Abstract
Among 413 Nigerian men who have sex with men and transgender women, retrospective testing for Mycoplasma genitalium revealed mostly asymptomatic infections of the anorectum (prevalence 36.8%, incidence 18.4 cases/100 person-years) and urogenital tract (12.4%, 4.0 cases/100 person-years). Risk factors included HIV and increasing number of sex partners.
Keywords: Mycoplasma genitalium, Acquired Immunodeficiency Syndrome, Sexual and Gender Minorities, Transgender Persons, Africa
SHORT SUMMARY
A study of Nigerian men who have sex with men and transgender women found that over one-third had anorectal Mycoplasma genitalium. HIV was independently associated with Mycoplasma genitalium infection.
INTRODUCTION
Laboratory investigations suggest that Mycoplasma genitalium (MG) infection of the urogenital tract or anorectum may facilitate HIV transmission through mucosal disruption(1, 2), recruitment of HIV-susceptible cells to the mucosal surface(3, 4), and direct enhancement of HIV replication(5–7). Limited human data support a causal relationship with HIV acquisition(8, 9). However, MG diagnostic capability is not readily available in resource-limited settings that bear a disproportionate burden of new HIV infections.
Urogenital MG appears to be more common in men than women and in resource-limited as compared to resource-rich settings(10). Prior studies in sub-Saharan Africa have identified urogenital MG in 3-10% of men tested(11–14). To our knowledge, no prior studies have evaluated anorectal MG in men who have sex with men (MSM) and transgender women (TGW) in sub-Saharan Africa despite the high prevalence of other STIs in these populations(15) and substantial stigma that limits healthcare engagement for STI diagnosis and treatment(16–18). We retrospectively tested stored specimens for anorectal and urogenital MG in cisgender MSM and TGW in Lagos, Nigeria.
MATERIALS AND METHODS
The Lagos site of TRUST/RV368, a community-engaged HIV/STI prevention and treatment cohort study, used respondent-driven sampling to recruit cisgender men and transgender women. Study staff worked with local advocacy groups and non-governmental organizations to recruit an initial small group of participants (“seeds”) from the MSM and TGW communities who lived in a variety of different neighborhoods and possessed a variety of sociodemographic characteristics. Each seed was provided three coupons to distribute to other potential participants in their communities and each newly-enrolled participant also received three coupons. To be eligible for enrollment, potential participants had to present a valid referral coupon, be ≥18 years old, and report anal intercourse with a male partner in the preceding 12 months(15). Incentives were provided for referrals (Naira 1500 [about USD 5]) and participation in visits (Naira 2000-3400 [about USD 6-11], depending on visit) every three months for up to 18 months. Medical history, solicited symptoms, and responses to a standardized demographic and behavioral questionnaire were documented. Questions about condom use at last anal sex with a male partner and number of anal sex partners in the preceding 12 months were asked at enrollment and after 9 and 15 months in the cohort. Prior questionnaire responses were carried forward as needed for visits that included retrospective MG testing.
At each visit, HIV testing was performed using clinician-collected fingerstick blood samples in a parallel algorithm of two rapid tests(19). Anorectal swab and voided urine specimens were self-collected at each visit and tested in real-time for Chlamydia trachomatis and Neisseria gonorrhoeae using the Aptima Combo 2® assay (Hologic, Bedford, MA, USA). For these analyses, anorectal swab and voided urine specimens from the first and last available visits underwent additional retrospective testing using the Aptima MG transcription-mediated amplification assay (Hologic, Bedford, MA, USA). MG testing was performed at only one anatomic site for some participants due to logistical reasons such as unavailability of stored specimen. Medical management of HIV, chlamydia, and gonorrhea was offered to participants in real-time. Participants were not notified of, or treated for, retrospectively-diagnosed MG.
Statistical analyses were conducted separately for anorectal and urogenital MG infections. Prevalence was calculated by dividing the number of participants with MG detected on the first sample tested by the number of participants tested. Among participants without prevalent disease, incidence was calculated by dividing the number of participants with MG on the second sample tested by the time elapsed since the first test, then standardized as a rate per 100 person-years (PY). Wald and exact 95% confidence intervals (CIs) were calculated for prevalence and incidence, respectively. Exact confidence intervals were also used for prevalence when fewer than five cases were observed within a group of interest. Prevalence was compared between groups using Student’s T or Fisher’s exact tests. Poisson regression with robust error variance was used to estimate risk ratios (RRs) and 95% CIs for pre-specified factors potentially associated with MG at each anatomic site(20). Multivariable models included all factors, including the presence or absence of infection with other STIs at the same anatomic site. Continuous variables, such as age and number of sexual partners, were categorized based on exploratory data analyses. Among participants with prevalent or incident MG, solicited symptoms from the first positive visit were tallied. Analyses were performed using Stata 15.0 (StataCorp LP, College Station, TX) and SAS 9.4 (SAS Institute Inc., Cary, NC).
