The endosomal sorting complex required for transport (ESCRT) plays a crucial role in the transportation and degradation of proteins. We determined that Vps27, a key protein of the ESCRT-0 complex, is required for the transport of the virulence factor laccase to the cell wall in Cryptococcus neoformans. Laccase activity was perturbed, as was melanin production, in vps27Δ strains. In the absence of VPS27, there was an accumulation of multivesicular bodies with vacuolar fragmentation and mistargeting of the vacuolar carboxypeptidase CPY/Prc1, resulting in an extracellular localization.
KEYWORDS: Cryptococcus neoformans, ESCRT, VPS27, attenuates virulence, laccase trafficking, multivesicular bodies (MVB)
ABSTRACT
The endosomal sorting complex required for transport (ESCRT) plays a crucial role in the transportation and degradation of proteins. We determined that Vps27, a key protein of the ESCRT-0 complex, is required for the transport of the virulence factor laccase to the cell wall in Cryptococcus neoformans. Laccase activity was perturbed, as was melanin production, in vps27Δ strains. In the absence of VPS27, there was an accumulation of multivesicular bodies with vacuolar fragmentation and mistargeting of the vacuolar carboxypeptidase CPY/Prc1, resulting in an extracellular localization. In addition, deletion of VPS27 resulted in a defect in laccase targeting of a Lac1-green fluorescent protein (GFP) fusion to the cell wall with trapping within intracellular puncta; this deletion was accompanied by reduced virulence in a mouse model. However, the actin cytoskeleton remained intact, suggesting that the trafficking defect is not due to defects in actin-related localization. Extracellular vesicle maturation was also defective in the vps27Δ mutant, which had a larger vesicle size as measured by dynamic light scattering. Our data identify cryptococcal VPS27 as a required gene for laccase trafficking and attenuates virulence of C. neoformans in a mouse intravenous (i.v.) meningitis model.
INTRODUCTION
Cryptococcus neoformans is an opportunistic pathogen that infects immunocompromised individuals such as those who suffer from HIV/AIDS (1). C. neoformans typically causes primary pulmonary cryptococcosis but can disseminate to the brain, initiating lethal meningoencephalitis. Cryptococcosis causes approximately 200,000 deaths annually in HIV/AIDS patients worldwide, which approaches the number of HIV/AIDS-related tuberculosis deaths (2). It is also a major cause of brain infections in solid organ transplant (SOT) recipients and infects a number of previously healthy individuals (3–5). In the United States, C. neoformans is currently the most common agent responsible for nonviral meningitis, due to reductions in bacterial meningeal infections from vaccinations (4, 6).
Upon gaining entry or reactivation in the host, C. neoformans establishes infection in the lung, where the infection is usually successfully treated, or it can disseminate to the central nervous system (CNS) (7). The virulence of C. neoformans depends on many factors, which include (i) the ability to survive at 37°C (8), (ii) a large polysaccharide capsule (9), and (iii) expression of virulence factors, which include phospholipase, urease, and laccase (10).
Laccase is an important virulence factor of C. neoformans and has roles in melanin formation, prevention of phagocyte Fenton reagents by iron oxidation, production of reactive neurocatecholamines, and immunomodulation of fungal prostaglandins (11–14). These functions serve to protect the fungus from the environment and reduce host cell immune response, and they have been shown to be essential for the neurotropism of the pathogen. Proper trafficking of laccase is important in neurotropism, since successful secretion of the enzyme to the cell wall during brain infection allows the enzyme access to neurological catecholamines and iron. Interestingly, a laccase-green fluorescent protein (GFP) fusion exhibited less cell wall targeting when the organism was recovered from the lung versus the brain of mice (15). Therefore, changes in the tissue environment affecting cellular targeting of proteins may serve to optimize fungal virulence within the brain, facilitating its neurotropism. Unfortunately, little is known about the cellular trafficking of virulence factors in C. neoformans, including that of laccase.
Several studies suggest that trafficking of laccase may proceed through the multivesicular trafficking pathway that is best known for its role in endocytosis of receptors after ligand binding. Previous studies suggest that the phosphatidylinositol 3-kinase (PI 3-kinase) Vps34 is involved in virulence, as well as in laccase expression (16), which was demonstrated again recently (17). The gene has also been implicated in the persistence of bacterial infections such as tuberculosis and salmonellosis by altering lipids of the host macrophage vacuoles, as well as infections by the fungus Candida albicans (18). Vps34 initiates the endosomal pathway by inositol lipid phosphorylation to yield membrane phosphatidylinositol 3-phosphate [PI(3)P] in order to recruit/position proteins for sorting to the vacuole via Vps27, which is a component of the ESCRT 0-III vacuolar sorting complexes involved in multivesicular body formation (Fig. 1A) (19). Vps27 interacts with Hse1 and Vps34 to make up ESCRT-0, which plays a crucial role in initiating the ESCRT pathway (20), as well as with the vacuolar-sorting protein Snf7, which is involved in polysaccharide secretion, capsular formation, and melanin formation in both C. neoformans and Cryptococcus gattii (21). Several phenotypes of ESCRT proteins in C. neoformans have been shown to exhibit typical endocytosis phenotypes involved in uptake of iron, as well as in pH tolerance (22, 23). Other global regulator pathways, such as the protein kinase A (PKA) pathway, have also been demonstrated to have roles in laccase localization, although the mechanisms remain less clear (24). In addition, while the majority of laccase in wild-type (WT) fungal cells is deposited on the cell wall (15), a minor fraction is found within extracellular vesicles (EVs) as well (25). Recently, the formation of EVs was found in other eukaryotes to be dependent on the ESCRT-MVB secretion pathway (26–29). Thus, in the present study, we assessed mutants of the ESCRT-0 initiation members VPS27, HSE1, and VPS34 for laccase activity, performed focused studies of laccase cellular localization and extracellular vesicle formation in mutants of VPS27, and VPS34, and assessed the role of VPS27 in murine virulence using an intravenous (i.v.) model which accentuates brain-related mortality (30) to corroborate the finding of a role in virulence of VPS34 (16). These findings demonstrate the role of the ESCRT-0 pathway in secretion of laccase in C. neoformans and support its role in the virulence of the fungus.
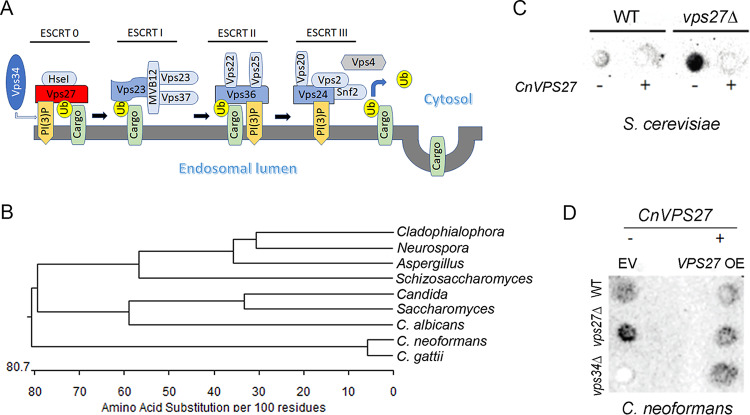
FIG 1.
Identification of a vacuolar protein sorting (vps) gene, VPS27, from Cryptococcus neoformans. (A) Scheme of the ESCRT subcomplexes 0 to III that mediate intraluminal sorting in the multivesicular body (MVB) pathway (adapted from Aufschnaiter and Büttner [19]). Ub, monoubiquitinates. (B) ClustalW comparison of protein sequences of closest matches of the Vps27 sequence: Aspergillus nidulans AN2071, C. albicans C1_00750C_A, Candida glabrata CAGL0105830g, Cladophialophora carrionii LCR 03922, Neurospora crassa NCU04015, S. cerevisiae YNR006W, Schizosaccharomyces pombe SPAC19A8.05c, C. neoformans CNAG 01267, and C. gattii I314_05320. (C) VPS27 overexpression restores CPY/Prc1 trafficking of S. cerevisiae or (D) C. neoformans. WT, vps27Δ, and vps34Δ strains were transformed with yellow fluorescent protein (YFP)-Vps27 (CnVPS27) containing a hygromycin marker under an actin promoter or empty vector (EV) control. Cells were grown to the mid-log phase, and 3.0 optical density at 600 nm (OD600) units of cells were spotted on 2% glucose, 2% bactopeptone, and 1% yeast extract (YPD) plates. Plates were overlaid with nitrocellulose sheets and incubated for 2 days. Nitrocellulose sheets were removed, washed, and subjected to dot blotting with anti-CPY/Prc1 antibody to assess carboxypeptidase Y mistrafficking to the cell wall.
