorescent qPCR products of human β-globin and ALDH2 gene amplification were next detected on a 1% agarose gel. Amplification of the human β-globin gene produced a clear band of 268 bp (Figure 4a, b), while amplification of the ALDH2 gene produced a clear band of 135 bp (Figure 4c, d), which were both of the expected size. There was no significant difference between fluorescent qPCR and agarose gel electrophoresis findings.
Figure 4.
Amplification the human β-globin gene and ALDH2 gene from FFPE tissues stored for different lengths of time. Amplification products of the human β-globin gene from DNA (a) and RNA (b). Lanes 1, 2, 7, and 8: FFPE tissues from 2010; lanes 3, 4, 9, and 10: FFPE tissues from 2015; lanes 5, 6, 11, and 12: FFPE tissues from 2017. Amplification products of the ALDH2 gene from DNA (c) and RNA (d). Lanes 13, 14, 19, and 20: FFPE tissues from 2010; lanes 15, 16, 21, and 22: FFPE tissues from 2015; lanes 17, 18, 23, and 24: FFPE tissues from 2017.
Discussion
The large numbers of FFPE tissue samples stored in hospital pathology departments are good sources of research materials. Although some PCR inhibitors are present in FFPE tissues, simple crude DNA or RNA extracts obtained with a limited number of complex extraction steps can still be used for PCR.10,11 The tissues stored in clinics first need to be fixed and dehydrated by formaldehyde before paraffin embedding. Formaldehyde is the smallest oxygen-containing organic compound that has high reactive activity. It provides methylene (-CH2-) to react with molecules containing hydroxyl (-OH), thiol (-SH), or amino (-NH2). Through interactions, free molecular chains are crosslinked and have adverse effects on DNA and RNA molecules. Storage of the tissues for long periods leads to the formation of extensive methylene cross-linking bridges between biological macromolecules, increasing the fragility of DNA and RNA chains and leading to more random breakage.12 The paraffin wax must be heated to about 62°C to embed tissues, which partially unlinks DNA and RNA, while residual traces of formaldehyde in tissues can methylate single strands of nucleic acid. These modified DNA and RNA strands are difficult to renaturate after cooling, leading to degradation.13,14
For accurate genetic analysis, it is necessary to obtain DNA and RNA of a sufficient quantity and purity. DNA extracted from paraffin-embedded tissue samples preserved for more than 1 year previously showed a different degradation phenomenon, with a lower quality to that extracted DNA from fresh tissue.15 However, it is difficult to obtain fresh–frozen tissue, and the costs of liquid nitrogen preservation are relatively high. 16 In contrast, FFPE samples can be preserved for long periods during which the DNA quality is unaffected.15,16 Typically, a longer storage time leads to increased degradation and reduces the lengths of sequence fragments that can be amplified. In a previous study, DNA was extracted from fresh FFPE samples then stored at –20°C for 3 years and compared with DNA extracted from FFPE samples that had been preserved for 3 years. Although there was no significant difference in the quality or concentration of DNA between the two methods,17,18 most DNA fragments extracted using the former technique were 100 to 500 bp while those extracted using the latter technique were mostly 100 bp long. This suggested an increase in the fragmentation of DNA fragments extracted from FFPE samples after 3 years of preservation. Similarly, Nam et al.19 evaluated the effect of sample storage time on RNA quality. FFPE samples were stored at room temperature for 1 month to 5 years and those stored for longer gave a lower RNA yield, while DNA and RNA degradation was high. A total of 58.3% of samples stored for 3 to 5 years could undergo amplification of the β-actin gene compared with 62.5% of samples stored for 1 month to 1 year. However, to date, little is known about whether paraffin samples that have been preserved for more than 5 years can be used to extract DNA and RNA simultaneously for PCR analysis.
The present study investigated whether the concentration and purity of extracted DNA and RNA from FFPE stored for long periods were sufficient for PCR amplification, to determine if this could be used for disease diagnosis. They concentration and purity were analyzed by spectrophotometry and agarose gel electrophoresis, but use of the Agilent Bioanalyzer and distribution value 200 RNA quality indexes would improve the accuracy of these measurements.
Reverse transcription was performed using oligo dT primers. If the 268-bp human β-globin amplicon was closer to the poly A tail than the 135-bp ALDH2 gene, then successful amplification of the shorter amplicon would require the RNA to be less degraded than amplification of the longer fragment. We observed no significant difference between the intensity of human β-globin and ALDH2 gene amplification products. However, this might be because of the small sample size.
FFPE tissues are often used in retrospective studies although they suffer from notable DNA and RNA degradation. In the present study, we found that long-term storage of FFPE tissues increased the level of DNA and RNA degradation and reduced the quantity of DNA and RNA extracted. Nevertheless, there was no significant difference in the purity of DNA or RNA extracted from FFPE tissues stored for different lengths of time, and more than 99% of samples yielded nucleic acids that could be used to amplify target gene fragments smaller than 300 bp.
