Prompt recognition of microbes by cells is critical to eliminate invading pathogens. Some cell-associated pattern recognition receptors (PRRs) recognize and respond to microbial ligands. However, others can respond to cellular perturbations, such as damage-associated molecular patterns (DAMPs). Nucleotide oligomerization domains 1 and 2 (NOD1/2) are PRRs that recognize and respond to multiple stimuli of microbial and cellular origin, such as bacterial peptidoglycan, viral infections, parasitic infections, activated Rho GTPases, and endoplasmic reticulum (ER) stress.
KEYWORDS: ER stress, NOD1, NOD2, pathogens
ABSTRACT
Prompt recognition of microbes by cells is critical to eliminate invading pathogens. Some cell-associated pattern recognition receptors (PRRs) recognize and respond to microbial ligands. However, others can respond to cellular perturbations, such as damage-associated molecular patterns (DAMPs). Nucleotide oligomerization domains 1 and 2 (NOD1/2) are PRRs that recognize and respond to multiple stimuli of microbial and cellular origin, such as bacterial peptidoglycan, viral infections, parasitic infections, activated Rho GTPases, and endoplasmic reticulum (ER) stress. How NOD1/2 are stimulated by such diverse stimuli is not fully understood but may partly rely on cellular changes during infection that result in ER stress. NOD1/2 are ER stress sensors that facilitate proinflammatory responses for pathogen clearance; thus, NOD1/2 may help mount broad antimicrobial responses through detection of ER stress, which is often induced during a variety of infections. Some pathogens may subvert this response to promote infection through manipulation of NOD1/2 responses to ER stress that lead to apoptosis. Here, we review NOD1/2 stimuli and cellular responses. Furthermore, we discuss pathogen-induced ER stress and how it might potentiate NOD1/2 signaling.
INTRODUCTION
Pattern recognition receptors (PRRs) are a consortium of innate immune molecules dedicated to quickly recognizing pathogens and alerting the immune system in order to control infections or cellular abnormalities. Five major classes of PRRs exist: (i) toll/Toll-like receptors (TLRs), (ii) nucleotide-binding domain, leucine-rich repeat-containing (NBD-LRR) proteins (NLRs); (iii) retinoic acid-inducible gene (RIG)-I-like receptors (RLRs); (iv) C-type lectin receptors (CLRs); and (v) DNA sensors (1–3). PRRs that alert cells to potential invading pathogens usually recognize very specific microbial molecules known as microbe-associated molecular patterns (MAMPs), e.g., TLR5 sensing of bacterial flagellin. A few PRRs respond to damage-associated molecular patterns (DAMPs), which are endogenous cellular ligands liberated during compromised cell health that act as danger signals. DAMPs, such as heat shock proteins and high-mobility group box 1 (HMGB1), can be produced during infections and activate the immune system similarly to MAMPs (2). In addition to recognizing MAMPs and DAMPs, the host can recognize distinct pathogen-induced processes which are considered to be common infection strategies that many pathogens employ to cause disease (4–7).
The NLR family contains over 20 intracellular receptors, including NOD1 (CARD4), NOD2 (CARD15), NLRCs (NLR family CARD domain containing), NLRPs (NLR family PVD domain containing), and NAIPs (NLR family apoptosis inhibitory protein) (3). Distinguishing characteristics of the NLR family are a central NOD (also known as NACHT), a C-terminal LRR (leucine-rich repeat) and an N-terminal protein-protein interaction domain (8, 9). NLR ligands are mainly of bacterial origin, although a few NLRs, e.g., NLRP3, respond to DAMPs. Uric acid and cholesterol crystals, ATP, oxidized mitochondrial DNA, reactive oxygen species (ROS), and endoplasmic reticulum (ER) stress are examples of NLR DAMPs (2, 10, 11). The founding members of the NLR family, NOD1 and NOD2 (NOD1/2), sense both MAMPs and DAMPs. NOD1/2 were initially described as bacterial peptidoglycan fragment receptors (12–15), but mounting evidence has revealed that NOD1 and/or NOD2 is activated during infections with peptidoglycan-deficient pathogens, including viruses (16–23), parasites (24–26), and fungi (27, 28). In addition, NOD1 and/or NOD2 participates in the detection of pathogen-induced processes such as the activation of Rho GTPases, ER stress, and the unfolded protein response (UPR), autophagy and mitophagy, disruption of calcium homeostasis, and cell death (29–32) (Table 1). Here, we review the details underlying NOD1 and NOD2 activation by diverse stimuli with an emphasis on pathogen-induced ER stress.
TABLE 1.
Diverse ligands stimulate NOD1 and/or NOD2 signaling
| Ligand | PRR | Role during infection | Reference(s) |
|---|---|---|---|
| iE-DAP | NOD1 | Activates NF-κB upon LRR ligation | 12, 13, 15 |
| MDP | NOD2 | Activates NF-κB upon LRR ligation | 14, 15 |
| Respiratory syncytial virus (RSV) | NOD2 | Activates type I IFN by ssRNA, requires MAVS; restricts viral replication | 19 |
| Vesicular stomatitis virus (VSV) | NOD2 | Activates type I IFN by ssRNA, requires MAVS; restricts viral replication | 19 |
| Influenza A virus (IAV) | NOD2 | Activates type I IFN by ssRNA, requires MAVS | 19 |
| NOD2 | Restricts viral pathogenesis | 17, 18 | |
| NOD2 | Restricts viral replication | 17 | |
| Human parainfluenza virus type 3 (HPIV3) | NOD2 | Activates type I IFN by ssRNA, requires MAVS | 19 |
| Human cytomegalovirus (HCMV) | NOD1 | Activates type I IFN, restricts viral replication | 21 |
| NOD2 | Activates type I IFN, restricts viral replication | 20, 160 | |
| Murine cytomegalovirus (MCMV) | NOD1 | Activates type I IFN, restricts viral replication | 21 |
| Hepatitis C virus (HCV) NS5B | NOD1 | Activates type I IFN by dsRNA, MAVS independent | 163 |
| Coxsackievirus B3 | NOD2 | Promotes virus replication and pathogenesis | 16 |
| Foot-and-mouth-disease virus (FMDV) | NOD2 | Activates type I IFN, restricts viral replication | 23 |
| Plasmodium berghei | NOD1/2 | Activated but parasite replication unaffected | 24 |
| Plasmodium falciparum | NOD2 | Activates NO production, hemozoin dependent | 25 |
| Trypanosoma cruzi | NOD1/2 | Activated during infection | 26 |
| NOD1 | Restricts parasite replication | 26 | |
| Aspergillus fumigatus | NOD1/2 | Activated, promote fungal replication by inhibiting fungal killing, Dectin-1 dependent | 27, 28 |
| Activated Rho GTPases | NOD1 | Constitutively active Rac1 and Cdc42, S. Typhimurium SopE activation of Rac1 and Cdc42 | 30 |
| NOD1 | S. flexneri, GEF-H1 and RhoA kinase dependent | 56 | |
| Endoplasmic reticulum (ER) stress | NOD1/2 | Thapsigargin-mediated activation | 29, 32, 42 |
| Calcium concn alterations | NOD1/2 | Thapsigargin, not tunicamycin; Ca2+ chelation, thapsigargin | 29, 32 |
| NOD1 | Thapsigargin; CaSR/PLC/IP3R agonists | 31 | |
| NOD1 | Binds RyR and increases cytosolic Ca2+ | 136 | |
| NOD2 | CaSR promotes MDP uptake | 73 |
NOD1 AND NOD2
Nod1 and Nod2 are highly similar yet distinct vertebral genes that may have resulted from an evolutionarily early gene duplication event (33). High interspecies amino acid conservation coupled with increased conservation of critical sequences important for mediating NOD1 or NOD2 protein/ligand molecular interactions reveals the importance of NOD1 and NOD2 for immunity (33). In addition to their central NOD and C-terminal LRR domains, NOD1 contains one N-terminal caspase activation and recruitment domain (CARD), while NOD2 contains two CARDs (34). The CARDs mediate protein-protein interactions, e.g., with adaptor protein receptor-interacting serine/threonine-protein kinase 2 (RIPK2). NOD domains contain a nucleotide binding site for ATPase activity and are important for oligomerization, while the LRR domains mediate ligand recognition (35). To maintain an inactive state in the absence of ligand, the LRR domain occludes the NOD (36). NOD1 is activated with moderate affinity (∼30 μM) (37) by γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP) moieties, which are produced by Gram-negative and a few Gram-positive bacteria (13, 15). NOD2 is activated with strong affinity (∼50 nM) (38) by muramyl dipeptide (MDP) moieties present in peptidoglycan from both Gram-positive and -negative bacteria (14, 15, 39). Peptidoglycan detection induces the oligomerization of NOD1 and NOD2 and recruitment of RIPK2, a NOD1/2-specific adaptor protein required for facilitating their proinflammatory response (40). RIPK2 consists of an N-terminal kinase domain, a central linker region and one CARD domain. The CARD of RIPK2 interacts with the CARDs of NOD1 and NOD2 leading to RIPK2 phosphorylation by leucine-rich repeat kinase 2 (LRRK2) and ubiquitination at multiple sites by several E3 ligases, such as the inhibitor of apoptosis proteins cIAP1, cIAP2, and XIAP (41). The phosphorylation and ubiquitination events promote interactions with TNF receptor-associated factors (TRAF) 2/5/6 (42) and activate the expression of nuclear factor-κB (NF-κB)- and mitogen-activated protein kinase (MAPK)-dependent genes involved in host immunity and pathogen clearance (43, 44).
Tissue expression of Nod1 and Nod2 is one distinguishing feature between these molecules. Nod1 is mostly ubiquitous throughout the body, while Nod2 expression is confined to monocytes, dendritic cells, epithelial cells, Paneth cells and intestinal stem cells (8, 44). However, Nod1 and Nod2 are interferon-stimulated genes (ISGs), and therefore their expression in tissues may only be detected under particular conditions, such as viral and bacterial infections (22). Furthermore, Nod2 mRNA expression in the murine heart was recently demonstrated during coxsackievirus B3 (CVB3) infection (16) and was also observed in vascular smooth muscle cells (45). This expanded distribution of Nod2 gene expression requires further confirmation but may be specific to the models or conditions used in these studies.
Membrane localization.
NOD1 and NOD2 are critical cytoplasmic innate immune molecules; however, significant evidence underscores a major role of membrane localization for NOD1/2 functions. NOD1/2 localizes to endosomes (46, 47) and the plasma membrane (48–53). NOD2 plasma membrane localization, which is mediated by the C-terminal end, is required for its response to MDP (48). Similarly, NOD1 localizes to the plasma membrane in an actin-dependent manner and accumulates at sites of Shigella flexneri attachment (49). Cell membrane localization via S-palmitoylation of NOD1 and NOD2 is required for peptidoglycan sensing (54). The signal that induces NOD1/2 to localize to membranes is unknown. Small Rho GTPases are known to regulate the actin cytoskeleton (55), and activation of Rac1, Cdc42 and RhoA by bacterial effector proteins results in NF-κB activation that is mediated by NOD1 and/or NOD2 (30, 56). Of note, bacterium-induced Rac1-mediated NF-κB activation also occurs via NOD1/2-independent mechanisms (57, 58). In addition, NOD2 bound to activated Arf GTPases (39), which are also involved in actin cytoskeletal dynamics but are distinct from Rho GTPases and associate with vesicular transport systems (59). Thus, perturbation of the actin cytoskeleton and the activation of small GTPases might be a signal for NOD1/2 to localize to membranes and initiate immune responses (30, 39, 51, 56).
Cell death.
