Abstract
Peanut allergy is a growing public concern; however, little is known about the immunological mechanism(s) that initiate the disease process. Our knowledge is also limited regarding the role of group 2 innate lymphoid cells (ILC2s) in regulating humoral immunity. To fill these major gaps in our knowledge, we investigated the immunological mechanisms involved in peanut allergen sensitization by using mouse models. To mimic environmental exposure in humans, naïve BALB/c mice were exposed to peanut flour by inhalation without any exogenous adjuvants. When exposed to peanut flour, naïve mice developed T follicular helper (Tfh) cells in their lung draining lymph nodes (dLNs) and produced IgE antibodies to peanuts. Mice deficient in IL-13 showed decreased numbers of Tfh cells and germinal center B cells and produced significantly less IgE antibodies. IL-13 was necessary and sufficient for induction of CD11c+ MHCIIhi dendritic cells that are implicated in Tfh cell development. Importantly, lung ILC2s served as a predominant early source of IL-13 when naive mice were exposed to peanut flour. Furthermore, mice that are deficient in lung ILC2s by bone marrow transfer from Rorasg/sg mice or by genetic manipulation produced significantly less IgE antibodies to peanuts compared to control mice. These findings suggest lung ILC2s that serve as a rapid source of IL-13 upon allergen exposure play a major role in Tfh cell development, IgE antibody production and initiation of peanut allergy.
INTRODUCTION
The late 1990s saw an increase in the prevalence of peanut allergies (1). Accidental ingestion of small amounts of peanuts can cause afflicted patients to experience severe clinical symptoms, including systemic anaphylaxis. The rise in peanut allergies has been accompanied by an increased burden on the medical care system along with considerable emotional and financial burdens for patients and their families (2). Therefore, it is essential to understand the immunologic mechanisms involved in initiation of peanut allergies and to develop effective and safe strategies to prevent and treat the disease.
Peanut allergies are associated with type 2 immune responses (3). The T follicular helper (Tfh) cells that are residing in lymph nodes (LNs) and specialized in providing B cell help are likely involved in production of IgE antibodies to airborne allergens (as opposed to conventional Th2-type CD4+ T cells) (4–7). More recently, a unique subset of IL-13-producing Tfh cells, called Tfh13 cells, has been implicated in the production of anaphylactic high-affinity IgE antibodies to inhaled allergens (8). However, we have limited knowledge of the mechanisms, the factors, and cell types involved in the development of these IgE-promoting Tfh cells.
The group 2 innate lymphoid cells (ILC2s) serve as a robust source of type 2 cytokines, particularly in the settings of innate immunity (9). ILC2s are found at epithelial barrier surfaces, such as lungs and intestine, where they play roles in mucosal inflammation, tissue homeostasis, and repair (10, 11). More recent studies suggest that ILC2s may promote and/or regulate adaptive immunity (12, 13). Studies also suggest that ILC2s are important in the development of human diseases (11). Indeed, dysregulated ILC2s have been implicated in allergic rhinitis, asthma, and chronic rhinosinusitis with nasal polyps (11). However, it remains to be determined whether ILC2s contribute to the regulation of humoral immunity and production of IgE antibodies.
This study investigated the immune responses that contribute to Tfh development and peanut allergen sensitization. Peanut proteins are readily detected in household dust at levels comparable to that of inhaled allergens like the house dust mite (14, 15). A dose-response relationship has been observed between environmental peanut exposure and the risk of peanut allergy (15, 16). Based on these pieces of epidemiologic evidence, we previously established a mouse model for peanut allergies by exposing naïve wild-type (WT) mice to peanut flour through the airways without any exogenous adjuvants (7). In this study, we found that IL-13 is necessary for the production of allergen-specific IgE antibodies. Furthermore, lung ILC2s that respond to IL-1 are likely to be the major and early source of IL-13 that promotes Tfh cell development and the sensitization process to peanuts.
MATERIALS AND METHODS
Mice
BALB/c, C57BL/6 (B6), B6.129S7-Il1r1tm1Imx/J (Il1r1−/−), B6.C3(Cg)-Rorasg/J (Rorasg/sg), B6.SJL-PtprcaPepcb/BoyJ (CD45.1), and C.129S7(B6)-Rag1tm1Mom/J (Rag1−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Il13egfp/egfp mice (IL-13-deficient mice) (17, BALB/c background) and Rorafl/flIl7r-Cre mice (13, B6 background) were maintained in specific pathogen-free conditions at Mayo Clinic (Rochester, MN). ILC2-deficient mice were generated by reconstituting lethally irradiated CD45.1 mice with 2–3 million bone marrow (BM) cells isolated from WT or Rorasg/sg littermates (18). Animals used in this study were female and ranged from 7 to 12 weeks of age. All protocols and procedures for the handling of the mice were reviewed and approved by the Mayo Institutional Animal Care and Use Committee, Mayo Clinic.
Allergens
Peanut flour was purchased from the Golden Peanut Company (Alpharetta, Ga), endotoxin was undetectable (<0.5 EU/mg flour) as previously described (7). Crude peanut extract (7) and Alternaria alternata extract (19) were purchased from Greer Laboratories (Lenoir, NC). Recombinant IL-1α was purchased from R&D Systems (Minneapolis, MN), and recombinant IL-13 was purchased from Biolegend (San Diego, Ca).
Mouse airway exposure
Naïve mice were lightly anesthetized using isoflurane, and exposed intranasally (i.n.) to 100 μg peanut flour in 50 μl sterile PBS or PBS alone, as previously described (7). For plasma antibody analysis, mice were exposed twice, 7 days apart, on days 0 and 7. Four weeks after the exposure, mice were lightly anesthetized with isoflurane for retro-orbital blood collection to analyze peanut-specific antibody levels. Shorter-term analyses of lungs or mediastinal lymph nodes (mLN) from peanut flour- or PBS-exposed mice were treated as described in the figure legends.
To determine the kinetics of plasma antibody after peanut flour or Alternaria exposure, naïve mice were exposed i.n. with 100 μg peanut flour plus 10 μg of endotoxin-free OVA, 100 μg Alternaria extract plus 10 μg of endotoxin-free OVA 19), 10 μg of OVA alone, or PBS once a week for 6 weeks. All exposure conditions were in a final volume of 50 μl PBS. Mice were bled retro-orbitally every 2 weeks under isoflurane anesthesia conditions for analysis of OVA-specific immunoglobulin levels.
For airway exposure to IL-13 plus OVA, naïve mice were exposed once with 100 ng IL-13 (Biolegend, San Diego, CA) plus 1 mg OVA in 50 μl PBS, 1 mg OVA alone, 100 μg peanut flour, or PBS. Four days later, mice were euthanized, and mLN were collected.
For airway exposure to IL-1α (R&D Systems, Minneapolis, MN), mice were exposed once i.n. with 10 ng IL-1α in 50 μl PBS, or PBS alone. Mice were euthanized 4.5 hours after the exposure, and lungs were collected. Alternatively, mice were exposed once i.n. with 50 μg or 100 μg Alternaria extract or 100 μg peanut flour in 50 μl PBS, and they were euthanized 3 hours or 6 hours later to collect lung specimens.
In vivo ILC2 depletion
To deplete ILC2s from mice, female Rag1−/− mice were administered intraperitoneally (i.p.) with either 250 μg anti-mouse Thy1.2 (30H12) or rat IgG2b isotype control (BioXCell, West Lebanon, NH) and simultaneously received i.n. dose at either 1/4 or 1/3 the i.p. dose 4 days or 2 days prior to exposure to peanut flour. Three hours after the exposure, mice were euthanized and lungs were collected.
Cytokine production in vitro
Day 11 mLN cells from WT or IL-13-deficient mice were cultured (400,000 cells/well) in complete RPMI medium (200 μl/well) with 100 μg/ml crude peanut extract for 4 days. Concentrations of IL-4 and IL-21 were measured using commercial ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
ELISA
Peanut- and OVA-specific IgE, IgG1, and IgG2a antibody levels in plasma specimens were analyzed by ELISA as previously described (7, 19). The levels of peanut- and OVA-specific IgG2b were analyzed similarly to peanut-specific IgG2a using anti-mouse IgG2b detection antibody (Invitrogen, Carlsbad, Ca).
For cytokine ELISA, lungs were processed as described previously (7, 20). Concentrations of IL-5 and IL-13 were determined from lung homogenates using commercial ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
FACS analysis
T- and B-cell populations from mLNs were analyzed by fluorescence-activated cell sorting (FACS) analysis, as described with minor modifications (19). CD45.2 antibody (Biolegend, San Diego Ca), and viability stain (Tonbo, San Diego Ca) were added to the stain to increase the accuracy of stained populations. DC populations from mLNs were analyzed by using previously described staining procedure (21) with antibodies against CD45.2, MHCII, CD11c, CD40, CXCR5, and CD11b (Biolegend, San Diego Ca). ILC2 populations were analyzed as described previously (20) with the additional stains to enhance specificity. Antibodies used were Lineage Antibody Cocktail (BD Biosciences, Franklin Lakes, NJ) and antibodies against CD45.2, CD4, CD8, CD19, CD11c, DX5, CD16/32, KLRG1, IL-1RL1(ST2), CD44 (Biolegend, San Diego, Ca), CD25 (BD Biosciences, Franklin Lakes, NJ), and ST2 (MD Biosciences (Oakdale MN). FACS analysis was performed on either a Canto X cytometer or a Fortessa X-20 cytometers (BD Biosciences, San Jose, Calif). Data were analyzed with FlowJo software (Tree Star, Ashland, Ore).
