Abstract
Objective: Long-term survivors (LS) of non-small cell lung cancer (NSCLC) without driver alterations, displaying an overall survival (OS) of more than 3 years, comprise around 10% of cases in several series treated with chemotherapy. There are classical prognosis factors for these cases [stage, Eastern Cooperative Oncology Group (ECOG), etc.], but more data are required in the literature. In this multi-center study, we focused on LS of advanced NSCLC with OS above 36 months to perform a clinical-pathological and molecular characterization.
Methods: In the first step, we conducted a clinical-pathological characterization of the patients. Afterwards, we carried out a genetic analysis by comparing LS to a sample of short-term survivors (SS) (with an OS less than 9 months). We initially used whole-genome RNA-seq to identify differentiating profiles of LS and SS, and later confirmed these with reverse transcription-polymerase chain reaction (RT-PCR) for the rest of the samples.
Results: A total of 94 patients were included, who were mainly men, former smokers, having adenocarcinoma (AC)-type NSCLC with an ECOG of 0–1. We obtained an initial differential transcriptome expression, displaying 5 over- and 33 under-expressed genes involved in different pathways: namely, the secretin receptor, surfactant protein, trefoil factor 1 (TFF1), serpin, Ca-channels, and Toll-like receptor (TLRs) families. Finally, RT-PCR analysis of 40 (20 LS/20 SS) samples confirmed that four genes (surfactant proteins and SFTP) were significantly down-regulated in SS compared to LS by using an analysis of covariance (ANCOVA) model: SFTPA1 (P = 0.023), SFTPA2 (P = 0.027), SFTPB (P = 0.02), and SFTPC (P = 0.047).
Conclusions: We present a sequential genetic analysis of a sample of NSCLC LS with no driver alterations, obtaining a differential RNA-seq/RT-PCR profile showing an abnormal expression of SF genes.
Keywords: Long-term survivors, non-small cell lung cancer, surfactant proteins
Introduction
Non-small cell lung cancer (NSCLC) is currently one of the cancers with the highest mortality rates worldwide. According to the literature, in the pre-immunotherapy era, the 5-year survival rate of this aggressive disease was only 16%. Indeed, metastatic NSCLC is currently an incurable disease, and both chemotherapy and immunotherapy have only a palliative role in its treatment1.
Chemotherapy for NSCLC is based on platinum for a first-line setting. A meta-analysis of 16 randomized controlled trials has demonstrated that platinum-based chemotherapy may have a better potential to prolong survival than the best supportive care for these patients2. However, the prognosis for patients with advanced NSCLC is still poor. The median survival of these patients is around 12 months, while the 1-year survival rate is just 30%–35%, with 4%–6% of them being long-term NSCLC survivors (LS) displaying a survival time of more than 2 years in several series3.
Epidermal growth factor receptor (EGFR) mutations (in 15%–17% of patients), anaplastic lymphoma kinase-echinoderm microtubule-associated protein-like (ALK-EML) (2%–5%) and ROS-1 (1%) translocation have been recently described in NSCLC patients. Target-specific treatments with tyrosine kinase inhibitors (TKIs) for these molecular alterations have demonstrated a significant benefit in both progression-free survival (PFS) and overall survival (OS), as well as a higher proportion of LS compared to chemotherapy4–6.
Recent developments in NSCLC diagnosis through next generation sequencing (NGS) techniques have allowed researchers to obtain more information about other potential treatable alterations (e.g., HER2, RET, MET, BRAF, and NTRK) that present in very low frequencies (1%–2%). In spite of their low prevalence, their identification is important because it allows treatment with certain targeted therapies that have a promising efficacy7.
A deeper analysis of LS patients without driver mutations that have undergone sequential chemotherapy treatments has rarely been explored in the literature. Why a small group of our patients without identified specific molecular alterations was able to reach an OS longer than 36 months is an unanswered question. Unknown molecular profiles involved in the response to chemotherapy, the tumor prognosis, or the impact of sequential local and systemic treatments could be some of the explanations. Previous studies about this group of patients without driver alterations include small numbers of patients and are focused on their clinical features alone, or alternatively on specific molecular findings. An integrative clinical–pathological and molecular analysis, as we propose in this research, has not been previously described.
Thus, in this study, we aimed for a complete characterization of advanced NSCLC LS displaying EGFR wild-type (wt) and non-translocated (nt) ALK/ROS1. We performed a thorough clinical and pathological treatment, and molecular characterization of a sample of advanced NSCLC patients from different institutions in Madrid who survived more than 36 months.
The primary objective of this study was to describe the basic clinical, pathological, and therapeutic characteristics in all the patients. In addition, we also analyzed both the OS and the PFS of all LSs included in the study.
A co-primary objective in this study was a molecular analysis of all the patients included. For this purpose, a sequential strategy of NGS (RNA-seq) and RT-PCR was used in order to identify specific molecular profiles of gene expression for these patients.
Materials and methods
Patients and samples
We retrospectively analyzed data from 94 patients at nine institutions in Madrid, Spain, who had been diagnosed with advanced NSCLC and had an EGFR wt/ALK-ROS1 nt genotype and an OS of at least 36 months. All these patients were selected from within a period of 14 years (from January 2002 to December 2016). We analyzed the clinical–pathological characteristics (age, sex, stage IIIB vs. IV, histology, smoking status, diabetes and cardiovascular disease, weight loss > 10%, symptoms at diagnosis, and sites of metastasis). Performance status (PS) was categorized from 0 to 4, according to the scale of the Eastern Cooperative Oncology Group (ECOG). Laboratory parameters [lactate dehydrogenase (LDH) and hemoglobin levels] and type of treatment administered (platinum-based treatment, metasectomies, chemotherapy lines, maintenance, grade 4 toxicity, and metformin intake) were also analyzed. Finally, PFS and OS data were also collected (Table 1).
Table 1.
