Abstract
Background: Kratom has a long history of traditional medicine use in Southeast Asia. Consumption of kratom products has also been reported in the US and other regions of the world. Pain relief is among many self-reported kratom effects but have not been evaluated in controlled human subject research. Methods: Kratom effects on pain tolerance were assessed in a randomized, placebo-controlled, double-blind study. During a 1-day inpatient stay, participants received a randomized sequence of kratom and placebo decoctions matched for taste and appearance. Pain tolerance was measured objectively in a cold pressor task (CPT) as time (seconds) between the pain onset and the hand withdrawal from the ice bath. Health status, vital signs, objective, and subjective indicators of withdrawal symptoms, self-reported data on lifetime kratom use patterns, and assessments of blinding procedures were also evaluated. Results: Twenty-six males with the mean (SD) age 24.3 (3.4) years were enrolled. They reported the mean (SD) 6.1 (3.2) years of daily kratom consumption. Pain tolerance increased significantly 1 hour after kratom ingestion from the mean (SD) 11.2 (6.7) seconds immediately before to 24.9 (39.4) seconds 1 hour after kratom consumption (F(2,53.7)=4.33, p=0.02). Pain tolerance was unchanged after consuming placebo drinks: 15.0 (19.0) seconds immediately before and 12.0 (8.1) seconds 1 hour after consumption of placebo (F(2,52.8)=0.93, p=0.40). No discomfort or signs of withdrawal were reported or observed during 10-20 hours of kratom discontinuation. Conclusions: Kratom decoction demonstrated a substantial and statistically significant increase in pain tolerance. Further rigorous research on kratom pain-relieving properties and a safety profile is needed.
Keywords: kratom, pain tolerance, plant medicine
Introduction
Consumption of the leaves of kratom tree (Mitragyna speciosa, Rubiaceae family) has a long history in Southeast Asia [1-3]. Whole leaves and their extracts have been consumed for their psychoactive properties or to self-manage or self-treat a broad range of conditions or ailments including pain, opioid withdrawal symptoms, and other conditions [4,5]. However, these self-reported beneficial kratom effects summarized in multiple peer-reviewed publications have not been evaluated in rigorously controlled clinical or laboratory research with human participants.
An increase in consumption of kratom based products has been also reported in recent years in the US and other countries due to claims of successful self-management of pain and opioid withdrawal symptoms [6-8]. Besides these alleged therapeutic effects, issues related to potential toxicity and fatal incidents that have been reportedly attributed to kratom products [2,9-11], as well as potential addictive properties of kratom or its active compounds are frequently debated within the scientific community and among regulatory agencies in the US and in other countries [12].
A substantial body of animal research has been conducted on mitragynine and 7-hydroxymitragynine, two of many active compounds identified in the kratom leaves [4]. Mitragynine was found to have unique morphine-like or opioid receptor agonist effects on guinea-pig ileum [13], and to have anti-nociceptive action via the supraspinal µ and δ opioid receptors in both in vivo and in vitro studies [14], while 7-hydroxymitragynine has been reported to have a high affinity for µ opioid receptors using receptor-binding assays and to inhibit electrically induced contraction through opioid receptors in guinea-pig ileum [15,16]. Experimental animal model studies have suggested possible analgesic properties of these chemical compounds [17,18]. Two recent studies also demonstrated a possibility of mitragynine crossing the blood-brain barrier (BBB) [19,20]. Yusof et al., additionally demonstrated that mitragynine has a higher capacity to cross the BBB than 7‐hydroxymitragynine, however, while in the brain, 7‐hydroxymitragynine may be more available for receptor binding than mitragynine [20]. While it has been reported that mitragynine and 7-hydroxymitragynine may act as partial agonists at opioid receptors [21], the structural and chemical properties of both mitragynine and 7-hydroxymitragynine are very different from all known opioids [16,21,22]. Furthermore, kratom leaves contain many additional compounds that have not been extensively evaluated [1,21-23], and presently there is no evidence to indicate which, if any, of these compounds cross the blood-brain barrier or have any potential analgesic or other medicinal properties.
Typically, only the kratom leaves are consumed, including chewing the whole leaves, ingesting or smoking dried and pulverized leaves, or drinking water extracts based on steeping or boiling of the leaf material [2-4,24]. In Malaysia, kratom is primarily consumed as a decoction, where the leaves are boiled for several hours and the resulting liquid is consumed several times throughout the day [5].
