Abstract
Oral herpes labialis, more commonly known as cold sores, are a common encountered viral infection involving herpes simplex virus-1 (HSV-1). Although relatively benign, these lesions can be both unsightly and clinically difficult to manage. Prescription standards of care and over-the-counter agents, such as docosonal, have often shown only limited efficacy in both decreasing lesional pain and reducing duration of lesional symptomology and are not without potential side effects. Despite some success with acute remediation, recurrent episodes often occur, with seemingly no imparted protection or suppression against future outbreaks. This case report involves the successful treatment of oro-facial herpes labialis with a synergistic botanical blend with marked reduction in symptoms, pain score, and lesion duration. Monitoring and evaluation post-treatment and application during future prodromal symptoms was also performed demonstrating additional reduction in the frequency of subsequent outbreaks. This case report supports the use of this treatment for prodromal and acute treatment of oro-facial herpes infection and appears to impart a reduction in the frequency of future outbreaks.
Keywords: Herpes labialis, HSV, cold sore, botanical, alternative
Introduction
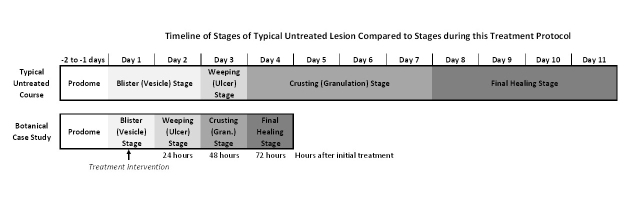
Herpes simplex virus 1 (HSV-1) is the causative agent associated with oro-facial herpes labialis commonly referred to as cold sores. Cold sores are an ailment that is experienced by over 40% of the US population [1,2] with current seroprevalence of HSV-1 estimated to be 53.9% [3,4]. Subsequent to infection, viral persistence remains following acute fulminant infection with HSV-1 via uptake into trigeminal nerve ganglia. This “trigeminal seeding” causes the initial viral infection to develop into chronic latency and provides the etiology to future reactivation eruptions [5]. Cold sores have a well-documented disease process that starts with a prodrome stage of 1-2 days in which paresthesia is commonly felt locally within the nerve region prior to eruption. This is followed by approximately 2 days of the eruption stage when there is a visible raised vesicular lesion with area erythema and swelling often present. The next stage lasts typically for 1-2 days and is the weeping or ulceration stage in which the lesion is most contagious with shedding of the replicated viral material. The contagious ulcer becomes granulated over the course of the next stage which lasts for usually 4 days. Following the granulation stage, another 4 days of epidermal remodeling encompasses the final healing process. This process is illustrated in Figure 2.
Clinically, cold sores represent a difficult condition to resolve with a relatively limited number of treatment options available. In many cases, some degree of urgency or pressure is on the physician for the lesions to be resolved in a short period of time due to the unsightly nature of the lesions and the subsequent social ignominy they cause to the patient. Severity of the lesions size, recurrence rate, and pain often varies from patient to patient. Standard treatment protocols for oro-facial HSV infections include over-the-counter creams such as docosanol and pharmaceutical anti-viral agents such as acyclovir [6,7]. These standard treatments options have mixed degrees of success and do not appear to reduce recurrence rates. Botanical-based therapeutic options represent a growing, albeit still under-utilized treatment alternative to conventional strategies. Despite a significant body of in vitro evidence demonstrating botanical-based activity against HSV-1, limited research or published documentation has been reported applying in vitro work in clinical settings [8,9]. This case report illustrates a successful treatment protocol using a botanical blend in the treatment of recurrent cold sores.
Case Presentation
A 41-year-old female patient presented with a diagnosis of oro-facial herpes labialis (cold sore). Gross inspection revealed a visible, fluid-filled vesicle on the vermillion border of her right lower lip (Figure 1). The patient reported the lesion had appeared within 24 hours prior to the time of intake and noted a medical history of having outbreaks regularly for the past 20 years with a frequency of approximately one outbreak per month to her best recollection of the preceding 3 years. In the past, the patient had self-treated her recurrent cold sore eruptions topically with over-the-counter docosanol 10% cream, but reported limited success with no subsequent decrease in future outbreak reoccurrence post-application.
Figure 1.