Clinical, pathological and treatment collected data from all the LS patients included
| Clinical-pathological characteristics | n = 94 pts | % |
|---|---|---|
| Age, ≥ 65/< 65 years | 55/39 | 58.5/41.4 |
| Gender, M/F | 67/27 | 71.2/27.7 |
| Smoking status, yes/no | 85/9 | 90.4/9.6 |
| Diabetes mellitus, yes/no | 7/87 | 7.4/92.5 |
| Vascular disease, yes/no | 13/81 | 13.8/86.1 |
| Stage, IV/IIIB pleural effusion | 83/11 | 88.3/11.7 |
| Pathology, LC/adeno/SQ/others | 7/60/21/6 | 7.4/63.8/22.3/6.3 |
| ECOG, 0/1/≥ 2 | 32/57/5 | 34/60.6/5.3 |
| Cough at diagnosis, yes/no | 53/41 | 56.3/43.6 |
| Pain at diagnosis, yes/no | 60/34 | 63.8/36.1 |
| Hemoptysis at diagnosis, yes/no | 9/85 | 90.4/9.5 |
| Dyspnea, yes/no | 25/69 | 26.6/73.4 |
| > 10% Weigh loss, yes/no | 14/80 | 14.8/85.1 |
| Metastasis number location, 0/1/2/> 3 | 7/45/25/16 | 7.5/48.3/2 6.8/17.2 |
| Brain metastasis, yes/no | 13/80 | 13.9/86 |
| Adrenal metastasis, yes/no | 5/88 | 5.3/94.6 |
| Metastasis surgery, yes/no | 35/59 | 37.2/62.7 |
| Platinum based regimen, yes/no | 92/2 | 97.8/2.1 |
| Maintenance, yes/no | 45/49 | 47.8/52.1 |
| - 2nd Line, yes/no | 72/22 | 23.4/76.5 |
| - 3rd Line, yes/no | 53/41 | 56.3/43.6 |
| - 4th Line, yes/no | 38/55 | 40.8/59.1 |
| - 5th Line, yes/no | 26/67 | 27.9/72 |
| Grade IV toxicity, yes/no | 7/86 | 7.5/92.4 |
| LDH levels > 1.5UNL//< 1.5ULN | 29/65 | 30.8/69.1 |
| Metformine intake, yes/no | 16/70 | 18.6/81.4 |
| Hemoglobin levels, ≥ 12/< 12 | 22/72 | 23.4/76.6 |
ECOG, Eastern Cooperative Oncology Group; M/F, male/female; LDH, lactate dehydrogenase; LC, lung cancer; LS, long-term survivor SQ, squamous.
For the molecular analysis, a control sample of 25 patients, including short-term survivors (SS) defined as having an OS less than 9 months, was also included. Histology, gender, PFS, and OS were among the clinical data collected from the SS patients.
This study was approved by our Ethics Committee, and all the patients signed an informed consent form during the recruitment process.
Molecular analysis
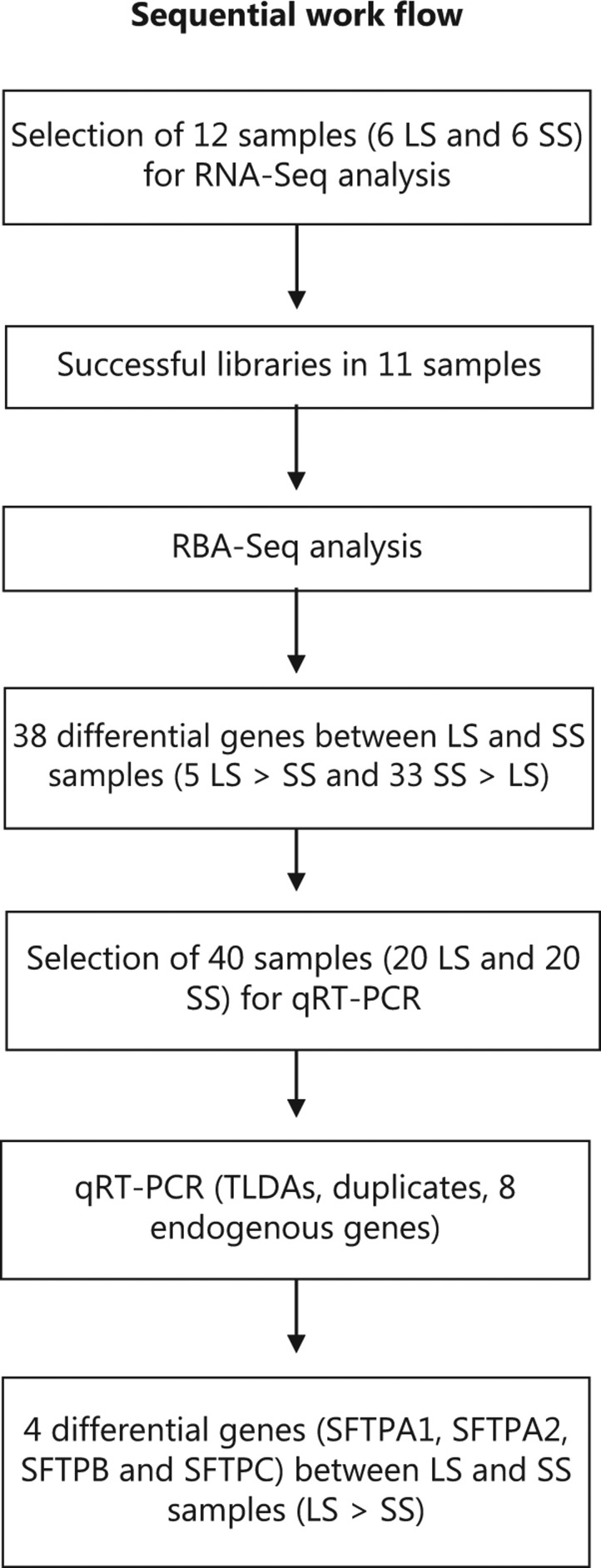
Molecular analysis was carried out with a sequential process comprising an initial untargeted whole-genome screen for differentially-expressed genes through RNA-seq technology, followed by a targeted screen with quantitative (q)RT-PCR using the genetic profile previously obtained (Figure 1).
Figure 1.
Sequential molecular analysis: indirect screening through RNA-Seq and further directed screening through reverse transcription-polymerase chain reaction (RT-PCR).
Untargeted screening of candidate genes: for the initial RNA-seq experiment, 11 samples were selected, namely six samples from LS patients and five samples from SS. Successful libraries were obtained in all the 11 samples.
Targeted screening: For the quantitative PCR study (qRT-PCR), 114 samples were collected were collected, but we were not able to use most of the samples due to their poor quality or the lack of associated clinical data. We therefore performed the qPCR study with a subset of 40 samples (20 from LS patients and 20 from SS patients).
Institutional approval from our Ethics Committee was obtained for conducting the study. The ethical aspects are in accordance with Resolution CNS 196/96 and its complementary resolution. The samples were analyzed by two independent pathologists.
Construction of RNA-seq libraries and analysis
To identify the differentially expressed genes between the tumors of the LS and SS patients, a previous exploratory study using the RNA-seq technology was carried out. RNA sequencing (RNA-seq), also called whole transcriptome shotgun sequencing (WTSS), is a developed approach to transcriptome profiling that uses NGS. RNA-seq can reveal the presence and quantity of RNA in a biological sample at a given moment. RNA-seq provides a far more precise measurement of the levels of transcripts and their isoforms than other methods.