To evaluate previously reported potential beneficial effects of consuming kratom leaf preparations on pain, a randomized, placebo-controlled, double-blind, pilot study was conducted. The study assessed changes in pain tolerance, other physiologic responses, and changes in potential withdrawal signs or symptoms during an initial discontinuation of kratom use followed by consumption of kratom or placebo decoctions in a controlled laboratory setting. The study enrolled individuals experienced with kratom through their long-term, daily, habitual kratom consumption who were otherwise healthy. The study design aimed to closely approximate the frequency and amounts of kratom consumed in natural settings, and the process of preparing the study active decoction followed recipes and methods observed in previous field research in Malaysia [2,5]. Objective pain measurements obtained through repeated administration of a cold pressor task (CPT) [25,26], and other objective and subjective standardized assessments were employed in the study.
Methods
The study protocol and procedures were reviewed and approved by the Institutional Review Board of Universiti Sains Malaysia (USM) and registered at ClinicalTrials.gov: NCT03414099.
Study Hypothesis and Sample Size Estimation
It was hypothesized that consumption of an active kratom decoction will result in a statistically significant increase in pain tolerance (the primary outcome) measured as the time difference (in seconds) between the pain onset after a hand immersion in ice water bath and the hand withdrawal from the ice water bath during the CPT. No controlled human studies on kratom related pain effects have been published to date. Consequently, no prior data were available to estimate the potential effect size of the hypothesized pain tolerance increase. The sample size of 20-30 participants was determined to have > 80% power to detect large effects (Cohen’s d’=0.6 or larger) for the proposed within-subject pilot study comparing kratom vs placebo at two-sided alpha level of 0.05.
Participant Screening and Enrollment
Because no prior well-controlled, clinical studies collecting objective, physiological data on kratom safety profile in humans were conducted, the study design included a restrictive set of inclusion and exclusion criteria and an extensive screening protocol to safeguard that no participants without an extensive prior kratom experience were exposed to kratom during the laboratory procedures and that potential risks due to the study participation were minimized. Consequently, a sample of individuals with a long history of a frequent, daily kratom consumption who were otherwise healthy, based on extensive and objective health evaluation was targeted for the study enrollment. Participants were recruited in areas of Penang state in Malaysia with a high prevalence of kratom use identified in previous research. Study personnel travelled to locations where kratom using individuals live, socialize, buy, and consume kratom. They provided information about the study and handed out a contact phone number for those who were interested in study participation. Additionally, participants who took part in the study were asked to provide the study contact phone number to other individuals in their communities. Those who expressed interest in the study were briefed about objectives and procedures and those who were interested in participation were referred to a voluntary, free health screening to evaluate their overall health status. The health screening was conducted by the medical personnel of an independent ambulatory clinic who were not part of the study. The health screening protocol included an interview, a physical evaluation, and collection of blood and urine samples to assess the overall health status, current and past use of psychoactive substances, including opioids, amphetamine type stimulants (ATS), benzodiazepines, marijuana, ketamine, and alcohol; HIV and syphilis status; chronic liver disease (e.g. cirrhosis, hepatitis A, B, C, non-alcoholic fatty liver disease); coronary heart disease and diabetes; and histories of psychological, psychiatric, or neurological problems and pain conditions.
The study eligibility criteria were: female or male individuals, 18-years-old or older, with at least 11 years of education, who reported daily kratom intake during the past 12 months. The exclusion criteria were: a positive urine drug test for any of the tested substances (opioids, ATS, benzodiazepines, marijuana, ketamine); a history of significant psychological or neurological problems, current or past alcohol problems; history of medical conditions, including liver disease (e.g. cirrhosis, hepatitis A, B, C, non-alcoholic fatty liver disease), coronary heart disease, diabetes, chronic pain, and psychiatric disorders; HIV or syphilis infection.