RESULTS
Identification and mutation of a cryptococcal homolog of the ESCRT component Vps27.
ClustalW analysis (EMBL-EBI database; http://www.ebi.ac.uk/Tools/clustalw/index.html) comparing closely related fungi showed strong similarity of the C. neoformans Vps27 putative protein sequence to that of closely related VPS27 members (Fig. 1B). In addition, a BLAST analysis of the cryptococcal genomic database (https://fungidb.org) yielded a single annotated putative protein sequence of 744 amino acids (CNAG_02167) having close identity (31.3%) to the Saccharomyces cerevisiae Vps27 protein sequence (YNR006W). The cryptococcal VPS27 gene successfully complemented an S. cerevisiae vps27Δ mutant that mistargeted a vacuolar carboxypeptidase (CPY/Prc1) to the extracellular space by restoration of its intracellular localization after heterologous expression of C. neoformans VPS27 (31) (Fig. 1C), suggesting that C. neoformans VPS27 is a functional homolog of S. cerevisiae VPS27. In addition, the C. neoformans vps27Δ strain was a phenocopy of the S. cerevisiae vps27Δ strain in that CPY/Prc1 was aberrantly trafficked to the extracellular space; this aberrant trafficking was partially reduced after complementation with WT C. neoformans VPS27 (Fig. 1D). Interestingly, the C. neoformans vps34Δ strain exhibited no detectable CPY/Prc1 activity and demonstrated some activity after VPS27 complementation. C. neoformans VPS34 expresses a highly conserved class III PI 3-kinase (16) that is responsible in eukaryotes for phosphorylation of VPS27 and initiation of ESCRT-0-dependent trafficking (19). The more severe and opposite phenotype of the C. neoformans vps34 mutant may be due to its role in multiple functions, which include both CPY sorting and other processes such as autophagy (32). Taken together, these data demonstrate that VPS27 is epistatic to VPS34 and has a role in the ESCRT-0 pathway in C. neoformans.
Loss of ESCRT-0 components is associated with growth defects at alkaline pH and under high salt conditions.
In addition to ESCRT-0 complex mutant vps34Δ and vps27Δ strains generated in our laboratory, we assessed the role in growth of HSEI and ESCRT-II VPS25 as a comparator using relevant mutants from the Cryptococcus neoformans Genome Open Reading Frame (ORF) Knockout Collection version 1.0 (33). We found that all of the strains grew equally well on 2% glucose, 2% bactopeptone, and 1% yeast extract (YPD) agars at pH 6 and pH 7 (Fig. 2A, left and middle panels). However, the vps27Δ, vps34Δ, hseIΔ, and vps25Δ mutants demonstrated reduced growth compared with that of the WT strain on YPD at pH 8, indicating that these ESCRT components were required for robust growth at alkaline pH (Fig. 2A, right panel). Moreover, the vps27Δ, vps34Δ, hseIΔ, and vps25Δ mutants showed reduced growth on YPD agar supplemented with either 1.5 M NaCl, 1.5 M CaCl2, or 1.5 M KCl, and the vps34Δ mutant was defective in YPD after LiCl supplementation, but all grew well after calcium supplementation (Fig. 2B). In addition, these mutants grew well on YPD agar with 1.5 M sorbitol, indicating that salt rather than osmotic stress caused growth defects. We next examined the contributions of the representative ESCRT components to growth on low-iron yeast nitrogen base (YNB) medium with 150 μM bathophenanthroline disulfonate (BPS), as described previously (23). The wild-type (WT) strain grew on low-iron YNB with all iron sources. The vps27Δ, vps34Δ, hseIΔ, and vps25Δ mutants grew like the WT strain on low-iron YNB medium supplemented with 100 μM FeCl3 or 2 μM hemoglobin (Fig. 2C). Taken together, these results indicate that the vps27Δ, vps34Δ, hseIΔ, and vps25Δ mutants share phenotypes with the C. neoformans ESCRT-0, -I, -II, and -III mutants, as described previously (23).
FIG 2.
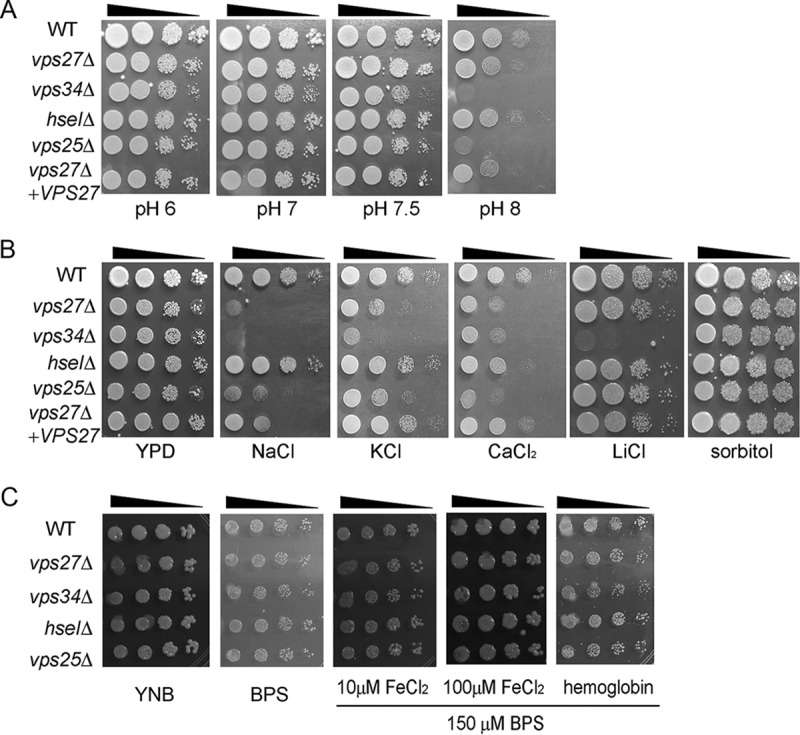
Susceptibility of ESCRT mutants to alkaline pH and salt stress. (A) Cells (OD600 = 1) from 10-fold serial dilutions of the indicated strains were spotted on buffered YPD plates at the indicated pH, and plates were incubated at 30°C for 2 to 7 days. (B) Tenfold serial dilutions of cells (OD600 = 1) of the indicated strains were spotted onto solid YPD without or with 1.5 M NaCl, 1.5 M KCl, 250 mM CaCl2, 200 mM LiCl, or 1.5 M sorbitol, and plates were incubated at 30°C for 4 to 9 days. (C) The growth of ESCRT mutants and the WT strain was tested on yeast nitrogen base (YNB) and YNB plus bathophenanthroline disulfonate (BPS) supplemented with either hemoglobin or FeCl3 (10 μM or 100 μM) at pH 7.0.
