Abstract
Background:
Although lower-complexity cardiac malformations constitute the majority of adult congenital heart disease (ACHD), the long-term risks of adverse cardiovascular events and relationship with conventional risk factors in this population are poorly understood. We aimed to quantify the risk of adverse cardiovascular events associated with lower-complexity ACHD that is unmeasured by conventional risk factors.
Methods:
A multi-tiered classification algorithm was used to select individuals with lower-complexity ACHD and individuals without ACHD for comparison amongst >500,000 British adults in the UK Biobank (UKB). ACHD diagnoses were sub-classified as “isolated aortic valve (AoV)” and “non-complex” defects. Time-to-event analyses were conducted for primary endpoints of fatal or non-fatal acute coronary syndrome (ACS), ischemic stroke, heart failure (HF), and atrial fibrillation, and a secondary combined endpoint for major adverse cardiovascular event (MACE). Maximum follow-up time for the study period was 22 years using retrospectively and prospectively collected data from the UKB.
Results:
We identified 2,006 individuals with lower-complexity ACHD and 497,983 unexposed individuals in the UKB (median [IQR] age at enrollment 58 [51,63]). Of the ACHD-exposed group, 59% were male; 51% were current or former smokers; 30% were obese; 69%, 41%, and 7% were diagnosed or treated for hypertension, hyperlipidemia, and diabetes respectively. After adjustment for 12 measured cardiovascular risk factors, ACHD remained strongly associated with the primary endpoints, with hazard ratios (HR) ranging from 2.0 (95% confidence interval [CI] 1.5–2.8, p<0.001) for ACS to 13.0 (95% CI 9.4–18.1, p<0.001) for HF. ACHD-exposed individuals with ≤2 cardiovascular risk factors had a 29% age-adjusted incidence rate of MACE in contrast to 13% in non-ACHD individuals with ≥5 risk factors.
Conclusions:
Individuals with lower-complexity ACHD had higher burden of adverse cardiovascular events relative to the general population that was unaccounted for by conventional cardiovascular risk factors. These findings highlight the need for closer surveillance of patients with mild to moderate ACHD and further investigation into management and mechanisms of cardiovascular risk unique to this growing population of high-risk adults.
Keywords: adult congenital heart disease, adverse events complication, acute coronary syndrome, atrial fibrillation, heart failure, ischemic stroke
Introduction
For children born with congenital heart disease, surviving to adulthood is now the norm. With the adult congenital heart disease (ACHD) population exceeding the population of affected children1, the burden of morbidity and mortality has shifted towards acquired diseases of adulthood. Population-based and institutional ACHD registries demonstrate a high prevalence of coronary artery disease (CAD)2–4, stroke5,6, heart failure7,8, arrhythmia9,10, and associated other cardiometabolic conditions11–13 in the ACHD population, specifically among those with more complex lesions.
While adults with lesions of high complexity (e.g., single ventricles) increasingly receive comprehensive cardiovascular care in ACHD clinics, those with simpler lesions (e.g., isolated septal or valvular defects) have measurably less consistent participation in specialized clinics and research studies14,15, and thus the risk of cardiac complications in the latter population is not well understood. Furthermore, the contribution of traditional risk factors to acquired cardiovascular disease has been difficult to characterize owing to limitations in population-scale ascertainment of risk factors in regionally fragmented systems of care for this population.
Here, we assess the relationship between lower complexity ACHD and cardiovascular disease risk using the UK Biobank, a large prospective population-based cohort of over 500,000 individuals with long-term follow-up. Specifically, we characterize the burden of cardiovascular risk factors and disease incidence in a real-world ACHD population primarily comprised of lower-complexity cardiac malformations and quantify the risk for adverse cardiovascular events associated with ACHD that is unaccounted for by traditional cardiovascular risk factors.
Methods
The data for this study is publicly available to registered investigators of the UK Biobank, and the analytic methods are made available online by the authors at https://github.com/priestlab/Circulation_2019_Saha.
Study population
The study cohort was derived from the UK Biobank (UKB), a large prospective cohort study with comprehensive health data from over 500,000 volunteer participants in the United Kingdom aged 37–73 years at recruitment in 2006–2010. The cohort has previously been described in detail16,17. Studies using the UKB are exempt from Institutional Review Board approval as the data is de-identified and publicly available.
Classification of Adults with Congenital Heart Disease
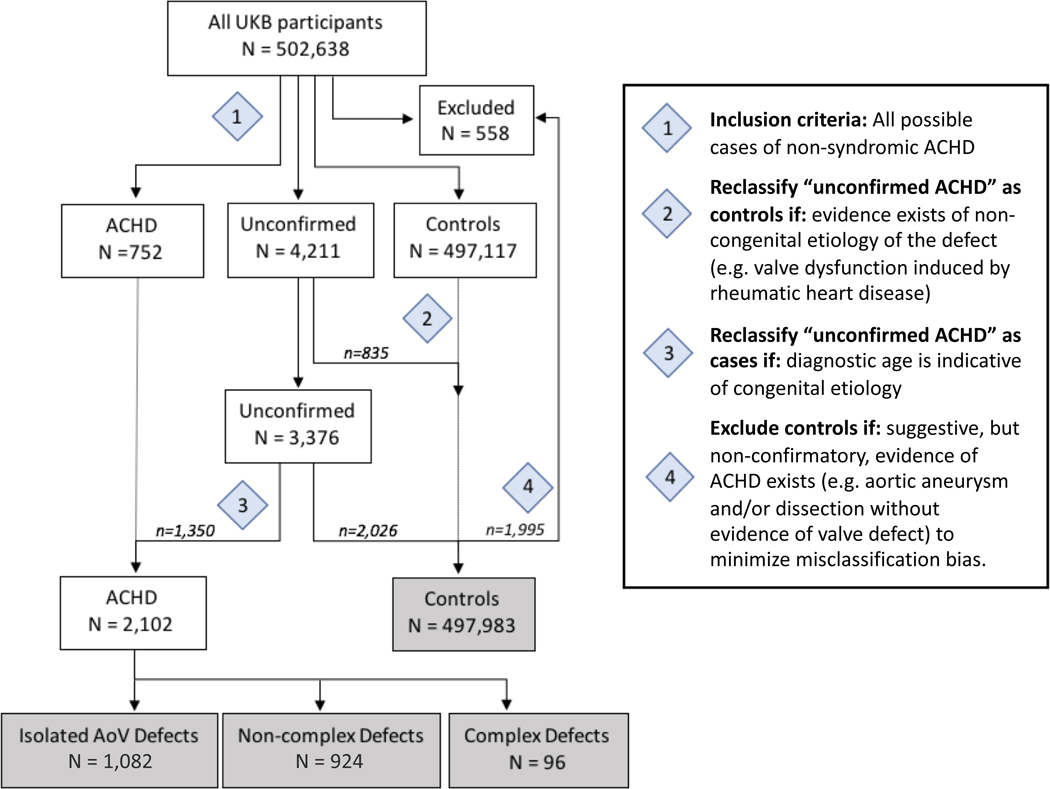
Congenital heart disease was defined as any structural cardiac abnormality, inclusive of bicuspid aortic valve, present in the heart or adjoining great vessels from birth in the absence of syndromic illness (i.e., syndromes characterized by extra-cardiac or neurocognitive manifestations in addition to cardiac malformations). A multi-step classification algorithm (Figure 1, Supplemental Tables 1–4, Supplemental Methods) was developed to ascertain 2,102 individuals with non-syndromic ACHD and 497,983 non-ACHD comparison individuals using hospital encounter statistics (HES) and self-reported medical history in the UKB.