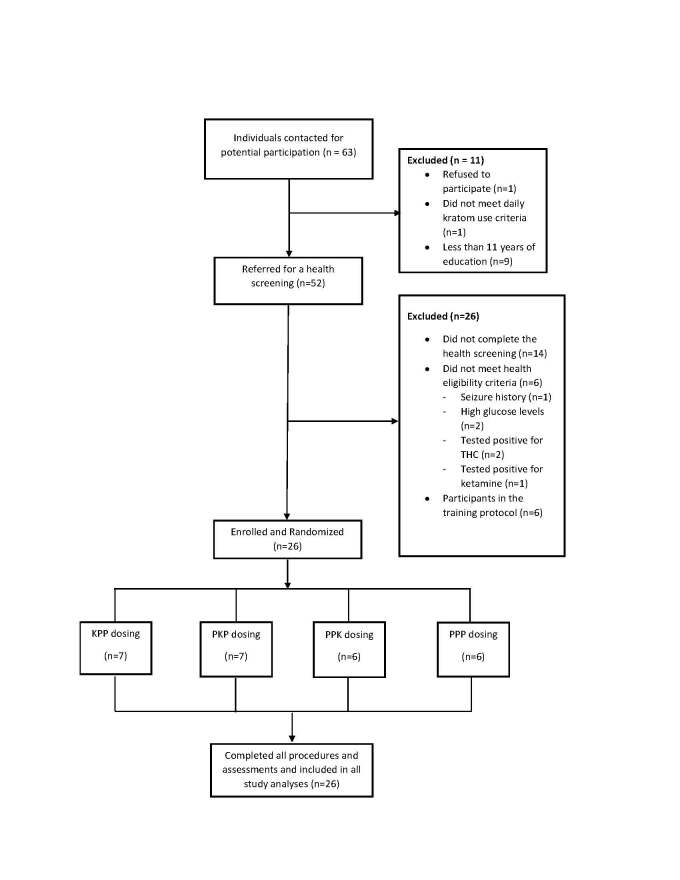
Sixty-three individuals were contacted: one refused to participate, one did not meet criteria for daily kratom use, one had a history of seizure, nine had less than 11 years of education, two had high glucose levels, two tested positive for tetrahydrocannabinol (THC), one tested positive for ketamine, 14 individuals did not complete the health screening, and six individuals were part of a training protocol: 26 male participants were enrolled (see Figure 1 for the CONSORT diagram). Despite extensive outreach efforts, no females who expressed interest in study participation were identified.
Figure 1.
The CONSORT diagram illustrating participant flow in the study.
The study protocol included a training phase aimed to train the research personnel on all laboratory and assessment procedures planned and specified in the research protocol. The current study is the first study enrolling human subjects where kratom has been administered under placebo controlled, double-blind conditions, and where the cold pressor task (CPT) and other assessments were used with kratom using individuals. During the training phase, six non-kratom users (USM students) were engaged to test the CPT and the paper-and-pencil assessments, and six male kratom using participants were enrolled where the full study procedures were implemented. The research personnel’s performance was evaluated before commencing the enrollment of the randomized study sample.
Study Procedures
Eligible participants were admitted for an overnight stay at an inpatient USM ward). Upon arrival they signed a voluntary written informed consent. Participants were not allowed to bring kratom or other psychoactive substances to the ward. Upon admission urine toxicology tests for morphine, methamphetamine, amphetamine, THC, benzodiazepine, methadone, and ketamine were performed and all participants were interviewed about amounts and times of kratom consumption on each day during 7 days prior to the study admission using the Timeline Follow Back (TLFB) method [27,28]. They were also asked questions about their lifetime patterns of kratom use. All participants were admitted during afternoon hours (approximately 5 pm) and spent the night in the ward. They were not allowed to leave the ward unaccompanied, but they could smoke cigarettes outside of the ward at the designated area.
Blood Samples Collection
On the next day, at ~6:30 am, participants were cannulated for blood drawing on their non-dominant hand. After each draw the veins were kept patent (1-2 ml of heparinized saline, 10 units/mL). Blood samples (1 ml) were collected 15 times. The vacutainer tubes were gently inverted several times to prevent clotting and centrifuged for 15 minutes at 3,000 rpm to separate the plasma from the red blood cells. Plasma was transferred to cryovials and stored at -20°C. The samples were analyzed using liquid chromatography mass spectrometry (LCMS/MS) to obtain mitragynine pharmacokinetic profiles. Instrumentation limitations precluded measurement of other active compounds in the kratom leaves and in the blood samples. Mitragynine pharmacokinetic findings from the current study will be reported in a separate publication.
Kratom and Placebo Drinks
Kratom leaves used in preparation of the study decoction were obtained from a plantation. A member of the research team travelled to the plantation and observed the leaf collection. Ten kilograms of the freshly collected leaves were purchased. The leaves were transported to a laboratory at the USM where they were washed in cold water to remove particles, dust, dirt, or insects. Subsequently, the leaves were dried for 48 hours at room temperature (~26°C) and then dried in an oven at 37°C for 24 hours.
Kratom drinks approximating mitragynine concentration levels found in field decoctions were prepared by mixing the prepared leaf material with water and boiling it on a low heat. The mixture was then strained to remove the sediment and stored at 4°C in a locked refrigerator. To assess mitragynine concentration, decoction was analyzed using high-performance liquid chromatography-ultra violet (HPLC-UV) and high-performance liquid chromatography-diode array detector (HPLC-DAD) [29].
To match for taste and appearance, the placebo decoction was prepared using the same procedures that were used in preparation of the kratom decoction but with botanical materials obtained from vegetables in the Cucurbitaceae family that are frequently cultivated and consumed in Malaysia and do not contain any known active compounds identified in the kratom plant. To further mask potential taste differences, both the kratom and placebo decoctions were flavored with a sugar syrup.
