Key Points
Question
Is RBFOX1 associated with brain amyloidosis, as measured by positron emission tomographic imaging, in early and preclinical Alzheimer disease?
Findings
In this genetic association study, a meta-analysis of amyloid positron emission tomographic imaging data collected on 4314 participants in 6 studies noted genome-wide significant associations with single-nucleotide variants in a novel locus, RBFOX1, as well as in APOE. In addition, reduced expression of RBFOX1 appeared to be associated with increased amyloid burden and global cognitive decline during life.
Meaning
In this study, RBFOX1 appeared to be a novel locus associated with positron emission tomographic imaging–derived brain amyloidosis and may be involved in the pathogenesis of Alzheimer disease.
Abstract
Importance
Genetic studies of Alzheimer disease have focused on the clinical or pathologic diagnosis as the primary outcome, but little is known about the genetic basis of the preclinical phase of the disease.
Objective
To examine the underlying genetic basis for brain amyloidosis in the preclinical phase of Alzheimer disease.
Design, Setting, and Participants
In the first stage of this genetic association study, a meta-analysis was conducted using genetic and imaging data acquired from 6 multicenter cohort studies of healthy older individuals between 1994 and 2019: the Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease Study, the Berkeley Aging Cohort Study, the Wisconsin Registry for Alzheimer’s Prevention, the Biomarkers of Cognitive Decline Among Normal Individuals cohort, the Baltimore Longitudinal Study of Aging, and the Alzheimer Disease Neuroimaging Initiative, which included Alzheimer disease and mild cognitive impairment. The second stage was designed to validate genetic observations using pathologic and clinical data from the Religious Orders Study and Rush Memory and Aging Project. Participants older than 50 years with amyloid positron emission tomographic (PET) imaging data and DNA from the 6 cohorts were included. The largest cohort, the Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease Study (n = 3154), was the PET screening cohort used for a secondary prevention trial designed to slow cognitive decline associated with brain amyloidosis. Six smaller, longitudinal cohort studies (n = 1160) provided additional amyloid PET imaging data with existing genetic data. The present study was conducted from March 29, 2019, to February 19, 2020.
Main Outcomes and Measures
A genome-wide association study of PET imaging amyloid levels.
Results
From the 4314 analyzed participants (age, 52-96 years; 2478 participants [57%] were women), a novel locus for amyloidosis was noted within RBFOX1 (β = 0.61, P = 3 × 10−9) in addition to APOE. The RBFOX1 protein localized around plaques, and reduced expression of RBFOX1 was correlated with higher amyloid-β burden (β = −0.008, P = .002) and worse cognition (β = 0.007, P = .006) during life in the Religious Orders Study and Rush Memory and Aging Project cohort.
Conclusions and Relevance
RBFOX1 encodes a neuronal RNA-binding protein known to be expressed in neuronal tissues and may play a role in neuronal development. The findings of this study suggest that RBFOX1 is a novel locus that may be involved in the pathogenesis of Alzheimer disease.
This genetic association study examines amyloid levels through positron emission tomographic imaging in individuals with or at risk for Alzheimer disease.
Introduction
Alzheimer disease (AD) is a complex polygenic disease with high heritability. Genome-wide association studies (GWAS) have identified more than 25 risk loci that highlight amyloid processing, lipid metabolism, endocytosis, and innate immunity as important biological factors in the development of AD.1,2 While much of the genetic work on AD has focused on clinical diagnosis as the primary outcome, AD is heterogeneous and has a long preclinical phase when brain amyloid deposition accumulates before the onset of cognitive impairment.3
The development of amyloid positron emission tomographic (PET) imaging tracers has provided a biomarker for diagnosis and risk assessment enabling in vivo detection of fibrillar amyloid-β before the onset of symptoms.4 The approval by the US Food and Drug Administration of additional ligands facilitated the application of amyloid PET imaging in clinical practice and in research.5 Advancing this biomarker, Jack et al6 proposed a model in which brain amyloid-β deposition precedes the onset of neurodegeneration and cognitive dysfunction. This model also implied that an amyloid-β biomarker, such as PET imaging, could identify individuals at the highest risk for AD long before the diagnosis. Several previous genetic investigations of brain amyloidosis using amyloid PET imaging have found an association with the APOE locus.7,8,9,10,11 However, to our knowledge, there has been no consistent confirmation of other loci.
Therapeutic efforts have begun to shift focus toward identifying and treating individuals in the preclinical phase of disease before onset of neurodegeneration and cognitive decline. Using a PET biomarker of brain amyloidosis to screen participants, the Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease (A4 Study) clinical trial screened more than 4000 asymptomatic older individuals with amyloid PET imaging, of whom 1169 had elevated amyloid levels and were eligible for a prevention trial.12,13 Clinical information and DNA from these at-risk, asymptomatic study participants provided an opportunity to identify novel genetic associations with brain amyloidosis during the preclinical phase of disease. In addition, the analyses of such data could provide insight into the mechanisms underlying cerebral amyloid accumulation.
