Proteus mirabilis, a frequent uropathogen, forms extensive biofilms on catheters that are infamously difficult to treat. To explore the mechanisms of biofilm formation by P. mirabilis, we performed in vivo transposon mutagenesis. A mutant with impaired biofilm formation was isolated. The mutant was found to have Tn5 inserted in the zapD gene, encoding an outer membrane protein of the putative type 1 secretion system ZapBCD.
KEYWORDS: CpxR, zapD, biofilm, Proteus mirabilis
ABSTRACT
Proteus mirabilis, a frequent uropathogen, forms extensive biofilms on catheters that are infamously difficult to treat. To explore the mechanisms of biofilm formation by P. mirabilis, we performed in vivo transposon mutagenesis. A mutant with impaired biofilm formation was isolated. The mutant was found to have Tn5 inserted in the zapD gene, encoding an outer membrane protein of the putative type 1 secretion system ZapBCD. zapBCD and its upstream zapA gene, encoding a protease, constitute an operon under the control of CpxR, a two-component regulator. The cpxR mutant and zapA mutant strains also had a biofilm-forming defect. CpxR positively regulates the promoter activities of zapABCD, cpxP, and cpxR. An electrophoretic mobility shift assay revealed that CpxR binds zapA promoter DNA. The loss of zapD reduced CpxR-regulated gene expression of cpxR, zapA, cpxP, and mrpA, the mannose-resistant Proteus-like (MR/P) fimbrial major subunit gene. The restoration of biofilm formation in the zapD mutant with a CpxR-expressing plasmid reinforces the idea that CpxR-mediated gene expression contributes to zapD-involved biofilm formation. In trans expression of zapBCD from a zapBCD-expressing plasmid also reestablished the biofilm formation ability of the cpxR mutant to a certain level. The zapD and cpxR mutants had significantly lower protease activity, adhesion, and autoaggregation ability and production of exopolysaccharides and extracellular DNA (eDNA) than did the wild type. Finally, we identified copper as a signal for CpxR to increase biofilm formation. The loss of cpxR or zapD abolished the copper-mediated biofilm upshift. CpxR was required for copper-induced expression of zapA and cpxR. Taken together, these data highlight the important role of CpxR-regulated zapD in biofilm formation and the underlying mechanisms in P. mirabilis.
INTRODUCTION
Urinary tract infections (UTIs) afflict a large proportion of the human population, and catheter-associated urinary tract infections (CAUTIs) are among the most common nosocomial infections, causing an enormous health and financial burden worldwide (1–3). The management of catheterized patients is frequently complicated by infections in which the formation of biofilms is a key feature (3, 4).
Bacterial biofilms are ubiquitous multicellular aggregates which are usually attached to biotic or abiotic surfaces in which bacteria are embedded in a self-produced extracellular polymeric matrix composed of mainly polysaccharides, proteins, lipids, and extracellular DNA (eDNA) (5–7). Bacteria within biofilms are refractory to host immune responses and antibiotic treatment (8), and biofilm formation has been associated with the virulence of a number of pathogens (9). Biofilm formation plays important roles in the colonization of catheters, implants, and other medical devices, frequently leading to chronic infections, such as UTIs (9).
The two-component signal transduction system (TCS) is the predominant strategy used by bacteria to adapt to fluctuating environments. The CpxAR system is one of the most widespread TCS in Gram-negative bacteria in response to different signals and has been shown to regulate protein folding and degrading factors participating in relieving envelope stress (10–12). The Cpx system appears to play a key role in regulating the virulence potential of a number of pathogens (11, 13). The Cpx system is composed of the histidine kinase CpxA and the cytoplasmic response regulator CpxR. Autophosphorylation of CpxA in response to environmental cues results in the phosphorylation of CpxR, a transcriptional regulator triggering the expression of over 100 downstream genes in Escherichia coli (14). The envelope of bacteria functions as both a sensor of external conditions and a physical barrier from environmental insults. Envelope stress-sensing systems are likely critical for Proteus mirabilis to detect and respond to potentially fatal environments during the course of infections (15). Notably, the CpxAR TCS appears to have contrasting effects on the pathogenesis of different bacterial species, even among strains (12). The human pathogens Yersinia pestis and uropathogenic E. coli require CpxAR for full virulence (13, 16). CpxR of enteropathogenic E. coli (EPEC) regulates the synthesis of bundle-forming pili and adherence to epithelial cells (17). Interestingly, it was reported that the induction of Cpx leads to defects in the virulence of EPEC (18). In Haemophilus ducreyi, the constitutive activation of CpxRA also attenuated in vivo virulence, consistent with what was observed during experimental infection with Vibrio cholerae (11).
P. mirabilis is an important pathogen of the urinary tract, especially in patients with indwelling urinary catheters (2, 19). It is difficult to eliminate once established in the catheterized urinary tract, often causing chronic infections. Several virulence factors of P. mirabilis, including flagellin-mediated swarming motility, hemolysin, urease, cell invasiveness, ZapA metalloprotease, and adherence to the urothelium mediated by fimbriae, have been demonstrated to contribute to infections (2, 19, 20). Among Proteus mirabilis fimbriae, mannose-resistant Proteus-like (MR/P) fimbriae were implicated in adherence and biofilm formation (19). The formation of P. mirabilis biofilms on catheters has been well documented (15, 21, 22). P. mirabilis CAUTIs are exacerbated with crystalline biofilm formation, in particular, by the potent urease activity, frequently leading to catheter blockage and other clinical complications. Although notable progress has been achieved by dedicated researchers in a number of areas, the mechanisms underpinning the biofilm formation of P. mirabilis remain elusive. A P. mirabilis rcsB (encoding the two-component regulator RcsB) mutant is deficient in its ability to form biofilms (23). Polyphosphate kinase (PPK) (24), nitrogen metabolism, and efflux systems (21) are involved in biofilm formation of P. mirabilis. In this regard, efflux constitutes an important waste management process in the challenged biofilm community. In addition, phosphate-specific transport (Pst) system also has a role in the biofilm formation of P. mirabilis, with the pstS mutant displaying a significant biofilm-forming defect (25).
For shedding light on how P. mirabilis successfully establishes CAUTIs, the mechanisms of biofilm formation require further investigation. In this study, we employed random transposon mutagenesis to identify genes associated with biofilm formation in P. mirabilis. A mutant exhibiting impaired biofilm formation was found to have a disrupted zapD gene, which may encode an outer membrane protein of the putative type 1 secretion system (T1SS) (26). The zapABCD operon was subject to the control of two-component regulator CpxR. We investigated the underlying mechanisms of the CpxR-regulated zapD in biofilm formation and identified copper as a signal for CpxR to modulate biofilm formation. This is the first report revealing a P. mirabilis CpxR-regulated T1SS, ZapBCD, participating in biofilm formation. These investigations provided new insight into the underlying mechanisms of biofilm formation in P. mirabilis, as well as the potential strategies for intervention in the clinically relevant biofilm formation.
RESULTS
Isolation of a biofilm-deficient zapD mutant of P. mirabilis.
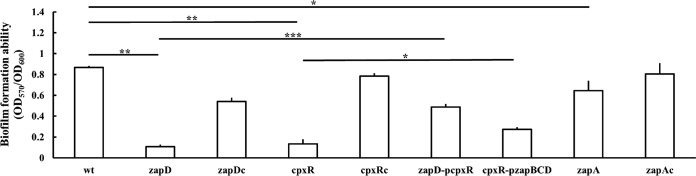
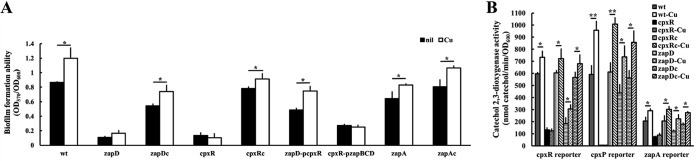
The formation of biofilms plays a key role in bacterial colonization and infection. To explore the mechanisms of biofilm formation of P. mirabilis, we undertook in vivo Tn5-transposon mutagenesis, as described previously (27), to isolate the biofilm-deficient mutants. Three mutants exhibiting impaired biofilm formation by the crystal violet staining method were isolated from about 7,000 transconjugants. One of the mutants with the most significantly reduced ability to form biofilm compared to the wild-type P. mirabilis N2 was selected for further investigation. We obtained the nucleotide sequence flanking the mini-Tn5 from the cloned mutant DNA fragment containing the mini-Tn5 with a kanamycin resistance marker. The zapD gene was found to be disrupted by Tn5 after searching for the sequences we obtained in the P. mirabilis released genome sequence (https://www.ncbi.nlm.nih.gov/). We constructed a zapD knockout mutant exhibiting no growth defect compared to the wild type, and the impaired biofilm formation of the zapD mutant was confirmed (Fig. 1). zapD gene encodes an outer membrane protein, constituting a putative T1SS with ZapB and ZapC (26).