Statistical analysis
Data are presented as mean ± SEMs for the number of mice or experiments indicated. Statistical significance of differences between the various treatment groups was assessed by using a Student’s t-test (for two group comparison) or ANOVA with post-hoc Tukey test (for multiple group comparison). A p-value of less than 0.05 was considered statistically significant.
RESULTS
IL-13 is required for the production of peanut-specific IgE
To mimic environmental exposure to peanut allergens in house dust (14–16), we previously established a mouse model of peanut allergy in which naïve wild-type (WT) BALB/c or C57BL/6 mice are exposed intranasally (i.n.) to peanut flour (without exogenous adjuvants) weekly for 4 weeks (7). Exposed mice produced peanut-specific IgE antibodies, and importantly both production of peanut-specific IgE antibodies and acute anaphylaxis were dependent on Tfh cells rather than conventional Th2 cells (7). We extended these previous studies and investigated the mechanisms that initiate the development of Tfh cells and IgE-antibody production. Naïve WT BALB/c mice were exposed i.n. to peanut flour only twice, on days 0 and 7, and antibody plasma levels were examined on day 34 (Fig. 1A). These mice produced peanut-specific IgE antibodies, as shown by a titration curve of individual plasma samples (Fig. 1B, black circles). The animals also produced IgG1, IgG2a, and IgG2b isotypes of peanut-specific antibodies.
Figure 1.
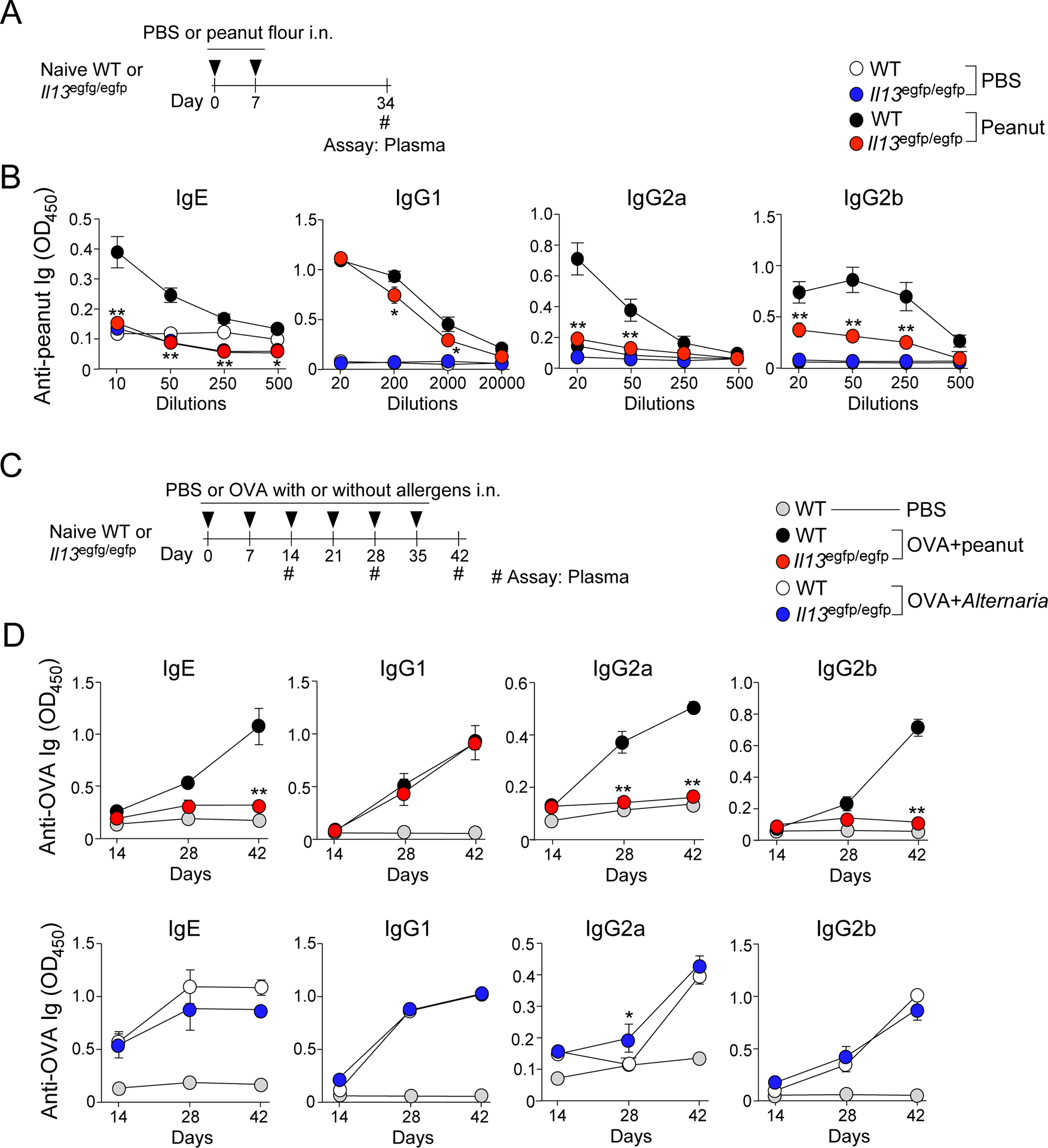
IL-13 is necessary for the production of peanut-specific IgE, IgG2a, and IgG2b antibodies. A. Wild-type (WT) and Il13egfp/egfp (IL-13-deficient) BALB/c mice were intranasally (i.n.) exposed to peanut flour on days 0 and 7. Blood was assessed for peanut-specific antibodies on day 34. B. Day 34 blood antibody titers for peanut-specific IgE, IgG1, IgG2a, and IgG2b from IL-13-deficient or WT mice described in Fig. 1A. C. WT and IL-13-deficient mice were exposed i.n. to peanut flour + OVA, Alternaria extract + OVA, or PBS once a week for 6 weeks. Blood samples were taken every 2 weeks and assessed for peanut-specific antibodies. D. Kinetics of plasma antibody titers for peanut-specific IgE, IgG1, IgG2a, and IgG2b from IL-13-deficient or WT mice described in Fig. 1C. Data represent two experiments using three mice per group. Error bars represent mean ± SEM. *P<0.05 and **P<0.01 compared to WT mice treated similarly.
After antigenic stimulation, signals from IL-4 or IL-13 from conventional Th2-type CD4+ T cells are considered necessary for the IgE class switch in B cells (22). IL-13 also affects the production of other Ig subtypes (23, 24). Therefore, to examine whether IL-13 contributes to the development of peanut-specific IgE antibodies, we exposed naïve IL-13-deficient (Il13egfp/egfp) BALB/c mice i.n. to peanut flour by using the protocol described in Fig. 1A. IL-13-deficient mice showed an approximately 50-fold decrease in the plasma levels of peanut-specific IgE antibodies compared to WT mice (Fig. 1B, red circles). Similar reductions in antibody levels were observed in IgG2a and IgG2b isotypes, and slight decrease was observed in the IgG1 isotype.
We next examined whether the requirement for IL-13 is reproduced in the model when mice are exposed to allergens multiple times for a prolonged period to mimic exposure in humans. We also addressed whether IL-13 deficiency might result in a global defect in antibody production. Weekly for up to 6 weeks, WT mice or IL-13-deficient mice were exposed i.n. to ovalbumin (OVA) with either an extract of the common fungal allergen, Alternaria (4), or peanut flour (Fig. 1C). As reported previously (19), we used this strategy to spike these allergens with OVA and analyze humoral immune responses to the OVA antigen in order to compare the immunologic responses to Alternaria extract and peanut flour by minimizing the variability in the antigenicity of these allergens and to overcome the technical difficulty in detecting Alternaria-specific IgE antibodies in mouse plasma. Consistent with the shorter exposure model (Fig. 1B), the IL-13-deficient mice exposed to peanut flour had significantly decreased plasma levels of OVA-specific IgE, IgG2a, and IgG2b antibodies compared to WT mice (p<0.05); the levels of OVA-specific IgG1 antibodies were roughly comparable between the two strains (Fig. 1D). In contrast, mice exposed to Alternaria extract showed no apparent differences for any isotypes between WT and IL-13-deficient mice. These findings suggest that IL-13-deficient mice are not intrinsically impaired for humoral immunity and that the dependency of IgE antibody production on IL-13 likely reflects the biochemical properties of allergens.