Figure 1.
Classification Algorithm Flowchart for Ascertaining Non-syndromic Adult Congenital Heart Disease (ACHD) in UK Biobank
Diagnostic codes (ICD9, ICD10, OPCS-4, and self-reported data) for defining case-control groups are given in Supplemental Tables 1–4. Individuals identified with complex defects were excluded from further analysis in this study.
Individuals with ACHD were further organized into mutually exclusive subgroups based on lesion (Figure 1, Supplemental Table 5). The “isolated aortic valve (AoV) defects” subgroup (n = 1,082), which represents the largest uniform subtype of ACHD in the UKB, is comprised of individuals with congenital aortic insufficiency and/or stenosis (ICD9: 746.3, 746.4, ICD10: Q23.0, Q23.1) or inferred through ancillary diagnostic evidence to have an AoV defect (Supplemental Methods, Supplemental Figure 1). “Complex” defects (n = 96), adapted in part from the definition of “severe” congenital heart disease by Marelli and colleagues18, represents lesions with high likelihood of cyanosis and is comprised of single ventricles, transposition complex, truncus arteriosus, tetralogy of Fallot, atrioventricular canal defect, and total anomalous pulmonary venous return. All remaining lesions, the majority being left-to-right shunt defects, were categorized as “non-complex” defects (n = 924). Only the isolated AoV defects and non-complex defects subgroups – representing mild to moderate ACHD – were considered for analysis in this study.
Outcome phenotype definitions
Cardiovascular outcomes were defined using ICD-9, ICD-10, and OPCS-4 codes from the HES and death registries as well as self-reported non-cancer illnesses and operations; definitions were adapted in part from previously described methods (Supplemental Methods). The primary endpoints for survival analysis were first occurrence of fatal or non-fatal acute coronary syndrome (ACS), ischemic stroke, heart failure (HF), and atrial fibrillation (AF) respectively. In secondary and sensitivity analyses, a combined endpoint of major adverse cardiovascular event (MACE), defined as first occurrence of any of the primary endpoints, was used. Owing to the availability of hospital admissions data predating the enrollment period, the follow-up period for each individual in this study spanned from 10 years prior to enrollment in the UKB until occurrence of a primary endpoint, death, or censorship from UKB. This allowed for a maximum of 22 years of follow-up.
Continuous covariates used in fully adjusted multivariable Cox regression analyses were age, Townsend deprivation index (a validated measure of socioeconomic status in the United Kingdom)19, body mass index, systolic blood pressure and diastolic blood pressure. Categorical covariates used in Cox regression were sex, ethnicity (Caucasian, non-Caucasian), smoking status (current, former, never), alcohol consumption (daily, weekly, seldom), physical activity (low versus high, where “low” is defined as not satisfying WHO recommendation of at least three days per week of vigorous intensity activity or five days per week of moderate intensity exercise), family history of cardiovascular disease, history of hypertension and/or use of anti-hypertensive medications, history of hyperlipidemia diagnosis and/or use of statins, and history of diabetes diagnosis and/or use of insulin (Supplemental Methods, Supplemental Table 6). Covariates were measured at the time of enrollment and considered to be stable proxy measures for the span of the follow-up period (Supplemental Methods, Supplemental Table 7).
Statistical Analysis
Statistical analyses were performed using the ‘survival’ and ‘survminer’ statistical packages in R version 3.4.3. Sociodemographic, lifestyle, and cardiometabolic covariates were compared acros8s groups using Chi-squared tests for categorical variables and Wilcoxon rank sum test for continuous variables (assuming non-normal distributions).
Cumulative incidence curves were generated for each of the primary endpoints. A multi-state model20 was utilized to take into account the competing risk of death and potential mediation effects between the primary endpoints. Multivariable Cox proprotional hazards regression was then used to estimate hazard ratios (HR) and 95% confidence intervals (CI) after adjusting for continuous and categorical covariates mentioned previously. Adherence of survival analyses to the underlying proportional hazards assumption was assessed using the Schoenfeld residuals test.
Individuals were then stratified into three categories of cardiovascular risk based on the number present out of eight classical CV risk factors (male sex, current/former smoking status, obesity [i.e. BMI >30], low physical activity, family history of cardiovascular disease, diagnosis/treatment for hypertension, diagnosis/treatment for hypercholesterolemia, diagnosis/treatment for diabetes). Low (≤2), intermediate (=3–4), and high (≥5) number of cardiovascular risk factors were equivalent to risk tertiles. Direct standardization was employed to estimate age-adjusted incidence rates for MACE at each level of cardiovascular risk over the 22-year follow up period. Cox proportional hazards regression was used to estimate HRs for MACE associated with ACHD at each stratum of cardiovascular risk, adjusted for age, ethnicity, and Townsend deprivation index.
Sensitivity Analyses
Given the known risk of acquiring cardiac morbidity acutely in the perioperative setting, a sensitivity analysis was performed on the multi-state Cox regression models for each primary endpoint in which individuals who had undergone cardiac surgery within one year prior to the event of interest were excluded from analysis (Supplemental Table 8).
Sensitivity analyses were undertaken to assess for potential misclassification of ACHD cases resulting from inference of ACHD using non-specific diagnostic codes (Supplemental Tables 9–12). In sensitivity analysis A (Supplemental Table 9), HRs for the isolated AoV defects group were estimated with both inclusion and exclusion of diagnostic codes specific for congenital aortic insufficiency and/or stenosis to assess for the effect of inferred cases on the estimates. In sensitivity analysis B (Supplemental Table 10), the isolated AoV defects group was restricted to include inferred cases who were ascertained to have a valve defect using an earlier diagnostic age cutoff (aortic stenosis/insufficiency diagnosed at <50 years and/or aortic valve replacement at <55 years), thereby increasing the likelihood that the cause of valvular disease was due to a congenital malformation and not acquired calcific valvular disease (Supplemental Figure 1). In sensitivity analysis C (Supplemental Table 11), individuals who had undergone aortic valve replacement procedures were excluded from the cohort to assess for selection bias since aortic valve replacement was a qualifying inclusion criterion for inference of isolated AoV defects. In sensitivity analysis D (Supplemental Table 12), individuals who had an unspecified non-complex defect (i.e. heart surgery prior to age 18 and/or non-specific diagnostic code for congenital heart disease) were excluded from analysis to assess for misclassification bias in the non-complex defects subgroup.