In conclusion, FFPE tissue samples stored for up to 8 years can be used for the analysis of small fragments of DNA and RNA. However, preliminary investigations would be beneficial before genetic analysis to select the most appropriate FFPE samples according to the size of the target gene. The use of FFPE tissue specimens provides a rapid, economical, and practical method for the routine detection of DNA and RNA in clinical settings.
Authors’ contributions
Qing-qing Yi and Rong Yang designed the study, Dong-yu Liang collected the data, Jun-feng Shi analyzed the data, Shuang Sha and Nai-yan Zeng reviewed the article, Qing Chang agreed to submit the article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Nature Science Foundation of China (Grant nos. 81670968 and 81702284) and partially supported by the Agricultural and Social Research Projects of Jiading, Shanghai (Grant no. JDKW-2018-W21).
ORCID iD
Qing Chang https://orcid.org/0000-0002-7440-8629
References
- 1.Etzel BM, Gerth M, Chen Y, et al. Mutation analysis of tumor necrosis factor alpha-induced protein 3 gene in Hodgkin lymphoma. Pathol Res Pract 2017; 213: 256–260. [DOI] [PubMed] [Google Scholar]
- 2.Durham AL, Caramori G, Chung KF, et al. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res 2016; 167: 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015; 27: 15–26. [DOI] [PubMed] [Google Scholar]
- 4.Guyard A, Boyez A, Pujals A, et al. DNA degrades during storage in formalin-fixed and paraffin-embedded tissue blocks. Virchows Arch 2017; 471: 491–500. [DOI] [PubMed] [Google Scholar]
- 5.Daugaard I, Kjeldsen TE, Hager H, et al. The influence of DNA degradation in formalin-fixed, paraffin-embedded (FFPE) tissue on locus-specific methylation assessment by MS-HRM. Exp Mol Pathol 2015; 99: 632–640. [DOI] [PubMed] [Google Scholar]
- 6.Hara M, Nakanishi H, Takahashi S, et al. Relationship between DNA degradation ratios and the number of loci detectable by STR kits in extremely old seminal stain samples. Leg Med (Tokyo) 2015; 17: 391–393. [DOI] [PubMed] [Google Scholar]
- 7.Ghatak S, Sanga Z, Pautu JL, et al. Coextraction and PCR based analysis of nucleic acids from formalin-fixed paraffin-embedded specimens. J Clin Lab Anal 2016; 29: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun SM, Sung CO, Jeon H, et al. Next-generation sequencing using S1 nuclease for poor-quality formalin-fixed, paraffin-embedded tumor specimens. J Mol Diag 2018; 20 : 802–811. [DOI] [PubMed] [Google Scholar]
- 9.Zugazagoitia J, Rueda D, Carrizo N, et al. Prospective clinical integration of an amplicon-based next-generation sequencing method to select advanced non-small cell lung cancer patients for genotype-tailored treatments. Clin Lung Cancer 2018; 19: 65–73.e7. [DOI] [PubMed] [Google Scholar]
- 10.Jakovski Z, Ajanovska RJ, Stankov A, et al. Comparative study of two dna extraction methods in different tissues and conditions of degradation. Forensic Sci Int: Gen Suppl Ser 2015; 5: e403–e404. [Google Scholar]
- 11.Sidova M, Tomankova S, Abaffy P, et al. Effects of post-mortem and physical degradation on RNA integrity and quality. Biomol Detect Quantif 2015; 5: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary A, Mambo E, Sanford T, et al. Evaluation of an integrated clinical workflow for targeted next-generation sequencing of low-quality tumor DNA using a 51-gene enrichment panel. BMC Med Genomics 2014; 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malentacchi F, Pazzagli M, Simi L, et al. SPIDIA-DNA: an external quality assessment for the pre-analytical phase of blood samples used for DNA-based analyses. Clin Chim Acta 2013; 424: 274–286. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Korenková V, Sjöback R, et al. Biomarkers for monitoring pre-analytical quality variation of mRNA in blood samples. PLoS One 2013; 9: e0163125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakimoto Y, Tanaka M, Kamiguchi H, et al. MicroRNA stability in FFPE tissue samples: dependence on GC content. PLoS ONE 2016; 11: 387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghatak S, Lallawmzuali D, Lalmawia RS, et al. Mitochondrial D-loop and cytochrome oxidase C subunit I polymorphisms among the breast cancer patients of Mizoram, Northeast India. Curr Genet 2014; 60: 201–212. [DOI] [PubMed] [Google Scholar]
- 17.Qian XY, Wang S, Shen YC, et al. Methodology comparison and influence factors analysis of epidermal growth factor receptor mutation detection. Zhonghua Yi Xue Za Zhi 2015; 95: 106–111. (In Chinese) [PubMed] [Google Scholar]
- 18.Watanabe M, Hashida S, Yamamoto H, et al. Estimation of age-related DNA degradation from formalin-fixed and paraffin-embedded tissue according to the extraction methods. Exp Ther Med 2017; 14: 2683–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Kim Y, Cha HK, et al. Cell death-associated ribosomal RNA cleavage in postmortem tissues and its forensic applications. Mol Cells 2017; 40: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]