All participants provided written informed consent prior to enrollment. The study was approved by institutional review boards at the Nigerian Ministry of Defense, Abuja, Nigeria; Walter Reed Army Institute of Research, Silver Spring, MD, USA; and all collaborating institutions.
RESULTS
Using samples collected from May 2014 through July 2016, 413 participants were screened retrospectively for MG. Participants had median age 23 (interquartile range [IQR] 20-26) years, 63 (15.3%) self-identified as TGW, and 290 (70.2%) were HIV-infected (Supplemental Table 1).
Among 408 participants tested for anorectal MG, there were 150 prevalent cases (36.8%, 95% CI 32.1-41.6%), including disproportionately more among HIV-infected participants (Figure 1A). Anorectal MG prevalence among cisgender MSM was 36.2% (95% CI 30.9-41.4%), among TGW 43.6% (95% CI 31.2-55.9%), and among persons with unknown/other gender identity 28.6% (11.8-45.3%; p=0.37). The prevalence of anorectal MG among participants reporting one or more symptoms was 35.4% (95% CI 23.8-47.0%) and among those reporting no symptoms was 37.0% (95% CI 31.9-42.1%, p=0.80). After a median of 1.01 (IQR 0.34-1.41) years, 52 (45.6%) of 114 participants with a second test had persistent or repeated infection. Incident anorectal MG was observed in 31 participants at a median of 0.88 (IQR 0.42-1.44) years after their first negative test (19.1 [95% CI 13.4-26.0] cases/100PY). Anorectal incidence among cisgender MSM was 22.7 (95% CI 15.5-31.3) cases/100PY and among TGW was 9.7 (95% CI 2.0-25.7) cases/100PY (p<0.001).
Figure 1. Prevalence of Mycoplasma genitalium, Stratified by Other Sexually Transmitted Infections.
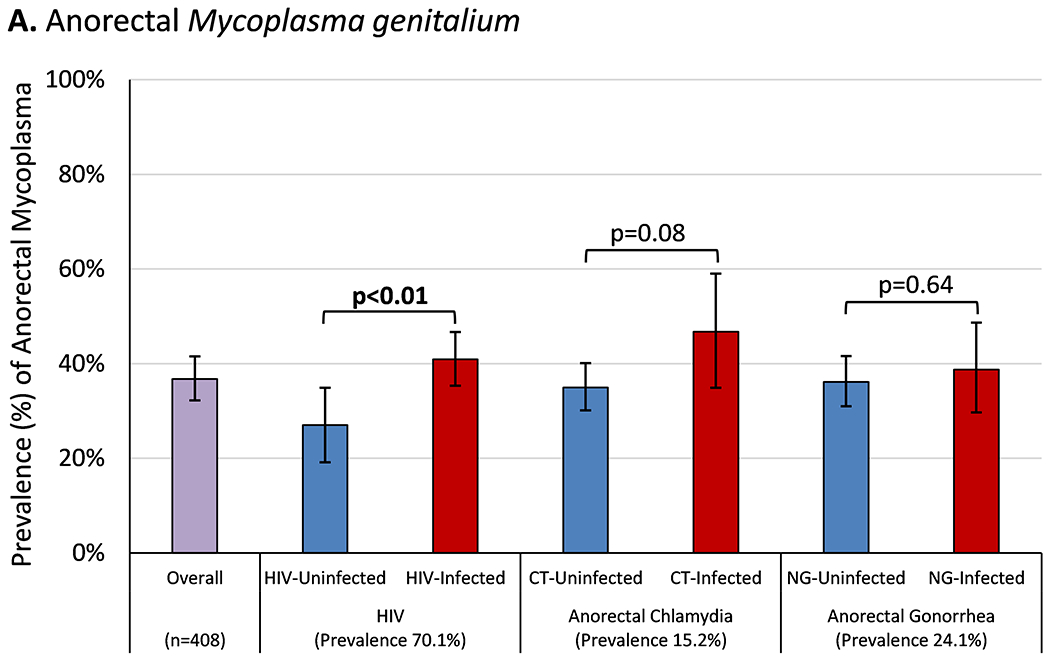
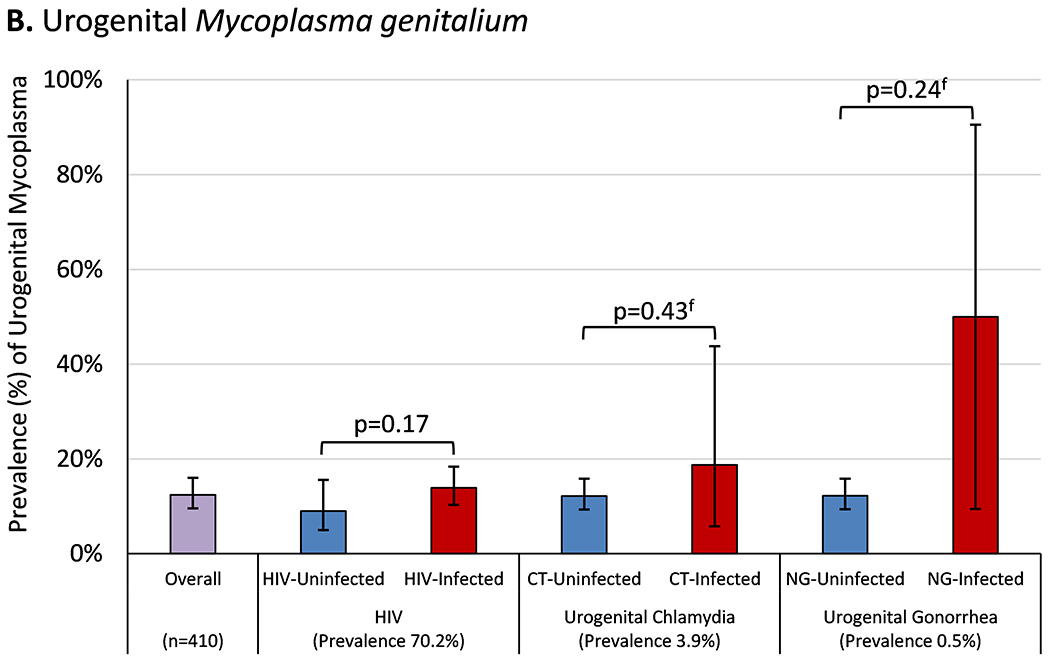
