In some women, sexually transmitted Chlamydia trachomatis may ascend to infect the endometrium, leading to pelvic inflammatory disease. To identify endometrial innate immune components that interact with Chlamydia, we introduced C. trachomatis into mouse endometrium via transcervical inoculation and compared the infectious yields in mice with and without immunodeficiency. Live C. trachomatis recovered from vaginal swabs or endometrial tissues peaked on day 3 and then declined in all mice with or without deficiency in adaptive immunity, indicating a critical role for innate immunity in endometrial control of C. trachomatis infection.
KEYWORDS: Chlamydia trachomatis, endometrial resistance, innate immunity, innate lymphoid cells
ABSTRACT
In some women, sexually transmitted Chlamydia trachomatis may ascend to infect the endometrium, leading to pelvic inflammatory disease. To identify endometrial innate immune components that interact with Chlamydia, we introduced C. trachomatis into mouse endometrium via transcervical inoculation and compared the infectious yields in mice with and without immunodeficiency. Live C. trachomatis recovered from vaginal swabs or endometrial tissues peaked on day 3 and then declined in all mice with or without deficiency in adaptive immunity, indicating a critical role for innate immunity in endometrial control of C. trachomatis infection. Additional knockout of interleukin 2 receptor common gamma chain (IL-2Rγc) from adaptive immunity-deficient mice significantly compromised the endometrial innate immunity, demonstrating an important role for innate lymphoid cells (ILCs). Consistently, deficiency in IL-7 receptor alone, a common gamma chain-containing receptor required for ILC development, significantly reduced endometrial innate immunity. Furthermore, mice deficient in RORγt or T-bet became more susceptible to endometrial infection with C. trachomatis, suggesting a role for group 3-like ILCs in endometrial innate immunity. Furthermore, genetic deletion of gamma interferon (IFN-γ) but not IL-22 or antibody-mediated depletion of IFN-γ from adaptive immunity-deficient mice significantly compromised the endometrial innate immunity. Finally, depletion of NK1.1+ cells from adaptive immunity-deficient mice both significantly reduced IFN-γ and increased C. trachomatis burden in the endometrial tissue, confirming that mouse ILCs contribute significantly to endometrial innate immunity via an IFN-γ-dependent effector mechanism. It will be worth investigating whether IFN-γ-producing ILCs also improve endometrial resistance to sexually transmitted C. trachomatis infection in women.
INTRODUCTION
Chlamydia trachomatis is a leading cause of sexually transmitted bacterial infections in women (1), and some infected women may develop uterine endometrial infection, leading to complications such as pelvic inflammatory disease (PID) and infertility (2). In order to successfully infect endometrial epithelial cells in the uterus, sexually transmitted C. trachomatis organisms have to both pass through the cervical barrier and overcome antimicrobial mechanisms in endometrium. Thus, both the cervical barrier and endometrial resistance may contribute to host immunity against chlamydial ascending infection.
Although endometrial epithelial cells are the major target cells of the ascending chlamydial organisms, immune cells in both intraepithelial space and lamina propria may regulate epithelial cell susceptibility to chlamydial infection. Innate lymphoid cells (ILCs) have been shown to provide early resistance to microbial infections to prevent microbes from establishing stable colonization in mucosal tissues (3–6). Illuminating the mechanisms by which ILCs restrict bacterial infection is a hot area of investigation (7, 8), since the mechanistic information may be used to improve human health (9). For example, information on whether and how ILCs may contribute to endometrial immunity against chlamydial infection may be useful for developing strategies to enhance endometrial resistance to infection and to attenuate endometrial inflammatory pathologies. Although previous studies showed that conventional natural killer (cNK) cells play important roles in chlamydial infection (10–18), those studies did not differentiate cNK cells from non-NK ILCs. We recently showed that a mutant strain of Chlamydia muridarum was inhibited by ILCs in the mouse colon (19), suggesting that chlamydial intracellular infection can be regulated by ILCs. However, it remains unknown whether and how ILCs can contribute to host defense against genital tract infection with C. trachomatis. Thus, the goal of the current study was to use C. trachomatis infection of the mouse genital tract as a model to investigate whether and how mouse genital ILCs interact with chlamydial organisms.
ILCs consist of both the killer ILCs (also traditionally called cNK cells) and the helper-like ILCs, the latter of which are further categorized into ILC1s, ILC2s, and ILC3s according to their surface molecules and their cytokines and transcriptional factors (20, 21). ILC1s express transcriptional factor T-bet and secrete gamma interferon (IFN-γ) (22), while ILC2s express GATA3 and secrete interleukin 5 (IL-5) and IL-13 (23), and ILC3s express retinoic acid receptor-related orphan receptor γt (RORγt) and secrete IL-22 and IL-17 (24–26). ILC3s are most heterogeneous, with some being positive for CCR6 (defined as lymphoid tissue inducer [LTi]), while others are negative for CCR6. The CCR6-negative ILC3s can be either negative or positive for natural cytotoxicity receptor 1 (NCR1) or NKp46 (27). Furthermore, some ILC3s can be induced by local tissue-derived cytokines, such as IL-12 and/or IL-15, to upregulate T-bet and to secrete IFN-γ (28, 29). The T-bet- and RORγt-coexpressing ILCs are designated ILC3-like or ex-ILC3 (28, 29). Various ILC3-to-ILC1 transition subpopulations have been detected in different mucosal tissues (30). Mouse uterine tissues are known to contain all ILC subtypes in addition to NK cells (31). However, it is not clear whether ex-ILC3s can be induced in mouse genital mucosal tissues in response to C. trachomatis infection. Furthermore, it remains unknown whether and how the endometrial ILCs play any significant roles in controlling sexually transmitted Chlamydia infection.
