Key Points
Question
Does intranasally delivered insulin provide a feasible and effective treatment for adults with mild cognitive impairment or Alzheimer disease dementia?
Findings
In this randomized clinical trial of 289 adults with mild cognitive impairment or Alzheimer disease dementia, no cognitive or functional benefits were observed with intranasal insulin treatment compared with placebo over a 12-month period in the primary analyses. The study execution and interpretation of results were complicated by issues with the intranasal delivery device.
Meaning
The results of this study suggest that further investigation is needed with intranasal delivery devices that have been reported to increase insulin levels in the central nervous system, which might better determine the therapeutic benefit of intranasal insulin for the treatment of persons with mild cognitive impairment and Alzheimer disease dementia.
Abstract
Importance
Insulin modulates aspects of brain function relevant to Alzheimer disease and can be delivered to the brain using intranasal devices. To date, the use of intranasal insulin to treat persons with mild cognitive impairment and Alzheimer’s disease dementia remains to be examined in a multi-site trial.
Objective
To examine the feasibility, safety, and efficacy of intranasal insulin for the treatment of persons with mild cognitive impairment and Alzheimer disease dementia in a phase 2/3 multisite clinical trial.
Design, Setting, and Participants
A randomized (1:1) double-blind clinical trial was conducted between 2014 and 2018. Participants received 40 IU of insulin or placebo for 12 months during the blinded phase, which was followed by a 6-month open-label extension phase. The clinical trial was conducted at 27 sites of the Alzheimer’s Therapeutic Research Institute. A total of 432 adults were screened, and 144 adults were excluded. Inclusion criteria included adults aged 55 to 85 years with a diagnosis of amnestic mild cognitive impairment or Alzheimer disease (based on National Institute on Aging–Alzheimer Association criteria), a score of 20 or higher on the Mini-Mental State Examination, a clinical dementia rating of 0.5 or 1.0, and a delayed logical memory score within a specified range. A total of 289 participants were randomized. Among the first 49 participants, the first device (device 1) used to administer intranasal insulin treatment had inconsistent reliability. A new device (device 2) was used for the remaining 240 participants, who were designated the primary intention-to-treat population. Data were analyzed from August 2018 to March 2019.
Interventions
Participants received 40 IU of insulin (Humulin-RU-100; Lilly) or placebo (diluent) daily for 12 months (blinded phase) followed by a 6-month open-label extension phase. Insulin was administered with 2 intranasal delivery devices.
Main Outcomes and Measures
The primary outcome (mean score change on the Alzheimer Disease Assessment Scale–cognitive subscale 12) was evaluated at 3-month intervals. Secondary clinical outcomes were assessed at 6-month intervals. Cerebrospinal fluid collection and magnetic resonance imaging scans occurred at baseline and 12 months.
Results
A total of 289 participants (155 men [54.6%]; mean [SD] age, 70.9 [7.1] years) were randomized. Of those, 260 participants completed the blinded phase, and 240 participants completed the open-label extension phase. For the first 49 participants, the first device used to administer treatment had inconsistent reliability. A second device was used for the remaining 240 participants (123 men [51.3%]; mean [SD] age, 70.8 [7.1] years), who were designated the primary intention-to-treat population. No differences were observed between treatment arms for the primary outcome (mean score change on ADAS-cog-12 from baseline to month 12) in the device 2 ITT cohort (0.0258 points; 95% CI, −1.771 to 1.822 points; P = .98) or for the other clinical or cerebrospinal fluid outcomes in the primary (second device) intention-to-treat analysis. No clinically important adverse events were associated with treatment.
Conclusions and Relevance
In this study, no cognitive or functional benefits were observed with intranasal insulin treatment over a 12-month period among the primary intention-to-treat cohort.
Trial Registration
ClinicalTrials.gov Identifier: NCT01767909
This randomized clinical trial examines the safety, efficacy, and feasibility of intranasal insulin for the treatment of persons with mild cognitive impairment and Alzheimer disease dementia.
Introduction
Insulin, a peptide produced by pancreatic β cells, is best known for its role in peripheral glucose homeostasis. It also has multifaceted implications for brain function. Insulin readily crosses the blood-brain barrier and may also be produced by brain neurogliaform cells.1 As summarized in a recent review,2 although brain glucose metabolism is not dependent on insulin, insulin can alter glucose use through classic interactions with neuronal glucose transporter type 4 (GLUT4) in key cognitive circuits and through its promotion of glycogen uptake in astrocytes, processes thought to be important at times of high energy demand. Insulin also enhances synaptic viability and dendritic spine formation as well as modulating levels of key neurotransmitters, such as dopamine.3 Of particular relevance, insulin protects against the synaptotoxic effects of the amyloid beta (Aβ) peptide and changes its clearance.4,5,6 Through these mechanisms, insulin may be associated with memory and other cognitive functions. Insulin also plays an important role in vascular function through its association with vasoreactivity, lipid metabolism, and inflammation.7
Reduced brain and cerebrospinal fluid (CSF) insulin levels or activity have been documented in Alzheimer disease (AD), although these reductions have not been observed in all studies.8,9 Markers of insulin resistance, a condition in which defective insulin signaling prevents further initiation of its normal functions in target tissues, have been detected in neuronally derived exosomes and in brain tissue from adults with Alzheimer disease. These indices have been associated with hyperphosphorylated tau in CSF and brain tissue10,11 and with increased deposition of Aβ12,13; however, again, these associations have not been found in all studies.14
Given the many important functions of insulin in the brain and the evidence of associations between brain insulin dysregulation and AD pathology, restoring brain insulin function may provide therapeutic benefit for adults with AD or its prodrome, amnestic mild cognitive impairment (MCI). One such strategy is to increase insulin availability to the brain through intranasal insulin administration. In preclinical studies, insulin and associated peptides delivered intranasally bypass the blood-brain barrier and reach the brain via olfactory and trigeminal perivascular channels without raising peripheral insulin or lowering blood glucose.15,16 In a pilot clinical trial, daily intranasal administration of 20 IU and 40 IU of insulin for 4 months to 105 participants with AD or MCI preserved performance on the Alzheimer Disease Assessment Scale–cognitive subscale 12 (ADAS-cog-12) compared with placebo and enhanced cerebral glucose metabolism assessed with 18F-fluorodeoxyglucose positron emission tomography.17,18 The present study aimed to extend these findings in a longer and larger multisite randomized double-blinded clinical trial designed to assess the efficacy of intranasal insulin on cognition, function, and AD biomarkers as well as the safety and feasibility of the intranasal delivery method.