FIG 1.
Biofilm formation of wild-type P. mirabilis, mutants, and respective complemented strains. The OD600 of LB overnight bacterial cultures in each microtiter was determined, and the biofilms were stained with crystal violet (1%). The OD570s of the extracts from the crystal violet-stained biofilms by acetone and alcohol were determined to calculate the OD570/OD600, representing the ability of biofilm formation. The data are the averages and standard deviations of the results from three independent experiments. Significant differences were determined by using a one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). wt, wild type; zapD, zapD mutant; zapDc, zapD-complemented strain; cpxR, cpxR mutant; cpxRc, cpxR-complemented strain; zapD-pcpxR, zapD mutant with the cpxR-expressing plasmid; cpxR-pzapBCD, cpxR mutant with the zapBCD-expressing plasmid; zapA, zapA mutant; zapAc, zapA-complemented strain.
Sequence analysis of the zapABCD locus of P. mirabilis.
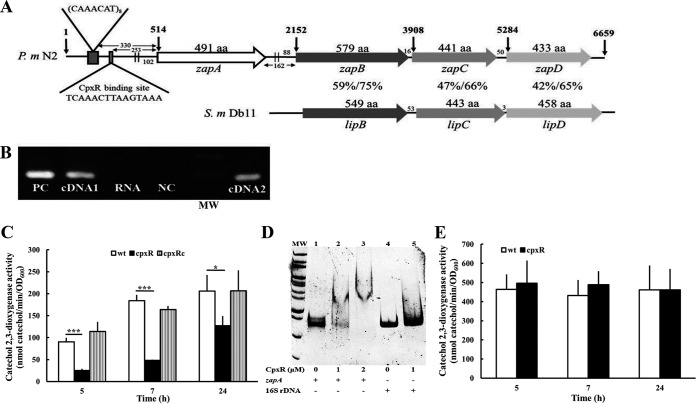
We amplified the entire DNA fragment (6,659 bp, Fig. 2A) containing zapA, zapB, zapC, zapD, and the upstream region of zapA with the KOD DNA polymerase of high fidelity and efficiency using primers designed from P. mirabilis HI4320 (Table 1), and the sequence of the product was determined. The upstream region contains 8 copies of the heptameric sequence CAAACAT in a direct tandem repeat, as described previously (26), and a putative CpxR binding site, comprising 330 and 253 bases, respectively, from the start codon of zapA (Fig. 2A). Analysis of the upstream DNA sequences of zapA and zapB by Neural Network Promoter Prediction (https://www.fruitfly.org/seq_tools/promoter.html) and Prediction of bacterial promoters (BPROM; Softberry) revealed a possible promoter of both zapA and zapB (Fig. 2A). This indicates that zapABCD and zapBCD are likely to be transcribed as a polycistronic message. The deduced amino acid sequences of ZapA, ZapB, ZapC, and ZapD have 98.8 to 100% identity and 99 to 100% similarity to the corresponding sequences of P. mirabilis strains HI4320 and BB2000. Through analysis of the amino acid sequences, ZapA, ZapB, and ZapC are the putative metalloprotease, type 1 secretion ATP-binding protein, and a membrane fusion protein, respectively. The ZapB, ZapC, and ZapD proteins show homology to the Serratia marcescens type 1 secretion system LipB (59% identity, 75% similarity), LipC (47% identity, 66% similarity), and LipD (42% identity, 65% similarity), respectively (Fig. 2A). To verify the possibility of zapABCD constituting an operon, we performed reverse transcription using one primer annealing to the zapD gene and the other to zapB. Then, we used the cDNA to amplify a zapA fragment. A predicted product of 172 bp occurred by using either zapD or zapB cDNA as the template (Fig. 2B). The result indicates that the zapA, zapB, zapC, and zapD genes form an operon in P. mirabilis.
FIG 2.
P. mirabilis zapABCD locus and the zapABCD operon regulated by CpxR. (A) Genomic organization of the zapABCD locus in P. mirabilis. Contained within a 6,659-bp region are four open reading frames (ORFs), zapA, zapB, zapC, and zapD. Amino acid numbers are listed on the top of each ORF. The vertical arrow indicates the nucleotide number within this locus. The two parallel lines upstream of zapA and zapB indicate the RpoD binding site. The CpxR binding site and CAAACAT repeat sequence preceding the zapA start codon are indicated. The nucleotide numbers of the intergenic space and the nucleotide distance of the RpoD binding site to the zapA and zapB start codon are also shown. Percent amino acid identity and similarity between P. mirabilis N2 ZapB/C/D and the corresponding LipB/C/D of S. marcescens Db11 by the BLAST analysis are shown. (B) The zapABCD forms an operon. We diluted overnight bacterial cultures and incubated the cultures for another 5 h at 37°C. Total RNA was prepared, and reverse transcription was carried out, with one primer annealing to zapD and the other to zapB. The cDNA product was used to amplify a zapA DNA fragment. For control reactions, RNA without reverse transcriptase (no RT) or genomic DNA was used as the template. The representative result of three independent experiments is shown. PC, genomic DNA as a positive control; cDNA1, cDNA from zapD; RNA, RNA without reverse transcriptase as a negative control; NC, ddH2O negative control; MW, molecular weight (size markers); cDNA2, cDNA from zapB. (C) The zapA promoter activities of the wild-type, cpxR mutant, and cpxRc strains. Bacterial cells with the zapA reporter plasmid were grown overnight, diluted, and incubated for 5, 7, and 24 h at 37°C before the XylE activity was measured as described previously. (D) Electrophoretic mobility shift assay of purified P. mirabilis CpxR with the zapA promoter fragment. The DNA fragments (0.1 μg) of the zapA promoter (373 bp) or 16S rRNA gene promoter (380 bp) obtained by PCR were incubated with the indicated concentrations of the CpxR protein. After protein-DNA complex formation, the samples were resolved on a 5% nondenaturing polyacrylamide gel. The representative result of three independent experiments is shown. Arrow, protein-DNA complex; lane MW, size markers; lanes 1 to 3, zapA promoter DNA with CpxR; lanes 4 and 5, 16S rRNA gene promoter DNA with CpxR. (E) The zapB promoter activities of wild-type and cpxR mutant strains were determined as described in panel C. (C and E) The data are the averages and standard deviations of the results from three independent experiments. Significant differences were determined by using Student's t test (*, P < 0.05; ***, P < 0.001). wt, wild type; cpxR, cpxR mutant; cpxRc, cpxR-complemented strain.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′–3′)a | Purpose |
|---|---|---|
| cpxA-KOup-R | TCTAGAGCGCTGACAAACTGTTTATC | cpxA knockout |
| cpxA-KOdown-F | TCTAGATAAGCTATCTAAAAGTCGCC | cpxA knockout |
| cpxA-KOdown-R | ATAAGGAGCTTAAGGTTGCC | cpxA knockout |
| cpxR-KOup-F | AAACGCAAAAAAAAGCCCCTCTCC | cpxR knockout |
| cpxR-KOup-R | TCTAGATTCGCGATCGTCATCCAC | cpxR knockout |
| cpxR-KOdown-F | TCTAGACGTGGCCGTGGTTATCTT | cpxR knockout |
| cpxR-KOdown-R | GCGGTTACTGTTAGGGTT | cpxR knockout |
| zapA-KOup-F | GGACATAATGAACTTCAAGGTG | zapA knockout |
| zapA-KOup-R | TCTAGAGCGGTATCATAATCAAAAGAAGGT | zapA knockout |
| zapA-KOdown-F | TCTAGATGCTGGCGAAGCAACTCTTA | zapA knockout |
| zapA-KOdown-R | TCCATACATTGATCACTTGCTCA | zapA knockout |
| zapD-KOup-F | TTCATCTACTGAAAATAGAG | zapD knockout |
| zapD-KOup-R | TCTAGATAGAGATCAGACAATGTAAC | zapD knockout |
| zapD-KOdown-F | TCTAGATACCAATACTCTAGATATTG | zapD knockout |
| zapD-KOdown-R | GTTTAGTTATTGGCTATGCT | zapD knockout |
| I-out primer | GAGCTCGAATTCGGCCTAG | Identifying Tn5-inserted genes |
| O-out primer | CCTGCAGGCATGCAAGCTTC | Identifying Tn5-inserted genes |
| cpxR protein-F | GGATCCATGCATAAAATCTTATTAGTGG | Purification of CpxR protein |
| cpxR protein-R | TTAAGTTATTGAAACCATAAGACTCGAG | Purification of CpxR protein |
