Trogocytosis is part of an emerging, exciting theme of cell-cell interactions both within and between species, and it is relevant to host-pathogen interactions in many different contexts. Trogocytosis is a process in which one cell physically extracts and ingests “bites” of cellular material from another cell. It was first described in eukaryotic microbes, where it was uncovered as a mechanism by which amoebae kill cells. Trogocytosis is potentially a fundamental form of eukaryotic cell-cell interaction, since it also occurs in multicellular organisms, where it has functions in the immune system, in the central nervous system, and during development.
KEYWORDS: cell death, complement, Entamoeba, Francisella, macrophages, neutrophils, phagocytosis, Trichomonas, trogocytosis
ABSTRACT
Trogocytosis is part of an emerging, exciting theme of cell-cell interactions both within and between species, and it is relevant to host-pathogen interactions in many different contexts. Trogocytosis is a process in which one cell physically extracts and ingests “bites” of cellular material from another cell. It was first described in eukaryotic microbes, where it was uncovered as a mechanism by which amoebae kill cells. Trogocytosis is potentially a fundamental form of eukaryotic cell-cell interaction, since it also occurs in multicellular organisms, where it has functions in the immune system, in the central nervous system, and during development. There are numerous scenarios in which trogocytosis occurs and an ever-evolving list of functions associated with this process. Many aspects of trogocytosis are relevant to microbial pathogenesis. It was recently discovered that immune cells perform trogocytosis to kill Trichomonas vaginalis parasites. Additionally, through trogocytosis, Entamoeba histolytica acquires and displays human cell membrane proteins, enabling immune evasion. Intracellular bacteria seem to exploit host cell trogocytosis, since they can use it to spread from cell to cell. Thus, a picture is emerging in which trogocytosis plays critical roles in normal physiology, infection, and disease.
INTRODUCTION
Trogocytosis (trogo-: nibble) is an underappreciated theme in eukaryotic biology that is gaining ground (Fig. 1) (1, 2). In this process, one cell physically extracts and ingests “bites” of cellular material from another cell. Trogocytosis contrasts with phagocytosis (phago-: devour), where one cell ingests another cell in its entirety. Trogocytosis has been distinguished from other mechanisms for cell-cell exchange, such as nanotubes or exosomes, by its requirement for direct contact between living cells (3–5), its fast time frame (3, 6), and its transfer of intact proteins (7, 8). Since the underlying molecular mechanism has not been fully defined, it is not clear if all examples of trogocytosis that have been described represent the same, conserved molecular process, or if they represent multiple distinct mechanisms. If trogocytosis is a unified molecular process, it is likely to be fundamental to eukaryotic biology, as it is seen in at least three supergroups.
FIG 1.
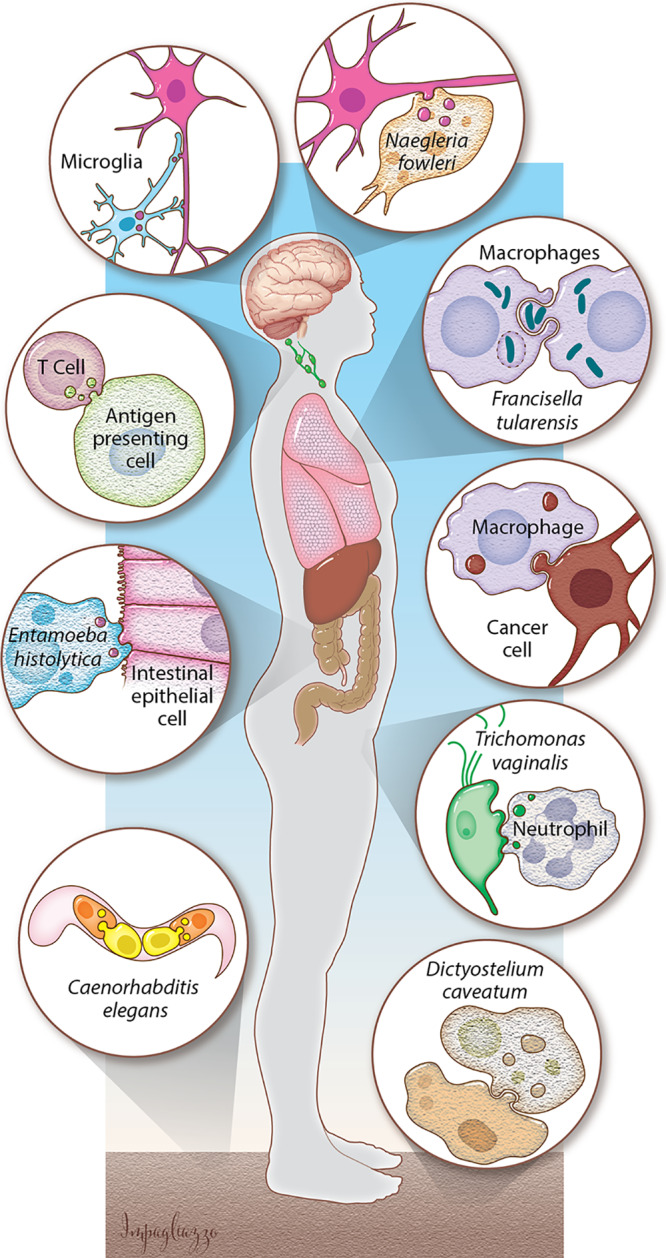
Trogocytosis is a broad, developing concept. In the central nervous system, microglia use trogocytosis to remodel neuronal synapses, and the parasite N. fowleri kills human cells through trogocytosis. Immune cells take bites out of other human cells. Bacteria such as F. tularensis exploit trogocytosis/merocytophagy to spread between cells. Macrophages can perform trogocytosis to kill antibody-opsonized cells. E. histolytica kills human cells by performing trogocytosis. Neutrophils kill T. vaginalis through trogocytosis. Primordial germ cells in C. elegans are nibbled by endodermal cells. D. caveatum kills other Dictyostelium species through trogocytosis. (Courtesy of Anita Impagliazzo, reproduced with permission.)
Trogocytosis was first described in microbes in the late 1970s to mid-1980s, where microbes were seen using trogocytosis to attack and kill other cells (9–12). Later, trogocytosis was seen between mammalian immune cells. Since the early 2000s (3, 13, 14), trogocytosis by immune cells has been actively studied. In immune cells, trogocytosis has been characterized as a benign form of cell-cell interaction, without cell death (3, 15). Within the last 5 years, trogocytosis has expanded broadly. Trogocytosis has now been detected in many different cell types, including cells of the nervous system (16) and embryonic cells (17). Its functions have broadened to include remodeling of one cell by another (16, 17), cell-cell spread of intracellular bacteria (18), and killing of microbes by immune cells (19). Trogocytosis can result in display of acquired membrane proteins by the nibbling cell, a process that can enable microbial immune evasion when acquired host proteins are displayed (20). In light of these recent paradigm changes, here we will discuss the wide-ranging biology of trogocytosis, its underlying mechanism, the display of membrane proteins acquired through trogocytosis, and the major outstanding questions about this process.
BIOLOGY OF TROGOCYTOSIS
Trogocytosis is used by microbes for cell killing.