In the current study, we took advantage of the fact that C. trachomatis infection is inhibited by innate immunity in the mouse genital tract (32) and introduced C. trachomatis directly into mouse endometrium via transcervical inoculation to evaluate the potential roles of ILCs in endometrial innate immunity. We found that live C. trachomatis recovered from vaginal swabs or endometrial tissues peaked on day 3 and then rapidly declined in all mice with or without adaptive immunity, demonstrating that C. trachomatis infection is inhibited by mouse endometrial innate immunity. In addition to lack of adaptive immunity, removal of IL-2 receptor common gamma chain (IL-2Rγc) significantly increased C. trachomatis yields in the endometrial tissues collected on day 3, demonstrating a critical role for ILCs in endometrial innate immunity, since all lymphoid cells, including ILCs, are dependent on IL-2Rγc. Mice deficient in RORγt or T-bet exhibited significantly increased endometrial susceptibility to C. trachomatis, suggesting a role for group 3-like ILCs in endometrial innate immunity, since RORγt is a signature transcriptional factor of ILC3s, and ILC3s can be induced to upregulate T-bet for IFN-γ production. Genetic deletion of IFN-γ from C57BL mice or antibody depletion of IFN-γ from Rag1−/− mice significantly compromised the endometrial innate immunity against C. trachomatis infection. Most importantly, depletion of ILCs from adaptive immunity-deficient mice both significantly reduced IFN-γ and increased C. trachomatis burden in the endometrial tissue. Thus, we have demonstrated that a subset of ILC3s may promote mouse endometrial immunity against C. trachomatis infection via IFN-γ-dependent mechanism. These mouse model-based observations should encourage future studies to investigate whether IFN-γ-producing ILCs improve endometrial resistance to sexually transmitted C. trachomatis infection in women.
RESULTS
Innate but not adaptive immunity inhibits human C. trachomatis in mouse endometrium during the first week following transcervical inoculation.
To determine the contributions of innate versus adaptive immunity to inhibition of C. trachomatis infection along the course of genital tract infection, we compared live C. trachomatis yields recovered from vaginal swabs from mice with and without Rag1 deficiency (Rag1-deficient mice lack adaptive immunity) following transcervical inoculation (Fig. 1). Compared to wild-type C57BL/6J mice, mice lacking adaptive immunity developed an enhanced overall vaginal shedding course (P < 0.05), indicating a significant role for mouse genital adaptive immunity in limiting human chlamydial infection. However, careful comparison of the shedding courses revealed that the difference in the live-organism shedding levels between mice with and without adaptive immunity was mainly restricted to the period from day 10 to 56. Starting on day 10, wild-type mice reduced live-organism titers to a minimal level, although a complete clearance was not achieved until day 56, while adaptive immunity-deficient mice continuously maintained significantly higher levels of live-organism shedding in the same period (P < 0.05). These observations indicated the role of adaptive immunity only during the infection stage when adaptive immunity was fully developed. In contrast, the live-organism shedding courses within the first week were very similar between mice with and without adaptive immunity (P > 0.05). Both groups of mice permitted an initial growth of C. trachomatis, allowing live-organism yields to peak on day 3, followed by steady declining. These observations demonstrate that innate immunity is able to limit C. trachomatis growth in the mouse genital tract within the first week after infection (when adaptive immunity is not fully developed or is genetically defective). This conclusion was further validated by the observation that mice with or without adaptive immunity displayed similar levels of live C. trachomatis organisms in different genital tract tissues harvested on day 3 after transcervical inoculation (Fig. 2).
FIG 1.
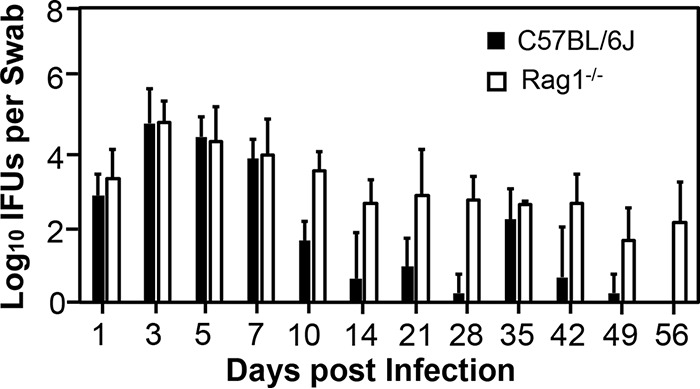
Comparison of live C. trachomatis yields in vaginal swabs between mice with and without adaptive immunity following transcervical inoculation. C57BL/6J mice without (solid bars) or with (open bars) deficiency in adaptive immunity or Rag1 (Rag1−/−) were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and were then monitored for live-organism recovery from the genital tract by taking vaginal swabs on days 1, 3, 5, 7, 10, and 14 and weekly thereafter. The number of live organisms recovered from each swab at each time point was expressed as log10 IFU, and group means and standard deviations are shown. n = 4 for each data point from two independent experiments. P values were >0.05 (Wilcoxon rank sum) between the two groups of mice when IFU from the first 7 days were compared and <0.05 between the two groups when IFU from days 10 to 56 or days 1 to 56 were compared.
FIG 2.
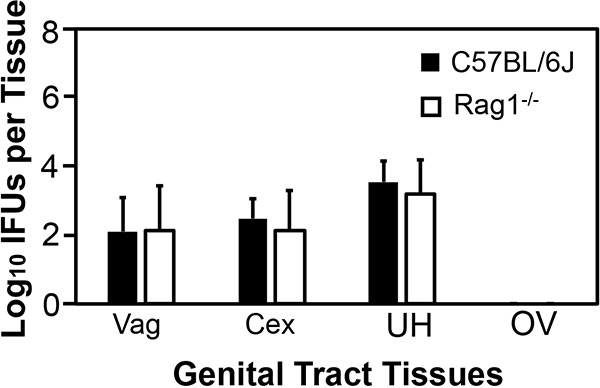
Comparison of live C. trachomatis yields in genital tract tissues between mice with or without adaptive immunity following transcervical inoculation. C57BL/6J mice without (solid bars; n = 7) or with (open bars; Rag1−/−; n = 6) deficiency in adaptive immunity were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation. All mice were monitored for live-organism recovery from the genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct or ovary (OV). The tissues were harvested on day 3 after transcervical inoculation. The number of live organisms recovered from each tissue is expressed as log10 IFU, and group means and standard deviations are shown. Data are from two independent experiments. P was >0.05 (Wilcoxon rank sum test) between C57BL/6J and Rag1−/− mice when IFU from any given tissue were compared.