Bar height represents the percentage of study participants diagnosed with (A) anorectal or (B) urogenital Mycoplasma genitalium on the first tested specimen, stratified by the presence or absence of co-infection with other sexually transmitted infections. Error bars represent Wald 95% confidence intervals. Comparisons between participants with and without each co-infection were made using Student’s t-test or Fisher’s exact test (for rare events, indicated by f) with statistically significant p-values (p≤0.05) shown in bold. The prevalence of each sexually transmitted infection was calculated by dividing the number of cases detected by the total number of participants retrospectively tested for Mycoplasma genitalium at each anatomic site.
Abbreviations: HIV, human immunodeficiency virus; CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae
Among 410 participants tested for urogenital MG, there were 51 prevalent cases (12.4%, 95% CI 9.2-15.6%) without variation by HIV, chlamydia, or gonorrhea status (Figure 1B). Urogenital MG prevalence among cisgender MSM was 14.1% (95% CI 10.3-17.9%), among TGW 4.8% (95% CI 1.0-13.5%), and among persons with unknown/other gender identity 10.3% (2.2-27.4%; p=0.12). The prevalence of urogenital MG among participants reporting one or more symptoms was 11.8% (95% CI 4.1-19.4%) and among those reporting no symptoms was 12.6% (95% CI 9.0-16.1%, p=0.85). After a median of 0.80 (IQR 0.38-1.38) years, 19 (65.5%) of 29 with a second test had persistent or repeated infection. Incident urogenital MG was observed in 10 participants at a median of 0.92 (IQR 0.40-1.43) years after their first negative test (4.0 [95% CI 2.2-7.5] cases/100PY). Urogenital incidence among cisgender MSM was 4.6 (95% CI 2.0-8.8) cases/100PY and among TGW was 1.8 (95% CI 0.0-9.5) cases/100PY (p<0.001).