NOD1 and NOD2 were initially implicated to play a role in cell death. NOD1 and NOD2, as well as other members of the NLR family, share homology with apoptotic protease activating factor 1 (APAF-1), a major regulator of apoptosis (60–63). Upon binding to cytochrome c that is released from mitochondria in response to certain apoptotic stimuli, APAF-1 associates with procaspase-9 via CARD-CARD interaction, resulting in formation of the apoptosome which leads to cell death. NOD1 can bind several caspases, including caspase-9 (60). It was proposed that NOD2 can bind to caspase-1 to induce interleukin-1β (IL-1β) secretion (64). Furthermore, administration of iE-DAP induces apoptosis in murine hearts, suggesting that NOD1 activation stimulates cell death (65). However, several reports showed that Nod1- and/or Nod2-deficient cells have increased cell death, arguing for a protective role of NOD1 and NOD2 (66). In Nod1-deficient cells, Shigella-induced cell death increased, indicating that NOD1 protects nonmyeloid cells from apoptosis during Shigella infection (66). Recent work characterizing the role of NOD2 in vascular smooth muscle cells revealed that tunicamycin-induced endoplasmic reticulum (ER) stress was resolved in Nod2-sufficient cells, whereas ER stress-induced cell death was increased in Nod2-deficient cells (45). One ER stress-induced cell death pathway depends on caspase-12. In resting cells, procaspase-12 is localized to the ER and bound to TRAF2. Upon ER stress, procaspase-12 is cleaved by m-calpain, which is activated by the release of calcium from the ER (67, 68). TRAF2 dissociates from procaspase-12 and is recruited to IRE1α leading to Jun N-terminal kinase (JNK) and NF-κB activation (69). Activated caspase-12 subsequently cleaves caspase-9, which in turn cleaves and activates the executioner caspase-3 to induce cell death (67). Caspase-12-deficient mice are resistant to ER stress-induced apoptosis, but their cells still undergo apoptosis in response to other stimuli, supporting the observation that caspase-12 activation is only mediated by ER stress (67). Interestingly, caspase-12 competes with TRAF6 binding to RIPK2 in response to Citrobacter rodentium infection, thereby inhibiting RIPK2 ubiquitination and NF-κB activation (70). One possible explanation for the controversial role of NOD signaling in cell death could be caspase-12-mediated control. In the event of low to moderate ER stress, the cell attempts to restore homeostasis, and caspase-12 inhibits immune responses. However, in the event of severe ER stress, caspase-12 will activate caspase-9 and caspase-3 to execute cell death. An excess in calcium flux might be the trigger that determines whether the cell will remain viable or undergo apoptosis.
Calcium homeostasis.
Perturbation in calcium homeostasis is a major cause of ER stress, and infection-triggered calcium signaling can be considered a DAMP. Many pathogens can stimulate calcium influx through the plasma membrane or via release from the ER leading to changes in intracellular calcium concentrations (71). Thapsigargin is an inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), resulting in depletion of ER calcium and an increase in cytosolic calcium (72). Thapsigargin-induced ER stress resulted in NOD1/2-dependent inflammatory responses, whereas ER stress induced by tunicamycin, which inhibits protein glycosylation, resulted in NOD1/2-independent inflammatory responses, suggesting that calcium flux contributes to NOD1/2 responses to ER stress (29). It was recently proposed that increases in cytosolic and extracellular calcium concentrations induce endocytosis of trace levels of contaminating peptidoglycan found in the cell culture additive fetal calf serum (FCS), as well as in mouse serum thereby activating NOD1/2 signaling (32). Similarly, it was suggested that activation of the plasma membrane-localized calcium-sensing receptor (CaSR) increased macropinocytosis, which facilitated the uptake of extracellular MDP and activated NOD2 signaling (73). An alternative explanation is that activation of CaSR results in phospholipase C (PLC) activation which generates inositol triphosphate (IP3), the ligand for the 1,4,5-triphosphate receptor (IP3R), suggesting activation of IP3R may contribute to NOD1/2 activation by extracellular calcium stimulation. Calcium flux from the ER is mediated by IP3R and the ryanodine receptors (RyRs). Induction of the CaSR/PLC/IP3R pathway significantly contributes to NOD1-mediated immune responses (31). In mice with postmyocardial infarction, NOD1 modulated intracellular calcium mishandling. It was demonstrated that NOD1 binds RyR, thereby increasing its phosphorylation and activation, which led to increased cytosolic calcium (74). The precise mechanism of how NOD1 activation influences calcium flux and whether the increase in intracellular calcium facilitates the uptake of trace levels of peptidoglycan needs to be further elucidated.
Autophagy.
Macroautophagy (referred to here as “autophagy”) is a self-degradative process for balancing cellular homeostasis to promote survival. It plays an important role in the removal of damaged organelles, protein aggregates, misfolded proteins, and pathogens. The UPR and autophagy are intimately related as ER stress can directly induce autophagy. The transcription factors CHOP and ATF4, which are downstream of PERK, activate autophagy gene transcription. Furthermore, activation of JNK results in autophagosome formation. On the other hand, impaired autophagy can activate the UPR (75). Interestingly, NOD1 and NOD2 directly interact with ATG16L1 to regulate autophagy by recruiting ATG16L1 to the cell membrane at the site of bacterial invasion, which promotes autophagic killing of bacteria (50). The Crohn’s disease-associated Nod2-3020insC mutation showed impaired autophagy and was unable to recruit ATG16L1 to the plasma membrane. Similarly, the Atg16l1 mutation associated with Crohn’s disease was unable to induce autophagy when cells were stimulated with MDP (50). Whether NOD2 and NOD1 play important roles in autophagy induced in the absence of peptidoglycan remains to be elucidated. However, NOD2 and RIPK2 are required for mitophagy in cells infected with influenza A virus (IAV) (18). Mitophagy is a form of autophagy targeting mitochondria that have been damaged during infection or stress (76). Mice deficient for Nod2 and Ripk2 were hypersusceptible to IAV-induced pathogenesis due to defective mitophagy. The damaged mitochondria in Ripk2−/− bone marrow-derived dendritic cells (BMDCs) produced increased levels of ROS, leading to activation of the NLRP3 inflammasome and uncontrolled inflammation compared to wild-type BMDCs (18). These results suggest that NOD2 and RIPK2 play important roles in host defense in the absence of peptidoglycan, although microbiota-derived contaminating peptidoglycan fragments that activate NOD2 during viral infections cannot be fully excluded.
ER stress.
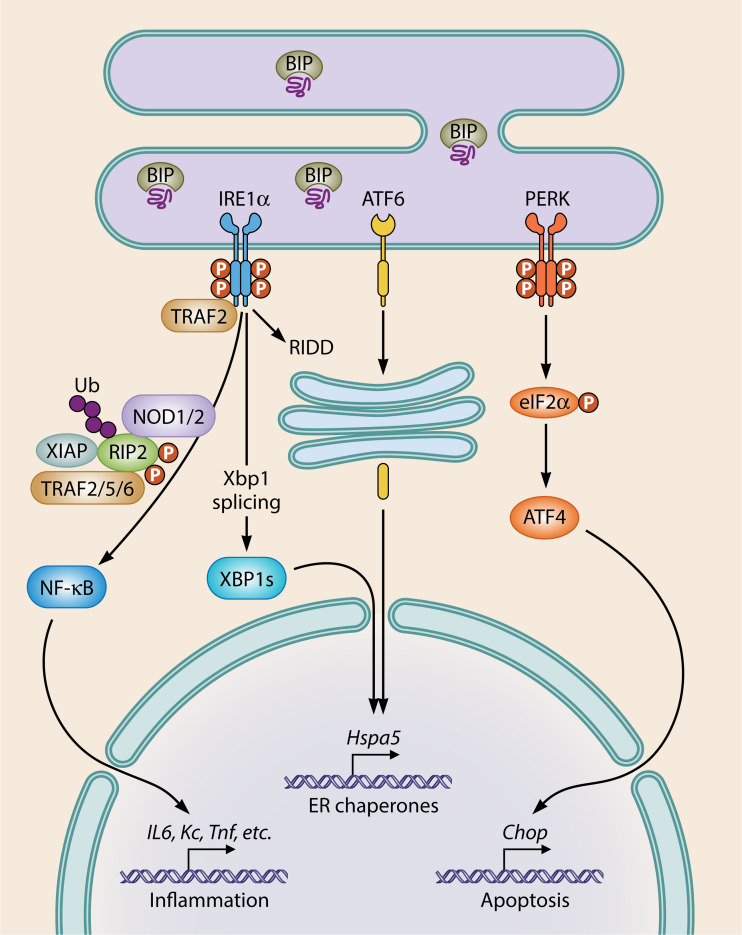
ER stress activates NOD1/2 to mount a proinflammatory response (29, 32, 42). ER stress occurs when a cellular perturbation disrupts the ER or its capacity to properly process and fold proteins. The unfolded protein response (UPR) is initiated to help the cell restore homeostasis. Three UPR pathways exist which are activated by separate ER stress transmembrane receptors, including inositol-requiring protein 1 (IRE1α), activating transcription factor 6 (ATF6), and protein kinase R-like ER kinase (PERK) (77) (Fig. 1). Each receptor is held inactive by the master ER chaperone binding immunoglobulin protein (BIP; also known as 78-kDa glucose-regulated protein, GRP78, encoded by Hspa5). When ER stress occurs and misfolded proteins accumulate, BIP dissociates from IRE1α, ATF6, and PERK, initiating their activation (77). Each protein signals distinctly to influence cellular physiology in order to cope with ER stress. IRE1α dimerizes, autophosphorylates, and splices X-box binding protein-1 (Xbp1) mRNA to produce a functional XBP1s transcription factor, as well as initiates regulated IRE1α-dependent decay (RIDD), which degrades mRNAs in order to reduce protein burdens (78). BIP dissociation from ATF6 reveals a Golgi localization signal that targets ATF6 to the Golgi apparatus, where it is cleaved, translocates to the nucleus, and functions as a transcription factor (77). PERK, like IRE1α, dimerizes and autophosphorylates. PERK then phosphorylates eukaryotic initiation factor 2α (eIF2α) to inhibit translation of most proteins aside from activating transcription factor 4 (ATF4), which regulates C/EBP homologous protein (CHOP; also known as GADD34). CHOP is a major regulator of apoptosis in response to ER stress (79). As a whole, these three pathways lead to ER chaperone and immune gene regulation, ER-associated degradation (ERAD), survival versus apoptotic responses depending on the magnitude of ER stress, lipid synthesis, autophagy, and redox regulation (77).
FIG 1.
ER stress induces the UPR. Transmembrane ER stress sensors IRE1α, ATF6, and PERK are activated upon dissociation of BIP, an ER chaperone that binds to misfolded proteins. UPR promotes restoration of homeostasis through upregulation of genes involved in multiple pathways related to global cellular physiology. If unresolvable, UPR induces apoptosis mediated via PERK. NOD1/2 are activated downstream of IRE1α resulting in proinflammatory cytokine production.
ER stress promotes inflammation and is thus often considered a DAMP (80) since ER stress is linked to PRR-associated immune responses (81). Some NLRs sense and respond to ER stress (29, 31, 32, 82). NLRP3 senses tunicamycin-induced ER stress through an IRE1α-dependent mechanism that initiates mitochondrial production of ROS. It was proposed that ROS production leads to recruitment of NLRP3 to mitochondrial membranes, the release of mitochondrial contents, and activation of the canonical NLRP3 inflammasome (82). Receptor interacting protein 1 (RIP1) is required for NLRP3 sensing of ER stress (83). Similarly, using both Nod1/2−/− and Ripk2−/− mice/cells compared to the wild type, NOD1/2 required RIPK2 to sense and respond to ER stress (29, 32, 42). Upon ER stress, NOD1/2 were selectively activated downstream of IRE1α (29). Thus, NOD1 and NOD2 can sense the DAMP ER stress and elicit immune gene expression; however, the exact mechanism of NOD1/2 sensing of ER stress remains to be elucidated. A possible mechanism might involve ER stress-mediated sampling of the extracellular environment for peptidoglycan fragments, and NOD1/2-dependent antimicrobial responses may be a result of ER stress-induced uptake of peptidoglycan (32). This mechanism assumes that peptidoglycan is present in the extracellular milieu, and it was found in trace levels in both mouse serum and cell culture FCS tested in this study; however, the authors of that study did not assess whether the concentrations of trace peptidoglycan detected in sera were sufficient to activate NOD1/2 (32). Thus, additional research is needed to determine whether potential contaminating peptidoglycan taken up during times of ER stress is responsible for NOD1/2 activation, and caution should be taken when making conclusions about mechanisms underlying peptidoglycan-independent NOD1/2 activation. An alternative, peptidoglycan-independent mechanism of ER stress-mediated NOD1/2 activation is that NOD1 and NOD2 are recruited to the ER stress-activated IRE1α receptor in complex with TRAF2 at the ER membrane, which is mediated by the major TRAF2-binding motifs present in NOD1 and NOD2 to initiate their signaling and antimicrobial responses (84).