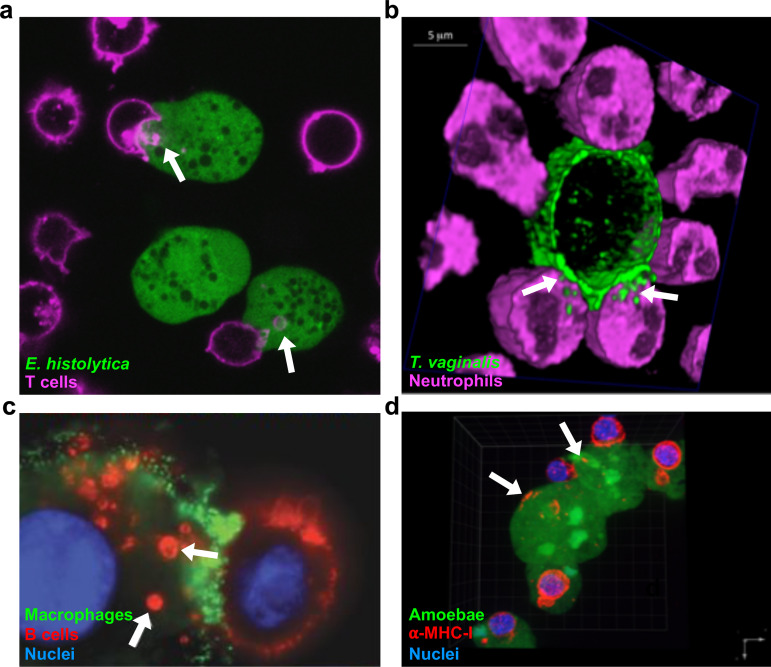
Trogocytosis was first described in eukaryotic microbes, where it was uncovered as a mechanism by which amoebae kill other eukaryotic cells. However, it has been studied in only a few microbes, and the molecular details are limited. The “brain-eating” amoeba Naegleria fowleri appears to kill mammalian cells by nibbling them (9). The term “trogocytosis” was coined for the first time to describe this process (9). It was later shown that the predatory soil amoeba Dictyostelium caveatum kills D. discoideum by “nibbling” (10). In addition to these studies, there are descriptions of pathogens, including Acanthamoeba and Hartmannella, that nibble on host cells (11, 12). More recently, it was shown that Entamoeba histolytica performs trogocytosis to kill human cells (Fig. 2a) (21). Trogocytosis was required for invasion of explanted mouse intestinal tissue by E. histolytica, suggesting relevance to pathogenesis (21).
FIG 2.
Examples of trogocytosis within and between species. (a) E. histolytica kills human cells through trogocytosis. E. histolytica is stained with cell tracker green, and human Jurkat T cell membranes are stained with DiD (pink). Arrows, ingested bites. (b) Neutrophils kill T. vaginalis through trogocytosis. T. vaginalis membranes are stained with streptavidin-488 (green), and neutrophils are stained with cell tracker deep red (pink). Arrows, ingested bites. (c) Macrophages can perform trogocytosis to kill antibody-opsonized cells. Macrophages are stained with anti-CD45 (green), Raji B cells are opsonized with trastuzumab (red), and nuclei are stained with Hoechst stain (blue). Arrows, ingested bites. (d) E. histolytica acquires and displays human cell membrane proteins through trogocytosis. E. histolytica is stained with cell tracker green, human anti-MHC-I is shown in red, and nuclei are stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Arrows, acquired MHC-I. (Reprinted from references 20, 41, and 96 with permission.)
While all examples of trogocytosis by microbes involve amoebae, it is important to recognize that amoebae are not a phylogenetic group. “Amoeba” is a morphology that is found in many branches of the eukaryotic tree. The amoebae that perform trogocytosis belong to several eukaryotic supergroups, supporting the idea that trogocytosis may be fundamental to eukaryotes.
Trogocytosis is used for cell-cell communication and cell killing in the immune system.
(i) Immune cells use trogocytosis for cell-cell communication and cell signaling. In multicellular organisms, trogocytosis was first seen in mammalian immune cells, where nibbling occurs at the immunological synapse (3). This was characterized by the transfer of cell membrane proteins from one cell to another (13). In the immune cell literature, the term “trogocytosis” has been used broadly, making it not entirely clear if different studies describe the same process. Some studies have simply defined trogocytosis as the acquisition of membrane and membrane proteins from another cell, without resolving the subcellular localization, while other studies have defined trogocytosis as the internalization of material acquired from another cell. Here we will refer to immune cell trogocytosis in both of these ways that it has been defined.
Instead of a cell-killing mechanism, immune cell trogocytosis has historically been described as a benign form of cell-cell communication (3, 15) that can serve to modulate the immune response (14). Protein transfer between immune cells was initially detected in studies that used major histocompatibility complex (MHC)-mismatched mice (22, 23), and other studies suggested that antigen could be transferred from macrophages to lymphocytes (24, 25). Later, the transfer of MHC-I molecules from antigen-presenting cells to T cells was observed, and acquisition of MHC-I peptide complexes was linked to T cell fratricide, suggesting that trogocytosis could modulate the immune response (14). Many groups reported transfer of antigen and plasma membrane proteins from donor cells to T cells (6, 26–28). In the early 2000s, this process was named trogocytosis (13), making this the second time that the term trogocytosis was coined, following the original definition of the term in studies of N. fowleri (9). Since then, trogocytosis has been seen in T cells, B cells (29), NK cells (30), dendritic cells (31), macrophages (32), neutrophils (5), and basophils (4), and the transfer of many different types of molecules has been reported.
It is not entirely clear which cellular components are transferred during immune cell trogocytosis. The accepted view is that only membrane and membrane proteins are transferred, without intracellular components. This is based on a few studies that used fluorescent cytoplasmic dyes and flow cytometry and that did not detect cytoplasm transfer (6, 8). Microscopy would be a more sensitive assay, although even with microscopy, cytoplasm transfer is more difficult to detect than membrane transfer (21). Thus, the use of sufficiently bright cytoplasmic markers, together with microscopy, would be the best way to resolve this issue. Supporting the idea that cytoplasm might be transferred, recent microscopy data appear to show neutrophils acquiring cytoplasmic calcein dye during trogocytosis (33). Likewise, cytoplasmic bacteria spread between macrophages through a process that resembles trogocytosis, which results in transfer of bacteria together with cytoplasmic calcein dye and cell membrane (18). Finally, transfer of carboxyfluorescein succinimidyl ester (CFSE)-labeled cytoplasmic proteins from herpes simplex virus (HSV)-infected monocyte-derived dendritic cells to plasmacytoid dendritic cells has been observed (34). Membrane proteins were also transferred, consistent with trogocytosis (34). More studies are needed to resolve which cellular components are transferred, but it appears likely that immune cell trogocytosis involves the transfer of intracellular components.
(ii) Neutrophils use trogocytosis to kill parasites. Although immune cell trogocytosis has historically been thought of as a benign form of cell-cell interaction, it has become clear that it can also be used for cell killing. A recent study revealed that neutrophils can perform trogocytosis to kill parasites (Fig. 2b) (19). Neutrophils killed Trichomonas vaginalis in a dose- and contact-dependent manner (19). Canonical mechanisms by which neutrophils kill microbes include phagocytosis, secretion of antimicrobial peptides, and release of neutrophil extracellular traps (NETs) (35). Surprisingly, these mechanisms were not involved in the killing of T. vaginalis (19). Instead, neutrophils surrounded T. vaginalis parasites and killed them by taking bites. Interestingly, neutrophils performed trogocytosis to nibble live parasites and performed phagocytosis to engulf dead parasites (19). This is similar to E. histolytica, which nibbles live human cells and performs phagocytosis to engulf dead human cells (21). The discovery of neutrophil trogocytosis adds a new weapon to the arsenal of neutrophil cell-killing mechanisms and shows that trogocytosis is relevant to infection.
(iii) Macrophages and neutrophils use trogocytosis to kill cancer cells. Trogocytosis by macrophages and neutrophils has recently been linked to cell killing in the context of antibody therapy for cancer. The general principle of antibody therapy is that binding of antibodies to the surface of a cancer cell can directly downregulate growth factors or lead to cancer cell death via several mechanisms: cell-mediated cytotoxicity, complement-dependent cytotoxicity, or phagocytosis (36). Trogocytosis has a known role in interfering with antibody therapy, since nibbling can remove both antigens and therapeutic antibodies such as anti-CD20 (e.g., the leukemia treatment rituximab) from the cancer cell surface, allowing the cancer cell to evade therapy (37, 38). This has been called “shaving.” Dosing regimens have been developed to attempt to minimize the detrimental shaving effect of trogocytosis (39, 40).