Innate lymphoid cells contribute to mouse endometrial innate immunity.
To determine whether ILCs play any roles in innate immunity control of C. trachomatis infection in the female mouse genital tract, we compared live C. trachomatis yields between adaptive immunity-deficient mice with and without IL-2 receptor common gamma chain (IL-2Rγc) following transcervical inoculation (Fig. 3). Mice deficient in both Rag2 and IL-2Rγc were highly susceptible to C. trachomatis infection. The live C. trachomatis organism yields recovered from either vaginal swabs or genital tract tissues collected on day 3 were significantly higher in mice deficient in both Rag2 and IL-2Rγc. Significant differences were found in uterus/uterine horn or endometrial tissues, vaginal tissues, and vaginal swabs but not cervix tissue. Since C. trachomatis was directly inoculated into the endometrium via transcervical inoculation, the increased susceptibility in multiple tissues might be due to descending infection caused by the infectious chlamydial organisms from endometrial tissues. Since lymphoid but not myeloid cells are dependent on IL-2Rγc, the above observations have demonstrated critical roles of ILCs in endometrial innate immunity.
FIG 3.
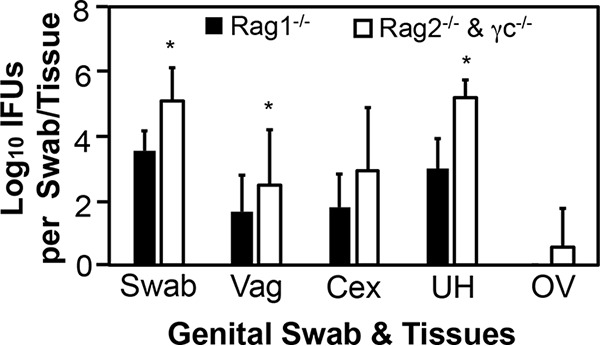
Comparison of live C. trachomatis yields between adaptive immunity-deficient mice with or without IL-2Rγc following transcervical inoculation. Mice deficient in Rag1 (Rag1−/−; solid bars) or in both Rag2 and IL-2Rγc (Rag2−/− & γc−/−; open bars) were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and then monitored for live-organism recovery from both vaginal swabs and genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct or ovary (OV). All swabs and tissues were harvested on day 3 after inoculation. The number of live organisms recovered from each swab or tissue is expressed as log10 IFU, and group means and standard deviations are shown. n = 5 for each data point, and the data are from two independent experiments. *, P < 0.05 (Wilcoxon rank sum test).
Endometrial innate immunity is dependent on IL-7R, RORγt, and T-bet.
Mice deficient in IL-7 receptor, an IL-2Rγc-containing receptor for lymphoid but not myeloid cell development, developed significantly higher yields of live C. trachomatis in endometrium on day 3 (Fig. 4), again confirming a significant role for ILCs in endometrial innate immunity. To further characterize the responsible ILCs, we compared live C. trachomatis yields between mice with and without deficiency in transcription factor T-bet or RORγt following transcervical inoculation (Fig. 5). Mice deficient in either T-bet or RORγt exhibited significantly higher live C. trachomatis yields in endometrial tissue (uterus or uterine horn) collected on day 3 after inoculation. It is worth noting that the increase was more obvious in mice with RORγt deficiency, suggesting that the responsible ILCs are highly dependent on RORγt and that they may belong to the group 3 ILCs.
FIG 4.
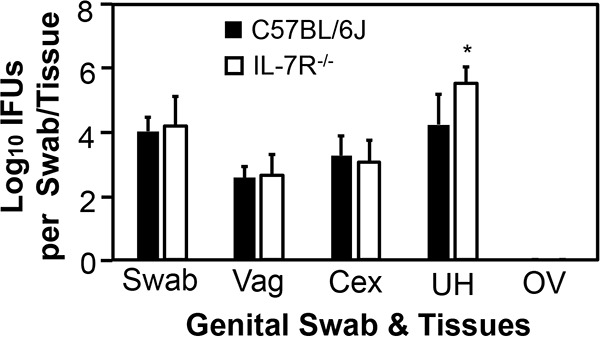
Comparison of live C. trachomatis yields between mice with or without deficiency in IL-7 receptor following transcervical inoculation. Mice without (solid bars) or with (IL-7R−/−; open bars) IL-7 receptor deficiency were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and then monitored for live-organism recovery from both swabs and genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct or ovary (OV). All swabs and tissues were harvested on day 3 after inoculation. The number of live organisms recovered from each swab or tissue is expressed as log10 IFU, and group means and standard deviations are shown. n = 4 for each data point and the data were from two independent experiments. *, P < 0.05 (Wilcoxon rank sum test).
FIG 5.
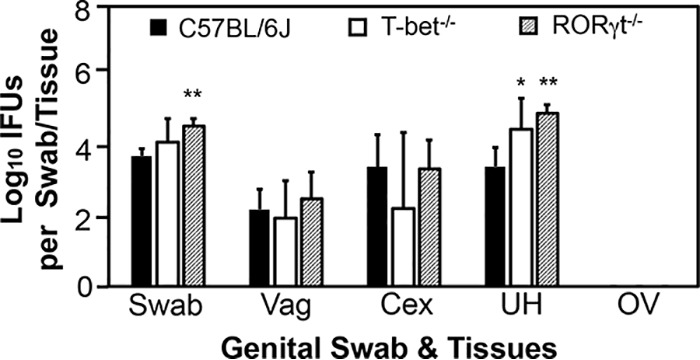
Comparison of live C. trachomatis yields between mice with or without deficiency in T-bet or RORγt following transcervical inoculation. Mice without (solid bars) or with deficiency in transcriptional factors T-bet (T-bet−/−; open bars) or RORγt (RORγt−/−; hatched bars) were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and then monitored for live-organism recovery from both swabs and genital tract tissues, including vagina (Vag), cervix (Cex), uterine/uterine horn (UH), and oviduct/ovary (OV). All swabs and tissues were harvested on day 3 after inoculation. The number of live organisms recovered from each swab or tissue was expressed as log10 IFU, and group means and standard deviations are shown. n = 5 for each data point, and the data are from two independent experiments. *, P < 0.05, and **, P < 0.01, for comparison of the indicated gene-deficient group with the wild-type group (Wilcoxon rank sum test).
