Abstract
Objectives
To investigate the effect of tumor necrosis factor ligand-related molecule 1A (TL1A) on the intestinal mucosal barrier in mice with chronic colitis.
Methods
Male TL1A-overexpressing transgenic mice and male C57BL/6 wild-type mice were used to establish a dextran sodium sulfate (DSS)-induced colitis model. The expression of occludin and claudin-1 was observed. Bacterial distribution in the intestinal mucosa and Th9/interleukin (IL)-9 expression were detected. In vitro co-culture systems of naive CD4+ T cells and Caco-2 cells were established and TL1A was added. Changes in transepithelial electrical resistance and IL-9 expression were measured. CD4+IL-9 cells were detected by flow cytometry.
Results
DSS mice showed a significant down-regulation of occludin and claudin-1 compared with controls. Expression levels of occludin, zonulin-1, and claudin-1 in the Caco-2+TGF-β+IL-4+TL1A group were significantly lower than in the Caco-2+TGF-β+IL-4 group. Bacterial distribution was clearly disordered in the DSS group. Transmembrane resistance of the Caco-2+TGF-β+IL-4+TL1A group was significantly lower and IL-9 expression significantly higher than in the Caco-2+TGF-β+IL-4 group.
Conclusions
TL1A overexpression promotes destruction of the intestinal mucosal barrier in mice with chronic colitis. The underlying mechanism may be associated with the promoting role of TL1A in Th9/IL-9 expression, which further destroys the mucosal barrier.
Keywords: Inflammatory bowel disease, tumor necrosis factor-like ligand 1A, interleukin-9, intestinal mucosa barrier, chronic colitis, dextran sodium sulfate
Introduction
Inflammatory bowel disease (IBD) is a group of intestinal inflammatory diseases including ulcerative colitis (UC) and Crohn’s disease (CD). The incidence rate is increasing annually, but the pathogenesis is unclear.1,2 However, it is thought that environmental factors such as diet and lifestyle act on subjects with genetic susceptibility to initiate an intestinal immune response with the involvement of gut microbiota to produce a variety of pro-inflammatory factors.3–5 Destruction of the intestinal mucosal barrier is an important pathological change of IBD. This barrier is composed of a monolayer of columnar epithelial cells formed by intestinal epithelial cells and tight junction (TJ) proteins.6,7 Proinflammatory cytokines released during intestinal inflammation can inhibit the invasion of microorganisms by activating immune cells and affecting the expression, distribution, and composition of TJ proteins, thereby influencing the function of the intestinal mucosal barrier.8
Th9 cells are a newly-defined member of the T helper subset, also known as interleukin-9 (IL-9)-secreting T helper cells.9 Several recent studies have revealed the important pathogenic role of Th9 cells in allergic and autoimmune diseases. Expression of the receptor of IL-9 is elevated in intestinal epithelial cells in patients with active UC.10,11 Consistently, the mRNA expression of IL-9 and interferon regulatory factor 4 in T cells is increased in the lamina propria of active UC patients. In dextran sulfate sodium (DSS)-induced and oxazolone-induced experimental colitis mice, application of the IL-9 antibody significantly attenuates inflammation of the intestinal mucosa, indicating the potential therapeutic effect of IL-9 antibodies.12,13
Tumor necrosis factor-like ligand 1A (TL1A) is a newly-discovered tumor necrosis factor (TNF) family member. TL1A binds death domain receptor 3 (DR3) to regulate the function of T cells, natural killer cells, and natural killer T cells to synergistically stimulate lymphocyte proliferation and cytokine production, thereby playing an important role in asthma, rheumatoid arthritis, and IBD.14,15
Although Th9 and its cytokine IL-9 were shown to be highly expressed in IBD, their effects on permeability of the intestinal mucosa and the role of TL1A in chronic colitis have rarely been reported.14,16,17 Therefore, the present study used lymphocyte-specific TL1A-overexpressing transgenic mice (LCK-CD2-TL1A-GFP-transgenic) to construct a DSS-induced chronic experimental colitis model, and the Caco-2 cell line to establish an in vitro intestinal epithelial cell barrier model. The effects of TL1A on the intestinal mucosal barrier and its mechanism in chronic experimental colitis were explored at both the animal and cell level, and our findings could act as a reference for the study of chronic colitis.
Materials and methods
Establishment of a chronic colitis mouse model
All animal procedures were carried out in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Chinese Academy of Sciences Animal Care and Use Committee. Male wild-type C57BL/6 mice aged 8 to 10 weeks were obtained from the Laboratory Animal Center of Hebei Medical University (Qualification Certificate No. 911102) and male transgenic (Tg) mice overexpressing lymphocyte-specific TL1A were acquired from the Inflammatory Bowel Disease Center and the Immunobiology Research Institute (Cedars-Sinai Medical Center, Los Angeles, CA, USA).
