Version Changes
Revised. Amendments from Version 1
Edits following review mainly includes changes (i.e. corrections, clarifications, and extended discussion) to the text in the results and discussion sections, but also updated figures 3 and figure 4.
Abstract
Background: Despite technological advances, how specific cell types are involved in brain function remains shrouded in mystery. Further, little is known about the contribution of different ion channel currents to cell excitability across different neuronal subtypes and their dendritic compartments in vivo. The picture that we do have is largely based on somatic recordings performed in vitro. Uncovering dendritic ion channel current contributions in neuron subtypes that represent a minority of the neuronal population is not currently a feasible task using purely experimental means.
Methods: We employ two morphologically-detailed multi-compartment models of a specific type of inhibitory interneuron, the oriens lacunosum moleculare (OLM) cell. The OLM cell is a well-studied cell type in CA1 hippocampus that is important in gating sensory and contextual information. We create in vivo-like states for these cellular models by including levels of synaptic bombardment that would occur in vivo. Using visualization tools and analyses we assess the ion channel current contribution profile across the different somatic and dendritic compartments of the models.
Results: We identify changes in dendritic excitability, ion channel current contributions and co-activation patterns between in vitro and in vivo-like states. Primarily, we find that the relative timing between ion channel currents are mostly invariant between states, but exhibit changes in magnitudes and decreased propagation across dendritic compartments. We also find enhanced dendritic hyperpolarization-activated cyclic nucleotide-gated channel (h-channel) activation during in vivo-like states, which suggests that dendritically located h-channels are functionally important in altering signal propagation in the behaving animal.
Conclusions: Overall, we have demonstrated, using computational modelling, the dynamical changes that can occur to ion channel mechanisms governing neuronal spiking. Simultaneous access to dendritic compartments during simulated in vivo states shows that the magnitudes of some ion channel current contributions are differentially altered during in vivo-like states relative to in vitro.
Keywords: Hippocampus, interneuron, computational neuroscience, ion channels, dendrites, synapses.
Introduction
Since the days of Hodgkin & Huxley 1– 4, there have been tremendous advances in techniques to probe cellular activities 5– 11. However, the “gold standard” of electrophysiological experiments — patch-clamp recordings — is one of the most difficult and laborious types of experiments to perform in live animals 10. Although neurons have been recorded intracellularly in vivo 12– 15, inhibitory interneuron subtypes are more often recorded juxtacellularly 16– 19. This is because inhibitory interneurons represent a small percentage of the neuronal population, which makes it more difficult to access and patch them relative to pyramidal cells – though there are some studies in barrel cortex where interneuron patch clamp recordings have been obtained 20, 21. Patch-clamp experiments are vitally beneficial to our understanding since they can provide clear signals of single-cell and single-channel activity at a high temporal resolution. Using this technique in combination with ion channel blockers helps uncover the ion channel mechanisms through which cell excitability is governed. Because in vivo patch-clamp recordings of interneurons are so difficult to perform, not much is known about their ion channel current contribution profiles in vivo or how they might differ from in vitro. Moreover, attempting to experimentally assess different ion channel currents in dendritic compartments of interneuron subtypes carries with it a risk for loss of time and resources in return for only a small amount of data. Indeed, most experiments focus on attaining neuronal recordings in somata, since the comparatively thinner dendrites and axons are more difficult to patch.
Using a combination of biochemical and electrophysiological techniques, it has been shown that neurons, especially inhibitory neurons, can be characterized into many different cellular classes 22– 27. In the CA1 region of the hippocampus, inhibitory interneurons represent about 10–15% of neurons 28, and because they are more diverse than the excitatory cell population, they are also more difficult to isolate and record. As such, a lot less is known about their activities in vivo and the ion channel mechanisms governing their excitability, although we note that there have been several studies using calcium imaging or extracellular recording techniques to uncover interneuron firing or activation patterns during behaviour (e.g. see 16– 19, 29, 30). One such interneuron type whose firing patterns have been characterized experimentally in awake and behaving animals, is the oriens lacunosum moleculare (OLM) cell 17, 18, 31. In CA1, OLM cells have somata and dendrites in the stratum oriens/alveus, and receive inputs from local pyramidal cells 32, bistratified cells 32, interneuron specific 3 cells 33, and long-range projecting GABAergic inputs from medial septum 33, among others. They have axons projecting to the stratum lacunosum moleculare where they synapse onto the distal dendrites of pyramidal cells, which allows them to gate the flow of sensory information from entorhinal cortex 32, 34. Though OLM cell (both putative and confirmed) intrinsic properties have been studied extensively in vitro 35– 41 and computationally 42– 47, much remains unknown about the ion channel current profiles across their dendritic trees as well as how ion channel mechanisms affect OLM cell firing in vivo during behaviour.
Previous experimental studies have shown that neurons exhibit excitability differences in vivo compared to in vitro, due to the effects of synaptic bombardment causing “high-conductance states” 12, 15, 48, 49. Though these experiments have mostly focused on recordings from the excitatory cell population, there is some evidence that interneurons show similar differences in vivo versus in vitro 50. With morphologically-detailed cellular models containing biophysical ion channel mechanisms it is possible to create in vivo-like states by including a plethora of excitatory and inhibitory synaptic inputs 12, 51, 52. With such models, one can easily probe and record multiple ion channel current types concurrently across different dendritic compartments. In doing so, one can predict how ion channel current contributions may change between in vitro and in vivo-like states as well as across dendritic compartments.
In this work we use two morphologically-detailed models of OLM cells and bombard them with synaptic inputs so as to create in vivo-like states. We use these models to determine somatic and dendritic ion channel current contributions to excitability that may occur in the behaving animal. In doing so, we highlight changes in dendritic excitability, ion channel current contributions and co-activation patterns relative to in vitro states. Specifically, we find enhanced dendritic hyperpolarization-activated cyclic nucleotide–gated (HCN) channel activation during in vivo-like states, which suggests a specific role for these channels in altering signal propagation in the behaving animal.
Methods
Neuron models
We use two previously developed multi-compartment models of OLM cells (i.e. cell 1 and cell 2) 47. Each model was developed using a morphological reconstruction and electrophysiological dataset obtained from the same cell, including I H current recordings, which predicted that models with somatodendritic I H, rather than just somatic I H, best matched the data. Thus, the passive and active properties of each of the two models are specific to cell 1 or cell 2 and are not identical. For cell 1 and cell 2, the surface areas are 29,378.1 μm 2 & 35,158.5 μm 2, the input resistances are 360.1 MΩ & 490.2 MΩ, and the membrane time constants are 22.6 ms & 32.0 ms, respectively. Electrotonic distances are shown in Figure 1A, and for example, we note that cell 2 exhibits electrotonic distances that are up to twice as large as those seen in cell 1 ( Figure 1A) indicating more signal attenuation in cell 2. These models were developed in NEURON 53 and codes for the models are available on https://github.com/FKSkinnerLab/OLMng. Simulations performed for the present paper are run using NEURON 7.5 as a module in Python 2.7.
Figure 1. Cell 2 is more compartmentalized than cell 1, and F-I relationship is linear past rheobase.

A: Electrotonic distance [i.e. decay of a 1 mV signal; log(voltage upstream/voltage downstream)] for voltage flowing into the soma (Vin; top) and voltage flowing out of the soma (Vout; bottom). B: Relationships between holding current and resulting spike rates in cell 1 and 2 models in vitro (red), as compared to the experimental data upon which they were optimized (blue).
Ion channel types in our OLM cell models include: hyperpolarization-activated cyclic nucleotide-gated ( H), transient sodium ( NaT), fast and slow delayed rectifier potassium ( Kdrf, Kdrs), A-type potassium ( KA), M-type potassium ( M), T- and L-type calcium ( CaT, CaL), and calcium-dependent potassium ( KCa) channels. Equations describing them are given in the Appendix of 43, but specifically for H and NaT channel mechanisms, they are given in 47. Maximal conductances in soma ( s), axon ( a) or dendrites ( d) are represented respectively as G H, G NaT, G Kdrf, G Kdrs, G KA, G M, G CaT, G CaL, G KCa, as given in Table 1. Note that I { channel} is used to refer to the corresponding ion channel currents of the various ion channel types. Other parameters for activation, inactivation and time constants are given in 43 or 47 as specified above.