Endometrial innate immunity requires IFN-γ but not IL-22.
To determine the effector mechanisms by which ILCs mediate mouse endometrial resistance to C. trachomatis infection, we compared live C. trachomatis yields between wild-type mice and mice deficient in IFN-γ or IL-22 following transcervical inoculation (Fig. 6). Mice with deficiency in IFN-γ but not IL-22 had significantly increased susceptibility to C. trachomatis infection in endometrium, indicating a critical role for IFN-γ but not IL-22 in mouse endometrial innate immunity against C. trachomatis infection. The role of IFN-γ in mediating ILC anti-chlamydial activity was further validated by the observation that depletion of IFN-γ from the adaptive immunity-deficient Rag1−/− mice significantly increased the infectious yields of C. trachomatis in endometrial tissue (Fig. 7). Most importantly, depletion of NK1.1+ cells from Rag1−/− mice both significantly reduced IFN-γ and increased C. trachomatis burden in the endometrial tissue on day 7 after transcervical inoculation (Fig. 8), demonstrating that NK1.1+ ILCs contribute significantly to endometrial innate immunity via an IFN-γ-dependent effector mechanism, and the impact of ILCs on endometrial susceptibility to chlamydial infection is measurable for 7 days after infection.
FIG 6.
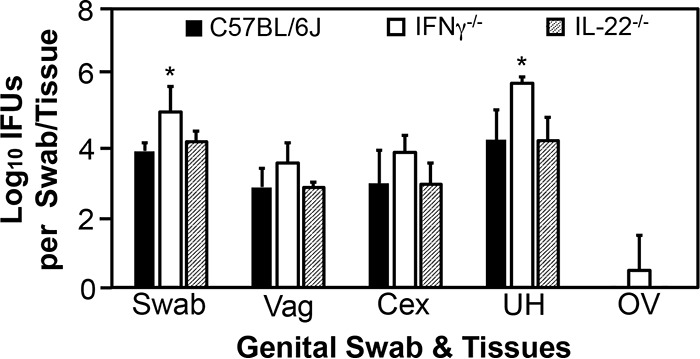
Comparison of live C. trachomatis yields between mice with or without deficiency in IFN-γ or IL-22 following transcervical inoculation. Mice without (solid bars; n = 10) or with (open bars; IFN-γ−/−; n = 4) or IL-22 (hatched bars; IL-22−/−; n = 7) deficiency in IFN-γ were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and then monitored for live-organism recovery from both swabs and genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct/ovary (OV). All swabs and tissues were harvested on day 3 after inoculation. The number of live organisms recovered from each swab or tissue is expressed as log10 IFU, and group means and standard deviations are shown. Data are from two independent experiments. *, P < 0.05 (Wilcoxon rank sum test).
FIG 7.
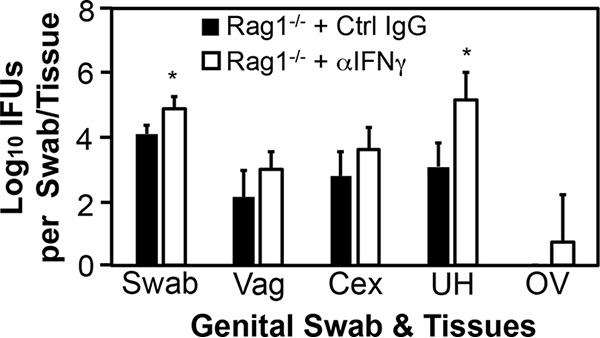
Comparison of live C. trachomatis yields between adaptive immunity-deficient mice with or without depletion of IFN-γ following transcervical inoculation. Adaptive immunity-deficient mice without (Rag1−/− + Ctrl IgG; solid bars) or with (Rag1−/− + αIFN-γ; open bars) depletion of IFN-γ were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation and then monitored for live-organism recovery from both swabs and genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct or ovary (OV). All swabs and tissues were harvested on day 3 after inoculation. The number of live organisms recovered from each swab or tissue is expressed as log10 IFU, and group means and standard deviations are shown. n = 4 for each data point, and data are from two independent experiments. *, P < 0.05 (Wilcoxon rank sum test).
FIG 8.
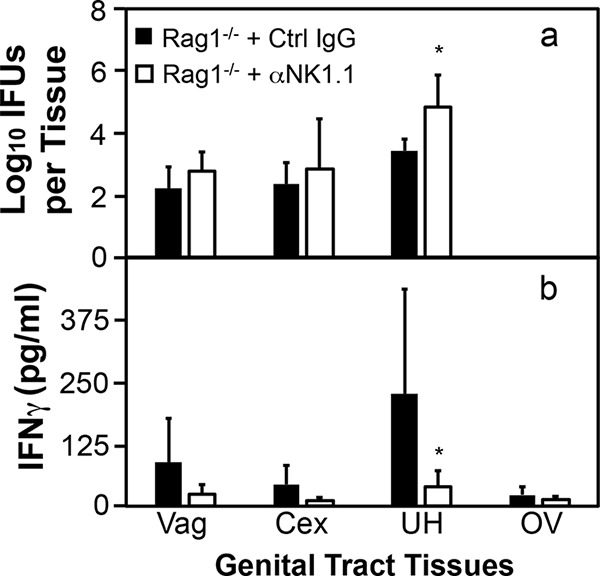
Comparison of live C. trachomatis yields between adaptive immunity-deficient mice with or without depletion of NK1.1+ cells following transcervical inoculation. Adaptive immunity-deficient mice without (Rag1−/− + Ctrl IgG; solid bars) or with (Rag1−/− + αNK1.1; open bars) depletion of NK1.1+ cells were infected with 5 × 106 IFU of C. trachomatis serovar D organisms via transcervical inoculation. The infected mice were then monitored for live-organism recovery (a) and IFN-γ production (b) from genital tract tissues, including vagina (Vag), cervix (Cex), uterus or uterine horn (UH), and oviduct or ovary (OV). All tissues were harvested on day 7 after inoculation. Group means and standard deviations for numbers of live organisms recovered from each tissue (expressed as log10 IFU) and IFN-γ measured from the same tissues are shown. n = 4 to 5 for each data point from two independent experiments. *, P < 0.05 (Wilcoxon rank sum test).