Methods
In this genetic association study, participant data were acquired during the screening process in the A4 Study.12,13 We also included other cohort studies: the Alzheimer Disease Neuroimaging Initiative (ADNI), the Berkeley Aging Cohort Study, the Wisconsin Registry for Alzheimer’s Prevention (WRAP), the Biomarkers of Cognitive Decline Among Normal Individuals: the BIOCARD cohort, and the Baltimore Longitudinal Study of Aging (BLSA). Vanderbilt University and Columbia University institutional review boards approved the data analyses. The present study was conducted from March 29, 2019, to February 19, 2020. This study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guideline for genotyping, population stratification, haplotype modeling, Hardy-Weinberg equilibrium, and replication.14 We also describe how the participant data were selected, how quantitative traits were harmonized before analyses, the statistical methods used, and the sources of data.
The A4 Study clinical trial began screening in 2014, recruiting healthy adults aged 65 to 85 years with amyloid PET imaging.12,13 The ADNI study was launched in 2003 and has included more than 1500 participants aged 55 to 90 years with normal cognition, mild cognitive impairment, or AD. In 2001, WRAP began recruiting participants aged 40 to 65 years who had a parent with autopsy-confirmed or clinically verified AD.15,16 The BIOCARD study enrolled middle-aged participants who were cognitively intact; 75% of the participants had a first-degree relative with AD. The study began in 1995, stopped in 2005, and was reestablished in 2009, with annual clinical and cognitive assessments.17 The neuroimaging substudy of the BLSA began in 1994 and included participants without dementia aged 59 to 85 years who had up to 10 years of prospective data collection at baseline.18 Amyloid imaging with PET and carbon 11 Pittsburgh Compound B (C11PiB) was introduced into the study in 2005.19 The Berkeley Aging Cohort Study began enrolling cognitively normal individuals recruited from the local community in 2005. For the amyloid PET imaging GWAS, we filtered each data set to individuals older than 50 years who had amyloid PET imaging (either C11PiB or florbetapir) and genetic data available for analysis. Informed consent was obtained from participants in each study.
To validate genetic findings, we used autopsy data from the Religious Orders Study and Rush Memory and Aging Project (ROS/MAP), which were 2 harmonized longitudinal studies enrolling older adults without dementia who underwent annual clinical evaluations and organ donation at death.20 Both studies were approved by an institutional review board of Rush University Medical Center. All participants in ROS/MAP signed an informed consent, an Anatomical Gift Act form, and a repository consent that allows their data to be repurposed. The Rush Alzheimer Disease Center resource sharing hub (https://www.radc.rush.edu/) and the Accelerating Medicines Partnership–AD Knowledge Portal (syn3219045) provided access to the data and are available on request with a data use agreement.
Genotyping was performed in each study on different platforms. Data from all cohorts underwent a quality control21 process to filter variants not successfully genotyped (missing >5%), out of Hardy-Weinberg equilibrium (P > 1 × 10−6), or with low minor allele frequency (<1%). Participants were excluded for poor genotypic efficiency (missing >1% of variants) if reported and genotyped sex differed if cryptic relatedness was identified (removed second-degree or closer relatives) or if large-scale differences in ethnicity/race were identified by principal component detection. After these filters, imputation was performed using the European samples from the HRC r1.1.2016 reference panel (Build 37 Assembly 19) and SHAPEIT phasing on the Michigan imputation server.22 Postimputation genotype data were filtered for imputation quality (R2 >0.9) and minor allele frequency (<1%). A summary of the quality control process performed on each data set is reported in eTable 1 in the Supplement.
Amyloid PET Imaging Acquisition
Protocols for amyloid acquisition differed by site (eTable 2 in the Supplement). The A4 Study is a large, multisite trial with florbetapir F 18 (18F) amyloid PET imaging data acquired 50 to 70 minutes postinjection. ADNI 18F-florbetapir and C11PiB data were acquired using a dynamic 3-dimensional scan on various scanner platforms with four 5-minute frames acquired 50 to 70 minutes postinjection. Berkeley C11PiB data were acquired using a full dynamic protocol for 90 minutes (35 total frames) in a scanner (ECAT EXACT HR+ PET; Siemens). BIOCARD and BLSA C11PiB data were acquired on a scanner (GE Advance; GE Healthcare) using a 70-minute dynamic protocol. Similarly, WRAP C11PiB data were acquired on a scanner using a dynamic 70-minute protocol (ECAT EXACT HR+; Siemens). In all studies, images were reconstructed, averaged, spatially aligned, interpolated, and smoothed using study-specific pipelines. Mean standard uptake value ratio and distribution volume ratio calculations varied by site; all sites used whole or gray matter cerebellum as the reference region.
Harmonization of Amyloid Data
Harmonization was performed from composite cortical values within each site. To ensure that all amyloid values were on the same scale, we applied a gaussian mixture model23 using a modification of a recently developed harmonization algorithm.24 Gaussian mixture models were estimated among individuals who were cognitively normal within each cohort, and the mean (SD) was applied to the entire sample. In all cases, a 2-component model fit the data, confirming that global amyloid PET imaging followed a bimodal distribution reflecting amyloid-negative and amyloid-positive groups. Mean standard uptake value ratios were scaled and normalized using the mean and SD estimated from the predicted amyloid-negative gaussian distribution. The harmonization appropriately overlaid all data sets onto a common scale (eFigure 1 in the Supplement). As noted in the original harmonization manuscript, C11PiB has a larger dynamic range compared with 18F-florbetapir ligands, including a higher ceiling and wider distribution, particularly among amyloid-positive individuals.24 Consistently, we observed higher values among the harmonized C11PiB samples. An alternative approach to harmonization is to use the characteristics of both gaussian distributions to transform all C11PiB values to 18F-florbetapir values.24 As a sensitivity analysis, we performed harmonization using this full transformation and compared results.