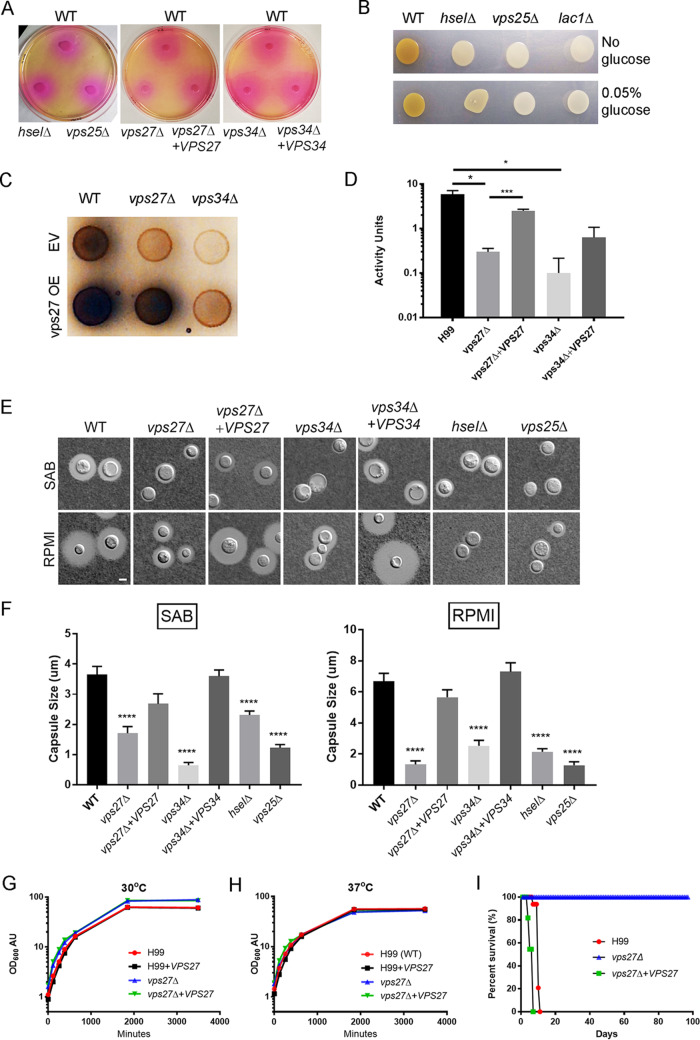
Mutant strains of the ESCRT-0 gene demonstrate reduced levels of laccase and polysaccharide capsule, and vps27Δ mutant strains demonstrated a further reduction in urease activity, as well as attenuated virulence in a mouse meningoencephalitis model.
Loss of ESCRT proteins reduces attachment of capsule polysaccharide to the cell wall and melanin formation (22, 23), and we therefore tested whether mutants in other ESCRT components shared these phenotypes. Urease activity demonstrated by a zone of phenol red-detectable pH change in the presence of urea was reduced slightly in the vps27Δ strains; otherwise, no changes were evident in the vps34Δ, hseIΔ, and vps25Δ mutant strains (Fig. 3A). Assessment of virulence-related phenotypes of C. neoformans found a significant reductions in laccase activity in vps27Δ, vps34Δ, hseIΔ, and vps25Δ strains compared to that in the WT strain, evidenced by reduced melanin pigment formation after incubation of the fungus in the presence of norepinephrine (Fig. 3B) and using a quantitative laccase assay (34). The WT strain produced 5.9 ± 2.2 U/liter of extracellular laccase while, WT + VPS27, vps27Δ, vps34Δ, and vps34Δ + VPS27 strain cells displayed 7.1 ± 2.5 (P < 0.05), 0.3 ± 0.1 (P < 0.05), 0.1 ± 0.2 (P < 0.05), and 1.9 ± 0.6 (P < 0.05) U/liter of extracellular laccase, respectively (Fig. 3C and D). Enzyme activity of the vps34Δ strain was partially recovered to wild type by VPS27 overexpression similar to that of the mistargeting of CPY/Prc1. As expected, the WT cells produced a large capsule on 10% Sabouraud (SAB) or RPMI media, while deletion of VPS27, VPS34, HSEI, and VPS25 caused a marked reduction in capsule size (Fig. 3E and F). This suggests differences in secretory pathways between laccase, urease, and capsule, with small effects on urease specific to VPS27.
FIG 3.
Virulence-related phenotypes of cryptococcal vps27Δ strains and VPS27 are required for mammalian virulence. (A) Indicated strains were assayed for urease by incubation on Christenson’s media according to Materials and Methods. (B) Indicated strains were assayed for laccase by melanin formation according to Materials and Methods. (C) Indicated strains were assayed for laccase by melanin formation according to Materials and Methods. WT; wild type, EV; empty vector, OE; overexpressor. (D) For quantitative analysis of laccase activity, a colorimetric assay was used as described previously (34), using the laccase substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sufonic acid) according to Materials and Methods. Error bars indicate standard error of the mean (SEM), N = 3 independent experiments. *P < 0.05, ***P < 0.005. (E) Indicated strains were incubated on a 1:10 dilution of Sabouraud (SAB) or RPMI agar for 2 days at 30°C and examined by India ink microscopy. Bar, 5 μm. (F) Results (n > 20 cells) ± SEM, Student’s t test, ****P < 0.001. Wild-type H99, vps27Δ, VPS27 overexpressor, and VPS27 complemented strains were grown to the mid-log phase, resuspended to an OD600 of 0.1, and grown in a shaking incubator at (G) 30°C or (H) 37°C. (I) ND40 mice were intravenously infected with 106 wild-type H99, vps27Δ, or vps27Δ + VPS27 complemented strain cells. P < 0.0001.
VPS27 did not affect growth of C. neoformans. The growth of vps27Δ was not affected at 30°C (Fig. 3G) or at 37°C (Fig. 3H). Since growth rates of the vps27Δ, overexpressor, and complementary strains were equivalent both at 30°C and 37°C to that of the WT strain, we assessed for virulence using a mouse meningoencephalitis model. For these experiments, 1 × 106 cells of wild-type H99, vps27Δ, or complementary strains were injected into the lateral tail vein of ND40 mice. All mice that died showed evidence of meningoencephalitis by observable head swelling and positive fungal cultures (>105 CFU/brain), as described previously (30). As shown in Fig. 3I, the vps27Δ strains exhibited a significant decrease in virulence relative to those of the wild-type control and complementary strain. Recovered brains from mice infected with the vps27Δ strains were sterile. This suggests that ESCRT-0 function is required for virulence in a mouse meningoencephalitis model, in addition to its function described in pulmonary disease (20).
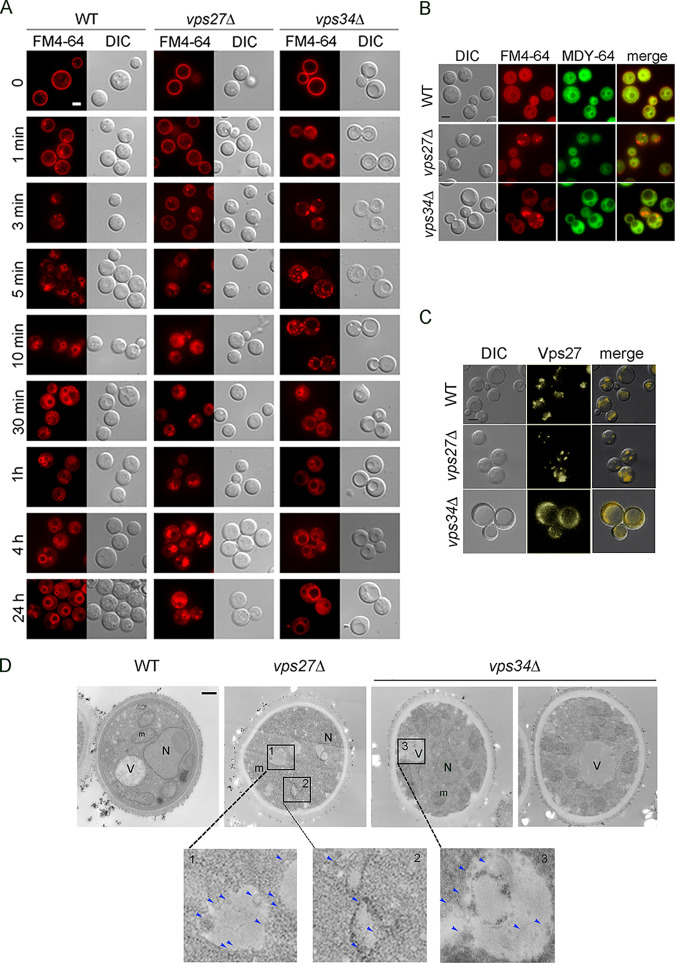
Deletion of VPS27 gene results in reduced endocytic vesicles and increased intracellular multivesicular bodies.