| SphI-cpxR-repo-F | GCATGCTGTCCCACTCCTCATTCGGT | Construction of cpxR reporter |
| PstI-cpxR-repo-R | CTGCAGAATTCGCGATCGTCATCCAC | Construction of cpxR reporter |
| SphI-cpxP-repo-F | GCATGCAATTCGCGATCGTCATCCAC | Construction of cpxP reporter |
| PstI-cpxP-repo-R | CTGCAGTGTCCCACTCCTCATTCGGT | Construction of cpxP reporter |
| SphI-zapA-repo-F | GCATGCCAAACATCAAACATCAAAC | Construction of zapA reporter |
| PstI-zapA-repo-R | CTGCAGCTCCAGATATAAATTTAGG | Construction of zapA reporter |
| SphI-zapB-repo-F | GCATGCTGCTGGCGAAGCAACTCTTA | Construction of zapB reporter |
| PstI-zapB-repo-R | CTGCAGGTGGTATTCCTGATTTAATTTGTCT | Construction of zapB reporter |
| cpxR over-F | CACGTCCATCATGCATACCG | Cloning cpxR into pGEM-T Easy and cpxA knockout |
| cpxR over-R | GGCGAATATACGCGCTGACA | Cloning cpxR into pGEM-T Easy |
| zapA over-F | ATGTCTATCTTTATCCTCAATGGC | Cloning zapA into pGEM-T Easy |
| zapA over-R | CCTAACTATGACTTAAACAATAAAATC | Cloning zapA into pGEM-T Easy |
| zapBCD over-F | GTGAAAATAGACGCTTCTTTAAAACC | Cloning zapBCD into pGEM-T Easy |
| zapBCD over-R | CGCAAAGCCCTATAACCTGATTA | Cloning zapBCD into pGEM-T Easy and amplifying zapABCD locus |
| zapA RT-F | GGTGACTTATTCATTCCC | zapABCD operon checking |
| zapA RT-R | CATTCCATCAACATTCCC | zapABCD operon checking |
| zapD RT-R | GTGGGGTCATGTTTAATCGC | zapABCD operon checking |
| zapB RT-R | TGGCAGCTTGGATCACTTT | zapABCD operon checking |
| mrpA RT-F | GGTTCTTTAGGCATTGAAGG | mrpA real-time PCR |
| mrpA RT-R | TCATTGTTACCATCACGCAG | mrpA real-time PCR |
| zapA sequence-F | TAAGGATTACATTGTAGTCC | Amplifying zapABCD locus |
Underlined sequences represent enzyme restriction sites.
Given that the zapB, zapC, and zapD genes are likely cotranscribed as a single transcript and that ZapB, ZapC, and ZapD constitute a T1SS, we speculated that complementation of the zapD mutation should be carried out with the entire ZapBCD to restore the wild-type phenotype. As expected, we found that transformation of the zapD mutant with a zapBCD-expressing plasmid but not a zapD-expressing plasmid (data not shown) restored biofilm formation although not to the wild-type level (Fig. 1, zapDc complemented strain versus the wild-type strain).
CpxR regulates the promoter activity of P. mirabilis zapABCD but not zapB.
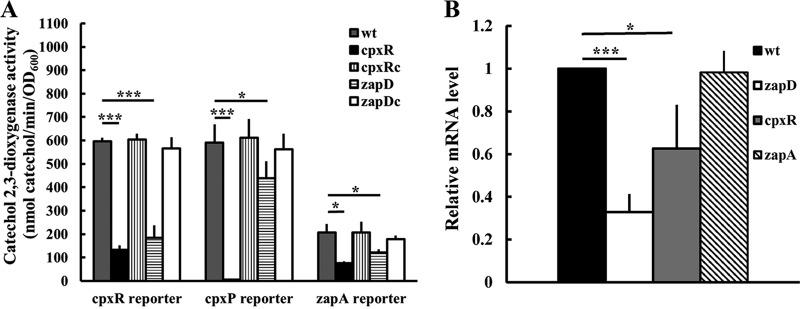
In view of an operon made up of zapABCD (Fig. 2B) and a putative CpxR binding site (TCAAACTTAAGTAAA) located in the zapABCD promoter region (Fig. 2A), we constructed a cpxR mutant exhibiting no growth defect compared to the wild type and the cpxR-complemented strain (cpxRc) to examine if CpxR regulates the expression of zapABCD by the reporter assay. We found that the cpxR mutant displayed significantly lower zapA promoter activity than did the wild-type and cpxR-complemented (cpxRc) strains (Fig. 2C). However, no difference in zapB promoter activity was observed between the wild type and cpxR mutant (Fig. 2E). The wild type and cpxR mutant had similarly higher zapB promoter activity at 5, 7, and 24 h than zapA promoter activity (Fig. 2C and E). Furthermore, an electrophoretic mobility shift assay (EMSA) showed that CpxR could bind to the zapA promoter region but not the irrelevant promoter DNA (Fig. 2D). The results indicate that CpxR could bind the zapA promoter to control the transcription of zapA but not zapB. It has been known that cpxR and cpxP belong to the CpxR regulon (12, 14). Accordingly, we found that the cpxR and cpxP promoter activities were downregulated in the cpxR mutant compared to the wild-type and cpxRc strains, being almost nil for the cpxP promoter (Fig. 3A). Notably, the cpxR mutant still had CpxR-independent cpxR promoter activity (Fig. 3A).
FIG 3.
The loss of P. mirabilis zapD affected the expression of genes regulated by CpxR. (A) Shown is the expression of cpxR, cpxP, and zapA in the wild-type, zapD and cpxR mutant, and respective complemented strains. The promoter activity was determined as described in Fig. 2C. Only results after incubation for 24 h were shown. The data are the averages and standard deviations of the results from three independent experiments. Significant differences were determined by using Student's t test (*, P < 0.05; ***, P < 0.001). (B) The mrpA mRNA level in the wild type and zapD, cpxR and zapA mutants. Total RNA was isolated from bacterial cells at 5 h after seeding onto LB agar plates and then subjected to real-time RT-PCR for mRNA measurement. The mRNA levels were normalized using the gyrB gene as the internal control. The value obtained for the wild-type cells at 5 h after seeding was set as 1, and other data are presented relative to this value. The data are averages and standard deviations of the results from three independent experiments. Significant differences were determined by using Student's t test (*, P < 0.05; ***, P < 0.001). wt, wild type; zapD, zapD mutant; cpxR, cpxR mutant; zapDc, zapD-complemented strain; cpxRc, cpxR-complemented strain; zapA, zapA mutant.
The cpxR mutant of P. mirabilis displays impaired biofilm formation and reduced mrpA mRNA levels, and zapD deletion decreases the expression of genes regulated by CpxR.
Since the loss of cpxR affected the expression of zapABCD (Fig. 2C), we tested the role of cpxR in biofilm formation. We found that the deletion of cpxR caused a severe defect in biofilm formation compared to the wild-type and cpxR-complemented (cpxRc) strains (Fig. 1). According to the regulation of zapABCD (zapD) expression by CpxR (Fig. 2C) and the contribution of zapD in biofilm formation (Fig. 1), in trans expression of zapBCD from a zapBCD-expressing plasmid could restore the biofilm formation ability of the cpxR mutant to a certain extent (Fig. 1). Knowing that the Cpx TCS affects the expression of pili (17) and that MR/P fimbriae are involved in P. mirabilis biofilm formation (28), we examined the mRNA level of mrpA and found that the mrpA mRNA amount was significantly lower in the cpxR mutant than in the wild type (Fig. 3B). To our surprise, a significant reduction in mrpA mRNA levels of the zapD mutant occurred (Fig. 3B). Therefore, we performed the reporter assay to examine if the loss of zapD affected the CpxR signaling pathway. We found that the CpxR-driven cpxR, cpxP, and zapA promoter activities were all reduced in the zapD mutant relative to the wild-type and zapDc strains (Fig. 3A). Based on the finding that zapD and cpxR were required for biofilm formation (Fig. 1), the decreased expression of cpxR in the zapD mutant suggests that certain CpxR-regulated biofilm-promoting factors could contribute to zapD-mediated biofilm formation. Hence, we transformed the cpxR-expressing plasmid into the zapD mutant and found that the biofilm defect of the zapD mutant was restored in a similar way as the cpxR mutant with the cpxR-expressing plasmid, albeit not to the same extent (Fig. 1). This reinforces the idea that CpxR deficiency could account for the biofilm defect of zapD mutant to a certain extent.