RNA-seq libraries were generated from tumor samples embedded in paraffin. Previously, by means of a hematoxylin and eosin (H&E) staining, it was determined that the samples were composed of at least 80% tumor cells. These libraries were prepared according to the instructions of the NEBNext Ultra Directional RNA Library Prep kit for Illumina (Illumina, San Diego, CA, USA), following the protocol of the Poly(A) mRNA Magnetic Isolation Module to focus sequencing towards the 3′ end of transcripts. The input yield of total RNA to start the protocol was around 1 μg, as estimated by the Agilent 2100 Bioanalyzer using an RNA 6000 Nano LabChip kit (Agilent Technologies, Santa Clara, CA, USA). RNA integrity number (RIN) values were low, consistent with the characteristics of formalin-fixed paraffin-embedded (FFPE) samples, and so RNA fragmentation time was reduced accordingly. The library amplification phase included within the citied protocol was performed by applying a PCR of 18 cycles. The libraries obtained by these means were validated and quantified using an Agilent 2100 Bioanalyzer and a DNA 7500 LabChip kit (Agilent Technologies, Santa Clara, CA, USA). An equimolecular pool of libraries was prepared, cleaned using AmpureBeads (Beckman) and finally titrated by qPCR using the Kapa-SYBR FAST qPCR kit for LightCycler480 (Kapa Biosystems Inc., Wilmington, MA, USA) and a reference standard for quantification (Parque Científico de Madrid, Madrid, Spain). The pool of libraries was denatured prior to being seeded on a NextSeq flow cell at a density of 2.2 pM for clustering. Samples were then sequenced using a NextSeq™ 500 High Output Kit (Illumina, San Diego, CA, USA), in a format of 1 × 75 single read sequencing. An average of 41 ± 14 millions of pass-filter reads was obtained for the group of 11 samples, which were used for further filtering and bioinformatics analysis. Sequences were processed to remove possible artefacts and were then aligned against the human genome using the Bowtie aligner.
RNA extraction and qPCR
The sections of FFPE tumors from the samples of the 119 patients (94 LS + 25 SS) were reviewed by two expert pathologists, as mentioned previously. A total of 40 samples were randomly selected after excluding cases with insufficient tumor cells or incomplete clinical data. More than 80% enrichment of the tumor cells was ensured, when necessary, by subsequent macro dissection with the use of a safety blade and a new confirmatory staining by H&E. The RNA was extracted from 10 to 15.5 μm sections using the AllPrep DNA/RNA FFPE Kit, an RNA purification kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Then qRT-PCR was performed using the LightCycler® 480 sequence detection system (Roche Diagnostics, Penzberg, Germany) according to the manufacturer’s instructions. After its design, the TaqMan Low Density Arrays (TLDA) were manufactured and supplied by AnyGenes (AnyGenes, Paris, France).
Statistical analysis
Survival was recorded from the first day of treatment to the date of death or last follow-up, and the survival curves were calculated according to the Kaplan–Meier method. Description of qualitative data was made by using absolute frequencies and percentages and quantitative data by mean and standard deviation (SD).
In the untargeted screening of genes through RNA-seq statistical analysis, genes were assigned and differential expression [as fragments per kilobase of transcript per million mapped reads (FPKM) values] between LS and SS patients was determined by using EdgeR (Suite G-PRO, Biotechvana, Valencia, Spain).
In the case of the targeted screening of genes by qRT-PCR, analysis of covariance (ANCOVA) models were used to test the association of gene expression with survival group, using sex, age, histology, and stage as adjustment variables. Gene expressions were normalized by subtracting the average gene expression of three control genes: GAPDH, ACTB and TBP. A total of 41 genes were thereby tested, and the P-value of the gene expression variable was adjusted for multiple tests using the Bonferroni method to avoid inflation of Type I error. A significance level of 0.05 was used with two-tailed tests. The R 3.5.1 software was used throughout all the calculations.
Results
Clinical-pathological data
Table 1 shows all the data collected concerning the clinical-pathological analysis for all 94 patients. All of the patients (hereafter pts) selected had been previously diagnosed with NSCLC with an OS of at least 36 months (m). All of them had been diagnosed and treated at one of nine institutions in Madrid: Infanta Sofía University Hospital (8/94 pts, 8.5%); Ramón y Cajal University Hospital (13/94 pts, 14.8%); San Carlos University Hospital (11 pts, 12.7%), Gregorio Marañón University Hospital (17 pts, 19.1%), 12 de Octubre University Hospital (30 pts, 32.9%); Alcorcón Foundation Hospital (8 pts, 8.5%), Príncipe de Asturias University Hospital (3 pts, 3.1%), La Paz University Hospital (2 pts) and Torrejón University Hospital (2 pts).
Regarding age, of the 94 patients, 55 (58.5%) were 65 years or older. Sixty-seven patients (71%) were male and 84 out of 94 (89.4%) had smoked or were current smokers. Comorbidities (diabetes and vascular disease) were infrequent: 7 pts (7%) and 13 pts (14%), respectively. Concerning performance status, we found ECOG 0 in 32 pts. (34%) and 1 in 57 pts (61%). Only 12 pts (14%) had weight loss greater than 10% at presentation. Cough (41 pts, 44%), followed by pain (34 pts, 36%), dyspnea (25 pts, 27%) and hemoptysis (9 pts, 9%) were the main symptoms, and the majority of LDH as well as hemoglobin levels were normal in the majority of patients.
Adenocarcinoma (AC) was the most common pathological subgroup (60 pts, 65%), followed by squamous (21 pts, 22%), large cell carcinoma (7 pts, 7%) and “other subtypes” (6 pts, 6%). One or two metastatic sites at diagnosis were described in 44 pts (47%) and 29 pts (31%), respectively, and only 13 pts (14%) had brain metastasis and 5 pts (5%) had adrenal metastasis.
First-line chemotherapy based on platinum was administrated in 92 pts (98%), and maintenance was used only in 41 pts (44%). Local treatment (metasectomies and/or radiation), was done in 35 pts (38%). Grade 4 toxicity was detected in 7 pts (7%).
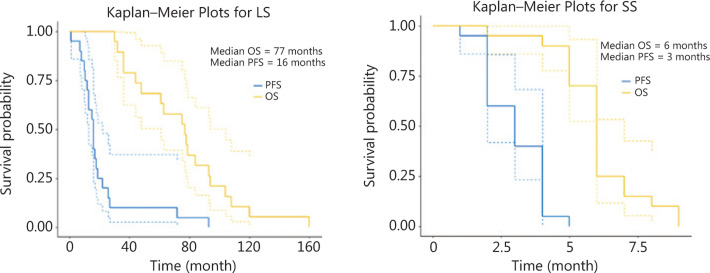
Finally, a descriptive survival analysis was performed. The medians of PFS and OS were 16 months (14 m–32 m) and 77 months (36 m–80 m), respectively (Figure 2).
Figure 2.
Progression free survival (PFS) and overall survival (OS) Kaplan–Meyer curve from advanced non-small cell lung cancer (NSCLC) long survivors (LS) and short survivors (SS) participating in the study.
Among cases of SS included for the molecular comparative analysis, 14/26 were AC, 10 were squamous and 2 were large-cell carcinoma. Median PFS and OS were 3 months (1 m–4 m) and 6 months (4 m–9 m), respectively (Figure 2).
Molecular analysis: potential involvement of surfactant proteins in long-term survival of NSCLC patients
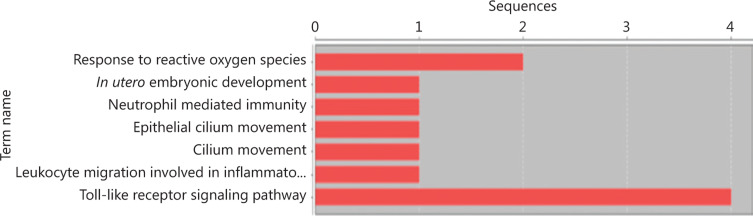
In this study, we have carried out a sequential molecular analysis (Figure 1). An initial NGS exploration was carried out on a limited and representative sample of six LS and five SS, according to sex, histology, and age, and a good quality of the sample embedded in the paraffin block. In this primary analysis, we obtained a significant differential transcriptome expression between samples from six LS and five SS, including five over-expressed genes in LS compared to SS and 33 over-expressed genes in SS compared to LS (Table 2). These genes were involved in different cellular pathways: the secretin receptor, surfactant protein, TFF1, the serpin family, the Ca-binding protein channel and the Toll-like receptor (TLRs) family (Figure 3).
