Abstract
Pseudomonas aeruginosa (P. aeruginosa) is a major nosocomial pathogen of increasing relevance to human health and disease, particularly in the setting of chronic wound infections in diabetic and hospitalized patients. There is an urgent need for chronic infection models to aid in the investigation of wound pathogenesis and the development of new therapies against this pathogen. Here, we describe a protocol that uses delayed inoculation 24 hours after full-thickness excisional wounding. The infection of the provisional wound matrix present at this time forestalls either rapid clearance or dissemination of infection and instead establishes chronic infection lasting 7–10 days without the need for implantation of foreign materials or immune suppression. This protocol mimics a typical temporal course of post-operative infection in humans. The use of a luminescent P. aeruginosa strain (PAO1:lux) allows for quantitative daily assessment of bacterial burden for P. aeruginosa wound infections. This novel model may be a useful tool in the investigation of bacterial pathogenesis and the development of new therapies for chronic P. aeruginosa wound infections.
Keywords: Immunology and Infection, Issue 156, Pseudomonas, Pseudomonas aeruginosa, wound model, chronic infection, wound infection, bioluminescence imaging
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a Gram-negative rod-shaped bacterium with increasing relevance to human health and disease. It is responsible for extensive morbidity and mortality in nosocomial settings, particularly involving wound infections in immunocompromised patients1,2. The emergence of multidrug-resistant strains of this pathogen has provided further impetus for investigation into factors contributing to P. aeruginosa virulence, mechanisms of P. aeruginosa antibiotic resistance, and new methods for prevention and treatment of this deadly infection3. As such, the need for animal models of chronic wound infection as tools for investigating these research questions has never been greater.
Unfortunately, many animal models of P. aeruginosa infection tend to simulate acute infection with rapid resolution of infection or rapid decline due to sepsis4,5, which does not adequately simulate the oftentimes chronic nature of these infections. To address this drawback, some models utilize the implantation of foreign bodies such as agar beads, silicone implants, or alginate gels6,7,8. Other models use mice that are immunocompromised due to advanced age, obesity, or diabetes, or through pharmacological means such as cyclophosphamide-induced neutropenia9,10,11,12. However, either the use of foreign materials or immune compromised hosts likely alters the local inflammatory process, making it difficult to gain an understanding of the pathophysiology involved in chronic wound infections in hosts with otherwise normal immune systems.
We have developed a chronic model of P. aeruginosa wound infection in mice that involves delayed inoculation with bacteria after excisional wounding. Delayed inoculation allows for experiments assessing bacterial burden extending out to at least 7 days. This model opens up new opportunities for investigating both pathogenesis and new treatments of P. aeruginosa chronic infections.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) at Stanford University.
1. Preparation and growth of bacteria
Conduct all work with P. aeruginosa and animals with BSL-2 precautions per the researcher’s institutional biosafety committee and animal use committee guidelines. Do all steps described here involving P. aeruginosa, including mouse inoculation, in a biosafety cabinet.
The luminescent PAO1:lux strain of P. aeruginosa is available from our lab by request. Streak PAO1, stored as frozen glycerol stock, on Lysogeny Broth (LB) agar. For the luminescent PAO1:lux strain, LB agar should contain selective antibiotics (100 μg/mL carbenicillin and 12.5 μg/mL kanamycin). Grow at 37 °C overnight in a bacterial incubator.
Pick an isolated colony and grow overnight at 37 °C in 3 ml LB medium, pH 7.4. For luminescent strains, broth should contain 100 μg/mL carbenicillin. Grow under shaking, aerobic conditions.
2. Procedure preparation
Have all personnel performing surgery wear a clean gown/Lab coat, face mask, hair net, and gloves.
Autoclave all surgical tools, including scissors and forceps. Use aseptic technique to sterilize tools between animals.
Clean the surgical table with ethanol and prepare a clean surgical field.
3. Hair removal
- Anesthetize 8–12 week old C57BL/6J mice using 1%–3% isoflurane. Investigators should follow their institution’s veterinary staff guidelines for anesthesia when using isoflurane.
- Start anesthesia by delivering 1%–3% isoflurane and adjust oxygen flow rate to 1.5 L/min. Place the mouse in the induction chamber.