Methods
The study was initiated by the Alzheimer Disease Cooperative Study Group of the University of California, San Diego, and completed by the Alzheimer’s Therapeutic Research Institute of the University of Southern California (P.A., director) together with the principal investigator (S.C.) at the Wake Forest School of Medicine. The clinical trial protocol is available in Supplement 1. Twenty-seven sites of the Alzheimer’s Therapeutic Research Institute participated in the clinical trial (eTable 1 in Supplement 2). The study was conducted in accordance with the Guideline for Good Clinical Practice19 and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Study protocols and informed consent were approved by the institutional review boards at the individual study sites and at the University of California, San Diego, the University of Southern California, and the Wake Forest School of Medicine. Written informed consent was obtained from participants and study partners. The study was conducted under local institutional review board supervision.
Study participant screening for the device 1 cohort occurred between January 23, 2014, and August 14, 2014. Screening was then paused (although study visits for enrolled participants continued) while identification and implementation of device 2 occurred. Screening resumed for the device 2 cohort on November 24, 2015, and the final open-label extension visit occurred on December 11, 2018. All participants used the device to which they were originally assigned throughout their participation.
Participants and Study Design
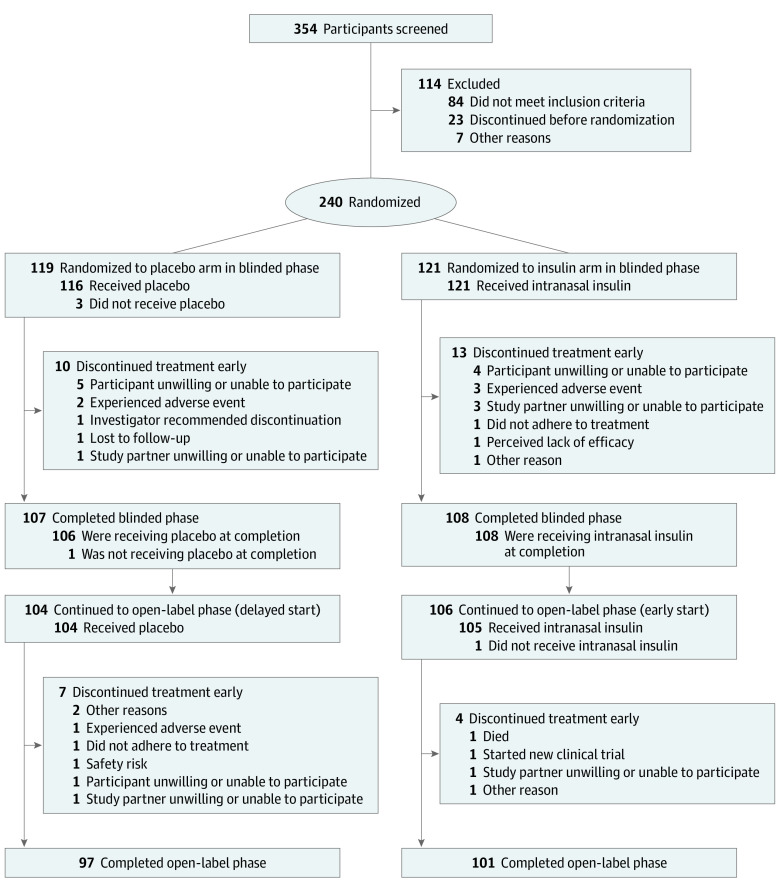
A total of 432 adults were screened, and 144 adults were excluded; 289 participants were randomized. For the first 49 participants, device 1 was used to administer intranasal insulin treatment. Because device 1 had inconsistent reliability, device 2 was used for the remaining 240 participants, who were designated the primary intention-to-treat (ITT) cohort. Of those, 121 participants were randomized to receive intranasal insulin (insulin arm), and 119 participants were randomized to receive placebo (placebo arm). The device 1 ITT cohort (n = 49) and the combined (devices 1 and 2) ITT cohort (n = 289) were considered secondary cohorts.
Eligible adults were those aged 55 to 85 years with diagnoses of amnestic MCI or AD (based on criteria from the National Institute on Aging–Alzheimer Association), scores of 20 and higher on the Mini-Mental State Examination (MMSE; score range, 0-30, with lower scores indicating poorer cognitive performance), global clinical dementia ratings of 0.5 or 1.0, and logical memory-delayed scores within a specified education-adjusted range. The stable use of medications approved for the treatment of AD was allowed. Persons with diabetes requiring medication were excluded, as were persons who had received insulin within 1 year of the screening visit. Additional exclusionary criteria are listed in eTable 2 in Supplement 2.