Table 2.
RNA-Seq analysis: Significant overexpression in LS compared to SS samples and CS compared to LS
| Expression | P | Gene name | Gene description | Chromosome name | HGNC symbol |
|---|---|---|---|---|---|
| LS >> CS | 2.93E-06 | SCTR | Secretin receptor | 2 | SCTR |
| LS >> CS | 1.99E-08 | SFTPA1 | Surfactant protein A1 | 10 | SFTPA1 |
| LS >> CS | 2.37E-08 | SFTPA2 | Surfactant protein A2 | 10 | SFTPA2 |
| LS >> CS | 1.03E-06 | SFTPB | Surfactant protein B | 2 | SFTPB |
| LS >> CS | 6.09E-10 | SFTPC | Surfactant protein C | 8 | SFTPC |
| CS >> LS | 2.11E-05 | AKR1B10 | Aldo-keto reductase family 1 member B10 | 7 | AKR1B10 |
| CS >> LS | 1.92E-05 | C10orf99 | Chromosome 10 open reading frame 99 | 10 | C10orf99 |
| CS >> LS | 2.10E-05 | C9orf84 | Chromosome 9 open reading frame 84 | 9 | C9orf84 |
| CS >> LS | 2.00E-05 | CAPSL | Calcyphosine like | 5 | CAPSL |
| CS >> LS | 2.25E-05 | CXCL6 | C-X-C motif chemokine ligand 6 | 4 | CXCL6 |
| CS >> LS | 4.22E-06 | DBT | Dihydrolipoamide branched chain transacylase E2 | 1 | DBT |
| CS >> LS | 1.70E-05 | DNAI1 | Dynein axonemal intermediate chain 1 | 9 | DNAI1 |
| CS >> LS | 1.37E-05 | DNAJC19 | DnaJ heat shock protein family (Hsp40) member C19 | 3 | DNAJC19 |
| CS >> LS | 6.96E-06 | FAM3D | Family with sequence similarity 3 member D | 3 | FAM3D |
| CS >> LS | 1.18E-05 | FAM83B | Family with sequence similarity 83 member B | 6 | FAM83B |
| CS >> LS | 1.55E-05 | FSTL1 | Follistatin like 1 | 3 | FSTL1 |
| CS >> LS | 1.78E-09 | GJB3 | Gap junction protein beta 3 | 1 | GJB3 |
| CS >> LS | 1.45E-05 | KIF20A | Kinesin family member 20A | 5 | KIF20A |
| CS >> LS | 8.19E-06 | KRT14 | Keratin 14 | 17 | KRT14 |
| CS >> LS | 6.18E-06 | KRT16 | Keratin 16 | 17 | KRT16 |
| CS >> LS | 9.35E-07 | KRT17 | Keratin 17 | 17 | KRT17 |
| CS >> LS | 8.98E-06 | KRT5 | Keratin 5 | 12 | KRT5 |
| CS >> LS | 1.48E-05 | LCE3D | Late cornified envelope 3D | 1 | LCE3D |
| CS >> LS | 2.50E-06 | PDS5B | PDS5 cohesin associated factor B | 13 | PDS5B |
| CS >> LS | 1.70E-08 | PPBP | Pro-platelet basic protein | 4 | PPBP |
| CS >> LS | 9.44E-09 | S100A7 | S100 calcium binding protein A7 | 1 | S100A7 |
| CS >> LS | 7.25E-06 | SAA2 | Serum amyloid A2 | 11 | SAA2 |
| CS >> LS | 8.20E-06 | SAA2-SAA4 | SAA2-SAA4 | 11 | SAA2-SAA4 |
| CS >> LS | 4.59E-06 | SAA4 | Serum amyloid A4, constitutive | 11 | SAA4 |
| CS >> LS | 7.54E-07 | SBSN | Suprabasin | 19 | SBSN |
| CS >> LS | 1.32E-08 | SERPINB4 | Serpin family B member 4 | 18 | SERPINB4 |
| CS >> LS | 1.22E-05 | SPRR2B | Small proline rich protein 2B | 1 | SPRR2B |
| CS >> LS | 8.37E-06 | SPRR2F | Small proline rich protein 2F | 1 | SPRR2F |
| CS >> LS | 1.23E-05 | SPRR2G | Small proline rich protein 2G | 1 | SPRR2G |
| CS >> LS | 2.10E-07 | STYK1 | Serine/threonine/tyrosine kinase 1 | 12 | STYK1 |
| CS >> LS | 2.21E-05 | TFF1 | Trefoil factor 1 | 21 | TFF1 |
| CS >> LS | 1.36E-06 | TGM3 | Transglutaminase | 20 | TGM3 |
| CS >> LS | 2.03E-05 | UBE4B | Ubiquitination factor E4B | 1 | UBE4B |
CS, short survivors; LS, long-term survivors; SS; short-term survivors.
Figure 3.
Histogram with number of sequences with GO annotations for ontology “Biological Process” in RNA-Seq analysis.
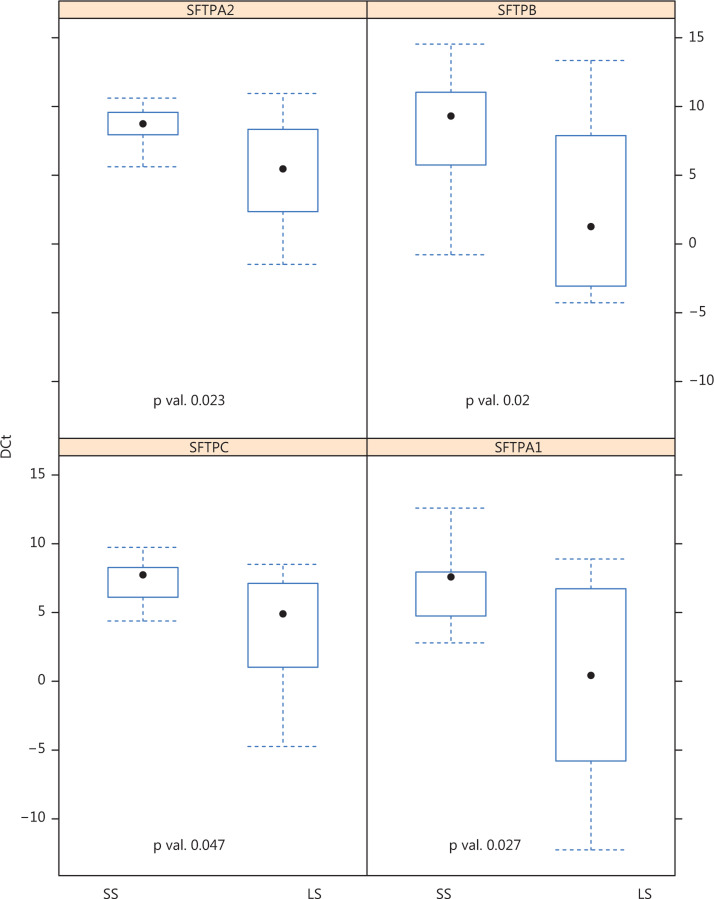
Finally, we conducted a confirmatory qRT-PCR of the previously-identified differentially expressed genes in 40 samples of both SS (20) and LS (20). By using ANCOVA models to analyze the data, it was determined that only four genes codifying for surfactant proteins were significantly down-regulated in SS compared to LS: SFTPA1 (P = 0.023), SFTPA2 (P = 0.027), SFTPB (P = 0.02), and SFTPC (P = 0.047) (Figure 4).