To examine the effects of vps27Δ and vps34Δ on the kinetics of endocytotic vesicle trafficking, we followed the internalization of the amphiphilic dye FM4-64, which, in fungal cells, is taken up by endocytosis and transported to the vacuolar membrane over defined time periods that can be followed by lipophilic dyes (35, 36). A time course of FM4-64 lipophilic dye uptake in WT, vps27Δ, and vps34Δ cells demonstrated that within 1 min, bright fluorescence was associated with the cell membrane (Fig. 4A). FM4-64 transferred effectively to the vacuole membrane in WT cells within 5 min, preceded by the formation of small intracellular vesicles at 1 to 3 min. In contrast the vps27Δ mutant strain had irregular formation of intracellular vesicles over 3 to 30 min, and clear vacuolar localization was only consistently evident after multiple hours. Similarly, the vps34Δ mutant displayed delayed vacuolar delivery compared to that of the WT, but some cells showed vacuolar localization at 5 to 30 min, shorter than the time for the vps27Δ mutant strain. In addition, the vacuoles of the vps27Δ strain were fragmented and remained irregular, as shown at the 4-h and 24-h time points, whereas beginning at 10 to 30 min, the vps34Δ strain displayed large vacuoles that remained stable. Statistical analysis of FM4-64 staining from 10 cells of each strain at 3 min demonstrated that the vps27Δ and vps34Δ strains contained an average of 1.0 ± 1.0 and 1.0 ± 1.0 cytoplasmic puncta, respectively (mean of 20 representative cells), whereas the strain carrying the WT had an average of 5 ± 2 vesicles (P < 0.05). Delayed FM4-64 delivery to the vacuole was confirmed at the early time points using the MDY-64 stain-labeling vacuoles (37), which colocalized with FM4-64 in WT cells but not in the vps27Δ cells. Diffuse staining of MDY-64 in the vps34Δ strains made this less useful as a colocalizing stain in this mutant, but the clear demonstration of a vacuolar location on differential interference contrast (DIC) supported a mistargeted location of the FM4-64 (Fig. 4B). Formation of Vps27-populated vesicles also appeared dependent on the Vps34 PI 3-kinase, as fluorescently labeled Vps27-yellow fluorescent protein (YFP) fusion formed a more diffuse pattern in the vps34Δ strain rather than distinct puncta, as in the WT strain (Fig. 4C). Taken together, these data revealed that VPS27 is involved in effective endocytosis and formation of typical yeast vacuoles.
FIG 4.
Deletion of the VPS27 gene results in the production of multivesicular bodies (MVB). (A) Time course of FM4-64 uptake in living cells; indicated strains were visualized with a fluorescein isothiocyanate (FITC) filter and merged with differential interference contrast (DIC) Nomarski images at the indicated time points for cell contour and visualization of vacuolar compartments. Bar, 5 μm. (B) Indicated cells were incubated in ASN without glucose with indicated dyes and observed by fluorescence or DIC microscopy. Bar, 5 μm. (C) Indicated strains were visualized with an FITC filter and merged with DIC Nomarski images for cell contour and visualization of the Vps27-YFP. (D) Electron microscopy of wild-type H99, vps27Δ, or vps34Δ strains of C. neoformans. Arrows point to multivesicular bodies (MVBs). Bar, 600 nm.
As the ESCRT pathway has been associated with endocytosis via the MVB pathway, we next performed a more focused study on representative vps27Δ and vps34Δ ESCRT-0 mutants for cytoplasmic trapping of MVBs by transmission electron microscopy (TEM). As shown in Fig. 4D, while the WT strain displayed normal cellular architecture, the vps27Δ and vps34Δ strains produced a number of cytoplasmic vesicles with smaller intravesicular vesicles typical of MVBs (38). Quantification of MVBs/cell in 10 independent cell sections demonstrated increased numbers of MVBs in the ESCRT-0 mutants (WT, vps27Δ, and vps34Δ = 1.5 ± 0.5, 5.6 ± 1.6, and 3 ± 0.9 MVBs/cell, respectively; P < 0.05).
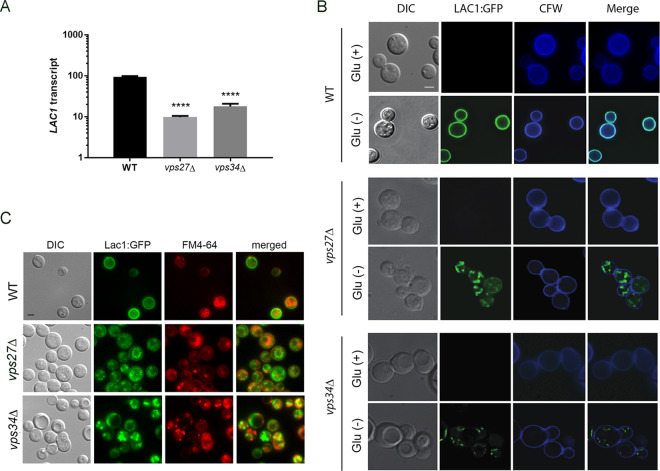
Trafficking of laccase is defective in vps27Δ and vps34Δ strains.
Because laccase is predominantly a cell wall-associated virulence factor, we hypothesized that reduced laccase activity in the vps27Δ-expressing strains may be due in part to aberrant trafficking of the laccase protein. Reverse transcription-quantitative PCR (qRT-PCR) analysis of LAC1 RNA obtained from cells induced for 3 h under starvation conditions showed reduced laccase transcription in both the vps27Δ and vps34Δ strains compared to that in the WT (Fig. 5A), suggesting the alteration may include a transcriptional defect. To determine the effect of VPS27 and VPS34 on laccase trafficking, vps27Δ and vps34Δ strains were transformed with a plasmid expressing an N-terminal GFP-laccase fusion under the native laccase promoter (GFP-Lac1). As shown in Fig. 5B, GFP-laccase fluorescence in the WT strain was repressed under glucose conditions, as reported previously, and was derepressed under starvation conditions; protein also localized to the cell wall, as evidenced by colocalization with calcofluor dye after 3 h of induction (11). However, the vps27Δ GFP-Lac1 and vps34Δ GFP-Lac1 strains exhibited defective trafficking with detection of cytoplasmic puncta over the same time period. Use of the lipophilic vesicle stain FM4-64 demonstrated that the GFP-laccase fluorescence was not found within FM4-64-stained vesicles (Fig. 5C). These data suggest that the ESCRT-0 pathway is critical to transcription, as well as to cellular targeting of laccase to the cell wall of C. neoformans.
FIG 5.
Localization of Vps27 and laccase. (A) Expression levels of LAC1/18S rRNA after 3 h of incubation under starvation conditions. Error bars indicate SEM, n = 3 independent experiments. ****, P < 0.001 (Student’s t test). Error bars represent standard deviation. (B) C. neoformans cells expressing Lac1-GFP were incubated in YPD (Glu+) and ASN without glucose (Glu−) and observed by fluorescence (Lac1, calcofluor white) or differential interference contrast (DIC) microscopy. Bar, 5 μm. (C) Indicated cells expressing Lac1-GFP were incubated in ASN without glucose (Glu−) with FM4-64 and observed by fluorescence or differential interference contrast (DIC) microscopy. Bar, 5 μm.
Laccase trafficking is actin dependent.
To assess the effect of actin on laccase trafficking, WT cells were again assessed for cellular localization of laccase-GFP fusion protein in the presence of latrunculin A (LatA), which inhibits actin polymerization and is required for endocytosis (39). Cells were grown to the mid-log phase in glucose-rich medium, washed, and incubated in the presence of 200 μg/ml LatA for 1, 2, and 6 h or in equivalent amounts of dimethyl sulfoxide (DMSO) solvent alone (1% final concentration) in glucose-depleted minimal medium (asparagine salts) for the indicated times and visualized under DIC Nomarski and fluorescein isothiocyanate (FITC) filters. As shown in Fig. 6A, Lac1-GFP was clearly targeted to the periphery of cells in the absence of LatA, but it was localized to intracellular puncta in the presence of LatA. To assess for a possible overlap between ESCRT-0 function and actin formation, actin patches were assessed in the representative mutants. Actin patches were stained with FITC-phalloidin overlaid with DIC which demonstrated normal formation of actin patches in wild-type, vps27Δ and vps34Δ, overexpressor, and complemented cells (Fig. 6B). Thus, it appears that laccase trafficking to the periphery is dependent on both actin polymerization and the ESCRT-0 pathway but is not dependent on actin localization.
FIG 6.