The study used a double-blind placebo-controlled design, in which participants were randomized on a 1:1 basis to receive 40 IU of intranasal insulin (Humulin-RU-100; Lilly) or placebo (diluent) daily for 12 months (blinded phase) followed by a 6-month open-label extension phase. Participants were randomized using a covariate-adaptive algorithm implemented centrally that considered MMSE score, apolipoprotein E ε4 allele (APOE ε4) carriage status, study site, sex, and age based on research indicating that these factors may be associated with treatment response.17,20
Intranasal Delivery Devices
The study was initiated using device 1 (ViaNase; Kurve Technology), which had been used in the pilot study and other studies of persons with AD.17,21 Modifications of device 1 for this clinical trial included the addition of an electronic timer. Participants pressed a switch that turned on the device, engaging a pump that released a nebulized stream of insulin through a nose piece into a nostril for 20 seconds, after which the device switched off. The process was then repeated in the other nostril. However, the electronic timer malfunctioned in some devices, requiring the clinical trial coordinating center, site personnel, and participants to expend time and effort to replace devices. It was determined that these malfunctions were unacceptably frequent, and a decision was made to switch to device 2 (I109 Precision Olfactory Delivery; Impel NeuroPharma).
Device 2 had not been used previously in studies of persons with AD but had demonstrated good reliability in delivering specified doses of insulin to the olfactory cleft, which is thought to be a key portal for nose-to-brain delivery. Device 2 used a liquid hydrofluoroalkane propellant to eject a metered dose of insulin through a nose tip without electronic assistance when a switch was depressed. Participants were instructed to insert the device tip into a nostril aligned along the nasal bridge and depress the switch to release the insulin. The process was then repeated in the other nostril.
Cognitive and Clinical Outcomes
The primary outcome was the participant mean score change on the ADAS-cog-12, which was administered at baseline and at 3-month intervals. The ADAS-cog22 is a psychometric instrument that evaluates memory, attention, reasoning, language, orientation, and praxis. The ADAS-cog-12 version used in the current study included assessment of delayed word recall, which is a measure of episodic memory.23 Scores from the original portion of the test ranged from 0 (best) to 70 (worst), and the number of items not recalled (ranging from 0-10 items) was added, for a maximum score of 80. A higher score indicated cognitive worsening.
Secondary functional outcomes, which included the mean change in the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment (ADL-MCI; score range, 0-53, with lower scores indicating worse function) score and the Clinical Dementia Rating scale Sum of Boxes (CDR-SB; score range, 0-18, with higher scores indicating worse cognition and daily function) score, were administered at 6-month intervals, as was a memory composite evaluation (defined as the sum of z scores from the Free and Cued Selective Reminding Test [score range, 0-48, with higher scores indicating better performance]24 and immediate and delayed story recall).25
CSF and Blood Collection
Lumbar puncture was conducted in the morning after an overnight fast before initiation of the study drug and at month 12. Cerebrospinal fluid was immediately frozen upright on dry ice for at least 20 minutes before being packaged and shipped overnight (also frozen on dry ice) to the central biomarker laboratory. Alzheimer disease biomarkers Aβ42 and Aβ40, total tau protein, and tau phosphorylated at threonine 181 (tau p-181) were measured using the Meso Scale Discovery platform (Meso Scale Diagnostics). Cerebrospinal fluid insulin was measured with an ultrasensitive enzyme-linked immunosorbent assay (Mercodia), which had a lower limit of detection of 0.15 mU/L.
Using standard protocols, nonfasting blood samples were collected and assessed for glucose (at baseline, month 6, and month 12) and hemoglobin A1c (at baseline and month 12) by a central laboratory that was certified by the Central Laboratory Improvement Amendments. Blood was also collected for APOE genotyping using established protocols.
Magnetic Resonance Imaging
Magnetic resonance imaging scans were conducted at screening and month 12 on 1.5-T or 3.0-T scanners that passed the study’s qualification procedures. Most participants (n = 280) were scanned by the same scanner at both times. Images were checked for quality and adherence to scanning protocols. The 3-dimensional T1-weighted data sets that passed quality checks were corrected for spatial distortion and intensity variation. The imaging protocol included a localizer scan followed by a high-resolution 3-dimensional T1-weighted structural series scan (magnetization prepared rapid gradient echo [MP-RAGE] or inversion recovery spoiled gradient echo [IR-SPGR]), a T2-weighted series scan (fluid attenuation inversion recovery [FLAIR]), a diffusion-weighted scan, and a gradient recalled echo scan. The volumetric analysis procedure included corrections for gradient nonlinearities and intensity nonuniformity.26,27,28
Baseline and follow-up data sets for each participant were spatially coregistered using rigid-body registration followed by nonlinear registration and neuroanatomic parcellation to quantify entorhinal and hippocampal volumes normalized to intracerebroventricular volume (ICV; expressed as percentage of ICV) on a participant-by-participant basis.
Safety and Adherence
At each study visit, any occurrence of an adverse event (AE) was reviewed and documented, and concomitant medications were recorded. Physical and neurological examination as well as routine laboratory testing occurred at screening, month 6, month 12, and month 18. A standard 12-lead resting electrocardiogram was performed at screening. The study was monitored by an independent data and safety monitoring board whose members reviewed the safety data every 3 months throughout the study.
Participant adherence was defined as the total number of doses received, as recorded in the participant’s study diary, divided by the expected number of doses (4 doses multiplied by the number of days) for both the blinded phase and the open-label phase.
Statistical Analysis
Prospective power was based on pilot estimates to detect a mean (SD) difference of 2.3 (5.7) points between the ADAS-cog-12 score at baseline and 12 months (primary outcome measure) in 2 groups. Assuming an attrition rate of 25%, we required a sample of 239 participants to detect a treatment effect of insulin with 80% power at a 2-tailed α level of 5%. The specified covariates for treatment outcomes included participant score on the MMSE (≤25 vs >25 points), study site, APOE ε4 carriage status, sex, and age (≤70 years vs >70 years). To achieve optimal balance between 2 treatment groups, a covariate-adaptive randomization strategy was used.
Efficacy analyses of all primary and secondary outcomes were conducted in a modified ITT population, which included all randomized participants who had at least 1 postbaseline assessment. Before database lock, the Alzheimer’s Therapeutic Research Institute Biostatistical Core specified the device 2 ITT cohort as the primary cohort and the device 1 ITT cohort and the combined ITT cohort as secondary cohorts. The ADAS-cog-12 and CDR-SB tests with missing item scores were imputed using a proration strategy. We used a serial gatekeeping procedure to maintain an overall experimentwise type 1 error rate of 5% for the 4 outcome hypotheses (mean score change on the ADAS-cog-12, CDR-SB, ADL-MCI, and memory composite) of the device 2 cohort.