Results
Clinical Characteristics of Study Population
We identified 2,006 participants with mild to moderate ACHD, comprising 1,082 participants with isolated AoV defects and 924 with non-complex defects, and 497,983 individuals for comparison from the UKB. Cardiac MRI data from 8 standard anatomical sequences were available for >20,000 individuals in the UKB; of 32 ACHD cases with available imaging data, 29 displayed clear confirmatory visual evidence of the assigned ACHD diagnosis yielding a specificity of 91% for our ACHD classification algorithm (Supplemental Figure 2).
The socio-demographic, lifestyle, and cardiometabolic characteristics shown in Table 1 demonstrate a higher burden of CV risk factors among the ACHD cohort, including male predominance (59% versus 46%, p<0.001), slightly higher material deprivation (median Townsend Deprivation Index −1.74 versus −2.14, p<0.001), and a higher proportion of current or former smokers (51% versus 46%, p<0.001). Several cardiometabolic risk factors were more prevalent in the exposed versus the unexposed individuals, such as obesity (30% versus 24%, p<0.001), hypertension (69% versus 55%, p<0.001), hyperlipidemia (41% versus 19%, p<0.001), and diabetes (6.7% versus 3.8%, p<0.001). Both the isolated AoV and non-complex defects subgroups followed the composite ACHD trends, with the exception of a similar proportion of men (46%) and a lower rate of current or former smoking (44%) in the non-complex defects group relative to the comparison group. The isolated AoV defects group generally displayed a higher frequency of risk factors than the non-complex defects group. There were no significant differences between the ACHD subgroups and the comparison group in age at enrollment, ethnicity, alcohol consumption, physical activity, and family history of cardiovascular disease.
Table 1.
Study Participant Characteristics at Enrollment
| CONTROL | ALL ACHD | ISOLATED AoV DEFECT | NON-COMPLEX | p value* | |
|---|---|---|---|---|---|
| n† | 497,983 | 2,006 | 1,082 | 924 | |
| Demographics | |||||
| Age at Enrollment (median [IQR]) | 58 [50, 63] | 58 [51, 63] | 59 [53, 64] | 57 [49, 63] | 0.007 |
| Male sex (%) | 226,574 (45.5) | 1,175 (58.6) | 747 (69.0) | 428 (46.3) | <0.001 |
| Caucasian Ethnicity (%) | 468,429 (94.2) | 1,920 (95.9) | 1,044 (96.8) | 876 (94.8) | 0.002 |
| Townsend Deprivation Index (median [IQR]) | −2.14 [−3.65, 0.54] | −1.74 [−3.39, 1.38] | −1.53 [−3.30, 1.49] | −1.92 [−3.44, 1.23] | <0.001 |
| Behavioral Risk Factors | |||||
| Smoking (%) | <0.001 | ||||
| Never | 271,321 (54.8) | 982 (49.4) | 468 (43.8) | 514 (55.9) | |
| Current | 52,473 (10.6) | 223 (11.2) | 133 (12.5) | 90 (9.8) | |
| Former | 171,277 (34.6) | 782 (39.4) | 467 (43.7) | 315 (34.3) | |
| Alcohol consumption (%) | 0.008 | ||||
| Seldom | 152,844 (30.8) | 666 (33.4) | 322 (29.9) | 344 (37.4) | |
| Daily | 100,874 (20.3) | 422 (21.1) | 254 (23.6) | 168 (18.2) | |
| Weekly | 242,782 (48.9) | 909 (45.5) | 500 (46.5) | 409 (44.4) | |
| Physical Activity (%) | 0.067 | ||||
| High | 245,532 (52.6) | 924 (50.4) | 509 (51.7) | 415 (48.9) | |
| Low | 221,486 (47.4) | 909 (49.6) | 476 (48.3) | 433 (51.1) | |
| Cardiometabolic Risk Factors | |||||
| Positive Family History of CVD (%) | 361,706 (74.4) | 1,482 (75.7) | 796 (75.3) | 686 (76.1) | 0.234 |
| Systolic Blood Pressure (median [IQR]) | 137 [125, 150] | 137 [125, 150] | 139 [128, 152] | 134 [121, 147] | 0.566 |
| Diastolic Blood Pressure (median [IQR]) | 82 [76, 89] | 80 [73, 88] | 81 [74, 88] | 79 [73, 87] | <0.001 |
| BMI (median [IQR]) | 26.7 [24.1, 29.9] | 27.5 [24.7, 30.9] | 27.8 [25.2, 31.4] | 26.9 [24.2, 30.2] | <0.001 |
| Obesity (%) | 120,854 (24.4) | 590 (29.8) | 349 (32.7) | 241 (26.4) | <0.001 |
| Diagnosed/Treated for Hypertension (%) | 272,727 (55.0) | 1,361 (68.8) | 824 (77.4) | 537 (58.9) | <0.001 |
| Diagnosed/Treated for Hyperlipidemia (%) | 92,536 (18.7) | 811 (41.1) | 538 (50.6) | 273 (29.9) | <0.001 |
| Diagnosed/Treated for Diabetes (%) | 18,709 (3.8) | 135 (6.7) | 95 (8.8) | 40 (4.3) | <0.001 |
ACHD = adult congenital heart disease, AoV = aortic valve, CVD = cardiovascular disease, BMI = body mass index, IQR = interquartile range
P values given for comparison between Controls and All ACHD.
Number of participants do not sum to group totals due to missing values.