Figure 4.
Genes obtained by reverse transcription-polymerase chain reaction RT-PCR with statistical significance resulting after RNA-Seq profile confirmation in two opposite samples with 20 long survivors (LS advanced lung cancer patients) and 20 short survivors (SS advanced lung cancer patients).
Discussion
New treatment options are available for NSCLC, such as targeted therapy, immunotherapy, and chemo–immunotherapy combinations. However, the LS in advanced settings who were treated with chemotherapy previous to the immunotherapy era comprise a small subset of the NSCLC patients, characterized by a median OS of around 14–18 months. As we previously mentioned, the 5-year survival rates of these patients vary from 9% to 15%2.
In this retrospective study, we have included a significant sample of very LS of NSCLC without EGFR mutations or ALK/ROS1 translocations, and with a median OS of 36 months, at nine hospitals in Madrid. This is the sample of patients who lived the longest with these characteristics reported in the reviewed literature.
Patient-related factors
In our study, 55/94 (58%) of the patients were 65 years or older, most were male (67/94, 71%) and the proportion of non-smokers was very low (9/94, 9.6%). However, regarding prognosis factors, females classically have a better prognosis; lung cancer incidence and prevalence have been described to be higher among men than women. In addition, EGFR, ALK and ROS1 alterations (excluded here) are more prevalent among women than men. Age is a controversial prognostic factor in lung cancer. Whereas different studies did not find a significant relation between age and long-term survival8, other authors consider older age to be a poor prognostic factor, mainly in those patients with potentially resectable disease9. This finding could be explained by comorbidities and tolerability to treatments, including surgery, in elderly patients compared to younger ones. However, in our sample, similar rates among patients both older and younger than 65 years were found, suggesting that it is probable that age is not a differential feature for becoming a LS in this setting.
As previously mentioned, the majority of patients included were current or former smokers, very similar to other series of lung cancer LS10.
In this study, most of the patients had neither diabetes (87/94, 92.5%) nor vascular diseases (81/94, 86%) indicating a very fit population with no serious comorbidities. Inal et al.11 found that diabetes is a poor prognosis factor in lung cancer in multivariate analysis. Given the low rate of diabetic patients in our sample, this would suggest that diabetes could play a negative role in a lung cancer patient’s outcome. Research concerning metformin and lung cancer is emerging. Different authors have concluded that metformin intake in diabetic patients diagnosed with NSCLC could improve their prognosis12. Studies of metformin intake in combination with targeted therapies such as anti-EGFR inhibitors or antiangiogenic drugs (e.g., bevacizumab) have shown a synergistic effect. In our study, most of our patients did not take metformin (74/94, 79%), but all of our diabetic patients took metformin as their main antidiabetic treatment (7/7). In addition, 11 patients were treated with metformin without a diagnosis of type-2 diabetes because of a pre-diabetic condition. The small number of patients in this subgroup precludes the analysis of the impact in survival of metformin intake.
PS is a classical prognosis factor in lung cancer, and several researchers have concluded that a poor PS is related to a poor prognosis13. In our sample, only 5 out of 94 patients (5.3%) had an ECOG of 2, whereas 60% of patients had ECOG 1 and 34% had ECOG 0, suggesting that a good initial PS could be a potentially favorable prognostic factor.
In addition, in our sample we have observed different symptoms at presentation: cough, pain, hemoptysis, and dyspnea. However, no studies have been reported correlating these factors to a poorer prognosis. Walter et al.14 described a group of NSCLC patients where hemoptysis was reported by 21.6% as an initial symptom associated with cancer, and chest/shoulder pain was the only initial symptom with a significantly shorter diagnostic interval for cancer compared with non-cancer diagnoses (P = 0.003). In our LS series, hemoptysis at diagnosis was very infrequent (9/94 patients, 9.5%) followed by dyspnea (26%), cough (56%), and pain (63%). These data are similar to most of the studies focused on collecting symptoms of lung cancer at presentation: cough is the most common manifestation, followed by weight loss, dyspnea, and chest pain15.
Regarding weight loss, several authors have reported that it is a clear factor in poor prognosis. Sahin et al.16 found that a low nutritional index was clearly related to shorter OS, and Crvenkova et al.17 found that weight loss > 10% was a poor and independently poor prognosis factor for long-term survival after therapy. Illa et al.18 reported that nutritional risk screening is a significant predictor of tumor response. Therefore, according to their data, early detection of malnutrition could be important for the prognosis of cancer patients as well as for planning effective supportive care. In our series, the majority of patients (85%) at diagnosis had not lost more than 10% of their basal weight. These findings were in accordance with those of previous authors who correlated a long survival with a good nutritional status. However, other recent studies failed to demonstrate this hypothesis. For example, in stage IV patients with lung ACs, authors found that pre-diagnosis weight loss had a negative impact on PFS, but no such effect was noticed on OS19.
Tumour-related factors
Histology has been classically discussed as a prognosis factor. In this study, we have found that AC was the most common pathological subgroup (60 pts, 65%) followed by squamous (SC) (21 pts, 22%), and large cell carcinoma (LCC) (7 pts, 7%). In a large review with more than 20,000 patients of early lung cancer who underwent lobectomy, 872 were diagnosed with adenosquamous carcinoma (ASC), 7,888 with squamous cell (SC), and 12,601 with AC20. Shi found that survival after lobectomy for stage I and II disease was significantly reduced in ASC and SC compared to AC (P < 0.0001). ASC also had a significantly increased hazard ratio of 1.35 and 1.27 relative to AC and SC, respectively21. However, currently it is difficult to know if the pathology subtype itself involves a worse prognosis, or if molecular alterations associated with them could induce the outcome of these lung cancer patients. In our work, LS patients have an AC subtype, which is consistent with the literature conferring a better outcome22.
The number of metastasis sites has been studied as a potential feature associated with long-term survival. Other series of LS showed that a single metastasis location, as opposed to more than one location, was associated with a better outcome (62% vs. 41%, P = 0.008). In our sample, most of the patients have one or two metastasis sites (44 patients, 47%, and 29 patients, 31%, respectively), in agreement with the previous literature. The brain is a very common site of metastasis in patients with NSCLC, and we found only 13 patients (14%) with a single brain metastasis and 5 (5%) with adrenal metastasis who had undergone surgery23,24. Moscetti’s study found three cases (16.7%) of stage IV, 5-year treated with resection associated with improvement of long-term survival25.