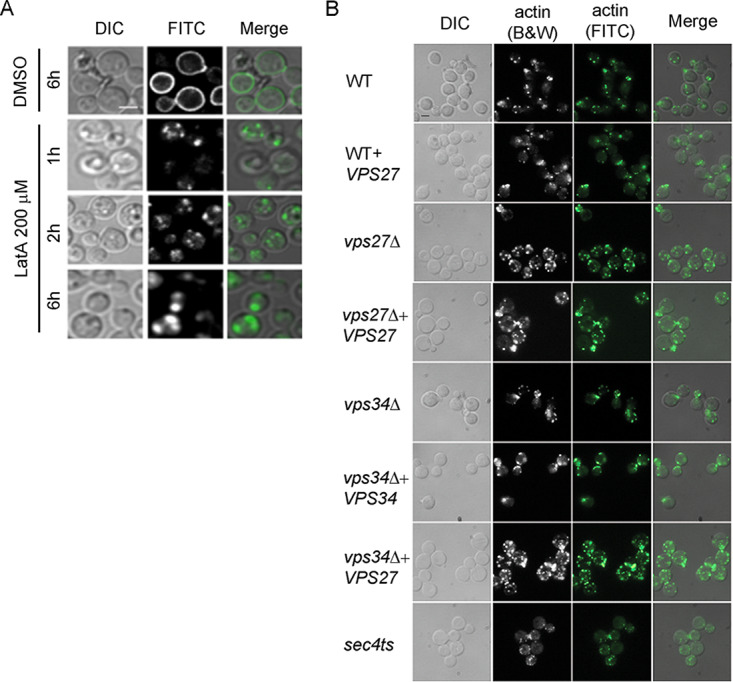
Laccase trafficking is actin polymerization dependent. (A) Wild-type H99 cells were transformed with overexpression Lac1-GFP constructs under an actin promoter containing the URA5 marker. Cells were grown to the mid-log phase in glucose-rich medium, washed, and transferred to glucose-depleted minimal medium (pH = 7.5) containing 200 mM LatA. Cells were incubated in a shaking incubator for indicated times and visualized under DIC Nomarski and FITC filters. Merged figures show cell contour in relation to the GFP-laccase localization intracellularly or on the cell wall. Bar, 5 μm. (B) Actin patches were stained with FITC-phalloidin overlaid with DIC to show normal formation of actin patches in indicated cells. Bar, 5 μm.
Quantification and sizing of extracellular vesicles.
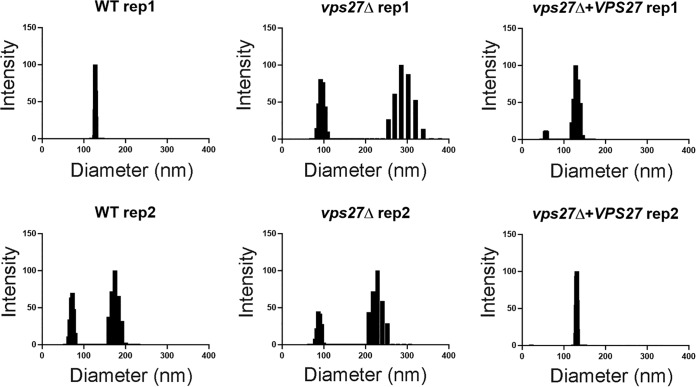
Formation of intraluminal MVB is required not only for endocytosis but also for the formation of EVs (40). Thus, we quantified the distributed size and overall EV-associated lipids which demonstrated the presence of EVs in the vps27Δ mutant but with EVs of an increased size greater than 200 nm in the vps27Δ cells with smaller EVs in both the WT and the VPS27-complemented cells (Fig. 7). Secreted EV-associated lipids were also assayed and found to be present in the vps27Δ strain and did not differ between the mutant and either the WT or VPS27-complemented strain (WT = 2.3 ± 0.04; vps27Δ = 8.1 ± 4.3; vps27Δ + VPS27 = 5.8 ± 3.9; P = 0.27, mean ± standard deviation [SD]) (data not shown).
FIG 7.
Size distribution of extracellular vesicles of indicated strains measured by dynamic light scattering according to Materials and Methods. Data correspond to two independent experiments.
DISCUSSION
The present studies extend our analysis of laccase secretion and cell wall targeting in Cryptococcus species and identified a role for the ESCRT pathway in cell wall targeting of this important virulence factor. Since production of melanin pigments and immune-modulating molecules requires exogenous substrates, cell wall localization of laccase is thought to provide more efficient substrate-enzyme interactions (15, 41). Production of immunomodulating oxylipids at the cell wall by laccase may also be more effective at the fungal-host interface, where laccase-specific prostaglandin E2 inhibits induction of protective Th17 differentiation, worsening mortality (42). Recent work supports a role for ESCRT in the formation of EVs in eukaryotes, best understood in mammalian systems (43). In this process, rather than formation of vesicles from the plasma membrane, EVs have their lipid biogenesis from MVBs that are also utilized for endocytosis vesicle delivery of plasma membrane cargo to the vacuole for degradation. These studies clarify understanding of our previous work demonstrating that iSEC6 knockdown strains of C. neoformans exhibited both defective laccase trafficking and trapped intracellular MVBs, suggesting a role for the MVB pathway in secretion of laccase (25). These data also suggest a linkage of ESCRT to the SEC6 secretory pathway in that the ESCRT-0 mutants vps27Δ and vps34Δ are phenocopies of the iSEC6 mutants in regard to both laccase mistargeting and the trapping of MVB structures within the cytoplasm. In addition, ESCRT-0-related deficits in laccase secretion and capsule production shared with those of endocytosis related to iron and heme uptake, as previously described (23), link these important virulence traits through the ESCRT pathway. ESCRT mutants of C. neoformans had previously been shown to exhibit avirulence in a intranasal lung model; this was also demonstrated in the present studies using a meningoencephalitis model that, in addition to lung inoculation, results in direct brain infection by intravenous inoculation, resulting in simultaneous brain and lung inoculation followed by exponential growth in multiple tissues and death by meningoencephalitis (44). We also identified a role for actin polymerization in laccase secretion, which has been implicated previously in endosomal recycling and transport (45) and which has a fungicidal effect in C. neoformans (46), although actin polymerization itself was not altered by ESCRT, as we observed by intact actin patches in the vps27Δ mutant. The conserved presence of ESCRT pathways in other fungi such as Candida albicans, the plant pathogen Fusarium graminearum, and model fungus Aspergillus nidulans (47–49) also suggests that ESCRT could be an important pathway in multiple fungal pathogens, suggesting that broad therapeutic interventions may be achieved by targeting the ESCRT pathway.
Trafficking studies demonstrated that C. neoformans ESCRT-0 plays an evolutionarily conserved role in vacuolar targeting of cargos exemplified by the vacuolar carboxypeptidase CPY/Prc1 and in the formation of intact vacuolar structures described previously in ascomycete yeast (50, 51). The ESCRT pathway was first established in S. cerevisiae yeast model systems and mammalian systems to be involved in recruiting proteins from the endosomal compartment to the vacuole for degradation or in recycling proteins to internal compartments such as the Golgi (52). The strong sequence and functional identity with the S. cerevisiae homolog may be important, as several structural units, including the Vps23-interacting motif, are divergent between yeast and mammalian proteins, suggesting possibilities for antifungal drug targeting (53). It is interesting that laccase trafficking defects have been demonstrated in host lung macrophages, suggesting a host defense role in limiting the ESCRT pathway that could affect multiple virulence pathways (15). The regulatory pathway derived from protein kinase A (PKA) has been proposed to act in concert with ESCRT to activate the Rim101 regulatory pathway in response to the environment, resulting in cell wall and capsular maturation (54). Thus, changes in laccase trafficking in host macrophages could be a result of such host environmental inputs.