Loss of P. mirabilis zapA affects biofilm formation.
It has been shown that T1SS-encoding genes are usually situated adjacent to the genes encoding proteins secreted by this system (29). Previous work has shown that mutations in zapB, zapC, or zapD adversely affect ZapA metalloprotease activity, implying that these gene products are involved in ZapA secretion (30). Moreover, a metalloprotease was shown to contribute to biofilm formation in Serratia spp. (31). Therefore, we constructed the zapA mutant exhibiting no growth defect compared to the wild-type and zapA-complemented (zapAc) strains to evaluate the biofilm formation ability. As shown in Fig. 1, the loss of zapA causes a decrease in biofilm formation compared to the wild-type and zapAc strains.
Thus, we concluded that CpxR signaling pathway could modulate biofilm formation through regulating the expression of zapABCD (i.e., T1SS ZapBCD and ZapA) and mrpA.
Loss of P. mirabilis zapD or cpxR decreases extracellular protease activity, production of eDNA and EPS, adhesion to the uroepithelial cells, and autoaggregative ability.
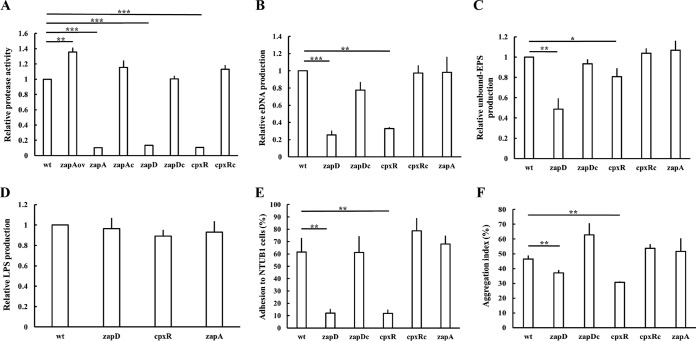
Extracellular polymeric substances of biofilms are composed of exopolysaccharide (EPS), lipopolysaccharides (LPS), eDNA, and proteins (5–7). Seeing that we found that zapD, cpxR, and zapA were involved in biofilm formation (Fig. 1), we assessed extracellular protease activity, the production of eDNA, EPS, and LPS, adhesion to the uroepithelial cells, and autoaggregative ability in the wild-type, related mutants, and complemented strains. We found that the protease activities of the zapA, zapD, and cpxR mutants were dramatically low relative to the wild type and their respective complemented strains (Fig. 4A). In addition, the zapA-overexpressed strain exhibited higher protease activity than did the wild type (Fig. 4A).
FIG 4.
Extracellular protease activity, production of eDNA, EPS, and LPS, adhesion to urothelial cells, and autoaggregation ability in wild-type P. mirabilis and related strains. (A) Extracellular protease activity. The overnight bacterial culture mixed with the azocasein solution was incubated at 37°C for 24 h, and then trichloroacetic acid (15%) was added. After centrifugation, the IgA protease activity in the supernatant was determined. (B) eDNA production. The eDNA amount was measured using the supernatant from overnight bacterial cultures, as described in Materials and Methods. (C) EPS production. The unbound EPS level was determined using overnight bacterial cultures. Bacterial cells in CPG broth were incubated for 24 h at 37°C. After centrifugation, the unbound EPS in the supernatant was precipitated and quantified by the phenol-sulfuric acid method. (D) LPS production. LPS extraction and analysis were performed as described in Materials and Methods. Equal amounts of wild-type and mutant cells were subject to the Purpald assay to determine the LPS concentration. (E) Adhesion to urothelial cells. Overnight bacterial cells were applied to NTUB1 cells cultured to confluent monolayers at the MOI of 10. After incubation for 1 h at 37°C, NTUB1 cells were lysed to determine bacterial CFU. The cell adhesion ability was the percentage of viable bacteria that adhered to the NTUB1 cells versus the total inoculum. (F) Autoaggregation ability. Overnight bacterial cultures were washed and resuspended for determining the initial OD660. We then centrifuged the cell suspensions at 2,600 rpm for 2 min, and the OD660 of the supernatant was measured. We calculated the aggregation index as described in Materials and Methods. (A to D) The value obtained for the wild-type cells was set as 1, and the other data are presented relative to this value. The data are the averages and standard deviations of the results from three independent experiments. Significant differences were determined by using Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). wt, wild type; zapD, zapD mutant; zapDc, zapD-complemented strain; cpxR, cpxR mutant; cpxRc, cpxR-complemented strain; zapA, zapA mutant; zapAc, zapA-complemented strain; zapAov, zapA-overexpressed strain.
The zapD mutant produced lower levels of eDNA and EPS than did the wild-type and zapD-complemented strains (Fig. 4B and C). In view of the positive regulation of zapABCD expression by CpxR, the production of eDNA and EPS in the cpxR mutant was found to follow a similar trend as the zapD mutant (Fig. 4B and C). The cpxR mutant harboring the cpxR-expressing plasmid restored the secretion of eDNA and EPS to the wild-type level (Fig. 4B and C). The data suggest that ZapD participates in the secretion of EPS and eDNA. No difference in LPS production was observed between the wild type and either of the mutants (Fig. 4D). It has been known that bacterial adhesion is an important initial step in biofilm formation (10, 32) and that EPS and eDNA are important bacterial adhesion molecules (7, 32–35). Knowing that the loss of zapD or cpxR affected biofilm formation and decreased the secretion of EPS and eDNA, the adhesion ability to urothelial cells (NTUB1 cells) was assessed. As expected, both mutants had a lower adhesion ability than those of the wild-type and respective complemented strains (Fig. 4E). In addition, the autoaggregative ability which is associated with fimbria-mediated adherence, representing another indicator for biofilm formation (36, 37), was determined. A lower autoaggregative ability was observed in both zapD and cpxR mutants than the wild-type and the corresponding complemented strains (Fig. 4F). These data indicate that the loss of zapD or cpxR reduces the biofilm-forming factors, including the production of EPS and eDNA, adhesion to urothelial cells, and autoaggregation ability. The production of eDNA, EPS, and LPS, adhesion to the uroepithelial cells, and autoaggregative ability were also evaluated in the zapA mutant. As shown in Fig. 4B to F, these biofilm-associated traits of zapA mutant were similar to those of the wild type. In addition, the loss of zapA had no effect on the mrpA mRNA level (Fig. 3B).
Copper induces biofilm formation in the wild type but not the cpxR and zapD mutants.
Copper has been reported to trigger CpxAR-mediated gene expression (38, 39) and to be associated with biofilm formation (40). We found that CpxR regulated the expression of the zapABCD operon (Fig. 2C), zapD loss decreased cpxR promoter activity (Fig. 3A), and both zapD and cpxR affected biofilm formation (Fig. 1). We then examined the effect of copper on biofilm formation in the wild type and related strains. Copper increased biofilm formation in the wild-type, zapA mutant, and complemented strains of zapD, cpxR, and zapA (Fig. 5A). Copper had no significant effect on the biofilm formation of the cpxR and zapD mutants (Fig. 5A). Notably, the zapD mutant with the cpxR-expressing plasmid behaved as the wild type in response to copper, but the cpxR mutant with the zapBCD-expressing plasmid was unresponsive to copper (Fig. 5A). These data imply that CpxR is required for responding to copper to enhance biofilm formation by triggering the production of biofilm formation factors, such as MR/P fimbriae, ZapA, and ZapBCD-associated eDNA and EPS. Moreover, we examined the effect of copper on the transcription of zapA, cpxP, and cpxR in the wild-type strains and in the cpxR and zapD mutants and their respective complemented strains by a reporter assay. As expected, the cpxR mutant was unresponsive to copper in the transcription of zapA, cpxP, and cpxR (Fig. 5B). Interestingly, we found that copper could still elicit the transcription of zapA, cpxP, and cpxR in the zapD mutant, as is the case for the wild-type and complemented strains (Fig. 5B).
FIG 5.