- Pinch the mouse’s toe to assess depth of anesthesia. When the mouse no longer responds to stimulation, remove it from the induction chamber and place it on the surgical bench with its nose in the isoflurane nose cone.
- Apply ocular lubricant to both eyes.
Weigh the mouse to obtain a baseline pre-procedure weight.
Place the mouse in prone position. Inject the mouse subcutaneously with pre-warmed sterile 0.9% sodium chloride, 250 μL at each flank for a total of 500 μL.
Shave the dorsal area of the mouse using an electric shaver. Shaving should occur at a different location than the surgical station to prevent hair contamination of the wound.
Apply a thin layer of hair removal lotion. Let the lotion sit for 20–60 s. Remove the hair and excess lotion with gauze moistened in warm water. Following hair removal, proceed to the excisional wounding procedure.
4. Full thickness excisional wound surgery
Inject sustained release buprenorphine 0.6–1 mg/kg subcutaneously using a 25 G needle at the mid-dorsal area of the mouse. Slow release buprenorphine provides pain relief over 48–72 h.
Disinfect the surgical site. Wipe the dorsal surface with a sterile betadine swab. Wipe excess betadine with a sterile alcohol swab. This should be performed 3 times (alternating between betadine and alcohol), swabbing by moving from the center in a circular manner to the edge. Allow the area to air dry.
Create a drape surrounding the surgical site using sterile gauze or plastic cling wrap.
Stretch skin taut caudally. Use a sterile 6-mm diameter skin biopsy punch to make an initial incision through the left dorsal epidermis. Repeat on the right dorsal epidermis.
Use forceps to tent the skin from the center of the left outlined wound area. Excise the epidermal and dermal layers using scissors. Repeat on the right outlined wound area to create symmetrical excisional wounds.
Wash wounds with 50 μL of sterile saline. Allow the surgical site and surrounding skin to air dry. Then, cover the wounds and dorsum with a transparent film dressing.
Place the mouse back in a clean cage. House 1 animal per cage.
Place the cage on a heating pad and monitor until the mouse wakes up.
When performing the above surgery on multiple animals, use a hot bead sterilizer to clean all surgical instruments between animals.
Allow 24 h for the mice to recover from the surgical procedure and for formation of a provisional wound matrix over the wounds prior to proceeding to inoculation with bacteria.
5. Inoculation with P. aeruginosa
Dilute overnight PAO1:lux culture to OD600 = 0.05 in 75 mL of LB media containing 100 μg/mL carbenicillin and grow the bacteria until the culture is in early exponential phase (OD600 ≈ 0.3). This should take approximately 2–3 h.
Dilute PAO1:lux in PBS to a concentration of (7.5 ± 2.5) × 102 CFU/mL. Be sure to prepare excess inoculum to ensure sufficient volume and to allow for plating after the experiment. If transporting between facilities (i.e. from the lab to the vivarium), use double containment in a leak proof box clearly marked Biohazard.
Perform all work with P. aeruginosa and mice using approved personal protective equipment in an Animal Biosafety Level 2 (ABSL-2) approved biological safety cabinet (BSC). Reusable equipment such as the weighing scale should be covered with cling wrap to prevent contamination.
Anesthetize using 3% isoflurane as described above. Weigh mouse and record the weight. Inject the mouse subcutaneously with pre-warmed sterile 0.9% sodium chloride, 250 μL at each flank for a total of 500 μL.
If the mouse’s transparent film dressing has come off overnight, remove any resulting scab carefully and put on a new dressing.
Use a 500 μL tuberculin 27 G safety cap syringe to inject 40 μL of the PAO1:lux suspension through the transparent film dressing into each wound. Different mice should be used for non-inoculated/PBS wound controls in order to prevent cross-contamination from the contralateral side.
Place the mouse back in its cage on a heating pad and monitor until it wakes up. All mice should be housed individually in separate cages to prevent cross-contamination.
Provide high calorie nutritional supplement paste sandwiched between food pellets on the floor of the cage.
Use the remaining inoculum to streak an LB agar plate. Count colonies to confirm the number of bacteria administered.
6. In vivo imaging of infected wounds
Follow BSL-2 containment protocols for transport of mice to and from the imaging instrument, including use of a secondary container. Be careful not to transfer or drop any animal bedding during the transfer of the mouse to the induction chamber or imaging instrument.