Treatment-related factors
Chemotherapy is the standard first treatment for NSCLC with ALK/ROS1 nt and EGFR wt genotypes. A platinum-based regime is the most common treatment in advanced lung cancer, associated with an OS of 10–14 months. Maintenance strategy has improved survival in those patients with response after induction26. First-line chemotherapy with a platinum-based regime was administered to 92 patients (98%); however, maintenance therapy was only administered to 41 patients (47%). No differences among platinum combinations have been demonstrated27, although different authors demonstrated a preferred combination such as AC and cisplatin-pemetrexed and squamous and cisplatin-gemcitabine28. In this series, only half of the patients (45 pts, 47%) received maintenance therapy, and therefore, we cannot affirm that this strategy is related to a better outcome.
Local treatments like metasectomies were performed in 35 patients (38%). The brain is a very common site of metastasis in patients with NSCLC. As we previously mentioned, only 13 patients (14%) had brain metastasis while 5 (5%) had adrenal metastasis and in all cases had undergone surgery. In accordance with this, Hsiung et al.29 confirmed these findings in another study with patients with resected brain metastasis, whole brain radiation or chemotherapy, who lived longer compared to those who did not receive such treatment.
Grade 4 toxicity was detected in only 7 patients (8%). Hardy et al.30 concluded that the administration of chemotherapy agents for NSCLC was associated to short- and long-term toxicities, and that they could be related to a worse survival. However, they did not find a relation between the intensity and the survival. There are no studies positing chemotherapy toxicity as a predictor of long-term survival, although, obviously, treatment is withdrawn or doses are reduced in patients with severe toxicity to chemotherapy, with a potential negative impact on their survival.
Finally, in this sample, we estimated a median PFS of 16 months and a median OS of 77 months, which is even better than other LS series, such as that of Skaug et al.31 with a median survival of 2.9 years (ranging from 2 to 5.1 years), 4.1% being still alive after 36 months and 0.41% reaching over 5 years of OS.
Molecular analysis
We further enhanced the current study by including a molecular analysis in order to identify differentially expressed genes in our sample of LS. We performed a whole-genome RNA-seq analysis followed by confirmatory qRT-PCR.
As previously described, a molecular exploration was carried by including all the patients with an adequate sample for an NGS analysis. In the first step, we selected 11 patients (6 LS and 5 SS) displaying an extremely different OS (> 36 months vs. < 9 months, respectively). They were representative of the rest of the patients (balanced histology and centers and good-quality pathologist specimens). We selected RNA-seq for this initial approximation to do a massive differential expression analysis. A significant differential profile between LS and SS was obtained (Figure 3 and Table 2), with 5 genes over-expressed in LS compared to SS and 33 over-expressed in SS compared to LS in transcriptome expression. Different families of relevant bronchial mucosae genes were involved in these differences. For example, secretin receptor genes were over-expressed in LS compared to SS samples, and its role in lung cancer has been described by some authors. Korner et al.32 found wild-type and splice-variant secretin receptors in bronchopulmonary carcinoid, suggesting that these genes may play a role in peritumoral lung pathophysiology. Other authors also have analyzed secretin receptors’ alterations in different tumors (gastrointestinal, lung, and pancreatic cancer), describing a relevant implication for cancer physiopathology33,34. Trefoil factor 1 (TFF1) was also one of the genes involved in this differential expression among LS and SS. Trefoil factor family (TFF) domain peptides are thought to be involved in epithelial restitution of mucosa. It has been hypothesized that TFFs are also expressed on mucosal surfaces of the respiratory tract with inflammation, as well as in some cases with AC. These are very important in lung mucosa definitions; thus, an association of TFFs with bacteria was found in several studies and may contribute to the anti-microbial mucociliary system35. This fact could drive a special interest in new immune treatments and their interaction with respiratory microbiota. An ongoing analysis by our group is currently analyzing these genes and their relation to immunotherapy.
Septins are a family of cytoskeleton-related proteins with 14 members that associate and interact with actin and tubulin. In our study, septin family genes are also detected with a potential role in these differences among LS and SS. Several authors have described an established relation between septin genes and tumorigenesis35. Liu et al.36 found that SEPT2, SEPT8, SEPT9, and SEPT11 were consistently up-regulated and SEPT4 and SEPT10 were down-regulated in most cancer types investigated. Alterations in septin protein expression were found in many tumors, such as biliary cancer, hepatocarcinoma, ovarian, colorectal, and urological cancer; however, this is the first study describing a role of these genes in lung cancer37.
Regarding calcium-binding protein channels and lung cancer, some authors had associated dysregulations of genes codifying ion-channels with a cancer outcome. For example, in a genome-wide DNA methylation analysis, Bulk et al.38 identified the KCa 3.1 channel gene (KCNN4) promoter as being hypomethylated in an aggressive NSCLC cell line, suggesting the existence of more aggressive phenotype cells. Alterations in the Ca channel (activated chloride channel-2, CLCA2) were also detected in RT-PCR with circulating tumor cells (CTC) in patients with lung AC39. Therefore, according to our findings as well as those of others in the literature, cellular Ca2+ channels could be an important target in lung cancer treatment.
TLRs are a well-known family of pattern recognition receptors that play a key role in the host immune system. Some authors have found a correlation between rs4986791 polymorphism of a protein of this family (TLR4) and lung cancer40. Li et al.41 also concluded that TLR4 activation promoted the immune system evasion of lung AC.
In summary, the RNA-seq results of our first analysis are biologically consistent, and all the genes dysregulated in our samples have been previously described as having a potential role in carcinogenesis or cancer progression.
As we previously mentioned, we made a second confirmatory analysis with RT-PCR of the 55 genes over- and under-expressed in the RNA-seq analysis, included in the different families previously described: secretin receptor, surfactant protein, TFF1, serpin family, Ca-binding protein channel and TLR family. In this second analysis, 40 samples were used (20 LS and 20 SS). In this case, only several subtypes of genes codifying for surfactant proteins demonstrated a significant differential expression between the two groups in the ANCOVA models after correcting for multiple tests: SFTPA1 (P = 0.023), SFTPA2 (P = 0.027), SFTPB (P = 0.02), and SFTPC (P = 0.047) (Figure 4).