Table 1. Location and conductance values for ion channel types.
|
Conductance
type |
Distribution
location |
Cell 1
G (pS/µm 2 ) |
Cell 2
G (pS/µm 2 ) |
|---|---|---|---|
| G NaT, s | soma | 70.99 | 75.09 |
| G NaT, d | dendrites | 99.48 | 64.68 |
| G NaT, a | axon | 66.42 | 140.89 |
| G Kdr f, s | soma | 115.47 | 91.15 |
| G Kdr f, d | dendrites | 50.49 | 52.52 |
| G Kdr f, a | axon | 155.97 | 144.03 |
| G Kdrs, s | soma | 0.0054 | 0.0070 |
| G Kdrs, d | dendrites | 0.0038 | 0.0062 |
| G Kdrs, a | axon | 0.0082 | 0.0024 |
| G KA | soma, dendrites | 76.08 | 110.18 |
| G Ca L | dendrites | 47.19 | 22.01 |
| G CaT | dendrites | 1.01 | 3.74 |
| G KCa | dendrites | 0.14 | 7.08 |
| G M | soma, dendrites | 0.14 | 0.18 |
| G H | soma, dendrites | 0.1063 | 0.0608 |
Synapse model
For the synapse model, we use NEURON’s built-in Exp2Syn function, which models synaptic current as a two-state kinetic scheme.
Where i is the synaptic current, G is the maximal synaptic conductance, E is the reversal potential, V is the membrane potential, weight is the synaptic weight, factor is a NEURON process that is used to normalize the peak synaptic conductance to the value of weight, t is time, τ r is the rise time, and τ d is the decay time.
Target input populations to OLM cell models
The input populations to OLM cells that we model include interneuron specific 3 (IS3) cell inputs, GABAergic long-range projecting inputs from medial septum (MS), bistratified (BIS) cell inputs, and local pyramidal (PYR) cell inputs, and, in the absence of specific constraints, distribute them randomly across all dendritic compartments. We focus on these input populations because enough details of EPSCs and IPSCs onto OLM cells have been previously reported in the literature (IS3 cells & MS: see 33; BIS & PYR cells: see 32).
Focusing first on the IS3 cell and MS inputs 33, we note that these were recorded under minimal stimulation using voltage-clamp of OLM cells at +10 mV. Note that there is a junction potential correction of +15.4 mV (personal communication from L. Topolnik, Laval University, QC) such that the holding current is actually nearer to -5.4 mV. This is near the reversal potential of excitatory synapses (0 mV) and so would silence current generated from excitatory synapses, leaving only minimally evoked IPSCs from IS3 cell or MS inputs. For BIS and PYR cell inputs to OLM cells 32, these were not recorded under minimal stimulation (and so these currents could be generated from multiple synaptic inputs) and only the holding potential for recording excitatory inputs is reported (-60 mV; i.e. holding potential while recording BIS cell inputs is not reported). The target features of the EPSCs and IPSCs reported in the literature are summarized in Table 2.
Table 2. Target EPSC and IPSC features for inputs to OLM cells.
| Input Type | Amplitude | Rise Time | Decay Time | Reference |
|---|---|---|---|---|
| IS3 | 13.9 ± 2.0 pA | *1.6 ± 0.2 ms | 12.0 ± 0.9 ms | 33 |
| MS | 23.0 ± 2.3 pA | *1.1 ± 0.2 ms | 12.1 ± 1.1 ms | 33 |
| BIS | **16.9 pA | ***1.35 ms | ***12.05 | 32 |
| PYR | ****-12.14 pA | *****2.4 ms | *****12.7 ms | 32 |
*20–80% rise time of IPSC
**Peak IPSC amplitude is 67.6 ± 7.8 pA, but this is not with minimal stimulation. If assuming 4 synapses per connection (as per in 51), this means 16.9 pA per synapse.
***Not reported, so the values chosen are midway between the values for IS3 and MS inputs since the BIS amplitude falls about midway between the two.
****Peak EPSC amplitude is -109.3 ± 8.7 pA, but this is not with minimal stimulation. If assuming 9 synapses per connection (as per in 51), this means -12.4 pA per synapse.
*****Not reported, so values used are those that were reported for excitatory inputs from Fimbria Fornix.
Also note that E rev , exc is assumed to be equal to 0 mV and that E rev , inh should be -87.1 mV, if taking directly from what is reported experimentally with liquid junction potential correction 33. For E rev , inh, if assuming that there is voltage decay from the soma to the synapse when measuring reversal potential 29, one needs to take a more depolarized value, so we use -80 mV as the inhibitory reversal potential instead. We note that there are other types of inputs to OLM cells that have been reported in the literature (e.g., serotonergic receptors 54, metabotropic glutamate receptors 55, 56, cholinergic receptors 57– 59, additional complexities in NMDA/AMPA/Kainate receptors 60, 61, and TRPV1 receptors 62), but in the absence of particular constraints, we opted to not include them at this time.
Synaptic optimizations
We set the rise and decay time constants to those reported in the literature ( Table 2) and we optimized the synaptic weight parameters. Here, we describe the program that was written to perform this task. Incrementally, for each compartment, we increase the weight until the amplitude of the synaptic current that is generated is approximately equivalent to the target value obtained from the literature. Since past a certain distance from the soma, the electrotonic distance can create an exponential increase in the synaptic weight needed to generate large enough current amplitudes 29, we simply stop the optimizations after 100 iterations of increasing the weight. From our previous experience, letting the algorithm optimize to larger weights than this simply generates synaptic conductance predictions outside of the realm of reality when considering single-receptor conductance values and the maximum numbers of receptors per synapse seen experimentally 29. During these optimizations we assume that all voltage-gated channels are blocked and we set the leak reversal potential to the voltage-clamped holding potential of the model (0 mV when fitting IPSCs, and -60 mV when fitting to EPSCs). Following the optimizations, the synaptic conductances for each input type have increasing values with distance from soma ( G PYR = 0.00020 to 0.00082 µS; G MS = 0.00024 to 0.00132 µS; G IS 3 = 0.00018 to 0.00068 µS; G BIS = 0.00021 to 0.00100 µS), most likely due to the effects of electrotonic decay.
Generating in vivo-like states
In previous work using IS3 cell multi-compartment models 51, we performed high-resolution parameter searches in parallel on the Neuroscience Gateway (NSG) for high-performance computing 63 to find input parameter combinations (i.e. numbers of excitatory and inhibitory synapses and incoming spike rates) that could generate in vivo-like (IVL) states. We applied a similar methodology here for creating IVL states for the OLM cell models.
As done previously 51, we range excitatory spike rates from 0 to 30 Hz (resolution: 5 Hz), and inhibitory spike rates from 0 to 100 Hz (resolution: 10 Hz). We estimate maximal ranges for excitatory and inhibitory synapses based on findings from 64, scaled by the lengths and numbers of compartments in the OLM cell models (cell 1: 0 to 4641 excitatory synapses and we use a resolution of 35 synapses, 0 to 1989 inhibitory synapses with a 24-synapse resolution; cell 2: 0 to 6012 excitatory synapses with a 45-synapse resolution, 0 to 3006 inhibitory synapses with a 36-synapse resolution). Also, for every addition of inhibitory synapses, one third are assigned as IS3 cell inputs, another third are assigned as MS inputs, and a final third are assigned as BIS cell inputs (i.e. 8 IS3, 8 MS and 8 BIS synapses for cell 1; 12 IS3, 12 MS and 12 BIS synapses for cell 2). Note that the total numbers of synapses taken from 64 are numbers estimated for calbindin-expressing (CB+) cells. While certain subtypes of OLM cells express CB, this marker is also broadly expressed in several other cell types 22. As well, the CB+ morphological reconstructions shown in 64 do not appear to carry resemblances to OLM cell morphologies. Nonetheless, these estimates are still used because they are the only estimates that can reasonably be linked to synaptic densities in OLM cells. We note that when synapses are added we assume common inputs (i.e. each presynaptic spike train is assigned to multiple synapses, that is, akin to cells forming multiple synaptic connections between each other - we use 7 excitatory synapses per connection and 8 inhibitory synapses per connection for cell 1, and 9 excitatory synapses per connection and 12 inhibitory synapses per connection for cell 2).