Induce anesthesia of the mouse with inhaled 1%–3% isoflurane in an induction chamber as described in step 3.1.
Once the mouse is anesthetized, place it in prone position in the imaging chamber of an optical imaging system with the nose in the isoflurane nose cone.
Open the software program.
The acquisition parameters will vary based on the number of animals imaged simultaneously and intensity of bioluminescence. The basic parameters to set include exposure time, binning, f/stop, and field of view (FOV). Our default starting settings are exposure time 30 seconds, binning low (2), f/stop 1.2, and FOV 25. Adjust these settings as needed depending on the researcher’s needs.
- Analyze luminescence data using an imaging program (see Table of Materials). Luminescence will be represented as a pseudocolor image overlaid on a color photograph of the mice.
- Create a region of interest (ROI) at the wound site and measure the average flux (photons/second) detected. Note that data can also be reported as radiance (photons/second/cm2/steradian), but as long as the distance of the imaging platform from the camera remains constant between imaging, flux is sufficient.
- Measure background by creating a ROI at a random area on the imaging platform. Subtract the background number of photons/second.
- Export the data to a spreadsheet for further analysis.
Perform imaging as described above as often as daily to track infection progression.
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| 0.9% Sodium Chloride injection | Hospira | 2484457 | |
| 18 G × 1 sterile needle | BD | 305195 | |
| 25 G × 1 1/5 sterile needle | BD | 305127 | |
| Alcohol swab | BD | 326895 | |
| Aura Imaging Software | Spectral Instruments Imaging | n/a | |
| Betadine | Purdue Frederick Company | 19-065534 | |
| Buprenorphine SR LAB | Zoopharm | n/a | |
| C57BL/6J male mice | The Jackson Laboratory | 000664 | |
| Disposable biopsy punch, 6mm | Integra | 33–36 | |
| Fine scissors - Tungsten Carbide | Fine Science Tools | 14568-09 | |
| Glass Bead Dry Sterilizer | Harvard Apparatus | 61-0183 | |
| Granulated Agar | Fisher BioReagents | BP9744 | |
| Heating Pad | Milliard | 804879481218 | |
| Insulin syringe with 28 G needle | BD | 329461 | |
| Lago X Imaging System | Spectral Instruments Imaging | n/a | |
| LB broth | Fisher BioReagents | BP1426 | |
| Leur-Lok 1 mL syringe | BD | 309628 | |
| Mini Arco Animal Trimmer | Wahl Professional | 919152 | |
| Nair Hair Removal Lotion with Baby Oil | Church and Dwight | n/a | Available at any pharmacy |
| Octagon Forceps | Fine Science Tools | 11041-08 | |
| Petri dish | Falcon | 351029 | |
| Phosphate Buffered Saline (PBS) 1x | Corning | 21-040-CV | |
| Press and Seal Cling Wrap | Glad | n/a | |
| SafetyGlide Insulin syringe with 30 G needle | BD | 305934 | |
| Safetyglide Insulin syringe, 1/2 mL, 30 G × 5/16 TW | BD | 305934 | |
| Scale | Ohaus Scout Pro | SP202 | |
| Supplical Nutritional Supplement | Henry Schein Animal Health | 29908 | |
| Tegaderm, 6 cm × 7 cm | 3M | 1624W |
7. Postoperative management
Monitor mice according to guidelines set by the researcher’s IACUC protocol. We monitor all mice daily for the first 4 days, then every other day until the end of the experiment. Weigh the mice once per day for the first 4 days post-surgery in a BSC. Inject 250 μL of 0.9% sodium chloride subcutaneously on post-infection days 1 and 2.
Check for signs of pain/distress in the mice, including hunched posture, scruffy coat, lethargy, difficulty breathing, facial grimace, and weight loss.
If animals display signs of deteriorating health, consult with a veterinarian. Any mouse that appears to show worsening signs of pain/distress and a weight loss of 20% or greater should be euthanized.
8. Wound excision
At the end of the experiment, sacrifice the mice using CO2 inhalation, followed by cervical dislocation. Dispose of the animal carcass according to the institution’s ABSL-2 protocols.
Excise wound beds using sterile scissors and forceps in a BSC. Place each wound bed in 1 mL sterile PBS in a 1.5 mL polypropylene tube. Mince the wound tissue with scissors. All wounds should be treated as ASBL-2, even if they are not considered infected.