PATHOGEN-INDUCED ER STRESS SENSING AND NOD1/2
Intracellular pathogens, including bacteria, protozoan parasites, and viruses, are well known to elicit ER stress (81, 85). ER stress can be a result of increased protein translation due to replication of the invading pathogen or modification of ER membranes, e.g., to establish viral replication complexes, as is the case for many RNA viruses (85, 86). Moreover, extracellular bacteria that attach to mammalian cells also induce ER stress through the introduction of toxins or effector proteins into the host cytosol via bacterial secretion systems (85, 87). Whether pathogens specifically activate single arms of the UPR or if pathogen-induced cellular disruptions cause ER stress differs among pathogens. Detecting ER stress during microbially induced pathogenesis is a good strategy for cells to initiate early, broad antimicrobial immune responses that allow cells to combat infections.
Bacteria.
As sensors of peptidoglycan fragments, NOD1 and NOD2 are activated by many pathogenic bacteria (44, 88). NOD1 promotes immune clearance of Pseudomonas aeruginosa (89), Campylobacter jejuni (90), Clostridium difficile (91), Legionella pneumophila (92–94), Helicobacter pylori (95, 96), Chlamydia pneumoniae (97), Citrobacter rodentium (98), Haemophilus influenzae (99), Listeria monocytogenes (100–102), Salmonella enterica Typhimurium (103–105), Shigella flexneri (50), and Streptococcus pneumoniae (106). NOD2 protects against infections with Citrobacter rodentium (22, 98), Legionella pneumophila (92), Listeria monocytogenes (100–102, 107), Salmonella enterica Typhimurium (103–105), and Staphylococcus aureus (108). Peptidoglycan recognition by NOD1 and/or NOD2 during bacterial infection initiates NF-κB-dependent proinflammatory responses; however, whether bacterium-induced ER stress is also sensed by NOD1/2 during infection is beginning to be explored. Indeed, NF-κB activation during Brucella abortus infection was increased in wild-type versus Nod1/2−/− mice (29), which required the B. abortus effector VceC that induces ER stress (109). In addition, S. Typhimurium exploits the UPR to promote intracellular replication, and ER stress enhanced NOD1-dependent NF-κB activation (31, 110). ER stress mediated by bacterial infections is increasingly appreciated as a commonality during infection; some bacteria produce toxins or inject effectors into host cells that impact ER stability, and others induce rearrangements of ER membranes for replicative purposes (85, 87). Thus, NOD1/2 sensing of ER stress as a pathogen-induced process may promote UPR-dependent innate immune responses that can influence bacterial infections (Fig. 2).
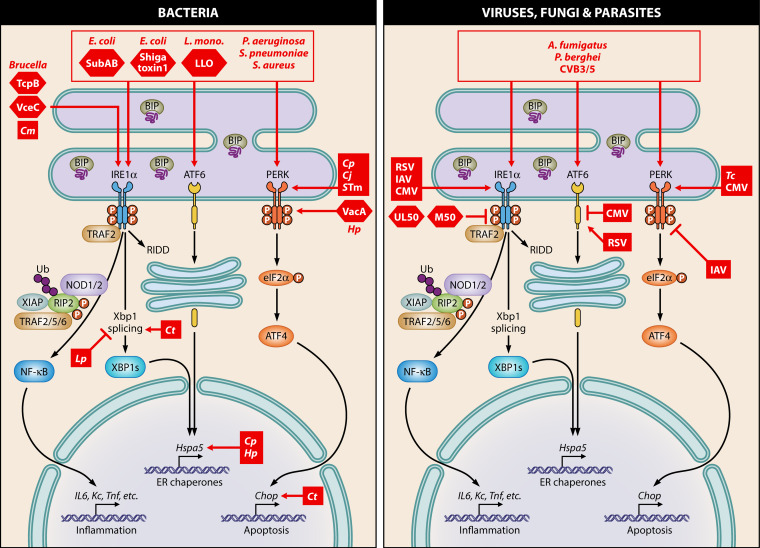
FIG 2.
Pathogen-induced ER stress and the convergence with NOD1/2 signaling. Bacteria (left panel) and viruses, fungi, and parasites (right panel) differentially induce ER stress, which may be sensed by NOD1/2. Pathogens or pathogen-encoded proteins are depicted in red. RSV, respiratory syncytial virus; IAV, influenza A virus; CMV, cytomegalovirus (proteins UL50 and M50); CVB, coxsackievirus B; Cm, Chlamydia muridarum; Lp, Legionella pneumophila; Ct, Chlamydia trachomatis; Cp, Chlamydia pneumoniae; Hp, Helicobacter pylori; Cj, Campylobacter jejuni; STm, Salmonella Typhimurium; Tc, Trypanosoma cruzi; L. mono., Listeria monocytogenes; LLO, listeriolysin O; SubAB, subtilase toxin AB.
To establish intracellular replicative compartments, Brucella significantly alters ER structure (85, 87) using its TcpB protein, which stabilizes microtubules, localizes to the ER, and also induces UPR (111, 112). Furthermore, Brucella abortus effector VceC triggers ER stress by inducing inflammatory cytokines in an IRE1α-dependent manner (109). VceC localizes to the ER and binds to host BIP, suggesting that VceC sequesters BIP from ER stress receptors, leading to activation of the UPR (109). Furthermore, the UPR is critical for B. abortus replication (113–116). Proinflammatory gene expression following UPR activation by B. abortus infection required VceC and NOD1/2, which exacerbated infection and disease outcomes. Furthermore, this effect was independent of peptidoglycan sensing by NOD1/2 and unveiled a novel role for NOD1/2 in detecting and responding to ER stress in an IRE1α-dependent manner (29). Therefore, B. abortus exploits the UPR and NOD1/2 to establish a successful infection.
Like Brucella spp., Legionella and Chlamydia spp. establish intracellular replication compartments that either are derived from the ER or come in contact with it, respectively (117, 118). Legionella pneumophila encodes translation elongation inhibitors to block host translation, which are required to inhibit IRE1α-mediated splicing of Xbp1 in a MyD88-dependent manner (119). Given that L. pneumophila inhibits the IRE1α/ER stress pathway, it might be preventing NOD1/2 stimulation via IRE1α, since NOD1/2 restrict L. pneumophila infection (92–94). In one report, Chlamydia pneumoniae upregulated Hspa5 and activated PERK, but IRE1α and ATF6 pathways were unaffected (120). However, a different study showed that Xbp1 splicing and Chop expression increased during Chlamydia trachomatis infection (121). Chlamydia muridarum infection induced Il6 gene expression, which was reduced when IRE1α was inhibited; this effect was dependent on RIPK2 (29). The differences in UPR activation may lie in the Chlamydia species used or timing of infection. Overall, ER stress is evident during infection with Chlamydia, and Chlamydia is known to stimulate NOD1 and/or NOD2 during infection (122, 123).
NOD1 is also stimulated during infection with extracellular bacteria, including Helicobacter pylori (96, 124), a result of peptidoglycan delivery to host cell cytosol mediated by the type IV secretion system of H. pylori. Moreover, mice deficient for Nod1 are more susceptible to H. pylori stomach colonization (96). To help protect against H. pylori infection, NOD1 promotes anti-bacterial type I interferon (IFN) signaling (9). Helicobacter induces ER stress to various degrees for all three UPR pathways in mice and humans (125). H. pylori effector VacA activated PERK, which increased Chop expression and ultimately apoptosis (126) or autophagic cell death (127) in human gastric cell lines. No changes in the IRE1α or ATF6 pathways were observed, but Hspa5 was upregulated (126). In addition, increased ER stress induced by thapsigargin in VacA-expressing cells further enhanced VacA-mediated autophagic cell death (127). Since Helicobacter upregulates ER stress and NOD1 is activated by H. pylori, then NOD1 may act as a sensor for H. pylori-induced ER stress to limit infection. If so, this would likely occur through a mechanism independent of VacA since IRE1α stimulation was not observed by introduction of this effector alone (126). Another possibility is that NOD1 signaling may rely on other ER stress receptors independently of IRE1α during H. pylori infection.
Bacterial pore-forming toxins can have potent effects on targeted host cells, and UPRs elicited by bacterial pore-forming toxins are protective in mice (128). Escherichia coli subtilase toxin SubAB activated all three arms of the UPR (129) by cleavage of BIP (130, 131), which led to apoptosis (131). However, proinflammatory responses to SubAB appear unaffected by NOD1/2 (32). Another E. coli cytotoxin, Shiga toxin 1, caused calcium release from the ER, which resulted in apoptosis in monocytes (132). Similarly, Listeria monocytogenes toxin listeriolysin O induced all three UPR arms, and the ER stressors tunicamycin and thapsigargin reduced L. monocytogenes loads (133), which indicates that the UPR facilitates antibacterial effects. UPR stimulation by L. monocytogenes also likely occurs through depletion of ER calcium (134, 135). Since ER stress responses to pore-forming toxins ameliorate toxin-induced damage (128) and NOD1 senses calcium fluxes (31, 32, 136), perhaps this occurs through the IRE1α/NOD1/NF-κB axis. Clostridium difficile, which encodes a pore-forming toxin, is restricted by NOD1 and also induces ER stress (137–139). Thus, NOD1 might sense pore-forming toxin-mediated ER stress to mount protective immune responses, which would aid in alleviation of the potent effects of intracellular toxins.
Several other bacteria restricted by NOD1 and/or NOD2 signaling induce ER stress during infections. Campylobacter jejuni activated the PERK pathway and led to an upregulation of CHOP, but XBP1s and ATF6 upregulation was not observed. In addition, siRNA depletion of Ire1α, Atf6, or Perk increased intracellular C. jejuni loads. Furthermore, C. jejuni invasion was inhibited by the UPR induced by thapsigargin and tunicamycin treatment (140). Altogether, these data indicate that the UPR inhibits C. jejuni infection. NOD1 (but not NOD2) restricts C. jejuni infection in intestinal epithelial cells (90) and therefore may be involved in ER stress-mediated restriction of C. jejuni infection. Other bacteria that induce ER stress and are also inhibited by NOD1 or NOD2 include P. aeruginosa (141–145), Staphylococcus aureus (146, 147), S. Typhimurium (148), and S. pneumoniae (149). Additional studies are necessary to determine a link between bacterium-induced ER stress and NOD1/2 antibacterial effects.
Fungi.
NOD1 senses and elicits proinflammatory responses during Aspergillus fumigatus infection of corneal epithelial cells (150) and promotes invasive aspergillosis, a disease induced by A. fumigatus (27). Similarly, NOD2 promotes A. fumigatus pathogenicity (28). Nod1−/− mice were less susceptible to A. fumigatus, and bone marrow-derived macrophages (BMDMs) from Nod1−/− mice killed A. fumigatus more efficiently in an ROS-dependent manner. A similar, yet distinct, mechanism for NOD2 enhancement of invasive aspergillosis occurs. Peripheral blood mononuclear cells (PBMCs) from human donors with the Nod2 polymorphism 1007insC and Nod2-deficient PBMCs had decreased proinflammatory cytokines yet increased fungal killing in an ROS-independent manner during A. fumigatus infection. Like Nod1, during A. fumigatus infection (27), Nod2 deficiency led to an upregulation of Dectin-1 (28), which is a CLR that promotes uptake of A. fumigatus (151), thereby enhancing phagocytosis of A. fumigatus (27, 28). Therefore, A. fumigatus exploits the PRR Dectin-1 to gain access to host cells in a NOD1/2-dependent fashion.