Tfh cells and germinal center B cells are reduced in IL-13-deficient mice
We and others found previously found that the production of IgE antibodies in response to peanut exposure is dependent on Tfh cells, but not on conventional Th2 cells (7, 8). To elucidate the roles for IL-13 in the development of Tfh cells, WT or IL-13-deficient mice were exposed i.n. to peanut flour on days 0 and 7, and the cellular composition of the lung dLNs, namely mLNs, was examined on day 11 (Fig. 2A). IL-13-deficient mice exposed to peanut flour demonstrated a marked reduction in overall cellularity compared to exposed WT mice (Fig. 2B). FACS analyses showed ~70% reduction in the total numbers of CD3+CD4+ T cells and B220+ B cells in IL-13-deficient mice relative to WT mice. In contrast, when exposed to peanut flour, the IL-13-deficient mice developed distinct populations of mature CXCR5+PD-1+ Tfh cells (Fig. 2C) and FAS+PNA+ GC B cells (Fig. 2D) that were roughly comparable to those in WT mice. Indeed, when quantified, no differences were observed in the frequency of Tfh cells and GC B cells between IL-13-deficient and WT mice. Nonetheless, the total numbers of Tfh cells and GC B cells in mLNs were decreased by 60–70% in IL-13-deficient mice due to the decreased total cellularity of the mLNs in these mice.
Figure 2.
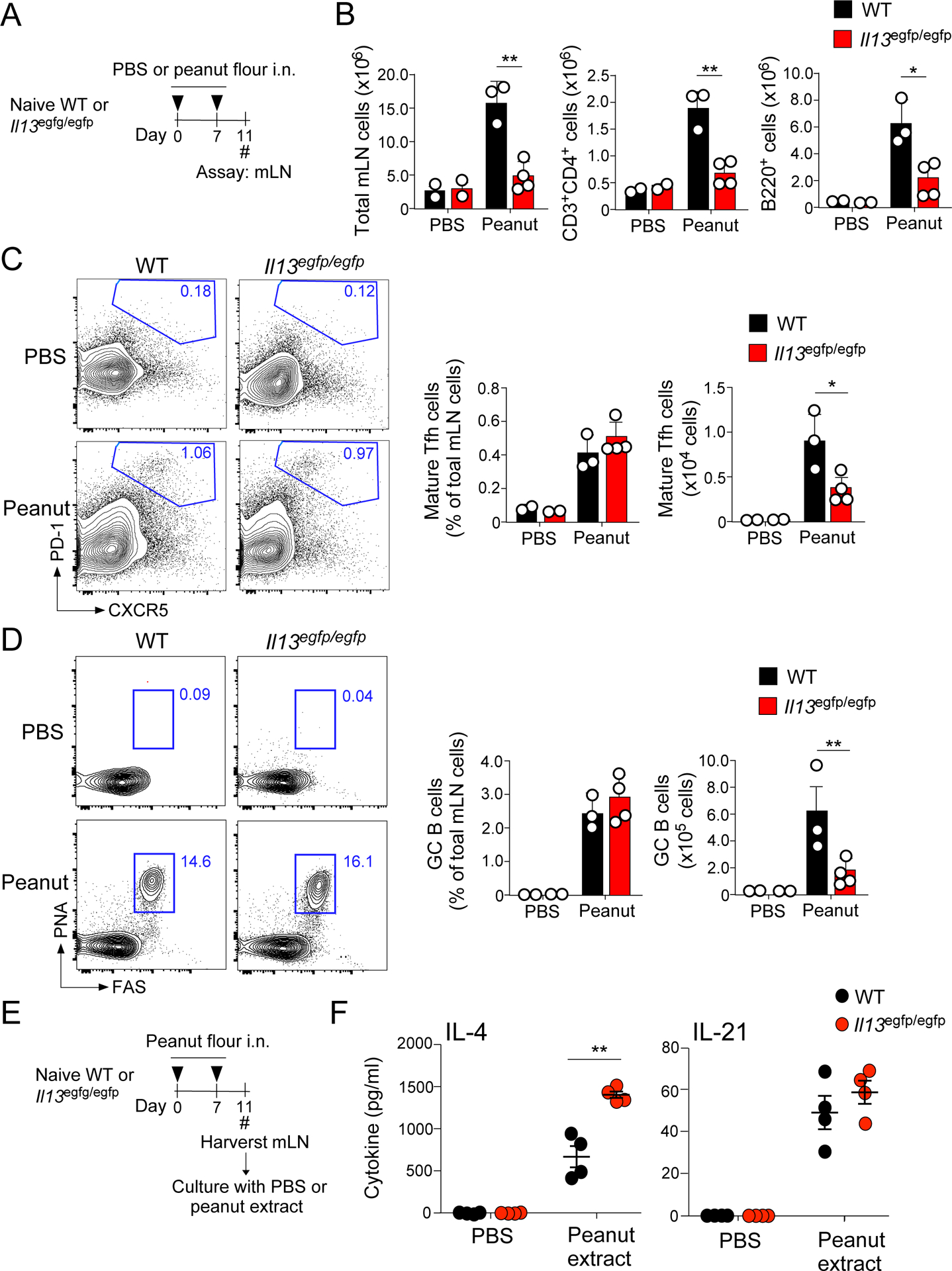
Functional Tfh and germinal center (GC) B cells develop at significantly lower numbers in IL-13-deficient mice. A. WT and IL-13-deficient BALB/c mice were exposed i.n. to peanut flour or PBS twice (on days 0 and 7) and mediastinal lymph nodes (mLN) were analyzed on day 11. B. Day 11 mLN cell counts of total mLNs, CD3+CD4+ T cells, and B220+ B cells. C. Sample flow plots and analysis of mature Tfh cells (CD3+CD4+CXCR5+PD-1+). Cells were gated on the CD45+CD3+CD4+ cell population. D. Sample flow plots and analysis of GC B cells (B220+ PNA+FAS+). Cells were gated on the CD45+B220+ cell population. E. WT and IL-13-deficient BALB/c mice were exposed i.n. to peanut flour or PBS (on days 0 and 7), and mLNs were collected on day 11. The cells were normalized at 2×106 cells/ml, cultured with PBS or crude peanut extract, and assessed for Tfh cytokines. F. IL-4 and IL-21 analysis from samples described in Fig. 2E. Error bars represent mean ± SEM. *P<0.05 and **P<0.01 between the groups indicated by horizontal lines. Each circle represents individual mouse.
To examine whether the functions of Tfh cells are affected in the absence of IL-13, we analyzed their capacity to produce B cell-stimulating cytokines, including IL-4 and IL-21 (25). Mice were exposed i.n. to peanut flour on days 0 and 7, and day 11 mLNs were cultured with crude peanut extract in vitro (Fig. 2E). To compensate for the decreased total cellularity of mLNs in IL-13-deficient mice, we normalized the cell numbers to 2.0×106 cells/ml. The mLN cells from WT mice produced IL-4 and IL-21 in an antigen-specific manner (Fig. 2F). The capacity to produce IL-4 and IL-21 was not compromised in mLN cells from IL-13-deficient mice. Rather, IL-13-deficient mice produced significantly more IL-4 compared to WT mice (Fig. 2F, p<0.01). These findings suggest that IL-13 likely affects cellularity of the Tfh and GC B cell populations but is probably not involved in the differentiation or function of Tfh cells. This interpretation is also consistent with the fact that IL-13-deficiency does not affect the levels of peanut-specific IgG1 antibodies (Fig. 1), which is also dependent on Tfh cells (7).
IL-13 is indispensable for generating CD11c+MHCIIhi dendritic cells
IL-13 is known to induce MHCII expression on macrophages, and IL-13 can affect the dynamics of dendritic cells (DCs) in mice exposed i.n. to a cysteine protease papain (10, 12). Given the decreased numbers of Tfh cells and GC B cells in IL-13-deficient mice, we hypothesized that IL-13 affects the dynamics of DCs. To test this, WT and IL-13-deficient mice were acutely exposed i.n. to peanut flour on days 0 and 1, and mLNs were examined on day 4 (Fig. 3A). In WT mice, the overall cellularity of mLNs increased in mice that were exposed to peanut flour compared to mice exposed to PBS (Fig. 3B, p<0.01). In contrast, in IL-13-deficient mice, no increase in mLN cellularity was observed, suggesting a vital role for early IL-13 in initiating innate immunity in response to peanut flour exposure.
Figure 3.