All study participants consumed three decoction drinks throughout the study day: at 7 am, 10 am, and 1 pm. Only one of the three drinks contained active kratom, or all three drinks were placebo decoctions (PPP randomization sequence). The randomization sequences with the active kratom decoction were as follows: active kratom decoction as the first drink followed by two placebo drinks (KPP); active kratom decoction as the second drink in the sequence (PKP); or active kratom decoction as the third drink in the sequence (PPK). The assignment of kratom and placebo drinks consumption sequences was random for all participants. A randomization sequence generated by the study statistician was provided to the USM study pharmacist who prepared both the active kratom and placebo drinks. All kratom and placebo drinks were served in non-transparent identical cups. The participants and the study personnel were blinded about the drinks randomization sequence.
Assessments of the Blinding Procedures
To assess whether participants can differentiate between the active and placebo drinks based on taste, they were asked to rate the kratom potency/strength of all consumed drinks using a 100 mm visual analogue scale (VAS) immediately after consuming each drink. The same VAS assessment was conducted 30 minutes later to assess whether participants can differentiate between the active and placebo drinks based on other subjectively perceptible effects of kratom vs placebo.
The Cold Pressor Task (CPT)
CPT is a laboratory procedure to induce pain experience safely and without long lasting effects in laboratory and clinical research settings [20,21]. During the CPT, participants were asked to immerse their dominant hand into a 10-liter ice-water bath. Water temperature was maintained between 2.5°C and 3.5°C and recorded for every CPT task.
After immersing their hand in the ice bath, participants were asked to report verbally when they first started feeling pain (pain onset outcome) and to keep their hand immersed for as long as they can (for up to 5 minutes maximum). The times from when the participant immersed his hand until he reported pain and the time when the hand was withdrawn from the ice water bath were recorded. The primary outcome was pain tolerance, defined as the difference (in seconds) between the pain onset and the hand withdrawal from the ice bath.
For each participant, the CPT was performed repeatedly 10 times throughout the study: immediately before ingesting each drink and at 1-hour intervals thereafter. Immediately after, each CPT participant was also asked to rate the level of unpleasantness of the CPT using a 100mm VAS scale.
Physiology and Withdrawal Assessments
Because no standardized assessments of potential kratom withdrawal symptoms are currently available, the Clinical Opioid Withdrawal Scale (COWS) [30] was administered four times: before each drink and at 120-minute intervals thereafter. The vital signs, including body temperature, blood pressure, heart rate, and respiratory rate were obtained during admission, at baseline before the first drink and at 120-minute intervals thereafter. The study safety parameters were: body temperature within 36.5 – 37.5°C, systolic blood pressure 90 – 140 mm Hg, diastolic blood pressure 60 – 90 mm Hg, and heart rate 60 – 100 beats/minute. The study protocol stipulated that if vital signs fall out of the safety parameters range, a medical doctor will evaluate the participant. Participants were also asked to report any discomfort or unusual symptoms.
Upon the study completion, urine samples were tested for morphine, amphetamine type stimulants (ATS), methadone, benzodiazepines, THC, and ketamine. Each participant received RM350 (~80 USD) as a compensation for time spent in the study.
Data Analyses
CPT data were log-transformed to correct for skewness and were analyzed using the mixed models with drink type (kratom vs placebo), time following drink administration (0, 1, 2 hours), dosing sequence (first, second, or third) and the interaction between drink type and time as within-subject effects and randomization sequence (KPP, PKP, PPK, and PPP) as the between-subject factors. Random subject effects and the autoregressive structure of the errors were used to model the correlation of repeated observations within individuals. Best-fitting variance-covariance structure for each model was selected using Akaike’s Information Criterion (AICC). Significant interaction effects were followed by post-hoc F-tests of simple effects and t-tests of pairwise comparisons, whereas significant main effects were explained by performing all pairwise least square mean comparisons corresponding to the Fisher’s Least Significant Difference (LSD) approach [31].
The CPT unpleasantness assessments were analyzed using the same analytical approach described above. The results of VAS evaluating the maintenance of the blinding procedures were compared between the kratom and placebo drinks using an analysis of variance method (t-tests). All analyses were performed by authors RG and MCH using the SAS 9.4 and SPSS 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) statistical packages.
Results
Between May and August 2018, 26 male Malay participants were enrolled. Their mean (SD) age was 24.3 (3.4) years. They reported the mean (SD) of 6.1 (3.2) years history of daily kratom consumption and consuming kratom drinks multiple times during each of the seven days prior to their study participation.