In contrast to the detrimental effects of trogocytosis, in which therapeutic antibodies are removed, new studies have shown that trogocytosis can also result in cancer cell death. Three-dimensional microscopy approaches revealed that macrophages kill trastuzumab antibody-opsonized HER2-breast cancer cells through trogocytosis (Fig. 2c) (41). Increasing the IgG1 affinity for the Fcγ receptor (FcγR) caused higher levels of trogocytosis and cell death, supporting that cell killing was dependent on binding of the therapeutic antibody by the macrophage FcγR (41). In another key study, Kupffer cells, specialized macrophages in the liver, killed invariant natural killer (iNKT) cells through trogocytosis (42). Kupffer cells grabbed and ripped the trailing edge of iNKT cells that moved over them, causing iNKT cell death (42). Further experimentation showed that iNKT opsonization with the antibody CXCR3-173 was necessary for cell killing, together with iNKT movement and Kupffer cell FcγR (42). This was described as antibody-dependent fragmentation since the cell fragments were potentially larger than most immune cell trogocytosis bites (42), but there is no clear size cutoff that specifically defines trogocytosis. Together, these studies show that various kinds of macrophages can perform trogocytosis to kill cancer cells.
Neutrophils also engage trogocytosis to kill cancer cells (33). Killing of cancer cells by neutrophils required an antibody such as trastuzumab, together with CD11b/CD18 interaction (33). Conjugate formation was independent of the CD47-SIRPα “don’t-eat-me” signal that is overexpressed on cancer cells, and blocking the CD47-SIRPα interaction enhanced conjugate formation (33). The proportion and accumulation of trogocytosis events correlated with the lytic or necrotic cell death of antibody-opsonized cancer cells. That study used the term “trogoptosis” to refer to trogocytosis that results in cell death (33).
Trogocytosis is used to remodel cells in the nervous system.
Trogocytosis has expanded beyond the immune system, and the known functions of trogocytosis are also broadening. Moving beyond cell-cell communication and cell killing, new examples of trogocytosis in the nervous system and during embryonic development have added cellular remodeling to the repertoire of trogocytosis.
(i) Microglia use trogocytosis to remodel synapses. In the nervous system, microglia shape and prune neuronal cells through trogocytosis (16). Microglia are motile glial cells that remodel neuronal synapses to create the mature synaptic connections (43). Microglia were previously thought to remodel synapses by using phagocytosis (43). In a study that used correlative light and electron microscopy (CLEM) techniques, microglia were seen directly contacting dendritic spines and ingesting presynaptic structures (16). Using this technique, the spine encapsulations that were previously thought to involve phagocytosis were recognized to be apposition events, rather than ingestion events (16). When ingestion occurred, the small size of the ingested material was consistent with trogocytosis, rather than phagocytosis. Time-lapse imaging further showed that trogocytosis occurred briefly and rapidly and required contact with filopodia (dendritic membrane protrusions) (16).
(ii) Astrocytes use trogocytosis to remodel axons. Beyond microglia, there are examples of apparent cell nibbling by astrocytes, which are central nervous system glial cells (44). Astrocytes have been shown to nibble parts of neurons in the myelination transition zone (45). Astrocytes ingested bites containing mitochondria from retinal ganglion cell axon protrusions in the optic head nerve (46). These mitochondria were further digested in a mitophagy-independent manner within the Lamp1+ lysosome of the astrocyte, as seen through terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) and MitoFISH (46). Interestingly, astrocytes capable of ingesting axon protrusions were seen throughout the central nervous system, hinting that cell nibbling might occur in other sites, beyond the myelination transition zone (46). Astrocytes are also crucial to shortening the myelinated axons of the Xenopus laevis optic nerve during late metamorphosis (47). The entrapment of myelin protrusions that have been seen is morphologically similar to trogocytosis, and expressing dominant negative forms of genes involved in astrocyte phagocytosis caused deficits in myelin clearance (47). Taking these examples together, astrocytes appear to perform cell nibbling to remodel the size and organelle composition of neurons.
Trogocytosis is used to remodel cells during embryonic development.
Trogocytosis has also been found to play a role in cellular remodeling during embryonic development in Caenorhabditis elegans and X. laevis. Primordial germ cells attach to intestinal precursor cells for proper gastrulation (48). In C. elegans, these primordial germ cells develop “lobes,” which later disappear in a manner that suggests they have been nibbled (49). Through confocal microscopy, the neighboring endodermal cells were found to nibble and ingest the lobes. Through the removal of these lobes/bites, the primordial germ cells became remodeled, since the number of mitochondria, cell body volume, and cellular composition were changed (17). Interestingly, the mitochondria removed from primordial germ cells were oxidant rich (17). Thus, trogocytosis may allow primordial germ cells to dispense with organelles that are damaging or no longer needed.
During X. laevis gastrulation, endodermal cells were shown to move in an amoeboid-like manner and to elongate and have undulating membranes (50). Interestingly, the formation of double-membraned vesicles also occurred and culminated in the retraction, remodeling, and reabsorption of the trailing edge of the endodermal cell (50). This process involving ingestion of cellular material by another cell has been interchangeably called both macropinocytosis and transendocytosis, and it resembles trogocytosis morphologically. Together, the examples in C. elegans and X. laevis show that endodermal cells, a cell type previously not linked to ingestion, have an important role in performing trogocytosis for development of gastrulating cells.
Trogocytosis is exploited by intracellular pathogens.
Fitting with its potentially fundamental role in eukaryotic biology, intracellular pathogens exploit trogocytosis. Francisella tularensis and Salmonella enterica serovar Typhimurium reside in the macrophage cytoplasm and can transfer from one macrophage to another through trogocytosis (18, 51). In this scenario, plasma membrane, cytoplasm, and live bacteria from an infected cell were transferred to a new cell via a bite of ingested material (18). After trogocytosis occurred, bacteria resided in double-membraned vesicles that contained both donor and recipient cell membranes, and the bacterial type VI secretion system was required for escape from this compartment (51). The process was initially referred to as trogocytosis (18) and was subsequently renamed “merocytophagy” (51). The authors proposed that while trogocytosis might involve recycling of acquired material, merocytophagy involves trafficking of acquired material for endocytic degradation. Since both F. tularensis and S. Typhimurium can spread from cell to cell through merocytophagy/trogocytosis, it is possible that this may apply more broadly to other infections.
Eukaryotic intracellular pathogens also engage trogocytosis. Red blood cells infected with Plasmodium falciparum transferred membrane material and malaria antigens to endothelial cells in an actin-dependent manner (52). This increased the immune response to endothelial cells and opened endothelial cell intercellular junctions, both of which have potentially detrimental implications for cerebral malaria (52).
THE MOLECULAR MECHANISM UNDERLYING TROGOCYTOSIS
Despite its widespread occurrence, the molecular mechanism underlying trogocytosis has not yet been well defined in any organism. Without an established molecular mechanism, it not clear if the wide variety of cell nibbling scenarios that have been described are all examples of the same, conserved molecular process. Since it is not clear if there is a single, unified, underlying trogocytosis molecular mechanism, here we will organize our discussion of the molecular mechanism by cell types and organisms.
The molecular mechanism underlying trogocytosis in multicellular organisms.
(i) Trogocytosis versus phagocytosis.