The effect of copper on biofilm formation and expression of genes regulated by CpxR in the wild-type P. mirabilis, mutants, and respective complemented strains. (A) Biofilm formation. The bacterial strains used were the same as in Fig. 1. The diluted overnight bacterial cultures were inoculated into the LB-containing microtiter wells with or without copper (1 mM). The OD600 of each well was obtained after overnight incubation, and the biofilm formation ability was determined as described in Fig. 1. nil, no-copper control. (B) Expression of genes regulated by CpxR. The bacterial strains used were the same as those in Fig. 3A. The promoter activity was determined as described in Fig. 2C in the presence and absence of copper (1 mM). Only results after incubation for 24 h were shown. The data are averages and standard deviations of the results from three independent experiments. (A and B) Significant differences were determined by using two-way ANOVA with Tukey’s multiple-comparison test (*, P < 0.05; **, P < 0.01).
DISCUSSION
The pathogenesis of UTIs caused by P. mirabilis is not fully understood at present. The hallmarks of these infections include the formation of stones in the bladder and kidneys and biofilm formation on urinary catheters (2, 15, 19, 22). The exact mechanisms of biofilm formation in P. mirabilis have remained an open question until now. In this study, we uncovered a CpxR-regulated zapABCD operon participating in the biofilm formation of uropathogenic P. mirabilis. Several lines of evidence support the notion. First, the biofilm formation was deficient in zapD, cpxR, and zapA mutants, using the crystal violet staining assay or confocal electron microscopy, relative to the wild type (Fig. 1; see also Fig. S1 in the supplemental material). Second, the expression of MrpA fimbriae was lower in the zapD and cpxR mutants than in the wild type (Fig. 3B). Accordingly, both mutants had worse adhesion to urothelial cells and less aggregation ability than did the wild type and the respective complemented strains. Third, both the zapD and cpxR mutants produced smaller amounts of biofilm-forming factors, eDNA, EPS, and ZapA than the wild-type and the respective complemented strains (Fig. 4). Fourth, CpxR regulates the zapABCD promoter activity and binds to the zapA promoter (Fig. 2C and D).
The CpxAR TCS affects the expression of hundreds of genes with a plethora of functions, including biofilm formation and virulence (11, 12, 41). The contribution of the CpxAR TCS to biofilm formation appears to vary among species. For example, Actinobacillus pleuropneumoniae CpxR regulates the expression of the pga operon (synthesizing the major biofilm matrix polysaccharide polygalacturonic acid [PGA]) through rpoE to facilitate biofilm formation (42). CpxR-mediated expression of curli and fimbrial operons is required for biofilm formation in Salmonella enterica serovar Enteritidis (43). In contrast, E. coli CpxR-P can directly bind to the fimA promoter, thus negatively affecting the production of type 1 fimbriae and biofilms (44). S. marcescens CpxR negatively regulates biofilm formation by directly inhibiting the expression of PrtA (31). For the first time, we found that the P. mirabilis CpxR-regulated zapABCD genes (encoding T1SS and ZapA protease) participate in biofilm formation, which corresponds with the role of CpxAR TCS in the regulation of virulence factors. Recently, our unpublished data also showed that CpxR was involved in urinary tract colonization.
The transition from the planktonic growth mode to biofilm formation involves several distinct steps (45). After initial attachment, the microcolonies form and grow to form larger aggregates. As this happens, many extracellular matrix substances, such as polysaccharides, proteins, and eDNA adding strength to the structural scaffold, are secreted. The colonies eventually develop into mature biofilms with mushroom-shaped structures. The release of eDNA could adjust the hydrophobicity of the bacterial cell surface to interact with the substratum or neighboring cells, thus assisting adhesion (7, 33). Overproduction of the exopolysaccharide has been shown to result in enhanced bacterial cell aggregation and adhesion to microtiter wells during biofilm plate assays (34). It was inferred that exopolysaccharides have an important role in adhesion, which is critical for initiation and maintenance of the biofilm structure (34). MR/P fimbriae were shown to promote initial biofilm formation, with the fimbriae conferring an aggregative phenotype (36). Aggregation might permit more adherence with bacteria piling on top of each other. It was suggested that a metalloprotease may modulate biofilm formation dependent on the catalytic activity or structural property harbored by it (31). Accordingly, we noticed that biofilm-deficient zapD and cpxR mutants had lower extracellular protease activity, production of eDNA and EPS, adhesion to the uroepithelial cells, and autoaggregative ability than those of the wild type (Fig. 4). As for the zapA mutant, the biofilm-forming factors, except the extracellular protease activity, were similar to those of the wild type (Fig. 4). Because we observed that ZapA expression was not associated with P. mirabilis autolysis (data not shown) as was shown to be the case for PrtA metalloprotease in S. marcescens (31), we can rule out the possibility that ZapA-dependent liberation of eDNA could act to modulate the formation or maintenance of P. mirabilis biofilms. Further studies are needed to reveal the scenarios of zapA involvement in the biofilm-forming process. Previously, we demonstrated that RpoE positively modulated the expression of MR/P fimbriae by regulating mrp genes (46). The findings of positive regulation of RpoE by CpxR in P. mirabilis (data not shown) and the presence of the RpoE binding site instead of CpxR in the upstream region of mrp operon raise the possibility that CpxR may regulate mrpA expression through RpoE.
It is noteworthy that the deletion of zapD resulted in significantly lower expression of cpxR and CpxR-regulated cpxP and zapA (Fig. 3A). How could the loss of a T1SS outer membrane protein affect the signal transduction of a TCS? Based on the fact that the Cpx pathway plays a key role in the regulation of adhesion-induced gene expression in a process requiring the outer membrane lipoprotein NlpE (10) and that ZapD participated in the production of adhesion factors such as EPS and eDNA (Fig. 4), we attribute the decreased cpxR expression in the zapD mutant to the deficiency of adhesion factors, eDNA, and EPS. Accordingly, the zapD mutant exhibited a significantly lower adhesion ability to the bladder urothelial (NTUB1) cells than did the wild-type and zapDc strains (Fig. 4E). It is likely that the deletion of zapD could affect the production of adhesion molecules whereby the Cpx activation and subsequent expression of biofilm-related genes (mrpA and zapABCD) are attenuated. The finding that the biofilm-related phenotypes and CpxR-regulated gene expression in the zapD mutant followed trends similar to those observed in the cpxR mutant (Fig. 1, 3, and 4) reinforces the notion. Further studies to compare the CpxR activation in the wild type and nlpE mutant in the presence of EPS or eDNA will be required to prove the notion.
T1SS is widespread in Gram-negative bacteria, especially in pathogenic bacteria, for the secretion of adhesins, lipases, proteases, peroxidase, bacteriocin, the swimming-related SwmA protein, or pore-forming toxins in one step across two membranes into the extracellular space (31, 47–49). This implies that T1SS plays important physiological roles. In this regard, Serratia spp. (31) and Pseudomonas spp. (48) produce T1SS-dependent adhesion proteins to enable the cells to attach to the surface in initiating biofilm formation. T1SS substrates are usually defined by the presence of several nonapeptide sequences with the consensus GGxGxDxUx, where x can be any amino acid and U is a large hydrophobic amino acid (49). These motifs specifically bind divalent metal and are implicated in posttranslocation folding (49). Analysis of the amino acid sequence of P. mirabilis N2 ZapA revealed four nonapeptide repeats located in the C-terminal end and the last four residues of DFIV as the hallmark signature for a T1SS substrate (30). Proteus sZapA, Serratia PrtA (31), and Erwinia chrysanthemi PrtC (50) are all serralysin metalloproteases secreted by T1SS. Genes encoding the protein substrates are usually located in the operon coding for the corresponding export proteins (29), as is the case for the P. mirabilis zapABCD genes, with the exception of S. marcescens LipBCD versus PrtA. A zapABCD gene locus also exists in the genomes of Proteus vulgaris and Proteus penneri with high amino acid identity with that of P. mirabilis. The corresponding gene arrangement in Erwinia chrysanthemi, prtCDEFEC, has 48, 48, 38, and 33% amino acid identity to ZapA, ZapB, ZapC, and ZapD, respectively. T1SS also participates in the assembly and secretion of exopolysaccharides (32). Bacterial eDNAs were shown to be released by autolysis, secreted vesicles, or the type IV secretion system (7). For the first time, we revealed that the P. mirabilis T1SS is associated with the production of eDNA and EPS, the matrix components of biofilms.
We found that CpxR regulated the promoter activity of zapABCD but not zapBCD, consistent with the lack of a CpxR binding site in the region upstream of zapB. We found the binding sites of sigma factor N (RpoN) and its enhancer binding protein (QseF) (51) in the zapB promoter region. The qseF mutant of P. mirabilis displayed a severe biofilm defect (data not shown). Studies are undertaken to characterize the RpoN/QseF-mediated expression of zapB. A putative CpxR binding site is also found in the upstream of zapABCD-like locus of P. mirabilis BB2000/HI4320, P. vulgaris, and P. penneri, but it is not the case for prtCDEFEC.