Mice were divided into four groups: Control/WT group, Control/Tg group, DSS/WT group, and DSS/Tg group (n = 10 per group). DSS was used to establish a mouse model of chronic experimental colitis.18 Briefly, mice were administered distilled water containing 2.5% DSS on days 1 to 5, 8 to 12, 15 to 19, and 22 to 26, and were given distilled water at other times. There were four cycles, and body weight changes and the disease activity index (DAI) were monitored on days 1, 3, and 5 of each cycle. Mice were sacrificed on the 29th day by cervical dislocation. Colons were removed and collected from the cecum to the anus, and some of the intestinal tissue was cut into approximately 5-mm lengths. These were embedded in paraffin and fixed with 4% paraformaldehyde for 12 to 24 h. Remaining intestinal tissue was prepared for extracting cells. The intestinal mucosa, mesenteric lymph nodes (MLN), and spleen were quickly isolated in a sterile environment.
Histopathological analysis
Sections of colon tissue (4 µm) were cut from the paraffin blocks and stained with hematoxylin and eosin. Pathological changes of intestinal tissue were observed under a light microscope and scored according to previous studies.19
Immunohistochemistry analysis
Intestinal mucosa tissue was placed on a glass slide and a drop of PBS was added. The tissues were incubated with primary antibodies against CD4, IL-9, occludin, or claudin-2 (Abcam, Cambridge, MA, USA; dilution 1:500) at 4°C overnight and washed twice with PBS. They were then incubated with a goat anti-rabbit IgG (H+L) Superclonal™ secondary antibody, Alexa Fluor® 555 conjugate (Gibco BRL Life Technologies Inc., Gaithersburg, MD, USA; dilution 1:1000) and rinsed three times with PBS. Finally, DAPI antifade solution was added and slides were observed.
Fluorescent in situ hybridization (FISH)
FISH was performed as previously described.20 EUB338 (Guangzhou Exon Biotechnology Co., Ltd) was used as the probe to detect bacterial distribution in the intestinal mucosa. Paraffin-embedded sections of intestinal mucosa were deparaffinized in xylene, hydrated through a series of aqueous ethanol solutions, and rinsed in PBS. Subsequently, lysozyme solutions were added for digestion at 37°C for 10 minutes, then sections were fixed with 4% paraformaldehyde for 5 minutes, washed with PBS, and air dried. EUB338 was diluted with hybridization buffer at a ratio of 1:50 to 200, denatured at 88°C for 5 minutes, and renatured at 37°C for 5 minutes. The diluted probe was dropped onto the dried samples and covered with a cover glass, then sections were blocked with rubber cement in the dark at 37°C overnight. The next day, the rubber cement was removed and 2× saline sodium citrate (SSC) was used to elute the coverslip. Slides were then washed three times with 2× SSC (10 minutes each time), dehydrated with a graded ethanol series, and air dried. 4′, 6′-diamidino-2-phenylindole dihydrochloride (DAPI)-antifade solution was added and incubated for 20 minutes at room temperature. Subsequently, the slides were visualized under a fluorescence microscope and photographed.
Bacterial translocation assay
All MLN were resected, weighed, and homogenized. Aliquots of the tissue homogenate were plated onto agar medium and cultured for 24 hours in a 37°C incubator. The number of colonies was counted according to colony dilution counts and converted into bacteria per gram of tissue, which was shown as colony forming units. Each sample was cultured in duplicate.
Extraction of spleen naïve CD4+ T cells
Isolated spleens were cut into pieces and ground on a 200-µm mesh sterile filter to obtain grinding fluid. After centrifugation at 700 × g for 5 minutes, red blood cell lysis buffer (Sigma-Aldrich, St Louis, MO, USA) was added to the pellet with a liquid/solid ratio of 10:1 and allowed to stand for 3 minutes. After centrifugation at 700 × g for 5 minutes, the precipitated cells were washed two to three times with PBS and resuspended in PBS. Subsequently, all CD4-positive lymphocytes were sorted using CD4 magnetic beads (Abcam).
Co-culture of Caco-2 cells with naïve CD4+ T cells
Extracted naïve CD4+ T cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 complete medium supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Rockford, IL, USA), and the cell suspension was prepared after passaging 2 to 3 times. Caco-2 cells (Pinuosai Biotechnology Co., Ltd., Wuhan, China) were cultured in RPMI 1640 complete medium containing 10% FBS at 37°C with 5% CO2, passaged two to three times in the logarithmic phase, and divided into four groups: Caco-2 group, Caco-2+TGF-β+IL-4 group, Caco-2+TGF-β+IL-4+anti-IL-9 group, and Caco-2+TGF-β+IL-4+TL1A group. Cells in the Caco-2 group were cultured alone; for the Caco-2+TGF-β+IL-4 group, naïve CD4+ T cells and Caco2 cells were co-cultured at a ratio of 50:1, and final concentrations of 20 pg/ml of transforming growth factor (TGF)-β and 10 pg/ml of IL-4 were added to simulate the differentiation process into Th9 cells; for the Caco-2+TGF-β+IL-4+anti-IL-9 group, naïve CD4+ T cells and Caco2 cells were co-cultured and TGF-β, IL-4, and a final concentration of 20 pg/ml of IL-9 were added; and for the Caco-2+TGF-β+IL-4+TL1A group, naïve CD4+ T cells and Caco2 cells were co-cultured and TGF-β, IL-4, and a final concentration of 10 pg/ml of TL1A were added.