DISCUSSION
Using a series of gene knockout mice in combination with various antibody depletion schemes in C. trachomatis infection of female mouse endometrium model, we have demonstrated a significant role for ILCs in endometrial innate immunity. The experimental evidence has led us to conclude that ILCs are an essential component of endometrial innate immunity. First, live C. trachomatis organisms recovered from mouse genital tracts were reduced after a day 3 peak in the absence of adaptive immunity, indicating a role for innate immunity in restricting C. trachomatis infection. Second, deficiency in IL-2Rγc in adaptive immunity-deficient mice significantly increased mouse endometrial susceptibility to C. trachomatis infection on day 3 following transcervical inoculation, indicating a significant role for ILCs in endometrial innate immunity. This is because IL-2Rγc is required for development of all lymphoid cells, including ILCs. Rag-deficient mice do not have regular lymphocytes but maintain normal ILCs. The increased susceptibility to chlamydial infection in mice deficient in both Rag and IL-2Rγc must be caused by lack of ILCs. Third, C. trachomatis replication is rescued in the endometrial tissue of C57 mice deficient in only IL-7R. IL-7R is required for differentiation and maturation of all lymphoid cells but not myeloid cells. Thus, even on a wild-type mouse background, the role of lymphoid cells in endometrial resistance to chlamydial infection can be demonstrated. Since the resistance was measured on day 3 (3 days is too short a time for naive mice to develop adaptive immunity), we can attribute this resistance to innate lymphoid cells. Fourth, mice deficient in either RORγt or T-bet had significantly lower endometrial innate immunity against chlamydial infection, suggesting that the responsible ILCs may belong to a subset of ILC3s. Fifth, genetically deleting IFN-γ from C57BL/6J mice or antibody-mediated depletion of IFN-γ from adaptive immunity-deficient mice significantly compromised endometrial resistance to human chlamydial infection, suggesting that the responsible ILC3s may be induced to secrete IFN-γ for their contribution to endometrial innate immunity. Finally, depletion of NK1.1+ cells from adaptive immunity-deficient mice both significantly reduced IFN-γ and increased C. trachomatis yields in the endometrium, demonstrating that NK1.1+ ILCs can inhibit chlamydial infection in mouse endometrium via an IFN-γ-mediated effector mechanism. The mechanistic information may be useful in guiding the design of future clinical studies.
Although cNK cells were shown in previous studies to play important roles in innate immunity against chlamydial infection in both the airway and the genital tract (10–18), those studies did not attempt to differentiate cNK cells from other ILCs. Thus, the relative contribution of distinct ILC subsets to the mouse genital tract innate immunity against chlamydial infection remains unclear. The use of RORγt knockout mice in the current study allowed us to differentiate cNK from non-cNK cells, since it is well established that cNK cells are independent of RORγt and they are defined as NK1.1+ RORγt− cells (33, 34). Although NK1.1+ RORγt+ cells have been identified from intestinal lamina propria, these cells are LTi-like ILC3 cells and derived from a non-NK lineage (28). We have now demonstrated that endometrial resistance to chlamydial infection is highly dependent on RORγt, indicating a critical role for ILC3s in endometrial innate immunity. Furthermore, the endometrial immunity is mediated by innate IFN-γ, since depletion of IFN-γ from Rag1−/− mice (adaptive immunity-deficient mice) significantly reduced the endometrial immunity. The innate IFN-γ is likely produced by NK1.1+ RORγt+ ILC3s, since depletion of NK1.1+ cells from Rag1−/− mice both blocked IFN-γ production and increased endometrial susceptibility to chlamydial infection. Although NK1.1+ RORγt+ ILC3s have been shown to produce IL-22 (33, 34), these cells may also be able to produce IFN-γ if they are induced to differentiate into so-called ex-ILC3s. We propose that NK1.1+ RORγt+ ILC3s may be induced to coexpress T-bet to produce IFN-γ by endometrial tissue cytokines in response to chlamydial infection. It is worth noting that although ex-ILC3s are frequently detected in the gastrointestinal tract, it is not clear whether these cells can be induced in the genital tract. Thus, the current study has provided the first experimental evidence suggesting that transcervical inoculation with C. trachomatis may be able to induce ex-ILC3s in the female genital tract mucosal tissue. C. trachomatis infection of endometrial epithelial cells may stimulate endometrial myeloid cells to secrete cytokines IL-12 and/or IL-15. These cytokines may induce ILC3s to differentiate into ex-ILC3s for IFN-γ secretion. This hypothesis is consistent with our recent observation that C. muridarum colonization may be able to induce ILC3s to secrete IFN-γ in the mouse colon (19). ILC3s have been shown to play significant roles in protecting the epithelial barrier against bacterial infection (29). Efforts are under way to further characterize the female genital tract ILC3 activation pathways and functional properties.