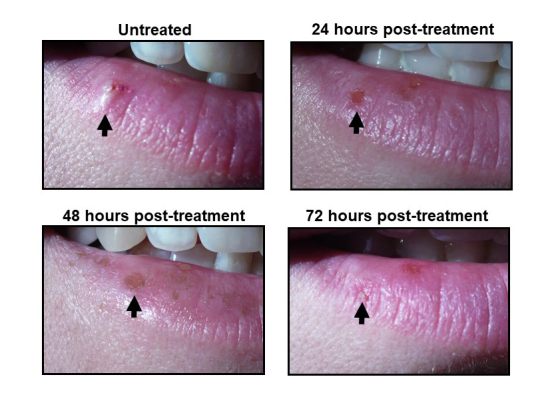
Photographs of herpes labialis lesions during treatment. Photos show the status of a herpes labialis lesions prior to treatment (untreated), and 24-, 48-, and 72-hours post-treatment.
Therapeutic Intervention and Treatment Protocol
Due to the patient’s previously unsuccessful attempts with docosanol 10% cream, alternative botanical-based therapies were investigated for the treatment of this patient. A botanical gel containing Hypericum perforatum (2.5%, commonly referred to as St. John’s wort), Lavandula officinalis (10%, commonly referred to as lavender), Glycyrrhiza glabra (2.5%, commonly referred to as licorice), Melissa officinalis at (6%, commonly referred to as lemon balm), Eleutherococcus senticosus (4%, commonly referred to as Siberian ginseng) and Sarracenia (mixed species) (25%, commonly referred to as the purple pitcher plant) suspended in a Verbase gel (obtained from PCCA) was chosen due to the anecdotal and historical antiviral, anti-inflammatory, and analgesic properties associated with these botanicals [8-13]. In addition, the gel formulation contained allantoin and glycerin, which are known to improve skin healing. Explicit instructions were given to the patient including detailed application instructions. These included: a) wash the area with warm water only (no soap) and pat dry, b) beginning at the outer edge of the area, apply a very thin layer of the gel until the lesion is covered uniformly, and 3) reapply the gel five times daily (especially after facial washing, eating, or drinking), rewashing the area prior to bedtime and once again in the morning. Repeat the instructions for each application. The patient was informed to not apply any cosmetics or other lip balms to the area. The patient was asked to document any side effects or concerns and to return to clinic for photographic documentation of the lesion in the days following. Additional instructions were also provided to the patient for recurrent outbreaks where the patient was to reapply the gel immediately upon feeling any prodromal effects (described to the patient as any unexplained “tingling sensations” on the lips). The patient was asked to document the per-month frequency of occurrence of acute fulminant cold sore presentations in the months following initial treatment.
Outcome
Figure 1 visually documents the timeline of the acutely treated cold sore outbreak. Day 0 shows the untreated raised 4 mm lesion at the vermillion border of the right lower lip. Day 0 can be identified as consistent with the vesicle stage of the cold sore disease process. Treatment was implemented immediately following photography of the lesion on Day 0. Day 1 shows an ulcerated surface as part of the “weeping” or ulceration stage of the disease process. Within the 24 hours of treatment, from Day 0 to Day 1, there was a visible reduction of excrescence with some hyperpigmented or darkened regions of the tissue. Day 2 of treatment (48 hours post-treatment) shows both additional hyperpigmentation and further reduction and/or complete resolution of lesion excrescence with the ulcerated skin undergoing granulation. Treatment Day 3 (72 hours post-treatment) shows the final healing stages of epidermal repair with almost complete lesion resolution with a small degree of residual granulation tissue present.
Figure 2 shows a comparison of a typical cold sore disease process timeline compared to the disease process documented in this case. In contrast to a typical cold sore disease course, the use of this treatment led to lesion resolution in 70% less time and visually, which is often a concern for patients, resolved noticeable gross pathology with 48-72 hours post-application.
Figure 2.
Timeline of herpes labialis resolution. Upper timeline illustrates the typical stages of an untreated HSV-1 lesion. Bottom timeline illustrates the observed stages of resolution of the subject treated in this case report.
Localized pain was charted during the ensuing hours post-initial application using a Numerical Rating Scale (NRS-11). Figure 3 shows the localized pain at the time of intake being described as moderate 6/10 pain. Within 1 hour of application, pain became mild in nature rated at 1/10 and by 6 hours following gel application, the patient described the pain as nearly negligible.
Figure 3.