After controlling for other factors, more anorectal MG was observed with increasing number of partners for receptive anal sex, HIV infection, and anorectal chlamydia (Table 1). More urogenital MG was observed with increasing number of partners for insertive anal sex and with HIV infection.
Table 1.
Analyses of Factors Associated with Anorectal and Urogenital Mycoplasma genitalium Infection
| Anorectal Mycoplasma genitalium |
Urogenital Mycoplasma genitalium |
|||
|---|---|---|---|---|
| Unadjusted Risk Ratio (95% CI) | Adjusted Risk Ratio (95% CI) | Unadjusted Risk Ratio (95% CI) | Adjusted Risk Ratio (95% CI) | |
| Age | ||||
| < 22 years | Reference | Reference | Reference | Reference |
| 22-30 years | 0.79 (0.62-1.00) | 0.85 (0.67-1.07) | 0.98 (0.61-1.57) | 0.93 (0.56-1.54) |
| > 30 years | 0.68 (0.43-1.08) | 0.71 (0.44-1.12) | 0.78 (0.35-1.74) | 0.64 (0.28-1.46) |
| Gender Identity | ||||
| Cisgender Man | Reference | Reference | Reference | Reference |
| Transgender Woman | 1.05 (0.78-1.43) | 0.90 (0.66-1.28) | 0.43 (0.16-1.20) | 0.41 (0.16-1.06) |
| Other/Unknown | 0.80 (0.44-1.44) | 0.73 (0.42-1.28) | 0.81 (0.30-2.18) | 0.82 (0.34-2.02) |
| Sexual Orientation | ||||
| Gay/Homosexual | Reference | Reference | Reference | Reference |
| Bisexual† | 0.83 (0.66-1.05) | 0.90 (0.71-1.14) | 0.94 (0.58-1.54) | 0.71 (0.44-1.15) |
| Condom Use at Last Anal Sex | ||||
| Yes | Reference | Reference | Reference | Reference |
| No‡ | 0.92 (0.73-1.15) | 0.87 (0.69-1.09) | 0.94 (0.65-1.36) | 0.90 (0.61-1.35) |
| # of Partners for Receptive Anal Sex | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1-2 | 1.34 (0.98-1.85) | 1.26 (0.92-1.74) | 1.30 (0.72-2.36) | 1.17 (0.67-2.06) |
| 3 or more | 1.72 (1.27-2.32) | 1.59 (1.16-2.18) | 1.04 (0.58-1.87) | 0.89 (0.50-1.59) |
| # of Partners for Insertive Anal Sex | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1-2 | 0.93 (0.74-1.18) | 0.97 (0.77-1.23) | 2.51 (1.37-4.61) | 2.64 (1.40-4.96) |
| 3 or more | 0.82 (0.64-1.06) | 0.88 (0.68-1.56) | 2.96 (1.55-5.67) | 3.52 (1.77-6.98) |
| HIV Status | ||||
| Uninfected | Reference | Reference | Reference | Reference |
| Infected | 1.46 (1.08-1.98) | 1.37 (1.01-1.86) | 1.48 (0.81-2.69) | 1.84 (1.01-3.36) |
| Chlamydia Status* | ||||
| Uninfected | Reference | Reference | Reference | Reference |
| Infected | 1.40 (1.08-1.82) | 1.36 (1.04-1.76) | 1.32 (0.73-2.39) | 1.22 (0.68-2.21) |
| Gonorrhea Status* | ||||
| Uninfected | Reference | Reference | Reference | Reference |
| Infected | 1.18 (0.93-1.50) | 0.98 (0.78-1.24) | 2.35 (0.65-8.50) | 3.24 (0.70-14.92) |
Unadjusted and adjusted Poisson regression models with generalized estimating equations, clustered by participant, and robust error variance were used to calculate relative risk and 95% confidence intervals for factors associated with Mycoplasma genitalium infection at each anatomic site tested. Adjusted models included all factors listed in the table. Statistically significant associations (p≤0.05) are shown in bold.
The “bisexual” category of sexual orientation also includes two participants who reported “transgender” and one “queer.”
The “no” category of condom use at last anal sex with a male partner includes one participant who responded “don’t know” when asked about condom use.