Effects of TL1A on the Caco-2 and naive CD4+ T cell co-culture system
Flow cytometry analysis of Caco-2 cell apoptosis
Cells in each group were cultured in the same incubator. To assess the rates of cell proliferation and apoptosis, the Annexin-PE/7-ADD-ECD flow cytometry kit (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) was used according to the manufacturer’s instructions. Results were analyzed by flow cytometry.
Observation of cell morphology under a light microscope
Cells in each group were cultured for 48 hours, then morphological changes were observed under a Nikon TMS light microscope (Nikon Corp. Instruments Co., Sendai, Japan).
Effect of TL1A on the permeability of the Caco-2 cell monolayer
Measurement of monolayer transepithelial electrical resistance (TEER)
Cells in each group were added to transwell chambers at a density of 4 × 105 per 1 mL (200 µL/per well), and 600 µL of RPMI 1640 culture medium was added to the bottom of the chamber. The culture medium was changed after 24 hours of inoculation and was then changed every other day to monitor TEER. TEER was measured by a Millcell ERS meter (Millipore, Billerica, MA, USA) as previously described.21 Briefly, the two electrodes of the resistance meter were immersed in liquid to measure the resistance of the monolayer at the top and the bottom of the chamber at 37°C. The standard TEER value = (measured value – blank control)/0.33 cm2.
Western blotting analysis of occludin, zonulin-1, and claudin-1 expression in Caco-2 cells
Cells in each group were cultured in the same incubator for 48 hours. Afterwards, Caco2 cells were collected and washed 2 to 3 times with PBS. Lysis buffer was added and total protein was extracted then quantified using a Micro BCA Protein Assay kit (Thermo Fisher Scientific). Total protein was then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking with 0.1% (v/v) goat serum in PBS for 30 minutes at room temperature, the membranes were incubated for 30 minutes at room temperature with primary antibodies against CD4, IL-9, occludin, or claudin-2 (Abcam; dilution 1:500). After three washes in PBS, cells were incubated with goat anti-rabbit IgG (H+L) Superclonal™ secondary antibody, Alexa Fluor® 555 conjugate (Gibco BRL Life Technologies Inc.; (diluted 1:1000 in PBS) for a further 30 minutes. After washing, data analysis was performed by Quantity One software.
Enzyme-linked immunosorbent assay (ELISA) detection of IL-9 expression in the culture medium
IL-9 expression was determined using an ELISA kit (Abcam) according to the manufacturer’s instructions.
Immunofluorescence analysis of cellular IL-9 expression
Cells in each group were cultured in the same incubator for 48 hours. Immunofluorescence was used to detect IL-9 expression as described above. Briefly, after incubation with the primary antibody against IL-9 (Abcam; dilution 1:500) at 4°C overnight and with the secondary antibody (Abcam; dilution 1:1000) at 37°C for 1 hour, DAPI was used for nuclear staining. Images were then observed and acquired under a fluorescence microscope.
Flow cytometry detection of the CD4+IL9+ (Th9) cell proportion
Cells in each group were cultured in the same incubator for 12, 24, and 48 hours. Suspended lymphocytes were extracted, washed 2 to 3 times with PBS, and 10 µl of CD4-fluorescein isothiocyanate (Becton, Dickinson and Co.) was added. After incubating for 15 minutes in the dark and washing twice with PBS, cells were incubated with PI-IL9 (Becton, Dickinson and Co.) in the dark for 15 minutes and washed twice with PBS. The cell proportion of CD4+IL9+ (Th9) was detected by flow cytometry.
Statistical analysis
All data analysis was performed using SPSS v.18.0 software (SPSS Inc., Chicago, IL, USA). Values are presented as means ± standard deviation. Statistical comparisons were made by one-way analysis of variance for quantitative variables and the SNK (q) test for categorical variables. A P value less than 0.05 was considered statistically significant.
