Abstract
Historic concerns about the cardiovascular and neuropsychiatric side effects of smoking-cessation pharmacotherapy have in part limited their use. We sought to evaluate whether depressive symptoms are associated with active smoking among survivors of stroke and myocardial infarction (MI). To do this, we performed a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (2005–2016). We included participants ≥20 years old with prior stroke or MI and any history of smoking. Symptoms of depression, at survey participation, were ascertained using the Patient Health Questionnaire-9. Active smoking was defined using self-report and, secondarily, with cotinine measures. We used logistic regression to evaluate the association between depression and active smoking after adjusting for demographics, smoking-related medical conditions, and health-related behaviors. We found that, among stroke and MI survivors with any history of smoking, 37.9% (95% CI, 34.5–41.3%) reported active smoking and 43.8% (95% CI, 40.3–47.3%) had biochemical evidence of smoking. Rates of active smoking were similar for stroke and MI survivors. Twenty-one percent screened positive for depression. In adjusted models, depression was associated with active smoking in the combined group of stroke and MI survivors (odds ratio, 2.28; 95% CI, 1.24–4.20) and in stroke survivors (odds ratio, 2.97; 95% CI, 1.20–7.38). Tests of heterogeneity by event type did not reveal an interaction. Findings were similar when using cotinine measures. We conclude that symptoms of depression were associated with active smoking among stroke and MI survivors. Stroke and MI survivors with symptoms of depression may require targeted smoking-cessation interventions.
Keywords: epidemiology, risk factors, secondary prevention, myocardial infarction, cerebrovascular disease/stroke, smoking, depression
1. Introduction
Smoking cessation after stroke and myocardial infarction (MI) is associated with a decreased risk of recurrent vascular events and mortality.1–4 Over the last two decades, several smoking-cessation pharmacotherapies have become available and are endorsed by secondary prevention guidelines.5, 6 Until recent publication of reassuring data,7 two of these effective smoking-cessation treatments – varenicline and bupropion – carried warnings cautioning against use in patients with depression. Such warnings have been associated with decreases in smoking cessation drug prescriptions,8 which may further compound the smoking-cessation challenges faced by individuals with depression.9, 10
Prior studies suggested that depression is associated with persistent smoking in the general population.10, 11 Prior studies suggested this may be the case for stroke and MI survivors in the United States as well.12–18 However, these studies typically assessed for depression at the time of stroke or MI-related hospitalization, used only self-reported smoking as their outcome measure, and were not population-based.
Whether symptoms of depression are associated with active smoking in stroke and MI survivors at the population level in the United States is not clear. Therefore, we sought to evaluate the association in a population-based, nationally-representative United States dataset while using validated measures of depression in addition to both self-reported and biochemical measures of smoking. Specifically, in this analysis of contemporary data from NHANES, we sought to test the hypothesis that symptoms of depression are associated with active smoking among non-institutionalized survivors of stroke and MI in the United States.
2. Methods
2.1. Study Design
We performed a cross-sectional study using continuous survey data from six biannual survey cycles spanning 2005 to 2016. NHANES data are collected by the United States National Center for Health Statistics, Centers for Disease Control and Prevention using a multistage, probability cluster survey method to generate nationally-representative statistics for the US population. Participants undergo standardized home interview followed by physical examination and biological specimen collection in mobile examination centers.19 Data collection for NHANES was approved by the National Center for Health Statistics Research Ethics Review Board, and written informed consent was obtained from participants. The data that support the findings of this study are publicly available at https://www.cdc.gov/nchs/nhanes. Analytic methods will be made available upon reasonable request from the corresponding author. The Weill Cornell institutional review board exempted this analysis from review.
2.2. Population
We included adult participants 20 years of age and older who reported a prior stroke or MI and any history of tobacco smoking (≥100 cigarettes during lifetime) (Figure). We did not include participants without any history of tobacco smoking because they were not asked subsequent questions regarding tobacco use, and because participants without any smoking history are by definition not active smokers. In NHANES, participants were asked whether and when they had a prior stroke or MI. For example, participants were asked, “Has a doctor or other health professional ever told you that you had a stroke?” We categorized participants who reported both stroke and MI based on the more recent event. The self-reported measures of stroke and MI are reasonably accurate in the United States general population and have been used in prior epidemiological studies using data from NHANES.20–22 Ischemic and hemorrhagic stroke are not differentiated in NHANES. We excluded participants under age 20, pregnant participants, and those with missing smoking data. We chose the age cut-off based on the target age of relevant NHANES survey questions regarding tobacco smoking.
Figure. Participant flow diagram for primary analysis of active smoking in survivors of stroke and myocardial infarction.