Demographic and baseline characteristics of the 2 treatment groups were compared using a Fisher exact test for categorical variables and a Wilcoxon rank sum test for continuous variables. The mixed model of repeated measures was used for the primary outcome analysis and all secondary outcome analyses. The dependent variable of the mixed model of repeated measures was the change from baseline at each follow-up visit. The model used time as a categorical variable and included fixed effects for treatment, treatment-by-time interactions, baseline outcome, sex, age, baseline MMSE score, and APOE ε4 carriage status. A compound symmetric correlation and heterogeneous variance with respect to time were assumed. Because some participants may not have adhered to the protocol visit schedule, rules were applied to assign actual visits to analysis visits.
Safety analyses were conducted on the ITT population, which included all randomized participants. The Fisher exact test was used to compare frequencies of AEs, and the Wilcoxon rank sum test was used to compare the change from baseline at each follow-up visit of vital signs and the laboratory test results between treatment groups at each follow-up visit.
Magnetic resonance imaging and CSF biomarker outcomes were analyzed using a linear mixed-effects model, with fixed effects for time from baseline as a continuous variable and with treatment-by-time interactions, sex, age, baseline MMSE score, and APOE ε4 carriage status as covariates. All statistical analyses were performed with R software, version 3.4.2 (R Project for Statistical Computing), and results were reported as point estimates with 95% CIs. A P value of .05 was considered statistically significant. Data were analyzed from August 2018 to March 2019.
Results
Participants
A total of 354 participants were screened for the primary (device 2) ITT cohort, 114 of whom did not meet eligibility requirements, resulting in 240 participants (123 men [51.3%]; mean [SD] age, 70.8 [7.1] years). Of those, 121 participants were randomized to the insulin arm, and 119 participants were randomized to the placebo arm; among both arms of the device 2 cohort, a total of 215 participants completed the blinded phase, and 198 participants completed the open-label extension phase, with similar discontinuation rates in both arms (Figure 1). No differences were observed in demographic characteristics between the assigned groups in the blinded or open-label extension phases for the device 2 cohort (Table). Amyloid positivity (defined as CSF Aβ42 levels <600 pg/mL) was observed for 134 of 145 participants in the device 2 cohort who received a baseline lumbar puncture.
Figure 1. CONSORT Diagram for the Primary (Device 2) Cohort .
Table. Characteristics of the Primary (Device 2) Cohort.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Blinded phase | Open-label phase | |||||
| Placebo | Insulin | Total | Placebo | Insulin | Total | |
| Participants, No. | 119 | 121 | 240 | 104 | 106 | 210 |
| Sex | ||||||
| Male | 61 (51.3) | 62 (51.2) | 123 (51.3) | 53 (51.0) | 57 (53.8) | 110 (52.4) |
| Female | 58 (48.7) | 59 (48.8) | 117 (48.7) | 51 (49.0) | 49 (46.2) | 100 (47.6) |
| Age, mean (SD), y | 71.1 (6.8) | 70.5 (7.4) | 70.8 (7.1) | 71.0 (7.0) | 70.3 (7.3) | 70.7 (7.2) |
| Education, mean (SD), y | 16.3 (2.9) | 16.1 (2.6) | 16.2 (2.8) | 16.4 (2.8) | 16.1 (2.7) | 16.3 (2.8) |
| Race | ||||||
| American Indian or Alaskan native | 0 | 0 | 0 | 0 | 0 | 0 |
| Asian | 4 (3.4) | 1 (0.8) | 5 (2.1) | 4 (3.8) | 1 (0.9) | 5 (2.4) |
| Black | 6 (5.0) | 3 (2.5) | 9 (3.7) | 4 (3.8) | 2 (1.9) | 6 (2.8) |