To identify IVL states using simulated voltage recordings, we use a previously designed IVL metric 51 - see Equation 2. This metric uses threshold values based on experimental values found in the literature to establish whether the average subthreshold membrane potential ( ), the standard deviation of the subthreshold membrane potential ( σ V m), and the interspike interval coefficient of variation ( ISICV), are large enough for a given state to be considered IVL 12, 14, 15, 48, 65, 66. It also uses the average spike amplitude ( ), to establish whether the model is entering an overly-excited state of depolarization block. Here we use a depolarization threshold value ( ; -70.588 mV) tailored to the resting potential of OLM cells and assuming an approximate 3 mV shift in vivo. We used this assumption in previous work 51 and it is based on the shift in baseline voltage seen in CA1 place cells in vivo during place field traversals 14. We also add a spike rate criterion to ensure that the spike rate is between 3 and 25 Hz since this is known for OLM cells in vivo 17, 18. The threshold values for membrane potential standard deviation ( σ V m), interspike interval coefficient of variation ( ISICV), and spike amplitude ( S amp) are the same as the values used previously for IS3 cell models 51.
Given this, an IVL metric score of 4 would indicate that the input parameter combination produces an IVL state. Our parameter search yielded a variety of different excitatory/inhibitory input solution sets that could generate IVL states. As well, we observed an input parameter distribution towards low values across all input parameters, and more IVL scenarios were generated whilst in inhibitory-dominant regimes (not shown).
Choosing in vivo-like (IVL) states. We chose representative IVL states from those generated by running ten randomized (i.e., of synaptic locations and presynaptic spike times as done previously 51) simulations for each set of input parameters that generated an IVL state, until one was found to be consistently IVL. A state is considered to be consistently IVL if the IVL metric is 4 in at least five out the ten simulations, and the other five simulations have IVL metrics of at least 3. We focused on sampling from a subset of the parameter space with low inputs (i.e., sparse enough) so as to allow cells to have larger input resistances and be more sensitive to additional rhythmically-timed inputs as previously found to be required 51.
In running the full parameter searches and then choosing representative IVL states from the low input subset of the parameter space we found that the amount of input was still far larger than numbers of inputs seen previously in our other models 51, corresponding to a more reduced input resistance and therefore a reduced sensitivity to additional inputs. To find even sparser input parameter sets that produce IVL states we sampled at a finer resolution (i.e. at a resolution of 1 synapse for numbers of synapses and 1 additional spike for spike rates) from low input parameter value sets that generate similar excitatory/inhibitory balances to the IVL states found in the full parameter search. This balance is as given by our EI Metric calculation of Equation 3 (i.e. cell 1: -42685 to -8785 synapses × Hz; cell 2: -65700 to -9990 synapses × Hz). Once we identified parameter sets that fell within these EI metric ranges, we sampled the parameter combinations in order from the lowest total input to the highest total input (i.e. see Total input calculation in Equation 3), and simulated each of these possible combinations of input parameters until another consistently IVL state was found.
where N E is number of excitatory synapses, N I is number of inhibitory synapses, f E is excitatory spike frequency, and f I is inhibitory spike frequency. The rationale with this approach is that IVL states should still be generated so long as the balance between excitation and inhibition falls within the right range. Following this, we found IVL states for both models where the total input is reduced (cell 1: 12,938.6 vs. 39,250 inputs; cell 2: 14,510.5 vs 46,350 inputs), which ultimately corresponds to input resistances that would allow enough sensitivity to additional inputs, as based on our previous work 51. The resulting input parameter values for cell 1 are 1268 excitatory synapses firing at 1.6 Hz and 1254 inhibitory synapses firing at 8.7 Hz, and for cell 2, they are 1503 excitatory synapses firing at 1.5 Hz and 1532 inhibitory synapses firing at 8 Hz.
Approach and data analysis
To analyze and compare how the ion channel current contributions differ under in vitro (‘isolated slice preparation with synapses blocked’) and in vivo (’behaving animal’) conditions, we use the following approach with the two OLM cell models:
-
1.
Run the models without synapses (i.e., in vitro state) at two different somatic holding currents above rheobase (60 pA and 120 pA). Compute the resulting spike rate and determine the slope ( m) and intercept ( b) of the line between the two data points of spike rates. Linear extrapolation of these values from just two data points is justified since the F-I curves are fairly linear above rheobase ( Figure 1B).
-
2.
Run the models using the input parameter set that produced the chosen IVL state with a given set of random seeds and measure the spike rate ( f IVL) of the resulting spike train. Use f IVL together with m and b calculated previously to compute the holding current ( I hold) necessary to elicit a similar spike rate in the in vitro state:
-
3.
Choose a different set of random seeds and repeat step 2 ten times.
To consider comparisons in different locations, we choose five different recording sites in each model, which include soma and four increasingly distant dendritic compartments (i.e. soma, D1, D2, D3, and D4; Figure 2). The diameters of these locations are: 9.84 μm (soma), 1.92 μm (D1), 0.82 μm (D2), 0.94 μm (D3), and 0.75 μm (D4) for cell 1; 4.44 μm (soma), 1.26 μm (D1), 1.01 μm (D2), 0.74 μm (D3), and 0.60 μm (D4) for cell 2.
Figure 2. Dendritic spiking is deteriorated in the in vivo-like context.
Currentscape plots 67 are shown above the shape plots in A (cell 1) and B (cell 2). The dots in the shape plots indicate the recording sites (S = Soma; D1–D4 = Dendrite 1–4). All recording sites were chosen such that they are along the same dendritic path. In each currentscape plot, the top trace is the voltage trace (y-axis scale bar = -50 mV [horizontal dashed line] to -20 mV), the filled-in black traces above and below the coloured plots are the total outward and inward currents respectively (dotted lines = ±0.5 pA, ±5 pA, ±50 pA), and the coloured plots show the percent contributions of each individual outward (top half of the plot) and inward (bottom half of the plot) current (see colour references in the legend on the right) to the total outward and inward currents. For each recording site location we show one in vitro currentscape plot (top) and one corresponding IVL currentscape plot (bottom), in the last second of simulation time (time axis = 9 s to 10 s). Distance values above each set of in vitro/IVL currentscape plots indicate the recording site distance from soma.
To analyze the total area under the current traces we use the numpy.trapz(current_trace,time_vector) function in Python. For outward currents, more positive values indicate larger currents, and for inward currents, more negative values indicate larger currents.
To compute cross-correlations between two time series (i.e. currents and/or voltage traces; a1 and a2), we first normalize the signals in order to generate cross-correlation magnitudes between -1 and 1, as follows:
a1 = (a1 - numpy . mean (a1)) / ( numpy .std (a1)
* len(a1))
a2 = (a2 - numpy . mean (a2)) / numpy . std (a2) xcorr = numpy . correlate (a1 ,a2 , mode =’full ’)
Note that all inward currents are reversed in polarity for these cross-correlations (i.e. from negative to positive), since their “activation” is reversed with respect to the polarity of voltage and outward current activation. This step allows us to better interpret the cross-correlation plots. Note that I L, though mostly an inward current at baseline, is not reversed in polarity since it becomes an outward current during spikes. Analyses of total area under the current traces and cross-correlations across current and/or voltage traces are done using the last 9 seconds of 10 second-long simulations.