The present studies also provide insight into laccase-associated EV transport. EVs are conserved secreted vesicles present in diverse eukaryotes, including in bacteria, fungi, and mammalian cells (55); these vesicles range in size from 20 to 500 nm in diameter (56). Diverse pathogen EVs carry a large number of cellular proteins, RNA, and virulence factors and can contribute to antibiotic resistance, and biofilm formation (27). Viral pathogens have also been shown to usurp components of the ESCRT pathway—HIV Gag1p binds to downstream components of the VPS27 ESCRT complexes such as ALIX1, whose normal function is required for exosome budding (57). Isolation of EVs from C. neoformans in previous studies identified multiple proteins within the lipid structures, including laccase, and their presence was associated with extracellular as well as cell wall laccase localization (58). EV biosynthesis has been most extensively studied in mammalian systems, in which they are thought to originate from MVBs, budding off from the Golgi rather than from the plasma membrane and fusing with the plasma membrane to release their cargo (59). The present study used EM to identify retained MVB structures in ESCRT mutants, as well as aberrantly enlarged EVs suggestive of defective maturation, which are consistent with data showing cellular transport of laccase through these structures (25, 58). The present study further associates expression of ESCRT with SEC6-dependent transport in C. neoformans, as mutation of both pathways resulted in (i) defective laccase secretion, (ii) trapping of intracellular laccase in vesicles, and (iii) visualization by EM of retained MVBs. Another interesting finding was that mutation of ESCRT-0 members also showed reductions in laccase transcription. This could be related to ESCRT-related trafficking or to turnover of laccase coactivators. For example, another EV-associated factor, Ssa1, was previously found to transcriptionally coactivate the C. neoformans LAC1 gene in association with the heat shock transcription factor HSF1 (34), and cellular levels have been reported to be dependent on ESCRT members (39). In addition, since iron uptake is an important inducer of laccase activity but a repressor of capsule production (60), and both processes are facilitated by ESCRT member expression, these two linked physiological systems may serve to provide a mechanism of cross-regulation by ESCRT member regulation. Another interesting observation was that urease activity was less affected than polysaccharide/protein secretion after deletion of VPS27. Urease is not predicted to have a required signal peptide for conventional protein secretion (http://www.cbs.dtu.dk/services/SignalP/), which could help to distinguish conventional from nonconventional secretory pathways via the ESCRT pathway.
In summary, the present study implicates the ESCRT-0 pathway in trafficking of the laccase cryptococcal virulence factor and serve to link important virulence traits, such as iron uptake, and virulence factors such as laccase.
MATERIALS AND METHODS
Ethics statement.
All experimental procedures were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the Intramural Research Program of the NIAID, NIH (protocol no. LCIM12E). All experimental studies were approved by the relevant NIAID Animal Care and Use Committee per the Guide for the Care and Use of Laboratory Animals from the National Research Council of the National Academies (Washington, DC).
Fungal and mouse strains, plasmids, and media.
The wild-type C. neoformans ATCC 208821 (H99) strain was a gift from J. Perfect (Duke University, Durham, NC) and was the host strain for the vps27Δ mutation and the fluoroorotic acid (FOA)-resistant mutants generated for plasmid transformations. The following plasmids were transformed into the H99 strains. The S. cerevisiae BY4741 (ATCC 201388) wild-type strain and the vps27Δ (ATCC 4005381) mutation are from the yeast deletion collection (61). Cells were grown on enhanced yeast peptone dextrose (YPD) complete medium (catalog no. Y2075; USBiological, Salem, MA) or asparagine salts synthetic medium (ASN) without uracil (2% glucose, 1 g/liter asparagine, 10 mM sodium phosphate [pH 6.5], and 0.25 g/liter MgSO4) for FOA mutants. For starvation experiments, cells were grown on asparagine salts synthetic medium without the addition of glucose. Norepinephrine (NE) plates were made in asparagine salts synthetic medium without glucose and with the addition of 10 mM norepinephrine bitartrate salt. Norepinephrine laccase activity medium was made as previously described by Williamson (62). Biolistic recovery medium consisted of YPD supplemented with 1.0 M sorbitol. Deletion strains were grown on YPD supplemented with 50 μg/ml nourseothricin (NAT), and complementation strains were plated on YPD with 200 μg/ml hygromycin B (catalog no. 10687-010; Life Technologies, Carlsbad, CA). The vps25Δ and hse1Δ strains came from the Cryptococcus neoformans Genome ORF Knockout Collection Version 1.0 (CnKOv1,0). The Madhani laboratory at the University of California–San Francisco generated deletions in approximately 1,200 open reading frames (ORFs) (i.e., about 20% of the genome ORFs) of strain H99 (serotype A, C. neoformans var. grubii) of this organism.
Generation of VPS27 deletion and complemented strains.
Disruption of VPS27 in C. neoformans (CNAG_02167) was constructed by homologous recombination. The ORF of VPS27 was disrupted with the Nat gene (plasmid was a gift from J. K. Kwon-Chung, Bethesda, MD). All primers listed are oriented 5′ to 3′ for conventional purposes. The uppercase nucleotides are homologous to VPS27 genomic DNA. The lowercase nucleotides are homologous to the NAT sequence on the plasmid. The following primers were used for VPS27 disruption mutant generations in C. neoformans: (i) VPS27 1000 up forward primer, CCT CGG TGG ATT CGA TTT TGG CG; (ii) reverse A1′, gct agt ttc tac atc tct tcc gtg GAT GGA TGA TTA CTG GAG GAC GGg c; (iii) NAT forward primer, CAC GGA AGA GAT GTA GAA ACT AGC; (iv) NAT reverse primer, GAG GAT GTG AGC TGG AGA GCG GCG; (v) NAT C forward, cgc cgc tct cca gct cac atc ctc CTC AAA TAC GTG GGA AAT CTG GCC AG; and (vi) VPS27 1000 down reverse primer, GCA TCT GAA GAG TGG GAA AAG C. PCR was used with PFU Ultra polymerase (catalog no. 600394; Agilent, Wilmington, DE). The first PCR consisted of paired primer sets 1 and 2, 3 and 4, and 5 and 6 on C. neoformans genomic DNA, NAT plasmid, and C. neoformans genomic DNA, respectively. The PCR products were then mixed at a 1:1:1 ratio and subjected to overlap PCR. The resulting overlap PCR product was used to transform H99 strains using a standard biolistics protocol using gold beads as described by Toffaletti et al. (63). Single colonies were verified by diagnostic PCR with the VPS27 1,500-nucleotide up primer TAC TCG TCC TCA AAT GTG GCA TCG and the NAT reverse primer (4) from the primers used for disruption of VPS27. To complement the vps27Δ mutant strain, a 2.7-kb genomic fragment encompassing the full open reading frame (ORF) of VPS27 plus 1 kb of the 5′ promoter region and 3′ UTR was PCR amplified using a primer set of vps27-Mlu-1000s (ATA CGA CGC GTT CAC CTT GAC AAT GAA ACC C) and vps27-Mlu-1000a (ATT CGA CGC GTC ACA AGA TAT GTC TCC AGA G). The PCR product was digested with MluI and ligated into a modified pORA vector containing the hygromycin resistance gene under the control of a cryptococcal actin promoter. This construct was digested with MluI and introduced into vps27Δ mutant cells by biolistics. Transformants were selected on hygromycin-containing asparagine salts synthetic medium (ASN) agar plates. Heterologous genomic insertion of the complement was verified by PCR (see Fig. S1 in the supplemental material).
Plasmid construction.
Plasmids were generated by restriction enzyme cloning into pBluescript II KS (Stratagene). URA5 or NAT or HYG reporter genes were cloned into the SacI site. VPS27 genomic DNA 1,500 bp up from the start codon and 1,500 bp down from the stop codon were PCR amplified and cloned into the BamHI site. For constitutive expression plasmids, the actin promotor was cloned unidirectionally by NotI and XbaI sites into the pBluescript II KS containing the URA5 reporter gene. YFP was cloned unidirectionally downstream and in frame with the actin promoter using the XbaI and SpeI sites. VPS27 plus 1,100 bp down from the stop codon was cloned into the SpeI site. Sequences were verified by sequencing. For complementation of the VPS27 genomic DNA, 1,500 nucleotides upstream and downstream were cloned into pBluescript II KS at the BamHI site, and hygromycin B was cloned into the SacI site.
Growth curves.
Cells were grown to the mid-log phase and resuspended to an optical density at 600 nm (OD600) of 0.1 and grown in a shaking 30°C or 37°C incubator. Cell aliquots were measured at indicated times over a 2-day period to generate growth curves.
Immunofluorescence and florescence microscopy.