The mean (SD) score on the COWS at baseline at ~ 7 am (before the kratom or placebo drinks were given) was 0.5 (0.8). The remaining COWS scores collected throughout the study day were: 0.4 (0.6), 0.4 (0.6), and 0.5 (0.5) respectively. One participant had one diastolic blood pressure reading of 58 mm Hg while receiving placebo and one of 57 mm Hg when he received kratom. One participant who received only placebo drinks had one diastolic blood pressure reading of 95 mm Hg. One participant had one diastolic blood pressure reading of 58 mm Hg on the night of admission. One participant had one diastolic blood pressure reading of 93 mm Hg at ~8 hours after receiving the kratom drink. A medical doctor evaluated each of these participants and concluded that it was safe for them to continue with the study.
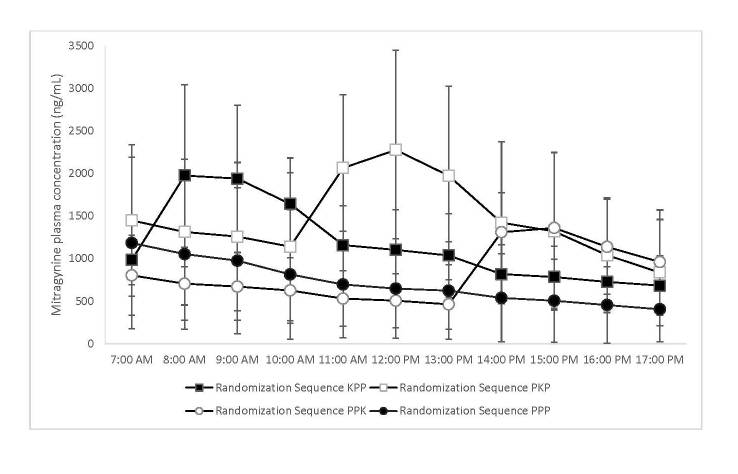
The means of mitragynine plasma concentrations for all randomization groups are shown in Figure 2.
Figure 2.
Hourly means of mitragynine plasma concentrations for each of the four study randomization groups. The error bars represent 95% confidence intervals.
Twenty participants received one kratom and two placebo drinks: seven received the active kratom decoction in first dosing sequence (~ at 7 am, KPP), seven received the active kratom decoction in second dosing sequence (~ at 10 am, PKP), six received the active kratom decoction in third dosing sequence (~ at 1 pm, PPK). The remaining six participants received three placebo drinks (PPP).
The means (SD) of the reported strengths on the VAS for the active kratom and placebo drinks were 57 (27) mm versus 52 (26) mm respectively when evaluated immediately upon consumption (p=0.5) and 58 (23) mm vs 46 (24) mm respectively when evaluated 30 minutes after the drinks consumption (p=0.09).
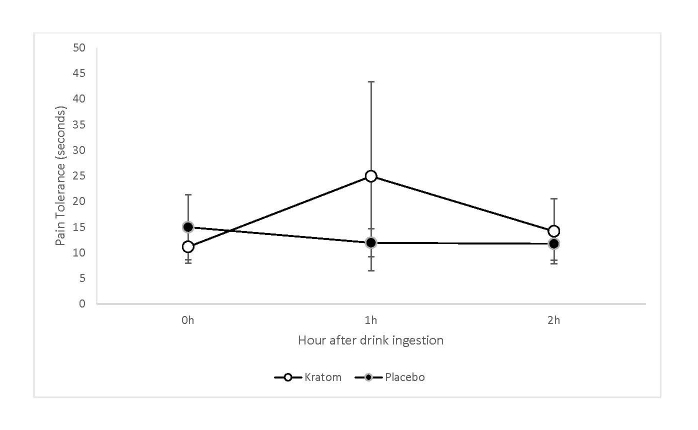
Pain tolerance increased significantly from the mean (SD) 11.2 (6.7) seconds when measured immediately before kratom consumption to the mean (SD) 24.9 (39.4) seconds at 1 hour after consuming the kratom drinks. In contrast, the mean (SD) pain tolerance was 15.0 (19.0) seconds when measured immediately before consuming the placebo drinks and 12.0 (8.1) seconds at 1 hour after consuming the placebo drinks (see Figure 3).
Figure 3.
The means of pain tolerance, in seconds, immediately before the drink consumption and at 1 and 2 hours after consumption for kratom and placebo drinks. The error bars represent 95% confidence intervals.
There was a significant interaction between the drink type and time after consumption (F(2,65.5)=3.20, p=0.05) with significant changes in pain tolerance after kratom consumption. After consuming the active kratom drinks, pain tolerance was significantly higher at 1 hour compared to 0 hour (t(68.3)=2.77, p=0.007) and compared to 2 hours (t(50)=2.06, p=0.04), with no significant differences between 0 hour and 2 hours (t(51.8)=1.04, p=0.30). After consuming the placebo drinks, none of the pairwise differences of the three timepoints were significant (all p-values > 0.20). The pain tolerance difference at 1 hour after the drink consumption between kratom and placebo was not statistically significant (p=0.12).