Results
TL1A overexpression aggravates intestinal inflammation in chronic experimental colitis mice
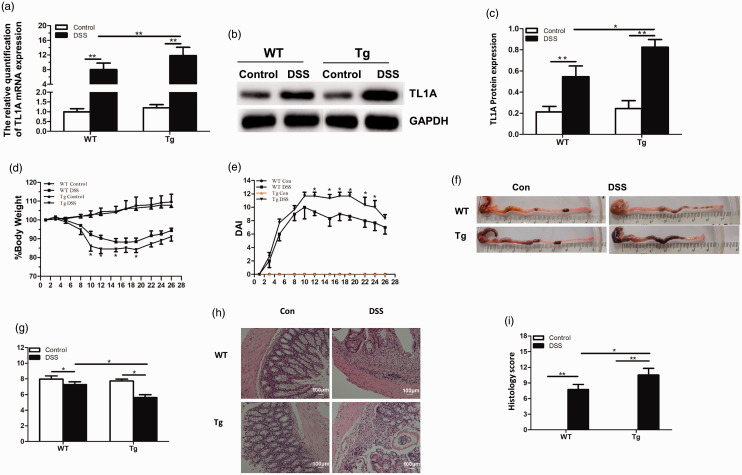
After construction of the DSS chronic colitis model, quantitative reverse transcription PCR and western blotting showed transgenic mice overexpressed TL1A (Figure 1a–c), which was evidence of their successful construction. Additionally, the DSS group showed higher expression of TL1A compared with the control group, indicating that TL1A is involved in chronic colitis development. Weight loss in the DSS/Tg group (83.9% ± 1.3%) was more pronounced than in the DSS/WT group (87.1% ± 2.1%) (Figure 1d). Additionally, DAI scores including weight loss, stool status, and fecal occult blood were higher in the DSS/Tg group than in the DSS/WT group, and significantly so on days 12, 15, 17, 19, 22, and 24 (P < 0.05; Figure 1e).
Figure 1.
TL1A overexpression aggravates intestinal mucosa inflammation in chronic experimental colitis mice. (a) TL1A mRNA expression detected by quantitative reverse transcription PCR analysis. (b, c) TL1A protein expression detected by western blot analysis. (d, e) Body weight and disease activity index (DAI) changes in each group. (f, g) Samples of colon and colon length in each group. (h, i) HE staining (magnification ×100) and histological score in each group. *P<0.05, **P<0.01, ***P<0.001.
Compared with the control group (Control/WT and Control/Tg), mice in DSS groups (DSS/WT and DSS/Tg) showed hyperemia of the colonic mucosa, edema and thickening of the intestinal wall, and a significantly shorter colon length (P < 0.05). The colon length of the DSS/Tg group was significantly shorter than that of the Control/Tg group (3.10 ± 0.26 vs 0.00±0.00, respectively; P < 0.05), while that of the DSS/Tg group was significantly shorter than the DSS/WT group (P < 0.05; Figure 1f, g). Additionally, the colonic morphological score of the DSS/Tg group was significantly higher than in the DSS/WT group (3.10 ± 0.26 vs 2.05 ± 0.27, respectively; P < 0.05).
Compared with the Control/WT group, the DSS/WT group presented with colonic mucosa defects, a reduced number of goblet cells, damaged or absent glands, and high lymphocyte infiltration in the mucosa, submucosa, and muscle layer. The pathological score was significantly higher in the DSS/WT group than the Control/WT group (8.25 ± 0.50 vs 0.00 ± 0.00, respectively; P < 0.05), significantly higher in the DSS/Tg group than in the Control/Tg group (12.50 ± 0.58 vs 0.00 ± 0.00, respectively; P < 0.05), and significantly higher in the DSS/Tg group than in the DSS/WT group (12.50 ± 0.58 vs 8.25 ± 0.50, respectively; P < 0.05; Figure 1h, i). DSS/Tg and DSS/WT groups showed similar levels of damage.
TL1A overexpression aggravates the intestinal mucosal barrier injury in chronic experimental colitis mice
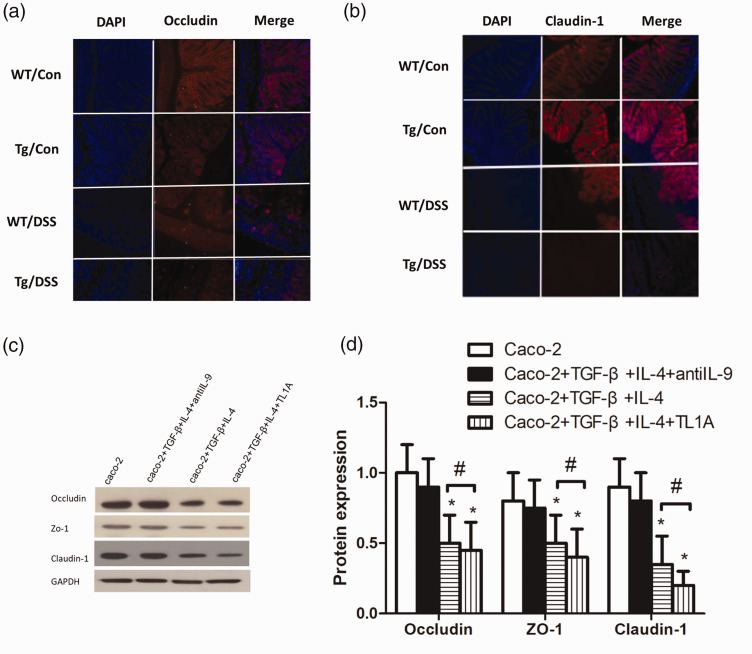
DSS/Tg and DSS/WT groups showed significantly down-regulated expression of occludin and claudin-1 in mucosa tissues compared with Control/WT and Control/Tg groups, and their protein distribution was loose and disordered. Levels of occludin and claudin-1 were significantly lower in the /Tg group than in the DSS/WT group (Figure 2a, b).