To visualize the contribution of the different ion channel mechanisms to the voltage dynamics, we take advantage of currentscape plots (e.g. Figure 2), a recent visualization technique that plots the percent current contributions to the total inward or outward currents 67. Additional relevant code for running simulations and plotting the results shown in this paper is available online at https://github.com/FKSkinnerLab/OLM_IVLCurrents.
Results
While it is clear that the intense synaptic bombardment present in vivo relative to quiescent in vitro states changes a cell’s response, conferring advantageous computational properties 48, how the different ion channel types present in different locations contribute has not been explored. In creating in vivo-like (IVL) states for our computational OLM cell models, as described in the Methods, we are now in a position to compare differences between in vitro and IVL states from the perspective of ion channel currents in somata and dendrites. As well, we can explore how the different ion channel mechanisms might contribute to cell excitability in vivo.
Dendritic ion channel current contribution profiles change substantially during IVL states relative to in vitro states
To ensure that comparable firing rates exist in the in vitro and IVL states of the models, we inject an appropriate amount of current into the soma of the in vitro models (i.e., OLM cell models without any synaptic inputs), as described in the Methods. The ion channel current contribution changes between in vitro and IVL states for cell 1 and cell 2 are shown in Figure 2 A and B respectively. These changes are shown in somatic compartments as well as across dendritic compartments at locations specified above the plots and as indicated in the reconstructed cell schematics. This is shown through the use of currentscape plots 67 where each color represents the percent of the total inward or outward current that each channel contributes across time. In the in vitro cases, across both models there is a shift in the I Na/ I L balance (pink/brown in Figure 2) with distance from soma, where in more distal dendritic compartments I Na contributions become narrower. However, once moved to the IVL scenario, this shift in I Na contributions is no longer apparent. This is likely because somatic current injections decay with distance from soma and thus recruit I Na in distal dendrites less, whereas in the IVL state, distal dendritic compartments are bombarded directly with synaptic inputs, and thus engage dendritic I Na more directly. A similar observation can be made with the I Kdrf contributions. To see this more clearly, we plot the somatic (S) and a dendritic (D4) location on an expanded axis so that the effect of the synaptic bombardment in dendritic regions can be seen with the voltage fluctuations ( Figure 3, left). The increase in I Na (pink) and I Kdrf (orange) in dendritic locations in the IVL scenario relative to in vitro is very apparent, clearly due to direct synaptic inputs in dendritic regions to activate dendritic ion channels, which would not be the case in vitro. In particular, I Na can persist because of less inactivation, and in cell 2, more depolarized states without spiking occur.
One stark difference between cell 1 and cell 2 is that I M (purple) contributions are almost non-existent in cell 2 dendrites during IVL states. When looking at the voltage traces for those compartments where I M is not contributing, we see that spikes fail to form, with large depolarizations occurring instead. Since I M relative contributions appear largest during interspike intervals (see 43), it is not surprising that its contribution is minimized when there is no spiking and interspike intervals are not present. We further note that cell 2 has an almost two-fold larger maximal electrotonic distance than cell 1 ( Figure 1A), which helps explain why spikes propagate less in the distal dendrites of cell 2 relative to cell 1.
In general, it is clear from looking at the voltages and current contributions that dendritic compartments are more de-correlated from each other and the soma when the model is in an IVL state (i.e. relative to the corresponding in vitro state). This could partially be due to the high-conductance effects of synaptic bombardment where it suppresses input sensitivity and can drown out the magnitude of unitary inputs. As a result, these smaller amplitude signals do not propagate as far, and the different morphological compartments may appear more decorrelated from each other.
Only I H is suppressed during spikes and consistently enhanced during in vivo-like (IVL) states
In looking at the ion channel current traces in a relative comparison using currentscape visualization for soma vs D4 ( Figure 3, left), and in actual values for soma and all dendritic locations ( Figure 3, right), for IVL vs in vitro both cell 1 and cell 2, it is clear that all ion channel types become sharply activated during spikes, except for I H (cyan) which is suppressed during spikes. I L (brown) is also different but from the perspective that it is primarily an inward current at resting potentials, but sharply transitions into an outward current, specifically during spikes. This is the case across both IVL and in vitro states as well as in soma and in the furthest dendritic location (D4), which makes sense given the biophysical characteristics of these channels.
Figure 3. Example current recordings.
Recordings show the last 200 milliseconds of a ten second simulation from an example pair of IVL and in vitro simulations for cell 1 ( A) and cell 2 ( B) with currentscape plots on the left (S and D4 only) and current traces on the right (IVL: dotted lines; in vitro: solid lines). The colour code for the currents is the same as the legend in Figure 2 but fainter coloured lines show current traces from further dendritic compartments from the soma (S to D4).
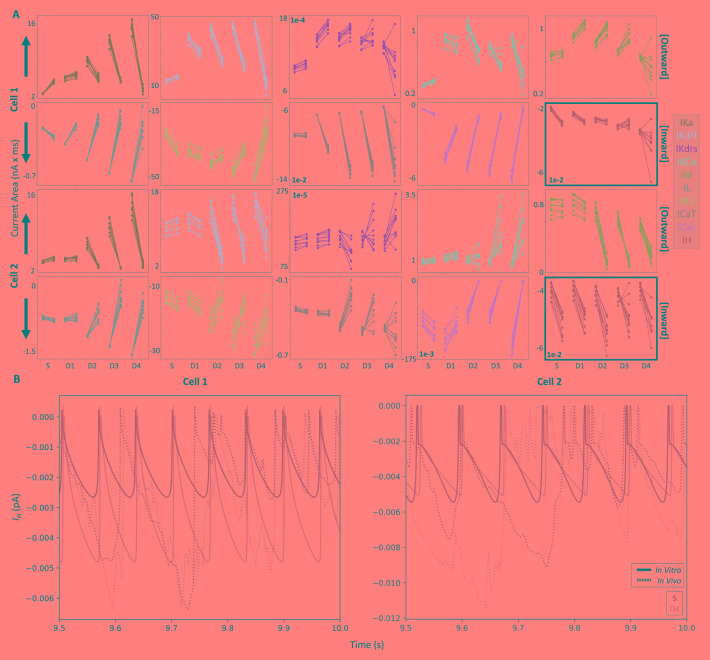
In looking at changes in the total current (i.e. area under the ion channel current traces; Figure 4A) across the two models, four currents changed differently in cell 1 and cell 2 going from the in vitro to the IVL state. These include I CaT (increases dendritically in cell 1, but decreases dendritically in cell 2), I M (increases in cell 1, but decreases dendritically in cell 2), as well as I KCa & I Kdrs (decrease dendritically in cell 1, but increase in cell 2). We already observed and discussed this difference in I M contributions changes in the previous section (i.e. the currentscape plots in Figure 2), and it is possible that the other three ion channel currents show this difference for similar reasons (i.e. a larger suppression of dendritic spike propagation in cell 2 during IVL states), since these differences are not readily observable in the somatic compartments Figure 4A).
Figure 4. In vitro vs in vivo-like total changes in currents.
A) Each line (10 re-randomizations = 10 lines for each recording site) connects in vitro results (left dots) with their corresponding IVL results (right dots). In each plot, from left to right along the x-axis, we plot soma, D1, D2, D3, and D4 (same recording sites shown in Figure 2). Note that outward currents are shown in the top rows (larger contributions = more positive values) and inward currents are shown in the bottom rows (larger contributions = more negative values). As such, the arrows indicate the direction of larger current magnitudes for each row of currents. Also note that the I H plot is highlighted with thicker borders. B) Example of I H traces from the S (darker cyan) and D4 (lighter cyan) compartments during the last 500 ms of IVL (dotted lines) and in vitro (solid lines) simulations.