Several studies provide evidence that membrane disturbance and oxidative stress are associated with biofilm formation. First, a loss of tat, encoding a twin-arginine translocation (52) pathway with important physiological functions for the bacterial envelope, impairs outer membrane integrity and triggers biofilm formation (52). Second, CpxAR can respond to a cell envelope stress caused by sub-MICs of penicillin G by significantly upregulating the production of matrix components to induce biofilm formation (53). Third, the antibiotic tropodithietic acid (TDA) induces oxidative stress responses in Vibrio vulnificus and switches bacterial cells to a biofilm lifestyle (54). In addition, a number of studies implicate copper stress genes in envelope functions, including biofilm formation (14, 55). Copper exposure seems to induce changes in bacterial membrane composition (40), and the biofilm matrix polysaccharides were shown to be able to attenuate the reactive oxygen species (ROS) generated by copper exposure (56). Recent studies also showed that copper-responsive genes regulated by CpxAR are involved in the maintenance of homeostasis for copper and the envelope, as well as in protection from oxidative stresses (38, 39). In this study, we found that Cu-induced biofilm formation is CpxR dependent. The induction of the CpxR regulon may be a reflection of the disrupted membrane functions and/or ROS production caused by copper exposure.
In summary, we demonstrated that CpxR-regulated zapD expression is involved in the biofilm formation of uropathogenic P. mirabilis. CpxR positively regulates the expression of MR/P fimbriae, ZapA protease, and ZapBCD, and thus the production of ZapBCD-associated EPS and eDNA, to facilitate biofilm formation. This is the first study providing evidence to support the idea that CpxR-regulated T1SS participates in biofilm formation in P. mirabilis. This study indicates that the CpxR pathway and T1SS could be potential therapeutic targets for preventing UTIs by P. mirabilis. We are still at some distance from a systematic understanding of the biofilm formation in P. mirabilis, and there are many details to be discovered before strategically acting upon them.
MATERIALS AND METHODS
Bacterial strains, plasmids, reagents, and growth conditions.
The bacterial strains and plasmids in this study are listed in Table 2. All chemicals were obtained from Sigma-Aldrich, unless otherwise indicated, and the primer sequences are listed in Table 1. Bacteria were routinely cultured in Luria-Bertani (LB) broth at 37°C. L swarm minus (LSW−) agar was used to prevent swarming motility. Ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (100 μg/ml), tetracycline (20 μg/ml), or cupric sulfate (CuSO4) was added to the medium as needed.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| P. mirabilis | ||
| wt | Wild-type N2, Rifr Tetr | Clinical isolate |
| cpxR | wt derivative, cpxR knockout mutant, Kmr | This study |
| cpxRc | cpxR mutant containing cpxR in pGEM-T Easy, cpxR-complemented strain, Kmr Ampr | This study |
| cpxR-pzapBCD | cpxR mutant with the zapBCD-expressing pGEM-T Easy | |
| zapA | wt derivative, zapA knockout mutant, Kmr | This study |
| zapAc | zapA mutant containing zapA in pGEM-T Easy, cpxR-complemented strain, Kmr Ampr | This study |
| zapAov | wt containing zapA in pGEM-T Easy, zapA-overexpressed strain, Ampr | This study |
| zapD | wt derivative, zapD knockout mutant, Kmr | This study |
| zapDc | zapD mutant containing zapBCD in pGEM-T Easy, Kmr Ampr | This study |
| zapD-pcpxR | zapD mutant with the cpxR-expressing pGEM-T Easy | |
| E. coli | ||
| DH5α | fhuA2 lacΔU169 phoA glnV44 thi-1 hsdR17 Φ80′ lacZΔM15 gyrA96 recA1 relA1 endA1 | Invitrogen |
| S17-1 λ pir | λ pir lysogen of S17-1 [thi pro hsdR− hsdM+ recA RP4 2-Tc::Mu-Km::Tn7 (Tpr Smr)], permissive host able to transfer suicide plasmids requiring the Pir protein by conjugation to recipient cells | 27 |
| Plasmids | ||
| pGEM-T Easy | High-copy-number TA cloning vector, Ampr | Promega |
| pUT-Km1 | Suicide plasmid requiring the Pir protein for replication and containing a mini-Tn5 cassette, Kmr gene | This study |
| pACYC184-xylE | pACYC184 containing xylE, Cmr | This study |
Rifr, rifampin resistance; Tetr, tetracycline resistance; Kmr, kanamycin resistance; Ampr, ampicillin resistance; Smr, streptomycin resistance; Tpr, trimethoprim resistance; Cmr, chloramphenicol resistance.
Transposon mutagenesis and identification of the mutated gene.
P. mirabilis mini-Tn5 kanamycin (Km) mutagenized mutants were isolated as described previously (27). Biofilm-deficient mutants were identified by the crystal violet staining assay. Chromosomal DNA was extracted from the mutants and partially digested with AluI, and fragments over 4 kb were cloned into SmaI-digested pGEM-4Z (Promega, USA). Following the transformation of E. coli DH5α cells, Km-resistant Tn5 Km-containing clones were isolated. By PCR using I-out and O-out primers (Table 1) and subsequent sequencing, we obtained the nucleotide sequences flanking the Tn5 cassette. The disrupted gene appeared by searching for the sequence we obtained in the P. mirabilis released genome sequence (NC_010554.1). The amino acid sequence comparison was conducted by the BLAST analysis (https://www.ncbi.nlm.nih.gov/).
Biofilm formation assay.
Biofilm analysis was determined as described previously (27), with some modifications. The diluted overnight bacterial cultures were inoculated into wells of polystyrene microtiter plates with or without copper (1 mM) in LB broth. After overnight incubation at 37°C, the optical density at 600 nm (OD600) of bacterial cultures in each well was determined. After being washed and air dried, cells in each well were stained with crystal violet (1%). The crystal violet-stained biofilms were extracted with a mixture of acetone and 95% alcohol (1:4). The OD570 of the extract was determined using a microplate reader. The value of OD570/OD600 representing biofilm formation was calculated to adjust the growth.
zapABCD operon analysis.
Overnight bacterial cultures were diluted 100-fold in LB broth and incubated for 5 h at 37°C. Total RNA was prepared, and reverse transcription was carried out, with one primer annealing to zapD (zapD RT-R in Table 1) and the other to zapB (zapB RT-R in Table 1). Then, the cDNA product was used to amplify the zapA DNA fragment. For control reactions, RNA without reverse transcriptase and genomic DNA were used as the template for negative and positive control, respectively.
Construction of mutants and complemented strains.
Sequences flanking the zapD gene were amplified by PCR using the zapD-KOup-F/zapD-KOup-R and zapD-KOdown-F/zapD-KOdown-R primer pairs (Table 1), and cloned into the pGEM-T Easy (Promega) to generate pGzapDup and pGzapDdn. pGzapDup was digested with SalI/XbaI, and the zapDup fragment was ligated to the SalI/XbaI-digested pGzapDdn to produce the pGzapDupdn plasmid. A kanamycin resistance (Kmr) cassette was inserted to the XbaI-digested pGzapDupdn plasmid to generate pGzapDupdn-Km. The plasmid was cleaved with SalI/SphI, and the Kmr cassette-containing DNA fragment was ligated into SalI/SphI-digested pUT/mini-Tn5 (Km) (27) to generate pUTzapD-Km. Gene inactivation mutagenesis by homologous recombination and confirmation of mutants with double-crossover events were performed as described previously (27). The cpxR and zapA mutants were obtained in the same way using the respective primer pairs (Table 1). For the construction of complemented strains or a zapA-overexpressed strain, we cloned the fragments containing full-length genes amplified by PCR into pGEM-T Easy (Promega). The plasmids were transformed into the respective mutants for complementation or the wild type for overexpression.
Electrophoretic mobility shift assay.
We performed an EMSA using purified His-tagged CpxR and zapA promoter DNA. The zapA promoter fragment was PCR amplified using the SphI-zapA-repo-F/PstI-zapA-repo-R primer pair (Table 1). We incubated the amplified product (0.1 μg) with His-CpxR in a 10-μl binding buffer (150 mM KCl, 10 mM MgCl2, 30 mM Tris-HCl [pH 9]). After incubation for 30 min at room temperature, the reaction mixtures were loaded onto a 5% nondenaturing polyacrylamide gel buffered with Tris-acetate-EDTA, and then the gel was subject to electrophoresis at 100 V for 2 h and subsequent staining with ethidium bromide (EtBr) for observation.