Figure 2.
TL1A aggravates the intestinal mucosal barrier injury. (a) Immunofluorescence of occludin. (b) Immunofluorescence of claudin-1. (c, d) The expression of occludin, zonulin-1, and claudin-1 in Caco-2 cells. *P<0.05 indicates the comparison with the Caco-2 group at the same time point. #P<0.05 indicates the comparison between Caco-2+TGF-β+IL-4+TL1A and Caco-2+TGF-β+IL-4 groups at the same time point.
The expression of tight junction proteins in monolayer Caco-2 cells after the addition of TL1A
To further verify the effect of TL1A on mucosal barrier function, monolayer Caco-2 cells were used to simulate the mucosal barrier, and the expression levels of occludin, zonulin-1, and claudin-1 were detected by western blotting (Figure 2c, d). After culture for 48 hours, there were no significant differences between the expression levels of occludin, zonulin-1, or claudin-1 in the Caco-2+TGF-β+IL-4+antiIL-9 group and Caco-2 group (P > 0.05). However, occludin, zonulin-1, and claudin-1 expression was significantly lower in Caco-2+TGF-β+IL-4 and Caco-2+TGF-β+IL-4 +TL1A groups than in the Caco-2 group (P < 0.05), and significantly lower in the Caco-2+TGF-β+IL-4+TL1A group than in the Caco-2+TGF-β+IL-4 group (P < 0.05).
TL1A overexpression promotes bacterial translocation in chronic experimental colitis mice
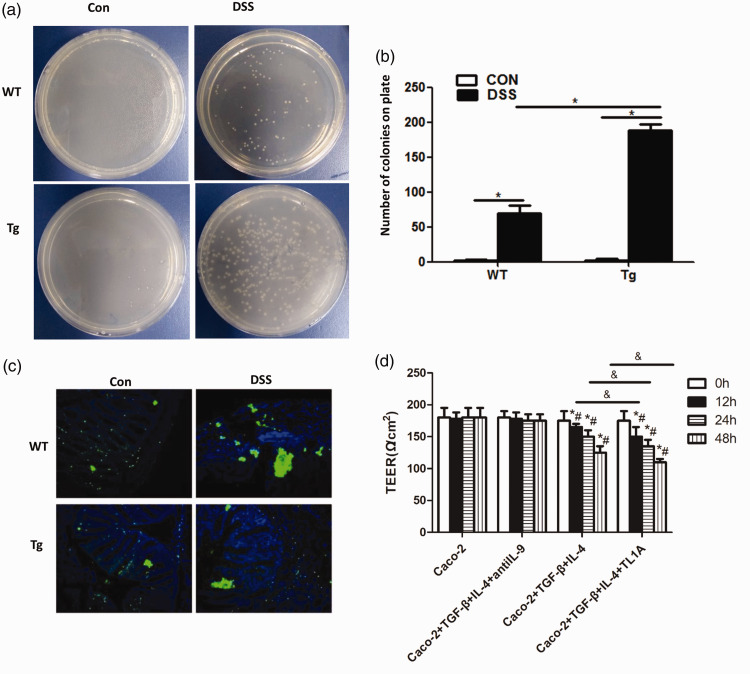
Bacterial translocation in chronic colitis mice was next detected (Figure 3a, b), and the number of bacterial colonies shown to be significantly higher in the DSS/WT group than in the Control/WT group (69.17 ± 11.14 vs 1.83 ± 1.17, respectively; P < 0.01). There were also significantly more bacterial colonies in the DSS/Tg group than in the Control/Tg group (188.33 ± 8.17 vs 2.5 ± 1.52, respectively; P<0.01), and in the DSS/Tg group compared with the DSS/WT group (188.33 ± 8.17 vs 69.17 ± 11.14, respectively; P < 0.01).
Figure 3.
Effects of TL1A on bacterial translocation. (a, b) The effects of TL1A on bacterial translocation in the mouse intestinal mucosa. (c) Detection of bacterial distribution in the intestinal mucosa by the EUB338 probe. (d) TEER results. *P<0.05 indicates the comparison at 0 h of the same group. #P<0.05 indicates the comparison with the Caco-2 group at the same time point. &P<0.05 indicates the comparison between Caco-2+TGF-β+IL-4+TL1A and Caco-2+TGF-β+IL-4 groups at the same time point.