A. fumigatus activates all three UPR axes (Fig. 2), which required mitochondrial ROS production (152, 153). Interestingly, ER stress inhibition using 4-phenylbutyric acid (4-PBA) during A. fumigatus infection in an asthma mouse model reduced pulmonary inflammation, ROS generation, ER calcium depletion, and NLRP3 inflammasome activation (153). Thus, ER stress mediated by A. fumigatus infection leads to damaging inflammatory responses. This may involve NOD1/2 sensing of A. fumigatus-induced ER stress directly or possibly by regulating A. fumigatus-induced mitophagy.
Parasites.
Little is known regarding the role of NOD1/2 during infections with protozoan parasites; however, NOD1 and/or NOD2 is activated by a few parasites. Plasmodium berghei infection activated NOD1/2, but parasite replication or disease outcome was unaffected by NOD1/2 signaling (24). A related malarial parasite, Plasmodium falciparum, induced nitric oxide, which required NOD2, RIPK2, and the P. falciparum toxin hemozoin (25). Both NOD1 and NOD2 are required for NF-κB-dependent cytokine responses during Trypanosoma cruzi infection; however, NOD1, but not NOD2, restricted T. cruzi infection in mice (26). ER stress sensing by NOD1/2 may be a plausible mode of sensing and responding to parasitic infections. Indeed, P. berghei infection in the liver and brain activates and requires the IRE1α/JNK, ATF6, and PERK branches of the UPR (Fig. 2) (154–156). Proapoptotic factors, proinflammatory cytokines, and neuronal cell death were decreased in P. berghei-infected mice that were administered a JNK inhibitor (157). Thus, P. berghei induction of ER stress likely activates NOD1/2 proinflammatory pathways in both the liver and the brain. ER stress is critical for establishing infection in the liver, and NOD1/2 may therefore benefit P. berghei infection in the liver. ER stress is linked with inflammation at both infection sites (154, 157); however, potential NOD1/2 sensing of P. berghei-induced ER stress may lead to detrimental inflammatory effects in the brain, resulting in neuronal cell death. Indeed, NOD1/2 proinflammatory responses have been associated with hyperinflammation that can have adverse effects on cells (85). Whether NOD1/2 sense Plasmodium-induced ER stress and whether NOD1/2 may have organ-specific effects that result in differential disease outcomes warrant more investigation.
T. cruzi also causes ER stress as measured by assessing the PERK pathway. Inhibition of ER stress in T. cruzi-infected mice relieves cardiomyopathy and Chagas disease sequelae, which is consistent with decreased apoptosis and cardiac inflammation. Furthermore, T. cruzi infection reduced mitochondrial antioxidant gene expression, which was reversed with an ER stress inhibitor (158). NOD1/2 activation may respond to parasite-induced ER stress during T. cruzi infection and contribute to inflammation, leading to cardiomyopathy and the development of Chagas disease. However, early recognition of ER stress during T. cruzi infection by NOD1 may control parasite replication prior to disease onset (26).
Altogether, ER stress mostly promotes cell death during parasite infections suggesting that if NOD1/2 sense parasite-induced ER stress, then they likely contribute to a hyper-inflammatory response that could have detrimental effects by inducing killing of infected cells. Since NOD1 protects mice against T. cruzi infection (26), then perhaps early sensing and responses by NOD1/2 restrict parasite replication prior to later stages in disease in which NOD1/2 might contribute to cell death and adverse inflammatory effects.
Viruses.
NOD1 and/or NOD2 is activated during some viral infections and is mostly antiviral. NOD2 protects mice/cells against respiratory syncytial virus (RSV) (19), vesicular stomatitis virus (VSV) (19), IAV (17, 18, 159) and foot-and-mouth-disease virus (FMDV) (23) in a type I IFN-dependent manner (17, 19, 23, 159). Furthermore, both NOD1 and NOD2 are upregulated by and restrictive to human and mouse cytomegalovirus (HCMV and MCMV, respectively), which required type I IFN as well (20, 21, 160). Interestingly, single nucleotide polymorphisms in Nod1 correlated with the prevalence of HCMV infection in humans (21), further indicating its antiviral effects. A few viruses have countermeasures to inhibit NOD1/2 signaling, including FMDV and human immunodeficiency virus 1 (HIV-1). Although NOD2 transcription increased during FMDV infection, the virus strongly inhibited protein expression (23). HIV-1 cleaves and inactivates RIPK2 (161), suggesting that NOD1/2 signaling may be antiviral to HIV-1; yet, this is undetermined.
NOD1/2 significantly impact viral infections through their interplay with the antiviral type I IFN pathway. NOD1/2 and type I IFN signaling have significant overlap and can regulate each other (11, 162). Indeed, Nod1 (163), Nod2 and Ripk2 are ISGs (22) and are required for strong induction of type I IFN signaling (9, 19, 164). Introduction of single-stranded RNA (ssRNA) into cells to mimic viral infection led to NOD2-dependent, not NOD1, type I IFN responses (19). Sabbah et al. (19) revealed that mitochondrial antiviral signaling protein (MAVS) was required for the NOD2-mediated type I IFN response to ssRNA. Ectopically expressed NOD2 and MAVS interacted, and NOD2 localization to mitochondrial membranes increased upon RSV infection (19). Furthermore, NOD2 enhanced type I IFN and proinflammatory responses during infection with CMV by an unclear mechanism (20), since the DNA virus CMV does not have an RNA intermediate. NOD2 interacted with antiviral dsRNA-activated 2′-5′-oligoadenylate synthetase type 2 (OAS2), which enhances RNase L activity (165). RNase L is an endoribonuclease whose cleavage products initiate type I IFN signaling during viral infection (166). Hepatitis C virus (HCV) RNA-dependent RNA polymerase NS5B induced NOD1 expression in hepatocytes and IFN-β was decreased upon NOD1 inhibition (163). In contrast, NOD2 inhibited type I IFN signaling by directly interacting with RIG-I to negatively regulate RIG-I-induced type I IFN expression (167). Thus, NOD1/2 are intimately linked to type I IFN signaling, and yet how they regulate each other is largely unclear.
The mechanisms underlying NOD1/2 sensing of viruses are mostly elusive. NOD2 may interact with ssRNA (19). However, additional biochemical analyses are required to determine whether the interaction of NOD2 with ssRNA is direct or indirect to help understand the mechanism of NOD2 stimulation by ssRNA. Moreover, the double-stranded RNA mimetic poly(I·C) elicited strong inflammatory cytokine expression (Il8, Tnfα, and Ifnβ), which was reduced using a dominant negative Nod1 or cells depleted of Nod1 or Ripk2. NOD1 bound directly to poly(I·C), which was independent of the NOD1 LRR domain (163). Therefore, these studies indicate that NOD1/2 could be involved in direct sensing of viral single-stranded or double-stranded RNA, but more research is necessary to fully confirm these possibilities.
An alternative hypothesis for NOD1/2 sensing of viral infection could be that NOD1/2 are broadly sensing ER stress induced during viral infections or are involved in ER stress-mediated cellular processes such as autophagy and mitophagy (18, 29, 47, 50, 168). ER stress induction by viruses is a well-known phenomenon and usually occurs by ER membrane rearrangements induced by several viruses that establish replication complexes on the ER, disruption of ER-Golgi trafficking and/or by the large influx of viral proteins that need to be processed and folded (85, 86). Of the viruses known to be restricted by NOD1/2, RSV, IAV, and CMV stimulate ER stress (Fig. 2). FMDV is a member of the Picornaviridae family; other members of this family induce ER stress (169), but this has not been shown for FMDV specifically. RSV, IAV, and CMV uniquely affect ER stress pathways, but the IRE1α pathway that was implicated in NOD1/2 signaling (29) is activated by all three (170–175).
RSV infection causes ER stress as evidenced by ATF6 and IRE1α activation, and IRE1α inhibits RSV replication independent of Xbp1 (170). IRE1α-dependent restriction of RSV replication may occur by viral mRNA degradation via the RIDD pathway downstream of IRE1α (176), which has not been evaluated. Alternatively, NOD1/2 acting downstream of IRE1α may be responsible for restricting RSV replication.
HCMV differentially regulates the UPR (175). PERK and IRE1α were activated during CMV infection, whereas ATF6 was suppressed, and ER stress-inducing drugs inhibit CMV infection (173, 175, 177). PERK is critical for HCMV replication (178). HCMV inhibited IRE1α (177), and MCMV inhibited Xbp1 splicing by IRE1α (179). In addition, XBP1s target EDEM was downregulated by HCMV (175), further suggesting that CMV actively inhibits the IRE1α pathway. Indeed, cytomegalovirus (CMV) proteins M50 and UL50 downregulate IRE1α (180). However, XBP1 is important for CMV gene expression (173). It is likely that IRE1α signaling at different stages of CMV infection can lead to various effects on CMV replication. Therefore, ER stress induced by CMV likely activates IRE1α signaling early, but CMV then inhibits IRE1α signaling later in infection once enough M50 and UL50 have been generated. Thus, CMV may block ER stress-activated IRE1α signaling to inhibit antiviral NOD1/2 signaling.
ER stress induction by IAV has been studied rather extensively but may vary based on cell types and timing of infection. IRE1α is activated during IAV infection (171, 172, 174, 181), but the effects of IRE1α on IAV infection are unclear. Inhibition of IRE1α blocks IAV protein synthesis and replication (171), suggesting that ER stress is critical for IAV replication. In addition, knockdown of the ER stress chaperone ERp57 (also known as Grp58), critical for proper folding of IAV hemagglutinin (HA) protein, reduced IAV replication (172, 182). IRE1α is activated by HA, which upregulates ERAD and restricts IAV replication (174). However, this indicates that ER stress can restrict IAV infection. In addition, poorly glycosylated HA from pandemic-like IAVs, compared to commonly circulating human-adapted strains, causes significant ER stress and a heightened proinflammatory immune response which correlated with increased IAV pathogenesis. The IRE1α and PERK pathways were upregulated by a pandemic IAV strain, and ER stress inhibition reduced the production of proinflammatory cytokines (183). IAV replication was unaffected by ER stress in this study; however, pathogenesis induced by IAV increases with more ER stress. Lastly, IAV inhibits PERK phosphorylation, and chemical activation of PERK is antiviral (184). Overall, ER stress pathways are differentially regulated by IAV and result in various outcomes. One common theme is the induction of IRE1α, which can lead to NOD1/2 activation (29). Since NOD2 and RIPK2 have been shown to inhibit IAV infection (17, 18, 159), early detection of virus-induced ER stress by NOD1/2 could reduce IAV pathogenesis through upregulation of immune responses. Analysis of NOD1 and further analyses of NOD2 during IAV infection would be helpful to distinguish whether there is a role for IAV-induced ER stress in activating NOD1/2.
Despite its previously described antiviral role, NOD2 enhanced CVB3 replication and myocarditis in a CVB3-induced myocarditis mouse model. CVB3-induced apoptosis and NLRP3 activation were reduced in the absence of Nod2, which correlated with reduced myocarditis and production of proinflammatory mediators (16). NLRP3 was previously shown to contribute to CVB3 myocarditis (185), and it appears to be regulated by NOD2 in this infection model (16). How NOD2 promotes CVB3 replication and pathogenesis is unknown when it generally inhibits other viruses. Interestingly, CVB induces all three arms of UPR, which are important for CVB replication (186–188). Knockdown of each ER stress pathway modestly reduced CVB3 replication, indicating that ER stress response proteins are required for efficient CVB3 replication (187). CHOP was critical for CVB3 to establish myocarditis and replicate efficiently, and proinflammatory cytokine expression was significantly reduced with an ER stress inhibitor in CVB3-infected mice (186). CVB5 activated IRE1α and PERK in pancreatic β cells, which enhanced CVB5 replication. Inhibition of JNK, which is downstream of IRE1α, reduced CVB5-induced apoptosis and replication (188). Furthermore, a soluble factor produced by CVB3-infected cardiomyocytes induced ER stress in cardiac infiltrating macrophages leading to enhanced CVB3 pathogenesis, but replication was not assessed. In this study, inhibition of ER stress reduced cardiac macrophage production of proinflammatory cytokines and alleviated CVB3-induced myocarditis (189). CVB3 also induced Nod2 expression, which is important for viral infection (16). Therefore, CVB likely activates NOD2, and possibly NOD1, signaling by inducing ER stress, and this benefits CVB through promotion of infection by an unknown mechanism.