Incubate on a shaker at 300 rpm for 2 h at 4 °C. Vortex each tube for 10 s and serially dilute the bacterial effluent in PBS. Plate the diluted bacterial effluent on LB agar to enumerate the bacterial burden.
Consider wounds infected if luminescent signal in the wound is above background luminescence and more bacteria are detected in the wound effluent than in wounds inoculated with PBS as control.
Representative Results
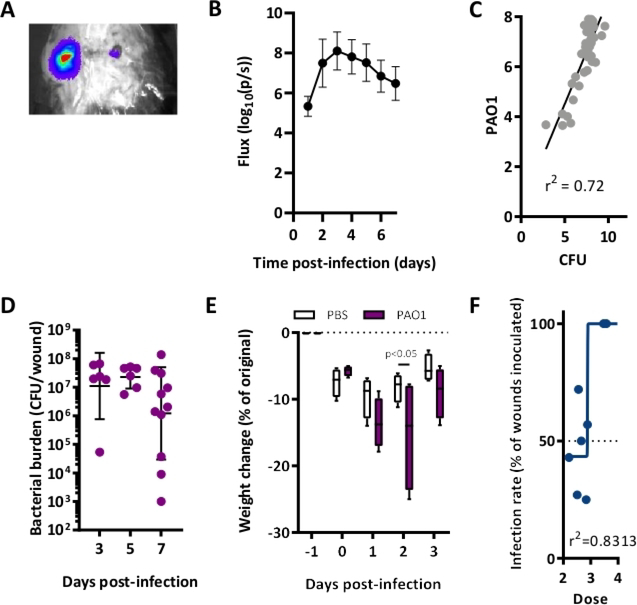
Using a luminescent strain of PAO1 with a plasmid encoding the luxABCDE reporter system (PAO1:lux), we performed excisional wounding on mice, inoculated these wounds with planktonic P. aeruginosa 24 h later, and measured bacterial burden over time (Figure 1 and Figure 2). A representative image obtained using an imaging optical system demonstrates that this model results in detectable luminescence (Figure 3A). Infection peaked at day 3 post-inoculation and persisted 7 days post-inoculation based on both bioluminescence and colony counts (Figure 3B–C). Using this model, we are able to reliably generate wounds lasting 7–10 ays depending on the strain of bacteria and the mouse background. Culture of the bacteria isolated from the wound showed that quantified CFU/wound correlated with detected luminescence (Figure 3D). Finally, despite demonstrable and quantifiable P. aeruginosa infection, mice survived for at least 7 days. Though there was initial rapid weight loss immediately after infection, saline injections and supplemental nutrition resulted in restoration of weight (Figure 3E). Finally, we calculated the inoculation dose at which 50% of wounds would become sustainably infected with PAO1. The calculated IC50 value was ~7.7 × 102 CFU/mL. Doses higher than 104 CFU/mL resulted in 100% infection rate (Figure 3F). These results were adapted from previously published data13.
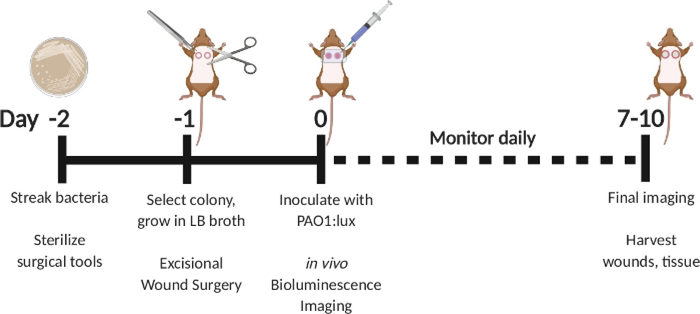
Figure 1: Schematic depicting the excisional full-thickness wound infection model with delayed inoculation with bioluminescent P. aeruginosa.
Streak PAO1:lux on day −2. Select a colony on day −1 and grow overnight in LB broth. Perform excisional wound surgery on day −1. On day 0, inoculate with PAO1:lux by injecting into the wound bed through the transparent film dressing. Perform bioluminescence imaging after inoculation. Repeat imaging as often as daily through day 7–10. On day 7–10, sacrifice animals and harvest wounds.