It is presently unclear how much of the trogocytosis mechanism is distinct from the phagocytosis mechanism. It is possible that trogocytosis is essentially failed phagocytosis, where a bite is ingested instead of an entire cell. However, when a macrophage fails to perform phagocytosis because the target is too large, it does not ingest bites. Rather, the macrophage attempts to surround the target in a futile process called “frustrated phagocytosis” (53, 54). Therefore, when phagocytosis fails due to the excessive size of a target cell, trogocytosis is not the outcome. If trogocytosis does represent a failure of phagocytosis in other scenarios, it would likely necessitate the use of scission machinery in order to physically extract a bite from a live target cell, an act that is likely to require mechanical force. Thus, even if trogocytosis does represent an outcome of failed phagocytosis, it is still likely to require a scission mechanism that is not a normal feature of phagocytosis. Fitting with this idea, and outlined in detail below, trogocytosis requires proteins involved in membrane bending and scission (17, 55) and a small GTPase (56), none of which normally have roles in engulfment and internalization of target cells during phagocytosis.
Trogocytosis does not represent a random failure of phagocytosis, as it occurs in specific situations. In some organisms, trogocytosis is performed to nibble live cell targets, while phagocytosis is performed to engulf dead cell targets (19, 21). As outlined below, expression of engineered receptors can induce macrophages to nibble on target cells that they would normally ingest through phagocytosis (57). Expression of these receptors in nonprofessional phagocytes can induce them to perform trogocytosis (57), further supporting that trogocytosis not simply a failure of a phagocyte to fully ingest a target. Potential distinctions between trogocytosis and phagocytosis are highlighted in more detail in the sections that follow.
(ii) The phagocytosis mechanism in immune cells.
Phagocytosis by immune cells provides a general starting point for discussion of the mechanism of trogocytosis. The molecular mechanism of phagocytosis is the most well studied in the case of FcγR-mediated phagocytosis (58). Once opsonized target cells are bound by FcγR, clustering of FcγR triggers their intracellular phosphorylation by Src kinases (59). Phosphorylated FcγR then recruits Syk kinase, leading to the activation of lipid-modifying enzymes (e.g., phosphatidylinositol 3-kinase [PI3K] and phospholipase C), kinases (e.g., protein kinase C [PKC]), and small GTPases (e.g., Rac and Cdc42), resulting in actin reorganization and pseudopod extension (59). The phagocytic cup extends to surround the target cell until the target is fully engulfed and ultimately contained within a phagosome (58). Finally, actin is depolymerized, and the phagosome matures into a phagolysosome for degradation of its contents (60).
(iii) The trogocytosis mechanism in immune cells.
In general, immune cell trogocytosis requires cell-cell contact mediated by receptor-ligand interactions, actin, and PI3K. In specific cell types, roles for TC21, RhoG, Src, Syk intracellular calcium, and myosin light-chain kinase have also been defined. All of these proteins are also involved in phagocytosis, with the exception of TC21.
immune cell trogocytosis is initiated either by the formation of the immunological synapse or by engagement of Fcγ receptors (Fig. 3a). Trogocytosis in T cells, B cells, and natural killer cells occurs with formation of the immunological synapse (7, 61). Cytotoxic lymphocytes acquire antigenic peptides and plasma membrane fragments from target cells through engagement of the T cell receptor (6), and the T cell receptor then becomes internalized after acquisition of antigen (14). Trogocytosis has also been described in models where phagocytes recognize antibody-coated target cells through engagement of their Fcγ receptors (41, 62).
FIG 3.
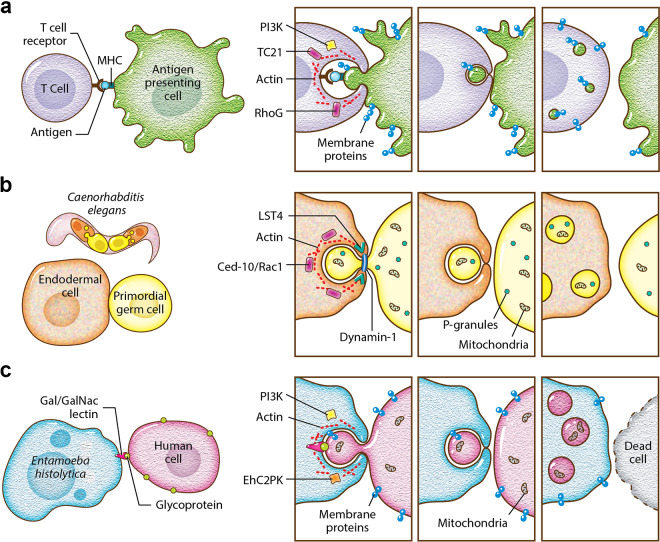
The molecular mechanism underlying trogocytosis. (a) T cell trogocytosis. T cell receptors engage with antigen bound by MHC. The small GTPases TC21 and RhoG play roles in trogocytosis, along with PI3K and actin. Membrane proteins from the antigen-presenting cell are ultimately displayed on the T cell. The cells separate and remain viable. (b) C. elegans endodermal cell trogocytosis. The small GTPase CED-10/Rac1 plays a role in trogocytosis, along with actin, Lst-4/SNX9, and dynamin-1. Lst-4/SNX9 has a role in membrane bending, and dynamin-1 has a role in membrane scission. Some P-granules and mitochondria are removed from the primordial germ cell. The cells separate without cell death, and after trogocytosis, the primordial germ cell is smaller and contains fewer P-granules and mitochondria. (c) E. histolytica trogocytosis. Glycoproteins on the human cell surface are engaged by the Gal/GalNAc lectin. The kinase EhC2PK plays a role in trogocytosis, together with PI3K and actin. Membrane proteins from the human cell are ultimately displayed on the amoeba. The cells separate once the human cell is killed. (Courtesy of Anita Impagliazzo, reproduced with permission.)
TC21 and RhoG appear to be required for T cell trogocytosis, and only RhoG has a known role in phagocytosis (56). This conclusion is based on a study that found that T cells were capable of phagocytosis of latex beads along with internalization of the T cell receptor. Ingestion of beads required actin, PI3K, TC21, and RhoG (Fig. 3a) (56). The molecules that were required for bead ingestion were inferred to be required for trogocytosis, and consistent with this, trogocytosis of membrane fragments and MHC-II molecules was dependent on PI3K, TC21, and RhoG (56). Actin along with Src, Syk, and PI3K have all been implicated in T cell trogocytosis (29). Similarly, actin, Src, and Syk were involved in MHC-II transfer from dendritic cells to basophils (4).
There is potentially a role for receptor engagement in activating either trogocytosis or phagocytosis (57). A family of chimeric antigen receptors were engineered to direct macrophages to perform phagocytosis (CAR-Ps). These CAR-Ps had an extracellular antibody fragment that recognized target cell antigens and an intracellular signaling domain, such as the intracellular domain from Megf10 or FcγR, that contained immunoreceptor tyrosine-based activation motifs (ITAMs) phosphorylated by Src family kinases (57). These CAR-Ps caused macrophages to primarily perform trogocytosis, instead of phagocytosis, to ingest target cells. Enrichment of phosphotyrosine at the synapse between cells increased trogocytosis of target cells. Expression of CAR-Ps in nonprofessional phagocytes, like fibroblasts, led them to nibble (57). These results suggest that specific ligand interactions are important for the initiation of either trogocytosis or phagocytosis (57).
When immune cells use trogocytosis as a cell-killing mechanism, it appears to require the same machinery as benign immune cell trogocytosis. Neutrophil trogoptosis (killing of cancer cells via neutrophil trogocytosis) required a reduction in CD47-SIRPα interaction, together with CD11b/CD18 conjugate formation (33). Additionally, Syk, intracellular calcium, PI3K, and myosin light-chain kinase were required (33). During neutrophil killing of T. vaginalis, neutrophil serine proteases were involved in trogocytosis but not phagocytosis, potentially working together with granules (19). Anti-human iC3b and anti-human immunoglobulin bound to parasites, indicating a role for human serum components such as antibodies and complement (19). Additionally, blocking the neutrophil FcγR inhibited killing of T. vaginalis, underscoring that killing was dependent on FcγR engagement (19).
