Abstract
Background: Phytoadaptogens are considered to be herbal medicines with a multi-target effect that strengthen organ systems compromised by stress. Although animal and laboratory studies have identified numerous molecular targets associated with adaptogenic activity, the non-specific characteristic of these herbal medicines has meant there is no known methods to accurately determine efficacy of adaptogens in humans. This critical review of the evidence aims to identify domains which have been used to measure the effect of adaptogens in humans, in order to create pathways for translating laboratory, animal, and clinical studies on adaptogens into practical applications in the future. Methods: EMBASE, AMED, PubMed, Cochrane Library, and WHO ICTRP databases were searched for randomized trials which examined known physiological actions of adaptogens. Results: Twenty-four studies were identified and critically appraised using the Jadad scale. The findings identified three broad categories of outcome measures, including cognitive, mood and biological measures. Conclusions: There was a great heterogeneity in data making it difficult to draw conclusions as to the most effective measurement tools to capture the holistic activity in humans. Cognitive measures hold promise as a reliable measurement tool when used in conjunction with other relevant tools. Further investigation is necessary to determine the most appropriate and diverse tools to measure the complex multi-target action of adaptogens.
Keywords: Adaptogens, Stress adaptation, Physiological adaptation, Herbal medicine, Medicinal plants
Introduction
Phytoadaptogens (often referred to as “adaptogens”) are a class of herbal medicine commonly used by herbalists to assist in reducing the negative impact that chronic stress has on health [1]. The most recent definition describes phytoadaptogens as stress response modifiers that non-specifically increase an organism’s resistance to various stressors (physical, chemical, and biological), thereby promoting adaptation and survival [2]. They are considered to strengthen organ systems compromised by stress and normalize body functions in the face of stress [3].
The term “adaptogen” dates back about 70 years to investigations into a synthetic compound (dibasol) found to have this effect by a Russian toxicologist, N. V. Lazarev [4]. It was later defined more precisely and attributed to herbal medicines by herbalists Brekhman and Dardymov [5] who noted that the concept had been preceded by folk medicine of long standing. The herbalists defined this action as having a non-specific response therefore increasing the power of resistance against multiple stressors, having a normalizing effect, irrespective of the nature of the pathology, and being non-toxic [5]. Both the original definition and the more recent definitions derived from laboratory findings are relatively vague with no specific or measurable domains that could be used to standardize the concept by regulatory bodies. The vague nature of adaptogen definitions may relate to the deficit of current clinical research due to there being a vast array of possible approaches to measuring a non-specific and poorly understood herbal action, and no consensus having been reached on the most appropriate approach.
In the 1960s, the Union of Soviet Socialist Republics (USSR) drove a targeted research direction into the study of plant adaptogens with extensive research (over 1000 studies) being published, primarily on Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. over the following 20 years [6]. Due to security measures within the former USSR these papers published in Russian journals and conference proceedings were not accessible to the public, were never translated, and have mostly remained inaccessible to Western researchers. Given the modernity of the term adaptogen, there is no discussion of adaptogens within traditional texts in the context of the current terminology. However, herbal medicines with adaptogenic qualities have a long history of traditional use in many cultures and various parts of the world [2]. Herbs exhibiting adaptogenic qualities – and subsequently recognized as adaptogens today – were often listed as tonics traditionally [4]. As such, the cross-over of herbal concepts “tonic” and “adaptogen” may represent the link between traditional use and modern terminology. Prior to the “birth” of the adaptogenic term and concept by Lazarev, some of the herbs considered to have adaptogenic qualities were traditionally described as tonics, which can be seen in the State Pharmacopoeia of the USSR [4].