Unadjusted Cumulative Incidence of Cardiovascular Morbidity
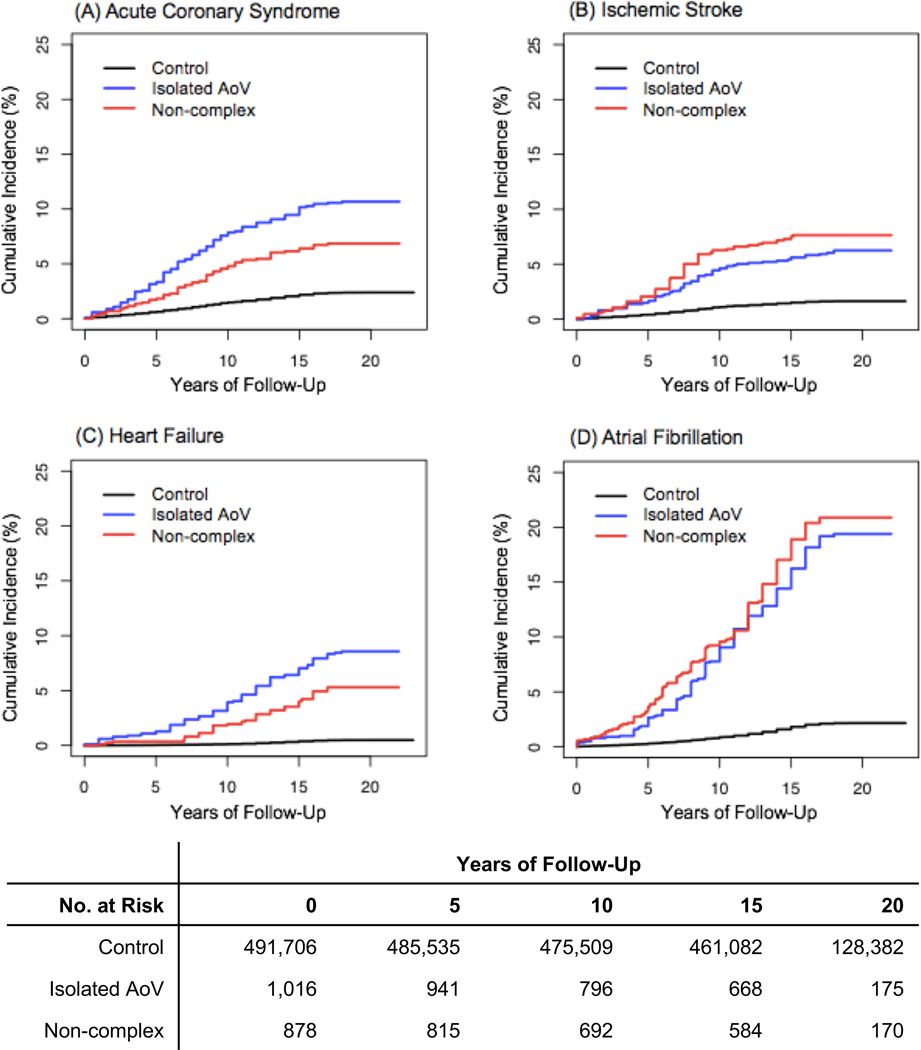
Over a median of 20 years of follow-up centered on the time of enrollment into the UKB, the unadjusted incidence of all primary endpoints occurred at a higher rate for ACHD-exposed individuals than non-ACHD comparison individuals (Figure 2). By the end of the follow-up period, 5–12% of the ACHD cohort had suffered incident ACS, ischemic stroke, and/or HF and 20% had suffered from AF, compared to less than 5% incidence of all endpoints for the non-ACHD population in this cohort. After adjusting for only sociodemographic factors (age, sex, ethnicity, and Townsend deprivation), the non-complex ACHD subgroup demonstrated a significantly higher hazard of AF (p = 0.00003) and ischemic stroke (p=0.007) than the isolated AoV defects subgroup. There was no significant difference in incidence rates of ACS and HF between the two ACHD subgroups.
Figure 2.
Cumulative Incidence of Acute Coronary Syndrome, Ischemic Stroke, Heart Failure, and Atrial Fibrillation in Adults with Congenital Heart Disease Compared to General UKB Population
Panels A-D illustrate the unadjusted cumulative incidence of acute coronary syndrome, ischemic stroke, heart failure, and atrial fibrillation in individuals with isolated AoV defects, non-complex defects, and without ACHD (controls).
AoV = aortic valve; ACHD = adult congenital heart disease; No. = Number
Multivariable-Adjusted Multi-State Cox Regression Analysis
After adjustment for common cardiovascular risk factors (i.e., age, sex, ethnicity, Townsend Deprivation index, smoking status, alcohol consumption, physical activity, family history of cardiovascular disease, BMI, hypertension, hyperlipidemia, diabetes), ACHD exposure continued to be strongly associated with the primary endpoints (Table 2). Sensitivity analyses did not suggest that incident events were temporally related to perioperative risk (Supplemental Table 8) or bias in classification (Supplemental Tables 9–12). The outcome with highest risk was HF, demonstrating no significant difference (p=0.75) between individuals with isolated AoV defects (adjusted HR [aHR] 12.2 [95% CI 9.5–15.6], p<10−16) and with non-complex defects (aHR 13.0 [95% CI 9.4–18.1], p<10−16). Ischemic stroke and AF continued to demonstrate higher hazard in the non-complex defects subgroup (ischemic stroke: aHR 5.1 [95% CI 3.9–6.6], p<10−16; AF: aHR 13.0 [95% CI 11.1–15.2], p<10−16) than in the isolated AoV defects subgroup (ischemic stroke: aHR 2.2 [95% CI 1.7–2.9], p=1.3 × 10−8; AF: aHR 7.5 [95% CI 6.4–8.7], p<10−16), p-value for comparison significant only for AF. Strikingly, the risk of ACS displayed similar aHR in both ACHD subgroups (isolated AoV: aHR 2.1 [95% CI 1.7–2.5], p=1.5 × 10−11; non-complex: aHR 2.0 [95% CI 1.5–2.8], p=8.7 × 10−6), p=0.95 for comparison.
Table 2.
Multivariable-Adjusted Hazard Ratios for Cardiovascular Events in Adults with Congenital Heart Disease
| Unadjusted* | Adjusted† | ||||
|---|---|---|---|---|---|
| No. Events/No. At Risk‡ | HR (95% CI) | P value | HR (95% CI) | P value | |
| Acute Coronary Syndrome (ACS) | |||||
| Controls (No ACHD) | 11,666/491,706 | 1 | ref | 1 | ref |
| Isolated AoV defects | 108/1,016 | 3.9 (3.2–4.7) | <10−16 | 2.1 (1.7–2.5) | 1.5 × 10−11 |
| Non-complex defects | 60/878 | 3.7 (2.9–4.8) | <10−16 | 2.0 (1.5–2.8) | 8.7 × 10−6 |
| Ischemic Stroke | |||||
| Controls (No ACHD) | 7,991/491,706 | 1 | ref | 1 | ref |
| Isolated AoV defects | 63/1,016 | 3.7 (2.9–4.8) | <10−16 | 2.2 (1.7–2.9) | 1.3 × 10−8 |
| Non-complex defects | 67/878 | 6.0 (4.7–7.6) | <10−16 | 5.1 (3.9–6.6) | <10−16 |
| Heart Failure (HF) | |||||
| Controls (No ACHD) | 2,333/491,706 | 1 | ref | 1 | ref |
| Isolated AoV defects | 86/1,016 | 17.9 (14.4–22.3) | <10−16 | 12.2 (9.5–15.6) | <10−16 |
| Non-complex defects | 46/878 | 16.1 (12.0–21.6) | <10−16 | 13.0 (9.4–18.1) | <10−16 |
| Atrial Fibrillation (AF) | |||||
| Controls (No ACHD) | 1,810/491,706 | 1 | ref | 1 | ref |
| Isolated AoV defects | 195/1,016 | 9.1 (7.9–10.5) | <10−16 | 7.5 (6.4–8.7) | <10−16 |
| Non-complex defects | 182/878 | 14.1 (12.2–16.3) | <10−16 | 13.0 (11.1–15.2) | <10−16 |
Adjusted for sociodemographic factors (age at enrollment, sex, ethnicity, and Townsend Deprivation Index).