Infection status for bacterial STIs was assessed at the same visit and the same anatomic site as the sample collection for Mycoplasma genitalium testing.
Among the 208 participants with prevalent or incident MG, 147 (70.7%) were infected at the anorectal site only, 27 (13.0%) at the urogenital site only, and 34 (16.3%) at both sites. Eight diagnoses were in participants screened at only one site (3 anorectal and 5 urogenital).
At sample collection, 28 (15.5%) of 181 participants with anorectal MG reported any symptom, with rash and fever/sweats being the most common (5.0% and 4.4%, respectively, Supplemental Table 2). The percentage who reported symptoms was similar among those with and without anorectal chlamydia co-infection (6.1% vs. 17.6%, p=0.12) and those with and without anorectal gonorrhea co-infection (18.6% vs. 14.5%, p=0.48). Symptoms were reported by 11 (18.0%) of 61 participants with urogenital MG, most commonly rash, genital ulcer, and/or pain around the anus (6.6% each). The percentage who reported symptoms was similar between those with and without urogenital chlamydia co-infection (25.0% vs. 17.5%, p=0.56). The one participant with concurrent urogenital MG and gonorrhea reported no symptoms.
DISCUSSION
MG was common in Nigerian MSM and TGW, particularly HIV-infected participants. The majority of infections in this study were detected at the anorectal site, which has not previously been studied in MSM and TGW in sub-Saharan Africa. Urogenital MG was marginally more common than the 3-10% prevalence reported in prior studies of African men(11–14). Notably, MG infections were substantially more common than both chlamydia and gonorrhea infections in this study. This relationship has also been observed in a few studies of those engaged in high-risk sexual practices in the developed world(21, 22).
The observed association of HIV with anorectal MG is consistent with previous reports from predominantly resource-rich settings(23, 24). Importantly, this finding was robust to analyses that controlled for potential behavioral confounders such as number of sexual partners, adding to the growing body of evidence supporting a biological mechanism for this association. In sub-Saharan Africa, an association between HIV and urogenital MG has been observed in multiple studies of mostly cisgender women(24), and this finding was replicated in our population of MSM and TGW. Even HIV-uninfected participants in this study had substantially higher prevalence and incidence of MG than has been previously reported in most settings(10).
Given the high prevalence of asymptomatic disease, potential for morbidity, and enhanced risk of HIV transmission, MG screening could be considered for at-risk individuals. However, resource limitations, unclear management of asymptomatic infections, and until recently the lack of a U.S. Food and Drug Administration-approved test have complicated the issue of whether this should be recommended(25). Even in resource-rich settings such as the United States, current guidelines recommend consideration of MG only in cases of persistent or recurrent urethritis, cervicitis, and pelvic inflammatory disease(26). At a minimum, MG should be considered in cases of urethritis and proctitis that fail to respond to conventional therapies, particularly in populations with a high burden of HIV, STIs, and frequent drug exposures that promote emergence of drug-resistant pathogens. Further research is required to inform the management of asymptomatic MG.
We characterized the prevalence and incidence of MG infection in a highly-marginalized population of Nigerian MSM and TGW. Partly because of the high prevalence of MG, few incident cases were detected, so prevalent and incident cases were combined for some analyses. Urogenital STIs, including MG, were less common than anorectal ones so statistical models were underpowered to identify associations between infections at the urogenital site. Genotypic testing for evidence of antimicrobial resistance was not undertaken and no isolates were available for phenotypic testing; while a few small studies have shown little or no fluoroquinolone or macrolide resistance among MG strains in African women(27–29), there is substantial evidence of increasing resistance among MG isolates globally(30). Characterizing the burden of antibiotic resistance in populations with a high burden of MG infection, such as the MSM and TGW in this study, should be a research priority. Self-administration of antibiotics is common in Nigeria and antibiotic treatment data were not consistently available for these analyses(31); antibiotic use may have influenced MG clearance, potential for reinfection, and led to an underestimation of MG incidence. The varying interval between MG tests may also have contributed to an underestimation of MG incidence, as infections may have gone undetected but inadvertently treated by antibiotic use for other indications during prolonged periods between tests. Findings from the Lagos metropolis may not be generalizable to MSM and TGW in other communities.