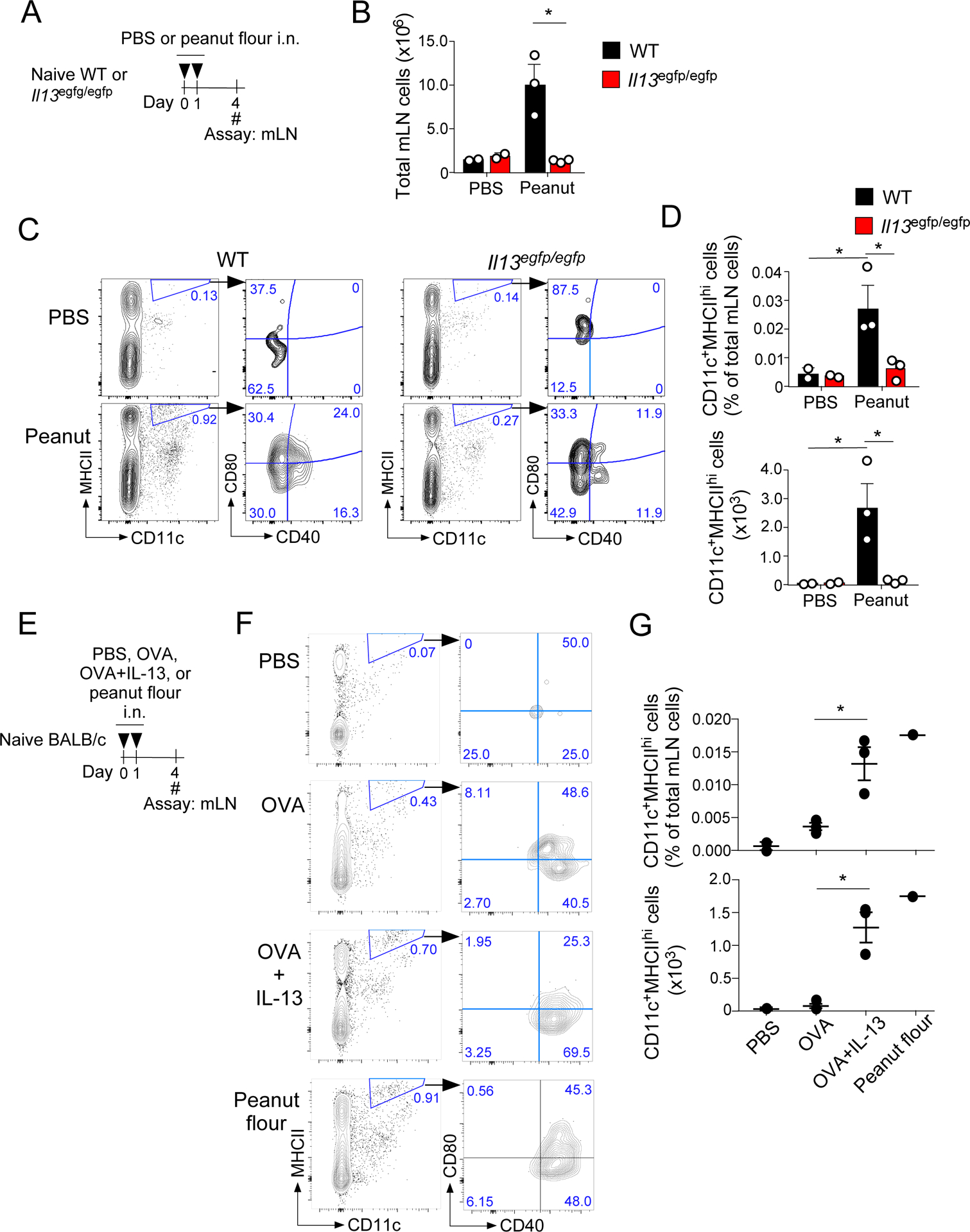
IL-13 deficiency results in reduced CD11c+MHCIIhi cells in mLNs after peanut flour exposure. A. WT and IL-13-deficient BALB/c mice were exposed i.n. to peanut flour or PBS on days 0 and 1. On day 4, mLN were harvested and analyzed by flow cytometry. B. Data represent total numbers of mLN cells in each treatment group. C. Representative gating strategy for each experimental group, preceded by gating on the CD45+, singlet, living, high FSC-A/SSC-A cell population. D. Data represents frequency and cell counts for the CD11c+MHCIIhi population. E. WT BALB/c mice were exposed i.n. to IL-13+OVA, OVA alone, peanut flour, or PBS for on days 0 and 1. On day 4, mLNs were harvested and analyzed by flow cytometry. F. Representative gating strategy for each experimental group, preceded by gating for CD45+, singlet, living cells G. Data represent frequency and cell counts of the CD11c+MHCIIhi population. Data are representative of two experiments of 2–4 mice each. *P<0.05 between the groups indicated by horizontal lines. Each circle represents individual mouse.
CD11c+MHCIIhi DCs have been associated with a migratory DC subtype that promotes Tfh cell differentiation (21). FACS analysis showed that CD11c+MHCIIhi DCs increased in mLNs of WT mice exposed to peanut flour (Fig. 3C); both the frequency and number of CD11c+MHCIIhi DCs increased significantly in WT mice exposed to peanut flour compared to mice exposed to PBS (Fig. 3D, p<0.05). In contrast, no increase in CD11c+MHCIIhi DCs was observed in IL-13-deficient mice (Fig. 3C and 3D). Furthermore, expression of co-stimulatory molecules, including CD40 and CD80, was also reduced in IL-13-deficient mice compared to WT mice that had been exposed to peanut flour (Fig. 3C). Additional FACS analysis of mLN from WT mice exposed to peanut flour reveal that the CD11c+MHCIIhi cell population also express CD11b+ and that ~50% of them express GC-homing receptor CXCR5 (Supplemental Fig. 1). We also compared the impact of IL-13 deficiency in mice exposed to peanut flour and Alternaria extract. When exposed to peanut flour, the frequency and total number of CD11c+MHCIIhi DCs significantly decreased in IL-13-deficient mice as compared to WT mice (Supplemental Fig. 2, p<0.05 and p<0.01). In contrast, no decrease in CD11c+MHCIIhi DCs was observed in IL-13-deficient mice when they were exposed to Alternaria extract, suggesting that the DC response induced by peanut flour is more strongly dependent on IL-13 than that induced by Alternaria extract.
To determine if IL-13 is sufficient to induce CD11c+MHCIIhi DCs, naïve WT mice were exposed i.n. to OVA alone, a combination of OVA plus IL-13 or peanut flour on days 0 and 1 (Fig. 3E). On day 4, as expected, mice exposed to peanut flour showed an increase in CD11c+MHCIIhi DCs that highly express CD40 and CD86 (Fig. 3F and 3G, Supplemental Fig. 3). Mice exposed to OVA plus IL-13 also showed a significant increase in CD11c+MHCIIhi DCs as compared to those mice exposed to OVA alone or PBS (p<0.01), suggesting that IL-13 in the presence of antigen is sufficient to induce the DC population. Altogether, these findings suggest that upon airway exposure to peanut flour, IL-13 is essential for mobilization of CD11c+MHCIIhi DCs to mLNs, which likely facilitate subsequent development of Tfh cells and GC B cells.
Lung ILC2s are the major source of IL-13 in response to peanut flour exposure
The prominent immunologic outcomes of IL-13-deficient mice motivated us to identify the cellular source of IL-13. To this end, naïve BALB/c mice were exposed i.n. to peanut flour once (Fig. 4A). Lung levels of IL-13 significantly increased in mice exposed to peanut flour compared to those exposed to PBS 3 hours post-exposure (Fig. 4B). At 6 hours post-exposure, the levels of IL-13 returned toward those of naïve mice. When naïve BALB/c mice were exposed to Alternaria extract, high concentrations of both IL-5 and IL-13 proteins were detected in lung homogenates at 3 hours, which remained detectable 6 hours post-exposure (Supplemental Fig. 4).
Figure 4.