Cells were grown to the mid-log phase in glucose-rich medium, washed, and transferred to glucose-depleted minimal medium (pH = 7.5) containing 200 mM LatA. Cells were incubated in a shaking incubator for the indicated times and visualized under DIC Nomarski and FITC filter microscopy.
Laccase activity assay.
To assess laccase activity by the production of melanin, age-matched strains were patched onto ASN agar without glucose and with 50 μg/ml norepinephrine. Plates were incubated at 30°C for 24 h and photographed. For quantitative analysis of laccase activity, a colorimetric assay was performed as described previously (34), using the laccase substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sufonic acid) (ABTS; Sigma, St. Louis, MO). The assay was performed in triplicate. One U is defined as 0.001 A415 in 1 h. Melanin production was assayed on plates by spotting 2.5 OD600 units of cells onto NE plates as described above. Cells were incubated at 30°C for 24 to 36 h to see production of the dark pigment deposited on the plates.
Capsule size measurements.
Capsule production was induced with SAB medium using the protocol previously described by van Duin et al. (64), and cells were grown for 24 or 36 h before resuspending the cells in India ink and visualizing under a DIC filter on a microscope.
Virulence studies.
Virulence studies were conducted according to a previously described intravenous mouse meningoencephalitis model (65) using 10 ND40 mice. Mouse health was monitored and moribund mice sacrificed in accordance with the NIH protocol guidelines. Mice were intravenously infected with 106 wild-type H99, vps27Δ, and VPS27 complement cells. Mice were followed and collected over 100 days for death.
Western blot analysis with anti-CPY/Prc1.
Cells were grown to the mid-log phase and 3.0 OD600 units of cells were spotted on YPD plates. Plates were overlaid with nitrocellulose sheets and incubated for 2 days. Nitrocellulose sheets were removed, washed, and subjected to Western blotting as follows: overnight block with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) and 0.1% Triton X-100. Blots were then washed with TBST and 0.1% Triton X-100 three times for 10 min each. Blots were subjected to 1 h of incubation with monoclonal anti-CPY/Prc1 antibody (Life Technologies) (1:5,000 dilution in TBST and 0.1%Triton X-100) washed three times in TBST and 0.1% Triton X-100 as described above and incubated with anti-mouse-horseradish peroxidase (HRP) antibody (Agilent) (1:10,000 in TBST and 0.1% Triton X-100) for 1 h, washed three times with TBST and 0.1% Triton X-100 for 10 min each, and subjected to HyGlo chemical development reagent to detect HRP by chemiluminescence. Blots were then visualized on a ChemiDoc XRS+ system (Bio-Rad).
Electron microscopy.
Transmission electron microscopy was used to visualize vesicles isolated from supernatants. Pellets obtained after washing and centrifugation at 10,000 × g were fixed in 2% glutaraldehyde in 0.1 M cacodylate at room temperature for 2 h and were then incubated overnight in 4% formaldehyde, 1% glutaraldehyde, and 0.1% phosphate-buffered saline. The samples were incubated for 90 min in 2% osmium, serially dehydrated in ethanol, and embedded in Spurr’s epoxy resin. Thin sections were obtained on a Reichert Ultracut microtome and stained with 0.5% uranyl acetate and 0.5% lead citrate. Samples were observed in a JEOL 1200 EX transmission electron microscope operating at 80 kV.
(i) Extracellular vesicle isolation. Fungal cells were precultured overnight in YPD (1% yeast extract, 2% peptone, and 2% dextrose) (Difco) liquid medium (with or without antibiotics) at 30°C under moderate shaking, starting from a single colony. Yeast cells were washed twice with 1× Dulbecco’s phosphate-buffered saline (DPBS) without calcium and magnesium (Corning, USA), counted, and inoculated at a final concentration of 5 × 104 cells/ml in 500 ml of minimal medium (MM; 15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, and 3 μM thiamine-HCl [pH 5.5]). After incubation for 5 days under the same conditions described above, the extracellular vesicles were isolated using the protocol previously described (66). Briefly, yeast cells were pelleted at 5,000 rpm (SLA rotor; Sorvall) for 30 min at 4°C. The supernatant was filtered through a 0.22-μm-pore filter (Millipore Sigma, USA) and pelleted at 100,000 × g for 1 h at 4°C with a slow brake with a very slow deacceleration speed (no brake) to avoid disruption of pellet (SW28 rotor; Beckman Coulter). The pellet was suspended in 1× DPBS and washed twice with the same buffer. The pellet (EVs) was suspended in approximately 500 μl of 1× DPBS and stored at −20°C until further analysis.
(ii) Sterol quantification. Fungal EVs are composed of sterols and defined by a lipid bilayered membrane. For this reason, the sterol content was used as an indirect indicator of vesicular secretion. Vesicle samples were evaluated using the quantitative fluorometric Amplex Red cholesterol assay kit (catalog no. A12216; Invitrogen) following the manufacturer’s instructions.
(iii) Dynamic light scattering. EVs suspended in a liquid phase exhibit Brownian motion. Therefore, illumination of samples (10 μl of vesicles in 100 μl of DPBS) with a laser monochromatic light translates into light-scattering fluctuations that were measured at a 90° angle in a 90Plus/BI-MAS multiangle particle sizing analyzer (Brookhaven Instruments). The dynamic light scattering (DLS) technique provides information on the size and heterogeneity of the sample. The average hydrodynamic diameter was obtained from 10 consecutive measurements of 1 min per run.
Statistics.
Error bars on graphs are standard errors of the means (SEMs), and statistical significance was established by Student’s t test. Statistical significance of differences in the mouse survival curves was assessed by Kruskal-Wallis analysis (analysis of variance [ANOVA] on ranks) using Prism. The graphed plots, curve fits, Pearson or Spearman correlations (r), and other statistical analyses were performed with Prism version 5.0a (GraphPad Software, San Diego, CA). All experiments were repeated at least three times for statistical analysis.
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the intramural research program of the NIAID, NIH.
All authors contributed to (i) the conception or design of the study, (ii) the acquisition, analysis, or interpretation of the data, and (iii) writing of the manuscript.