CONCLUDING REMARKS
The ER is a highly dynamic organelle that plays a major role in coordinating signaling pathways to ensure cellular homeostasis. Whether or not ER stress results in cell survival, inflammation, or cell death is determined by multiple different factors, such as the duration of ER stress, cell type, environmental and/or genetic factors, and infection with a variety of pathogens. NOD1 and NOD2 have been implicated in ER stress-induced inflammation either directly, by sensing the disruption in ER homeostasis, or indirectly, via ER stress-mediated processes such as autophagy, mitophagy and increases in intracellular calcium concentration. Are the functions of NOD1 and NOD2 solely to sense peptidoglycan, or do they have broader functions in the detection of pathogens? In this regard, it is of great interest to elucidate the underlying mechanisms of NOD1/2 activation by peptidoglycan-deficient pathogens. It is increasingly apparent that NOD1 and/or NOD2 is activated during parasitic, fungal, and viral infections, but whether sensing of ER stress by NOD1 and/or NOD2 is a common strategy to combat these infections remains to be elucidated.
Interestingly, evidence is accumulating for a role of ER stress-mediated inflammation and NOD1/2 signaling in a variety of chronic inflammatory disorders, such as inflammatory bowel disease, obesity, diabetes, rheumatoid arthritis, ankylosing spondylitis, and cardiovascular disease (190–197). What exactly are the roles of NOD1 and NOD2 in chronic inflammatory diseases? Do they recognize circulating peptidoglycan fragments which may be increased during chronic inflammation, is there an increase in intestinal microbiota translocation to various tissues, or can NOD1 and NOD2 sense ER stress and/or ER stress-mediated processes in the absence of peptidoglycan (198–200)? Targeting ER stress and the UPR pathways might offer opportunities for treating infectious diseases and chronic inflammatory diseases. However, a clearer understanding of the mechanisms that orchestrate ER stress-induced pathologies, whether activated by environmental and/or genetic factors or during infections, and a link with NOD1/2 signaling is required to safely modulate this process for the development of future therapeutics.
Biographies

Sharon K. Kuss-Duerkop received her B.S. in Biology from Angelo State University. Under the advisement of Dr. Julie Pfeiffer, Sharon completed her Ph.D. in Molecular Microbiology at the University of Texas Southwestern Medical Center in which she studied gastrointestinal influences on poliovirus infection. Her postdoctoral research characterizing the role of mTORC1 and mTORC2 during influenza virus infection was performed in Dr. Beatriz Fontoura’s laboratory at the University of Texas Southwestern Medical Center. Sharon became a Research Instructor at the University of Colorado Denver School of Medicine initially in the laboratory of Dr. Dohun Pyeon studying host restriction factors during human papillomavirus infection. She is currently a Research Instructor in the Keestra-Gounder laboratory, and her research over the last two years has focused on understanding how NOD1 and NOD2 respond to and influence viral infections.

A. Marijke Keestra-Gounder is an Assistant Professor at the University of Colorado Denver School of Medicine. She received her Ph.D. in microbiology and immunology at the Department of Infectious Diseases and Immunology, Utrecht University in the Netherlands, under the supervision of Dr. Jos van Putten. Her Ph.D. thesis project focused on the elucidation of the functions of Toll-like receptors (TLRs) of chicken to determine their ligand specificity and (dis)similarities with mammalian TLRs. During her postdoctoral training in the laboratory of Dr. Andreas Bäumler at the University of California at Davis, she investigated pathways of the innate immune system in response to Salmonella Typhimurium and Brucella abortus. Her research continues to focus on the activation of the host innate immune system and the roles of NOD1 and NOD2 during bacterial and viral infections.
REFERENCES
- 1.Ablasser A, Chen ZJ. 2019. cGAS in action: expanding roles in immunity and inflammation. Science 363:eaat8657. doi: 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- 2.Gong T, Liu L, Jiang W, Zhou R. 2020. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. 2015. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. 2013. Recognition of bacteria by inflammasomes. Annu Rev Immunol 31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 5.Asrat S, Davis KM, Isberg RR. 2015. Modulation of the host innate immune and inflammatory response by translocated bacterial proteins. Cell Microbiol 17:785–795. doi: 10.1111/cmi.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito C, Cabanes D, Sarmento Mesquita F, Sousa S. 2019. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell Mol Life Sci 76:1319–1339. doi: 10.1007/s00018-018-2992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance RE, Isberg RR, Portnoy DA. 2009. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. 2008. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol 83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. 2010. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest 120:1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keestra-Gounder AM, Tsolis RM. 2017. NOD1 and NOD2: beyond peptidoglycan sensing. Trends Immunol 38:758–767. doi: 10.1016/j.it.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutermarsh-Ott S, Eden K, Allen IC. 2016. Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure. J Gen Virol 97:825–838. doi: 10.1099/jgv.0.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 14.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 15.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem 278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 16.Tschope C, Muller I, Xia Y, Savvatis K, Pappritz K, Pinkert S, Lassner D, Heimesaat MM, Spillmann F, Miteva K, Bereswill S, Schultheiss HP, Fechner H, Pieske B, Kuhl U, Van Linthout S. 2017. NOD2 (nucleotide-binding oligomerization domain 2) is a major pathogenic mediator of coxsackievirus B3-induced myocarditis. Circ Heart Fail 10:e003870. doi: 10.1161/CIRCHEARTFAILURE.117.003870. [DOI] [PubMed] [Google Scholar]
- 17.Lupfer C, Thomas PG, Kanneganti TD. 2014. Nucleotide oligomerization and binding domain 2-dependent dendritic cell activation is necessary for innate immunity and optimal CD8+ T Cell responses to influenza A virus infection. J Virol 88:8946–8955. doi: 10.1128/JVI.01110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, Huang G, Green M, Kundu M, Chi H, Xavier RJ, Green DR, Lamkanfi M, Dinarello CA, Doherty PC, Kanneganti TD. 2013. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol 14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. 2009. Activation of innate immune antiviral responses by Nod2. Nat Immunol 10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor A, Forman M, Arav-Boger R. 2014. Activation of nucleotide oligomerization domain 2 (NOD2) by human cytomegalovirus initiates innate immune responses and restricts virus replication. PLoS One 9:e92704. doi: 10.1371/journal.pone.0092704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan YH, Roy S, Mukhopadhyay R, Kapoor A, Duggal P, Wojcik GL, Pass RF, Arav-Boger R. 2016. Role of nucleotide-binding oligomerization domain 1 (NOD1) and its variants in human cytomegalovirus control in vitro and in vivo. Proc Natl Acad Sci U S A 113:E7818–E7827. doi: 10.1073/pnas.1611711113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YG, Park JH, Reimer T, Baker DP, Kawai T, Kumar H, Akira S, Wobus C, Nunez G. 2011. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe 9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Zhu Z, Xue Q, Yang F, Cao W, Zhang K, Liu X, Zheng H. 2019. Foot-and-mouth disease virus antagonizes NOD2-mediated antiviral effects by inhibiting NOD2 protein expression. J Virol 93:e00124-19. doi: 10.1128/JVI.00124-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finney CA, Lu Z, LeBourhis L, Philpott DJ, Kain KC. 2009. Disruption of Nod-like receptors alters inflammatory response to infection but does not confer protection in experimental cerebral malaria. Am J Trop Med Hyg 80:718–722. doi: 10.4269/ajtmh.2009.80.718. [DOI] [PubMed] [Google Scholar]
- 25.Corbett Y, Parapini S, D’Alessandro S, Scaccabarozzi D, Rocha BC, Egan TJ, Omar A, Galastri L, Fitzgerald KA, Golenbock DT, Taramelli D, Basilico N. 2015. Involvement of Nod2 in the innate immune response elicited by malarial pigment hemozoin. Microbes Infect 17:184–194. doi: 10.1016/j.micinf.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva GK, Gutierrez FR, Guedes PM, Horta CV, Cunha LD, Mineo TW, Santiago-Silva J, Kobayashi KS, Flavell RA, Silva JS, Zamboni DS. 2010. Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J Immunol 184:1148–1152. doi: 10.4049/jimmunol.0902254. [DOI] [PubMed] [Google Scholar]
- 27.Gresnigt MS, Jaeger M,S, Malireddi RK, Rasid O, Jouvion G, Fitting C, Melchers WJG, Kanneganti TD, Carvalho A, Ibrahim-Granet O, van de Veerdonk FL. 2017. The absence of NOD1 enhances killing of Aspergillus fumigatus through modulation of Dectin-1 expression. Front Immunol 8:1777. doi: 10.3389/fimmu.2017.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gresnigt MS, Cunha C, Jaeger M, Goncalves SM, Malireddi RKS, Ammerdorffer A, Lubbers R, Oosting M, Rasid O, Jouvion G, Fitting C, Jong DJ, Lacerda JF, Campos A Jr, Melchers WJG, Lagrou K, Maertens J, Kanneganti TD, Carvalho A, Ibrahim-Granet O, van de Veerdonk FL. 2018. Genetic deficiency of NOD2 confers resistance to invasive aspergillosis. Nat Commun 9:2636. doi: 10.1038/s41467-018-04912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, de Jong MF, Kerrinnes T, Ravindran R, Luciw PA, McSorley SJ, Bäumler AJ, Tsolis RM. 2016. NOD1 and NOD2 signaling links ER stress with inflammation. Nature 532:394–397. doi: 10.1038/nature17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keestra AM, Winter MG, Auburger JJ, Frässle SP, Xavier MN, Winter SE, Kim A, Poon V, Ravesloot MM, Waldenmaier JFT, Tsolis RM, Eigenheer RA, Bäumler AJ. 2013. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 496:233–237. doi: 10.1038/nature12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendez JM, Kolora LD, Lemon JS, Dupree SL, Keestra-Gounder AM. 2019. Activation of the endoplasmic reticulum stress response impacts the NOD1 signaling pathway. Infect Immun 87:e00826-18. doi: 10.1128/IAI.00826-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molinaro R, Mukherjee T, Flick R, Philpott DJ, Girardin SE. 2019. Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress. J Biol Chem 294:9007–9015. doi: 10.1074/jbc.RA119.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyle JP, Mayle S, Parkhouse R, Monie TP. 2013. Comparative genomic and sequence analysis provides insight into the molecular functionality of NOD1 and NOD2. Front Immunol 4:317. doi: 10.3389/fimmu.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strober W, Murray PJ, Kitani A, Watanabe T. 2006. Signaling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 35.Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA. 2012. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem 287:23057–23067. doi: 10.1074/jbc.M112.344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maekawa S, Ohto U, Shibata T, Miyake K, Shimizu T. 2016. Crystal structure of NOD2 and its implications in human disease. Nat Commun 7:11813. doi: 10.1038/ncomms11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laroui H, Yan Y, Narui Y, Ingersoll SA, Ayyadurai S, Charania MA, Zhou F, Wang B, Salaita K, Sitaraman SV, Merlin D. 2011. l-Ala-γ-d-Glu-meso-diaminopimelic acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J Biol Chem 286:31003–31013. doi: 10.1074/jbc.M111.257501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimes CL, Ariyananda LDZ, Melnyk JE, O’Shea EK. 2012. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 134:13535–13537. doi: 10.1021/ja303883c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YC, Westcott NP, Griffin ME, Hang HC. 2019. Peptidoglycan metabolite photoaffinity reporters reveal direct binding to intracellular pattern recognition receptors and Arf GTPases. ACS Chem Biol 14:405–414. doi: 10.1021/acschembio.8b01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magalhaes JG, Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. 2011. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur J Immunol 41:1445–1455. doi: 10.1002/eji.201040827. [DOI] [PubMed] [Google Scholar]