In conclusion, clinicians should be aware that MG is common in Nigerian MSM and TGW. While most cases are asymptomatic, this pathogen must be considered in recurrent or refractory cases of genitourinary and anorectal infectious diseases. Judicious use of antibiotics in the community overall, as well as specifically for syndromic management of urethritis and proctitis, is necessary to mitigate the growing risk of antibiotic resistance. Longitudinal studies are needed to evaluate any potential causal relationship of asymptomatic anorectal MG with HIV transmission or acquisition. The cost-benefit relationship and other implications of expanded screening for MG and treatment of asymptomatic MG infection also require further exploration.
Supplementary Material
ACKNOWLEDGMENTS
The study team would like to thank the study participants for their valuable contributions to this research. The TRUST/RV368 Study Group includes Principal Investigators: Manhattan Charurat (IHV, University of Maryland, Baltimore, MD, USA), Julie Ake (MHRP, Walter Reed Army Institute of Research, Silver Spring, MD, USA); Co-Investigators: Sylvia Adebajo, Stefan Baral, Erik Billings, Trevor Crowell, George Eluwa, Charlotte Gaydos, Afoke Kokogho, Hongjie Liu, Jennifer Malia, Olumide Makanjuola, Nelson Michael, Nicaise Ndembi, Jean Njab, Rebecca Nowak, Oluwasolape Olawore, Zahra Parker, Sheila Peel, Habib Ramadhani, Merlin Robb, Cristina Rodriguez-Hart, Eric Sanders-Buell, Sodsai Tovanabutra; Institutions: Institute of Human Virology at the University of Maryland School of Medicine (IHV-UMB), University of Maryland School of Public Health (UMD SPH), Johns Hopkins Bloomberg School of Public Health (JHSPH), Johns Hopkins University School of Medicine (JHUSOM), U.S. Military HIV Research Program (MHRP), Walter Reed Army Institute of Research (WRAIR), Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), Henry M. Jackson Foundation Medical Research International (HJFMRI), Institute of Human Virology Nigeria (IHVN), International Centre for Advocacy for the Right to Health (ICARH), The Initiative for Equal Rights (TIERS), Population Council Nigeria, Imperial College London.
Funding
This work was supported by cooperative agreements between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040]; the National Institutes of Health [R01 MH099001, R01 AI120913, R01 MH110358]; Fogarty Epidemiology Research Training for Public Health Impact in Nigeria program [D43TW010051]; and the President’s Emergency Plan for AIDS Relief through a cooperative agreement between the Department of Health and Human Services/Centers for Disease Control and Prevention, Global AIDS Program, and the Institute for Human Virology-Nigeria [NU2GGH002099].
Conflicts of Interest: CG has received research support for Mycoplasma genitalium clinical trials from GenProbe/Hologic and SpeeDX. The other authors declare no relevant conflicts of interest.
Footnotes
Prior Presentation
This work was presented, in part, at IDWeek 2018 in San Francisco, CA, 3-7 October 2018.
Publisher's Disclaimer: Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
REFERENCES
- 1.Tully JG, Taylor-Robinson D, Rose DL, Furr PM, Graham CE, Barile MF. Urogenital challenge of primate species with Mycoplasma genitalium and characteristics of infection induced in chimpanzees. J Infect Dis. 1986;153(6):1046–54. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Wear DJ, Lo S. Mycoplasmal infections alter gene expression in cultured human prostatic and cervical epithelial cells. FEMS Immunol Med Microbiol. 2000;27(1):43–50. [DOI] [PubMed] [Google Scholar]
- 3.McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80(11):3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehon PM, McGowin CL. Mycoplasma genitalium infection is associated with microscopic signs of cervical inflammation in liquid cytology specimens. J Clin Microbiol. 2014;52(7):2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury MI, Munakata T, Koyanagi Y, Arai S, Yamamoto N. Mycoplasma stimulates HIV-1 expression from acutely- and dormantly-infected promonocyte/monoblastoid cell lines. Arch Virol. 1994;139(3-4):431–8. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki Y, Honda M, Makino M, Sasaki T. Mycoplasmas stimulate replication of human immunodeficiency virus type 1 through selective activation of CD4+ T lymphocytes. AIDS Res Hum Retroviruses. 1993;9(8):775–80. [DOI] [PubMed] [Google Scholar]
- 7.Phillips DM, Pearce-Pratt R, Tan X, Zacharopoulos VR. Association of mycoplasma with HIV-1 and HTLV-I in human T lymphocytes. AIDS Res Hum Retroviruses. 1992;8(11):1863–8. [DOI] [PubMed] [Google Scholar]