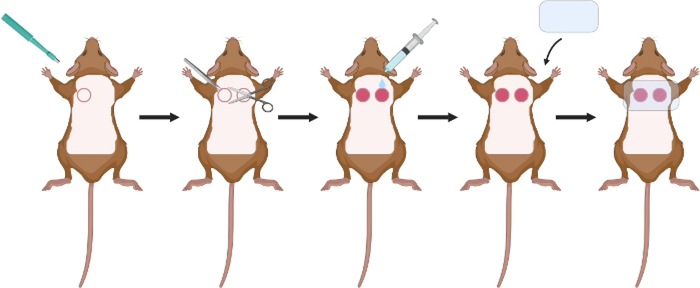
Figure 2: Schematic of excisional wound surgery.
After hair removal and sterilization of skin with alcohol and betadine, use a 6 mm biopsy punch to make an initial incision through the epidermis of the left and right dorsum. Use scissors and forceps to remove the dermal and epidermal layers. Wash with PBS. Cover with a clear dressing.
Figure 3: P. aeruginosa wound infection can be detected through 7 days of infection.
(A) Representative image of a mouse infected with PAO1:lux with bioluminescence overlay on excisional wounds. (B) Luminescent signal reflecting wound bacterial burden. N = 6 wounds. Inoculation: 105 CFU/mL PAO1. (C) Linear regression analysis of in vivo luminescent signal and bacterial CFU of PAO1:lux-infected wounds, collected 4–7 days post-inoculation with 105 CFU/mL to allow for a range of bacterial burdens. (D) Bacterial burden in CFU/wound over time in mice infected with 105 CFU/mL PAO1:lux. (E) Weight change (relative to weight before wound excision surgery on T = −1) N = 4 mice/group. Depicted are boxplots for 5–95 percentile. Statistics are Two-way ANOVA corrected with Sidak multiple comparison. (F) Nonlinear regression analysis of wound infection rate used to calculate the IC50 for PAO1 three days post-inoculation. All graphs are representative of n≥3 experiments. This figure has been modified from Sweere et al. 201913.
Discussion
We have developed a novel delayed inoculation P. aeruginosa wound infection model. The strategy of delaying inoculation with bacteria until 24 h after excisional wounding enables the evaluation of wound infections over a 1-week timeframe. By using a luminescent strain of P. aeruginosa, it is possible to track infection progression throughout the infection course. The longer course of infection compared to other P. aeruginosa infection models will allow new opportunities for studying host-pathogen interactions and novel therapies targeting P. aeruginosa wound infections. For example, we have already used this model to demonstrate the role of Pf bacteriophage in stimulating an antiviral immune response that allows P. aeruginosa to evade the host immune system14.
The inclusion of 24 h of recovery time between the excisional wound surgery and bacterial inoculation is a critical step in this wound infection model. It allows the animals to recover from the wound surgery before being infected, which likely plays a role in their ability to survive through at least 7 days. Furthermore, it supports the formation of a provisional wound matrix prior to inoculation, which, in combination with the transparent film dressing, provides an environment conducive to biofilm formation. We performed a series of exploratory experiments during the development of this model looking at different amounts of time between wounding and inoculation. In our experience, immediate inoculation after wounding often results in sepsis and death. Conversely, inoculation at 48 hours and later time points led to unacceptable levels of heterogeneity between mice and between wounds on the same mouse. As evident in Figure 3A, there can still be some degree of expected heterogeneity in the bacterial load of infected wounds, even with the 24-h delayed inoculation. One way to compensate for this is to use multiple animals in each experiment.
A unique aspect of this protocol is the excision of two wounds independently rather than folding the skin down the midline and punching through to create two bilateral wounds, as is done in some other wound models. We found that this folding method was also effective for producing symmetrical wounds with clean margins. However, mice treated in this manner typically became septic quickly after bacterial inoculation and died. We believe that this may be because this folding method removes the dermal Panniculus carnosus - a thin layer of muscle underlying the skin of mice that is not present in humans. We speculate that this barrier may help prevent bacterial dissemination.
An important component of this protocol is sufficient hair removal from the surgical site, as excess hair could provide a nidus for superinfection and interferes with adherence of the transparent film dressing. We have found that shaving in addition to hair removal cream provides optimal hair removal with minimal skin irritation, though thoroughly washing off the cream with warm water is still important.