The EUB338 assay was then used to test the distribution of bacteria in the intestinal mucosa (Figure 3c). Bacterial distribution in the intestinal mucosa was clearly disordered and displaced in DSS/WT and DSS/Tg groups, with obvious bacterial clumping, compared with Control/WT and Control/Tg groups.
TL1A increases the permeability of Caco-2 cells
TEER was used at the cellular level to evaluate the effect of TL1A on the permeability of Caco-2 monolayer cells (Figure 3d). No significant changes were seen in the transmembrane resistance of Caco-2 and Caco-2+TGF-β+IL-4+antiIL-9 groups over time, or between the two groups. However, the transmembrane resistance of Caco-2+TGF-β+IL-4 and Caco-2+TGF-β+IL-4+TL1A groups significantly decreased over time, and was significantly lower than in the Caco-2 group (P < 0.05). TEER of the Caco-2+TGF-β+IL-4+TL1A group was significantly lower than that of the Caco-2+TGF-β+IL-4 group (P < 0.05).
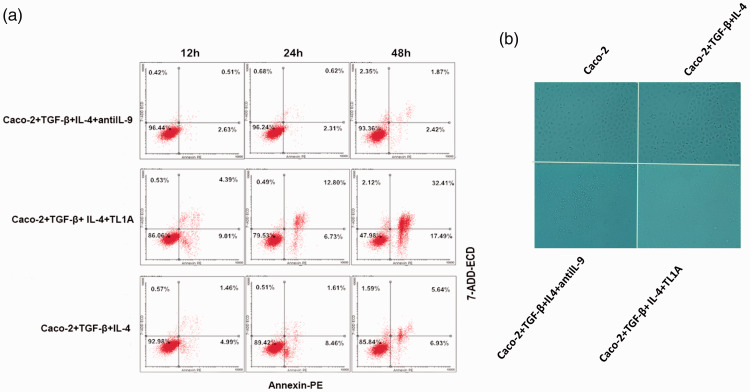
Apoptosis of monolayer Caco-2 cells after the addition of TL1A
The cell apoptosis assay was used to verify the effect of TL1A on apoptosis and the mucosal barrier (Figure 4a). Reduced cell viability was observed in the Caco-2+TGF-β+IL-4+TL1A group. Changes in the morphology of Caco-2 cells were observed under the light microscope (Figure 4b). After culture for 48 hours, normal cell morphology was seen in the Caco-2 and Caco-2+TGF-β+IL-4+antiIL-9 group, whereas notable morphological and monolayer cell structure changes were seen in cells of the Caco-2+TGF-β+IL-4+TL1A group.
Figure 4.
The effects of TL1A on the biological characteristics of monolayer Caco-2 cells. (a) Apoptosis of Caco-2 cells by flow cytometry. (b) Cell morphological changes of Caco-2 cells under a light microscope (magnification 200×). #P<0.05 indicates the comparison between Caco-2+TGF-β+IL-4+TL1A and Caco-2+TGF-β+IL-4 groups at the same time point.
TL1A overexpression promotes Th9/IL-9 expression in chronic experimental colitis mice
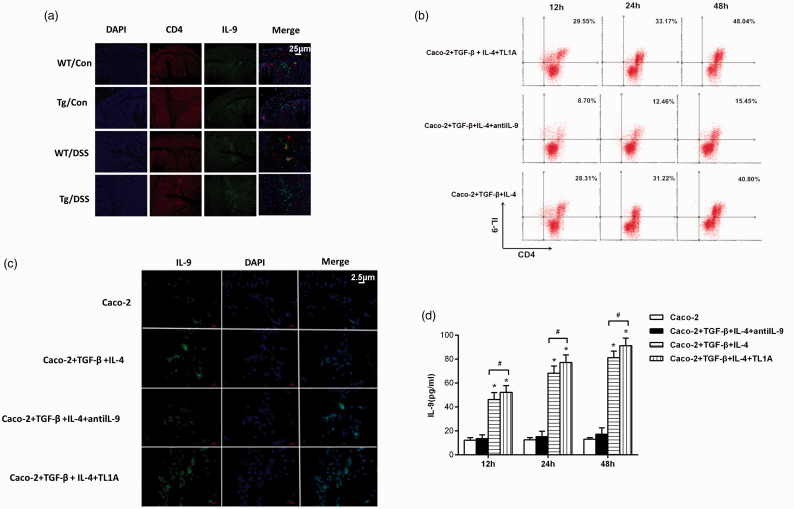
Tissue immunofluorescence (Figure 5a) showed that CD4+IL-9 expression was lower in the intestinal mucosa of Control/WT and Control/Tg mice, while CD4+IL-9 expression was higher in DSS/WT and DSS/Tg mice. CD4+IL-9 expression was higher in the DSS/Tg group than in the DSS/WT group.