| White | 109 (91.6) | 117 (96.7) | 226 (94.2) | 96 (92.3) | 103 (97.2) | 199 (94.8) |
| Ethnicity | ||||||
| Hispanic or Latino | 5 (4.2) | 5 (4.1) | 10 (4.2) | 2 (1.9) | 3 (2.8) | 5 (2.4) |
| Not Hispanic or Latino | 113 (95.0) | 116 (95.9) | 229 (95.4) | 101 (97.1) | 103 (97.2) | 204 (97.1) |
| Unknown or not reported | 1 (0.8) | 0 | 1 (0.4) | 1 (1.0) | 0 | 1 (0.5) |
| Diagnosis | ||||||
| AD | 73 (61.3) | 80 (66.1) | 153 (63.8) | 63 (60.6) | 71 (67.0) | 134 (63.8) |
| MCI | 46 (38.7) | 41 (33.9) | 87 (36.2) | 41 (39.4) | 35 (33.0) | 76 (36.2) |
| AD medications | ||||||
| No | 75 (63.0) | 70 (57.9) | 145 (60.4) | 69 (66.3) | 59 (55.7) | 128 (61.0) |
| Yes | 44 (37.0) | 51 (42.1) | 95 (39.6) | 35 (33.7) | 47 (44.3) | 82 (39.0) |
| APOE ε4 carriage status | ||||||
| No | 42 (35.3) | 42 (34.7) | 84 (35.0) | 39 (37.5) | 34 (32.1) | 73 (34.8) |
| Yes | 77 (64.7) | 79 (65.3) | 156 (65.0) | 65 (62.5) | 72 (67.9) | 137 (65.2) |
| Baseline ADAS-cog-12 score, mean (SD) | 24.73 (7.56) | 25.91 (8.28) | 25.33 (7.94) | 24.07 (7.30) | 25.34 (8.25) | 24.71 (7.80) |
| Screening MMSE score, mean (SD) | 24.84 (2.72) | 24.79 (2.75) | 24.82 (2.73) | 24.93 (2.73) | 24.95 (2.70) | 24.94 (2.71) |
| Baseline NPI score, mean (SD) | 6.67 (9.59) | 7.06 (7.69) | 6.86 (8.68) | 6.47 (9.91) | 7.17 (7.90) | 6.81 (8.96) |
| Baseline ADL-MCI score, mean (SD) | 39.77 (7.07) | 39.17 (7.77) | 39.46 (7.42) | 40.19 (6.85) | 39.09 (7.94) | 39.64 (7.42) |
| Screening CDR-SB score, mean (SD) | 3.35 (1.51) | 3.59 (1.51) | 3.47 (1.51) | 3.31 (1.53) | 3.56 (1.45) | 3.44 (1.49) |
Abbreviations: AD, Alzheimer disease; ADAS-cog-12, Alzheimer Disease Assessment Scale–cognitive subscale 12; ADL-MCI, Activities of Daily Living Scale for Mild Cognitive Impairment; APOE ε4, apolipoprotein E ε4 allele; CDR-SB, Clinical Dementia Rating scale Sum of Boxes; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
Outcomes, Biomarkers, and Imaging
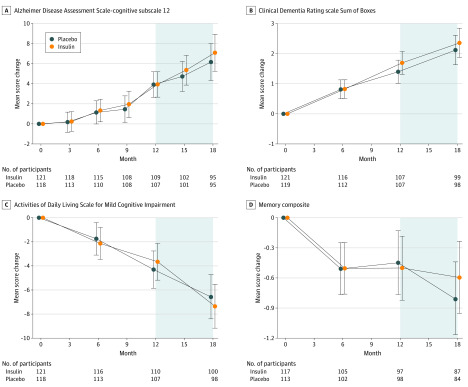
No differences were observed between treatment arms for the primary outcome (mean score change on ADAS-cog-12 from baseline to month 12) in the device 2 ITT cohort (0.0258 points; 95% CI, −1.771 to 1.822 points; P = .98) (Figure 2A). In addition, no differences were observed in performance on the CDR-SB, ADL-MCI, or memory composite tests (Figure 2B-D). In the open-label analyses for the device 2 cohort, participants originally randomized to the insulin arm (early start) and those randomized to the placebo arm (delayed start) did not differ in mean score change on the ADAS-cog-12 or on any other outcome at month 15 or month 18 (Figure 2).
Figure 2. Mean Score Changes From Baseline to Month 18 for the Primary (Device 2) Cohort .
The model used time as a categorical variable and included fixed effects for treatment, treatment-by-time interactions, baseline outcome, sex, age, baseline score on the Mini-Mental State Examination, and apolipoprotein E ε4 allele carriage status. The shaded region indicates the open-label phase. A, Alzheimer Disease Assessment Scale–cognitive subscale 12. B, Clinical Dementia Rating scale Sum of Boxes. C, Activities of Daily Living Scale for Mild Cognitive Impairment. D, Memory composite. Error bars indicate 95% CIs.
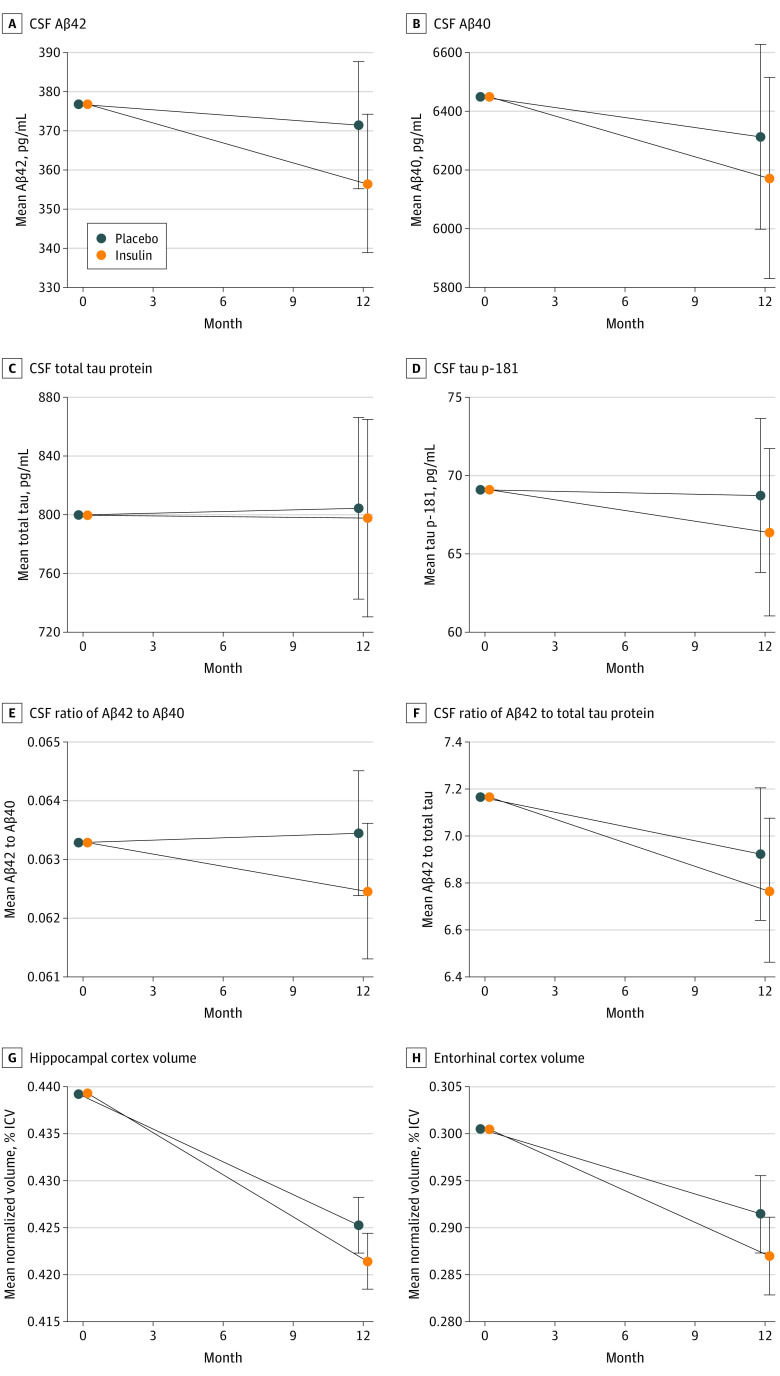
Cerebrospinal fluid Aβ42 and Aβ40, total tau protein, and tau p-181, and the ratios of Aβ42 to Aβ40 and Aβ42 to total tau did not differ between treatment arms for the device 2 cohort (Figure 3A-F). The CSF insulin levels were unchanged, as were the blood glucose and hemoglobin A1c values (eTable 3 and eFigure 1 in Supplement 2).