(iv) The trogocytosis mechanism in embryonic development.
As outlined below, there is new evidence for membrane bending and scission activities during embryonic trogocytosis. Dynamin and Lst-4 (SNX9) can deform and pinch membranes (63, 64). They have known roles during phagocytosis, after the target has been fully engulfed, where they aid in phagosome sealing and maturation (65, 66). In contrast, in newly defined roles in embryonic trogocytosis, these proteins localize to the site where a bite of material is being pinched (the “neck”), and they are required for excision and internalization of nibbled material (17, 55).
Aspects of the mechanism underlying Eph/ephrin trogocytosis have been defined. During development, cellular rearrangements such as repulsion (50, 67, 68) can be mediated by the removal of adhesive receptor-ligand Eph/ephrin complexes. These complexes can be internalized and effectively removed through trogocytosis, which has also been called transendocytosis (50). Internalization of Eph/ephrin through trogocytosis required Src/Tyr signaling (69), Rac GTPases, and the guanine nucleotide exchange factor Tiam2 (70). More recently, it was shown that Gulp-1 (CED-6) regulates Eph/ephrin trogocytosis (55). Gulp-1 is involved in recognizing and engulfing apoptotic cells (71), a well-established phagocytosis pathway in C. elegans. Cells lacking Gulp1 directly contacted each other and did not disengage, which decreased trogocytosis (55). Gulp-1 was recruited to Eph/ephrin clusters by Tiam2, and it further recruited the GTPase dynamin-2 for membrane scission and extraction of bites (55).
It appears that trogocytosis during C. elegans embryonic development follows a similar model. During trogocytosis of C. elegans primordial germ cells, Rac1, dynamin-1, and Lst-4 (SNX9) were required for removal and scission of bites (Fig. 3b) (17). Lst-4 is a sorting nexin, containing a lipid-binding PX domain and a BAR domain that functions in membrane bending (64). Thus, this fits with the model that includes membrane scission via dynamin that was established in the Eph/ephrin model and adds membrane-bending activities to this model. Rac1 induces actin polymerization, and in C. elegans, Rac1 has a role in trogocytosis that is independent from its role in phagocytosis (17). Rac1 is one of the players in Eph/ephrin trogocytosis (55), further linking the C. elegans and Eph/ephrin models.
The same working model may also hold true in X. laevis embryonic trogocytosis. In X. laevis, trogocytosis was linked with the endocytosis-associated Rab5 GTPase (72), as well as ephrin, which both accumulate in membrane clusters and localize to the trailing end of moving endodermal cells (50). Ephrin was also found in double-membraned vesicles, consistent with the Eph/ephrin model of trogocytosis (50). Additionally, injection of dominant negative Gulp-1 in the X. laevis gastrula significantly changed its normal developmental shape, pointing toward Gulp-1 regulation of endoderm migration and Eph/ephrin-dependent membrane uptake (50).
(v) The trogocytosis mechanism in the nervous system.
Microglia use trogocytosis to remodel neuronal synapses. In this process, there is a hint of a mechanism distinct from phagocytosis. Complement signaling is known to promote ingestion by microglia (73). Interestingly, mice without the complement receptor CR3 had no deficit in microglial trogocytosis, indicating that this pathway may have a specific role in phagocytosis and is not required for microglial trogocytosis (16).
Trogocytosis by astrocytes shares features with that by immune cells and embryonic cells. In order to perform trogocytosis of axonal protrusions with mitochondria, astrocytes upregulated the known phagocytic marker Mac2 (45), which requires stable γ-synuclein. Astrocytes involved in X. laevis myelination shortening during development expressed Rac1 to perform trogocytosis (47). Rac1 has a known role in phagocytosis and, as outlined above, is linked to trogocytosis during embryonic development. Astrocyte expression of Mfge8, a protein associated with immune cell phagocytosis (74), was also important in demyelination (47).
The molecular mechanism underlying trogocytosis in microbes.
Distinctions between trogocytosis and phagocytosis in microbes have been proposed but are not yet fully clear. Trogocytosis in N. fowleri involves actin (9). Beyond this information from N. fowleri, essentially all of the mechanistic studies of microbial trogocytosis have been carried out in E. histolytica.
E. histolytica performs trogocytosis to nibble live human cells and, in contrast, performs phagocytosis to engulf dead human cells (21). Since E. histolytica is capable of performing both trogocytosis and phagocytosis, it presents a useful model to compare and contrast these processes. In E. histolytica, both processes require actin, signaling initiated by the Gal/GalNAc lectin, PI3K, and the kinase EhC2PK (Fig. 3c) (21). It has been suggested that the kinase EhAGCK1 is specific to E. histolytica trogocytosis, while EhAGCK2 is involved in trogocytosis, phagocytosis, and pinocytosis (75). However, that study did not directly test for a trogocytosis defect (75) and used a human cell-killing assay that is confounded by amoebic protease activity (76). The EhAGCK1 knockdown mutants used in the study were generated using an approach that affects the expression of off-target genes (77). It has been suggested that E. histolytica phosphatidylinositol 3-phosphate (PI3P) membrane glycerophospholipid-binding proteins such as Vsps and SNXs might be involved in trogocytosis and phagocytosis (78). Further work is still needed to determine which mechanisms are specific to E. histolytica trogocytosis.
Inhibition of E. histolytica lysosome acidification led to a reduction in trogocytosis, which was consistent with an impairment in continued ingestion of human cell material (79). Phagocytosis was also inhibited, suggesting that lysosomes play a general role in both processes. Inhibition of amoebic cysteine proteases with E-64 resulted in a specific defect in trogocytosis and not phagocytosis (80). This fits with the finding that E-64 treatment inhibited human cell killing by E. histolytica (81). It is unclear if cysteine proteases play a specific role in degradation of material ingested during trogocytosis or if trogocytosis is especially sensitive to cysteine protease inhibition, as it appears to occur with faster kinetics than phagocytosis (20), and thus, ingested material may be trafficked to the lysosome more rapidly.
ANOTHER LAYER TO TROGOCYTOSIS: MEMBRANE PROTEIN DISPLAY
A feature of trogocytosis that has, until recently, only been described in immune cells is the transfer of membrane proteins from the donor cell membrane to the recipient cell membrane. This process modulates the immune response by allowing cells to take on and display new molecules. The mechanism of membrane protein transfer is still mostly unclear; however, in T cells it appears to be initiated at the immunological synapse.
Membrane protein display by immune cells.
Dendritic cells can acquire intact peptide-MHC complexes from other cells via trogocytosis and present them to lymphocytes (82) in a process termed “cross-dressing” (83, 84). Dendritic cells that acquire peptide-MHC complexes through trogocytosis are able to present them and stimulate T cells (15, 31). Acquisition of membrane proteins via trogocytosis can also suppress the immune response in the context of transplantation (85) and has also been well documented in regulatory T cells (86, 87). Acquisition of MHC-II molecules seems to enhance the suppressive activity of regulatory T cells through lymphocyte activation gene 3 (88, 89). Finally, acquisition of membrane proteins via trogocytosis appears to be a driver of the TH2 immune response. CD4+ T cells that performed trogocytosis were associated with a TH2 phenotype (90). Similarly, basophils that acquired peptide–MHC-II complexes via trogocytosis were able to stimulate peptide-specific naive CD4+ T cells in vitro, in a manner consistent with a TH2 phenotype (4).