IFN-γ but not IL-22 is sufficient for significantly reducing C. trachomatis infection in female mouse endometrium on day 3 after transcervical infection, demonstrating a critical role for IFN-γ as an effector in mediating endometrial innate immunity. Although it has been shown that C. trachomatis infection can be restricted by innate immunity in the mouse genital tract (32), the nature of the innate immunity is still unknown. The observations from the current study suggest that IFN-γ-secreting ILC3s may be a critical component of the innate immunity. Nevertheless, more studies may be required for fully demonstrating the role of IFN-γ-secreting ILC3s in endometrial innate immunity. It will be necessary to determine whether IFN-γ-producing RORγt+ ILC3s can be detected in the genital tissues of wild-type mice but not mice deficient in IFN-γ or RORγt. These IFN-γ-producing RORγt+ ILC3s can be further isolated for adoptive-transfer experiments. The transfer experiments will address whether these ILC3s are sufficient for mounting endometrial innate immunity in recipient mice deficient in IFN-γ or RORγt+ ILC3s. However, it has been difficult for us to achieve these goals due to a lack of sufficient IFN-γ-producing RORγt+ ILC3s that can be isolated from the female mouse genital mucosae. Although isolation of ILC3s from the gastrointestinal (GI) tract tissues is routine (35), the same protocol has not enabled us to acquire sufficient number of ILC3s from the female genital tract tissue. There may be multiple reasons for the difficulty. A primary reason may be the limited number of ILC3s available from the genital tract. This is because genital tract lumen is much less enriched in microbiota species than the lumen of the GI tract. Since mucosal ILC3s are known to play critical roles in maintaining the mucosal barrier function (36), fewer lumenal microbes may require or induce fewer ILC3s for preventing lumenal microbes from undergoing systemic spreading in the genital tract. Nevertheless, we are still making efforts to optimize the protocols for collecting sufficient IFN-γ-producing RORγt+ ILC3s for flow cytometry analysis and, furthermore, as donor cells for adoptive-transfer experiments.
It is worth noting that in Rag1−/− mice (lacking adaptive immunity), the C. trachomatis infectious yields recovered from vaginal swabs collected between days 10 and 56 after infection were always lower than those recovered from day 7. This observation suggests that innate immunity in Rag1−/− mice can maintain constant inhibition of C. trachomatis in the mouse genital tract. It will be interesting to investigate the mechanisms of the long-lasting innate immunity and to determine whether the same IFN-γ-producing ILC3s identified in the current study also contribute to the long-lasting innate immunity. Perry et al. previously correlated major histocompatibility (MHC) class II-independent IFN-γ production with inhibition of C. trachomatis serovar D in mouse genital tracts on day 18 after infection (37). It will be worth testing whether the MHC class II-independent IFN-γ is produced by ILCs.
The murine model has been extremely useful in understanding chlamydial immunology and pathogenesis. When C. muridarum, the chlamydial species that is highly adapted to mice, is used to infect the mouse genital tract, it readily overcomes the mouse innate immunity and induces robust pathology in the upper genital tract, similar to the pathology caused by C. trachomatis in women (38–41). Thus, the C. muridarum model is suitable for investigating chlamydial pathogenic mechanisms in the upper genital tract but not for investigating innate immune mechanisms. In contrast, when C. trachomatis, the species that is isolated from humans and not adapted to mice, is used to infect the mouse genital tract, it is significantly restricted by mouse innate immunity (32). Thus, in the current study, we took advantage of this phenotype to probe the innate immune components that are activated by and responsible for interacting with Chlamydia in the genital tract. We found that ILCs contribute to the endometrial innate immunity by secreting IFN-γ. This conclusion has been confirmed in multiple experimental settings, including C57BL/6J mice genetically deficient in IFN-γ and Rag1−/− mice depleted of IFN-γ or IFN-γ-producing ILCs. The next step is to test whether IFN-γ-producing ILCs can also promote endometrial resistance to C. trachomatis infection in women.
MATERIALS AND METHODS
Chlamydia and cell culture.
Wild-type Chlamydia trachomatis serovar D stock was initially obtained from Harlan Caldwell’s lab (42). The stocking organisms were amplified and passaged in HeLa cells (human cervical carcinoma epithelial cells; ATCC CCL-2) for 3 generations, and the amplified organisms were purified into elementary bodies (EBs) using discontinuous density centrifugation as previously described (43). The purified EBs were stored in aliquots at −80°C until use.
Mouse infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals endorsed by the National Institutes of Health (44). The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Mice used in the current study were 5- to 7-week-old female mice from Jackson Laboratories, Inc. (Bar Harbor, ME), as follows: wild-type mice (C57BL/6J; stock no. 000664) or mice deficient in IFN-γ (B6.129S7-Ifngtm1Ts/J [IFN-γ−/−]; no. 002287), recombination activating gene 1 (B6.129S7-Rag1tm1Mom/J [Rag1−/−]; no. 002216), recombination activating gene 2 plus interleukin 2 (IL-2) receptor common gamma chain (C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J [Rag2−/− γc−/−]; no. 014593), IL-7 receptor (B6.129S7-Il7rtm1Imx/J [IL-7R−/−]; no. 002295), T-bet (B6.129S6-Tbx21tm1Glm/J [T-bet−/−]; no. 004648), or RORγt (B6.129P2-Rorctm1Litt/J [RORγt−/−]; no. 007571).
All mice were infected with C. trachomatis EBs at 5 × 106 inclusion-forming units (IFU) per mouse via transcervical or intrauterine inoculation as described previously (45). Briefly, EBs were diluted in 10 μl of SPG (220 mM sucrose, 12.5 mM phosphate, 4 mM l-glutamic acid; pH 7.5) buffer and delivered directly into the uterus using a tube designed for transcervical inoculation (NSET60010; Braintree Scientific, Inc., Braintree, MA). After inoculation, mice were monitored for live organism shedding in vaginal swabs or sacrificed for titrating live organisms in designated organs and tissues.
Mouse treatments.
In some experiments, mice were treated with a rat anti-IFN-γ antibody (clone XMG1.2, IgG1/κ; Bio X Cell, West Lebanon, NH) or a mouse anti-NK1.1 antibody (clone PK136, IgG2a/κ; Bio X Cell). The control groups were similarly treated with rat IgG (code number 012-000-002) or normal mouse IgG (code number 015-000-002; both from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The antibody depletion treatment was carried out in Rag1−/− for assessing the roles of innate IFN-γ and NK1.1+ in controlling C. trachomatis infection in female mouse genital tract. The treatment began 5 days prior to transcervical infection (day −5), followed by treatments on days −3 and −1, day 1 after infection, and every other day thereafter. Each injection was administered intraperitoneally with 500 μg of IgG in 200 μl of phosphate-buffered saline (PBS) as described previously (46).