Across both models, many ion channel currents during IVL states relative to in vitro states also showed increased contributions near the soma but decreased contributions in the dendritic compartments, including I KA, I L, I Kdrf, I Na, I CaL. The only ion channel current that consistently showed increased contributions during IVL states across both models and all compartments was I H. This finding is not altogether surprising because I H is more active in subthreshold voltage ranges and further activated by inhibitory currents. To show in vitro and IVL state differences of I H more directly, we plot. I H from somatic and D4 compartments in Figure 4B, and the increase in total current becomes clear. That is, because the dendritic membrane potential is both bombarded by synaptic inputs and spends more time in the subthreshold voltage range relative to in vitro (such voltage differences can be seen in Figure 3, left), I H is more activated in dendrites during IVL states.
Timing of ion channel current activation relative to voltage
Below, we show results from analyzing the timing of ion channel currents using cross-correlations between each current trace and the corresponding location-specific voltage trace (i.e. soma or D4; Figure 5). Across all channel types, with the exception of I H, there were large narrow peaks with timescales in line with the duration of action potentials. Moreover, the lag time of the peaks are consistent across IVL vs in vitro states, as well as in soma vs dendrites, indicating that the relative timing of different ion channel currents is unchanged whilst under synaptic bombardment. Also across all dendritic ion channel currents in the IVL state, the cross-correlation timescale becomes broader, likely due to the general loss of spikes in more distal dendrites and longer timescale plateau-like potentials (i.e. as in the voltage trace plots in Figure 2). Some of the ion channel currents that were tightly linked with spiking had slightly non-zero peak lag times, indicating delayed activation (e.g. I Kdrf / I Kdrs / I KCa showing delayed rectification properties and I CaT / I CaL becoming more active in the after-spike hyperpolarization period). Others had peaks that were centered almost exactly at zero ( I K A, I Na, I M, and I L, which could all contribute to balancing action potential amplitude). In particular, I L and voltage cross-correlations were almost entirely symmetrical to the point where they appeared to be auto-correlations, indicating that I L is a good proxy for gauging changes in voltage. Finally, I H was unique in that it is the only ion channel current type that exhibited a negative cross correlation with spiking, which is in line with h-channels being activated during hyperpolarization and not spiking. Moreover, the cross-correlation between I H and voltage is even more negative in dendrites relative to soma, and during IVL states relative to in vitro states. This suggests that I H, which overall is enhanced during IVL states ( Figure 4), is comparatively more suppressed during spikes because the relative change in I H during a spike is larger in the IVL state.
Figure 5. Timing of currents relative to voltage.

Each plot shows the cross-correlations between the current traces and voltage traces using the last 9 seconds of each 10s trace. Note that all inward currents (right columns) are reversed in polarity for these cross-correlations (i.e. from negative to positive) in order to better interpret their activation periods with respect to voltage activation periods. Also note that we did not reverse the polarity for I L since it becomes an outward current during spikes ( Figure 3). For both cell 1 and cell 2 we run this analysis across 5 of the 10 re-randomizations of the IVL (red) and in vitro (blue) simulations, as well as across the somatic (darker tone) and D4 (lighter tone) compartments (see legend). The vertical and horizontal dashed lines in each plot are the zeroth axes (x-axis = -20 ms to 20 ms; y-axis = -1 to 1).
Timing of ion channel current activations relative to each other
Below, we show cross-correlations between every possible ion channel current combination in order to examine co-activation relationships ( Figure 6 and Figure 7). Across all cross-correlations, regardless of state, cell, or recording site, the peak lag time is preserved. While almost all cross-correlations exhibited peaks aligned very near zero, only I CaT consistently exhibited non-zero lag time peaks, indicating a delay in I CaT activation relative to other ion channel current activations. More specifically, most currents activate very close to when spikes are occurring ( Figure 5), but I CaT appears to exhibit a considerable delay relative to the timing of the other currents, and is more likely aligned to when the cell is undergoing after-spike hyperpolarization.
Figure 6. Timing of currents relative to each other (cell 1).
Cross-correlations are plotted in the same way as in Figure 5 (i.e. same legend but different cross-correlation pairs). The diagonal black dots, highlight channel auto-correlations (e.g. I K A * I K A). Everything above the diagonal is cross-correlated one way and everything below the diagonal is cross-correlated the opposite way (i.e. like a mirror; e.g. I K A * I Kdrf vs. I Kdrf * I K A). The vertical and horizontal dashed lines in each plot are the zeroth axes (x-axis = -20 ms to 20 ms; y-axis = -1 to 1).
Figure 7. Timing of currents relative to each other (cell 2).
Cross-correlations are plotted in the same way as in Figure 5 (i.e. same legend but different cross-correlation pairs). The diagonal black dots, highlight channel auto-correlations (e.g. I K A * I K A). Everything above the diagonal is cross-correlated one way and everything below the diagonal is cross-correlated the opposite way (i.e. like a mirror; e.g. I K A * I Kdrf vs. I Kdrf * I K A). The vertical and horizontal dashed lines in each plot are the zeroth axes (x-axis = -20 ms to 20 ms; y-axis = -1 to 1).
Also, of note, is the particular asymmetrical shape of any of the cross-correlations with I H. I H exhibited negative cross-correlation peaks with all of the other ion channel currents (i.e when the other currents increase in magnitude, I H decreases in magnitude). Specifically, this translates to I H showing decreased contributions during spikes, while all of the other ion channel currents show enhanced contributions during spikes ( Figure 5). The shape of I H cross-correlations are likely a result of the fact that I H possesses a longer and generally asymmetrical time course relative to the time course of currents that activate most strongly during spikes ( Figure 3).
When looking at differences between IVL (red) and in vitro (blue) states and across cellular compartments, we see that the amplitude of the peak cross-correlation is dependent on the cell ( Figure 6 and Figure 7). For example, with cell 1 ( Figure 6), where spiking is more easily propagated across dendritic compartments ( Figure 2A), we see that the ion channel currents that are activated during spiking do not show much change in the amplitude of the peak cross-correlations. However, for cell 2 ( Figure 7), where spiking is less easily propagated across dendritic compartments ( Figure 2B), the peak cross-correlations are decreased and broader in the IVL states. This was particularly the case for dendritic compartments, which exhibit broader cross-correlation peaks. Moreover, the somatic IVL cross-correlations are often closer in shape to the in vitro cross-correlations than they are to the dendritic IVL cross-correlations. One notable exception to this are the cross-correlations of either I M or I L with I H. In all cases, these cross-correlations with I H have more negative peak magnitudes whilst in IVL states (across both soma and dendrites), which parallels the cross-correlations between I H and voltage ( Figure 5). This may be related to the I H traces consistently being enhanced across compartments during IVL states ( Figure 4). As well, across many cross-correlations with I H (i.e. I Kdrf, I Kdrs, I KCa, I M, I L, I Na, and I CaT), IVL peak cross-correlations appear to be more negative in dendritic compartments than somatic compartments. This is in sharp contrast with other IVL state cross-correlations, which generally all exhibit larger cross-correlation peaks in somatic locations. This observation is seen more clearly with the cell 2 plots ( Figure 7). In summary this work predicts that, in vivo, dendritic I H is enhanced and as such, can be suppressed by a larger degree whenever other channels are more active. At the same time, this suggests that other dendritic ion channel currents show weaker co-activation between each other in vivo.
Altogether this suggests that I H activation is more prominent in dendritic compartments than in somatic compartments, as well as during IVL states where dendritic I H is more directly activated through synaptic bombardment. Comparatively, cross-correlations between other dendritic ion channel currents that are more active during spikes will have decreased co-activation during IVL states, potentially because of increased compartmentalization and a resultant decrease in the propagation of spikes across compartments.
Morphological compartments are more decorrelated with each other during in vivo-like states
Having observed a decrease in the dendritic propagation of spikes in the IVL context ( Figure 2), we can hypothesize that morphological compartments will become more de-correlated with each other during these states. As such, we further analyze channel current cross-correlations between the voltage and current traces in the soma and the corresponding traces in the D4 compartment ( in vitro = blue; IVL = red; Figure 8).
Figure 8. Relative timing of voltage and currents across soma and D4.

Each plot shows cross-correlations ( in vitro = blue; IVL = red) of a somatic current type or voltage cross-correlated against the same current type or voltage recorded in the D4 compartment. Cross-correlations are plotted in the same way as in Figure 5.