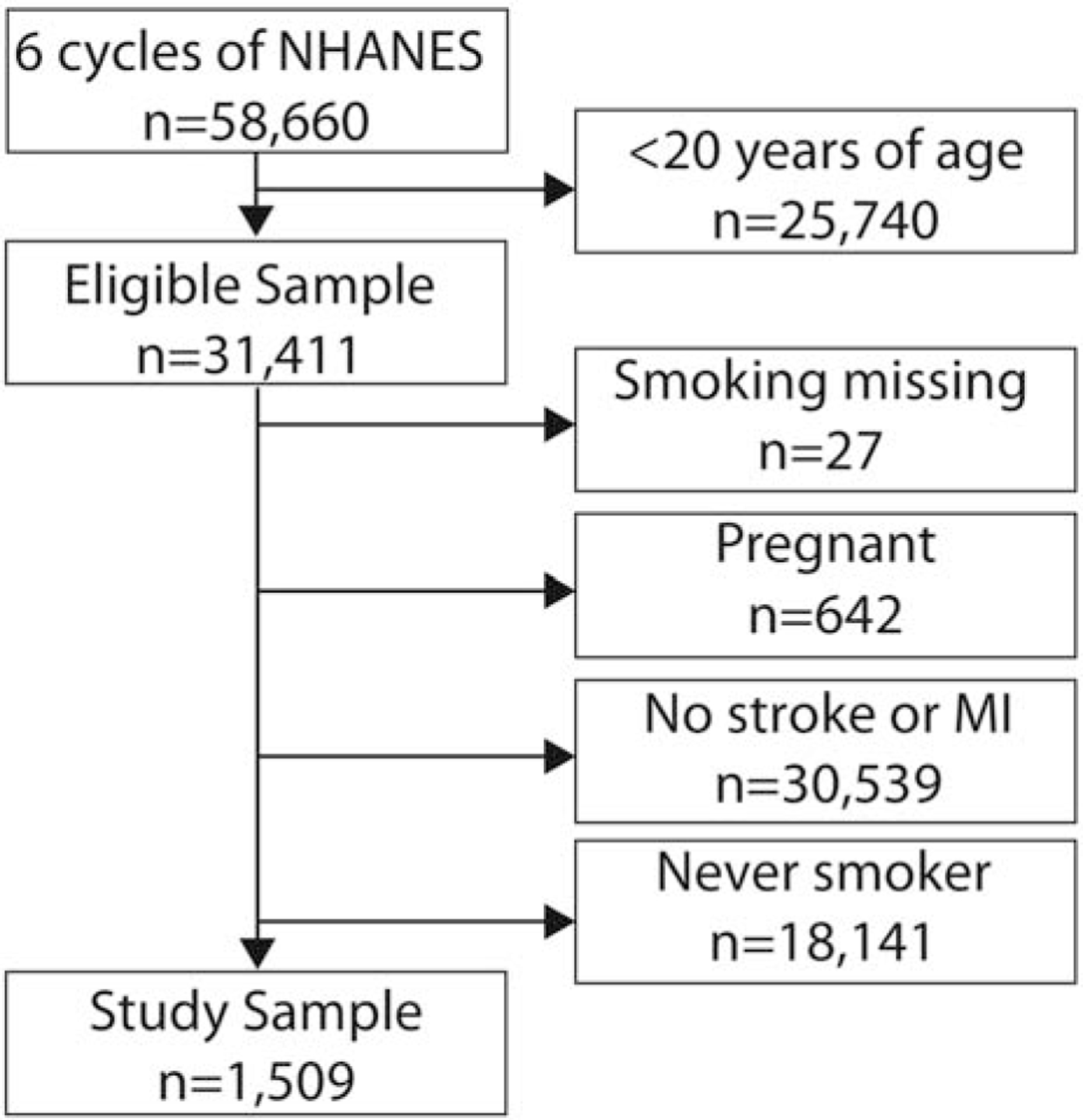
We excluded participants who were under the age of 20, did not report a prior stroke or myocardial infarction, had missing smoking data, were pregnant, and had never been regular cigarette smokers. Some participants had multiple reasons for exclusion. The primary analysis sample consisted of adult stroke and myocardial infarction survivors with any history of cigarette smoking. Abbreviations: MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey.
2.3. Measurements
Participants in NHANES were administered the Patient Health Quationnaire-9 (PHQ-9) scale. The PHQ-9 is a depression screening tool that generates a summed score between 0–27 from participants’ responses to questions regarding depression symptom frequency over the past two weeks. We defined having symptoms of depression, at the time of survey participation, as a score of 10 or greater on the Patient Health Questionnaire-9 scale. This cut-off has been validated in multiple settings. Specifically, this criteria performed well compared to structured clinical interview for depression, with an area under the curve of 0.96 among stroke survivors and an area under the curve of 0.95 among those with heart disease.23, 24 Pre-stroke/MI depression symptom status was not known.