Reporter assay.
For the XylE reporter assay, the promoter DNA fragment of the cpxR, cpxP, zapA, or zapB gene was amplified using primer pairs, with one containing SphI sequence and the other PstI (Table 1), and cloned into the pGEM-T Easy. We digested these promoter-containing plasmids with SphI and PstI, and the promoter-containing fragments were cloned to the xylE-carrying pACYC184 to construct different reporter plasmids. The wild-type, zapD and cpxR mutants, and complemented strains were transformed with respective reporter plasmids, prepared as follows. The promoter DNA fragment of the cpxR, cpxP, zapA, or zapB gene was amplified using primer pairs, with one containing SphI sequence and the other PstI (Table 1), and cloned into pGEM-T Easy. We digested these promoter-containing plasmids with SphI and PstI, and the promoter-containing fragments were cloned to the xylE-carrying pACYC184 to construct the different reporter plasmids. The XylE activity was measured as described previously (20).
Measurement of IgA protease activity.
IgA protease activity was determined as described previously (29). Overnight bacterial cultures (100 μl) were mixed with a 900-μl azocasein solution (0.5% azocasein, 2 mM CaCl2, 50 mM Tris [pH 8.0]) and incubated at 37°C for 24 h, and then trichloroacetic acid was added to 15%. After centrifugation (13,000 rpm, 5 min), the IgA protease activity in the supernatant was determined using a spectrophotometer at 440 nm.
Real-time reverse transcription-PCR.
To study the effect of cpxR, zapD, or zapA loss on the mrpA mRNA level, overnight bacterial cultures of the wild type and mutants were diluted 100-fold in LB broth and incubated for 5 h at 37°C. Total RNA was extracted, and real-time reverse transcription-PCR (RT-PCR) was carried out as described previously (20) to assess the mRNA levels normalized against the gyrB mRNA.
eDNA analysis.
The eDNA amount was determined as described previously (57). We added NaCl (to a concentration of 0.25 M) to overnight bacterial cultures (500 μl) and centrifuged the suspension after mixing. The supernatant was passed through a membrane filter (0.45 mm), and the eDNA in the filtrates was precipitated by a 2-fold volume of 95% ethanol. After centrifugation (13,000 rpm, 3 min), the precipitated eDNA was dissolved in double-distilled water (ddH2O), and the concentration was measured by spectrophotometry at 260 nm.
Unbound exopolysaccharide analysis.
The unbound EPS was determined as described previously (58). Overnight bacterial cultures were diluted 100-fold in CPG broth (1% peptone, 0.1% Casamino Acids, and 1% glucose) and incubated for 24 h at 37°C. Then, bacterial cultures were collected according to the formula OD600 (overnight culture) × volume (in ml) = 5, followed by adjusting the volume of each sample with phosphate-buffered saline (PBS) buffer. After centrifugation (8,000 rpm, 30 min), the unbound polysaccharide present in the culture supernatant was precipitated with a 3-fold volume of absolute ethanol at –20°C overnight. After centrifugation (12,000 rpm, 30 min), the precipitated polysaccharide was dissolved in water. The phenol-sulfuric acid method was used to quantify unbound EPS. After mixing a 200-μl sample with 5% phenol (100 μl) and sulfuric acid (500 μl) and keeping the sample in the dark for 30 min, we measured the unbound EPS by spectrophotometry at 490 nm against the glucose standard (5 to 200 μg/ml) curve.
Preparation and analysis of LPS.
We performed LPS extraction and analysis as described previously (27). Overnight bacterial cultures were subcultured and incubated again for 6 h at 37°C. Equal amounts of cells were washed with a buffer of MOPS [3-(N-morpholino)propanesulfonic acid]-MgSO4. An equal volume of MOPS buffer (20 mM MOPS [pH 6.9])-saturated phenol was added to resuspend the cells, and the suspension was incubated at 65°C for 30 min. The suspension was then kept on ice for 10 min and subjected to centrifugation. The top aqueous phases were collected, and ethanol was added to precipitate LPS. The LPS prepared from equal amounts of wild-type and mutant cells was subjected to the Purpald assay to determine the LPS concentration.
Cell adhesion.
We carried out the cell adhesion assay as described previously (20). Human NTUB1 bladder urothelial cells were cultured on 12-well cell culture plates (Corning) in a 10% fetal bovine serum-containing RPMI 1640 medium (Gibco) to confluence and washed with PBS before use. We centrifuged overnight bacterial cultures and resuspended them in RPMI 1640 medium, and the bacterial culture was applied to each well at the multiplicity of infection (MOI) of 10. Bacterial cells were brought into contact with urothelial cells by centrifugation, followed by incubation for 1 h at 37°C. We then washed the urothelial cells with PBS and lysed them with 1% Triton X-100 to determine the bacterial CFU. The cell adhesion ability was the percentage of viable bacteria that adhered to the NTUB1 cells versus the total inoculum.
Autoaggregation assay.
We performed the autoaggregation assay as described previously (37). After incubation in LB broth at 37°C overnight, we washed bacterial cells twice with a solution of 3 mM NaCl with 0.5 mM CaCl2 and resuspended them in the same solution. We measured the initial OD660 of each sample. Then, the sample was immediately centrifuged at 2,600 rpm for 2 min, and the OD660 of the supernatant was measured. We calculated the aggregation index as follows:
Data availability.
The nucleotide sequence of the zapABCD locus of P. mirabilis N2 has been submitted to GenBank and assigned the accession number MN317030.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants 101-2320-B-002-028 and 102-2320-B002-022 from the Ministry of Science and Technology, Taipei, Taiwan.
We thank Yeong-Shiau Pu (National Taiwan University Hospital) and Chishih Chu for providing NTUB1 cell line and pGFPuv plasmid, respectively. We thank Chih-Chieh Hsu (National Taiwan University, Graduate Institute of Oral Biology, School of Dentistry), who provided assistance in the CLSM examination.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
We disclose no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Foxman B. 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelling H, Nzakizwanayo J, Milo S, Denham EL, MacFarlane WM, Bock LJ, Sutton JM, Jones BV. 2019. Bacterial biofilm formation on indwelling urethral catheters. Lett Appl Microbiol 68:277–293. doi: 10.1111/lam.13144. [DOI] [PubMed] [Google Scholar]
- 4.Stickler DJ. 2014. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med 276:120–129. doi: 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- 5.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez de Aldecoa AL, Zafra O, González-Pastor JE. 2017. Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front Microbiol 8:1390. doi: 10.3389/fmicb.2017.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arciola CR, Campoccia D, Montanaro L. 2018. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 9.Römling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Akerlund B. 2014. Microbial biofilm formation: a need to act. J Intern Med 276:98–110. doi: 10.1111/joim.12242. [DOI] [PubMed] [Google Scholar]
- 10.Otto K, Silhavy TJ. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A 99:2287–2292. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raivio TL. 2014. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim Biophys Acta 1843:1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Vogt SL, Raivio TL. 2012. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett 326:2–11. doi: 10.1111/j.1574-6968.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 13.Debnath I, Norton JP, Barber AE, Ott EM, Dhakal BK, Kulesus RR, Mulvey MA. 2013. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun 81:1450–1459. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol 191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armbruster CE, Mobley HLT, Pearson MM. 8 February 2018, posting date. Pathogenesis of Proteus mirabilis infection. EcoSal Plus doi: 10.1128/ecosalplus.ESP-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. 2010. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun 78:773–782. doi: 10.1128/IAI.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevesinjac AZ, Raivio TL. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J Bacteriol 187:672–686. doi: 10.1128/JB.187.2.672-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leuko S, Raivio TL. 2012. Mutations that impact the enteropathogenic Escherichia coli Cpx envelope stress response attenuate virulence in Galleria mellonella. Infect Immun 80:3077–3085. doi: 10.1128/IAI.00081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armbruster CE, Mobley HL. 2012. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol 10:743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai YL, Chien HF, Huang KT, Lin WY, Liaw SJ. 2017. cAMP receptor protein regulates mouse colonization, motility, fimbria-mediated adhesion, and stress tolerance in uropathogenic Proteus mirabilis. Sci Rep 7:7282. doi: 10.1038/s41598-017-07304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holling N, Lednor D, Tsang S, Bissell A, Campbell L, Nzakizwanayo J, Dedi C, Hawthorne JA, Hanlon G, Ogilvie LA, Salvage JP, Patel BA, Barnes LM, Jones BV. 2014. Elucidating the genetic basis of crystalline biofilm formation in Proteus mirabilis. Infect Immun 82:1616–1626. doi: 10.1128/IAI.01652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norsworthy AN, Pearson MM. 2017. From catheter to kidney stone: the uropathogenic lifestyle of Proteus mirabilis. Trends Microbiol 25:304–315. doi: 10.1016/j.tim.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howery KE, Clemmer KM, Rather PN. 2016. The Rcs regulon in Proteus mirabilis: implications for motility, biofilm formation, and virulence. Curr Genet 62:775–789. doi: 10.1007/s00294-016-0579-1. [DOI] [PubMed] [Google Scholar]