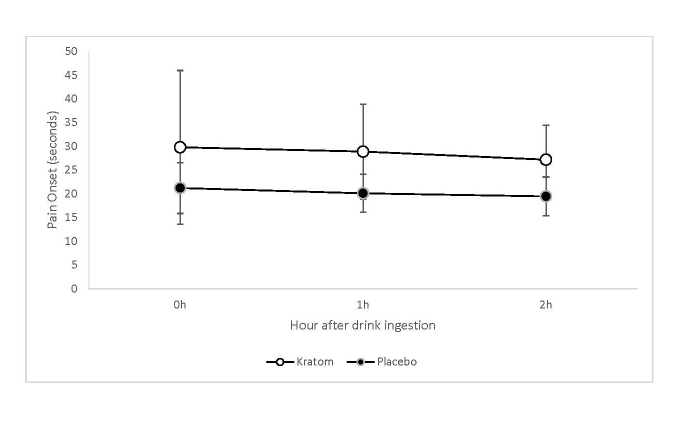
Pain onset showed a significant main effect of kratom vs placebo (F(1,48.1)=8.68, p=0.005), it was longer after consuming the active kratom drinks than after consuming the placebo drinks across all timepoints (see Figure 4). There was also a significant dosing sequence effect (F(2,47.9)=6.88, p=0.002). Pain onset was longer during the first dosing sequence than during the second and third sequences (t(52.5)=3.16, p=0.003 and t(40.2)=3.43, p=0.001 respectively). There were no differential changes in the pain onset after ingestion of active kratom versus placebo drinks.
Figure 4.
The means of pain onset, in seconds, immediately before the drink consumption and at 1 and 2 hours after consumption for kratom and placebo drinks. The error bars represent 95% confidence intervals.
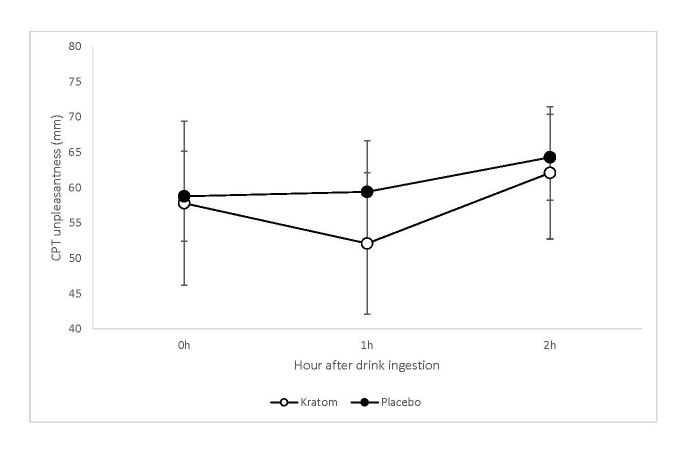
The mean (SD) rating of unpleasantness of the CPT decreased from 57.8 (24.7) mm immediately after (0 hour) to 52.1 (21.3) mm at 1 hour after consumption of the active kratom drinks. For the placebo drinks, the corresponding mean (SD) ratings were 58.8 (19.0) mm at 0 hour and 59.4 (21.6) mm 1 hour (see Figure 5). There was a significant main effect of time (F(2,108)=4.70, p=0.01) and a significant dosing sequence effect (F(2,45.4)=5.89, p=0.005). The mean CPT unpleasantness ratings went down from baseline to 1 hour (p=0.25) and then significantly up at 2 hours (p=0.003). The difference at 1 hour after the drink consumption between kratom and placebo on the unpleasantness rating was statistically significant (p=0.05). The mean CPT unpleasantness ratings were significantly higher during second and third dosing sequences than during the first dosing sequence (t(54.7)=3.03, p=0.004 and t(30.2)=3.17, p=0.004 respectively).
Figure 5.
The means of CPT unpleasantness immediately before the drink consumption and at 1 and 2 hours after consumption for kratom and placebo drinks. The error bars represent 95% confidence intervals.
Discussion
The current study found that in the enrolled cohort of kratom experienced individuals with long-term histories of daily kratom consumption, pain tolerance increased significantly 1 hour after active kratom drinks were consumed in laboratory settings. The ratings of unpleasantness of the CPT task decreased significantly 1 hour after consuming the active kratom drinks. The assessments of the blinding procedures indicated that participants were not able to distinguish between the placebo and the active kratom drinks based either on taste or other perceptual, subjective effects.
These study findings provide the first objectively measured evidence obtained in a controlled research with human subjects that are preliminarily supporting or confirming previously published reports of kratom pain relieving properties based on self-reports collected in observational studies.