Covariates included demographics, medical history of smoking-related conditions, and health-related behaviors. Demographic variables were age, gender, self-reported race-ethnicity, poverty, health insurance, and educational attainment. Race-ethnicity was categorized as White, Black, Hispanic, and Other; Asian Americans and Multi-racial individuals were included in Other given the small numbers of these individuals. Poverty was defined using the poverty-ratio index (ratio of family income to the local poverty threshold), by which an index of <100% denotes poverty. We categorized health insurance as uninsured, Medicaid, Medicare, private/commercial insurance, or other. We categorized educational attainment as ≤12th grade education versus any college or greater. We tabulated smoking-related conditions: hypertension, cancer, and pulmonary diseases; these conditions may prompt physicians to provide smoking-cessation interventions or increase individuals’ motivation to quit and were thus included as potential confounders. We defined hypertension as self-reported diagnosis, blood pressure > 140/90 mm Hg, or use of anti-hypertensive medications. Cancer and pulmonary diseases were defined using self-reported diagnoses. We also tabulated the health-related behaviors of physical inactivity, drug use, and heavy alcohol use. We defined physical inactivity as the absence of moderate or vigorous work or recreational physical activity in a typical week, or endorsing an average inactive physical activity level (Appendix). An affirmative response to questions regarding the use of marijuana, cocaine, heroin, or methamphetamines in the past 30 days constituted evidence of active drug use. Heavy alcohol use was defined as reporting greater than 14 drinks per week for men and greater than 7 drinks per week for women.25
The primary outcome was active smoking. We defined active smoking based on self-report and using biochemical measures. Participants in NHANES were asked if they were currently smoking at the time of survey; we categorized those with an affirmative response as active smokers. Secondarily, we defined active smoking with biochemical evidence using serum cotinine measures in participants who had measures available. Cotinine measures were available for participants who underwent examination (>90%) with the exception of participants with hemophilia or chemotherapy in the past 4 weeks. The laboratory methods are described in the NHANES manual.26 Cotinine is a nicotine metabolite with sufficiently long elimination half-life to reflect active tobacco exposure.27 We defined active smoking using race-specific cotinine thresholds that were developed to reflect active smoking rather than secondhand exposure and to account for racial differences in smoking habits and nicotine metabolism.28 The thresholds were: non-Hispanic white: > 4.85 ng/mL, non-Hispanic black > 5.92 ng/mL, Mexican-American > 0.84 ng/mL, and other including other Hispanic > 3.08 ng/mL.28 The sensitivity and specificity of these thresholds is greater than 95%.28
2.4. Statistical Analyses
We merged data for six biannual cycles into one 12-year pooled dataset and calculated survey-weighted estimates of demographic characteristics, comorbidities, and active smoking. NHANES cycle-specific weights were used to account for oversampling and nonresponse rates. We used survey procedures to generate descriptive statistics; participant characteristics were compared across groups using the chi-squared test and t-test as appropriate. Univariate associations between covariates and active smoking were individually assessed, and we used multivariable logistic regression to estimate the odds ratios (OR) and 95% CIs for the association between symptoms of depression and active smoking. We performed a test of interaction to assess whether the association between symptoms of depression and active smoking differed by event type, with a threshold of p=0.10 for interaction significance on the multiplicative scale. Models were adjusted incrementally for possible confounders. Model 1 was unadjusted. Model 2 was adjusted for demographics (age, gender, race-ethnicity, poverty, health insurance, and educational attainment), and Model 3 was additionally adjusted for smoking-related conditions (hypertension, cancer, and pulmonary disease) and health-related behaviors (physical inactivity, drug use, heavy alcohol use). We performed three post hoc analyses: first, we evaluated the association between depression and active smoking among stroke and MI survivors in two separate epochs: 2005–2010 and 2011–2016; second, we performed a sensitivity analysis in which we additionally adjusted Model 3 for whether participants reported having access to routine healthcare; third, we evaluated the association between depression and active smoking among adults without prior stroke or MI. In all analyses, we used sampling weights in regression models to account for the complex, multi-staged sampling design of NHANES. The threshold of statistical significance was set at α = 0.05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
The study sample consisted of 1,509 participants with a self-reported history of prior stroke or MI and any history of prior cigarette smoking (Figure), which represents 7,858,094 individuals after applying sample weights. These participants with a history of any smoking accounted for 63.2% of all participants with a prior stroke or MI; participants with stroke were more often women and less often white than those with MI but did not differ substantially in other attributes (Table 1). The mean age was 64.2 years (Standard error [SE], 0.4), and the average time since stroke or MI was 9.7 years (SE, 0.3).
Table 1.