- 8.Mavedzenge SN, Van Der Pol B, Weiss HA, et al. The association between Mycoplasma genitalium and HIV-1 acquisition in African women. AIDS. 2012;26(5):617–24. [DOI] [PubMed] [Google Scholar]
- 9.Vandepitte J, Weiss HA, Bukenya J, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect. 2014;90(7):545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SD, Gaydos C, Maclean I, et al. The effect of medical male circumcision on urogenital Mycoplasma genitalium among men in Kisumu, Kenya. Sex Transm Dis. 2012;39(4):276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapiga SH, Sam NE, Mlay J, et al. The epidemiology of HIV-1 infection in northern Tanzania: results from a community-based study. AIDS Care. 2006;18(4):379–87. [DOI] [PubMed] [Google Scholar]
- 13.Kaida A, Dietrich JJ, Laher F, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: implications for HIV prevention efforts. BMC Infect Dis. 2018;18(1):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leutscher P, Jensen JS, Hoffmann S, et al. Sexually transmitted infections in rural Madagascar at an early stage of the HIV epidemic: a 6-month community-based follow-up study. Sex Transm Dis. 2005;32(3):150–5. [DOI] [PubMed] [Google Scholar]
- 15.Keshinro B, Crowell TA, Nowak RG, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc. 2016;19(1):21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Hart C, Musci R, Nowak RG, et al. Sexual Stigma Patterns Among Nigerian Men Who Have Sex with Men and Their Link to HIV and Sexually Transmitted Infection Prevalence. AIDS Behav. 2018;22(5):1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Hart C, Nowak RG, Musci R, et al. Pathways from sexual stigma to incident HIV and sexually transmitted infections among Nigerian MSM. AIDS. 2017;31(17):2415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowell TA, Keshinro B, Baral SD, et al. Stigma, access to healthcare, and HIV risks among men who sell sex to men in Nigeria. J Int AIDS Soc. 2017;20(1):21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak RG, Mitchell A, Crowell TA, et al. Individual and Sexual Network Predictors of HIV Incidence Among Men Who Have Sex With Men in Nigeria. J Acquir Immune Defic Syndr. 2019;80(4):444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 21.Hakre S, Casimier RO, Danboise BA, et al. Enhanced Sexually Transmitted Infection Screening for Mycoplasma genitalium in Human Immunodeficiency Virus -Infected US Air Force Personnel. Clin Infect Dis. 2017;65(9):1585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gratrix J, Plitt S, Turnbull L, et al. Prevalence and antibiotic resistance of Mycoplasma genitalium among STI clinic attendees in Western Canada: a cross-sectional analysis. BMJ Open. 2017;7(7):e016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissessor M, Tabrizi SN, Bradshaw CS, et al. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin Microbiol Infect. 2016;22(3):260–5. [DOI] [PubMed] [Google Scholar]
- 24.Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS. 2009;23(5):611–20. [DOI] [PubMed] [Google Scholar]
- 25.Golden MR, Workowski KA, Bolan G. Developing a Public Health Response to Mycoplasma genitalium. J Infect Dis. 2017;216(suppl_2):S420–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 27.Ong JJ, Magooa MP, Chikandiwa A, et al. Prevalence and antimicrobial resistance of Mycoplasma genitalium infection among women living with HIV in South Africa: a prospective cohort study. Clin Infect Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Muller EE, Mahlangu MP, Lewis DA, Kularatne RS. Macrolide and fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Johannesburg, South Africa, 2007-2014. BMC Infect Dis. 2019;19(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay B, Dubbink JH, Ouburg S, et al. Prevalence and macrolide resistance of Mycoplasma genitalium in South African women. Sex Transm Dis. 2015;42(3):140–2. [DOI] [PubMed] [Google Scholar]
- 30.Martin DH, Manhart LE, Workowski KA. Mycoplasma genitalium From Basic Science to Public Health: Summary of the Results From a National Institute of Allergy and Infectious Disesases Technical Consultation and Consensus Recommendations for Future Research Priorities. J Infect Dis. 2017;216(suppl_2):S427–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowell TA, Hardick J, Lombardi K, et al. Asymptomatic lymphogranuloma venereum among Nigerian men who have sex with men. Sex Transm Infect. 2018;94(8):578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