Data on RNA sequencing from the dorsolateral prefrontal cortex of individuals participating in ROS/MAP were used for validation of candidate genes from the GWAS analysis. Details of the RNA sequencing methods have been published previously.25
Autopsy measures of β-amyloid were quantified in ROS/MAP using immunohistochemistry.26 Immunohistochemistry estimates of amyloid (anti-Aβ) were quantified from 8 brain regions, including the angular gyrus, hippocampus, entorhinal, inferior temporal, calcarine, middle frontal, superior frontal, and anterior cingulate cortices.
In ROS/MAP, a comprehensive neuropsychological protocol was completed at each study visit. For the present analysis, we leveraged both a global composite measure of cognition, quantified previously based on z scores from 17 total tests that assess 5 different cognitive domains (semantic memory, episodic memory, perceptual orientation, perceptual speed, and working memory)27 and the Mini-Mental State Examination.28
Additional human brain tissues from Vanderbilt University Medical Center were obtained from decedents with AD (n = 5) and age-matched controls (n = 5) after approval of the Vanderbilt University Medical Institutional Review Board. Fixed tissue was sectioned at 50 μm on a vibratome (Leica Biosystems) to produce floating sections. Antigen retrieval was performed by heating sections to 95 °C in a borate buffer for 20 minutes. Sections were photobleached for 48 hours using a light-emitting diode microarry (HTG Supply), blocked in bovine serum albumin, 4%, and incubated with the primary antibody (anti-RBFOX1; Atlas, 1:100; Cathepsin B; R&D, 1:500; or pan-neurofilament; Biolegend, 1:150) overnight. After washing, sections were incubated with a conjugated secondary antibody (Alexa Fluor; Abcam, 1:1000) for 4 hours and then were washed, counterstained with methoxy-X04 (100 μM; Tocris) to identify amyloid-β and tau aggregates, and mounted to slides (Prolong Glass Antifade Mountant; Invitrogen). Images were produced on a laser scanning confocal microscope (LSM710; Zeiss) using ×20 or ×63 objectives and a minimum resolution of at least 1024 × 1024 pixels. Images then were processed (ImageJ).29,30
Statistical Analysis
Genome-wide association studies were completed using PLINK, version 1.931 and R, version 3.6.2 (R Project for Statistical Computing), with additive coding and the harmonized continuous amyloid PET metric set as a quantitative outcome. Genome-wide association studies were completed in each cohort separately. Covariates included age, sex, and the first 3 principal components to account for unmeasured population stratification. Meta-analyses of all results were performed using the inverse-weighted method in METAL.32 Results were restricted to variants present in all cohorts. Significance was set a priori to P = 5 × 10−8. The R packages EasyStrata,33 qqman,34 and Metafor35 were used for data visualization, with additional variant-level visualization completed using LocusZoom.36
We used RNA sequencing data from ROS/MAP to validate candidate genes or loci. First, we assessed the association between gene expression and amyloid-β using linear regression. Immunohistochemistry measures of amyloid-β were square root transformed before analysis. Covariates included age at death, sex, and postmortem interval. For analyses of longitudinal cognitive performance, we performed a mixed-effects regression model with the same covariates. The interval (years prior to death) and intercept were entered as both fixed and random effects in all longitudinal models.
Results
Clinical data for the 3154 individuals in the A4 Study included those whose race/ethnicity was determined genetically to be non-Hispanic white (n = 2960), African American (n = 89), and Hispanic (n = 105). In addition, 6 amyloid PET data sets with participants of non-Hispanic white ethnicity (n = 1160) were analyzed (Table 1). Together, the participants ranged from age 52 to 96 years; 2478 of the participants (57%) were women. With the exception of the 2 ADNI cohorts, 99% of the participants had normal cognition; with those cohorts added, cognition was normal in 90% of the participants. Analysis of variance of each demographic variable indicated significant differences across the cohorts (Table 1). For example, percent women (F8,4305 = 10.8, P < .001), age (F8,4305 = 58.5, P < .001), and percent APOE-positive (F8,4305 = 3.2, P < .001) were significantly different between groups.
Table 1. Amyloid PET GWAS Participant Characteristics by Data Set.