Figure 3. Mean Change in Cerebrospinal Fluid and Neuroimaging Biomarkers From Baseline to Month 12 for the Primary (Device 2) Cohort.
The linear mixed-effects model used fixed effects for time from baseline as a continuous variable, and treatment-by-time interactions, sex, age, baseline score on the Mini-Mental State Examination, and apolipoprotein E ε4 allele carriage status as covariates. Aβ indicates amyloid beta; CSF, cerebrospinal fluid; ICV, intracerebroventricular volume; and tau p-181, tau phosphorylated at threonine 181. Error bars indicate 95% CIs.
Small but significant reductions in hippocampal volume were observed in neuroimaging results for the device 2 insulin arm (−0.0038% of ICV; 95% CI, −0.0073% to −0.0004% of ICV; P = .03), with similar reductions observed in entorhinal cortex volume (−0.0044% of ICV; 95% CI, −0.0093% to 0.0004% of ICV; P = .07) (Figure 3G and H).
Safety and Adherence
Equal numbers of AEs occurred in the placebo and insulin arms, and no differences were observed in the severity of AEs between the arms, with most AEs rated as mild (eTable 4 in Supplement 2). Seventeen percent of AEs were rated as possibly, probably, or definitely associated with the investigational drug (ie, intranasal insulin) in both groups. A total of 5% and 6% of AEs were rated as associated with the device in the placebo and insulin arms, respectively. Infections, injuries, respiratory disorders, and nervous system disorders were the most frequent AEs reported, which did not differ between groups.
An insignificantly higher rate of vascular disorders was observed in the insulin arm compared with the placebo arm (15 participants [12.4%] vs 6 participants [5.0%], respectively; P = .07). A median adherence rate of 92% (range, 0%-106% for insulin and 0%-103% for placebo) of scheduled doses was observed for both the insulin and placebo arms during the blinded phase, and a median adherence rate of 93% (range, 24%-168%) and 92% (range, 0%-108%) was observed for the insulin and placebo arms, respectively, for the open-label extension phase.
Secondary Analyses
For the secondary (device 1) ITT cohort, 78 participants were screened, and 49 participants (32 men [65.3%]; mean [SD] age, 71.9 [7.1] years) were randomized to the insulin (n = 24) or placebo (n = 25) arms. Of those, 45 participants completed the blinded phase, and 42 participants completed the open-label extension phase, with similar discontinuation rates in both arms (eFigure 2 in Supplement 2). Demographic characteristics were similar between groups (eTable 5 in Supplement 2).
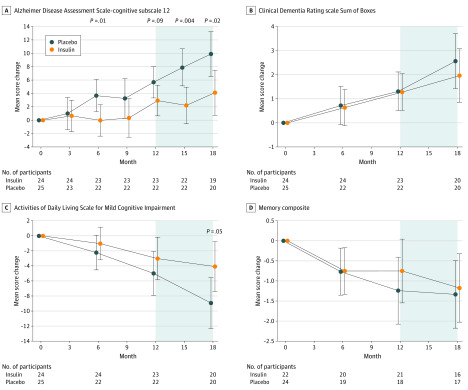
The insulin arms of the device 1 cohort indicated better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01) (Figure 4A). No differences between treatment arms were observed for other clinical or cognitive measures in the blinded phase (Figure 4B-D). In the open-label analyses, the early-start device 1 cohort had better ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; nominal P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; nominal P = .02) (Figure 4A) and better ADL-MCI scores at month 18 (4.85 points; 95% CI, 0.07-9.63 points; nominal P = .05) (Figure 4C) compared with the device 2 delayed-start participants. No differences were observed for other clinical or cognitive outcomes.
Figure 4. Mean Score Changes From Baseline to Month 18 for the Secondary (Device 1) Cohort .
The model used time as a categorical variable and included fixed effects for the treatment, the treatment-by-time interactions, baseline outcome, sex, age, baseline score on the Mini-Mental State Examination, and apolipoprotein E ε4 allele carriage status. The shaded region indicates the open-label phase. Error bars indicate 95% CIs.
Although individual biomarkers did not significantly differ between arms for the device 1 cohort, the ratios of Aβ42 to Aβ40 (0.004; 95% CI, 0.001-0.007; nominal P = .01) and Aβ42 to total tau (0.935; 95% CI, 0.093-1.778; nominal P = .03) increased (eFigure 3E and F in Supplement 2). For the device 1 cohort, small but significantly greater entorhinal cortex volume loss was observed for the insulin arm (−0.0114% of ICV; 95% CI, −0.0187% to −0.004% of ICV; P = .003), with no significant differences observed in hippocampal volume (eFigure 4A and B in Supplement 2).