Titrating live chlamydial organisms from mouse swabs and tissues.
For monitoring live-organism shedding from genital tract, vaginal swabs were taken on days 1, 3, 5, 7, and 10 after infection and weekly thereafter. Each swab was soaked in 0.5 ml of SPG buffer and mixed in a vortex mixer with glass beads to release infectious EBs for quantitation. For titrating live chlamydial organisms recovered from mouse genital tract tissues, tissue segments, including vagina, cervix, uterus or uterine horn, and oviduct or ovary, or as indicated for individual experiments, were harvested on designated days after inoculation, as specified for individual experiments. Each tissue segment was transferred to a tube containing 0.5 ml cold SPG buffer for homogenization and sonication. After centrifugation, the released live organisms in the supernatants were titrated on HeLa cells in duplicate as described previously (47). The total number of IFU per swab or tissue was converted to a log10 value for calculating the group mean and standard deviation at each time point. The detection limits of this titration method are 10 IFU per swab or tissue sample. This is because we always used 50 μl (from the total volume of 500 μl), starting with neat tissue homogenates without dilution followed by serial dilutions for a given sample. We counted the entire culture well for chlamydial inclusions when the inclusion density was low, allowing the detection of a single IFU per 50 μl.
Immunofluorescence assay.
An immunofluorescence assay was used for titrating live organisms as described previously (48). Briefly, HeLa cells grown on coverslips were fixed with paraformaldehyde (Sigma) and permeabilized with saponin (Sigma). After washing and blocking, the cell samples were subjected to a combination of antibody and chemical staining. Hoechst (blue; Sigma) was used to visualize nuclear DNA. A rabbit antichlamydial antibody (raised by immunization with serovar D EBs [data not shown]) and a goat anti-rabbit IgG conjugated with Cy2 (green; Jackson ImmunoResearch Laboratories, Inc.) were used to visualize chlamydial inclusions. The immunolabeled cultures were used for counting inclusions under an Olympus IX-81 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY).
Measurement of cytokines in mouse tissues using ELISA.
The enzyme-linked immunosorbent assay (ELISA) kit for measuring mouse IFN-γ from genital tissues was purchased from R&D Systems, Inc. (Minneapolis, MN; catalog no. MIF00). The tissues homogenized in SPG buffer were added with a protease inhibitor cocktail (catalog no. 78430, 100× stock; Thermo Fisher Scientific, Waltham, MA) at a 2× final concentration. The neat homogenates were 2-fold serially diluted with PBS containing 2× inhibitor cocktail, and the diluted samples were applied to 96-well plates precoated with a capture antibody. IFN-γ binding was detected with a detection antibody plus detection reagent. The absorbance at 450 nm was detected with a Synergy H4 microplate reader (BioTek, Winooski, VT), and the results were expressed as picograms per sample based on the standard curve obtained from the same plate.
Statistics.
The numbers of live organisms, expressed as IFU counts or as IFN-γ concentration in picograms per sample, were compared between two groups using a Wilcoxon rank sum test. Where there were more than two groups in a given study, analysis of variance (ANOVA) was performed, followed by a Wilcoxon rank sum test to further define the difference between any two groups. The Wilcoxon rank sum test has been used to compare both nonparametric and parametric data (49).
ACKNOWLEDGMENTS
This work was supported in part by grants from the U.S. National Institutes of Health (R01AI121989 and R01AI047997 to G.Z.) and Natural Science Foundation of China (31670178 to J.C.). H.X. is a recipient of the UT-Xiangya Medical Student Exchange Program.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2017. Sexually transmitted disease surveillance, 2016. U.S. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- 2.Brunham RC, Gottlieb SL, Paavonen J. 2015. Pelvic inflammatory disease. N Engl J Med 372:2039–2048. doi: 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenberg GF, Artis D. 2012. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37:601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britanova L, Diefenbach A. 2017. Interplay of innate lymphoid cells and the microbiota. Immunol Rev 279:36–51. doi: 10.1111/imr.12580. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos JG, Longman RS. 2020. Innate lymphoid cells link gut microbes with mucosal T cell immunity. Gut Microbes 11:231–236. doi: 10.1080/19490976.2019.1638725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim M, Kim CH. 2016. Colonization and effector functions of innate lymphoid cells in mucosal tissues. Microbes Infect 18:604–614. doi: 10.1016/j.micinf.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M. 2017. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brussow H. 2019. Probiotics and prebiotics in clinical tests: an update. F1000Res 8:F1000 Faculty Rev-1157. doi: 10.12688/f1000research.19043.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onsrud M, Qvigstad E. 1984. Natural killer cell activity after gynecologic infections with chlamydia. Acta Obstet Gynecol Scand 63:613–615. doi: 10.3109/00016348409155547. [DOI] [PubMed] [Google Scholar]
- 11.Williams DM, Schachter J, Grubbs B. 1987. Role of natural killer cells in infection with the mouse pneumonitis agent (murine Chlamydia trachomatis). Infect Immun 55:223–226. doi: 10.1128/IAI.55.1.223-226.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CT, Rank RG. 1998. Role of NK cells in early host response to chlamydial genital infection. Infect Immun 66:5867–5875. doi: 10.1128/IAI.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottenberg ME, Gigliotti Rothfuchs A, Gigliotti D, Ceausu M, Une C, Levitsky V, Wigzell H. 2000. Regulation and role of IFN-gamma in the innate resistance to infection with Chlamydia pneumoniae. J Immunol 164:4812–4818. doi: 10.4049/jimmunol.164.9.4812. [DOI] [PubMed] [Google Scholar]
- 14.Shekhar S, Peng Y, Gao X, Joyee AG, Wang S, Bai H, Zhao L, Yang J, Yang X. 2015. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. Eur J Immunol 45:2810–2820. doi: 10.1002/eji.201445390. [DOI] [PubMed] [Google Scholar]