- 41.Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, Knoefel WT, Reed JC. 2009. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci U S A 106:14524–14529. doi: 10.1073/pnas.0907131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan R, Liu Z. 2017. LRRK2 enhances Nod1/2-mediated inflammatory cytokine production by promoting Rip2 phosphorylation. Protein Cell 8:55–66. doi: 10.1007/s13238-016-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. 2014. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 44.Caruso R, Warner N, Inohara N, Nunez G. 2014. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon MY, Hwang N, Lee SJ, Chung SW. 2019. Nucleotide-binding oligomerization domain protein 2 attenuates ER stress-induced cell death in vascular smooth muscle cells. BMB Rep 52:665–670. doi: 10.5483/BMBRep.2019.52.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Maziere A, Klumperman J, Schlatter M, Delamarre L, Mellman I. 2014. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509:240–244. doi: 10.1038/nature13133. [DOI] [PubMed] [Google Scholar]
- 47.Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner LJ, Thomas BJ, Malosse C, Gantier MP, Casillas LN, Votta BJ, Bertin J, Boneca IG, Sasakawa C, Philpott DJ, Ferrero RL, Kaparakis-Liaskos M. 2014. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 15:623–635. doi: 10.1016/j.chom.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. 2005. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-κB activation in muramyl dipeptide recognition. J Cell Biol 170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kufer TA, Kremmer E, Adam AC, Philpott DJ, Sansonetti PJ. 2007. The pattern-recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell Microbiol 10:477–486. doi: 10.1111/j.1462-5822.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 50.Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nunez G, Girardin SE, Philpott DJ. 2010. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol 11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 51.Legrand-Poels S, Kustermans G, Bex F, Kremmer E, Kufer TA, Piette J. 2007. Modulation of Nod2-dependent NF-κB signaling by the actin cytoskeleton. J Cell Sci 120:1299–1310. doi: 10.1242/jcs.03424. [DOI] [PubMed] [Google Scholar]
- 52.Lipinski S, Grabe N, Jacobs G, Billmann-Born S, Till A, Hasler R, Aden K, Paulsen M, Arlt A, Kraemer L, Hagemann N, Erdmann KS, Schreiber S, Rosenstiel P. 2012. RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition. Proc Natl Acad Sci U S A 109:21426–21431. doi: 10.1073/pnas.1209673109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lecine P, Borg JP, Nunez G. 2005. A role for Erbin in the regulation of Nod2-dependent NF-κB signaling. J Biol Chem 280:40301–40309. doi: 10.1074/jbc.M508538200. [DOI] [PubMed] [Google Scholar]
- 54.Lu Y, Zheng Y, Coyaud E, Zhang C, Selvabaskaran A, Yu Y, Xu Z, Weng X, Chen JS, Meng Y, Warner N, Cheng X, Liu Y, Yao B, Hu H, Xia Z, Muise AM, Klip A, Brumell JH, Girardin SE, Ying S, Fairn GD, Raught B, Sun Q, Neculai D. 2019. Palmitoylation of NOD1 and NOD2 is required for bacterial sensing. Science 366:460–467. doi: 10.1126/science.aau6391. [DOI] [PubMed] [Google Scholar]
- 55.Hall A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 56.Fukazawa A, Alonso C, Kurachi K, Gupta S, Lesser CF, McCormick BA, Reinecker HC. 2008. GEF-H1-mediated control of NOD1-dependent NF-κB activation by Shigella effectors. PLoS Pathog 4:e1000228. doi: 10.1371/journal.ppat.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun H, Kamanova J, Lara-Tejero M, Galán JE. 2018. Salmonella stimulates proinflammatory signaling through p21-activated kinases bypassing innate immune receptors. Nat Microbiol 3:1122–1130. doi: 10.1038/s41564-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruno VM, Hannemann S, Lara-Tejero M, Flavell RA, Kleinstein SH, Galán JE. 2009. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog 5:e1000538. doi: 10.1371/journal.ppat.1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santy LC, Casanova JE. 2002. GTPase signaling: bridging the GAP between ARF and Rho. Curr Biol 12:R360–R362. doi: 10.1016/S0960-9822(02)00860-6. [DOI] [PubMed] [Google Scholar]
- 60.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Núñez G. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J Biol Chem 274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 61.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J Biol Chem 276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 62.Shakeri R, Kheirollahi A, Davoodi J. 2017. Apaf-1: regulation and function in cell death. Biochimie 135:111–125. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. 2008. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One 3:e2119. doi: 10.1371/journal.pone.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. 2008. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A 105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Velasco M, Prieto P, Terrón V, Benito G, Flores JM, Delgado C, Zaragoza C, Lavin B, Gómez-Parrizas M, López-Collazo E, Martín-Sanz P, Boscá L. 2012. NOD1 activation induces cardiac dysfunction and modulates cardiac fibrosis and cardiomyocyte apoptosis. PLoS One 7:e45260. doi: 10.1371/journal.pone.0045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carneiro LA, Travassos LH, Soares F, Tattoli I, Magalhaes JG, Bozza MT, Plotkowski MC, Sansonetti PJ, Molkentin JD, Philpott DJ, Girardin SE. 2009. Shigella induces mitochondrial dysfunction and cell death in nonmyeloid cells. Cell Host Microbe 5:123–136. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 68.Nakagawa T, Yuan J. 2000. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. 2001. Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 70.LeBlanc PM, Yeretssian G, Rutherford N, Doiron K, Nadiri A, Zhu L, Green DR, Gruenheid S, Saleh M. 2008. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 3:146–157. doi: 10.1016/j.chom.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Tran Van Nhieu G, Dupont G, Combettes L. 2018. Ca2+ signals triggered by bacterial pathogens and microdomains. Biochim Biophys Acta Mol Cell Res 1865:1838–1845. doi: 10.1016/j.bbamcr.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Foufelle F, Fromenty B. 2016. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect 4:e00211. doi: 10.1002/prp2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canton J, Schlam D, Breuer C, Gutschow M, Glogauer M, Grinstein S. 2016. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat Commun 7:11284. doi: 10.1038/ncomms11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Val-Blasco A, Piedras MJGM, Ruiz-Hurtado G, Suarez N, Prieto P, Gonzalez-Ramos S, Gómez-Hurtado N, Delgado C, Pereira L, Benito G, Zaragoza C, Domenech N, Crespo-Leiro MG, Vasquez-Echeverri D, Nuñez G, Lopez-Collazo E, Boscá L, Fernández-Velasco M. 2017. Role of NOD1 in heart failure progression via regulation of Ca2+ handling. J Am Coll Cardiol 69:423–433. doi: 10.1016/j.jacc.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 75.Song S, Tan J, Miao Y, Zhang Q. 2018. Crosstalk of ER stress-mediated autophagy and ER-phagy: involvement of UPR and the core autophagy machinery. J Cell Physiol 233:3867–3874. doi: 10.1002/jcp.26137. [DOI] [PubMed] [Google Scholar]
- 76.Gkikas I, Palikaras K, Tavernarakis N. 2018. The role of mitophagy in innate immunity. Front Immunol 9:1283. doi: 10.3389/fimmu.2018.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 78.Junjappa RP, Patil P, Bhattarai KR, Kim HR, Hj C. 2018. IRE1α implications in endoplasmic reticulum stress-mediated development and pathogenesis of autoimmune diseases. Front Immunol 9:1289. doi: 10.3389/fimmu.2018.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Guo Y, Tang J, Jiang J, Chen Z. 2014. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 80.Reverendo M, Mendes A, Arguello RJ, Gatti E, Pierre P. 2019. At the crossway of ER-stress and proinflammatory responses. FEBS J 286:297–310. doi: 10.1111/febs.14391. [DOI] [PubMed] [Google Scholar]
- 81.Galluzzi L, Diotallevi A, Magnani M. 2017. Endoplasmic reticulum stress and unfolded protein response in infection by intracellular parasites. Future Sci OA 3:FSO198. doi: 10.4155/fsoa-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM, O’Riordan MX. 2015. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43:451–462. doi: 10.1016/j.immuni.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao L, Lin H, Wen J, Sun Q, Gao Y, Xu X, Wang J, Zhang J, Weng D. 2018. The kinase receptor-interacting protein 1 is required for inflammasome activation induced by endoplasmic reticulum stress. Cell Death Dis 9:641. doi: 10.1038/s41419-018-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manes T, Glade K, Ziomek CA, Millan JL. 1990. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics 8:541–554. doi: 10.1016/0888-7543(90)90042-S. [DOI] [PubMed] [Google Scholar]
- 85.Smith JA. 2018. Regulation of cytokine production by the unfolded protein response: implications for infection and autoimmunity. Front Immunol 9:422. doi: 10.3389/fimmu.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Romero-Brey I, Bartenschlager R. 2016. Endoplasmic reticulum: the favorite intracellular niche for viral replication and assembly. Viruses 8:160. doi: 10.3390/v8060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Celli J, Tsolis RM. 2015. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat Rev Microbiol 13:71–82. doi: 10.1038/nrmicro3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mukherjee T, Hovingh ES, Foerster EG, Abdel-Nour M, Philpott DJ, Girardin SE. 2019. NOD1 and NOD2 in inflammation, immunity, and disease. Arch Biochem Biophys 670:69–81. doi: 10.1016/j.abb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 89.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, Plotkowski MC. 2005. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem 280:36714–36718. doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 90.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schüller S, Jones HE, Klein NJ, Núňez G, Wren BW, Bajaj-Elliott M. 2007. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol 9:2404–2416. doi: 10.1111/j.1462-5822.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 91.Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Nunez G, Inohara N. 2011. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol 186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 92.Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sonego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. 2010. The pattern recognition receptors Nod1 and Nod2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect 12:819–827. doi: 10.1016/j.micinf.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 93.Liu M, Haenssler E, Uehara T, Losick VP, Park JT, Isberg RR. 2012. The Legionella pneumophila EnhC protein interferes with immunostimulatory muramyl peptide production to evade innate immunity. Cell Host Microbe 12:166–176. doi: 10.1016/j.chom.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berrington WR, Iyer R, Wells RD, Smith KD, Skerrett SJ, Hawn TR. 2010. NOD1 and NOD2 regulation of pulmonary innate immunity to Legionella pneumophila. Eur J Immunol 40:3519–3527. doi: 10.1002/eji.201040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A, Boneca IG, Le Bourhis L, Reeve S, Smith IA, Hartland EL, Philpott DJ, Ferrero RL. 2010. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol 12:626–639. doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 96.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 97.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, Arditi M. 2009. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog 5:e1000379. doi: 10.1371/journal.ppat.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. 2011. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 99.Zola TA, Lysenko ES, Weiser JN. 2008. Mucosal clearance of capsule-expressing bacteria requires both TLR and nucleotide-binding oligomerization domain 1 signaling. J Immunol 181:7909–7916. doi: 10.4049/jimmunol.181.11.7909. [DOI] [PubMed] [Google Scholar]