| Characteristic | Mean (SD)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A4 NHW | A4 AA | A4 Hispanic | ADNI | ADNI | Berkeley | BIOCARD | BLSA | WRAP | |
| Amyloid acquisition | 18F-florbetapir | 18F-florbetapir | 18F-florbetapir | 18F-florbetapir | C11PiB | C11PiB | C11PiB | C11PiB | C11PiB |
| No. of participants | 2960 | 89 | 105 | 623 | 88 | 172 | 44 | 144 | 89 |
| Women, No. (%) | 1768 (60) | 63 (71) | 63 (60) | 279 (45) | 27 (31) | 101 (59) | 28 (64) | 91 (63) | 58 (65) |
| Normal cognition, No. (%) | 2960 (100) | 89 (100) | 105 (100) | 217 (33) | 63 (72) | 172 (100) | 44 (100) | 138 (96) | 87 (98) |
| Age, y | 71.4 (4.8) | 70.3 (4.6) | 71.9 (4.9) | 74.6 (7.6) | 76.5 (7.3) | 74.4 (6.4) | 70.8 (6.1) | 77.2 (7.9) | 67.3 (6.2) |
| APOE4 carriers, No. (%) | 1057 (36) | 33 (37) | 33 (31) | 255 (41) | 45 (51) | 48 (28) | 14 (32) | 39 (27) | 35 (39) |
| Amyloid (standardized) | 1.4 (2.5) | 0.49 (1.5) | 2.2 (4.5) | 2.7 (3.4) | 3.9 (3.0) | 1.8 (4.1) | 2.0 (4.5) | 3.9 (6.4) | 2.7 (5.1) |
| NC participants only | 1.4 (2.5) | 0.49 (1.5) | 2.2 (4.5) | 1.4 (2.8) | 2.2 (2.6) | 1.8 (4.1) | 2.0 (4.5) | 3.5 (6.1) | 2.3 (4.7) |
| AD participants only | NA | NA | NA | 5.2 (3.1) | 5.1 (2.7) | NA | NA | 15.3 (11.3) | NA |
Abbreviations: A4, Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease screening cohort; AA, African American; AD, Alzheimer disease; ADNI, Alzheimer Disease Neuroimaging Initiative; BIOCARD, Biomarkers of Cognitive Decline Among Normal Individuals cohort; BLSA, Baltimore Longitudinal Study of Aging; GWAS, genome-wide association studies; NA, not applicable; NC, normal cognition; NHW, non-Hispanic white; PET, positron emission tomographic; C11PiB, Pittsburgh Compound B; WRAP, Wisconsin Registry for Alzheimer’s Prevention.
Analysis of variance indicated significant differences (P < .001) across cohorts for all demographic categories.
Combining GWAS statistics and harmonized PET imaging amyloid data from each cohort, we completed a meta-analysis of all 6 studies to identify novel genetic associations with brain amyloid levels (n = 4314). We observed a robust association with brain amyloidosis at the APOE locus (top single-nucleotide variant [SNV; formerly SNP]: rs6857, β = 1.67, P = 5.79 × 10−132), similar in magnitude to previous reports.7,8,9,10,11 To determine whether other genes in the APOE region contributed to the association, we performed conditional analyses covarying for APOE ε4 and APOE ε2 status. All associations in the region were no longer significant (eFigure 2 in the Supplement).
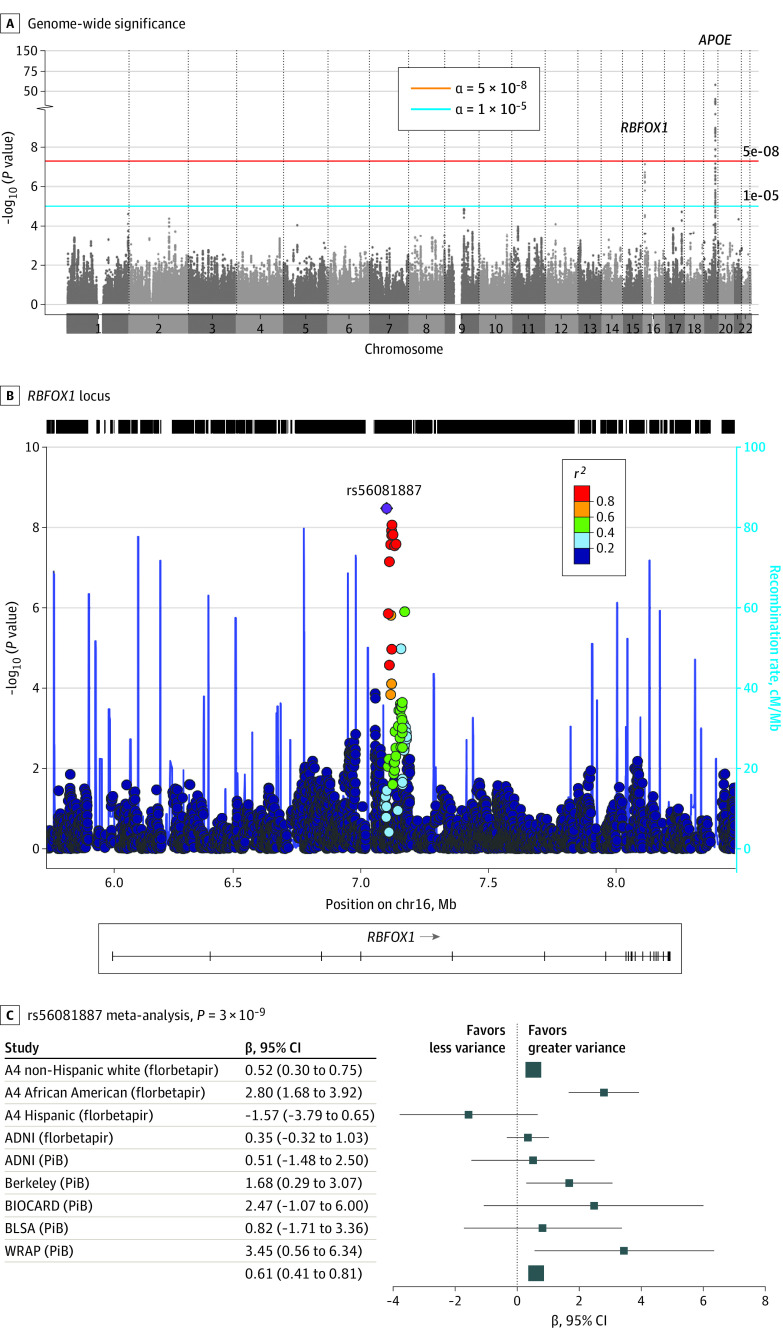
We observed a novel risk locus on chromosome 16p.13.3 (top SNV: rs56081887, β = 0.61, P = 3 × 10−9) that included RBFOX1 (Figure 1A and B). Ten SNVs within RBFOX1 reached genome-wide significance in meta-analysis; the top 2 are displayed in Table 2. RBFOX1 variants were associated with increased amyloid levels in all data sets except for Hispanic individuals in the A4 Study (Figure 1C); however, the small sample size of the Hispanic cohort and the observation that a higher proportion of amyloid-positive individuals were Hispanic (40%) compared with the African American cohort (16%) precluded firm conclusions. All genome-wide significant SNVs in RBFOX1 were in moderate to high linkage disequilibrium (non-Hispanic white r2 all >0.84; African American r2 all >0.53). Results for all variants with P < 19 × 10−5 are presented in eTable 3 in the Supplement. The corresponding QQ-plot is presented in eFigure 3 in the Supplement. There was no compelling evidence for an interaction with APOE ε4. Results were consistent when applying the alternative harmonization algorithm.