Another integral factor is nutritional support, hydration, and pain control during the first few days of the postsurgical and infection course. To address this, we administer 500 μL of subcutaneous 0.9% sodium chloride on day −1 and 0, then 250 μL on day 1 and 2. We also supply a multivitamin and caloric liquid gel supplement at the time of the excisional wound surgery. As demonstrated in Figure 3E, these measures allow for adequate recovery of weight by Day 3. With regard to analgesia, we have found that sustained release 0.5 mg/kg buprenorphine given prior to the excisional wound surgery provides sufficient pain control for 72 h, but additional doses can be given if warranted based on findings of distress in any individual mouse.
Though a model of P. aeruginosa wound infection extending to 7–10 days is a significant advantage over more acute models of infection, this still may not be sufficient for answering certain research questions involving more chronic infections. One reason for this limitation is that the luminescent signal of the PAO1:lux strain of P. aeruginosa peaks at day 2–3 (Figure 3B). After ~7 days the luciferase signal can become unreliable. For this reason, we quantify the bacteria by plating wound effluent on LB plates and counting CFUs in order to confirm infection of each wound at the end of the experiment. Another possible disadvantage of this model is the requirement for an in vivo imaging system, which some labs may not have access to. One way around this obstacle is to use non-luminescent wild type bacterial strains. This still allows the researcher to take advantage of this chronic infection model and, as indicated above, the bacteria CFUs can be quantified at the end of the experiment.
We have described a novel model of delayed inoculation P. aeruginosa wound infection that enables experiments extending to 7–10 days. This model will serve as a useful tool for evaluating the host-pathogen interaction with P. aeruginosa, as well as the development of new therapies. This model has the potential for multiple future directions, including adaptation for other wound pathogens such as Staphylococcus aureus, as well as polymicrobial infections including P. aeruginosa combined with other pathogens.
Acknowledgments
The pUT-Tn5-EM7-lux-Km1 luminescent construct vector was a gracious gift from J. Hardy. Schematics were created with BioRender.com. We thank the lab of G. Gurtner for their advice on the wound infection model. We also thank T. Doyle from the Stanford Center for Innovation in In Vivo Imaging for his technical expertise. This work was supported by grants R21AI133370, R21AI133240, R01AI12492093, and grants from Stanford SPARK, the Falk Medical Research Trust and the Cystic Fibrosis Foundation (CFF) to P.L.B. C.R.D was supported by T32AI007502. A Gabilan Stanford Graduate Fellowship for Science and Engineering and a Lubert Stryer Bio-X Stanford Interdisciplinary Graduate Fellowship supported J.M.S.
Footnotes
Disclosures
The authors have no competing financial interests to disclose.
References
- 1.Sen CK et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 17 (6), 763–771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serra R et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Review of Anti-infective Therapy. 13 (5), 605–613 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Obritsch MD, Fish DN, MacLaren R, Jung R Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 25 (10), 1353–1364 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Secor PR et al. Filamentous Bacteriophage Produced by Pseudomonas aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection In Vivo. Infection and Immunity. 85 (1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice SA et al. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. The ISME Journal. 3 (3), 271–282 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayes HK, Ritchie N, Irvine S, Evans TJ A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Scientific Reports. 6, 35838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gennip M et al. Interactions between polymorphonuclear leukocytes and Pseudomonas aeruginosa biofilms on silicone implants in vivo. Infection and Immunity. 80 (8), 2601–2607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trøstrup H et al. Pseudomonas aeruginosa biofilm aggravates skin inflammatory response in BALB/c mice in a novel chronic wound model. Wound Repair and Regeneration. 21 (2), 292–299 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Zhao G et al. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair and Regeneration. 20 (3), 342–352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. Journal of Immunolology. 190 (4), 1746–1757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watters C et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Medical Microbiology and Immunology. 202 (2), 131–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C, Kerrigan CL,Picard-Ami LA Cyclophosphamide-induced neutropenia: effect on postischemic skin-flap survival. Plastic and Reconstructive Surgery. 89 (6), 1092–1097 (1992). [PubMed] [Google Scholar]
- 13.Sweere JM et al. The immune response to Chronic Pseudomonas aeruginosa wound infection in immunocompetent mice. Advances in Wound Care. Published online 6 Aug 2019. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweere JM et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 363 (6434) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]