It is clear from these plots that ion channel current activity is considerably de-correlated during IVL states since the in vitro amplitudes of the cross-correlation peaks (blue) are considerably larger than the IVL amplitudes of the cross-correlation peaks (red). In the previous currentscape plots ( Figure 2) showing the in vitro cases, action potentials recorded in D1-D4 appeared to be the result of back-propagating action potentials generated near the soma. Here we show that this is indeed the case since the blue maximal peaks align at negative lag times ( Figure 8).
Interestingly, there is somewhat of a difference between cell 1 and cell 2 for the IVL cases ( Figure 8). While they both exhibited very diminished peaks compared to their corresponding in vitro cases, the lag time of the peaks was considerably different. Cell 1 exhibited more pronounced negative lag times, suggesting that spikes were being generated near to the soma and back-propagating to the D4 distal dendrite recording site (though slightly more slowly and with a considerably diminished amplitude compared to the in vitro spike back-propagation). On the other hand, cell 2 exhibited positive and broader peak lag times, suggesting that dendritic postsynaptic potentials are preceding somatic spikes with minimal spike back-propagation. We already know that spikes do not back-propagate easily into cell 2’s distal dendrites due to the combination of increased electrotonic distance ( Figure 1A) and synaptic bombardment ( Figure 2B). However since there is a positive peak, albeit small, we can presume that distal synaptic input are still coherently integrated such that they can lead to spikes at the soma in cell 2 ( Figure 8).
Although broad, IVL I H cross-correlations between soma and D4 were larger than the IVL cross-correlations between morphological compartments for other ion channel currents, suggesting that I H is possibly more resistant to the de-correlating effects of synaptic bombardment, possibly due to it being more active during subthreshold periods. This is intuitive given that the dendritic compartments during IVL states are more likely to be in subthreshold regimes due to dendritic plateau/complex spiking effects brought on by synaptic bombardment ( Figure 2).
Discussion
In this work we have computationally explored ion channel current contributions that are seen across different morphological compartments of an interneuronal cell type, the OLM cell, in in vitro and in vivo-like states. In doing so, we assessed the relative timing of ion channel current activation across pairs of ion channel types, across morphological compartments, and relative to voltage — a task that is not possible to perform experimentally in vivo, and that would be almost impossible to do in vitro. We found that the relative timing of ion channel current co-activation is preserved across states and locations. However, the magnitudes and relative contributions of the different ion channel currents are altered between states and across locations, and different morphological compartments become more de-correlated with each other during in vivo-like states. In particular we observe a consistent enhancement in I H across spatial compartments during in vivo-like states relative to in vitro, which could coincide with the de-correlation seen across morphological compartments.
Insights and interpretations
In this era of big data and channelopathies, it is highly desirable to be able to know and understand how different ion channel types might contribute to normal and pathological states. For example, a link between big potassium channels and epilepsy in Angelman syndrome was recently shown 68. In this ’tour de force’ experimental study, genetic, organoid and behavioural platforms were used to suggest this link. Computational modeling approaches as presented here could be harnessed to make such links and hypothesize others by examining particular ion channel types in cellular and networks states that are akin to in vitro and in vivo-like/behavioural states. Interestingly, in light of our results here, recent work has shown that blockage of HCN channels in OLM cells prevented the formation of type 2 theta rhythms (that emerge during immobile, anxiety-laden behavioural states) as controlled by OLM cells in ventral hippocampus 69.
We had noticed an enhancement of dendritic I H during in vivo-like states where subthreshold depolarizations are dominant. This follows since I H is a current that is most active during subthreshold and hyperpolarization regimes where it can be further activated by inhibitory perturbations 41, 70. In our models, our fitting required that synaptic conductance scales up with distance from soma, which could also contribute towards further enhancing dendritic I H. This is interesting considering the differential dendritic expression of I H in different cell types. For example, in layer 5 cortical pyramidal cells 71, 72 and CA1 hippocampal pyramidal cells 73 I H scales up in the apical dendrites with distance from soma. In OLM cells, our developed models provided support for h-channels being present in dendrites, but non-uniform distributions were not specifically examined 47. Our previous computational studies showed that dendritic I H in OLM cells can modulate the input frequencies at which they are preferentially recruited to spike 46. This suggests that if differential dendritic expression of I H were present in OLM cell dendrites, it could serve as a frequency modulator. Since our study here shows that dendritic I H is enhanced during in vivo-like states and operates in the subthreshold regime, it is likely to play a role in subthreshold signal propagation. Previous work has mostly highlighted that I H reduces signal propagation and enhances compartmentalization of dendrites by reducing the input resistance in principal neuron dendrites across hippocampus 74, 75, cortex 76, and basolateral amygdala 77. Expression and function of I H is also known to be modulated by long-term synaptic potentiation (LTP) mechanisms, where LTP can upregulate HCN channels, while at the same time suppress channel function 78. Altogether, this suggests that enhanced dendritic I H in vivo will contribute towards increasing dendritic compartmentalization. Since we do see a decrease in backpropagating action potentials ( Figure 2) and decreased cross-correlations between spatial compartments ( Figure 8) in the in vivo-like state, these may be a by-product of enhanced I H. However, it could also be due to a global effect across all of the synaptic and intrinsic currents that are enhanced in the in vivo-like state, which, together, all contribute towards decreasing the input resistance and suppressing the propagation of signals. We note that a more detailed sensitivity analysis may be warranted to better uncover these mechanisms, possibly using some of the visualization techniques made available through 67 to explore the full range through which currents may become rebalanced during conductance perturbations.
Limitations
In our in vivo-like states we did not try to directly simulate synaptic inputs linked to any particular behavioral paradigm (e.g. rhythmic or bursting inputs during theta or sharp waves). That is, we generated in vivo-like states that simulate the levels of synaptic bombardment that neurons might receive in vivo 12, 48. More specifically, we highlighted changes in ion channel current contribution profiles that may exist in vivo, and contextualized how these changes may affect the way in which individual neurons process behaviourally-relevant information. We note that in future work it will be informative to analyse current contribution profiles during these in vivo-like states and in the context of sensory-evoked stimuli, which have been shown to evoke dendritic spike events in pyramidal cells 73 and so would be interesting to study in the context of inhibitory interneurons.
In general, using computational simulations alone to investigate biophysical phenomena carries its own set of caveats. Though the models that we use are data driven, no model is ever truly complete 79, and degeneracies are to be expected 80. Moreover, many assumptions need to be made when constructing a morphologically-detailed multi-compartment model, such as types of ion channels to include and their distribution across the morphology of the model.
Conclusions
In summary, this work is a computational investigation into the dendritic ion channel contributions that govern OLM cell excitability in vivo. We highlight that the timing of ion channel currents relative to voltage and each other are invariant across states, though many undergo changes in their current output magnitudes. In particular dendritic I H is enhanced during in vivo-like states, which could indicate altered signal propagation in behaving animals relative to in vitro recordings. Finally, we show that during in vivo-like states, voltage and currents across compartments become more de-correlated relative to each, with a shift in the lag time of their maximal cross-correlation peaks. This was indicative of a loss of backpropagating action potentials, which made cross-correlations between subthreshold signals and spikes more apparent. Overall, this work shows a possible way to explore and gain insight into the coordination of ion channel currents that govern neuronal spiking in the “behaving animal”.
Data availability
Underlying data
Code for running simulations and plotting the results: https://github.com/FKSkinnerLab/OLM_IVLCurrents
Archived code as at time of publication: http://doi.org/10.5281/zenodo.3688619 81
License: GNU General Public License v3.0
Models of cell 1 and 2: https://github.com/FKSkinnerLab/OLMng
Archived models as at time of publication: http://doi.org/10.5281/zenodo.3689724 82
License: GNU General Public License v3.0
Acknowledgements
We thank M. Lankarany for reading and providing feedback on this manuscript.