To date, the concept of adaptogen has primarily been studied from physiological, pharmacological, and toxicological perspectives [6-9]. The latter laboratory data has been reviewed in 2017, identifying a range of key molecular and regulatory targets involved in adaptogenic activity including stress hormones such as cortisol and neuropeptide Y (NPY) and key mediators of the adaptive stress response including nitric oxide (NO), heat shock proteins (HSP), and the FOXO transcription factor [2]. Further, at least 88 of the 3516 genes identified as being regulated by adaptogens were closely associated with adaptive stress response and adaptive stress-response signaling pathways (ASRSPs), including neuronal signaling related to corticotropin-releasing hormone, cAMP-mediated, protein kinase A, and CREB; pathways related to signaling involving CXCR4, melatonin, nitric oxide synthase, GP6, Gαs, MAPK, neuroinflammation, neuropathic pain, opioids, renin–angiotensin, AMPK, calcium, and synapses; and pathways associated with dendritic cell maturation and G-coupled protein receptor–mediated nutrient sensing in enteroendocrine cells [10]. The pharmacological data builds on the identified need for well-designed clinical trials to demonstrate the efficacy of these traditional medicines by examining the contemporary understanding of the multi-faceted mechanism of action of adaptogens. Panossian [2] proposes that the multi-target action and shared use of receptor sites exhibited by adaptogens is an example of network pharmacology, and the typical reductionist pharmacological paradigm of one receptor-site for one drug does not apply to these medicines, an argument that has been echoed by the European Medicines Agency (EMA) [3]. An accepted clinically validated tool to measure this complex phytotherapeutic activity has not yet been developed.
While there is some research on individual aspects of certain adaptogenic herbs and some adaptogens are listed in internationally recognized traditional texts [11,12] modern evidence is lacking on adaptogenic activity and the knowledge base underpinning the use of adaptogens by Western herbalists is unclear. Knowledge translation from the former USSR data to the broader research world has commenced with two English language reviews examining in detail (from a laboratory perspective) two herbal medicines considered in Russia to be classical adaptogens [13,14], adding to the body of experimental data available. Traditional applications of a number of adaptogenic plants are also discussed in a review of medicinal plants of the Russian Pharmacopoeia [4], adding some traditional evidence to the body of knowledge Western researchers have collated on adaptogens. However, there remains a paucity of well-designed human clinical trials and a lack of understanding of the adaptogenic concept overall.
The process of defining the term adaptogen has been ongoing over many decades and there remains some confusion. In 2008, EMA published a reflection paper on this topic to establish the scope and interpretation of the term “adaptogen” to assess the feasibility of the acceptance of the term into pharmacological and clinical terminology for herbal and medicinal products [3]. The EMA review concluded that clinical data is insufficient, and the concept needs further clarification, also noting the necessity to work towards developing the tools to differentiate between herbal concepts (for example tonic and adaptogen) to facilitate standardization of these concepts. The US Food and Drug Administration (FDA) does recognize adaptogen as a functional term [15]. However, the term is recognized by the FDA as a “structure or function” claim on the basis that it is not a recognizable health or disease claim [15], echoing EMA comments on the unsuitability of the term (thus far) for clinical terminology. As such, there is a need for researchers to focus on identifying a relevant method to measure this action, that translates from laboratory to practical applications.
The majority of the earlier studies are either published in Russian and difficult to access and/or animal and in vitro studies. Given the complexity of the mechanism of action of adaptogens, animal and in vitro studies offer limited insight into the action and effect of adaptogens in humans. However, the expansiveness of the Russian literature needs to be considered in discussions of the contemporary understanding of phytoadaptogens. Russian health-regulatory authorities regard the term “adaptogen” as a functional term [13] and they have classified a number of herbal medicines including Panax ginseng C.A.Mey, Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. and Aralia elata var. mandshurica (Rupr. & Maxim.) J.Wen (syn. Aralia mandshurica Rupr. Et Maxim), Schisandra chinensis (Turcz.) Baill., Oplopanax elatus (Nakai) Nakai as classical adaptogens [13].
Despite some examples of adaptogen being used as a functional term, there appears to be a gap in knowledge translation between traditional understanding and use of adaptogens and clinical data, and between laboratory data and practical applications. In order for researchers to corroborate the practical use with modern evidence, more human clinical studies are needed. For this to proceed, a consensus on the most appropriate method of measuring the activity of adaptogens needs to be reached. The first step in achieving this is to identify those methods which have been used to date and analyze their efficacy and accuracy. A review of laboratory and animal studies [2] as well as other pre-clinical studies [16,17] have identified molecular targets and stress-related parameters relevant to measuring adaptogenic activity. The purpose of this review is to identify the domains that have been used to measure the effect and outcome of adaptogenic herbs in humans. It is the first review to analyze the methods of measurement of adaptogens used in human studies. An analysis of this data is necessary in order to determine the body of knowledge available, and which methods are the most suitable to give accurate insight into the activity of medicinal plants considered to be adaptogens. This is the preliminary work necessary to facilitate the translation of the body of laboratory and experimental data on adaptogens into practice, and to create valid methods of measuring adaptogenic activity to move forward with future clinical studies in this area of herbal medicine.