- 15.Hu VH, Luthert PJ, Derrick T, Pullin J, Weiss HA, Massae P, Mtuy T, Makupa W, Essex D, Mabey DC, Bailey RL, Holland MJ, Burton MJ. 2016. Immunohistochemical analysis of scarring trachoma indicates infiltration by natural killer and undefined CD45 negative cells. PLoS Negl Trop Dis 10:e0004734. doi: 10.1371/journal.pntd.0004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radomski N, Franzke K, Matthiesen S, Karger A, Knittler MR. 2019. NK cell-mediated processing of Chlamydia psittaci drives potent anti-bacterial Th1 immunity. Sci Rep 9:4799. doi: 10.1038/s41598-019-41264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim MJW, Rajagopalan S, Altmann DM, Boyton RJ, Sun PD, Long EO. 2019. Human NK cell receptor KIR2DS4 detects a conserved bacterial epitope presented by HLA-C. Proc Natl Acad Sci U S A 116:12964–12973. doi: 10.1073/pnas.1903781116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Gao X, Bai H, Joyee AG, Wang S, Yang J, Zhao W, Yang X. 2019. The important role of dendritic cell (DC) in iNKT-mediated modulation of NK cell function in Chlamydia pneumoniae lung infection. Mediators Inflamm 2019:4742634. doi: 10.1155/2019/4742634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koprivsek JJ, He Y, Song C, Zhang N, Tumanov A, Zhong G. 2019. Evasion of innate lymphoid cells-regulated IFNgamma responses by Chlamydia muridarum to achieve long-lasting colonization in mouse colon. Infect Immun 88:e00798-19. doi: 10.1128/IAI.00798-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl G, Colonna M, Di Santo JP, McKenzie AN. 2015. Innate lymphoid cells: a new paradigm in immunology. Science 348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H. 2018. Innate lymphoid cells: 10 years on. Cell 174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Y, Huntington ND, Belz GT, Seillet C. 2016. Type 1 innate lymphoid cell biology: lessons learnt from natural killer cells. Front Immunol 7:426. doi: 10.3389/fimmu.2016.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duerr CU, Fritz JH. 2016. Regulation of group 2 innate lymphoid cells. Cytokine 87:1–8. doi: 10.1016/j.cyto.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Killig M, Glatzer T, Romagnani C. 2014. Recognition strategies of group 3 innate lymphoid cells. Front Immunol 5:142. doi: 10.3389/fimmu.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montaldo E, Juelke K, Romagnani C. 2015. Group 3 innate lymphoid cells (ILC3s): origin, differentiation, and plasticity in humans and mice. Eur J Immunol 45:2171–2182. doi: 10.1002/eji.201545598. [DOI] [PubMed] [Google Scholar]
- 26.Withers DR, Hepworth MR. 2017. Group 3 Innate lymphoid cells: communications hubs of the intestinal immune system. Front Immunol 8:1298. doi: 10.3389/fimmu.2017.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng B, Shi S, Ashworth G, Dong C, Liu J, Xing F. 2019. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis 10:315. doi: 10.1038/s41419-019-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, Honig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. 2010. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klose CSN, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. 2013. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature 494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 30.Cella M, Gamini R, Secca C, Collins PL, Zhao S, Peng V, Robinette ML, Schettini J, Zaitsev K, Gordon W, Bando JK, Yomogida K, Cortez V, Fronick C, Fulton R, Lin LL, Gilfillan S, Flavell RA, Shan L, Artyomov MN, Bowman M, Oltz EM, Jelinsky SA, Colonna M. 2019. Subsets of ILC3-ILC1-like cells generate a diversity spectrum of innate lymphoid cells in human mucosal tissues. Nat Immunol 20:980–991. doi: 10.1038/s41590-019-0425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, Brosens JJ, Moffett A, Colucci F. 2015. Composition, development, and function of uterine innate lymphoid cells. J Immunol 195:3937–3945. doi: 10.4049/jimmunol.1500689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturdevant GL, Caldwell HD. 2014. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog Dis 72:70–73. doi: 10.1111/2049-632X.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E. 2009. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 34.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. 2009. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo X, Muite K, Wroblewska J, Fu Y-X. 2016. Purifiaction and adoptive transfer of group 3 gut innate lymphoid cells Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 36.Panda SK, Colonna M. 2019. Innate lymphoid cells in mucosal immunity. Front Immunol 10:861. doi: 10.3389/fimmu.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry LL, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell HD. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol 162:3541–3548. [PubMed] [Google Scholar]
- 38.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P Am J Obstet Gynecol 203:494.e7–494.e14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner C, Caldwell HD, Carlson JH, Graham MR, Kari L, Sturdevant GL, Tyler S, Zetner A, McClarty G. 2015. Chlamydia trachomatis virulence factor CT135 is stable in vivo but highly polymorphic in vitro. Pathog Dis 73:ftv043. doi: 10.1093/femspd/ftv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay S, Clark AP, Sullivan ED, Miller RD, Summersgill JT. 2004. Detailed protocol for purification of Chlamydia pneumoniae elementary bodies. J Clin Microbiol 42:3288–3290. doi: 10.1128/JCM.42.7.3288-3290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 45.Tang L, Yang Z, Zhang H, Zhou Z, Arulanandam B, Baseman J, Zhong G. 2014. Induction of protective immunity against Chlamydia muridarum intracervical infection in DBA/1j mice. Vaccine 32:1407–1413. doi: 10.1016/j.vaccine.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin H, He C, Koprivsek JJ, Chen J, Zhou Z, Arulanandam B, Xu Z, Tang L, Zhong G. 2019. Antigen-specific CD4(+) T cell-derived gamma interferon is both necessary and sufficient for clearing Chlamydia from the small intestine but not the large intestine. Infect Immun 87:e00055-19. doi: 10.1128/IAI.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2017. Nonpathogenic colonization with Chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman J. 2015. Biostatistics for practitioners: an interpretative guide for medicine and biology. Academic Press, New York, NY. [Google Scholar]