We declare that we have no conflicts of interest with the contents of this article.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute of Allergy and Infectious Diseases; National Heart, Lung, and Blood Institute, National Institutes of Health; or the United States Department of Health and Human Services.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Chayakulkeeree M, Perfect J. 2006. Cryptococcosis. Infect Dis Clin North Am 20:507–544. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratton EW, El Husseini N, Chastain CA, Lee MS, Poole C, Sturmer T, Juliano JJ, Weber DJ, Perfect JR. 2012. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One 7:e43582. doi: 10.1371/journal.pone.0043582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. 2013. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas PG. 2013. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 6.Castelblanco RL, Lee M, Hasbun R. 2014. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet Infect Dis 14:813–819. doi: 10.1016/S1473-3099(14)70805-9. [DOI] [PubMed] [Google Scholar]
- 7.Koyuncu OO, Hogue IB, Enquist LW. 2013. Virus infections in the nervous system. Cell Host Microbe 13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon-Chung KJ, Polacheck I, Popkin TJ. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol 150:1414–1421. doi: 10.1128/JB.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YC, Kwon-Chung KJ. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho C, Bocca AL, Casadevall A. 2014. The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol 87:1–41. doi: 10.1016/B978-0-12-800261-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. 2001. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69:5589–5596. doi: 10.1128/iai.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Tewari RP, Williamson PR. 1999. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun 67:6034–6039. doi: 10.1128/IAI.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon JR, Baldrian P, Murugesan K, Chang YS. 2012. Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microb Biotechnol 5:318–332. doi: 10.1111/j.1751-7915.2011.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu J, Olszewski MA, Williamson PR. 2013. Cryptococcus neoformans growth and protection from innate immunity are dependent on expression of a virulence-associated DEAD-box protein, Vad1. Infect Immun 81:777–788. doi: 10.1128/IAI.00821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waterman S, Hacham M, Panepinto J, Hu G, Shin S, Williamson P. 2007. Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infect Immun 75:714–722. doi: 10.1128/IAI.01351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G, Hacham M, Waterman SR, Panepinto J, Shin S, Liu X, Gibbons J, Valyi-Nagy T, Obara K, Jaffe HA, Ohsumi Y, Williamson PR. 2008. PI3K signaling of autophagy is required for starvation tolerance and virulenceof Cryptococcus neoformans. J Clin Invest 118:1186–1197. doi: 10.1172/JCI32053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D, Jang EH, Lee M, Kim SW, Lee Y, Lee KT, Bahn YS. 2019. Unraveling melanin biosynthesis and signaling networks in Cryptococcus neoformans. mBio 10:e02267-19. doi: 10.1128/mBio.02267-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckmann A, Kunkel W, Hartl A, Wetzker R, Eck R. 2000. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology 146:2755–2764. doi: 10.1099/00221287-146-11-2755. [DOI] [PubMed] [Google Scholar]
- 19.Aufschnaiter A, Buttner S. 2019. The vacuolar shapes of ageing: from function to morphology. Biochim Biophys Acta Mol Cell Res 1866:957–970. doi: 10.1016/j.bbamcr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol 4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- 21.Godinho RMdC, Crestani J, Kmetzsch L, Araujo GdS, Frases S, Staats CC, Schrank A, Vainstein MH, Rodrigues ML. 2014. The vacuolar-sorting protein Snf7 is required for export of virulence determinants in members of the Cryptococcus neoformans complex. Sci Rep 4:6198. doi: 10.1038/srep06198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu G, Caza M, Cadieux B, Chan V, Liu V, Kronstad J. 2013. Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence. Infect Immun 81:292–302. doi: 10.1128/IAI.01037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu G, Caza M, Cadieux B, Bakkeren E, Do E, Jung WH, Kronstad JW. 2015. The endosomal sorting complex required for transport machinery influences haem uptake and capsule elaboration in Cryptococcus neoformans. Mol Microbiol 96:973–992. doi: 10.1111/mmi.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J, Vogl AW, Kronstad JW. 2012. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol Microbiol 85:700–715. doi: 10.1111/j.1365-2958.2012.08134.x. [DOI] [PubMed] [Google Scholar]
- 25.Panepinto J, Komperda K, Frases S, Park Y, Djordjevic J, Casadevall A, Williamson P. 2008. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. [DOI] [PubMed]
- 26.Wu LG, Hamid E, Shin W, Chiang HC. 2014. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol 76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarnowski R, Sanchez H, Covelli AS, Dominguez E, Jaromin A, Bernhardt J, Mitchell KF, Heiss C, Azadi P, Mitchell A, Andes DR. 2018. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol 16:e2006872. doi: 10.1371/journal.pbio.2006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JM, Gould SJ. 2013. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans 41:277–282. doi: 10.1042/BST20120275. [DOI] [PubMed] [Google Scholar]
- 29.Hurley JH. 2008. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterman SR, Park YD, Raja M, Qiu J, Hammoud DA, O’Halloran TV, Williamson PR. 2012. Role of CTR4 in the virulence of Cryptococcus neoformans. mBio 3:e00285-12. doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harsay E, Schekman R. 2002. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol 156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kihara A, Noda T, Ishihara N, Ohsumi Y. 2001. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motaung TE. 2018. Cryptococcus neoformans mutant screening: a genome-scale’s worth of function discovery. Fungal Biology Rev 32:181–203. doi: 10.1016/j.fbr.2018.01.001. [DOI] [Google Scholar]
- 34.Zhang S, Hacham M, Panepinto J, Hu G, Shin S, Zhu X, Williamson P. 2006. The Hsp70 member, Ssa1 acts as a DNA-binding transcriptional co-activator in Cryptococcus neoformans. Mol Microbiol 62:1090–1101. doi: 10.1111/j.1365-2958.2006.05422.x. [DOI] [PubMed] [Google Scholar]
- 35.Vida TA, Emr SD. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND. 2000. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc 198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 37.Vachova L, Palkova Z. 2005. Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia. J Cell Biol 169:711–717. doi: 10.1083/jcb.200410064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babst M. 2011. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol 23:452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shields SB, Piper RC. 2011. How ubiquitin functions with ESCRTs. Traffic 12:1306–1317. doi: 10.1111/j.1600-0854.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessvik NP, Llorente A. 2018. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almeida F, Wolf JM, Casadevall A. 2015. Virulence-associated enzymes of Cryptococcus neoformans. Eukaryot Cell 14:1173–1185. doi: 10.1128/EC.00103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. 2012. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity 36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abels ER, Breakefield XO. 2016. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterman S, Hacham M, Hu G, Zhu X, Park Y, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, Singh N, Williamson P. 2007. Role of a CUF1-CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest 117:794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDonald E, Brown L, Selvais A, Liu H, Waring T, Newman D, Bithell J, Grimes D, Urbe S, Clague MJ, Zech T. 2018. HRS-WASH axis governs actin-mediated endosomal recycling and cell invasion. J Cell Biol 217:2549–2564. doi: 10.1083/jcb.201710051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopecka M, Yamaguchi M, Kawamoto S. 2014. The effects of the F-actin inhibitor latrunculin A on the pathogenic yeast Cryptococcus neoformans. Chemotherapy 60:185–190. doi: 10.1159/000377619. [DOI] [PubMed] [Google Scholar]
- 47.Xu W, Smith FJ Jr, Subaran R, Mitchell AP. 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol Biol Cell 15:5528–5537. doi: 10.1091/mbc.e04-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Q, Chen A, Zhang Y, Yuan M, Xie W, Zhang C, Zheng W, Wang Z, Li G, Zhou J. 2019. Component interaction of ESCRT complexes is essential for endocytosis-dependent growth, reproduction, DON production and full virulence in Fusarium graminearum. Front Microbiol 10:180. doi: 10.3389/fmicb.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galindo A, Calcagno-Pizarelli AM, Arst HN Jr, Peñalva MÁ. 2012. An ordered pathway for the assembly of fungal ESCRT-containing ambient pH signalling complexes at the plasma membrane. J Cell Sci 125:1784–1795. doi: 10.1242/jcs.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwaki T, Onishi M, Ikeuchi M, Kita A, Sugiura R, Giga-Hama Y, Fukui Y, Takegawa K. 2007. Essential roles of class E Vps proteins for sorting into multivesicular bodies in Schizosaccharomyces pombe. Microbiology 153:2753–2764. doi: 10.1099/mic.0.2007/006072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluter C, Lam KK, Brumm J, Wu BW, Saunders M, Stevens TH, Bryan J, Conibear E. 2008. Global analysis of yeast endosomal transport identifies the Vps55/68 sorting complex. Mol Biol Cell 19:1282–1294. doi: 10.1091/mbc.e07-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piper RC, Katzmann DJ. 2007. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren X, Hurley JH. 2011. Structural basis for endosomal recruitment of ESCRT-I by ESCRT-0 in yeast. EMBO J 30:2130–2139. doi: 10.1038/emboj.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. 2010. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coelho C, Casadevall A. 2019. Answers to naysayers regarding microbial extracellular vesicles. Biochem Soc Trans 47:1005–1012. doi: 10.1042/BST20180252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. 2015. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaturbhuj D, Patil A, Gangakhedkar R. 2018. PYRE insertion within HIV-1 subtype C p6-Gag functions as an ALIX-dependent late domain. Sci Rep 8:8897. doi: 10.1038/s41598-018-27162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Liu Y, Liu H, Tang WH. 2019. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobson ES, Compton GM. 1996. Discordant regulation of phenoloxidase and capsular polysaccharide in Cryptococcus neoformans. Med Mycol 34:289–291. doi: 10.1080/02681219680000491. [DOI] [PubMed] [Google Scholar]
- 61.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M’Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms RK, Veronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, Davis RW. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 62.Williamson PR. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol 176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Duin D, Cleare W, Zaragoza O, Casadevall A, Nosanchuk JD. 2004. Effects of voriconazole on Cryptococcus neoformans. Antimicrob Agents Chemother 48:2014–2020. doi: 10.1128/AAC.48.6.2014-2020.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camacho E, Vij R, Chrissian C, Prados-Rosales R, Gil D, O’Meally RN, Cordero RJB, Cole RN, McCaffery JM, Stark RE, Casadevall A. 2019. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J Biol Chem 294:10471–10489. doi: 10.1074/jbc.RA119.008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.