Adjusted for sociodemographic factors as in Unadjusted models, plus cardiovascular risk factors (smoking status, alcohol consumption, physical activity, family history of cardiovascular disease, body mass index, systolic and diastolic blood pressure adjusted for use of anti-hypertensive medication, hyperlipidemia, diabetes).
”No. At Risk” reflects total number of participants with non-missing values for event within pre-determined follow-up time.
ACHD = adult congenital heart disease; AoV = aortic valve; HR = hazard ratio
Stratification by Level of Cardiovascular Risk
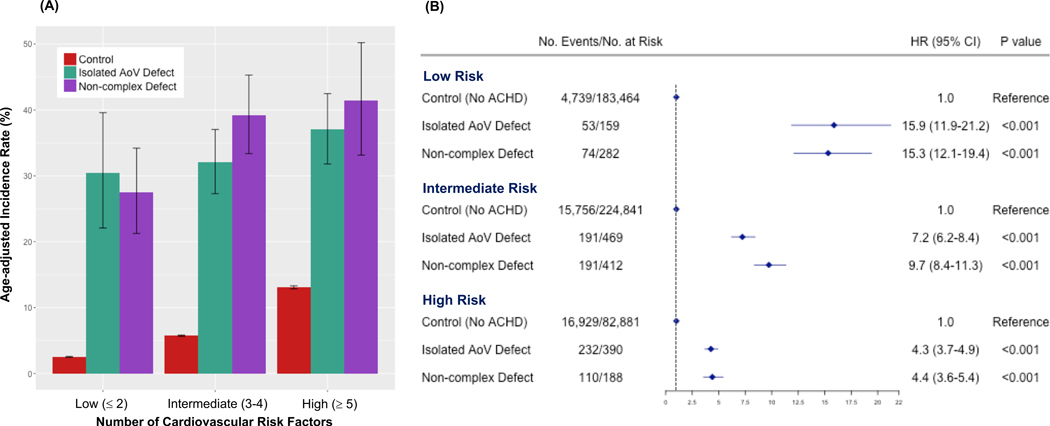
To compare the risk of MACE in individuals with and without ACHD across different levels of cardiovascular risk, we stratified all participants by the number of cardiovascular risk factors present into low (0–2), intermediate (3–4), or high (5–8) risk tertiles. When stratified by cardiovascular risk level, the ACHD population continued to show dramatically greater age-adjusted absolute incidence of MACE when compared to the comparison group in the same cardiovascular risk stratum (Figure 3A). Compared across risk strata, the absolute incidence of MACE for the ACHD subgroups in the lowest stratum of cardiovascular risk (30.5% [95% CI 22.1–39.6%] for isolated AoV and 27.5% [95% CI 21.2–34.2%] for non-complex) was more than double that of the comparison group at the highest level of cardiovascular risk (13.1% [95% CI 12.8–13.3%]). The risk of MACE relative to the non-ACHD comparison group was most pronounced among the two ACHD subgroups with ≤2 cardiovascular risk factors (aHR 15.9 [95% CI 11.9–21.2] for isolated AoV defects and aHR 15.3 [95% CI 12.1–19.4] for non-complex defects), whereas the relative risk was lower in the setting of ≥5 cardiovascular risk factors (aHR 4.3 [95% CI 3.7–4.9] for isolated AoV defects and aHR 4.4 [95% CI 3.6–5.4] for non-complex defects) (Figure 3B). Age-adjusted incidence by risk strata for individual primary endpoints are shown in Supplemental Figure 3.
Figure 3.
Absolute and Relative Risk of MACE associated with ACHD Stratified by Number of Cardiovascular Risk Factors
(A) Age-Adjusted Absolute Incidence Rate of MACE Stratified by Number of Cardiovascular Risk Factors (B) Age-, Ethnicity-, Sex- and Townsend Deprivation Index-adjusted Cox Regression for MACE stratified by Number of Cardiovascular Risk Factors
ACHD = adult congenital heart disease; MACE = major adverse cardiovascular event; AoV = aortic valve; No. = Number; HR = hazard ratio
The disparate risk estimates for MACE at different strata of cardiovascular risk suggested interaction between the cardiovascular risk factors and ACHD. Tests for interaction between the ACHD subgroups and individual CV risk factors (Supplemental Table 13) demonstrated that the presence of ACHD significantly modified the effects of sex, hypertension, and hyperlipidemia on the risk of MACE (significant at p<0.003 after Bonferroni correction). Furthermore, the estimated beta coefficients for the interaction terms between ACHD and the aforementioned risk factors were all less than zero, demonstrating that the effect of the risk factors was relatively smaller in the ACHD-exposed group than the unexposed comparison group. These results imply that traditional cardiovascular risk factors may have a less pronounced, albeit still significant, role in conferring risk for MACE in the ACHD versus non-ACHD population.
Discussion
In this population-based study of ACHD-associated risk of cardiovascular disease, we observed a substantial burden of adverse cardiovascular events in a cohort of older adults with simple to moderately complex ACHD. We discerned substantial residual risk for adverse cardiovascular events in the ACHD cohort that is disproportionate to the burden of known cardiovascular risk factors noted in this subgroup of the UKB. These findings provide further evidence for a cardiovascular disease risk profile unique to individuals with mild to moderate ACHD and highlight the need to extend preventive care practices beyond the management of conventional risk factors for this population.
The residual risk of adverse cardiovascular events after adjusting for conventional risk factors was considerably higher in individuals with ACHD compared to the general UKB population, ranging from a 2-fold increased risk of ACS to a 13-fold increased risk of HF. Our estimates are similar in magnitude to risk estimates derived from younger ACHD populations that were adjusted for only a small number of conventional cardiovascular risk factors21–24. At a median enrollment age of 58, the ACHD cohort – particularly individuals with isolated AoV defects – in the UKB had a higher frequency of current or former smokers, obesity, hypertension, hyperlipidemia, and diabetes than the rest of the UKB. Other ACHD cohorts comprising younger individuals (median age of 28–29) were observed to have higher rates of obesity, hypertension, and diabetes, indicating that such risk factors may be present from early adulthood5,22,25.
Despite an overall higher burden of cardiovascular risk factors in the ACHD cohort, we observed a significantly higher incidence of MACE in the ACHD subgroups than the general UKB population when comparing individuals at similar levels of cardiovascular risk. Among those with two or fewer cardiovascular risk factors, there was a 29% age-adjusted incidence of MACE in the ACHD cohort compared to only 3% incidence among the comparison group, suggesting that ACHD-associated risk mechanisms are key determinants of cardiovascular risk in the ACHD population irrespective of conventional risk factors. As has been hypothesized25,26,24, ACHD-associated factors may even modify the effect of conventional risk factors as suggested by the variation in relative risk at different risk levels. Notably, as the ACHD population in this study represents mild to moderate lesions – valvular and septal defects being the most common subtypes, these findings further emphasize the critical importance of surveillance of this highly prevalent ACHD subgroup which often lacks long-term clinical follow-up26,27 despite having higher risk of mortality and morbidity compared to the general population10,28,29.