Characteristics of Participants, Stratified by Vascular Event Type
| Characteristica | Stroke (nb=658; 3,270,323) | Myocardial infarction (nb=803; 4,302,393) | P-value |
|---|---|---|---|
| Age, mean (SE), years | 63.9 (0.62) | 64.7 (0.52) | 0.27 |
| Women | 48.0 (2.7) | 31.1 (2.1) | <0.0001 |
| Racec | 0.01 | ||
| White | 69 (2.6) | 78 (2.0) | |
| Black | 16 (1.5) | 9 (i-o) | |
| Hispanic | 7 (1.0) | 6 (0.9) | |
| Other | 8 (1.9) | 7 (1–3) | |
| No college education | 59 (2.6) | 59 (2.6) | 0.99 |
| Insuranced | 0.62 | ||
| Private | 45 (3–0) | 47 (2–5) | |
| Medicare | 31 (2.5) | 32 (2.2) | |
| Medicaid | 8 (1.4) | 6 (0.9) | |
| Other | 6 (1.4) | 6 (1.1) | |
| Uninsured | 9.4 (1.2) | 10 (1.5) | |
| Povertye | 29 (2.4) | 26 (2.0) | 0.36 |
| Hypertension | 78 (1.8) | 73 (2.0) | 0.10 |
| Pulmonary Diseases | 38 (2.2) | 34 (i-9) | 0.15 |
| Cancer | 23 (2.5) | 22 (1.8) | 0.82 |
| Physical Inactivity | 53 (2–3) | 44 (2–5) | 0.02 |
| Active drug use | 6 (1.1) | 5 (1–1) | 0.74 |
| Heavy alcohol use | 9 (1–9) | 12 (1.9) | 0.31 |
| Depressionf | 23 (2.5) | 19 (2.0) | 0.15 |
Abbreviations: SE, standard error.
Data are presented as percentage (standard error of percentage) unless otherwise specified. Percentages for any given characteristic may not sum to 100% because figures reflect
weighted estimates.
Numbers reported are the raw numbers of participants and corresponding weighted frequencies after applying sampling weights. Table includes 1,461 of 1,509 participants because event type could not be assigned for 48 participants with both prior stroke and myocardial infarction with unclear chronology.
Self-reported by participants or their surrogates.
Participants with both private insurance and Medicare were categorized as having private insurance. Those with both Medicare and Medicaid were categorized as having Medicare.
Poverty was defined using the poverty-ratio index (ratio of family income to the local poverty threshold), by which an index of <100% denotes poverty.
Symptoms of depression ascertained based on Patient Health Questionnaire –9 score of ≥10.
Overall, 37.9% (95% CI, 34.5–41.3%) of participants with prior stroke or MI and any history of smoking reported persistent active smoking at the time of NHANES participation. Serum cotinine measures were available for 94.1% of participants; among those with prior stroke or MI, 43.8% (95% CI, 40.3–47.3%) were classified as active smokers by this measure. There was good correlation between self-report and cotinine measures (Phi correlation coefficient, 0.85).
Active smoking was reported by 39.7% (95% CI, 35.1–44.4%) of stroke survivors and 35.2% (95% CI, 30.6–39.9%) of MI survivors. When active smoking was defined using validated serum cotinine measures, the active smoking rate was 43.7% (95% CI, 38.8–48.7%) among stroke survivors and 42.6% (95% CI, 38.0–47.3%) among MI survivors.
Complete PHQ-9 questionnaire data were available for 68.9% of participants. Participants with missing depression data were older and more often men, among other differences (Appendix Table A1). Overall, 20.5% (95% CI, 17.4–23.6%) screened positive for symptoms of depression; the proportion with positive depression screening among stroke and MI survivors did not differ (P=0.15). Participants screening positive for depression were younger, more often women, had lower markers of socioeconomic status, and less favorable health-related behaviors (Table 2).
Table 2.
Characteristics of Participants with History of Stroke or Myocardial Infarction, Stratified by Depressiona
| Characteristicb | Depressed (nc=240; 1,111,358) | Not Depressed (nc=766; 4,301,001) | P-value |
|---|---|---|---|
| Age, mean (SE), years | 57.0 (0.89) | 64.3 (0.64) | <0.0001 |
| Women | 54 (3–8) | 39 (2.o) | 0.001 |
| Raced | 0.02 | ||
| White | 66 (3.6) | 77 (2.2) | |
| Black | 17 (2.4) | 10 (1.1) | |
| Hispanic | 10 (1.9) | 6 (0.9) | |
| Other | 7 (2.1) | 7 (1–5) | |
| No college education | 68 (3.6) | 55 (2–2) | 0.005 |
| Insurancee | <0.0001 | ||
| Private | 25 (4.0) | 48 (2.3) | |
| Medicare | 39 (3–7) | 30 (2.3) | |
| Medicaid | 17 (3-o) | 6 (0.9) | |
| Other | 9 (2.0) | 6 (1.1) | |
| Uninsured | 10 (2.0) | 10 (1.5) | |
| Povertyf | 40 (4–5) | 27 (2.2) | 0.01 |
| Hypertension | 76 (3–6) | 77 (2–2) | 0.91 |
| Pulmonary Diseases | 59 (3–7) | 36 (2.0) | <0.0001 |
| Cancer | 19 (2–9) | 21 (2.2) | 0.58 |
| Active drug use | 15 (3–3) | 6 (1.0) | 0.008 |
| Heavy alcohol use | 17 (4–2) | 11 (2.1) | 0.24 |
| Physical Inactivity | 61 (4–7) | 44 (2.2) | 0.0001 |
Abbreviations: SE, standard error.