In a massive transcriptome analysis of normal tissue and lung cancer samples, the authors have defined a lung cancer-specific gene signature, containing SFTPA1 and SFTPA2 genes, which distinguished lung cancer from other cancer samples with a high predictive accuracy42. Additionally, Grageda et al.43 found in tumor and peri-tumor tissue that expression of SFTPA2 mRNA and total SP-A protein was significantly lower in cancerous tissue compared to adjacent NC tissue (P < 0.001), suggesting that SFTPA2 could be a biomarker for lung cancer diagnosis. Similar results were found by a Norwegian group while studying different markers in regional nodes and peripheral blood. They found SFTPA and SFTPC mRNAs to be potential markers, emphasizing a potential prognosis role of these proteins44. Some authors have demonstrated the over-expression of Pro-SFTPB in lung AC, but a prognosis value of these proteins has not been demonstrated45. Serum screening for lung cancer risk with Pro-SFTPB has been analyzed, indicating a potential value for lung cancer prediction46. Sin et al.47 also analyzed the role of SFTPC in stem cells in lung AC, and they found cancer stem cells in 52 out 57 samples; additionally, those with a bronchioalveolar phenotype SFTPC (+) were more differentiated and patients had a better prognosis compared to stem cells, which were related to a poorer prognosis. In our study, no alterations in SFTD were found, but a Japanese group found that SFTPD dysregulation could play a role in lung cancer48.
These surfactant proteins have been widely related to lung cancer identification in the literature However, less information about their role in cancer behavior has been described49. This is the first analysis demonstrating a correlation between lung cancer long-term survival and surfactant protein expression.
Therefore, among the strengths of this study we can highlight the following: the long follow-up period, the multi-institutional involvement and the relatively high number of long-term survival patients with advanced NSCLC included for clinical-pathological analysis. On the other hand, we must admit that we found a considerable decrease of valid cases for the molecular analysis because of tissue limitations. A further validation of this profile in a larger sample of patients is ongoing, including those LS treated with immunotherapy, to determine whether these results could also be applicable to a wider population. The role of these surfactant proteins in the bronchial immune system is widely known. A further validation of these profiles in lung cancer LS in the immunotherapy era is ongoing, primarily considering the potential role of surfactant proteins in immuno-regulation.
Conclusions
This is the largest multi-center study reported involving very LS (OS > 36 months) without EGFR and ALK/ROS1 alterations in an exhaustive clinical and pathological analysis. The majority of these patients were male smokers or former smokers with AC and ECOG 0–1. An additional comprehensive molecular analysis found a specific and differential molecular profile for this sample.
Acknowledgments
The authors would like to thank the following groups for aiding in the creation of this study: all the patients and their families for permitting the review of all the information included in this study, the “day hospital” workers from all the hospitals involved, the Carlos III Health Institute, the IMDEA Research Institute on Food & Health Sciences, the Spanish Ministry of Science (Plan Nacional I + D + i AGL2016-76736-C3), the Regional Government of Community of Madrid (S2018/BAA-4343), the Ramón Areces Foundation, the EU Structural Funds and the AECC (Spanish Association Against Cancer).Thanks to Scribendi editing and proofreading services for final manuscript review.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Abdel-Rahman O. Causes of death in long-term lung cancer survivors: a SEER database analysis. Curr Med Res Opin. 2017;33:1343–8. doi: 10.1080/03007995.2017.1322052. [DOI] [PubMed] [Google Scholar]
- 2.NSCLC Meta-Analysis Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AN, Simone CB 2nd, Jabbour SK. Risk factors and management of oligometastatic non-small cell lung cancer. Ther Adv Respir Dis. 2016;10:338–48. doi: 10.1177/1753465816642636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1405–11. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 7.Zugazagoitia J, Gómez-Rueda A, Jantus-Lewintre E, Isla D, Camps C, Ramos I, et al. Clinical utility of plasma-based digital next-generation sequencing in oncogene-driven non-small-cell lung cancer patients with tyrosine kinase inhibitor resistance. Lung Cancer. 2019;134:72–8. doi: 10.1016/j.lungcan.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Vijayvergia N, Shah PC, Denlinger CD. Survivorship in non-small cell lung cancer: challenges faced and steps forward. J Natl Compr Canc Net. 2015;13:1151–61. doi: 10.6004/jnccn.2015.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Gao F, Ma JL, Zhang XZ, Li Y, Song LP, et al. Analysis of the characteristics and prognosis of advanced non-small-cell lung cancer in older patients. Patient Prefer Adherence. 2015;9:1189–94. doi: 10.2147/PPA.S87069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riquet M, Rivera C, Pricopi C, Badia A, Arame A, Dujon A, et al. Clinical and paraclinical prognostic factors in non-small cell lung cancer surgery. Rev Pneumol Clin. 2015;71:264–74. doi: 10.1016/j.pneumo.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Inal A, Kaplan MA, Kucukoner M, Urakcı Z, Kılınc F, Isıkdogan A. Is diabetes mellitus a negative prognostic factor for the treatment of advanced non-small-cell lung cancer? Rev Port Pneumol. 2014;20:62–8. doi: 10.1016/j.rppneu.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Liu M, Huang Y, Wu K, Huang X, Zhao Y, et al. Metformin use and lung cancer risk in diabetic patients: a systematic review and meta-analysis. Dis Markers. 2019;2019:6230–634. doi: 10.1155/2019/6230162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remon J, Ahn MJ, Girard N, Johnson M, Kim DW, Lopes G, et al. Advanced-stage non-small cell lung cancer: advances in thoracic oncology 2018. J Thorac Oncol. 2019;14:1134–55. doi: 10.1016/j.jtho.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Walter FM, Rubin G, Bankhead C, Morris HC, Hall N, Mills K, et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer. 2015;112(Suppl. 1):S6–13. doi: 10.1038/bjc.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCannon J, Temel J. Comprehensive management of respiratory symptoms in patients with advanced lung cancer. J Support Oncol. 2012;10:1–9. doi: 10.1016/j.suponc.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Sahin C, Omar M, Tunca H. Weight loss at the time of diagnosis is not associated with prognosis in patients with advanced-stage non-small cell lung cancer. J BUON. 2015;20:1576–84. [PubMed] [Google Scholar]
- 17.Crvenkova S, Pesevska M. Important prognostic factors for the long-term survival in non-small cell lung cancer patients treated with combination of chemotherapy and conformal radiotherapy. J BUON. 2015;20:775–81. [PubMed] [Google Scholar]
- 18.Illa P, Tomiskova M, Skrickova J. Nutritional risk screening predicts tumor response in lung cancer patients. J Am Coll Nut. 2015;34:425–9. doi: 10.1080/07315724.2014.938789. [DOI] [PubMed] [Google Scholar]