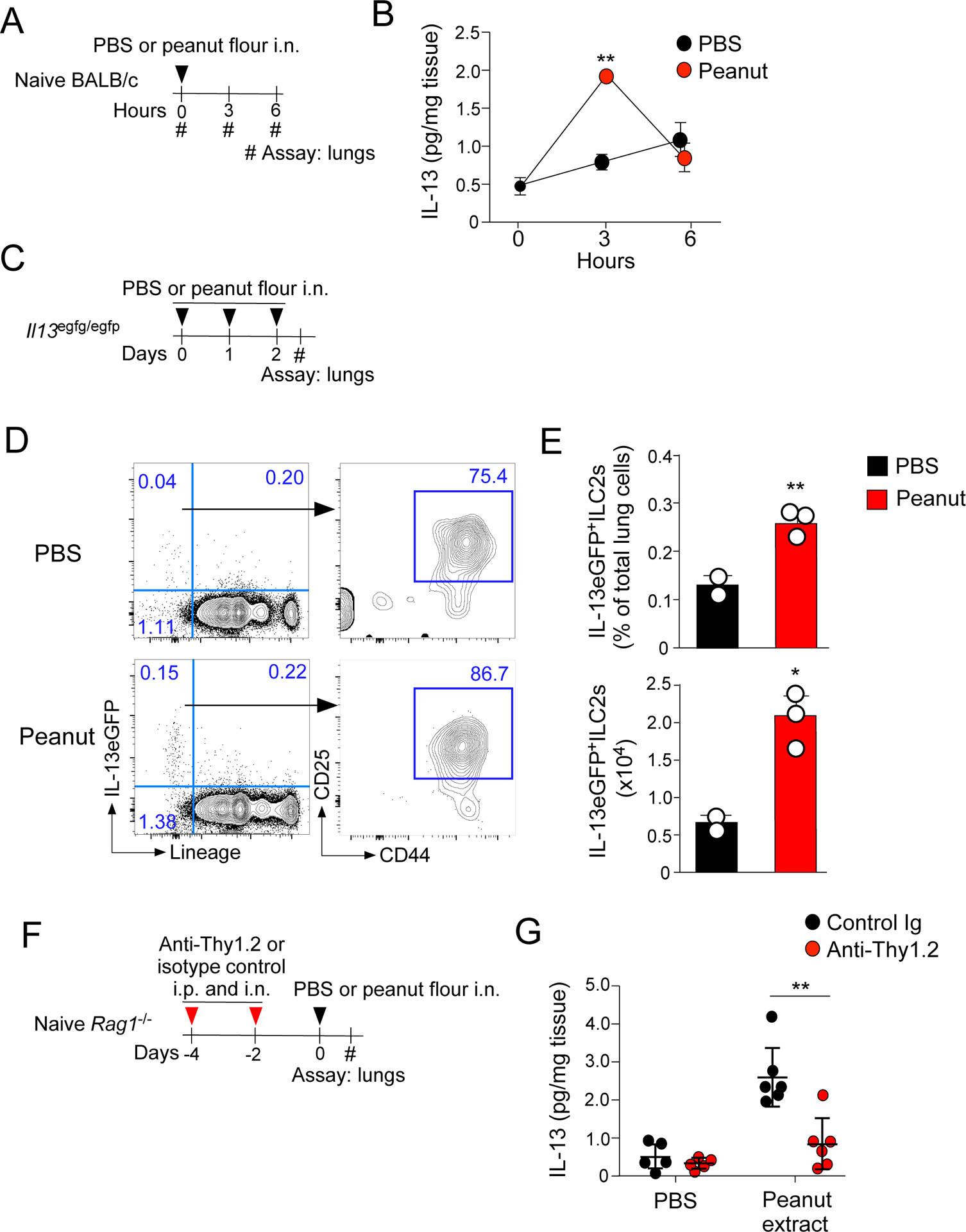
ILC2s are the primary source of IL-13 response immediately after airway PN exposure. A. WT BALB/c mice were exposed once to peanut flour or PBS, and lungs were harvested 3 or 6 hours later. B. IL-13 levels from lung lysate were assessed by ELISA. **P<0.01 compared to the mice exposed to PBS. C. Il13egfp/egfp mice were exposed i.n. to peanut flour or PBS for 3 consecutive days; in these experiments, Il13egfp/egfp mice were used to report IL-13 expression. Lungs were harvested after 24 hours and analyzed by flow cytometry. D. Representative gating strategy for each treatment group, preceded by gating for CD45+, singlet, living cells. E. Frequency and cell number of IL13eGFP+ ILC2s. Data are representative of two experiments for two PBS-exposed and four peanut flour-exposed Il13gfp/gfp mice. *P<0.05 and **P<0.01 compared to the mice exposed to PBS. F. To deplete ILC2s, Rag1−/− BALB/c mice were treated i.p. and i.n. with anti-Thy1.2-depleting antibody or isotype-control antibody on days −4 and −2. Mice were then exposed once i.n. to peanut flour or PBS. Three hours later, lungs were harvested and processed for ELISA analysis. G. IL-13 levels from lung lysates were assessed by ELISA. Data are representative of two experiments of 2–6 mice each. **P<0.01 between the groups indicated by horizontal lines. Error bars represent mean ± SEM. Each circle represents individual mouse.
Because the increase in IL-13 in naïve BALB/c mice was rapid and transient when mice were exposed to peanut flour, we suspected the involvement of lung ILC2s, which generally release type 2 cytokines quickly after airborne exposure to allergens or environmental insults (26). Although several cellular markers can be used to identify lung ILC2s, they are typically characterized by the lack of lineage markers (lineage− or lin−) as well as high expression of CD25 and CD44 (27). IL-13 reporter mice (Il13egfp/egfp) were exposed to peanut flour twice on days 0 and 1, and the lungs were analyzed 24 hours after the last exposure (Fig. 4C). We detected a significant increase in IL-13eGFP, primarily within the lineage− populations that highly expressed both CD25 and CD44, consistent with lung ILC2s (Fig. 4D and 4E, p<0.01 and p<0.05). No other cell populations, including any lineage-positive populations, appeared to express IL-13eGFP above background.
To verify the contribution of ILC2s in IL-13 production, we depleted the ILC2 population from Rag1−/− mice (deficient in mature T cells) by treating them with anti-Thy1.2 antibody (Fig. 4F) as previously published (28). When exposed i.n. to peanut flour, Rag1−/− mice produced comparable levels of IL-13 (Fig. 4G) as WT mice (Fig. 4B). Furthermore, depletion of ILC2s by anti-Thy1.2 antibody resulted in a significant decrease in the lung levels of IL-13 (Fig. 4G, p<0.01), suggesting that ILC2s provide an acute and major source of IL-13 in mice exposed to peanuts.
IL-13 release by ILC2s is dependent on IL-1R1 signaling
We previously found that airway sensitization to peanuts and production of IgE antibodies are dependent on signaling from the IL-1-family cytokines, in particular IL-1α/β (7). Therefore, we investigated whether IL-1α can induce IL-13 production from ILC2s. A significant increase in the lung levels of IL-5 and IL-13 were observed 4.5 hours after i.n. administration of IL-1α to naïve BALB/c mice (Fig. 5A and 5B). Furthermore, the lung levels of IL-13 after the peanut exposure were partially, but significantly, decreased in Il1r1−/− mice (Fig. 5D, p<0.05), suggesting that IL-1 is involved in IL-13 production by ILC2s.
Figure 5.
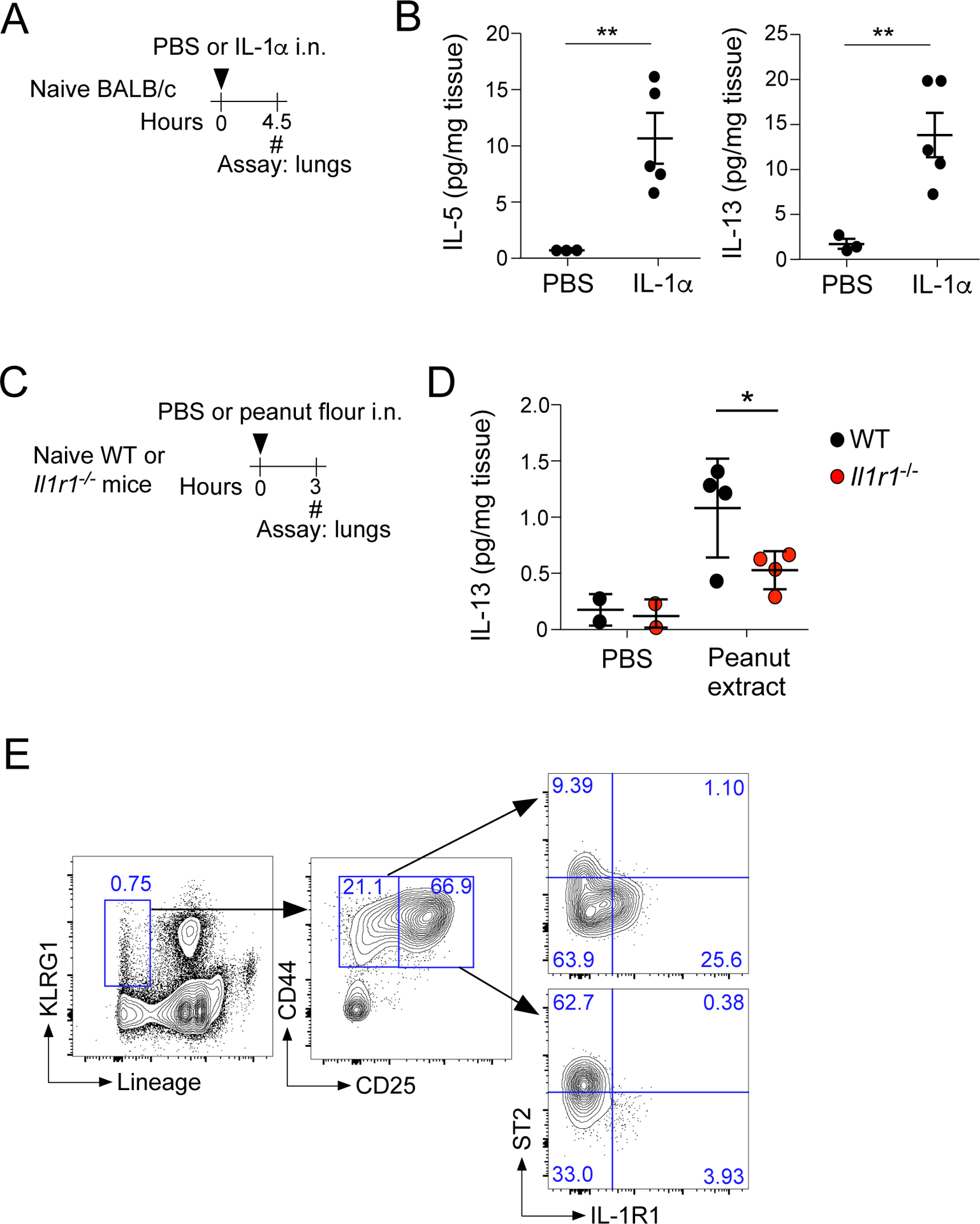
IL-13 release after peanut flour exposure is dependent on IL-1R1 signaling. A. Naïve BALB/c mice were exposed once i.n. to IL-1α or PBS, and lungs were harvested and analyzed 4.5 hours later. B. IL-5 and IL-13 levels measured by ELISA from lungs described in A. **P<0.01 between the groups indicated by horizontal lines. C. WT (C57B6/J) or Il1r1−/− mice were exposed once to peanut flour or PBS, and lungs were harvested 3 hours later. D. IL-13 levels from lung lysates were assessed by ELISA from mice described in C. *P<0.05 between the groups indicated by horizontal lines. E. Lungs from naïve mice were harvested and analyzed for lineage, KLRG1, CD44, and CD25 by flow cytometry. Gates were based on the CD45+, singlet, living cell population.