Figure 5.
The effects of TL1A on the differentiation of naïve CD4+ T cells into Th9 cells and the expression of IL-9. (a) Immunofluorescence staining of Th9/IL-9 in colon tissue (magnification 40×). (b) Proportion of Th9 cells by flow cytometry. (c) Immunofluorescence staining of Caco-2 cells (magnification 400×). (d) The expression of IL-9 in culture medium. *P<0.05 indicates the comparison with the Caco-2 group at the same time point. #P<0.05 indicates the comparison between Caco-2+TGF-β+IL-4+TL1A and Caco-2+TGF-β+IL-4 groups at the same time point.
The proportion of Th9 cells and expression of IL-9 after the addition of TL1A
A higher proportion of Th9 (CD4+IL9) cells was found in the Caco-2+TGF-β+IL-4+TL1A group (Figure 5b). Additionally, the expression of IL-9 was higher in the Caco-2+TGF-β+IL-4+antiIL-9 group than the Caco-2 group (Figure 5c), significantly higher in Caco-2+TGF-β+IL-4 and Caco-2+TGF-β+IL-4+TL1A groups than Caco-2 and Caco-2+TGF-β+IL-4+antiIL-9 groups, and significantly higher in the Caco-2+TGF-β+ IL-4+TL1A group than the Caco-2+TGF-β+IL-4 group (P<0.05).
The expression level of IL-9 was further detected in culture medium (Figure 5d). There were no significant differences between IL-9 expression between the Caco-2 group and Caco-2+TGF-β+IL-4+antiIL-9 group. However, levels were significantly higher in Caco-2+TGF-β+IL-4 and Caco-2+TGF-β+IL-4+TL1A groups compared with the Caco-2 group (P < 0.05), and in the Caco-2+TGF-β+IL-4+TL1A group compared with the Caco-2+TGF-β+IL-4 group (P < 0.05).
Discussion
The expression of TL1A is relatively low in most cell types, but it is significantly increased in the lamina propria of colonic mucosa in CD patients. The increased expression of TL1A in response to the activation of immune responses suggests it plays a vital role in the pathogenesis of IBD. In this study, mice with TL1A overexpression were used to establish an IBD model. We observed an increased severity of intestinal inflammation in the DSS/Tg group, including weight loss, higher DAI scores, and higher pathological scores, than in the DSS/WT group, which was consistent with a previous report.22 The upstream regulatory mechanisms of TL1A expression should be elucidated to understand its role in IBD.
The permeability of the intestinal mucosa can change with the progression of inflammation in IBD. Therefore, we studied the effects of TL1A overexpression on the intestinal mucosal barrier of mice. We demonstrated for the first time a strong decrease of occludin and claudin-1 expression in the DSS/Tg group, thus confirming the important relationship between TL1A and the dysregulated intestinal epithelial barrier. Additionally, structural damage such as disordered epithelial cells and an increased TJ gap between cells was observed in the DSS/Tg group. This was further supported by the observed decrease in zonulin-1, occluding, and claudin-1 expression in Caco-2 cells following the addition of TL1A.
An increased proportion of Th9 cells and increased IL-9 expression levels were observed in TL1A-overexpressing IBD mice after immunofluorescence staining, which was consistent with a previous study.16 Similar to another study,23 we also found that the synergistic stimulation of naïve CD4+ T cells by TGF-β and IL-4 could induce the production of Th9 cells. The proportion of Th9 cells was reduced after the addition of an anti-IL-9 antibody. The production of Th9 cells may further induce the apoptosis of Caco2 cells and promote the high expression of IL-9. Therefore, we speculate that TL1A enhances the expression of IL-9 by promoting the production of Th9 cells whose effects on permeability of the intestinal mucosa have rarely been reported.17
A previous study reported that IL-9 increased the apoptosis of epithelial cells in in vitro culture systems of oxazolone-induced colitis.24 In the present study, using the DSS-induced colitis model, a reduced survival rate and activity of Caco2 cells were observed after the addition of TL1A. Moreover, significant morphological changes and monolayer cell structure changes were observed in Caco2 cells following TL1A addition. Taken together, we speculate that the high expression of IL-9 may induce the apoptosis of Caco2 cells after TL1A is added.
A typical feature of apoptosis is increased membrane permeability, as indicated by the TEER assay in our study, and representative of reduced cellular transmembrane resistance. Furthermore, the destruction of a monolayer barrier structure of Caco2 cells was supported by the results of bacterial translocation and EUB338 assays. Additionally, IL-9 was found to control claudin and occludin expression in experimental colitis, suggesting that it directly controls barrier function.24–26 Similarly, the expression levels of key TJ proteins including occludin, zonulin-1, and claudin-1 decreased in line with the production of Th9 cells. Therefore, the destruction of the barrier structure appears to be associated with the low expression of TJ proteins.16
Conclusions
Collectively, in this study, we found that the overexpression of TL1A could promote the destruction of the intestinal mucosal barrier in IBD mice, and that its mechanism may be associated with the promoting role of TL1A in Th9/IL-9 expression, which further destroys the mucosal barrier.