Methods
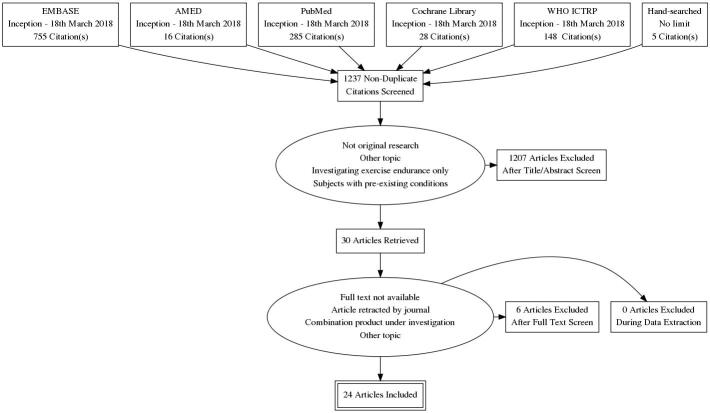
A database search was conducted to identify randomized clinical trials from the database’s inception to March 18th 2018 to identify domains that have been used to measure the effect and outcome of adaptogenic herbs in humans. On March 18th 2018, the following databases were searched: EMBASE (via OVID), AMED (via OVID), PubMed, Cochrane Library, and WHO ICTRP. Search terms (MeSH) were employed for: known physiological actions of adaptogens (adaptation, physiological/; stress, physiological/); herbal medicine (phytotherapy/; plants, medicinal/; herbal medicine/; plant extracts/); specific herbal medicines which were identified as adaptogens in traditional texts (rhodiola/; withania/; eleutherococcus/; panax/; ginseng.mp.; schisandra/ and astragalus plant/; astragalus membranaceus/); as well as a keyword search for the term adaptogen (adaptogen.mp). The search strategy is outlined in Table S1 (Appendix A). All articles were imported into Endnote [18], a bibliographic referencing software system. Twenty-two duplicates were identified and removed.
Articles were included if they were clinical trials reporting original research findings on individual herbal medicines with an adaptogenic action examining physical and mental endurance or physiological stress adaptation in healthy individuals. A data-extraction sheet was developed collaboratively between authors. This was pilot-tested on five randomly-selected included studies and agreed on by all authors. A critical analysis and narrative synthesis review was selected in order to capture those domains that have been used to date, to measure the outcome and effect of adaptogenic herbs. This method is considered most suitable where statistical meta-analysis is not feasible [19] and was implemented to critique the body of knowledge available rather than to statistically analyze results and efficacy. Articles were excluded if they were not in the English language, were not clinical trials, or were examining combinations of herbal medicines in a single treatment. Figure 1 outlines the methodological process of article selection. Articles were screened by title and abstract by one author (SG). Abstracts were analyzed by a second author (JW), and full texts agreed upon for selection. Bibliographic searching of included articles was also employed to identify additional material. Four additional articles were added at this stage. A summary of the characteristics of included articles is displayed in Table S2 (Appendix A).
Figure 1.
Process of article selection.
Critical Appraisal Analysis
On the basis of the review being limited to randomized controlled trials and that the majority of studies being reviewed predate the development of reporting guidelines, the Jadad Scale [20] was selected to assess the quality of each included study. The Jadad Scale is a simple, reliable, and validated tool for assessing scientific rigor of reports [20]. This tool has been used elsewhere [21,22] and contains one question on reporting of withdrawals, and two questions each on randomization and blinding where inappropriate methods can attract a negative score. Although not as detailed as other scales, the Jadad scale has advantages in the simplicity of assessment questions and ease of assessment performance, which is important when comparing trials of considerable heterogeneity, particularly when much of the literature predates established reporting standards. The Jadad scores focus on blinding, randomization, and appropriate description of withdrawals including point deduction where this has been inadequate, allows sufficient simplicity whilst retaining the features most important to studies of this topic. Table S3 (Appendix A) demonstrates the populated critical appraisal tool.
Results
A total of 24 articles were selected for review published worldwide between 1985 and 2014. Of the selected articles, 21 employed placebo-controlled methods, two were comparative parallel group studies, and one was an open-label study.