A recent study suggests that two populations of ILC2s are present in adult mouse lungs (29). IL-1α signal through a heterodimer composed of a common receptor, IL-1RAcP, and cytokine-specific receptors including IL-1R1 (for IL-1α and β) (30). KLRG1 expression on lineage− cells is a common gating strategy to identify mature ILC2s in mucosal tissues (31). Gating on the Lin−KLRG1+ population, two major cell populations of ILC2s were revealed based their expression levels of CD25 (Fig. 5E). The Lin− KLRG1+ CD44+CD25low population contained ST2+IL-1R1−ILC2s and ST2−IL-1R1+ILC2s while the Lin− KLRG1+ CD44+CD25high population mainly consisted of ST2+IL-1R1− ILC2s.
ILC2s are indispensable for the production of peanut-specific IgE
Given the IL-13 production by ILC2s in naïve mice exposed to peanut flour, we finally examined the contribution of ILC2s to generating peanut-specific IgE antibodies by using two models for ILC2-deficient mice. First, naïve C57BL/6 mice were lethally irradiated and reconstituted with bone marrow (BM) cells from WT or Rorasg/sg mice for 6 weeks as previously described (both C57BL/6 mice) (12, 32). Lung ILC2s were nearly absent in the lungs of mice reconstituted with Rorasg/sg BM, while CD4+ T cells in spleen were preserved (Fig. 6A and 6B). The mice that had received WT or Rorasg/sg BM were exposed i.n. to peanut flour twice, 7 days apart, and examined 4 weeks later similarly to Fig. 1A (Fig. 6C). While the mice reconstituted with WT BM produced a robust peanut-specific IgE, the IgE antibody levels were significantly reduced approximately 15-fold in mice that received Rorasg/sg BM (Fig. 6D, p<0.05). The plasma levels of IgG2a and IgG2b antibodies decreased over 20-fold and approximately 15-fold, respectively, in mice deficient in ILC2s.
Figure 6.
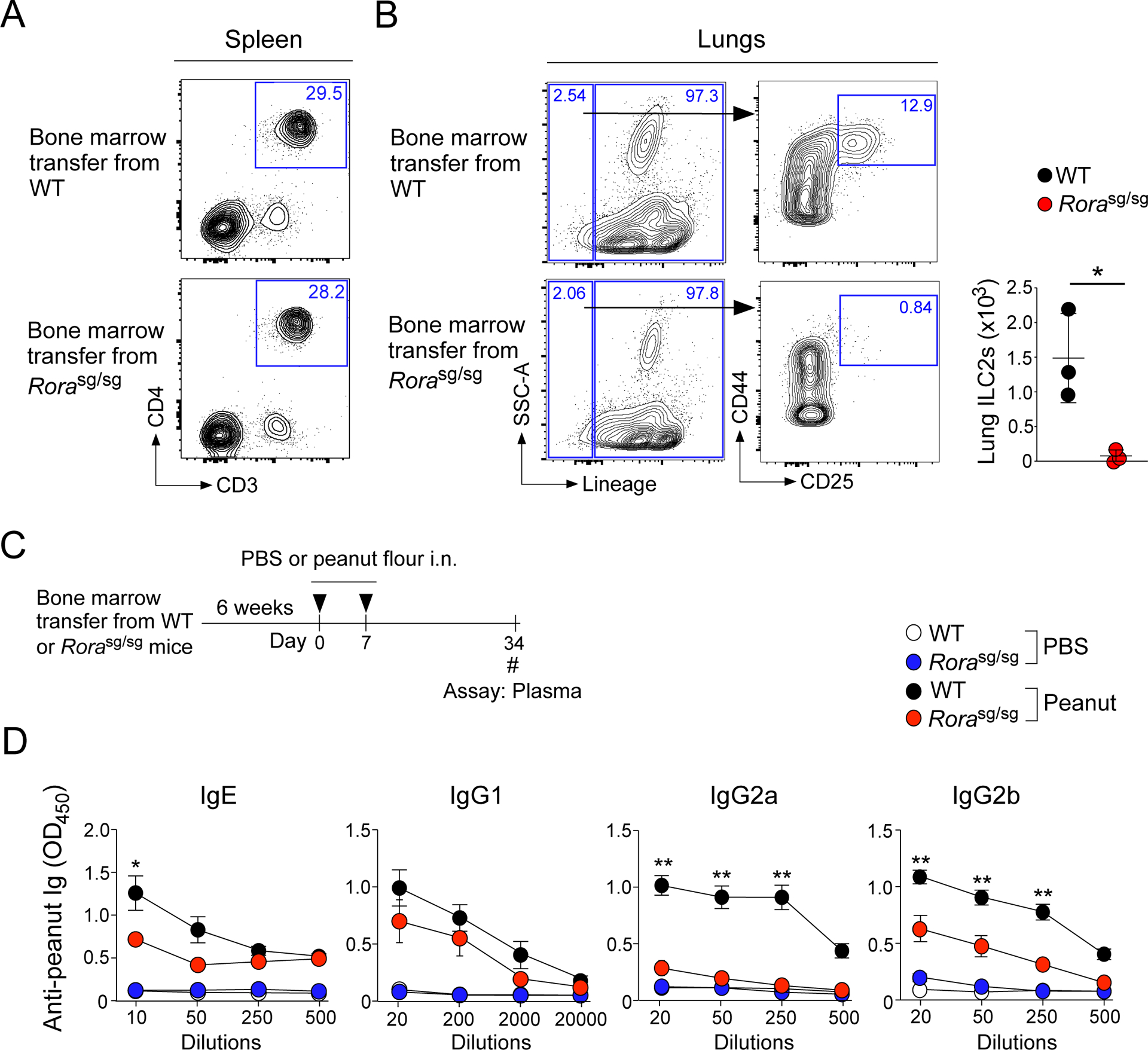
ILC2s are necessary for the production of peanut-specific-specific IgE, IgG2a and IgG2b antibodies. A and B. Verification of ILC2-deficiency in the bone marrow transfer model. ILC2-deficient mice were generated by reconstituting lethally irradiated CD45.1 congenic C57BL/6 mice with 2–3 million bone marrow cells isolated from WT or Rorasg/sg C57BL/6 littermates. The spleen (A) and lungs (B) of the mice were harvested 6 weeks later and analyzed by FACS. C. Naïve congenic C57BL/6 mice that had received WT and Rorasg/sg bone marrow were i.n. exposed to peanut flour or PBS twice, one week apart. Four weeks later, plasma was assessed for peanut-specific Igs. D. Day 34 blood antibody titers for peanut-specific IgE, IgG1, IgG2a, and IgG2b from mice described in Fig. 6C. Error bars represent mean ± SEM. Data are representative of two experiments using 3–7 mice each. *P<0.05 and **P<0.01 compared to mice received Rorasg/sg bone marrow cells and exposed to peanut flour.
Second, we verified the observation by using a genetic model. Previously, conditional deletion of Rora by Il7r-driven Cre (Rorafl/flIl7r-Cre) generated mice that are specifically deficient in ILC2s (13). Rorafl/flIl7r-Cre mice showed decreased frequency of CD11c+MHCIIhi DCs in mLNs as compared to Rorafl/fl littermate control mice (no Cre) when they were exposed to peanut flour (Supplemental Fig. 5). Importantly, the plasma levels of peanut-specific IgE antibody were significantly reduced in Rorafl/flIl7r-Cre mice as compared to control mice (Figure 7, p<0.01). Furthermore, the plasma levels of peanut-specific IgG1 and IgG2b antibodies also decreased in Rorafl/flIl7r-Cre mice. Altogether, these findings suggest that ILC2s are indispensable for IgE antibody production to peanut allergens.
Figure 7.