- 19.Chermiti Ben Abdallah F, Ben Saïd H, Chamkhi N, Ferchichi M, Chtourou A, Taktak S, et al. Assessment of nutritional status in patients with primary lung cancer. Tunis Med. 2013;91:600–4. [PubMed] [Google Scholar]
- 20.Cooke DT, Nguyen DV, Yang Y, Chen SL, Yu C, Calhoun RF. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg. 2010;90:943–8. doi: 10.1016/j.athoracsur.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Shi CL, Zhang XY, Han BH, He WZ, Shen J, Chu TQ. A clinical-pathological study of resected non-small cell lung cancers 2 cm or less in diameter: a prognostic assessment. Med Oncol. 2011;28:1441–6. doi: 10.1007/s12032-010-9632-y. [DOI] [PubMed] [Google Scholar]
- 22.Giroux Leprieur E, Lavole A, Ruppert AM, Gounant V, Wislez M, Cadranel J, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology. 2012;17:134–42. doi: 10.1111/j.1440-1843.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 23.Beitler AL, Urschel JD, Velagapudi SR, Takita H. Surgical management of adrenal metastases from lung cancer. J Surg Oncol. 1998;69:54–7. doi: 10.1002/(sici)1096-9098(199809)69:1<54::aid-jso11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24. Franceschi E, Brandes AA. Brain metastases from non-small-cell lung cancer: is there room for improvement? Expert Rev Anticancer Ther. 2012;12:421–3. doi: 10.1586/era.12.20. [DOI] [PubMed] [Google Scholar]
- 25.Moscetti L, Nelli F, Felici A, Rinaldi M, De Santis S, D’Auria G, et al. Up-front chemotherapy and radiation treatment in newly diagnosed non-small cell lung cancer with brain metastases: survey by Outcome Research Network for Evaluation of Treatment Results in Oncology. Cancer. 2007;109:274–81. doi: 10.1002/cncr.22399. [DOI] [PubMed] [Google Scholar]
- 26.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr, Brahmer JR, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3488–515. doi: 10.1200/JCO.2015.62.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiller JH, Cleary J, Johnson D. Lung cancer: review of the ECOG experience. Eastern Cooperative Oncology Group. Oncology. 1997;54:353–62. doi: 10.1159/000227718. [DOI] [PubMed] [Google Scholar]
- 28.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 29.Hsiung CY, Leung SW, Wang CJ, Lo SK, Chen HC, Sun LM, et al. The prognostic factors of lung cancer patients with brain metastases treated with radiotherapy. J Neuro-Oncol. 1998;36:71–7. doi: 10.1023/a:1005775029983. [DOI] [PubMed] [Google Scholar]
- 30.Hardy D, Cormier JN, Xing Y, Liu CC, Xia R, Du XL. Chemotherapy-associated toxicity in a large cohort of elderly patients with non-small cell lung cancer. J Thorac Oncol. 2010;5:90–8. doi: 10.1097/JTO.0b013e3181c0a128. [DOI] [PubMed] [Google Scholar]
- 31.Skaug K, Eide GE, Gulsvik A. Predictors of long-term survival of lung cancer patients in a Norwegian community. Clin Respir J. 2011;5:50–8. doi: 10.1111/j.1752-699X.2010.00200.x. [DOI] [PubMed] [Google Scholar]
- 32.Körner MU, Hayes GM, Carrigan PE, Rehmann R, Miller LJ, Reubi JC. Wild-type and splice-variant secretin receptors in lung cancer: overexpression in carcinoid tumors and peritumoral lung tissue. Mod Pathol. 2008;7:387–95. doi: 10.1038/modpathol.3801005. [DOI] [PubMed] [Google Scholar]
- 33.Sherman SK, Maxwell JE, Carr JC, Wang D, Bellizzi AM, O’Dorisio M, et al. Gene expression accurately distinguishes liver metastases of small bowel and pancreas neuroendocrine tumors. Clin Exp Metastasis. 2014;31:935–44. doi: 10.1007/s10585-014-9681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow BK. Molecular cloning and functional characterization of a human secretin receptor. Biochem Biophys Res Commun. 1995;22:204–11. doi: 10.1006/bbrc.1995.1957. [DOI] [PubMed] [Google Scholar]
- 35.dos Santos Silva E, Ulrich M, Döring G, Botzenhart K, Gött P. Trefoil factor family domain peptides in the human respiratory tract. J Pathol. 2000;2:133–42. doi: 10.1002/(SICI)1096-9896(200002)190:2<133::AID-PATH518>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Shen S, Chen F, Yu W, Yu L. Linking the septin expression with carcinogenesis. Mol Biol Rep. 2010;37:3601–8. doi: 10.1007/s11033-010-0009-2. [DOI] [PubMed] [Google Scholar]
- 37.Russell SE, Hall PA. Do septins have a role in cancer? Br J Cancer. 2005;93:499–503. doi: 10.1038/sj.bjc.6602753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulk E, Ay AS, Hammadi M, Ouadid-Ahidouch H, Schelhaas S, Hascher A, et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int J Cancer. 2015;137:1306–17. doi: 10.1002/ijc.29490. [DOI] [PubMed] [Google Scholar]
- 39.Man Y, Cao J, Jin S, Xu G, Pan B, Shang L, et al. Newly identified biomarkers for detecting circulating tumor cells in lung adenocarcinoma. Tohoku J Exp Med. 2014;234:29–40. doi: 10.1620/tjem.234.29. [DOI] [PubMed] [Google Scholar]
- 40.Kurt H, Ozbayer C, Bayramoglu A, Gunes HV, Degirmenci İ, Oner KS, et al. Determination of the relationship between rs4986790 and rs4986791 variants of TLR4 gene and lung cancer. Inflammation. 2012;39:166–71. doi: 10.1007/s10753-015-0235-9. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Yang W, Wu B, Liu Y, Li D, Guo Y, et al. KDM3A promotes inhibitory cytokines secretion by participating in TLR4 regulation of Foxp3 transcription in lung adenocarcinoma cells. Oncol Lett. 2017;13:3529–37. doi: 10.3892/ol.2017.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan N, Giraud V, Picard C, Nunes H, Dastot-Le Moal F, Copin B, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet. 2016;25:1457–67. doi: 10.1093/hmg/ddw014. [DOI] [PubMed] [Google Scholar]
- 43.Grageda M, Silveyra P, Thomas NJ, DiAngelo SL, Floros J. DNA methylation profile and expression of surfactant protein A2 gene in lung cancer. Exp Lung Res. 2015;41:93–102. doi: 10.3109/01902148.2014.976298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordgård O, Singh G, Solberg S, Jørgensen L, Halvorsen AR, Smaaland R, et al. Novel molecular tumor cell markers in regional lymph nodes and blood samples from patients undergoing surgery for non-small cell lung cancer. PLoS One. 2013;3:e621–53. doi: 10.1371/journal.pone.0062153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–9. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y, Jiang Z, Tong F, Li M, Yin X, Hu S, et al. Experimental study of peripheral-blood pro-surfactant protein B for screening non-small cell lung cancer. Acta Cir Bras. 2017;32:568–75. doi: 10.1590/s0102-865020170070000008. [DOI] [PubMed] [Google Scholar]
- 47.Sin DD, Tammemagi CM, Lam S, Barnett MJ, Duan X, Tam A, et al. Pro-surfactant protein B as a biomarker for lung cancer prediction. J Clin Oncol. 2013;20:4536–43. doi: 10.1200/JCO.2013.50.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang XY, Zheng BQ, Han BH, Huang JS, Geng Q, Xu HL, et al. Lung adenocarcinoma stem cell phenotypes and their correlation with patient prognosis. Zhonghua Zhong Liu Za Zhi. 2009;31:836–40. [PubMed] [Google Scholar]
- 49.Ishii T, Hagiwara K, Ikeda S, Arai T, Mieno MN, Kumasaka T, et al. Association between genetic variations in surfactant protein d and emphysema, interstitial pneumonia, and lung cancer in a Japanese population. COPD. 2012;9:409–16. doi: 10.3109/15412555.2012.676110. [DOI] [PubMed] [Google Scholar]