Because of laboratory assessment and instrumentation limitations, only mitragynine could be measured in the collected plasma samples and in the prepared drinks. Consequently, mitragynine concentration levels were used as approximate markers to ensure that the study active kratom decoction approximated the strength of drinks that are typically consumed in natural settings, and the patterns of mitragynine plasma concentrations obtained from the study participants were used as a verification of the randomization procedures and to document that no additional kratom was consumed during the study. As seen in Figure 2, the mitragynine plasma profiles peaks match the corresponding randomization sequences.
Large individual differences in pain responses, illustrated by the 95% CI error bars in Figure 3, and in mitragynine plasma levels, Figure 2, were observed. There were no measurable or discernible relationships between the mitragynine plasma profiles and pain responses profiles.
No adverse effects were observed in the study. All participants completed all study tasks and procedures, and none reported any discomfort or unusual symptoms. None of the participants reported withdrawal symptoms either using spontaneous self-report or had significant withdrawal symptoms based on the COWS scores. All urine toxicology screens conducted at the end of the testing day were negative.
All participants reported long histories of daily kratom consumption, with high frequency of daily consumption and substantial amounts consumed. It is not possible to quantify these reports into markers that could be used to approximate amounts of plant material or active ingredients consumed. However, despite the reported long duration and high levels of daily kratom consumption, during documented kratom discontinuation lasting from 10 to 20 hours, no participant reported or displayed discomfort, symptoms, or signs of potential withdrawal symptoms.
A substantial amount of misinformation has been published in literature and disseminated in media reports, creating a misconception that kratom is simply a dangerous opioid. Kratom is a plant that contains many alkaloids and other potentially active substances [1,21,23]. Psychoactive effects of consuming plant material are likely to result from synergistic interactions among many substances, including possible competing agonist and antagonist effects on opioid and other receptors [21].
Limitations
The enrolled cohort of participants resulted from the relatively restrictive inclusion/exclusion criteria of the current study and may not be representative of other groups of kratom users. Therefore, the generalizability of the study findings may be limited. However, the current study was aimed to objectively detect a signal under controlled laboratory conditions rather than to obtain a broadly generalizable evidence. Further research with larger and more diverse samples, including research enrolling kratom naïve participants is needed to obtain more generalizable evidence of kratom’s pain relieving properties.
The study was not able to enroll female participants despite strong outreach efforts. Kratom use among females in Malaysia is culturally restricted and less prevalent than among males and previous research in Malaysia encountered similar challenges in enrolling female participants [5,32]. The sample size was too small to evaluate potential factors contributing to the observed large data variability.
Instrumentation limitations precluded measurement of other active compounds in kratom leaves and in blood samples. Consequently, the full biochemical composition of the study kratom decoction and preparations consumed in natural settings are unknown. Further research is needed in this area.
The study design, including the lack of kratom specific and validated methods to assess withdrawal symptoms and the lack of a control, kratom-naïve group precluded longer range evaluations of potential kratom withdrawal symptoms that could emerge later than 10-20 hours after kratom discontinuation, and precluded evaluations of addictive potential of kratom.
Conclusions
The findings of increased pain tolerance resulting from consuming kratom decoction should be interpreted cautiously. These findings should be replicated in larger and more diverse samples to provide rigorous assessment of the observed effects. Extensive controlled studies are needed to fully evaluate currently debated potential beneficial and harmful effects of kratom and to determine its potential future therapeutic value for pain or other conditions. The study findings should not be interpreted as endorsing the use of kratom products for self-treatment of pain or other conditions. Furthermore, in the current study, kratom decoction was prepared under strictly controlled laboratory conditions using botanical materials inspected and identified using precise botanical markers by scientists with extensive experience studying the kratom plant. Products that are labeled as “kratom” or “containing kratom” that are purchased online or in stores are typically not prepared under such strictly controlled conditions. There have been reports of such products being adulterated with psychoactive substances or other chemicals or contaminated with pathogens [9,11,33,34].
Acknowledgments
The research was supported in part by the Ministry of Education of Malaysia under the HICoE Programme: 311.CDADAH.4401009.