Depression ascertained based on Patient Health Questionnaire –9 score of ≥ 10.
Data are presented as percentage (standard error of percentage) unless otherwise specified. Percentages for any given characteristic may not sum to 100% because figures reflect weighted estimates.
Numbers reported are the raw numbers of participants and corresponding weighted frequencies after applying sampling weights.
Self-reported by participants or their surrogates.
Participants with both private insurance and Medicare were categorized as having private insurance. Those with both Medicare and Medicaid were categorized as having Medicare.
Poverty was defined using the poverty-ratio index (ratio of family income to the local poverty threshold), by which an index of <100% denotes poverty.
The prevalence of depression among those reporting and denying active smoking was 32.8% and 12.1%, respectively. In unadjusted analyses, screening positive for depression was associated with active smoking, both self-reported (OR, 3.89; 95% CI, 2.58–5.86) and biochemically assessed (OR, 3.50; 95% CI, 2.32–5.27) among survivors of stroke and MI and for each vascular event type individually (Table 3). After adjusting for demographics, smoking-related conditions, and health-related behaviors, depression remained associated with self-reported active smoking for the combined group of stroke or MI survivors (OR, 2.28; 95% CI, 1.24–4.20) and among stroke survivors (OR, 2.97; 95% CI, 1.20–7.38). The direction of effect and effect sizes were similar in adjusted models using cotinine-based measures for the combined group (OR, 2.23; 95% CI, 1.18–4.19), but not significant for stroke survivors (OR, 2.58; 95% CI, 0.95–7.02) (Table 3). When an event type * depression interaction term was added to the most adjusted models, there was insufficient evidence of a multiplicative interaction between depression and vascular event type (P=0.19 for self-reported active smoking; P=0.25 for biochemically-assessed active smoking).
Table 3.
Association between Symptoms of Depression and Active Smoking among Survivors of Stroke and Myocardial Infarction
| Odds ratio (95% CI) of active smoking by depressiona | |||
|---|---|---|---|
| Stroke and Ml | Stroke | Ml | |
| Self-reported active smoking | |||
| Model 1 | 3.89 (2.58–5.86) | 3.57 (2.11–6.06) | 4.31 (2.37–7.85) |
| Model 2 | 2.32 (1.42–3.78) | 1.70 (0.88–3.28) | 2.96 (1.47–5.95) |
| Model 3 | 2.28 (1.24–4.20) | 2.97 (1.20–7.38) | 1.47 (0.63–3.46) |
| Biochemically-assessed active smoking | |||
| Model 1 | 3.50 (2.32–5.27) | 3.44 (1.82–6.49) | 3.70 (2.07–6.60) |
| Model 2 | 2.13 (1.31–3.48) | 1.52 (0.70–3.29) | 2.92 (1.45–5.86) |
| Model 3 | 2.23 (1.18–4.19) | 2.58 (0.95–7.02) | 1.66 (0.76–3.59) |
Abbreviations: MI, myocardial infarction.
Symptoms of depression ascertained based on Patient Health Questionnaire –9 score of ≥ 10. Model 1 is unadjusted. Model 2 is adjusted for age, gender, race-ethnicity, education, insurance, and poverty (defined using the poverty-ratio index). Model 3 is additionally adjusted for smoking-related illnesses (hypertension, cancer, pulmonary disease) and health-related behaviors (physical inactivity, drug use, heavy alcohol use) .
We performed three post hoc analyses (Appendix, Tables A2–4). First, we did not find a statistically significant interaction of study epoch (2005–2010 versus 2011–2016) in the association between depression and smoking; however, relationships appeared weaker in the later epoch. Second, additionally adjusting models for whether participants had access to routine care did not meaningfully change our results. Last, depression was associated with active smoking among participants without stroke or MI.
4. Discussion
In this analysis of nationally representative data for the United States, 38% of non-institutionalized survivors of stroke and MI with a prior smoking history reported active smoking. Serum cotinine measures revealed active smoking in 44% of this population, which was similar in stroke and MI survivors. Additionally, stroke and MI survivors with evidence of symptoms of depression were more likely to be active smokers.