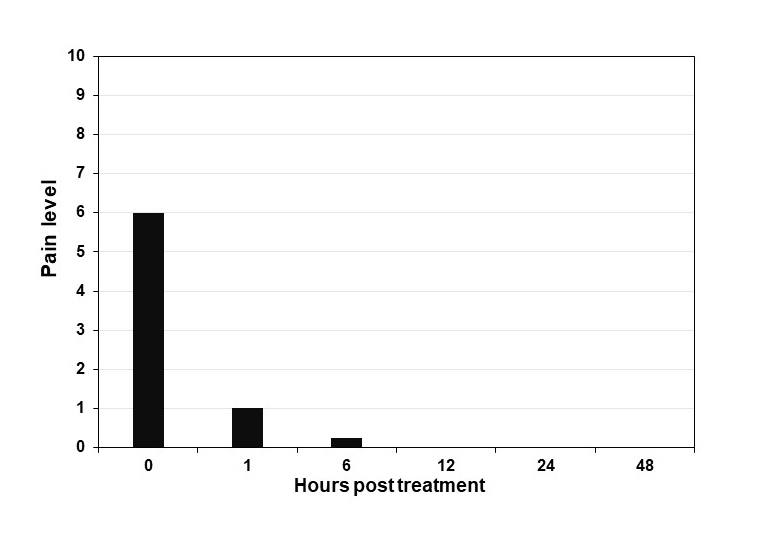
Pain level of herpes labialis lesion. Graph illustrates the pain level reported by the subject in this case report following hours post treatment. Pain scale ranges from 0-10 (10 maximum pain).
In terms of outbreak recurrence, in the subsequent months after the initial outbreak and the use of early interventional applications in the prodromal phase as described, the patient reported a dramatic decline in occurrence on a per-month basis (Figure 4). Longitudinally over the course of 5 years, the patient went from having a typical cold sore eruption at the average frequency of one time per month pre-treatment to currently having an outbreak frequency approximately once every 3 years.
Figure 4.
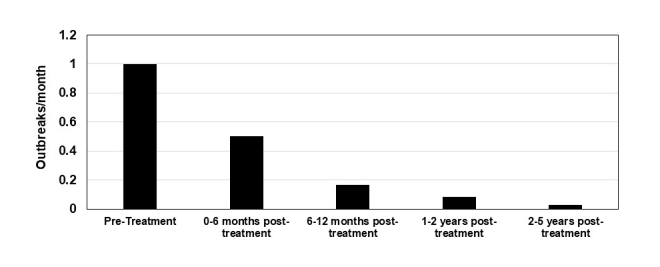
Frequency of recurrent herpes labialis outbreaks. The subject in this case was followed regarding the frequency of herpes labialis outbreaks over a 5-year period of treatment. Graph illustrates the number of outbreaks the subject in this case report recorded on a monthly basis.
In summation, positive outcomes were observed using the prescribed treatment protocols in which gross appearance revealed complete resolution of the lesion within 72 hours after initial treatment, localized pain experienced by the lesions was dramatically decreased within hours following treatment, and the recurrence rate of future episodes was shown to have a marked reduction.
Discussion
In the case presented, the use of the described synergistic botanical blend application provided the patient with an efficacious and rapid-acting alternative to a previously unsuccessful OTC cold sore remedy. The individual botanicals used in this blend have anecdotally and historically been used and previously been shown to inhibit HSV-1 replication and have therapeutic efficacy [8-13]. The strength of this treatment choice provided not only acute remediation of the lesion but also marked reduction in future recurrence and palliation to moderate levels of herpetic neuralgia. The reduction in pain may be partially explained by the inclusion of Sarracenia spp. in this blend which has previously been used in a partially-purified injectable solution for the management of neuropathic and cutaneous pain [14]. Viral inhibition responsible for the rapid resolution observed in this case was most likely attributable to the synergistic activity of the botanical blend targeting multiple points in the viral replication cycle. This rapid and efficacious reduction in acute viral loads may prevent or reduce viral “reseeding” into the trigeminal ganglia. Furthermore, the repeated, early treatment applications during subsequent prodromal symptoms likely provided additional decline to the remaining latent viral load within the ganglia and eventually may reach subclinical levels that were manageable by the immune system. As previously implicated, this potential decrease in latent viral loads was likely associated with the overall reduction in future recurrent outbreaks [15,16].