Figure 1. Association of 2 Single-Nucleotide Variants in the RBFOX1 Gene With Amyloid Levels.
A, Genome-wide significance (α = 5 x 10−8), suggestive (α = 1 x 10−5). Gene symbols for suspected genes within locus. B, Regional plots of the RBFOX1 locus. Points are colored by linkage disequilibrium with the top variant, denoted by the diamond shape. C, Associations across studies. Squares (point estimate) 95% CIs (line segments); size inversely related to the variance. chr, chromosome; cM/Mb, megabase; PiB, Pittsburgh compound B; Other abbreviations are expanded in note to Table 1.
Table 2. Top 2 Genome-Wide RBFOX1 Variantsa.
| Single-nucleotide variant | Chr:BP | MAF | Gene | Function | Meta-analysis | ||
|---|---|---|---|---|---|---|---|
| No. | β (95% CI) | P value | |||||
| rs56081887 | 16:6903160 | 0.09 | RBFOX1 | Intron | 4314 | 0.61 (0.41-0.81) | 3 × 109 |
| rs34860942 | 16:6919189 | 0.09 | RBFOX1 | Intron | 4314 | 0.59 (0.39-0.80) | 8 × 109 |
Abbreviations: BP, base pair; Chr, chromosome; MAF, minor allele frequency.
Top 2 outside of APOE.
To validate and augment genetic findings, we analyzed RNA sequencing data from the prefrontal cortex in 600 individuals from the ROS/MAP study (Table 3). Lower levels of RBFOX1 messenger RNA (mRNA) in prefrontal cortex were associated with a higher amyloid β burden (β = −0.008, P = .002) (eFigure 4 in the Supplement). Associations remained significant when covarying for differences in cell type composition across samples (eTable 4 in the Supplement). Lower RBFOX1 mRNA levels were also associated with poorer global cognitive performance at the final visit before death (β = 0.007, P = .006) and a faster rate of global cognitive decline across all study visits (β = 0.001, P = 4 × 10−5) (eFigure 5 in the Supplement). Expression of RBFOX1 explained 1.5% of the variance in cognitive trajectories beyond covariates and remained statistically significant when covarying for amyloid and tau, which explained 5% and 15% of variance in cognitive trajectories, respectively. When assessing the results of the Mini-Mental State Examination for clinical interpretation, an SD decrease in RBFOX1 was associated with an annual 0.2-point decrease in the Mini-Mental State Examination score.
Table 3. ROS/MAP Participant Characteristics.
| Characteristic | Brain tissue gene expressiona |
|---|---|
| No. of participants | 600 |
| Age at death, mean (SD), y | 88.61 (6.64) |
| Education, mean (SD), y | 16.48 (3.51) |
| Women, No. (%) | 384 (64) |
| Non-Hispanic white, No. (%) | 586 (98) |
| APOE4 carriers, No. (%) | 151 (25) |
| AD, No. (%) | 215 (36) |
| Postmortem interval, mean (SD), h | 6.83 (4.86) |
Abbreviations: AD, Alzheimer disease; ROS/MAP, Religious Orders Study and Rush Memory and Aging Project.
Gene expression data were collected from prefrontal cortex tissue of participants from ROS/MAP. Brain amyloid levels were measured using immunohistochemistry.