- 100.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. 2008. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot JP, Giovannini M, Coyle A, Bertin J, Namane A, Rousselle JC, Cayet N, Prevost MC, Balloy V, Chignard M, Philpott DJ, Cossart P, Girardin SE. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A 104:997–1002. doi: 10.1073/pnas.0609672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, Dittrich-Breiholz O, Kracht M, Hidmark A, Wigzell H, Rottenberg ME. 2009. Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner. Infect Immun 77:2908–2918. doi: 10.1128/IAI.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geddes K, Rubino S, Streutker C, Cho JH, Magalhaes JG, Le Bourhis L, Selvanantham T, Girardin SE, Philpott DJ. 2010. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect Immun 78:5107–5115. doi: 10.1128/IAI.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Bourhis L, Magalhaes JG, Selvanantham T, Travassos LH, Geddes K, Fritz JH, Viala J, Tedin K, Girardin SE, Philpott DJ. 2009. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect Immun 77:4480–4486. doi: 10.1128/IAI.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marijke Keestra A, Winter MG, Klein-Douwel D, Xavier MN, Winter SE, Kim A, Tsolis RM, Bäumler AJ. 2011. A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway. mBio 2:e00266-11. doi: 10.1128/mBio.00266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 108.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. 2009. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci U S A 106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Jong MF, Starr T, Winter MG, den Hartigh AB, Child R, Knodler LA, van Dijl JM, Celli J, Tsolis RM. 2013. Sensing of bacterial type IV secretion via the unfolded protein response. mBio 4:e00418-12. doi: 10.1128/mBio.00418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antoniou AN, Lenart I, Kriston-Vizi J, Iwawaki T, Turmaine M, McHugh K, Ali S, Blake N, Bowness P, Bajaj-Elliott M, Gould K, Nesbeth D, Powis SJ. 2019. Salmonella exploits HLA-B27 and host unfolded protein responses to promote intracellular replication. Ann Rheum Dis 78:74–82. doi: 10.1136/annrheumdis-2018-213532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith JA, Khan M, Magnani DD, Harms JS, Durward M, Radhakrishnan GK, Liu YP, Splitter GA. 2013. Brucella induces an unfolded protein response via TcpB that supports intracellular replication in macrophages. PLoS Pathog 9:e1003785. doi: 10.1371/journal.ppat.1003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Radhakrishnan GK, Harms JS, Splitter GA. 2011. Modulation of microtubule dynamics by a TIR domain protein from the intracellular pathogen Brucella melitensis. Biochem J 439:79–83. doi: 10.1042/BJ20110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Byndloss MX, Tsai AY, Walker GT, Miller CN, Young BM, English BC, Seyffert N, Kerrinnes T, de Jong MF, Atluri VL, Winter MG, Celli J, Tsolis RM. 2019. Brucella abortus infection of placental trophoblasts triggers endoplasmic reticulum stress-mediated cell death and fetal loss via type IV secretion system-dependent activation of CHOP. mBio 10:e01538-19. doi: 10.1128/mBio.01538-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guimaraes ES, Gomes MTR, Campos PC, Mansur DS, Dos Santos AA, Harms J, Splitter G, Smith JA, Barber GN, Oliveira SC. 2019. Brucella abortus cyclic dinucleotides trigger STING-dependent unfolded protein response that favors bacterial replication. J Immunol 202:2671–2681. doi: 10.4049/jimmunol.1801233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu N, Li Y, Dong C, Xu X, Wei P, Sun W, Peng Q. 2016. Inositol-requiring enzyme 1-dependent activation of AMPK promotes Brucella abortus intracellular growth. J Bacteriol 198:986–993. doi: 10.1128/JB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taguchi Y, Imaoka K, Kataoka M, Uda A, Nakatsu D, Horii-Okazaki S, Kunishige R, Kano F, Murata M. 2015. Yip1A, a novel host factor for the activation of the IRE1 pathway of the unfolded protein response during Brucella infection. PLoS Pathog 11:e1004747. doi: 10.1371/journal.ppat.1004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci 114:4637–4650. [DOI] [PubMed] [Google Scholar]
- 118.Derre I, Swiss R, Agaisse H. 2011. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog 7:e1002092. doi: 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hempstead AD, Isberg RR. 2015. Inhibition of host cell translation elongation by Legionella pneumophila blocks the host cell unfolded protein response. Proc Natl Acad Sci U S A 112:E6790–E6797. doi: 10.1073/pnas.1508716112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shima K, Klinger M, Schutze S, Kaufhold I, Solbach W, Reiling N, Rupp J. 2015. The role of endoplasmic reticulum-related BiP/GRP78 in interferon gamma-induced persistent Chlamydia pneumoniae infection. Cell Microbiol 17:923–934. doi: 10.1111/cmi.12416. [DOI] [PubMed] [Google Scholar]
- 121.Webster SJ, Ellis L, O’Brien LM, Tyrrell B, Fitzmaurice TJ, Elder MJ, Clare S, Chee R, Gaston JSH, Goodall JC. 2016. IRE1α mediates PKR activation in response to Chlamydia trachomatis infection. Microbes Infect 18:472–483. doi: 10.1016/j.micinf.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Packiam M, Weinrick B, Jacobs WR Jr, Maurelli AT. 2015. Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves “chlamydial anomaly. Proc Natl Acad Sci U S A 112:11660–11665. doi: 10.1073/pnas.1514026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zou Y, Lei W, He Z, Li Z. 2016. The role of NOD1 and NOD2 in host defense against chlamydial infection. FEMS Microbiol Lett 363:fnw170. doi: 10.1093/femsle/fnw170. [DOI] [PubMed] [Google Scholar]
- 124.Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. 2009. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol 183:8099–8109. doi: 10.4049/jimmunol.0900664. [DOI] [PubMed] [Google Scholar]
- 125.Baird M, Woon Ang P, Clark I, Bishop D, Oshima M, Cook MC, Hemmings C, Takeishi S, Worthley D, Boussioutas A, Wang TC, Taupin D. 2013. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell-autonomous manner. Lab Invest 93:112–122. doi: 10.1038/labinvest.2012.131. [DOI] [PubMed] [Google Scholar]
- 126.Akazawa Y, Isomoto H, Matsushima K, Kanda T, Minami H, Yamaghchi N, Taura N, Shiozawa K, Ohnita K, Takeshima F, Nakano M, Moss J, Hirayama T, Nakao K. 2013. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS One 8:e82322. doi: 10.1371/journal.pone.0082322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu P, Xue J, Zhang ZJ, Jia YP, Tong YN, Han D, Li Q, Xiang Y, Mao XH, Tang B. 2017. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis 8:3207. doi: 10.1038/s41419-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. 2008. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW. 2008. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol 10:1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 131.Morinaga N, Yahiro K, Matsuura G, Moss J, Noda M. 2008. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cell Microbiol 10:921–929. doi: 10.1111/j.1462-5822.2007.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee SY, Lee MS, Cherla RP, Tesh VL. 2008. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol 10:770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 133.Pillich H, Loose M, Zimmer KP, Chakraborty T. 2012. Activation of the unfolded protein response by Listeria monocytogenes. Cell Microbiol 14:949–964. doi: 10.1111/j.1462-5822.2012.01769.x. [DOI] [PubMed] [Google Scholar]
- 134.Gekara NO, Westphal K, Ma B, Rohde M, Groebe L, Weiss S. 2007. The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol 9:2008–2021. doi: 10.1111/j.1462-5822.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 135.Wadsworth SJ, Goldfine H. 1999. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun 67:1770–1778. doi: 10.1128/.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Val-Blasco A, Prieto P, Gonzalez-Ramos S, Benito G, Vallejo-Cremades MT, Pacheco I, González-Peramato P, Agra N, Terrón V, Delgado C, Martín-Sanz P, Boscá L, Fernández-Velasco M. 2017. NOD1 activation in cardiac fibroblasts induces myocardial fibrosis in a murine model of type 2 diabetes. Biochem J 474:399–410. doi: 10.1042/BCJ20160556. [DOI] [PubMed] [Google Scholar]
- 137.Sun C, Wang H, Chen S, Li Z, Li S, Wang J. 2014. Recombinant Clostridium difficile toxin B induces endoplasmic reticulum stress in mouse colonal carcinoma cells. Acta Biochim Biophys Sin (Shanghai) 46:973–981. doi: 10.1093/abbs/gmu091. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Ma Y, Sun CL, Li S, Wang JF. 2016. High mobility group Box1 protein is involved in endoplasmic reticulum stress induced by Clostridium difficile toxin A. Biomed Res Int 2016:1–7. doi: 10.1155/2016/4130834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang Y, Xu L, Yu L, Xu X, Feng C, Li J. 2019. NOX4 inhibition protects enteric glial cells against Clostridium difficile toxin B toxicity via attenuating oxidative and endoplasmic reticulum stresses. Free Radic Res 53:932–940. doi: 10.1080/10715762.2019.1649670. [DOI] [PubMed] [Google Scholar]
- 140.Tentaku A, Shimohata T, Hatayama S, Kido J, Nguyen AQ, Kanda Y, Fukushima S, Uebanso T, Iwata T, Mawatari K, Harada N, Takahashi A. 2018. Host cellular unfolded protein response signaling regulates Campylobacter jejuni invasion. PLoS One 13:e0205865. doi: 10.1371/journal.pone.0205865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van ‘T Wout EF, van Schadewijk A, van Boxtel R, Dalton LE, Clarke HJ, Tommassen J, Marciniak SJ, Hiemstra PS. 2015. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog 11:e1004946. doi: 10.1371/journal.ppat.1004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim S, Joe Y, Park SU, Jeong SO, Kim JK, Park SH, Pae HO, Surh YJ, Shin J, Chung HT. 2018. Induction of endoplasmic reticulum stress under endotoxin tolerance increases inflammatory responses and decreases Pseudomonas aeruginosa pneumonia. J Leukoc Biol 104:1003–1012. doi: 10.1002/JLB.3A0317-106RRR. [DOI] [PubMed] [Google Scholar]
- 143.Jiang F, Wang X, Wang B, Chen L, Zhao Z, Waterfield NR, Yang G, Jin Q. 2016. The Pseudomonas aeruginosa type VI secretion PGAP1-like effector induces host autophagy by activating endoplasmic reticulum stress. Cell Rep 16:1502–1509. doi: 10.1016/j.celrep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 144.Richardson CE, Kooistra T, Kim DH. 2010. An essential role for XBP-1 in host protection against immune activation in Caenorhabditis elegans. Nature 463:1092–1095. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martino MEB, Olsen JC, Fulcher NB, Wolfgang MC, O’Neal WK, Ribeiro CMP. 2009. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem 284:14904–14913. doi: 10.1074/jbc.M809180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abuaita BH, Burkholder KM, Boles BR, O’Riordan MX. 2015. The endoplasmic reticulum stress sensor inositol-requiring enzyme 1α augments bacterial killing through sustained oxidant production. mBio 6:e00705-15. doi: 10.1128/mBio.00705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim YM, Jin J, Choi JA, Cho SN, Lim YJ, Lee JH, Seo JY, Chen HY, Rha KS, Song CH. 2014. Staphylococcus aureus enterotoxin B-induced endoplasmic reticulum stress response is associated with chronic rhinosinusitis with nasal polyposis. Clin Biochem 47:96–103. doi: 10.1016/j.clinbiochem.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 148.Bel S, Pendse M, Wang Y, Li Y, Ruhn KA, Hassell B, Leal T, Winter SE, Xavier RJ, Hooper LV. 2017. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 357:1047–1052. doi: 10.1126/science.aal4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loose M, Hudel M, Zimmer KP, Garcia E, Hammerschmidt S, Lucas R, Chakraborty T, Pillich H. 2015. Pneumococcal hydrogen peroxide-induced stress signaling regulates inflammatory genes. J Infect Dis 211:306–316. doi: 10.1093/infdis/jiu428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Y, Wu J, Xin Z, Wu X. 2014. Aspergillus fumigatus triggers innate immune response via NOD1 signaling in human corneal epithelial cells. Exp Eye Res 127:170–178. doi: 10.1016/j.exer.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 151.Goyal S, Castrillon-Betancur JC, Klaile E, Slevogt H. 2018. The interaction of human pathogenic fungi with C-type lectin receptors. Front Immunol 9:1261. doi: 10.3389/fimmu.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee KS, Jeong JS, Kim SR, Cho SH, Kolliputi N, Ko YH, Lee KB, Park SC, Park HJ, Lee YC. 2016. Phosphoinositide 3-kinase-delta regulates fungus-induced allergic lung inflammation through endoplasmic reticulum stress. Thorax 71:52–63. doi: 10.1136/thoraxjnl-2015-207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee HY, Lee GH, Kim HR, Lee YC, Chae HJ. 2020. PI3Kδ controls endoplasmic reticulum membrane fluidity and permeability in fungus-induced allergic inflammation. Br J Pharmacol 177:1556–1567. doi: 10.1111/bph.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Inacio P, Zuzarte-Luis V, Ruivo MT, Falkard B, Nagaraj N, Rooijers K, Mann M, Mair G, Fidock DA, Mota MM. 2015. Parasite-induced ER stress response in hepatocytes facilitates Plasmodium liver-stage infection. EMBO Rep 16:955–964. doi: 10.15252/embr.201439979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anand SS, Babu PP. 2013. Endoplasmic reticulum stress and neurodegeneration in experimental cerebral malaria. Neurosignals 21:99–111. doi: 10.1159/000336970. [DOI] [PubMed] [Google Scholar]