Funding Statement
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC): Discovery Grant (RGPIN - 2016 - 06182) to FKS and Graduate Scholarship (CGSD2 - 504375 - 2017) to AGM.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Hodgkin AL, Huxley AF: The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952;116(4):473–496. 10.1113/jphysiol.1952.sp004718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodgkin AL, Huxley AF: Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116(4):449–472. 10.1113/jphysiol.1952.sp004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodgkin AL, Huxley AF, Katz B: Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952;116(4):424–448. 10.1113/jphysiol.1952.sp004716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgkin AL, Huxley AF: A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117(4):500–544. 10.1113/jphysiol.1952.sp004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali F, Kwan AC: Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: a review. Neurophotonics. 2020;7(1):011402. 10.1117/1.NPh.7.1.011402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim CK, Adhikari A, Deisseroth K: Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18(4):222–235. 10.1038/nrn.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luhmann HJ: Review of imaging network activities in developing rodent cerebral cortex in vivo. Neurophotonics. 2017;4(3):031202. 10.1117/1.NPh.4.3.031202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magrans de Abril I, Yoshimoto J, Doya K: Connectivity inference from neural recording data: Challenges, mathematical bases and research directions. Neural Netw. 2018;102:120–137. 10.1016/j.neunet.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 9. Sepehri Rad M, Choi Y, Cohen LB, et al. : Voltage and Calcium Imaging of Brain Activity. Biophys J. 2017;113(10):2160–2167. 10.1016/j.bpj.2017.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shuvaev AN, Salmin VV, Kuvacheva NV, et al. : Current advances in cell electrophysiology: applications for the analysis of intercellular communications within the neurovascular unit. Rev Neurosci. 2016;27(4):365–76. 10.1515/revneuro-2015-0047 [DOI] [PubMed] [Google Scholar]
- 11. Steinmetz NA, Koch C, Harris KD, et al. : Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Curr Opin in Neurobiol. 2018;50:92–100. 10.1016/j.conb.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Destexhe A, Paré D: Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol. 1999;81(4):1531–1547. 10.1152/jn.1999.81.4.1531 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez FR, Rahsepar B, White JA: Differences in the Electrophysiological Properties of Mouse Somatosensory Layer 2/3 Neurons In Vivo and Slice Stem from Intrinsic Sources Rather than a Network-Generated High Conductance State. eNeuro. 2018;5(2): pii: ENEURO.0447-17.2018. 10.1523/ENEURO.0447-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harvey CD, Collman F, Dombeck DA, et al. : Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461(7266):941–946. 10.1038/nature08499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monier C, Fournier J, Frégnac Y: In vitro and in vivo measures of evoked excitatory and inhibitory conductance dynamics in sensory cortices. J Neurosci Methods. 2008;169(2):323–365. 10.1016/j.jneumeth.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Lapray D, Lasztoczi B, Lagler M, et al. : Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15(9):1265–71. 10.1038/nn.3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katona L, Lapray D, Viney TJ, et al. : Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron. 2014;82(4):872–86. 10.1016/j.neuron.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varga C, Golshani P, Soltesz I: Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci U S A. 2012;109(40):E2726–34. 10.1073/pnas.1210929109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varga C, Oijala M, Lish J, et al. : Functional fission of parvalbumin interneuron classes during fast network events. eLife. 2014;3:e04006. 10.7554/eLife.04006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gentet LJ, Avermann M, Matyas F, et al. : Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65(3):422–35. 10.1016/j.neuron.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 21. Gentet LJ, Kremer Y, Taniguchi H, et al. : Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15(4):607–612. 10.1038/nn.3051 [DOI] [PubMed] [Google Scholar]
- 22. Harris KD, Hochgerner H, Skene NG, et al. : Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018;16(6):e2006387. 10.1371/journal.pbio.2006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang ZJ, Paul A: The diversity of GABAergic neurons and neural communication elements. Nat Rev Neurosci. 2019;20(9):563–572. 10.1038/s41583-019-0195-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klausberger T, Somogyi P: Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53–57. 10.1126/science.1149381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markram H, Toledo-Rodriguez M, Wang Y, et al. : Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- 26. Pelkey KA, Chittajallu R, Craig MT, et al. : Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev. 2017;97(4):1619–1747. 10.1152/physrev.00007.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tremblay R, Lee S, Rudy B: GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 2016;91(2):260–292. 10.1016/j.neuron.2016.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bezaire MJ, Soltesz I: Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23(9):751–785. 10.1002/hipo.22141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo X, Guet-McCreight A, Villette V, et al. : Synaptic Mechanisms Underlying the Network State-Dependent Recruitment of VIP-Expressing Interneurons in the CA1 Hippocampus. Cereb Cortex. 2020;30(6):3667–3685. 10.1093/cercor/bhz334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turi GF, Li WK, Chavlis S, et al. : Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning. Neuron. 2019;101(6):1150–1165.e8. 10.1016/j.neuron.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klausberger T, Magill PJ, Márton LF, et al. : Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421(6925):844–848. 10.1038/nature01374 [DOI] [PubMed] [Google Scholar]
- 32. Leão RN, Mikulovic S, Leão KE, et al. : OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15(11):1524–1530. 10.1038/nn.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chamberland S, Salesse C, Topolnik D, et al. : Synapse-specific inhibitory control of hippocampal feedback inhibitory circuit. Front Cell Neurosci. 2010;4: 130. 10.3389/fncel.2010.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siwani S, França ASC, Mikulovic S, et al. : OLMα2 Cells Bidirectionally Modulate Learning. Neuron. 2018;99(2):404–412.e3. 10.1016/j.neuron.2018.06.022 [DOI] [PubMed] [Google Scholar]
- 35. Hilscher MM, Nogueira I, Mikulovic S, et al. : Chrna2-OLM interneurons display different membrane properties and h-current magnitude depending on dorsoventral location. Hippocampus. 2019;29(12):1224–1237. 10.1002/hipo.23134 [DOI] [PubMed] [Google Scholar]
- 36. Kispersky TJ, Fernandez FR, Economo MN, et al. : Spike resonance properties in hippocampal O-LM cells are dependent on refractory dynamics. J Neurosci. 2012;32(11):3637–3651. 10.1523/JNEUROSCI.1361-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lien CC, Martina M, Schultz JH, et al. : Gating, modulation and subunit composition of voltage-gated K + channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538(Pt 2):405–419. 10.1113/jphysiol.2001.013066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maccaferri G, McBain CJ: The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol. 1996;497(Pt 1):119–130. 10.1113/jphysiol.1996.sp021754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martina M, Vida I, Jonas P: Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287(5451):295–300. 10.1126/science.287.5451.295 [DOI] [PubMed] [Google Scholar]