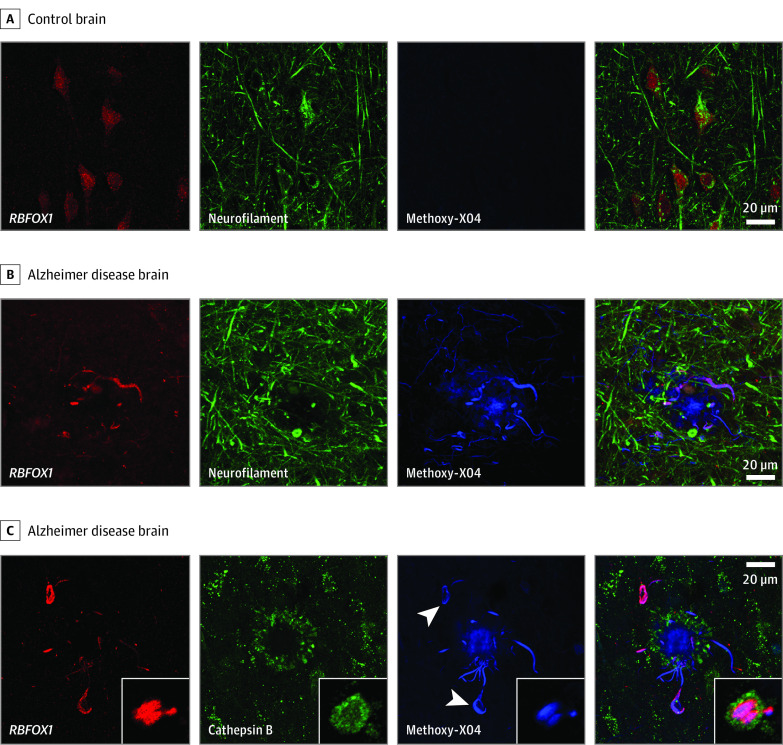
In the microscopic evaluation, RBFOX1 protein localized to neurons in control brains and colocalized with neuropil threads inside dystrophic neurites surrounding amyloid plaques in AD brains (Figure 2). In addition, we observed some colocalization of RBFOX1 with neurofibrillary tangles in AD. Both observations support a potential role for RBFOX1 in AD pathogenesis.
Figure 2. Microscopy of RBFOX1, Neuropil Threads, and Neurofibrillary Tangles .
A, In postmortem control human brain tissue, RBFOX 1 (red) is localized to neurons (neurofilament, green). B, In Alzheimer disease brain, RBFOX1 localizes to neuropil threads around β-amyloid plaques (methoxy-X04, blue). C, In Alzheimer disease brain, RBFOX1 is present in tau tangles (arrowheads) and neuropil threads running through dystrophic neurites (cathepsin B, green) surrounding β-amyloid plaques (methoxy-X04, blue). Insets: cross-section through a dystrophic neurite showing lysosomes (green) surrounding a core of tau (blue) on which RBFOX1 (red) is enriched. Scale bar is 20 μm. Width of inset box is 12 μm.
Discussion
The goal of this investigation was to examine the genetic basis of brain amyloidosis in preclinical AD. Using a collection of 6 publicly available data sets in a meta-analysis, we replicated the previously reported association between APOE and brain amyloidosis. In addition, we identified a novel locus on chromosome 16p13.3, RBFOX1, which encodes ataxin-2–binding protein, an RNA-binding protein. In support of the genetic findings reported herein, evidence for an association between variants in the RBFOX1 locus and AD were observed in an African American GWAS of AD (rs79537509, P = 5.3 × 10−7) (B. Kunkle, PhD, written communication, September 19, 2019), in a family-based study,37 and in a study of cerebral glucose metabolism in ADNI.38
Previous studies have used amyloid PET imaging to investigate the genetic basis of brain amyloidosis. A meta-analysis of 3 PET-PiB GWAS (n = 983) showed an association with APOE but no other genome-wide significant loci.8 In contrast, using 18F-florbetapir PET imaging within the ADNI cohort, 2 GWAS studies by Ramanan et al10,11 reported associations between brain amyloidosis and APOE and 2 other loci in a cross-sectional and longitudinal analysis, respectively: BCHE (butyrylcholinesterase) and IL1RAP (interleukin-1 receptor accessory protein). Although we observed an association for the BCHE SNV (rs509208, P = .007), the association was solely driven by the ADNI cohort. Therefore, neither previous locus was detected in the present study. The small sample size of previous studies likely limited the ability to detect the association with RBFOX1.
RBFOX1 encodes an RNA-binding protein expressed in muscle, heart, and neurons and is a member of the evolutionarily conserved Fox-1 family of RNA-binding proteins that bind to ataxin-2 and regulate alternative splicing.39 In addition, mammalian RBFOX1 is present in the cytoplasm where it binds to 3 prime untranslated regions of multiple mRNAs, regulating their stability.40 RBFOX1 is a highly conserved protein that can regulate splicing and transcriptional networks in human neuronal development, particularly in neuronal migration and synapse network formation within the cerebral cortex.40,41 In addition to a potential role as the binding protein for ataxin-2 in spinocerebellar ataxia type 2, deletions and other structural variants in the RBFOX1 gene increase the risk of generalized epilepsy, intellectual disability, autism spectrum disorder, and developmental disorders associated with aggression.42,43,44
While the exact mechanisms relating dysfunctional human RBFOX proteins with various neuropsychiatric disorders are not fully understood, there is evidence for multiple possible molecular causal pathways. Downregulation of RBFOX1 leads to destabilization of both nuclear and cytoplasmic mRNAs encoding for synaptic transmission proteins and loss of synaptic function in AD.45,46 RBFOX1 may regulate alternative splicing of APP,47 which may be particularly relevant to the amyloid associations observed in the present analysis. Alternatively, downregulation of RBFOX1 in AD may directly affect the stability and abundance of mRNAs that encode synaptic transmission proteins.45 Furthermore, because FOX1 and ataxin-2 are also present in the trans-Golgi network, a trafficking or recycling mechanism might be implicated. Clearly, additional experimental work will be needed to clarify the potential role of RBFOX1 in brain amyloidosis and AD dementia. Aberrant colocalization of disease-associated proteins has been previously reported in other neurodegenerative diseases, such as the TDP-43 protein in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.48 We found colocalization of the RBFOX1 protein not only just around amyloid plaques but also with neurofibrillary tangles. These results imply that the protein may play a general role in AD-related proteinopathy.