We provide contemporary estimates of the prevalence of active smoking among stroke and MI survivors in the United States based on self-report and cotinine measures. Our approach differs from other recent studies, which reported persistent smoking rates as high as 78% after stroke29 and 63% after MI.30 Such studies were not population-based and generally had brief duration of follow-up.13, 14, 16, 17, 29–36 Our use of complementary self-reported and biochemical smoking-status measures, and the population-based nature of our study sample, support the representativeness of our estimates for the United States. Our results can be compared to a historical analysis of NHANES data from 1988 to 1994, in which 225 (42%) of 530 stroke and MI survivors, with any history of smoking, reported active smoking.37 It is noteworthy that a similar proportion were active smokers in this 2005–2016 sample. The high, stable proportion of stroke and MI survivors with persistent smoking may in part be explained by very low rates of smoking-cessation aide prescriptions.8, 35 These data suggest that secondary prevention through aggressive smoking-cessation interventions are needed for stroke and MI survivors.
Our data demonstrate an association between screening positive for symptoms of depression and active smoking in a nationally-representative cohort of stroke and MI survivors with a history of prior smoking in the United States. Our findings are consistent with observations regarding the general population; those with depression appear to be more likely to smoke cigarettes and face greater challenges with cessation.10, 11 Our post hoc analysis in participants without stroke or MI were also consistent with these prior data. Additionally, an association between depression and smoking after MI was reported in several small studies,13–16 in addition to multicenter studies from Europe38 and Israel.1 However, population-level data for the United States were lacking, and data pertaining to stroke survivors were limited and conflicting.18, 31, 33 Two single-center studies of stroke survivors found depression to be associated with less cessation at 3 months,18, 33 whereas a national registry study did not find this to be the case at 1 year in South Korea.31 In our data, unadjusted models demonstrated the co-occurrence of depressive symptoms and active smoking. In adjusted analyses, screening positive for depression was associated with an increased odds of active smoking in the combined population of stroke and MI survivors, and for stroke survivors but not MI survivors, when analyzed separately. Differences in the nature of physical deficits and lifestyle limitations after stroke and MI may explain the discrepant associations. However, an important caveat is that formal tests of heterogeneity did not reveal an interaction in the association between depression and active smoking by event type, so the differences between stroke and MI may represent chance findings.
In contrast to studies that assessed depression at baseline, our cross-sectional study does not provide temporality, and therefore causality should not be inferred. Indeed, it is possible that smoking can lead to depression, as some studies in the general population have suggested.11 While baseline measures of depression would support temporality, the contemporaneous measurement of depression symptoms and smoking in our study better approximates routine clinical practice. The high rate of screening positive for symptoms of depression among active smokers is notable. Screening for depression in active smokers after stroke and MI may have clinical utility. Depression and other mood symptoms have been independently associated with reduced adherence to many secondary preventive therapies after stroke and MI.39, 40 Additionally, patients with depression have higher rates of recurrent stroke and poor outcomes after MI.41, 42 Therefore, in this context, irrespective of biological causality, our data suggest that stroke and MI survivors found to have depressive symptoms may require targeted interventions to improve overall secondary prevention, including smoking cessation. Although our data do not clarify whether targeting the depression or smoking itself would be beneficial, it is important to note that varenicline and bupropion appear safe for patients with psychiatric disease, and that psychiatric illness does not clearly impact the efficacy of these drugs.7, 43 Whether smoking-cessation aide prescriptions are less frequently given to stroke and MI survivors with depression requires study using reliable prescription or pharmacy claims data. In a post hoc analysis, we did not find clear evidence that the association between depression and smoking differed by epoch (2005–2010 versus 2011–2016); however, associations appeared weaker in the later epoch. Changes in access to treatment, for example related to improving insurance coverage of smoking-cessation treatments,44 may have had an impact, but this requires confirmation. Further investigation to determine the optimal approach for smoking cessation in stroke and MI survivors with depression is needed.
The key strengths of our analysis pertain to the nationally-representative nature of NHANES data and the availability of validated serum cotinine measures to corroborate results regarding self-reported active smoking. Several limitations warrant discussion. First, this was an observational, cross-sectional study such that the temporality of the association between depression and smoking cannot be assessed. Second, there was significant missingness of depression data. Given the sensitive nature of the depression screen, NHANES only performed the screen with English and Spanish speaking participants who did not have a proxy with them and who did not require an interpreter. Our results may therefore be less generalizable for older individuals, less independent individuals, and those facing language barriers. Third, because only non-institutionalized participants are included in NHANES, it is possible that our sample included participants with less severe strokes and heart disease. Fourth, some analyses may have been underpowered, particularly for tests of interaction. However, while formal heterogeneity by vascular event type (stroke versus MI) was not seen, the absence of a statistically significant multiplicative interaction may not necessarily imply homogeneity of effect, especially when sample size is limited. Fifth, our results reflect point estimates of active smoking and do not reflect cumulative exposure or longitudinal behavior. Sixth, survey data do not differentiate between ischemic and hemorrhagic stroke, so we cannot determine whether smoking behavior differs by stroke type. Seventh, we used the Patient Health Questionnaire-9 scale to ascertain symptoms of depression; our findings speak to the association between active symptoms of depression and smoking and likely do not generalize to those with successfully treated depression. Similarly, it must be noted that our exposure variable assesses symptoms of depression and not formal diagnoses. We did not have reliable antidepressant medication use data to include. Last, while the use of NHANES data facilitated comprehensive assessment of potential confounders from interviews and examination, there may be residual confounding in the association between depression and active smoking. Therefore, our findings may not necessarily support a causal association between depression and active smoking. Rather, the observed associations highlight the high co-occurrence of depressive symptoms and active smoking in this at-risk population; further study of biological determinants, mechanisms, and optimal treatments are needed.