Trends of studies included those examining dose-dependent changes of herbal medicines with adaptogenic qualities (n = 9), those comparing one herb to another herb (n = 3) and those examining a single dose (of one herb or of one compared to another) (n = 12). A total of nine articles were examining acute dosing, two examining sub-acute (up to 8 days) dosing, eight investigating chronic dosing, and two articles undertook a comparison of acute versus chronic dosing. The herbs examined in the included articles were: Panax ginseng C.A.Mey. (n = 9), Rhodiola rosea L. (n = 6), Ginkgo biloba L. (n = 3), Bacopa monnieri (L.) Wettst. (n = 2), Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (n = 2), Bryonia alba L. (n = 1), Panax quinquefolius L. (n = 1), Paullinia cupana Kunth (n = 1), Piper methysticum G.Forst. (n = 1), Schisandra chinensis (Turcz.) Baill. (n = 1), Valeriana officinalis L. (n = 1), and Withania somnifera (L.) Dunal (n = 1). The majority of studies used standardized extracts (n = 22) guaranteeing the content of active constituents and marker compounds of the extract, with two studies not sufficiently reporting on the extract used. Regarding safety, 12 studies did not report on adverse events and toxicology, eight studies reported no adverse events were observed, and three studies reported a minimal number of mild adverse events including headache and gastrointestinal disturbance relating to Rhodiola rosea L. and Panax ginseng C.A.Mey. No serious adverse effects were reported in any studies.
A significant proportion (58%) of the selected articles scored lower than 4 on the Jadad Scale (n = 14). Fifteen articles failed to describe the double-blinding methods, 15 articles failed to report the number of withdrawals, and nine articles did not describe the randomization methods. Three articles were of a superior standard with a score of 5. Results of critical appraisal on individual articles is displayed in Table S3 (Appendix A).
Articles reported three broad areas for outcome measures: cognitive tests (predominantly Cognitive Demand Battery (CDB) or tailored version) (n = 15), mood measures (n = 11), and biological measures (n = 7). Most of the articles included more than one of these measures or a combination of all three. Statistical significance was regarded as a p value of less than 0.05.
Outcome Measures
A tailored Cognitive Drug Research (CDR) computerized assessment battery or CDB was utilized in 12 articles to assess the effects of specific herbs on cognitive function and mental endurance in stressful situations. Seven of those studies used the Bond-lader Visual Analogue Scale (BL-VAS) [23-29] and two used other mood measures [30,31] in conjunction with the CDR battery. Six of those studies were examining Panax ginseng C.A.Mey. [24-27,29,32]. Similar cognitive tests (though not specifically CDR battery) were used in four articles examining Rhodiola rosea L. [31,33-35]. Five studies that used a tailored CDR battery also used biological measures [23,30,35-37]. These included blood pressure (BP) and heart rate (HR), salivary or serum cortisol testing, or a combination of both. Three studies used biological measures only [38-40], three studies used mood measures only [41-43], and two studies used mood measures in conjunction with a biological measures [44,45]. Table 1 outlines the outcome measures used across studies.
Table 1. Outcome measures used across studies.