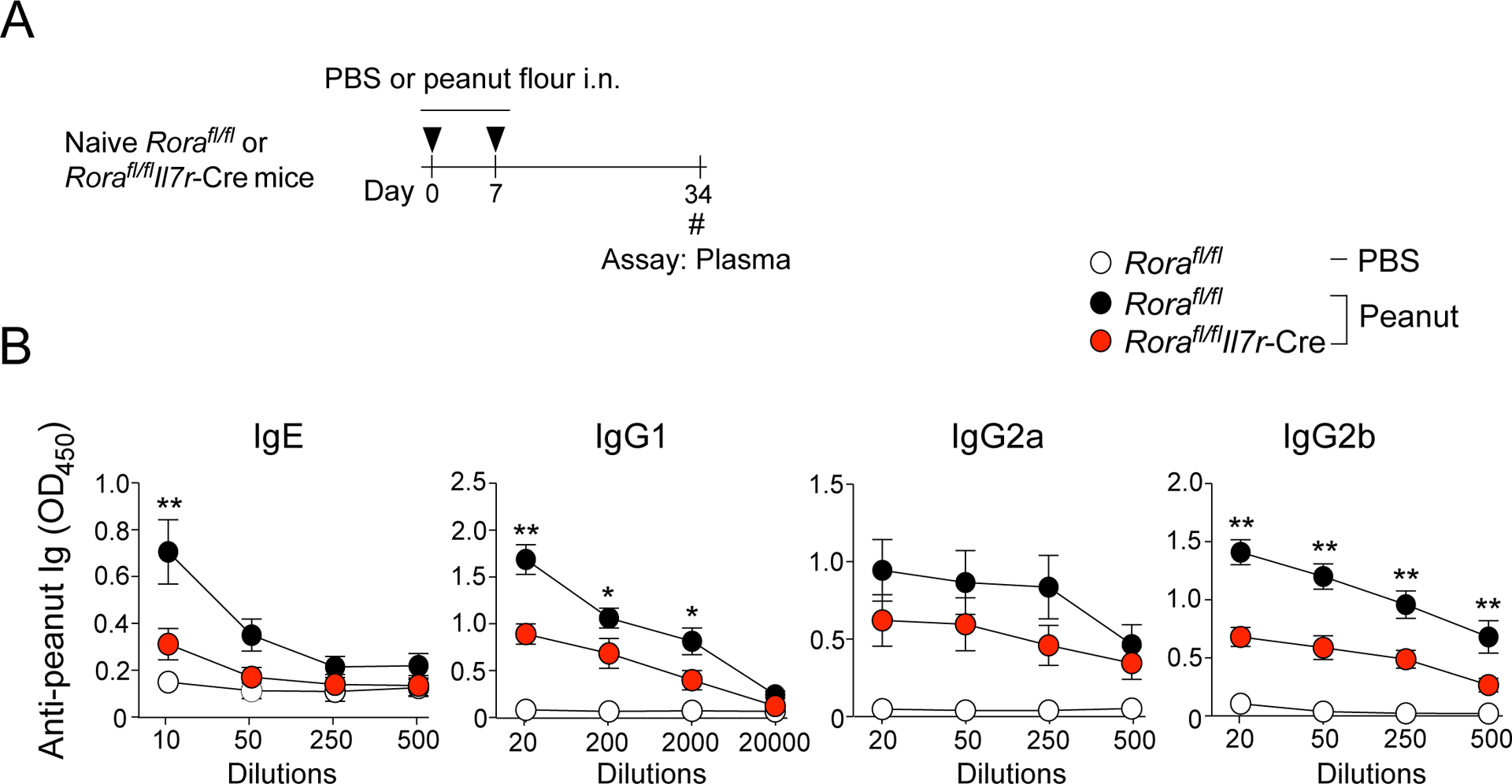
Mice deficient in ILC2s produce less peanut-specific IgE antibodies. A. Naïve Rorafl/flIl7r-Cre mice or Rorafl/fl littermate control mice were i.n. exposed to peanut flour or PBS twice, one week apart. Four weeks later, plasma was assessed for peanut-specific Igs. B. Day 34 blood antibody titers for peanut-specific IgE, IgG1, IgG2a, and IgG2b from mice described in Fig. 7A. Error bars represent mean ± SEM. Data are presented as a pool from two independent experiments, a total of 6–9 mice in each group. *P<0.05 and **P<0.01 compared to Rorafl/flIl7r-Cre mice exposed to peanut flour.
DISCUSSION
Our knowledge of the immunological processes responsible for IgE-type sensitization to peanut allergens is limited. Early work demonstrated that deletion of Th2 cell lineage-defining transcription factors, such as STAT6 or the prototypic Th2 cytokine IL-4, reduces the levels of total IgE antibodies (33, 34), leading to the notion that Th2 cells control the IgE antibody response. Nonetheless, more recent studies have demonstrated that Tfh cells, not conventional Th2 cells, are required for the production of allergen-specific IgE antibodies (4–6). However, the immunologic mechanisms involved in the development of these Tfh cells have been an enigma. We previously reported a model where naïve BALB/c mice exposed to peanut flour through the airways without any adjuvants became sensitized and developed acute and systemic anaphylaxis when challenged subsequently to peanut extract (7). In the current study, we expanded the model and clarified the sensitization process. One of the major findings included a novel role for IL-13-producing lung ILC2s that link innate immunity, Tfh cell and GC B cell expansion in dLNs, and IgE-type humoral immune response to inhaled peanut allergens. Furthermore, IL-13 was necessary and sufficient to mobilize CD11c+MHCIIhiDCs to the mLNs, which express GC-homing molecule CXCR5 and likely promote the development of Tfh cells (21).
Previous studies on the roles for IL-13 in IgE antibody production have been controversial. While IL-13-deficient mice had lower basal levels of serum IgE (35, 36), no differences were observed in serum levels of total IgE between IL-13-deficient mice and WT mice when they were infected with a helminth Nippostrongylus brasiliensis (35). Similarly, IL-13-deficient mice normally produced ovalbumin (OVA)-specific IgE antibody when they were immunized with intraperitoneal injection of OVA plus alum (37). In this study, we found that the need for IL-13 is likely to be dependent on the types of allergens because the IgE antibody production in response to the fungus Alternaria was not affected by IL-13-deficiency while that in response to peanut was nearly abolished (Fig. 1). Although investigation into the mechanisms to explain the difference is not a major focus of this study, we speculate that the pro-inflammatory environment that are created by exposure to Alternaria (38) likely activates lung DCs by several innate immune pathways, such as cytokines and toll-like receptors, potentially masking the contribution of IL-13. Indeed, Alternaria extract induced more robust and prolonged innate type 2 responses as compared to peanut flour (Supplemental Fig. 4). Furthermore, exposure to Alternaria extract mobilized CD11c+MHCIIhiDCs to the mLNs independently of IL-13 (Supplemental Fig. 2).
While the roles for ILC2s in innate immunity and tissue homeostasis have been well established (10), their roles in promoting or regulating adaptive immunity have only been recognized recently. For example, ILC2s and CD4+ Th2 cells may interact directly through cellular contact through MHCII and co-stimulatory molecules (27, 39, 40). Alternatively, ILC2s may indirectly promote adaptive immunity by activating DCs. For example, in mice exposed to protease papain, IL-13 derived from ILC2s induced DC migration to the lung dLNs, promoting differentiation of conventional Th2 cells and airway inflammation (12). Our observations add to this knowledge by linking ILC2s and humoral immunity. Indeed, ILC2-derived IL-13 significantly increased the pool of Tfh cells and GC B cells in dLNs, and facilitated the production of peanut-specific IgE antibodies. Conversely, depletion of ILC2s by using a BM transfer approach or a genetic approach significantly reduced the plasma levels of peanut-specific IgE.
The question remains as to how ILC2s are activated by exposure to peanut flour. Generally, epithelium-derived cytokines, such as IL-25, IL-33, and TSLP, activate ILC2s and initiate their production of type 2 cytokines (26). Nonetheless, no or minimal production of these epithelium-derived cytokines was observed in mice exposed to peanut flour (7). In the current study, we identified a subset of lung ILC2s that express IL-1 receptor IL-1R1 in the Lin−KLRG1+CD44+CD25low ILC2 population (Fig. 5). Furthermore, IL-1α alone was sufficient to induce IL-13 production, and IL-13 release in mice exposed to peanut was dependent on IL-1R1. Altogether, these observations are consistent with our previous findings where development of peanut-specific Tfh cells were dependent on IL-1R1 (7). Recently, two populations of ILC2s were identified in adult mouse lungs, including a novel IL-18Rα+ST2− population and a conventional IL-18Rα−ST2+ population (29). Therefore, depending on their subsets, the biological functions of ILC2s may include regulation of humoral immunity in mucosal organs in addition to their known functions in innate immunity and tissue homeostasis. Further studies are necessary to determine whether ST2+ILC2s, IL-1R1+ILC2s (or IL-18Rα+ILC2s) or both are necessary to promote IgE-type allergic sensitization to allergens. The cellular source for IL-1 that activate ILC2s also needs to be determined. Potential candidates include alveolar macrophages, which are a source of IL-1α in response to inhaled fine particles (41).