We also observed associations between variants in the APOE region and brain amyloidosis, consistent with previous reports leveraging autopsy measures of neuropathologic characteristics,49 cerebrospinal fluid biomarkers of amyloidosis,50 and PET biomarkers of amyloidosis.8,10,11 The locus surrounding APOE, chromosome 19q13.32, includes a number of potential genes, such as TOMM40, APOC1, and PVRL2 (eFigure 2 in the Supplement), but conditional analyses indicated that the genetic association was driven by APOE. APOE is thought to relate to AD through an amyloid clearance pathway, with APOE ε4 associated with earlier deposition of amyloid even during preclinical stages of disease.
Strengths and Limitations
The strengths of this study include the large sample size, the number of asymptomatic individuals allowing a focus on preclinical disease, and comprehensive validation analyses at the RNA and protein level. Study limitations include clinical heterogeneity across studies, overrepresentation of non-Hispanic white women with high levels of education, and our reliance on harmonized data acquired on different scanners and processed in different ways. Although we limited these factors statistically when possible, residual confounding cannot be ruled out.
Conclusions
To our knowledge, this is the largest GWAS of PET amyloid imaging; we report a novel genetic risk locus for brain amyloidosis within RBFOX1. Additional evidence at the transcript and protein level may further implicate RBFOX1 as a novel genetic risk locus for brain amyloidosis and a candidate for early progression in AD.
eTable 1. Summary of Imputation and Quality Control Measures Performed on Each Data set
eTable 2. Summary of Protocols for Amyloid Acquisition by Site
eTable 3. Top PET Amyloid Meta-analysis GWAS Associations (P < 1e-5)
eTable 4. RBFOX1 Brain Expression Associations With Amyloid Burden Adjusted for Cell-Type Composition
eFigure 1. Density Histogram of Normalized Amyloid PET Measures per Study
eFigure 2. Locus Zoom Plots of the APOE Region in Conditional Analyses
eFigure 3. QQ-Plot for the Meta-analysis of Amyloid PET Across All Cohorts
eFigure 4. Lower RBFOX1 Expression in the Prefrontal Cortex Was Associated With Higher Amyloid Burden
eFigure 5. Lower RBFOX1 Expression in the Prefrontal Cortex Was Associated With a Faster Rate of Global Cognitive Decline
References
- 1.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404-413. doi: 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunkle BW, Grenier-Boley B, Sims R, et al. ; Alzheimer Disease Genetics Consortium (ADGC); European Alzheimer’s Disease Initiative (EADI); Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE); Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES) . Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414-430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickers JC, Mitew S, Woodhouse A, et al. Defining the earliest pathological changes of Alzheimer’s disease. Curr Alzheimer Res. 2016;13(3):281-287. doi: 10.2174/1567205013666151218150322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306-319. doi: 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Rieves D, Ganley C. Brain amyloid imaging—FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367(10):885-887. doi: 10.1056/NEJMp1208061 [DOI] [PubMed] [Google Scholar]
- 6.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacher M, Porter T, Villemagne VL, et al. Validation of a priori candidate Alzheimer’s disease SNPs with brain amyloid-beta deposition. Sci Rep. 2019;9(1):17069. doi: 10.1038/s41598-019-53604-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Q, Nho K, Del-Aguila JL, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . Genome-wide association study of brain amyloid deposition as measured by Pittsburgh compound-B (PiB)-PET imaging. Mol Psychiatry. 2018. Published online October 25, 2018. doi: 10.1038/s41380-018-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhang Q, Chen F, et al. Genetic interactions explain variance in cingulate amyloid burden: an AV-45 PET genome-wide association and interaction study in the ADNI Cohort. Biomed Res Int. 2015;2015:647389. doi: 10.1155/2015/647389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanan VK, Risacher SL, Nho K, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain. 2015;138(pt 10):3076-3088. doi: 10.1093/brain/awv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanan VK, Risacher SL, Nho K, et al. ; Alzheimer’s Disease Neuroimaging Initiative . APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study. Mol Psychiatry. 2014;19(3):351-357. doi: 10.1038/mp.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperling RA, Rentz DM, Johnson KA, et al. The A4 Study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs13. doi: 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling RA, Donohue MC, Raman R, et al. ; A4 Study Team . Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. Published online April 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little J, Higgins JP, Ioannidis JP, et al. ; Strengthening the Reporting of Genetic Association Studies . Strengthening the Reporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009;6(2):e22. doi: 10.1371/journal.pmed.1000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245-249. doi: 10.1177/0891988705281882 [DOI] [PubMed] [Google Scholar]
- 16.Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer’s Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2017;10:130-142. doi: 10.1016/j.dadm.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert M, Soldan A, Gottesman R, et al. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimer Res. 2014;11(8):773-784. doi: 10.2174/156720501108140910121920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464-472. doi: 10.1093/cercor/10.5.464 [DOI] [PubMed] [Google Scholar]