5. Conclusion
In this nationally-representative sample, approximately 2 out of 5 stroke and MI survivors, with any prior history of smoking, were active smokers. These individuals who continued to smoke had a high rate of screening positive for depression, and screening positive for symptoms of depression was independently associated with active smoking among this population. Intensive smoking-cessation interventions for secondary prevention after stroke and MI deserve study, and further targeted smoking-cessation interventions may be necessary for stroke and MI survivors with symptoms of depression.
Supplementary Material
Highlights.
Smoking after stroke and myocardial infarction is associated with poor outcomes.
We assessed if depression is associated with smoking after stroke and MI in NHANES.
Depression was associated with self-reported and serum-based evidence of smoking.
Funding:
This work was supported by National Institute of Neurological Disorders and Stroke (grant T32NS07153) and National Institutes of Health StrokeNet (grant U24NS107237).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts-of-Interest/Disclosures:
Dr. Parikh: none. Dr. Salehi Omran: none. Dr. Kamel serves as co-PI for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Elkind serves as the Chairman of the Advisory Committee to the American Stroke Association and on the National, Founders Affiliate, and New York City boards of the American Heart Association. He receives royalties for chapters on stroke from UpToDate. Dr. Willey reports no disclosures.
References
- 1.Gerber Y, Rosen LJ, Goldbourt U, Benyamini Y, Drory Y, et al. Smoking status and long-term survival after first acute myocardial infarction a population-based cohort study. J Am Coll Cardiol. 2009;54:2382–2387 [DOI] [PubMed] [Google Scholar]
- 2.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: Meta-analysis of cohort studies. Archives of internal medicine. 2000;160:939–944 [DOI] [PubMed] [Google Scholar]
- 3.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Annals of internal medicine. 2002;137:494–500 [DOI] [PubMed] [Google Scholar]
- 4.Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89:1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72:3332–3365. [DOI] [PubMed] [Google Scholar]
- 6.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Sssociation. Stroke. 2014;45:2160–2236 [DOI] [PubMed] [Google Scholar]
- 7.Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520 [DOI] [PubMed] [Google Scholar]
- 8.Jarlenski M, Hyon Baik S, Zhang Y. Trends in use of medications for smoking cessation in medicare, 2007–2012. Am J Prev Med. 2016;51:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, et al. Past major depression and smoking cessation outcome: A systematic review and meta-analysis update. Addiction. 2013;108:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger AH, Kashan RS, Shpigel DM, Esan H, Taha F, Lee CJ, et al. Depression and cigarette smoking behavior: A critical review of population-based studies. Am J Drug Alcohol Abuse. 2017;43:416–431 [DOI] [PubMed] [Google Scholar]
- 11.Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: A systematic review. Nicotine Tob Res. 2017;19:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle F, Rohde D, Rutkowska A, Morgan K, Cousins G, McGee H. Systematic review and meta-analysis of the impact of depression on subsequent smoking cessation in patients with coronary heart disease: 1990 to 2013. Psychosom Med. 2014;76:44–57 [DOI] [PubMed] [Google Scholar]
- 13.Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S, et al. Predictors of smoking cessation after a myocardial infarction: The role of institutional smoking cessation programs in improving success. Archives of internal medicine. 2008;168:1961–1967 [DOI] [PubMed] [Google Scholar]
- 14.Holtrop JS, Stommel M, Corser W, Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome. J Hosp Med. 2009;4:E3–9 [DOI] [PubMed] [Google Scholar]
- 15.Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of internal medicine. 2008;168:186–191 [DOI] [PubMed] [Google Scholar]
- 16.Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: A predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2008;15:89–94 [DOI] [PubMed] [Google Scholar]
- 17.Suñer-Soler R, Grau-Martín A, Terceno M, Silva Y, Davalos A, Sánchez JM, et al. Biological and psychological factors associated with smoking abstinence six years poststroke. Nicotine Tob Res. 2018;20:1182–1188 [DOI] [PubMed] [Google Scholar]
- 18.Ballard J, Kreiter KT, Claassen J, Kowalski RG, Connolly ES, Mayer SA. Risk factors for continued cigarette use after subarachnoid hemorrhage. Stroke. 2003;34:1859–1863 [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES): questionnaires, datasets, and related documentation. [online]. Available at: https://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed December 1, 2019.