The number of AEs was almost identical between groups, and the AEs were comparable in severity, with most rated as mild and unassociated with the investigational drug or device (eTable 6 in Supplement 2). For the device 1 cohort, median adherence rates of 72% (range, 42%-95%) and 73% (range, 32%-97%) of scheduled doses were observed for the insulin and placebo arms, respectively, during the blinded phase, which increased to 86% (range, 44%-98% for early start and 0%-101% for delayed start) for both arms in the open-label phase.
The secondary (combined devices) ITT cohort comprised 289 participants (155 men [53.6%]; mean [SD] age, 70.9 [7.1] years) (eTable 7 in Supplement 2). Among this cohort, no significant effects were observed for any outcome measure during the blinded or open-label phases (eFigure 5 and eFigure 6 in Supplement 2), with the exception of reduced entorhinal cortex volume in the insulin arm at month 12 relative to baseline (−0.0055% of ICV; 95% CI, −0.0097% to −0.0014% of ICV; nominal P = .009) (eFigure 6H in Supplement 2). The AE profile was identical to that observed in the device 2 cohort.
Discussion
To our knowledge, this study is the first multisite phase 2/3 clinical trial of the use of intranasal insulin to treat persons with MCI and AD, and the study was designed to assess the efficacy of intranasal insulin on cognition and the safety and feasibility of the intranasal delivery method. In the primary analysis with the device 2 cohort, no differences were observed between the placebo and insulin arms at any time point for the primary outcome (mean score change on the ADAS-cog-12 from baseline to month 12) in either the blinded or open-label phases. In a similar manner, no differences were observed in other cognitive or functional outcomes or in CSF biomarkers. Small reductions in hippocampal volume, which were observed in the insulin arm, are of unclear clinical importance, given that both the insulin and placebo arms had similar cognitive, functional, and CSF profiles.
Feasibility, Adherence, and Safety
One goal of the clinical trial was to assess the feasibility of intranasal drug administration, a mode of delivery that purportedly can circumvent the blood-brain barrier, allowing brain access to molecules for which blood-brain barrier transport is impeded. Interpretation of the study results was complicated by the need to change delivery devices midtrial. Device 1, which was originally chosen for the clinical trial, had been used in previous studies of persons with MCI and AD and was reported to have good reliability and to be associated with improvements in cognition and cerebral glucose metabolism.17,21 However, a design adaptation introduced specifically for this clinical trial resulted in unreliable performance that required frequent replacement of devices and imposed feasibility challenges for clinical trial operations. Despite these challenges, participants using device 1 had adherence rates of 73% in the blinded phase and 86% in the open-label extension phase. Device 2, with which the study was completed, performed reliably with excellent adherence rates (>90%) but had not, to our knowledge, been used previously in clinical trials of persons with AD.
Although a detailed discussion of differences between the devices is beyond the scope of this article, it is notable that the devices use different delivery strategies, which may have altered their effectiveness in delivering insulin to the central nervous system. The results suggest that future studies using intranasal drug delivery devices should verify the devices’ ability to deliver compounds to the central nervous system directly through methods such as postadministration lumbar puncture or positron emission tomography.
Intranasal insulin was generally safe, with no differences in AE profiles between the insulin and placebo arms. This observation is consistent with a recent meta-analysis comprising 18 studies of 832 individuals who received human intranasal insulin treatment lasting between 21 days and 9.7 years, which reported no substantial safety issues or patterns of AEs other than transient local nasal rhinitis.29
Device 1 and Combined Cohorts
In the secondary analyses with the device 1 cohort, an advantage for the insulin arm on the ADAS-cog-12 was observed at the 12-month point, with nominally significant effects observed at 6 months and during the 6-month open-label extension phase. In addition, improved profiles were noted for the CSF biomarker ratios of Aβ42 to Aβ40 and Aβ42 to total tau protein for the device 1 insulin arm. Compared with individual CSF biomarkers, the Aβ42 to total tau ratio has indicated improved ability to estimate potential progression from MCI in research cohorts and has demonstrated the best sensitivity and specificity profile in a clinical setting.30,31 The Aβ42 to Aβ40 ratio also increased; low values of this ratio have been reported to be a more reliable factor associated with potential brain Aβ deposition assessed with positron emission tomography than CSF Aβ42 alone, possibly because the ratio corrects for individual differences in overall Aβ production and is more specific to AD.32 Caution must be used in interpreting the secondary analyses of the device 1 results; the improvements observed on the ADAS-cog-12 and biomarker profile in the device 1 cohort may be spurious given the small sample or may reflect a difference in the ability of the 2 devices to deliver insulin to the central nervous system, a possibility that can be tested in future validation studies.
Limitations
The study had limitations. One limitation was the feasibility challenge associated with device 1, which necessitated a midtrial switch to device 2. Interpretation of the primary results with device 2 were complicated by the fact that the device had not been used previously in persons with AD.
Conclusions
The analysis of the primary ITT cohort treated with device 2 found no benefits of intranasal insulin treatment for any outcome. Further investigation using reliable insulin delivery devices that have indicated the ability to elevate insulin in the central nervous system is needed to assess the therapeutic benefit of intranasal insulin for the treatment of MCI and AD.