- 156.Anand SS, Babu PP. 2011. c-Jun N terminal kinases (JNK) are activated in the brain during the pathology of experimental cerebral malaria. Neurosci Lett 488:118–122. doi: 10.1016/j.neulet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 157.Anand SS, Maruthi M, Babu PP. 2013. The specific, reversible JNK inhibitor SP600125 improves survivability and attenuates neuronal cell death in experimental cerebral malaria (ECM). Parasitol Res 112:1959–1966. doi: 10.1007/s00436-013-3352-0. [DOI] [PubMed] [Google Scholar]
- 158.Ayyappan JP, Lizardo K, Wang S, Yurkow E, Nagajyothi JF. 2019. Inhibition of ER stress by 2-aminopurine treatment modulates cardiomyopathy in a murine chronic Chagas disease model. Biomol Ther (Seoul) 27:386–394. doi: 10.4062/biomolther.2018.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J. 2012. Muramyl dipeptide induces NOD2-dependent Ly6Chigh monocyte recruitment to the lungs and protects against influenza virus infection. PLoS One 7:e36734. doi: 10.1371/journal.pone.0036734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kapoor A, Fan YH, Arav-Boger R. 2016. Bacterial muramyl dipeptide (MDP) restricts human cytomegalovirus replication via an IFN-β-dependent pathway. Sci Rep 6:20295. doi: 10.1038/srep20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wagner RN, Reed JC, Chanda SK. 2015. HIV-1 protease cleaves the serine-threonine kinases RIPK1 and RIPK2. Retrovirology 12:74. doi: 10.1186/s12977-015-0200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pashenkov MV, Dagil YA, Pinegin BV. 2018. NOD1 and NOD2: molecular targets in prevention and treatment of infectious diseases. Int Immunopharmacol 54:385–400. doi: 10.1016/j.intimp.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 163.Vegna S, Gregoire D, Moreau M, Lassus P, Durantel D, Assenat E, Hibner U, Simonin Y. 2016. NOD1 participates in the innate immune response triggered by hepatitis C virus polymerase. J Virol 90:6022–6035. doi: 10.1128/JVI.03230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. 2009. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dugan JW, Albor A, David L, Fowlkes J, Blackledge MT, Martin TM, Planck SR, Rosenzweig HL, Rosenbaum JT, Davey MP. 2009. Nucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol Immunol 47:560–566. doi: 10.1016/j.molimm.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malathi K, Dong B, Gale M Jr, Silverman RH. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morosky SA, Zhu J, Mukherjee A, Sarkar SN, Coyne CB. 2011. Retinoic acid-induced gene-I (RIG-I) associates with nucleotide-binding oligomerization domain-2 (NOD2) to negatively regulate inflammatory signaling. J Biol Chem 286:28574–28583. doi: 10.1074/jbc.M111.227942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. 2010. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 169.Jheng JR, Ho JY, Horng JT. 2014. ER stress, autophagy, and RNA viruses. Front Microbiol 5:388. doi: 10.3389/fmicb.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hassan I, Gaines KS, Hottel WJ, Wishy RM, Miller SE, Powers LS, Rutkowski DT, Monick MM. 2014. Inositol-requiring enzyme 1 inhibits respiratory syncytial virus replication. J Biol Chem 289:7537–7546. doi: 10.1074/jbc.M113.510594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hassan IH, Zhang MS, Powers LS, Shao JQ, Baltrusaitis J, Rutkowski DT, Legge K, Monick MM. 2012. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J Biol Chem 287:4679–4689. doi: 10.1074/jbc.M111.284695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roberson EC, Tully JE, Guala AS, Reiss JN, Godburn KE, Pociask DA, Alcorn JF, Riches DW, Dienz O, Janssen-Heininger YM, Anathy V. 2012. Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-beta release in lung epithelial cells. Am J Respir Cell Mol Biol 46:573–581. doi: 10.1165/rcmb.2010-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drori A, Messerle M, Brune W, Tirosh B. 2014. Lack of XBP-1 impedes murine cytomegalovirus gene expression. PLoS One 9:e110942. doi: 10.1371/journal.pone.0110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Frabutt DA, Wang B, Riaz S, Schwartz RC, Zheng YH. 2017. Innate sensing of influenza A virus hemagglutinin glycoproteins by the host endoplasmic reticulum (ER) stress pathway triggers a potent antiviral response via ER-associated protein degradation. J Virol 92:e01690-17. doi: 10.1128/JVI.01690-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Isler JA, Skalet AH, Alwine JC. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol 79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. 2009. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xuan B, Qian Z, Torigoi E, Yu D. 2009. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol 83:3463–3474. doi: 10.1128/JVI.02307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu Y, Pierciey FJ Jr, Maguire TG, Alwine JC. 2013. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog 9:e1003266. doi: 10.1371/journal.ppat.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qian Z, Xuan B, Chapa TJ, Gualberto N, Yu D. 2012. Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response. J Virol 86:6712–6723. doi: 10.1128/JVI.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stahl S, Burkhart JM, Hinte F, Tirosh B, Mohr H, Zahedi RP, Sickmann A, Ruzsics Z, Budt M, Brune W. 2013. Cytomegalovirus downregulates IRE1 to repress the unfolded protein response. PLoS Pathog 9:e1003544. doi: 10.1371/journal.ppat.1003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Numajiri Haruki A, Naito T, Nishie T, Saito S, Nagata K. 2011. Interferon-inducible antiviral protein MxA enhances cell death triggered by endoplasmic reticulum stress. J Interferon Cytokine Res 31:847–856. doi: 10.1089/jir.2010.0132. [DOI] [PubMed] [Google Scholar]
- 182.Solda T, Garbi N, Hammerling GJ, Molinari M. 2006. Consequences of ERp57 deletion on oxidative folding of obligate and facultative clients of the calnexin cycle. J Biol Chem 281:6219–6226. doi: 10.1074/jbc.M513595200. [DOI] [PubMed] [Google Scholar]
- 183.Hrincius ER, Liedmann S, Finkelstein D, Vogel P, Gansebom S, Samarasinghe AE, You D, Cormier SA, McCullers JA. 2015. Acute lung injury results from innate sensing of viruses by an ER stress pathway. Cell Rep 11:1591–1603. doi: 10.1016/j.celrep.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Landeras-Bueno S, Fernández Y, Falcón A, Oliveros JC, Ortín J. 2016. Chemical genomics identifies the PERK-mediated unfolded protein stress response as a cellular target for influenza virus inhibition. mBio 7:e00085-16. doi: 10.1128/mBio.00085-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang Y, Gao B, Xiong S. 2014. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol 307:H1438–H1447. doi: 10.1152/ajpheart.00441.2014. [DOI] [PubMed] [Google Scholar]
- 186.Cai Z, Shen L, Ma H, Yang J, Yang D, Chen H, Wei J, Lu Q, Wang DW, Xiang M, Wang J. 2015. Involvement of endoplasmic reticulum stress-mediated C/EBP homologous protein activation in coxsackievirus B3-induced acute viral myocarditis. Circ Heart Fail 8:809–818. doi: 10.1161/CIRCHEARTFAILURE.114.001244. [DOI] [PubMed] [Google Scholar]
- 187.Zhang HM, Ye X, Su Y, Yuan J, Liu Z, Stein DA, Yang D. 2010. Coxsackievirus B3 infection activates the unfolded protein response and induces apoptosis through downregulation of p58IPK and activation of CHOP and SREBP1. J Virol 84:8446–8459. doi: 10.1128/JVI.01416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Colli ML, Paula FM, Marselli L, Marchetti P, Roivainen M, Eizirik DL, Op de Beeck A. 2019. Coxsackievirus B tailors the unfolded protein response to favour viral amplification in pancreatic beta cells. J Innate Immun 11:375–390. doi: 10.1159/000496034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang H, Yue Y, Sun T, Wu X, Xiong S. 2017. Transmissible endoplasmic reticulum stress from myocardiocytes to macrophages is pivotal for the pathogenesis of CVB3-induced viral myocarditis. Sci Rep 7:42162. doi: 10.1038/srep42162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaser A, Blumberg RS. 2011. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology 140:1738–1747. doi: 10.1053/j.gastro.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mohan S, R PRM, Brown L, Ayyappan P, G RK. 2019. Endoplasmic reticulum stress: a master regulator of metabolic syndrome. Eur J Pharmacol 860:172553. doi: 10.1016/j.ejphar.2019.172553. [DOI] [PubMed] [Google Scholar]
- 192.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 193.Schertzer JD, Tamrakar AK, Magalhaes JG, Pereira S, Bilan PJ, Fullerton MD, Liu Z, Steinberg GR, Giacca A, Philpott DJ, Klip A. 2011. NOD1 activators link innate immunity to insulin resistance. Diabetes 60:2206–2215. doi: 10.2337/db11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Colbert RA, Tran TM, Layh-Schmitt G. 2014. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol 57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shen L, Li L, Li M, Wang W, Yin W, Liu W, Hu Y. 2018. Silencing of NOD2 protects against diabetic cardiomyopathy in a murine diabetes model. Int J Mol Med 42:3017–3026. doi: 10.3892/ijmm.2018.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crane AM, Bradbury L, van Heel DA, McGovern DP, Brophy S, Rubin L, Siminovitch KA, Wordsworth BP, Calin A, Brown MA. 2002. Role of NOD2 variants in spondylarthritis. Arthritis Rheum 46:1629–1633. doi: 10.1002/art.10329. [DOI] [PubMed] [Google Scholar]
- 197.González-Ramos S, Paz-García M, Rius C, Monte-Monge A, Rodríguez C, Fernández-García V, Andrés V, Martínez-González J, Lasunción MA, Martín-Sanz P, Soehnlein O, Boscá L. 2019. Endothelial NOD1 directs myeloid cell recruitment in atherosclerosis through VCAM-1. FASEB J 33:3912–3921. doi: 10.1096/fj.201801231RR. [DOI] [PubMed] [Google Scholar]
- 198.Chan KL, Tam TH, Boroumand P, Prescott D, Costford SR, Escalante NK, Fine N, Tu Y, Robertson SJ, Prabaharan D, Liu Z, Bilan PJ, Salter MW, Glogauer M, Girardin SE, Philpott DJ, Klip A. 2017. Circulating NOD1 activators and hematopoietic NOD1 contribute to metabolic inflammation and insulin resistance. Cell Rep 18:2415–2426. doi: 10.1016/j.celrep.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 199.Costa FR, Francozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Camara NO, de Zoete MR, Palm NW, Flavell RA, Silva JS, Carlos D. 2016. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 213:1223–1239. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh K, Han K, Tilve S, Wu K, Geller HM, Sack MN. 2018. Parkin targets NOD2 to regulate astrocyte endoplasmic reticulum stress and inflammation. Glia 66:2427–2437. doi: 10.1002/glia.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]