- 20.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 21.Lin MP, Ovbiagele B, Markovic D, Towfighi A. “Life’s simple 7” and long-term mortality after stroke. J Am Heart Assoc. 2015;4:e001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in myocardial infarction secondary prevention: The National Health and Nutrition Examination Surveys (NHANES), 1999–2012. J Am Heart Assoc. 2015;4:e001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams LS, Brizendine EJ, Plue L, Bakas T, Tu W, Hendrie H, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36:635–638 [DOI] [PubMed] [Google Scholar]
- 24.Haddad M, Walters P, Phillips R, Tsakok J, Williams P, Mann A, et al. Detecting depression in patients with coronary heart disease: A diagnostic evaluation of the PHQ-9 and HADS-D in primary care, findings from the UPBEAT-UK study. PLoS One. 2013;8:e78493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans [Online]. Available at: https://health.gov/dietaryguidelines/2015/. Accessed: March 1, 2019
- 26.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey Examination and Laboratory Procedures. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_MEC_Laboratory_Procedures_Manual.pdf. Accessed: March 1, 2019.
- 27.SRNT Subcomttee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159 [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248 [DOI] [PubMed] [Google Scholar]
- 29.Bak S, Sindrup SH, Alslev T, Kristensen O, Christensen K, Gaist D. Cessation of smoking after first-ever stroke: A follow-up study. Stroke. 2002;33:2263–2269 [DOI] [PubMed] [Google Scholar]
- 30.Colivicchi F, Mocini D, Tubaro M, Aiello A, Clavario P, Santini M. Effect of smoking relapse on outcome after acute coronary syndromes. Am J Cardiol. 2011;108:804–808 [DOI] [PubMed] [Google Scholar]
- 31.Lim YK, Shin DW, Kim HS, Yun JM, Shin JH, Lee H, et al. Persistent smoking after a cardiovascular event: A nationwide retrospective study in Korea. PLoS One. 2017;12:e0186872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suñer-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Dávalos A, et al. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43:131–136 [DOI] [PubMed] [Google Scholar]
- 33.Sienkiewicz-Jarosz H, Zatorski P, Baranowska A, Ryglewicz D, Bienkowski P. Predictors of smoking abstinence after first-ever ischemic stroke: A 3-month follow-up. Stroke. 2009;40:2592–2593 [DOI] [PubMed] [Google Scholar]
- 34.Ives SP, Heuschmann PU, Wolfe CD, Redfern J. Patterns of smoking cessation in the first 3 years after stroke: The South London stroke register. Eur J Cardiovasc Prev Rehabil. 2008;15:329–335 [DOI] [PubMed] [Google Scholar]
- 35.Pagidipati NJ, Hellkamp A, Thomas L, Gulati M, Peterson ED, Wang TY. Use of prescription smoking cessation medications after myocardial infarction among older patients in community practice. JAMA Cardiol. 2017;2:1040–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gall SL, Dewey HM, Thrift AG. Smoking cessation at 5 years after stroke in the north East Melbourne Stroke Incidence Study. Neuroepidemiology. 2009;32:196–200 [DOI] [PubMed] [Google Scholar]
- 37.Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population: Report from the Third National Health and Nutrition Examination Survey. Archives of internal medicine. 2001;161:1621–1628 [DOI] [PubMed] [Google Scholar]
- 38.Prugger C, Wellmann J, Heidrich J, De Bacquer D, De Backer G, Périer MC, et al. Readiness for smoking cessation in coronary heart disease patients across Europe: Results from the Euroaspire III survey. Eur J Prev Cardiol. 2015;22:1212–1219 [DOI] [PubMed] [Google Scholar]
- 39.Amann U, Kirchberger I, Heier M, Thilo C, Kuch B, Meisinger C. Medication use in long-term survivors from the MONICA/KORA myocardial infarction registry. Eur J Intern Med. 2018;47:62–68 [DOI] [PubMed] [Google Scholar]
- 40.Glader EL, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41:397–401 [DOI] [PubMed] [Google Scholar]
- 41.Yuan HW, Wang CX, Zhang N, Bai Y, Shi YZ, Zhou Y, et al. Poststroke depression and risk of recurrent stroke at 1 year in a Chinese cohort study. PLoS One. 2012;7:e46906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369 [DOI] [PubMed] [Google Scholar]
- 43.West R, Evins AE, Benowitz NL, Russ C, McRae T, Lawrence D, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in eagles. Addiction. 2018;113:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemaire RH, Bailey L, Leischow SJ. Meeting the tobacco cessation coverage requirement of the patient protection and affordable care act: State smoking cessation quitlines and cost sharing. Am J Public Health. 2015;105 Suppl 5:S699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