While the long-term risks of HF and arrhythmia associated with ACHD are well described8,30,31, morbidity from ACS and stroke in the ACHD population is a more recently recognized phenomenon due to increasing life expectancy32,33. In this study, the elevated risk of ACS and ischemic stroke associated with ACHD despite adjustment for atherosclerotic risk factors suggests a non-atherosclerotic etiology of cardiovascular disease. The development of cardiovascular disease may be related to a number of other factors including surgical interventions34, physiological stressors of early life35, intrinsic cellular dysfunction, and genetic predisposition30. Risk for ischemic stroke and AF have previously been associated with septal defects (with or without closure) and palliative shunt procedures4,28,36, suggesting a cardioembolic rather than atherosclerotic mechanism. Indeed, our study exhibits a greater risk of ischemic stroke and AF among those with non-complex defects (comprised primarily of septal defects) compared to those with isolated AoV defects, supporting the existing literature. In individuals with repaired coarctation of the aorta, predisposition for CAD has been associated with endothelial dysregulation, increased intimal/medial thickness, and an inflammatory cytokine profile37, all of which may be intrinsic to the congenital anomaly itself or a result of surgical intervention. Mechanisms of intra-operative inflammation resulting in vascular injury mediated by cytokines such as TGF-β1 have been proposed but require further investigation38. While reports on the prevalence of CAD in adults with aortic valve malformations are variable39, bicuspid aortic valve has been associated with higher incidence of CAD than expected for age40 and with underlying vasculopathies that predispose to CAD41. Variation at the TEX41 locus discovered through genome-wide association study has recently been associated with both bicuspid aortic valve and CAD42, further underscoring the potential for a shared genetic basis of congenital heart malformations and acquired cardiovascular disease. Further investigation is necessary to delineate these potential mechanisms of cardiovascular disease unique to ACHD.
There are several strengths to this study. The data are derived from a large population-based cohort with comprehensive and uniformly collected data on all participants, irrespective of diagnosis of congenital heart disease, thereby decreasing potential detection bias. Additionally, the breadth of clinical data available in the UKB allowed us to define and adjust for a range of sociodemographic, behavioral, and cardiometabolic potential confounding variables that were uniformly ascertained in ACHD and non-ACHD individuals alike. While the availability of baseline characteristics is greater than that available in most registry-based studies which often lack survey instruments, inclusion of covariates was restricted to cross-sectional data obtained at the time of enrollment (which occurred at different ages) thus potentially complicating interpretation. However, a sensitivity analysis assessing the potential bias introduced by time-static covariates showed no significant variation in results (Supplemental Table 7).
Our study shares limitations inherent to observational studies. As previously reported, the UKB is subject to a healthy volunteer bias43 and includes individuals born during an earlier era of congenital heart disease. In the UK, the first complete repair for Tetralogy of Fallot performed in an infant occurred in the early 1960’s, and with the UKB cohort born between 1931 and 1971, the majority of UKB participants predate the widespread availability of palliative procedures for complex ACHD. Thus, survivorship bias is also present as evidenced in the small number of ACHD participants classified as “complex” who were excluded from analysis. Furthermore, diagnoses and outcomes of interest managed only in an outpatient setting will not be well captured within the UKB or similar population-based registries44. As a result, our classification algorithm is primarily based upon hospital admission diagnoses which lack the specificity, sensitivity, and phenotypic detail available in institutional or practice-based ACHD registries45. While validation of our classification algorithm was limited by the inability to verify individual-level diagnoses with chart review, the sheer volume of control individuals for comparison dilutes any effects of false negative classifications of ACHD. Furthermore, while MRI was only available for 2% of all ACHD cases, visual inspection of available MRI data yielded a diagnostic positive predictive value of at least 91% (Supplemental Figure 2, supplemental media), providing a unique visual method of validation that is not possible in registry-based studies. Despite the limitations present, the classification algorithm ascertained a prevalence of ACHD of 4.2 per 1000 participants, which is reasonably within the range of previously published prevalence estimates ranging from 2.17 to 6.12 per 1000 adults10,46,47.
The potential for misclassification bias was most pronounced in the ascertainment of isolated AoV defects. Given the lack of a highly sensitive diagnostic code for congenital aortic valve malformations and the largely asymptomatic course in early life, the identification of these lesions is a challenge in any setting without imaging data. Although the majority of isolated AoV defects was inferred with non-imaging derived diagnostic information, we were able to increase our confidence in the classification through multiple sensitivity analyses (Supplemental Table 9–12) which demonstrated minimal changes in any estimates for cardiovascular outcomes. The roughly 1:1 ratio of isolated AoV defects to all other ACHD lesions as well as the predominance of men (68.9%) are also consistent with prior studies estimating the birth prevalence and sex ratio of bicuspid aortic valves48,49. While our classification algorithm does not allow for the identification of asymptomatic aortic valve defects, the literature suggests that most individuals with bicuspid aortic valves have acquired dysfunction by late adulthood3,50.
Conclusion
In this analysis of ~500,000 British individuals from the UKB, we found a substantially higher burden of cardiovascular risk factors and disease incidence in individuals with lower complexity ACHD than in the general population. Furthermore, we estimated significant residual risk of adverse cardiovascular events associated with ACHD after adjusting for conventional cardiovascular risk factors. These findings highlight the importance for establishing long-term follow-up in patients with simple to moderately complex ACHD and the need for further study into the mechanisms of cardiovascular disease specific to this rapidly growing population of high-risk adults.
Supplementary Material
Clinical Perspective.
What is new?
This study quantifies the risk of cardiovascular morbidity directly attributable to lower complexity congenital heart disease.
We identified 2,006 individuals with lower complexity congenital heart disease in the UK Biobank, a large prospective population-based cohort of over 500,000 individuals between 37 and 73 years of age with uniformly collected health information.
Individuals with congenital heart disease had a higher burden of cardiovascular risk factors and incidence of adverse cardiovascular events as compared to the general population.
After adjustment for conventional cardiovascular risk factors, the risk of adverse cardiovascular events remained 2 to 13 times higher in individuals with congenital heart disease.
What are the clinical implications?
Adults with “mild” congenital heart disease represent a large population of individuals previously considered to be at low risk for cardiovascular morbidity without need for long-term sub-specialized cardiovascular care.
This study demonstrates that adults with lower complexity congenital heart disease are at significant risk of acquiring cardiovascular morbidities relative to the general population, highlighting the need for long-term surveillance and further research on prevention and risk mechanisms of acquired cardiovascular disease in this population.