Membrane protein display and complement evasion by E. histolytica.
Extending membrane protein display beyond immune cells, E. histolytica acquires and displays human cell membrane proteins after performing trogocytosis (Fig. 2d) (20). Amoebic display of human cell membrane proteins was quantitatively inhibited when amoebae were treated with cytochalasin D, consistent with a requirement for trogocytosis (20). This suggests that the acquisition and display of membrane proteins potentially constitutes a conserved feature of trogocytosis.
The display of human cell membrane proteins by E. histolytica may impact many host-pathogen interactions. After performing trogocytosis, E. histolytica was protected from lysis by human serum (20). Protection was specific to trogocytosis, as it required actin and direct cell-cell contact, and amoebae were not protected after performing phagocytosis (20). The molecular mechanism by which amoebae become protected from complement by displaying human cell proteins is not yet clear. It is possible that complement regulatory proteins are displayed by amoebae and directly provide protection. Multiple factors may act together to protect amoebae from complement. Amoebic cysteine proteases have a role in cleavage of complement components (91–93), and the heavy chain of the Gal/GalNAc lectin can act as a CD59 mimic (94). Since trogocytosis occurs in other microbes, this strategy, in which acquired membrane proteins contribute to immune evasion, could potentially apply to other infections.
MAJOR QUESTIONS
Taking together the many diverse examples of trogocytosis in different organisms and scenarios, there are many outstanding questions about this process. A central question is the identity of the mechanism underlying trogocytosis. There are clearly shared features between trogocytosis and phagocytosis but also emerging hints that aspects of the trogocytosis mechanism are distinct. How does a cell “decide” to initiate trogocytosis or phagocytosis? Many cells are capable of both trogocytosis and phagocytosis, such as E. histolytica, neutrophils, or mammalian macrophages. There may be roles for receptor-ligand interactions, since expression of engineered receptors can induce macrophages to perform trogocytosis of target cells that they would normally ingest via phagocytosis (57). Additionally, in some cases, trogocytosis and phagocytosis are differentially performed during the ingestion of live and dead cells (19, 21), which have different surface ligands.
What is (or isn’t) ingested during trogocytosis? An overall theme is that cell membrane is transferred from one cell to another during trogocytosis. Additionally, in many different scenarios, cytoplasm, mitochondria, and other organelles are also transferred (9, 17, 21, 46). In immune cell trogocytosis, it is generally accepted that only cell membrane is transferred, but empirical evidence is likely insufficient to rule out the transfer of other cellular components. Conversely, in many cases, nuclei are not ingested during trogocytosis (11, 21). Is the acquisition and display of membrane proteins a universal feature of trogocytosis? While this occurs in immune cells and E. histolytica, it has not yet been investigated in other systems.
An almost totally unexplored area is the response of the cell that has been nibbled. Why does trogocytosis only sometimes result in cell death? This is especially relevant to cell types that are capable of nibbling with or without killing, such as macrophages and neutrophils. Are additional factors, such as toxins, needed to kill cells through trogocytosis? Since cell killing via trogocytosis requires direct contact, toxins would need to be specifically targeted to the cell that is being nibbled, or they could be generally secreted but result in the death of only cells that have been nibbled. In E. histolytica, there is a very large amount of polymerized actin at the site of interaction between the amoeba and the human cell (21), making secretion of toxins at this site less likely. When cells are killed by nibbling, why do they die? Is a cell death pathway activated, or do nibbled cells die due to the accumulation of physical damage? In some studies, it is clear that the nibbled cell initially retains membrane integrity and eventually loses membrane integrity after many bites have been taken, potentially because the nibbled cell has become too damaged to be repaired (9, 21). Conversely, when cells are not killed by trogocytosis, how do they retain cellular integrity? Fitting with the idea that cellular repair pathways might be activated in nibbled cells, an influx of extracellular calcium occurs in human cells nibbled by E. histolytica (21), and calcium influx is a trigger of plasma membrane repair (95).
SUMMARY
Trogocytosis is a broad, rapidly developing theme that is relevant to eukaryotic biology in general and to human biology, from normal physiology to disease. Trogocytosis applies to host-pathogen interactions in many contexts, including pathogens that nibble host cells, pathogens that acquire and display host proteins, bacteria that subvert trogocytosis, and pathogens that are attacked and killed by host trogocytosis. Given its apparently fundamental nature and relevance to disease, eukaryotic trogocytosis demands further investigation. It seems probable that more new examples of trogocytosis will be uncovered in the future. Quite a few fundamental questions have arisen, and many areas of trogocytosis are ready to be “chewed on” in future studies.
ACKNOWLEDGMENTS
We thank the members of our laboratory for helpful discussions and Anita Impagliazzo of Anita Impagliazzo Medical Illustration for the illustrations in Fig. 1 and 3.
A.B. was supported by a fellowship from the UC Davis Training Program in Biomolecular Technology. Work in our laboratory is supported by NIH grant 1R01AI146914 and a Pew Scholarship awarded to K.S.R.
Biographies

Akhila Bettadapur is a Ph.D. candidate at the University of California, Davis, in the Biochemistry, Molecular, Cellular, and Developmental Biology program with a Designated Emphasis in Biotechnology. She studies Entamoeba histolytica and trogocytosis, the process by which amoebae nibble and kill human cells. Before starting her Ph.D. studies in 2014, she was a research technician in plant genetics at the Howard Hughes Medical Institute at Stanford University. She received her B.S. in Microbiology, Immunology, and Molecular Genetics at the University of California, Los Angeles, in 2012.

Hannah W. Miller is a Ph.D. candidate at the University of California, Davis. She is currently investigating how Entamoeba histolytica uses trogocytosis to evade the host immune response. She received her bachelor’s degree in biology from Goshen College in 2010. She then joined Dr. Martin Prlic’s laboratory as a research technician in 2012 at the Fred Hutchinson Cancer Research Center in Seattle, WA, where she studied T cell activation. She began her studies in the Graduate Group in Immunology at the University of California, Davis, in 2015.

Katherine S. Ralston is an Assistant Professor at the University of California, Davis. Her laboratory studies interactions between Entamoeba histolytica and the human host, with a focus on trogocytosis, the process by which E. histolytica nibbles and kills human cells. She joined the UC Davis faculty in 2014 after she carried out postdoctoral studies on E. histolytica at the University of Virginia. She completed a Ph.D. at the University of California, Los Angeles, in 2009, where she focused on flagellar motility in Trypanosoma brucei.