Trial Protocol and Statistical Analysis Plan
eTable 1. Participating Trial Sites
eTable 2. Major Exclusionary Criteria
eTable 3. Glucose and Hemoglobin A1C Values for the Primary (Device 2) Cohort
eTable 4. Reported Adverse Events for the Primary (Device 2) Cohort
eTable 5. Descriptive Characteristics of the Secondary (Device 1) Blinded and Open-Label Cohort
eTable 6. Reported Adverse Events for the Secondary (Device 1) Cohort
eTable 7. Descriptive Characteristics of the Secondary (Combined Devices) Blinded and Open-Label Cohort
eFigure 1. CSF Insulin for Primary (Device 2) Cohort
eFigure 2. CONSORT Diagram for the Secondary (Device 1) Cohort
eFigure 3. CSF Biomarker Results for the Secondary (Device 1) Cohort
eFigure 4. Neuroimaging Results for the Secondary (Device 1) Cohort
eFigure 5. ADAS-cog-12, CDR-SB, ADL-MCI, and Memory Composite Results for the Combined Cohort
eFigure 6. CSF and Neuroimaging Biomarker Results for the Combined Cohort
Data Sharing Statement
References
- 1.Molnar G, Farago N, Kocsis AK, et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci. 2014;34(4):1133-1137. doi: 10.1523/JNEUROSCI.4082-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168-181. doi: 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology. 2018;136(Pt B):182-191. doi: 10.1016/j.neuropharm.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of abeta oligomers. Proc Natl Acad Sci U S A. 2009;106(6):1971-1976. doi: 10.1073/pnas.0809158106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt J, Wilcox KC, Tortelli V, et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol Biol Cell. 2017;28(20):2623-2636. doi: 10.1091/mbc.e17-06-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin W, Krishnan B, Taglialatela G. Chronic synaptic insulin resistance after traumatic brain injury abolishes insulin protection from amyloid beta and tau oligomer-induced synaptic dysfunction. Sci Rep. 2019;9(1):8228. doi: 10.1038/s41598-019-44635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBose-Boyd RA, Ye J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem Sci. 2018;43(5):358-368. doi: 10.1016/j.tibs.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Monte SM, Tong M, Daiello LA, Ott BR. Early-stage Alzheimer’s disease is associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J Alzheimers Dis. 2019;68(2):657-668. doi: 10.3233/JAD-180906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil-Bea FJ, Solas M, Solomon A, et al. Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):405-413. doi: 10.3233/JAD-2010-100795 [DOI] [PubMed] [Google Scholar]
- 10.Yarchoan M, Toledo JB, Lee EB, et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128(5):679-689. doi: 10.1007/s00401-014-1328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laws SM, Gaskin S, Woodfield A, et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep. 2017;7(1):9766. doi: 10.1038/s41598-017-09577-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekblad LL, Johansson J, Helin S, et al. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology. 2018;90(13):e1150-e1157. doi: 10.1212/WNL.0000000000005214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11(5):504-510. doi: 10.1016/j.jalz.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thambisetty M, Jeffrey Metter E, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70(9):1167-1172. doi: 10.1001/jamaneurol.2013.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep. 2019;9(1):2621. doi: 10.1038/s41598-019-39191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481-496. doi: 10.1016/j.neuroscience.2004.05.029 [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29-38. doi: 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craft S. Alzheimer disease: insulin resistance and AD—extending the translational path. Nat Rev Neurol. 2012;8(7):360-362. doi: 10.1038/nrneurol.2012.112 [DOI] [PubMed] [Google Scholar]
- 19.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1) June 10, 1996. https://ichgcp.net/
- 20.Claxton A, Baker LD, Wilkinson CW, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 2013;35(4):789-797. doi: 10.3233/JAD-122308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craft S, Claxton A, Baker LD, et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis. 2017;57(4):1325-1334. doi: 10.3233/JAD-161256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356-1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 23.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13-S21. doi: 10.1097/00002093-199700112-00003 [DOI] [PubMed] [Google Scholar]
- 24.Grober E, Sanders AE, Hall C, Lipton RB. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24(3):284-290. doi: 10.1097/WAD.0b013e3181cfc78b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craft S, Zallen G, Baker LD. Glucose and memory in mild senile dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1992;14(2):253-267. doi: 10.1080/01688639208402827 [DOI] [PubMed] [Google Scholar]
- 26.Arnold JB, Liow JS, Schaper KA, et al. Qualitative and quantitative evaluation of six algorithms for correcting intensity nonuniformity effects. Neuroimage. 2001;13(5):931-943. doi: 10.1006/nimg.2001.0756 [DOI] [PubMed] [Google Scholar]
- 27.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436-443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 28.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 29.Schmid V, Kullmann S, Gfrorer W, et al. Safety of intranasal human insulin: a review. Diabetes Obes Metab. 2018;20(7):1563-1577. doi: 10.1111/dom.13279 [DOI] [PubMed] [Google Scholar]
- 30.Tariciotti L, Casadei M, Honig LS, et al. Clinical experience with cerebrospinal fluid Aβ42, total and phosphorylated tau in the evaluation of 1,016 individuals for suspected dementia. J Alzheimers Dis. 2018;65(4):1417-1425. doi: 10.3233/JAD-180548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumurgier J, Hanseeuw BJ, Hatling FB, et al. Alzheimer’s disease biomarkers and future decline in cognitive normal older adults. J Alzheimers Dis. 2017;60(4):1451-1459. doi: 10.3233/JAD-170511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janelidze S, Zetterberg H, Mattsson N, et al. ; Swedish BioFINDER Study Group . CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3(3):154-165. doi: 10.1002/acn3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Participating Trial Sites
eTable 2. Major Exclusionary Criteria
eTable 3. Glucose and Hemoglobin A1C Values for the Primary (Device 2) Cohort
eTable 4. Reported Adverse Events for the Primary (Device 2) Cohort
eTable 5. Descriptive Characteristics of the Secondary (Device 1) Blinded and Open-Label Cohort
eTable 6. Reported Adverse Events for the Secondary (Device 1) Cohort
eTable 7. Descriptive Characteristics of the Secondary (Combined Devices) Blinded and Open-Label Cohort
eFigure 1. CSF Insulin for Primary (Device 2) Cohort
eFigure 2. CONSORT Diagram for the Secondary (Device 1) Cohort
eFigure 3. CSF Biomarker Results for the Secondary (Device 1) Cohort
eFigure 4. Neuroimaging Results for the Secondary (Device 1) Cohort
eFigure 5. ADAS-cog-12, CDR-SB, ADL-MCI, and Memory Composite Results for the Combined Cohort
eFigure 6. CSF and Neuroimaging Biomarker Results for the Combined Cohort
Data Sharing Statement