Acknowledgements:
This study is supported by data from the UK Biobank, applications 15860 (J.R.P.) and 13721 (E.I.). The interpretation and conclusions from the data contained herein do not represent those of the UK Biobank or Circulation.
Funding Sources
Funded by the National Institutes of Health (NIH) (K99HL130523 to J.R.P.) and the Sarnoff Cardiovascular Research Foundation (P.S.).
Footnotes
Disclosures:
None.
References
- 1.Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital Heart Defects in the United StatesClinical Perspective: Estimating the Magnitude of the Affected Population in 2010. Circulation. 2016;134:101–109. doi: 10.1161/CIRCULATIONAHA.115.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannakoulas G, Dimopoulos K, Engel R, Goktekin O, Kucukdurmaz Z, Vatankulu MA, Bedard E, Diller GP, Papaphylactou M, Francis DP, Di Mario C, Gatzoulis MA. Burden of Coronary Artery Disease in Adults With Congenital Heart Disease and Its Relation to Congenital and Traditional Heart Risk Factors. Am J Cardiol. 2009;103:1445–1450. doi: 10.1016/j.amjcard.2009.01.353. [DOI] [PubMed] [Google Scholar]
- 3.Yalonetsky S, Horlick EM, Osten MD, Benson LN, Oechslin EN, Silversides CK. Clinical characteristics of coronary artery disease in adults with congenital heart defects. Int J Cardiol. 2013;164:217–220. doi: 10.1016/j.ijcard.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Bokma JP, Zegstroo I, Kuijpers JM, Konings TC, van Kimmenade RRJ, van Melle JP, Kiès P, Mulder BJM, Bouma BJ. Factors associated with coronary artery disease and stroke in adults with congenital heart disease. Heart. 2017;104:574–580. doi: 10.1136/heartjnl-2017-311620. [DOI] [PubMed] [Google Scholar]
- 5.Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson P-O, Dellborg M. Ischemic Stroke in Children and Young Adults With Congenital Heart Disease. J Am Heart Assoc. 2016;5:e003071. doi: 10.1161/JAHA.115.003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in Adults With Congenital Heart Disease. Circulation. 2015;132:2385–2394. doi: 10.1161/CIRCULATIONAHA.115.011241. [DOI] [PubMed] [Google Scholar]
- 7.Pillutla P, Shetty KD, Foster E. Mortality associated with adult congenital heart disease: Trends in the US population from 1979 to 2005. Am Heart J. 2009;158:874–879. doi: 10.1016/j.ahj.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt AB, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S, Davidson WR, Earing MG, Ghoshhajra BB, Karamlou T, Mital S, Ting J, Tseng ZH. Congenital Heart Disease in the Older Adult: A Scientific Statement From the American Heart Association. Circulation. 2015;131:1884–1931. doi: 10.1161/CIR.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 9.Labombarda F, Hamilton R, Shohoudi A, Aboulhosn J, Broberg CS, Chaix MA, Cohen S, Cook S, Dore A, Fernandes SM, Fournier A, Kay J, Macle L, Mondésert B, Mongeon F-P, Opotowsky AR, Proietti A, Rivard L, Ting J, Thibault B, Zaidi A, Khairy P, AARCC. Increasing Prevalence of Atrial Fibrillation and Permanent Atrial Arrhythmias in Congenital Heart Disease. J Am Coll Cardiol. 2017;70:857–865. doi: 10.1016/j.jacc.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Wu M-H, Lu C-W, Chen H-C, Kao F-Y, Huang S-K. Adult Congenital Heart Disease in a Nationwide Population 2000–2014: Epidemiological Trends, Arrhythmia, and Standardized Mortality Ratio. J Am Heart Assoc. 2018;7:e007907. doi: 10.1161/JAHA.117.007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen NL, Marino BS, Woo JG, Thomsen RW, Videbœk J, Laursen HB, Olsen M. Congenital Heart Disease With and Without Cyanotic Potential and the Long‐term Risk of Diabetes Mellitus: A Population‐Based Follow‐up Study. J Am Heart Assoc. 2016;5:e003076. doi: 10.1161/JAHA.115.003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deen JF, Krieger EV, Slee AE, Arslan A, Arterburn D, Stout KK, Portman MA. Metabolic Syndrome in Adults With Congenital Heart Disease. J Am Heart Assoc. 2016;5: e001132. doi: 10.1161/JAHA.114.001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerman JB, Parness IA, Shenoy RU. Body Weights in Adults With Congenital Heart Disease and the Obesity Frequency. Am J Cardiol. 2017;119:638–642. doi: 10.1016/j.amjcard.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M, Alliance for Adult Research in Congenital Cardiology (AARCC) and Adult Congenital Heart Association. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61:2180–2184. doi: 10.1016/j.jacc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valente AM, Landzberg MJ, Gianola A, Harmon AJ, Cook S, Ting JG, Stout K, Kuehl K, Khairy P, Kay JD, Earing M, Houser L, Broberg C, Milliren C, Opotowsky AR, Webb G, Verstappen A, Gurvitz M, Alliance for Adult Research in Congenital Cardiology (AARCC) Investigators, Adult Congenital Heart Association (ACHA). Improving heart disease knowledge and research participation in adults with congenital heart disease (the Health, Education and Access Research Trial: HEART-ACHD). Int J Cardiol. 2013;168:3236–3240. doi: 10.1016/j.ijcard.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Medicine. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins R What makes UK Biobank special? Lancet. 2012;379:1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 18.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital Heart Disease in the General Population. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 19.Townsend P Poverty in the United Kingdom. London, UK: Allen Lane and Penguin Books; 1979. [Google Scholar]
- 20.Therneau T, Crowson C, Atkinson E. Multi-state models and competing risks. The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf. Published November 26, 2018. Accessed November 26, 2018.