- 40. Topolnik L, Chamberland S, Pelletier JG, et al. : Activity-dependent compartmentalized regulation of dendritic Ca 2+ signaling in hippocampal interneurons. J Neurosci. 2009;29(14):4658–4663. 10.1523/JNEUROSCI.0493-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zemankovics R, Káli S, Paulsen O, et al. : Differences in subthreshold resonance of hippocampal pyramidal cells and interneurons: the role of h-current and passive membrane characteristics. J Physiol. 2010;588(Pt 12):2109–2132. 10.1113/jphysiol.2009.185975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saraga F, Wu CP, Zhang L, et al. : Active dendrites and spike propagation in multi-compartment models of oriens-lacunosum/moleculare hippocampal interneurons. J Physiol. 2003;552(Pt 3):673–689. 10.1113/jphysiol.2003.046177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lawrence JJ, Saraga F, Churchill JF, et al. : Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26(47):12325–12338. 10.1523/JNEUROSCI.3521-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sekulić V, Lawrence JJ, Skinner FK: Using multi-compartment ensemble modeling as an investigative tool of spatially distributed biophysical balances: application to hippocampal oriens-lacunosum/moleculare (O-LM) cells. PLoS One. 2014;9(10):e106567. 10.1371/journal.pone.0106567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sekulić V, Chen TC, Lawrence JJ, et al. : Dendritic distributions of I h channels in experimentally-derived multi-compartment models of oriens-lacunosum/moleculare (O-LM) hippocampal interneurons. Front Synaptic Neurosci. 2015;7:2. 10.3389/fnsyn.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sekulić V, Skinner FK: Computational models of O-LM cells are recruited by low or high theta frequency inputs depending on h-channel distributions. eLife. 2017;6: pii: e22962. 10.7554/eLife.22962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sekulić V, Yi F, Garrett T, et al. : Somatodendritic HCN channels in hippocampal OLM cells revealed by a convergence of computational models and experiments. bioRxiv. 2019; 633941. 10.1101/633941 [DOI] [Google Scholar]
- 48. Destexhe A, Rudolph M, Paré D: The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4(9):739–751. 10.1038/nrn1198 [DOI] [PubMed] [Google Scholar]
- 49. Destexhe A: Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Curr Opin Neurobiol. 2011;21(5):717–725. 10.1016/j.conb.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 50. Fernandez FR, Noueihed J, White JA: Voltage-Dependent Membrane Properties Shape the Size But Not the Frequency Content of Spontaneous Voltage Fluctuations in Layer 2/3 Somatosensory Cortex. J Neurosci. 2019;39(12):2221–2237. 10.1523/JNEUROSCI.1648-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guet-McCreight A, Skinner FK: Using computational models to predict in vivo synaptic inputs to interneuron specific 3 (IS3) cells of CA1 hippocampus that also allow their recruitment during rhythmic states. PLoS One. 2019;14(1):e0209429. 10.1371/journal.pone.0209429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hay E, Segev I: Dendritic Excitability and Gain Control in Recurrent Cortical Microcircuits. Cereb Cortex. 2015;25(10):3561–3571. 10.1093/cercor/bhu200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carnevale NT, Hines ML: The NEURON Book. Cambridge University Press, Cambridge, UK; New York, 1 edition.2006. 10.1017/CBO9780511541612 [DOI] [Google Scholar]
- 54. Böhm C, Pangalos M, Schmitz D, et al. : Serotonin Attenuates Feedback Excitation onto O-LM Interneurons. Cerebral Cortex. 2015;25(11):4572–4583. 10.1093/cercor/bhv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Govindaiah G, Kang YJ, Lewis HES, et al. : Group I metabotropic glutamate receptors generate two types of intrinsic membrane oscillations in hippocampal oriens/alveus interneurons. Neuropharmacology. 2018;139:150–162. 10.1016/j.neuropharm.2018.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Losonczy A, Somogyi P, Nusser Z: Reduction of Excitatory Postsynaptic Responses by Persistently Active Metabotropic Glutamate Receptors in the Hippocampus. J Neurophysiol. 2003;89(4):1910–1919. 10.1152/jn.00842.2002 [DOI] [PubMed] [Google Scholar]
- 57. Haam J, Zhou J, Cui G, et al. : Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc Natl Acad Sci U S A. 2018;115(8):E1886–E1895. 10.1073/pnas.1712538115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lawrence JJ, Grinspan ZM, Statland JM, et al. : Muscarinic receptor activation tunes mouse stratum oriens interneurones to amplify spike reliability. J Physiol. 2006;571(Pt 3):555–562. 10.1113/jphysiol.2005.103218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmid LC, Mittag M, Poll S, et al. : Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron. 2016;92(1):114–125. 10.1016/j.neuron.2016.08.034 [DOI] [PubMed] [Google Scholar]
- 60. Hájos N, Acsády L, Freund TF: Target Selectivity and Neurochemical Characteristics of VIP-immunoreactive Interneurons in the Rat Dentate Gyrus. Eur J Neurosci. 1996;8(7):1415–1431. 10.1111/j.1460-9568.1996.tb01604.x [DOI] [PubMed] [Google Scholar]
- 61. Oren I, Nissen W, Kullmann DM, et al. : Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29(4):939–950. 10.1523/JNEUROSCI.3251-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hurtado-Zavala JI, Ramachandran B, Ahmed S, et al. : TRPV1 regulates excitatory innervation of OLM neurons in the hippocampus. Nat Commun. 2017;8:15878. 10.1038/ncomms15878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sivagnanam S, Majumdar A, Yoshimoto K, et al. : Introducing The Neuroscience Gate-way. volume 993 of CEUR Workshop Proceedings of CEUR Workshop Proceedings2013. Reference Source [Google Scholar]
- 64. Gulyás AI, Megias M, Emri Z, et al. : Total Number and Ratio of Excitatory and Inhibitory Synapses Converging onto Single Interneurons of Different Types in the CA1 Area of the Rat Hippocampus. J Neurosci. 1999;19(22):10082–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stevens CF, Zador AM: Input synchrony and the irregular firing of cortical neurons. Nat Neurosci. 1998;1(3):210–217. 10.1038/659 [DOI] [PubMed] [Google Scholar]
- 66. Softky WR, Koch C: The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci. 1993;13(1):334–350. 10.1523/JNEUROSCI.13-01-00334.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alonso LM, Marder E: Visualization of currents in neural models with similar behavior and different conductance densities. eLife. 2019;8: pii: e42722. 10.7554/eLife.42722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun AX, Yuan Q, Fukuda M, et al. : Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science. 2019;366(6472):1486–1492. 10.1126/science.aav5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mikulovic S, Restrepo CE, Siwani S, et al. : Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat Commun. 2019;9(1):3638. 10.1038/s41467-018-05907-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ascoli GA, Gasparini S, Medinilla V, et al. : Local control of postinhibitory rebound spiking in CA1 pyramidal neuron dendrites. J Neurosci. 2010;30(18):6434–6442. 10.1523/JNEUROSCI.4066-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Williams SR, Stuart GJ: Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83(5):3177–82. 10.1152/jn.2000.83.5.3177 [DOI] [PubMed] [Google Scholar]
- 72. Berger T, Senn W, Lüscher HR: Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol. 2003;90(4):2428–37. 10.1152/jn.00377.2003 [DOI] [PubMed] [Google Scholar]
- 73. Smith SL, Smith IT, Branco T, et al. : Dendritic Spikes Enhance Stimulus Selectivity in Cortical Neurons in Vivo. Nature. 2013;503(7474):115–20. 10.1038/nature12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Magee JC: Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18(19):7613–7624. 10.1523/JNEUROSCI.18-19-07613.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yamada-Hanff J, Bean BP: Activation of Ih and TTX-sensitive sodium current at subthreshold voltages during CA1 pyramidal neuron firing. J Neurophysiol. 2015;114(4):2376–2389. 10.1152/jn.00489.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berger T, Larkum ME, Lüscher HR: High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85(2):855–868. 10.1152/jn.2001.85.2.855 [DOI] [PubMed] [Google Scholar]
- 77. Park K, Lee S, Kang SJ, et al. : Hyperpolarization-activated currents control the excitability of principal neurons in the basolateral amygdala. Biochem Biophys Res Commun. 2007;361(3):718–724. 10.1016/j.bbrc.2007.07.064 [DOI] [PubMed] [Google Scholar]
- 78. Shah MM: Cortical HCN channels: function, trafficking and plasticity. J Physiol. 2014;592(13):2711–2719. 10.1113/jphysiol.2013.270058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Almog M, Korngreen A: Is realistic neuronal modeling realistic? J Neurophysiol. 2016;116(5):2180–2209. 10.1152/jn.00360.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marder E, Taylor AL: Multiple models to capture the variability in biological neurons and networks. Nat Neurosci. 2011;14(2):133–138. 10.1038/nn.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guet-McCreight A: FKSkinnerLabOLM_IVLCurrents: OLM_IVLCurrents version 1 (Version v1). Zenodo2020. 10.5281/zenodo.3688619 [DOI] [Google Scholar]
- 82. fskinner1 v, Guet-McCreight A: FKSkinnerLab/OLMng: OLMng version 1.0 (Version v1.0). Zenodo2020. 10.5281/zenodo.3689724 [DOI] [Google Scholar]