- 19.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807-815. doi: 10.1212/WNL.0b013e3181d3e3e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161-S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darst BF, Lu Q, Johnson SC, Engelman CD. Integrated analysis of genomics, longitudinal metabolomics, and Alzheimer’s risk factors among 1,111 cohort participants. Genet Epidemiol. 2019;43(6):657-674. doi: 10.1002/gepi.22211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284-1287. doi: 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mormino EC, Betensky RA, Hedden T, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study . Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760-1767. doi: 10.1212/WNL.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Properzi MJ, Buckley RF, Chhatwal JP, et al. Nonlinear distributional mapping (NoDiM) for harmonization across amyloid-PET radiotracers. Neuroimage. 2019;186:446-454. doi: 10.1016/j.neuroimage.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jager PL, Ma Y, McCabe C, et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci Data. 2018;5:180142. doi: 10.1038/sdata.2018.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406-412. doi: 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Petyuk VA, Tasaki S, et al. Association of cortical β-amyloid protein in the absence of insoluble deposits with Alzheimer disease. JAMA Neurol. 2019;76(7):818-826. doi: 10.1001/jamaneurol.2019.0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 29.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):529. doi: 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler TW, Kutalik Z, Gorski M, Lottaz C, Kronenberg F, Heid IM. EasyStrata: evaluation and visualization of stratified genome-wide association meta-analysis data. Bioinformatics. 2015;31(2):259-261. doi: 10.1093/bioinformatics/btu621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and Manhattan plots. Preprint. bioRxiv 005165. Posted online May 14, 2014. doi: 10.1101/005165 [DOI]
- 35.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 36.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336-2337. doi: 10.1093/bioinformatics/btq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herold C, Hooli BV, Mullin K, et al. Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer’s disease with OSBPL6, PTPRG, and PDCL3. Mol Psychiatry. 2016;21(11):1608-1612. doi: 10.1038/mp.2015.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong LL, Miao D, Tan L, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Genome-wide association study identifies RBFOX1 locus influencing brain glucose metabolism. Ann Transl Med. 2018;6(22):436. doi: 10.21037/atm.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auweter SD, Fasan R, Reymond L, et al. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 2006;25(1):163-173. doi: 10.1038/sj.emboj.7600918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada N, Ito H, Iwamoto I, Morishita R, Tabata H, Nagata K. Role of the cytoplasmic isoform of RBFOX1/A2BP1 in establishing the architecture of the developing cerebral cortex. Mol Autism. 2015;6:56. doi: 10.1186/s13229-015-0049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogel BL, Wexler E, Wahnich A, et al. RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum Mol Genet. 2012;21(19):4171-4186. doi: 10.1093/hmg/dds240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lal D, Trucks H, Møller RS, et al. ; EMINet Consortium; EPICURE Consortium . Rare exonic deletions of the RBFOX1 gene increase risk of idiopathic generalized epilepsy. Epilepsia. 2013;54(2):265-271. doi: 10.1111/epi.12084 [DOI] [PubMed] [Google Scholar]
- 43.Bill BR, Lowe JK, Dybuncio CT, Fogel BL. Orchestration of neurodevelopmental programs by RBFOX1: implications for autism spectrum disorder. Int Rev Neurobiol. 2013;113:251-267. doi: 10.1016/B978-0-12-418700-9.00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernàndez-Castillo N, Gan G, van Donkelaar MMJ, et al. RBFOX1, encoding a splicing regulator, is a candidate gene for aggressive behavior. Eur Neuropsychopharmacol. 2020;30:44-55. doi: 10.1016/j.euroneuro.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkallas R, Fish L, Goodarzi H, Najafabadi HS. Inference of RNA decay rate from transcriptional profiling highlights the regulatory programs of Alzheimer’s disease. Nat Commun. 2017;8(1):909. doi: 10.1038/s41467-017-00867-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JA, Damianov A, Lin C-H, et al. Cytoplasmic Rbfox1 regulates the expression of synaptic and autism-related genes. Neuron. 2016;89(1):113-128. doi: 10.1016/j.neuron.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam S, Suzuki H, Tsukahara T. Alternative splicing regulation of APP exon 7 by RBFox proteins. Neurochem Int. 2014;78:7-17. doi: 10.1016/j.neuint.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. doi: 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- 49.Beecham GW, Hamilton K, Naj AC, et al. ; Alzheimer’s Disease Genetics Consortium (ADGC) . Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deming Y, Li Z, Kapoor M, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI); Alzheimer Disease Genetic Consortium (ADGC) . Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 2017;133(5):839-856. doi: 10.1007/s00401-017-1685-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Imputation and Quality Control Measures Performed on Each Data set
eTable 2. Summary of Protocols for Amyloid Acquisition by Site
eTable 3. Top PET Amyloid Meta-analysis GWAS Associations (P < 1e-5)
eTable 4. RBFOX1 Brain Expression Associations With Amyloid Burden Adjusted for Cell-Type Composition
eFigure 1. Density Histogram of Normalized Amyloid PET Measures per Study
eFigure 2. Locus Zoom Plots of the APOE Region in Conditional Analyses
eFigure 3. QQ-Plot for the Meta-analysis of Amyloid PET Across All Cohorts
eFigure 4. Lower RBFOX1 Expression in the Prefrontal Cortex Was Associated With Higher Amyloid Burden
eFigure 5. Lower RBFOX1 Expression in the Prefrontal Cortex Was Associated With a Faster Rate of Global Cognitive Decline