Glossary
- CPT
cold pressor task
- BBB
blood-brain barrier
- ATS
amphetamine type stimulants
- TLFB
Timeline Follow Back
- VAS
visual analogue scale
- COWS
Clinical Opioid Withdrawal Scale
References
- Adkins JE, Boyer EW, McCurdy CR. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem. 2011;11(9):1165–75. [DOI] [PubMed] [Google Scholar]
- Singh D, Narayanan S, Vicknasingam B. Traditional and non-traditional uses of Mitragynine (Kratom): A survey of the literature. Brain Res Bull. 2016. September;126(Pt 1):41–6. [DOI] [PubMed] [Google Scholar]
- Suwanlert S. A study of kratom eaters in Thailand. Bull Narc. 1975. Jul-Sep;27(3):21–7. [PubMed] [Google Scholar]
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, et al. From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013. February;37(2):138–51. [DOI] [PubMed] [Google Scholar]
- Vicknasingam B, Narayanan S, Beng GT, Mansor SM. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010. July;21(4):283–8. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC. Update on the Pharmacology and Legal Status of Kratom. J Am Osteopath Assoc. 2016. December;116(12):802–9. [DOI] [PubMed] [Google Scholar]
- Anwar M, Law R, Schier J. Notes from the Field: Kratom (Mitragyna speciosa) Exposures Reported to Poison Centers - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016. July;65(29):748–9. [DOI] [PubMed] [Google Scholar]
- Grundmann O. Patterns of Kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63–70. [DOI] [PubMed] [Google Scholar]
- Ekar T, Kreft S. Common risks of adulterated and mislabeled herbal preparations. Food Chem Toxicol. 2019. January;123:288–97. [DOI] [PubMed] [Google Scholar]
- Gershman K, Timm K, Frank M, Lampi L, Melamed J, Gerona R, et al. Deaths in Colorado Attributed to Kratom. N Engl J Med. 2019. January;380(1):97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczuk AP, Łozak A, Zjawiony JK. Comprehensive methodology for identification of Kratom in police laboratories. Forensic Sci Int. 2013. December;233(1-3):238–43. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018. February;235(2):573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yano S, Horie S, Yamamoto LT. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci. 1997;60(12):933–42. [DOI] [PubMed] [Google Scholar]
- Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: salvia divinorum and Kratom. Clin Toxicol (Phila). 2008. February;46(2):146–52. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, et al. Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996;59(14):1149–55. [DOI] [PubMed] [Google Scholar]
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, et al. Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. J Med Chem. 2002. April;45(9):1949–56. [DOI] [PubMed] [Google Scholar]
- Reanmongkol W, Keawpradub N, Sawangjaroen K. Effects of the extracts from Mitragyna speciosa Korth. Leaves on analgesic and behavioral activities in experimental animals. Songklanakarin J Sci Technol. 2007;29:39–48. [Google Scholar]
- Sabetghadam A, Ramanathan S, Mansor SM. The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacognosy Res. 2010. May;2(3):181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WM, Mohamed Z, Alshawsh MA, Chik Z. Evaluation of pharmacokinetics and blood-brain barrier permeability of mitragynine using in vivo microdialysis technique. J Pharm Biomed Anal. 2017. September;143:43–7. [DOI] [PubMed] [Google Scholar]
- Yusof SR, Mohd Uzid M, Teh EH, Hanapi NA, Mohideen M, Mohamad Arshad AS, et al. Rate and extent of mitragynine and 7-hydroxymitragynine blood-brain barrier transport and their intra-brain distribution: the missing link in pharmacodynamic studies. Addict Biol. 2019. September;24(5):935–45. [DOI] [PubMed] [Google Scholar]
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, et al. Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J Am Chem Soc. 2016. June;138(21):6754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004. August;52(8):916–28. [DOI] [PubMed] [Google Scholar]
- Shellard EJ. The alkaloids of Mitragyna with special reference to those of Mitragyna speciosa, Korth. Bull Narc. 1974. Apr-Jun;26(2):41–55. [PubMed] [Google Scholar]
- Jansen KL, Prast CJ. Ethnopharmacology of kratom and the Mitragyna alkaloids. J Ethnopharmacol. 1988. May-Jun;23(1):115–9. [DOI] [PubMed] [Google Scholar]
- Edens J, Gil K. Experimental induction of pain: utility in the study of clinical pain. Behav Ther. 1995;26:197–216. [Google Scholar]
- Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain. 2004. May;5(4):233–7. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014. March;28(1):154–62. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996. September;42(1):49–54. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Ramanathan S, Murugaiyah V, Hamdan MR, Said MI, Lai CS, et al. A simple HPLC-DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci Int. 2013. March;226(1-3):183–7. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003. Apr-Jun;35(2):253–9. [DOI] [PubMed] [Google Scholar]
- Montgomery DC. Design and Analysis of Experiments. 10th ed. New York: John Wiley & Sons; 2019. [Google Scholar]
- Ahmad K, Aziz Z. Mitragyna speciosa use in the northern states of Malaysia: a cross-sectional study. J Ethnopharmacol. 2012. May;141(1):446–50. [DOI] [PubMed] [Google Scholar]
- Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011. May;35(4):242–7. [DOI] [PubMed] [Google Scholar]
- Voelker R. Kratom Investigation Concludes. JAMA. 2018. August;320(5):431. [DOI] [PubMed] [Google Scholar]