- 24.Peng L, Jiang Q, Pan JY, Deng C, Yu JY, Wu XM, Huang SH, Deng XY. 2016. Involvement of polyphosphate kinase in virulence and stress tolerance of uropathogenic Proteus mirabilis. Med Microbiol Immunol 205:97–109. doi: 10.1007/s00430-015-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’May GA, Jacobsen SM, Longwell M, Stoodley P, Mobley HLT, Shirtliff ME. 2009. The high-affinity phosphate transporter Pst in Proteus mirabilis HI4320 and its importance in biofilm formation. Microbiology 155:1523–1535. doi: 10.1099/mic.0.026500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker KE, Moghaddame-Jafari S, Lockatell CV, Johnson D, Belas R. 1999. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol Microbiol 32:825–836. doi: 10.1046/j.1365-2958.1999.01401.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang SS, Liu MC, Teng LJ, Wang WB, Hsueh PR, Liaw SJ. 2010. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob Agents Chemother 54:1564–1571. doi: 10.1128/AAC.01219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha SP, Pelayo JS, Elias WP. 2007. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol Med Microbiol 51:1–7. doi: 10.1111/j.1574-695X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 29.Fath MJ, Kolter R. 1993. ABC transporters: bacterial exporters. Microbiol Rev 57:995–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassif C, Cheek D, Belas R. 1995. Molecular analysis of a metalloprotease from Proteus mirabilis. J Bacteriol 177:5790–5798. doi: 10.1128/jb.177.20.5790-5798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruna RE, Molino MV, Lazzaro M, Mariscotti JF, García Véscovi E. 2018. CpxR-dependent thermoregulation of Serratia marcescens PrtA metalloprotease expression and its contribution to bacterial biofilm formation. J Bacteriol 200:e00006-18. doi: 10.1128/JB.00006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berne C, Ducret A, Hardy GG, Brun YV. 2015. Adhesins involved in attachment to abiotic surfaces by Gram-negative bacteria. Microbiol Spectr 3:MB-0018-2015. doi: 10.1128/microbiolspec.MB-0018-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das T, Sharma PK, Busscher HJ, van der Mei HC, Krom BP. 2010. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl Environ Microbiol 76:3405–3408. doi: 10.1128/AEM.03119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188:8213–8221. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maunders E, Welch M. 2017. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol Lett 364:fnx120. doi: 10.1093/femsle/fnx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen AM, Lockatell V, Johnson DE, Mobley HL. 2004. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect Immun 72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik A, Sakamoto M, Hanazaki S, Osawa M, Suzuki T, Tochigi M, Kakii K. 2003. Coaggregation among nonflocculating bacteria isolated from activated sludge. Appl Environ Microbiol 69:6056–6063. doi: 10.1128/aem.69.10.6056-6063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López C, Checa SK, Soncini FC. 2018. CpxR/CpxA controls scsABCD transcription to counteract copper and oxidative stress in Salmonella enterica serovar Typhimurium. J Bacteriol 200:e00126-18. doi: 10.1128/JB.00126-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K, Ishihama A. 2005. Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 40.Favre L, Ortalo-Magne A, Kerloch L, Pichereaux C, Misson B, Briand JF, Garnier C, Culioli G. 2019. Metabolomic and proteomic changes induced by growth inhibitory concentrations of copper in the biofilm-forming marine bacterium Pseudoalteromonas lipolytica. Metallomics 11:1887–1899. doi: 10.1039/c9mt00184k. [DOI] [PubMed] [Google Scholar]
- 41.Hunke S, Keller R, Muller VS. 2012. Signal integration by the Cpx-envelope stress system. FEMS Microbiol Lett 326:12–22. doi: 10.1111/j.1574-6968.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Liu F, Peng W, Yan K, Zhao H, Liu T, Cheng H, Chang P, Yuan F, Chen H, Bei W. 2018. The CpxA/CpxR two-component system affects biofilm formation and virulence in Actinobacillus pleuropneumoniae. Front Cell Infect Microbiol 8:72. doi: 10.3389/fcimb.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shetty D, Abrahante JE, Chekabab SM, Wu X, Korber DR, Vidovic S. 2019. Role of CpxR in biofilm development: expression of key fimbrial, O-antigen and virulence operons of Salmonella Enteritidis. Int J Mol Sci 20:5146. doi: 10.3390/ijms20205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matter LB, Ares MA, Abundes-Gallegos J, Cedillo ML, Yáñez JA, Martínez-Laguna Y, De la Cruz MA, Girón JA. 2018. The CpxRA stress response system regulates virulence features of avian pathogenic Escherichia coli. Environ Microbiol 20:3363–3377. doi: 10.1111/1462-2920.14368. [DOI] [PubMed] [Google Scholar]
- 45.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu Rev Microbiol 49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 46.Liu MC, Kuo KT, Chien HF, Tsai YL, Liaw SJ. 2015. New aspects of RpoE in uropathogenic Proteus mirabilis. Infect Immun 83:966–977. doi: 10.1128/IAI.02232-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delepelaire P. 2004. Type I secretion in Gram-negative bacteria. Biochim Biophys Acta 1694:149–161. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Guo S, Vance TDR, Stevens CA, Voets I, Davies PL. 2019. RTX adhesins are key bacterial surface megaproteins in the formation of biofilms. Trends Microbiol 27:453–467. doi: 10.1016/j.tim.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Spitz O, Erenburg IN, Beer T, Kanonenberg K, Holland IB, Schmitt L. 2019. Type I secretion systems-one mechanism for all? Microbiol Spectr 7:PSIB-0003-2018. doi: 10.1128/microbiolspec.PSIB-0013-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Létoffé S, Delepelaire P, Wandersman C. 1990. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J 9:1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riordan JT, Mitra A. 20 June 2017, posting date. Regulation of Escherichia coli pathogenesis by alternative sigma factor N. EcoSal Plus doi: 10.1128/ecosalplus.ESP-0016-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolata KM, Montero IG, Miller W, Sievers S, Sura T, Wolff C, Schluter R, Riedel K, Robinson C. 2019. Far-reaching cellular consequences of tat deletion in Escherichia coli revealed by comprehensive proteome analyses. Microbiol Res 218:97–107. doi: 10.1016/j.micres.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Hathroubi S, Fontaine-Gosselin SE, Tremblay YD, Labrie J, Jacques M. 2015. Sub-inhibitory concentrations of penicillin G induce biofilm formation by field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol 179:277–286. doi: 10.1016/j.vetmic.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Dittmann KK, Porsby CH, Goncalves P, Mateiu RV, Sonnenschein EC, Bentzon-Tilia M, Egan S, Gram L. 2019. Tropodithietic acid induces oxidative stress response, cell envelope biogenesis and iron uptake in Vibrio vulnificus. Environ Microbiol Rep 11:581–588. doi: 10.1111/1758-2229.12771. [DOI] [PubMed] [Google Scholar]
- 55.Macritchie DM, Raivio TL. 2009. Envelope stress responses. EcoSal Plus 3 doi: 10.1128/ecosalplus.5.4.7. [DOI] [PubMed] [Google Scholar]
- 56.Svenningsen NB, Martinez-Garcia E, Nicolaisen MH, de Lorenzo V, Nybroe O. 2018. The biofilm matrix polysaccharides cellulose and alginate both protect Pseudomonas putida mt-2 against reactive oxygen species generated under matric stress and copper exposure. Microbiology 164:883–888. doi: 10.1099/mic.0.000667. [DOI] [PubMed] [Google Scholar]
- 57.Godeke J, Paul K, Lassak J, Thormann KM. 2011. Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. ISME J 5:613–626. doi: 10.1038/ismej.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liaw SJ, Lai HC, Wang WB. 2004. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect Immun 72:6836–6845. doi: 10.1128/IAI.72.12.6836-6845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequence of the zapABCD locus of P. mirabilis N2 has been submitted to GenBank and assigned the accession number MN317030.