This case supports the use of a synergistic botanical blend as a clinical treatment of oro-facial herpes labialis in acute, prodromal, and neuralogic presentations. Moreover, this blend may also reduce recurrent episodes possibly due to a decrease in latent viral loads present in neural ganglia tissue. It must be noted that this is a single subject case report and resolution of symptoms may also be associated with the “moisturizing” nature of the gel base. Since completion of this case, three additional patients with herpes labialis were treated with this botanical formulation under physician supervision and observed similar rates of symptom resolution (data not shown). In addition, the decrease in the frequency of outbreaks observed for this subject could be due to other individual subject factors. Future investigations utilizing control samples (e.g. glycerin/allantoin) and conducting a larger scale study may contribute to additional therapeutic assessment and value of this botanical-based blend for the treatment of this and possibly other herpes virus infections which have limited efficacious treatments available to practitioners.
Glossary
- HSV-1
herpes simplex virus-1
Author Contributions
EON, GGR, AFK, TCT, EVL performed the clinical study and assessed the results. EON wrote the manuscript. JL was the principal investigator for the study.
References
- Embil JA, Stephens RG, Manuel FR. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. (1975);113(7):627-30. [PMC free article] [PubMed] [Google Scholar]
- Grout P, Barber VE. Cold sores-an epidemiological survey. J R Coll Gen Pract. 1976. June;26(167):428–34. [PMC free article] [PubMed] [Google Scholar]
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. O04. 4 Seroprevalence of Herpes Simplex Virus Types 1 and 2 United States, 1999–2010. Sex Transm Infect. 2013;89 Suppl 1:A33–33. [DOI] [PubMed] [Google Scholar]
- Gibson JJ, Hornung CA, Alexander GR, Lee FK, Potts WA, Nahmias AJ. A cross-sectional study of herpes simplex virus types 1 and 2 in college students: occurrence and determinants of infection. J Infect Dis. 1990. August;162(2):306–12. [DOI] [PubMed] [Google Scholar]
- Bader C, Crumpacker CS, Schnipper LE, Ransil B, Clark JE, Arndt K, et al. The natural history of recurrent facial-oral infection with herpes simplex virus. J Infect Dis. 1978. December;138(6):897–905. [DOI] [PubMed] [Google Scholar]
- Chi CC, Wang SH, Delamere FM, Wojnarowska F, Peters MC, Kanjirath PP. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst Rev. 2015. August;(8):CD010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmann J. The many challenges of facial herpes simplex virus infection. J Antimicrob Chemother. 2001. February;47 Suppl T1:17–27. [DOI] [PubMed] [Google Scholar]
- Denaro M, Smeriglio A, Barreca D, De Francesco C, Occhiuto C, Milano G, et al. Antiviral activity of plants and their isolated bioactive compounds: an update. Phytother Res. 2020. April;34(4):742–68. [DOI] [PubMed] [Google Scholar]
- Malekmohammad K, Rafieian-Kopaei M, Sardari S, Sewell RD. Effective antiviral medicinal plants and biological compounds against central nervous system infections: A mechanistic review. Curr Drug Discov Technol. 2019. July; 10.2174/1570163816666190715114741 [DOI] [PubMed] [Google Scholar]
- Denzler K, Huynh T, Jacobs B, Langland J. Melissa officinalis extract inhibits herpes simplex virus I glycoprotein B interaction with heparin sulfate. Herbal Medicine: Open Access. 2016;2(2):12. [Google Scholar]
- Arndt W, Mitnik C, Denzler KL, Waters RF, Jacobs BL, Rochon Y, et al. Rediscovery of a 19th century cure for smallpox. PLoS One. 2012;7(3):e32610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EO, Kozin AF, Ruiz G, Lasku A, Langland JO. Treatment of Athlete’s Plantar Warts Using a Botanical Blend: A Case Report. Altern Ther Health Med. 2017. May;23(3):51–4. [PubMed] [Google Scholar]
- Ferreira V, Jacobs BL, Denzler K, Waters R, Chamberlain R, Proefrock KJ, Langland JO. Treatment of Herpes Zoster with botanical interventions: case report. Naturopathic Doctor News & Review. 2015. https://ndnr.com/botanical-medicine/botanical-treatment-of-shingles-a-case-report/ [Google Scholar]
- Manchikanti L, Pampati V, Rivera JJ, Beyer C, Damron KS, Barnhill RC. Caudal epidural injections with sarapin or steroids in chronic low back pain. Pain Physician. 2001. October;4(4):322–35. [PubMed] [Google Scholar]
- Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2008. March;372(1):56–63. 10.1016/j.virol.2007.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NM, Poon DK, Tansky CS, Thompson RL. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998. July;72(7):5343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]