How can the knowledge derived from this study be translated to humans? Several mouse models for peanut allergies have been reported previously, which include models with a disinhibited mutation of the IL-4 receptor (42) and models using a combination of oral peanut exposure combined with mucosal adjuvants, such as cholera toxin (43–45). Although these models provide important insight, the immunologic mechanisms involved in the initiation and development of peanut allergies are difficult to assess due to genetic manipulation and the use of adjuvants. Additionally, the “Learning Early About Peanut” (LEAP) allergy study in humans has shown that oral exposure to peanut proteins is protective against developing a peanut allergy, implicating alternative routes for sensitization, such as the skin and airway (46). Peanut allergens have even been detected in household dust samples even if peanuts are not consumed by the occupants (14, 15). Although the route of allergen sensitization has not been fully established in the field, we hope that our model, which mimics environmental exposure to peanut allergen microparticles, is useful for a mechanistic understanding of the development of peanut allergies in humans.
In summary, we report that IL-13 plays a critical role in the development of Tfh cells and GC B cells in mice exposed to peanut flour. Previously, IL-13 or IL-13-specific receptor IL-13Rα1 was necessary to maintain steady-state levels of serum IgE, suggesting a role of IL-13 in linking innate immunity and IgE antibody production (35, 47). A recent report also implicated the role of a unique subset of Tfh cells that express IL-13, which leads to the production of high-affinity IgE antibodies (8). Together with the findings in this study, IL-13 that is produced by ILC2s, Tfh cells, or both may play a key role in allergen sensitization and IgE antibody production. While the role for IL-4 to mediate IgE class switch has been well established, more attention should be paid to the role of IL-13 in promoting the production of allergen-specific IgE antibodies. Several important questions remain. Are ILC2s required once IgE B cell memory has been established? What is the key target(s) of IL-13 during allergic sensitization, including DCs, Tfh cells, and GC B cells? Continued studies in this field will provide important insight into the immunological mechanisms involved in the initiation and development of allergies to peanuts and other allergens and help to develop novel strategies to prevent these diseases in humans.
Supplementary Material
Key points.
IL-13 is indispensable for the production of IgE antibodies to peanuts
Subsets of lung ILC2s produce IL-13 upon airborne exposure to peanut flour
Mice deficient in ILC2s produce lower titers of peanut-specific IgE antibodies
ACKNOWLEDGMENTS
We thank Ms. LuRaye S. Eischens (Mayo Clinic, Rochester, MN) for administrative help.
This work was supported by grants from the National Institutes of Health (R37AI71106, R01HL117823) and the Mayo Foundation. All the authors acknowledge no conflicts of interest.
Abbreviations:
- APCs
antigen-presenting cells
- B6
C57BL/6
- BAL
bronchoalveolar lavage
- dLNs
draining lymph nodes
- eGFP
enhanced green fluorescent protein
- FACS
fluorescence-activated cell sorting
- GC
germinal center
- HDM
house dust mite
- IL
interleukin
- ILC2
group 2 innate lymphoid cell
- i.n.
intranasally
- i.p.
intraperitoneally
- dLN
draining lymph node
- mLN
mediastinal lymph node
- LNs
lymph nodes
- OVA
ovalbumin
- SPF
specific pathogen-free
- Tfh
follicular helper T
- Th2
type 2 helper T
- Treg
regulatory T
- WT
wild-type
REFERENCES
- 1.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol 2010;126:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyer AA, Lau CH, Smith TL, Smith BM, Gupta RS. Pediatric emergency department visits and hospitalizations due to food-induced anaphylaxis in Illinois. Ann Allergy Asthma Immunol 2015;115:56–62. [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 2016;16:751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol 2017;139:300–13 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 2011;13:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble A, Zhao J. Follicular helper T cells are responsible for IgE responses to Der p 1 following house dust mite sensitization in mice. Clin Exp Allergy 2016;46:1075–82. [DOI] [PubMed] [Google Scholar]
- 7.Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, Kita H. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol 2018;142:1144–58 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, Joseph J, Gertie JA, Xu L, Collet MA, Grassmann JDS, Simoneau T, Chiang D, Berin MC, Craft JE, Weinstein JS, Williams A, Eisenbarth SC. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019;365, eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol 2018;18:121–33. [DOI] [PubMed] [Google Scholar]
- 10.Wallrapp A, Riesenfeld SJ, Burkett PR, Kuchroo VK. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol Rev 2018;286:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krabbendam L, Bal SM, Spits H, Golebski K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol Rev 2018;286:74–85. [DOI] [PubMed] [Google Scholar]
- 12.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014;40:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, Serrao EM, Haim-Vilmovsky L, Teichmann SA, Rodewald HR, Botto M, Vyse TJ, Fallon PG, Li Z, Withers DR, McKenzie ANJ. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018;48:1195–207 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trendelenburg V, Ahrens B, Wehrmann AK, Kalb B, Niggemann B, Beyer K. Peanut allergen in house dust of eating area and bed--a risk factor for peanut sensitization? Allergy 2013;68:1460–2. [DOI] [PubMed] [Google Scholar]
- 15.Brough HA, Santos AF, Makinson K, Penagos M, Stephens AC, Douiri A, Fox AT, Du Toit G, Turcanu V, Lack G. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 2013;132:630–8. [DOI] [PubMed] [Google Scholar]
- 16.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009;123:417–23. [DOI] [PubMed] [Google Scholar]
- 17.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010;464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo BC, Gold MJ, Hughes MR, Antignano F, Valdez Y, Zaph C, Harder KW, McNagny KM. The orphan nuclear receptor RORalpha and group 3 innate lymphoid cells drive fibrosis in a mouse model of Crohn’s disease. Sci Immunol 2016;1:eaaf8864. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol 2017;139:300–13 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartemes K, Chen CC, Iijima K, Drake L, Kita H. IL-33-Responsive Group 2 Innate Lymphoid Cells Are Regulated by Female Sex Hormones in the Uterus. J Immunol 2018;200:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, Collet MA, Yurieva M, Alsen S, Fogelstrand P, Walter A, Heath WR, Mueller SN, Yrlid U, Williams A, Eisenbarth SC. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol 2017;2: eaam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol 2003;3:721–32. [DOI] [PubMed] [Google Scholar]
- 23.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol 1998;102:165–9. [DOI] [PubMed] [Google Scholar]
- 24.Bost KL, Holton RH, Cain TK, Clements JD. In vivo treatment with anti-interleukin-13 antibodies significantly reduces the humoral immune response against an oral immunogen in mice. Immunology 1996;87:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity 2014;41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev 2018;286:37–52. [DOI] [PubMed] [Google Scholar]
- 27.Drake LY, Kita H. Group 2 innate lymphoid cells in the lung. Adv Immunol 2014;124:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011;12:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaedi M, Shen ZY, Orangi M, Martinez-Gonzalez I, Wei L, Lu X, Das A, Heravi-Moussavi A, Marra MA, Bhandola A, Takei F. Single-cell analysis of RORα tracer mouse lung reveals ILC progenitors and effector ILC2 subsets. J Exp Med 2019; 10.1084/jem/20182293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 2010;10:89–102. [DOI] [PubMed] [Google Scholar]
- 31.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, Kovats S. A Major Population of Functional KLRG1(−) ILC2s in Female Lungs Contributes to a Sex Bias in ILC2 Numbers. Immunohorizons 2018;2:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity 2012;37:463–74. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996;380:630–3. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature 1996;380:627–30. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity 1998;9:423–432. [DOI] [PubMed] [Google Scholar]
- 36.Fish SC, Donaldson DD, Goldman SJ, Williams CMM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol 2005;174:7716–24. [DOI] [PubMed] [Google Scholar]
- 37.Walter DM, McIntire J, Berry G, McKenzie ANJ, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 2001;167:4668–4675. [DOI] [PubMed] [Google Scholar]
- 38.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 2011;186:4375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie ANJ, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity 2014;41:366–74. [DOI] [PubMed] [Google Scholar]
- 40.Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol 2019;16:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda E, Ozasa K, Temizoz B, Ohata K, Koo CX, Kanuma T, Kusakabe T, Kobari S, Horie M, Morimoto Y, Nakajima S, Kabashima K, Ziegler SF, Iwakura Y, Ise W, Kurosaki T, Nagatake T, Kunisawa J, Takemura N, Uematsu S, Hayashi M, Aoshi T, Kobiyama K, Coban C, Ishii KJ. Inhaled Fine Particles Induce Alveolar Macrophage Death and Interleukin-1alpha Release to Promote Inducible Bronchus-Associated Lymphoid Tissue Formation. Immunity 2016;45:1299–310. [DOI] [PubMed] [Google Scholar]
- 42.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, Oettgen HC. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity 2014;41:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013;131:187–200 e1–8. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, Kim B, Waserman S, Reed J, Coyle AJ, Jordana M. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol 2007;179:6696–703. [DOI] [PubMed] [Google Scholar]
- 45.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol 2009;123:231–8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Loreno M, Plaut M, Lack G. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A 2008;105:7240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