Acknowledgements
The authors are grateful for the donation of transgenic mice by David Q. Shih and Stephan R. Targan (Cedars-Sinai Medical Center, USA).
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from the Natural Science Foundation of China (Grant No. 81600433).
ORCID iD
Xiaolan Zhang https://orcid.org/0000-0002-7100-8144
References
- 1.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014; 40: 843–854. DOI: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelsen JR, Baldassano RN, Artis D, et al. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2015; 1: 462–476. DOI: 10.1016/j.jcmgh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015; 1: 154–170. DOI: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courth LF, Ostaff MJ, Mailander-Sanchez D, et al. Crohn’s disease-derived monocytes fail to induce Paneth cell defensins. Proc Natl Acad Sci U S A 2015; 112: 14000–14005. DOI: 10.1073/pnas.1510084112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivinus-Nebot M, Frin-Mathy G, Bzioueche H, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 2014; 63: 744–752. DOI: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini RJ, Dowd SE, Galandiuk S, et al. The predominant site of bacterial translocation across the intestinal mucosal barrier occurs at the advancing disease margin in Crohn’s disease. Microbiology 2016; 162: 1608–1619. DOI: 10.1099/mic.0.000336. [DOI] [PubMed] [Google Scholar]
- 7.Assimakopoulos SF, Scopa CD, Charonis A, et al. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg 2004; 198: 748–757. DOI: 10.1016/j.jamcollsurg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Schoultz I, Keita AV. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells 2019; 8: pii: E193. DOI: 10.3390/cells8020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goswami R. Th9 cells: new member of T helper cell family. Methods Mol Biol 2017; 1585: 1–19. DOI: 10.1007/978-1-4939-6877-0_1. [DOI] [PubMed] [Google Scholar]
- 10.Cavallini C, Lovato O, Bertolaso A, et al. The TNF-family cytokine TL1A inhibits proliferation of human activated B cells. PLoS One 2013; 8: e60136. DOI: 10.1371/journal.pone.0060136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt E, Bopp T. Discovery and initial characterization of Th9 cells: the early years. Semin Immunopathol 2017; 39: 5–10. DOI: 10.1007/s00281-016-0610-0. [DOI] [PubMed] [Google Scholar]
- 12.Gerlach K, Hwang Y, Nikolaev A, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 2014; 15: 676–686. DOI: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 13.Nalleweg N, Chiriac MT, Podstawa E, et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015; 64: 743–755. DOI: 10.1136/gutjnl-2013-305947. [DOI] [PubMed] [Google Scholar]
- 14.Yuan A, Yang H, Qi H, et al. IL-9 antibody injection suppresses the inflammation in colitis mice. Biochem Biophys Res Commun 2015; 468: 921–926. DOI: 10.1016/j.bbrc.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Tan KB, Harrop J, Reddy M, et al. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene 1997; 204: 35–46. [DOI] [PubMed] [Google Scholar]
- 16.Richard AC, Tan C, Hawley ET, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol 2015; 194: 3567–3582. DOI: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008; 29: 947–957. DOI: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology 2008; 135: 552–567. DOI: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki MH, Vogel P, Malireddi RK, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 2011; 20: 649–660. DOI: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atherly T, Mosher C, Wang C, et al. Helicobacter bilis infection alters mucosal bacteria and modulates colitis development in defined microbiota mice. Inflamm Bowel Dis 2016; 22: 2571–2581. DOI: 10.1097/mib.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee T, Squillantea E, Gillespieb M, et al. Transepithelial electrical resistance is not a reliable measurement of the Caco-2 monolayer integrity in Transwell. Drug Deliv 2004; 11: 11–18. DOI: 10.1080/10717540490280345. [DOI] [PubMed] [Google Scholar]
- 22.Barrett R, Zhang X, Koon HW, et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol 2012; 180: 636–649. DOI: 10.1016/j.ajpath.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Y, Wang Z, Chang C, et al. Th9 cells and IL-9 in autoimmune disorders: pathogenesis and therapeutic potentials. Hum Immunol 2017; 78: 120–128. DOI: 10.1016/j.humimm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Weigmann B, Neurath MF. Th9 cells in inflammatory bowel diseases. Semin Immunopathol 2017; 39: 89–95. DOI: 10.1007/s00281-016-0603-z. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach K, McKenzie AN, Neurath MF, et al. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tissue Barriers 2015; 3: e983777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facco M, Cabrelle A, Calabrese F, et al. TL1A/DR3 axis involvement in the inflammatory cytokine network during pulmonary sarcoidosis. Clin Mol Allergy 2015; 13: 16. DOI: 10.1186/s12948-015-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]