REFERENCES
- 1.Dance A. 2019. Core concept: cells nibble one another via the under-appreciated process of trogocytosis. Proc Natl Acad Sci U S A 116:17608–17610. doi: 10.1073/pnas.1912252116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralston KS. 2015. Taking a bite: amoebic trogocytosis in Entamoeba histolytica and beyond. Curr Opin Microbiol 28:26–35. doi: 10.1016/j.mib.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista FD, Iber D, Neuberger MS. 2001. B cells acquire antigen from target cells after synapse formation. Nature 411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 4.Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. 2017. Trogocytosis of peptide–MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A 114:1111–1116. doi: 10.1073/pnas.1615973114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K-J, Wu C-H, Shen C-Y, Kuo Y-M, Yu C-L, Hsieh S-C. 2016. Membrane transfer from mononuclear cells to polymorphonuclear neutrophils transduces cell survival and activation signals in the recipient cells via anti-extrinsic apoptotic and MAP kinase signaling pathways. PLoS One 11:e0156262. doi: 10.1371/journal.pone.0156262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. 2001. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol 166:3645–3649. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 7.Wetzel SA, McKeithan TW, Parker DC. 2005. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol 174:80–89. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Vanherberghen B, Andersson K, Carlin LM, Nolte-'t Hoen ENM, Williams GS, Höglund P, Davis DM. 2004. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A 101:16873–16878. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown T. 1979. Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol 12:363–371. doi: 10.1099/00222615-12-3-363. [DOI] [PubMed] [Google Scholar]
- 10.Waddell DR, Vogel G. 1985. Phagocytic behavior of the predatory slime mold, Dictyostelium caveatum. Cell nibbling. Exp Cell Res 159:323–334. doi: 10.1016/s0014-4827(85)80006-9. [DOI] [PubMed] [Google Scholar]
- 11.Culbertson CG. 1970. Pathogenic Naegleria and Hartmannella (Acenthamoeba). Ann N Y Acad Sci 174:1018–1022. doi: 10.1111/j.1749-6632.1970.tb45623.x. [DOI] [PubMed] [Google Scholar]
- 12.Culbertson CG. 1971. The pathogenicity of soil amebas. Annu Rev Microbiol 25:231–254. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- 13.Joly E, Hudrisier D. 2003. What is trogocytosis and what is its purpose? Nat Immunol 4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 14.Huang J-F, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. 1999. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 15.Wakim LM, Bevan MJ. 2011. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT. 2018. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun 9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdu Y, Maniscalco C, Heddleston JM, Chew T-L, Nance J. 2016. Developmentally programmed germ cell remodelling by endodermal cell cannibalism. Nat Cell Biol 18:1302–1310. doi: 10.1038/ncb3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele S, Radlinski L, Taft-Benz S, Brunton J, Kawula TH. 2016. Trogocytosis-associated cell to cell spread of intracellular bacterial pathogens. Elife 5:e10625. doi: 10.7554/eLife.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer F, Ng SH, Brown TM, Boatman G, Johnson PJ. 2018. Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol 16:e2003885. doi: 10.1371/journal.pbio.2003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller HW, Suleiman RL, Ralston KS. 2019. Trogocytosis by Entamoeba histolytica mediates acquisition and display of human cell membrane proteins and evasion of lysis by human serum. mBio 10:e00068-19. doi: 10.1128/mBio.00068-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata Bhattacharya A, Petri WA Jr. 2014. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508:526–530. doi: 10.1038/nature13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cone RE, Sprent J, Marchalonis JJ. 1972. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc Natl Acad Sci U S A 69:2556–2560. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama M. 2015. Antigen presentation by MHC-dressed cells. Front Immunol 5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bona C, Robineaux R, Anteunis A, Heuclin C, Astesano A. 1973. Transfer of antigen from macrophages to lymphocytes. Immunology 24:831–840. [PMC free article] [PubMed] [Google Scholar]
- 25.Bona C, Anteunis A, Robineaux R, Astesano A. 1972. Transfer of antigenic macromolecules from macrophages to lymphocytes. Immunology 23:799–816. [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang I, Huang J-F, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. 2000. T cells can use either T cell receptor or Cd28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med 191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. 1999. Class II MHC/peptide complexes are released from APC and are acquired by T Cell responders during specific antigen recognition. J Immunol 163:5201–5210. [PubMed] [Google Scholar]
- 28.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. 2001. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 29.Hudrisier D, Aucher A, Puaux A-L, Bordier C, Joly E. 2007. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol 178:3637–3647. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. 2011. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci U S A 108:18360–18365. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaccorsi I, Morandi B, Antsiferova O, Costa G, Oliveri D, Conte R, Pezzino G, Vermiglio G, Anastasi GP, Navarra G, Münz C, Carlo ED, Mingari MC, Ferlazzo G. 2014. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol 192:824–832. doi: 10.4049/jimmunol.1301039. [DOI] [PubMed] [Google Scholar]
- 32.Pham T, Mero P, Booth JW. 2011. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS One 6:e14498. doi: 10.1371/journal.pone.0014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K, Schornagel K, Verkuijlen P, Janssen H, Halonen P, Lieftink C, Beijersbergen RL, Leusen JHW, Boelens JJ, Kuhnle I, van der Werff Ten Bosch J, Seeger K, Rutella S, Pagliara D, Matozaki T, Suzuki E, Menke-van der Houven van Oordt CW, van Bruggen R, Roos D, van Lier RAW, Kuijpers TW, Kubes P, van den Berg TK. 2018. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep 23:3946–3959. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 34.Megjugorac NJ, Jacobs ES, Izaguirre AG, George TC, Gupta G, Fitzgerald-Bocarsly P. 2007. Image-based study of interferongenic interactions between plasmacytoid dendritic cells and HSV-infected monocyte-derived dendritic cells. Immunol Invest 36:739–761. doi: 10.1080/08820130701715845. [DOI] [PubMed] [Google Scholar]
- 35.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 36.Gül N, van Egmond M. 2015. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res 75:5008–5013. doi: 10.1158/0008-5472.CAN-15-1330. [DOI] [PubMed] [Google Scholar]
- 37.Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren P, van de Winkel JGJ, Taylor RP. 2011. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol 187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 38.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. 2006. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol 176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 39.Taylor RP, Lindorfer MA. 2014. Analyses of CD20 monoclonal antibody-mediated tumor cell killing mechanisms: rational design of dosing strategies. Mol Pharmacol 86:485–491. doi: 10.1124/mol.114.092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boross P, Jansen JHM, Pastula A, van der Poel CE, Leusen J. 2012. Both activating and inhibitory Fc gamma receptors mediate rituximab-induced trogocytosis of CD20 in mice. Immunol Lett 143:44–52. doi: 10.1016/j.imlet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Velmurugan R, Challa DK, Ram S, Ober RJ, Ward ES. 2016. Macrophage-mediated trogocytosis leads to death of antibody-opsonized tumor cells. Mol Cancer Ther 15:1879–1889. doi: 10.1158/1535-7163.MCT-15-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liew PX, Kim JH, Lee W-Y, Kubes P. 2017. Antibody-dependent fragmentation is a newly identified mechanism of cell killing in vivo. Sci Rep 7:10515. doi: 10.1038/s41598-017-10420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu R, Shen Q, Xu P, Luo JJ, Tang Y. 2014. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sofroniew MV, Vinters HV. 2010. Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen JV, Soto I, Kim K-Y, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CO, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N. 2011. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A 108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis CO, Kim K-Y, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N. 2014. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A 111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills EA, Davis CO, Bushong EA, Boassa D, Kim K-Y, Ellisman MH, Marsh-Armstrong N. 2015. Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc Natl Acad Sci U S A 112:10509–10514. doi: 10.1073/pnas.1506486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chihara D, Nance J. 2012. An E-cadherin-mediated hitchhiking mechanism for C. elegans germ cell internalization during gastrulation. Development 139:2547–2556. doi: 10.1242/dev.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sulston JE, Schierenberg E, White JG, Thomson JN. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 50.Wen JW, Winklbauer R. 2017. Ingression-type cell migration drives vegetal endoderm internalisation in the Xenopus gastrula. Elife 6:e27190. doi: 10.7554/eLife.27190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele SP, Chamberlain Z, Park J, Kawula TH. 2019. Francisella tularensis enters a double membraned compartment following cell-cell transfer. Elife 8:e45252. doi: 10.7554/eLife.45252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jambou R, Combes V, Jambou M-J, Weksler BB, Couraud P-O, Grau GE. 2010. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PLoS Pathog 6:e1001021. doi: 10.1371/journal.ppat.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon GJ, Swanson JA. 1992. The macrophage capacity for phagocytosis. J Cell Sci 101:907–913. [DOI] [PubMed] [Google Scholar]