- 21.Lin Y-S, Liu P-H, Wu L-S, Chen Y-M, Chang C-J, Chu P-H. Major adverse cardiovascular events in adult congenital heart disease: a population-based follow-up study from Taiwan. BMC Cardiovasc Disord. 2014;14:38. doi: 10.1186/1471-2261-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billett J, Cowie MR, Gatzoulis MA, Muhll IFV, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case–control analysis. Heart. 2008;94:1194–1199. doi: 10.1136/hrt.2007.122671. [DOI] [PubMed] [Google Scholar]
- 23.Fedchenko M, Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Skoglund K, Dellborg M. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143–148. doi: 10.1016/j.ijcard.2017.06.120. [DOI] [PubMed] [Google Scholar]
- 24.Olsen M, Marino B, Kaltman J, Laursen H, Jakobsen L, Mahle W, Pearson G, Madsen N. Myocardial Infarction in Adults With Congenital Heart Disease. Am J Cardiol. 2017;120:2272–2277. doi: 10.1016/j.amjcard.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 25.Moons P, Van Deyk K, Dedroog D, Troost E, Budts W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612–616. doi: 10.1097/01.hjr.0000197472.81694.2b. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg JF. Long-term Follow-up of “Simple” Lesions—Atrial Septal Defect, Ventricular Septal Defect, and Coarctation of the Aorta. Congenit Heart Dis. 2015;10:466–474. doi: 10.1111/chd.12298. [DOI] [PubMed] [Google Scholar]
- 27.Jørgen Videbæk, Bækgaard Laursen Henning, Morten Olsen, Dan Eik Høfsten, Paaske Johnsen Søren. Long-Term Nationwide Follow-Up Study of Simple Congenital Heart Disease Diagnosed in Otherwise Healthy Children. Circulation. 2016;133:474–483. doi: 10.1161/CIRCULATIONAHA.115.017226. [DOI] [PubMed] [Google Scholar]
- 28.Nyboe C, Olsen MS, Nielsen-Kudsk JE, Hjortdal VE. Atrial fibrillation and stroke in adult patients with atrial septal defect and the long-term effect of closure. Heart. 2015;101:706–711. doi: 10.1136/heartjnl-2014-306552. [DOI] [PubMed] [Google Scholar]
- 29.Nyboe C, Karunanithi Z, Nielsen-Kudsk JE, Hjortdal VE. Long-term mortality in patients with atrial septal defect: a nationwide cohort-study. Eur Heart J. 2018;39:993–998. doi: 10.1093/eurheartj/ehx687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahed AC, Roberts AE, Mital S, Lakdawala NK. Heart Failure in Congenital Heart Disease: A Confluence of Acquired and Congenital. Heart Fail Clin. 2014;10:219–227. doi: 10.1016/j.hfc.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez FH, Moodie DS, Parekh DR, Franklin WJ, Morales DLS, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of Heart Failure–Related Hospitalization in Adults with Congenital Heart Disease in the United States. Congenit Heart Dis. 2013;8:513–519. doi: 10.1111/chd.12019. [DOI] [PubMed] [Google Scholar]
- 32.Lui GK, Rogers IS, Ding VY, Hedlin HK, MacMillen K, Maron DJ, Sillman C, Romfh A, Dade TC, Haeffele C, Grady SR, McElhinney DB, Murphy DJ, Fernandes SM. Risk Estimates for Atherosclerotic Cardiovascular Disease in Adults With Congenital Heart Disease. Am J Cardiol. 2017;119:112–118. doi: 10.1016/j.amjcard.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannakoulas G, Ntiloudi D. Acquired cardiovascular disease in adult patients with congenital heart disease. Heart. 2018;104:546–547. doi: 10.1136/heartjnl-2017-311997. [DOI] [PubMed] [Google Scholar]
- 34.Lui GK, Fernandes S, McElhinney DB. Management of Cardiovascular Risk Factors in Adults With Congenital Heart Disease. J Am Heart Assoc. 2014;3:e001076. doi: 10.1161/JAHA.114.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanetti D, Tikkanen E, Gustafsson S, Priest JR, Burgess S, Ingelsson E. Birthweight, Type 2 Diabetes Mellitus, and Cardiovascular Disease: Addressing the Barker Hypothesis With Mendelian Randomization. Circ Genom Precis Med. 2018;11:e002054. doi: 10.1161/CIRCGEN.117.002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karunanithi Z, Nyboe C, Hjortdal VE. Long-Term Risk of Atrial Fibrillation and Stroke in Patients With Atrial Septal Defect Diagnosed in Childhood. Am J Cardiol. 2017;119:461–465. doi: 10.1016/j.amjcard.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Brili S, Tousoulis D, Antoniades C, Aggeli C, Roubelakis A, Papathanasiu S, Stefanadis C. Evidence of vascular dysfunction in young patients with successfully repaired coarctation of aorta. Atherosclerosis. 2005;182:97–103. doi: 10.1016/j.atherosclerosis.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Justino H, Khairy P. Congenital Heart Disease and Coronary Atherosclerosis: A Looming Concern? Can J Cardiol. 2013;29:757–758. doi: 10.1016/j.cjca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Poggio P, Cavallotti L, Songia P, Minno AD, Ambrosino P, Mammana L, Parolari A, Alamanni F, Tremoli E, Minno MNDD. Impact of Valve Morphology on the Prevalence of Coronary Artery Disease: A Systematic Review and Meta‐Analysis. J Am Heart Assoc. 2016;5:e003200. doi: 10.1161/JAHA.116.003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boudoulas KD, Wolfe B, Ravi Y, Lilly S, Nagaraja HN, Sai-Sudhakar CB. The aortic stenosis complex: aortic valve, atherosclerosis, aortopathy. J Cardiol. 2015;65:377–382. doi: 10.1016/j.jjcc.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Roche SL, Silversides CK. Hypertension, Obesity, and Coronary Artery Disease in the Survivors of Congenital Heart Disease. Can J Cardiol. 2013;29:841–848. doi: 10.1016/j.cjca.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Helgadottir A, Thorleifsson G, Gretarsdottir S, Stefansson OA, Tragante V, Thorolfsdottir RB, Jonsdottir I, Bjornsson T, Steinthorsdottir V, Verweij N, Nielsen JB, Zhou W, Folkersen L, Martinsson A, Heydarpour M, Prakash S, Oskarsson G, Gudbjartsson T, Geirsson A, Olafsson I, Sigurdsson EL, Almgren P, Melander O, Franco-Cereceda A, Hamsten A, Fritsche L, Lin M, Yang B, Hornsby W, Guo D, Brummett CM, Abecasis G, Mathis M, Milewicz D, Body SC, Eriksson P, Willer CJ, Hveem K, Newton-Cheh C, Smith JG, Danielsen R, Thorgeirsson G, Thorsteinsdottir U, Gudbjartsson DF, Holm H, Stefansson K. Genome-wide analysis yields new loci associating with aortic valve stenosis. Nat Commun. 2018;9:987. doi: 10.1038/s41467-018-03252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson P-O, Skoglund K, Fedchenko M, Dellborg M. Atrial Fibrillation Burden in Young Patients With Congenital Heart Disease. Circulation. 2018;137:928–937. doi: 10.1161/CIRCULATIONAHA.117.029590. [DOI] [PubMed] [Google Scholar]
- 45.Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS. Limited Accuracy of Administrative Data for the Identification and Classification of Adult Congenital Heart Disease. J Am Heart Assoc. 2018;7:e007378. doi: 10.1161/JAHA.117.007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric Congenital Heart Disease: Burden of Disease and Predictors of Mortality. J Am Coll Cardiol. 2011;58:1509–1515. doi: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 47.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime Prevalence of Congenital Heart Disease in the General Population From 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 49.Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J. 2005;150:513–515. doi: 10.1016/j.ahj.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 50.Ward C Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.