- 54.Takemura R, Stenberg PE, Bainton DF, Werb Z. 1986. Rapid redistribution of clathrin onto macrophage plasma membranes in response to Fc receptor-ligand interaction during frustrated phagocytosis. J Cell Biol 102:55–69. doi: 10.1083/jcb.102.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong J, Gaitanos TN, Luu O, Huang Y, Gaitanos L, Lindner J, Winklbauer R, Klein R. 2019. Gulp1 controls Eph/ephrin trogocytosis and is important for cell rearrangements during development. J Cell Biol 218:3455–3471. doi: 10.1083/jcb.201901032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Martin N, Fernández-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A, Alarcón B. 2011. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity 35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, Vale RD. 2018. Chimeric antigen receptors that trigger phagocytosis. Elife 7:e36688. doi: 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flannagan RS, Jaumouillé V, Grinstein S. 2012. The cell biology of phagocytosis. Annu Rev Pathol 7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 59.Swanson JA, Hoppe AD. 2004. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol 76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- 60.Fairn GD, Grinstein S. 2012. How nascent phagosomes mature to become phagolysosomes. Trends Immunol 33:397–405. doi: 10.1016/j.it.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Osborne DG, Wetzel SA. 2012. Trogocytosis results in sustained intracellular signaling in CD4+ T cells. J Immunol 189:4728–4739. doi: 10.4049/jimmunol.1201507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. 2008. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol 181:8120–8132. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- 63.Shin N, Ahn N, Chang-Ileto B, Park J, Takei K, Ahn S-G, Kim S-A, Di Paolo G, Chang S. 2008. SNX9 regulates tubular invagination of the plasma membrane through interaction with actin cytoskeleton and dynamin 2. J Cell Sci 121:1252–1263. doi: 10.1242/jcs.016709. [DOI] [PubMed] [Google Scholar]
- 64.Bendris N, Schmid SL. 2017. Endocytosis, metastasis and beyond: multiple facets of SNX9. Trends Cell Biol 27:189–200. doi: 10.1016/j.tcb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. 1999. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med 190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marie-Anaïs F, Mazzolini J, Herit F, Niedergang F. 2016. Dynamin-actin cross talk contributes to phagosome formation and closure. Traffic 17:487–499. doi: 10.1111/tra.12386. [DOI] [PubMed] [Google Scholar]
- 67.Ventrella R, Kaplan N, Getsios S. 2017. Asymmetry at cell-cell interfaces direct cell sorting, boundary formation, and tissue morphogenesis. Exp Cell Res 358:58–64. doi: 10.1016/j.yexcr.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. 2010. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol 12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- 69.Zisch AH, Kalo MS, Chong LD, Pasquale EB. 1998. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]
- 70.Gaitanos TN, Koerner J, Klein R. 2016. Tiam–Rac signaling mediates trans-endocytosis of ephrin receptor EphB2 and is important for cell repulsion. J Cell Biol 214:735–752. doi: 10.1083/jcb.201512010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu QA, Hengartner MO. 1998. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell 93:961–972. doi: 10.1016/S0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 72.Lanzetti L, Palamidessi A, Areces L, Scita G, Fiore P. 2004. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 73.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng Y, Elkon KB. 2011. Autoimmunity in MFG-E8–deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest 121:2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Null S, Nakada-Tsukui K, Nozaki T. 2017. AGC family kinase 1 participates in trogocytosis but not in phagocytosis in Entamoeba histolytica. Nat Commun 8:101. doi: 10.1038/s41467-017-00199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tillack M, Nowak N, Lotter H, Bracha R, Mirelman D, Tannich E, Bruchhaus I. 2006. Increased expression of the major cysteine proteinases by stable episomal transfection underlines the important role of EhCP5 for the pathogenicity of Entamoeba histolytica. Mol Biochem Parasitol 149:58–64. doi: 10.1016/j.molbiopara.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Bracha R, Nuchamowitz Y, Anbar M, Mirelman D. 2006. Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica. PLoS Pathog 2:e48. doi: 10.1371/journal.ppat.0020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watanabe N, Nakada‐Tsukui K, Nozaki T. 2020. Two isotypes of phosphatidylinositol 3-phosphate-binding sorting nexins play distinct roles in trogocytosis in Entamoeba histolytica. Cell Microbiol 22. doi: 10.1111/cmi.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilmartin AA, Ralston KS, Petri WA. 2017. Inhibition of amebic lysosomal acidification blocks amebic trogocytosis and cell killing. mBio 8:e01187-17. doi: 10.1128/mBio.01187-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilmartin AA, Ralston KS, Petri WA. 2020. Inhibition of amebic cysteine proteases blocks amebic trogocytosis but not phagocytosis. J Infect Dis 221:1734–1739. doi: 10.1093/infdis/jiz671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faust DM, Marquay Markiewicz J, Danckaert A, Soubigou G, Guillen N. 2011. Human liver sinusoidal endothelial cells respond to interaction with Entamoeba histolytica by changes in morphology, integrin signalling and cell death. Cell Microbiol 13:1091–1106. doi: 10.1111/j.1462-5822.2011.01604.x. [DOI] [PubMed] [Google Scholar]
- 82.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. 2001. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol 166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 83.Yewdell JW, Haeryfar S. 2005. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu Rev Immunol 23:651–682. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- 84.Campana S, De Pasquale C, Carrega P, Ferlazzo G, Bonaccorsi I. 2015. Cross-dressing: an alternative mechanism for antigen presentation. Immunol Lett 168:349–354. doi: 10.1016/j.imlet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Chow T, Whiteley J, Li M, Rogers IM. 2013. The transfer of host MHC class I protein protects donor cells from NK cell and macrophage-mediated rejection during hematopoietic stem cell transplantation and engraftment in mice. Stem Cells 31:2242–2252. doi: 10.1002/stem.1458. [DOI] [PubMed] [Google Scholar]
- 86.McIntyre MSF, Young KJ, Gao J, Joe B, Zhang L. 2008. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol 181:2271–2275. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 87.Zhou G, Ding Z-C, Fu J, Levitsky HI. 2011. Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J Immunol 186:2148–2155. doi: 10.4049/jimmunol.1002917. [DOI] [PubMed] [Google Scholar]
- 88.Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. 2014. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS One 9:e86551. doi: 10.1371/journal.pone.0086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian D, Yang L, Wang S, Zhu Y, Shi W, Zhang C, Jin H, Tian Y, Xu H, Sun G, Liu K, Zhang Z, Zhang D. 2019. Double negative T cells mediate Lag3-dependent antigen-specific protection in allergic asthma. Nat Commun 10:4246. doi: 10.1038/s41467-019-12243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reed J, Wetzel SA. 2019. Trogocytosis-mediated intracellular signaling in CD4+ T cells drives TH2-associated effector cytokine production and differentiation. J Immunol 202:2873–2887. doi: 10.4049/jimmunol.1801577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reed SL, Ember JA, Herdman DS, DiScipio RG, Hugli TE, Gigli I. 1995. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J Immunol 155:266–274. [PubMed] [Google Scholar]
- 92.Reed SL, Gigli I. 1990. Lysis of complement-sensitive Entamoeba histolytica by activated terminal complement components. Initiation of complement activation by an extracellular neutral cysteine proteinase. J Clin Invest 86:1815–1822. doi: 10.1172/JCI114911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reed SL, Keene WE, McKerrow JH, Gigli I. 1989. Cleavage of C3 by a neutral cysteine proteinase of Entamoeba histolytica. J Immunol 143:189–195. [PubMed] [Google Scholar]
- 94.Braga LL, Ninomiya H, McCoy JJ, Eacker S, Wiedmer T, Pham C, Wood S, Sims PJ, Petri WA. 1992. Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. J Clin Invest 90:1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andrews NW, Corrotte M. 2018. Plasma membrane repair. Curr Biol 28:R392–R397. doi: 10.1016/j.cub.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 96.Mercer F, Johnson PJ. 2018. Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 34:683–693. doi: 10.1016/j.pt.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]