| Auddy et al. 2008 (Withania somnifera (L.) Dunal) | Benson et al. 2014 (Bacopa monnieri (L.) Wettst.) | Cardinal & Engels, 2001 (Panax ginseng C.A.Mey.) | Cropley et al. 2002 (Piper methysticum G.Forst. & Valeriana officinalis L. | D’Angelo et al. 1986 (Panax ginseng C.A.Mey.) | Darbinyan et al. 2000 (Rhodiola rosea L.) | De Bock et al. 2004 (Rhodiola rosea L.) | Downey et al. 2013 (Bacopa monnieri (L.) Wettst.) | ||
| Cognitive Measures | x | x | x | x | x | ||||
| Mood Measures | BL-VAS or VAS | x | x | ||||||
| SAM test | |||||||||
| MFI-20 | |||||||||
| HRQOL SF-36 | |||||||||
| STAI | x | ||||||||
| PANAS + POMS | x | ||||||||
| Other validated forms | x | ||||||||
| Biological Measures | BP + HR | x | x | x | |||||
| Cortisol testing | x | x | |||||||
| Edwards et al. 2012 (Rhodiola rosea L.) | Ellis & Reddy 2002 (Panax ginseng C.A.Mey.) | Facchinetti et al. 2002 Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.) | Jezova et al. 2002 (Ginkgo biloba L.) | Kennedy et al. 2004 (Panax ginseng C.A.Mey. & Paullinia cupana Kunth) | Kennedy et al. 2001 (Panax ginseng C.A.Mey.) | Kennedy et al. 2002 (Ginkgo biloba L. & Panax ginseng C.A.Mey.) | Olsson et al. 2009 (Rhodiola rosea L.) | ||
| Cognitive Measures | x | x | x | x | |||||
| Mood Measures | BL-VAS or VAS | x | x | x | |||||
| SAM test | |||||||||
| MFI-20 | x | ||||||||
| HRQOL SF-36 | x | ||||||||
| STAI | |||||||||
| PANAS + POMS | |||||||||
| Other validated forms | x | x | |||||||
| Biological Measures | BP + HR | x | x | ||||||
| Salivary cortisol | x | x | |||||||
| Panossian et al. 1999 (Schisandra chinensis (Turcz.) Baill. & Bryonia alba L.) | Reay et al. 2010 (Panax ginseng C.A.Mey.) | Schaffler et al. 2013 (Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.) | Scholey et al. 2010 (Panax quinquefolius L.) | Scholey & Kennedy 2002 (Ginkgo biloba L. & Panax ginseng C.A.Mey.) | Shevtsov et al. 2003 (Rhodiola rosea L.) | Spasov et al. 2000 (Rhodiola rosea L.) | Sunram-Lea et al. 2005 (Panax ginseng C.A.Mey.) | ||
| Cognitive Measures | x | x | x | x | x | x | x | x | |
| Mood Measures | BL-VAS or VAS | x | x | x | |||||
| SAM test | x | ||||||||
| MFI-20 | x | ||||||||
| HRQOL SF-36 | |||||||||
| STAI | x | ||||||||
| PANAS + POMS | |||||||||
| Other validated forms | x | x | |||||||
| Biological Measures | BP + HR | x | x | ||||||
| Cortisol testing | x | x |
BL-VAS: Bond-Lader Visual Analogue Scale; VAS: Visual Analogue Scale; SAM: Self-Assessment Manikin; MFI: Multi-dimensional Fatigue Inventory; HRQOL SF: Health-related Quality of Life Short Form; STAI: State-Trait Anxiety Inventory; PANAS: Positive Affect-Negative Affect Scale; POMS: Profile of Mood States inventory; BP: Blood Pressure; HR: Heart Rate. NB: x indicates which measures were utilized in each study.
Cognitive Measures
The CDR system is a reliable and validated set of computerized testing designed to assess cognitive function [46]. The CDR battery includes the tests described in Table 2. The studies utilizing this outcome measure have used these tests, or a tailored version including a selection of these tests.
Table 2. Tests available in the CDR system.
| Attention | Simple reaction time |
| Choice reaction time | |
| Digit vigilance | |
| Executive Function and Working Memory | Rapid visual information processing |
| Semantic reasoning | |
| Logical reasoning | |
| Articulatory working memory | |
| Spatial working memory | |
| Episodic Secondary Memory | Word recall |
| Word recognition | |
| Picture recognition | |
| Face recognition | |
| Motor Control | Joystick tracking task |
| Tapping task | |
| Postural stability task | |
| Psychophysical Thresholds | Critical flicker fusion (with and without pupil size control) |
Note. Adapted from “The Value of Assessing Cognitive Function in Drug Development” by K.A. Wesnes, 2000, Dialogues in Clinical Neuroscience, 2(3), p.185. Copyright © 2000 LLS.
Among the studies examining Panax ginseng C.A.Mey. using the CDR battery all found significant improvements in cognitive function in four or more independent and objective measures. One placebo-controlled, double-blind, crossover study examining different dosages found significant improvement in cognition (specifically quality of memory) at 400mg of ginseng, but no significant difference at 200mg or 600mg [26]. On the contrary, a decrement in speed of memory was found at 200mg dosage at the 4-hour time point. Another randomized, placebo-controlled trial found a significant improvement in cognitive function after acute dosing (one dose), but no significant improvement after 7 days of treatment [27].
One double-blind, placebo-controlled cross-over study used the cognitive measures (in the form of a Multitasking Framework (MTF)) to examine the effect of Bacopa monnieri (L.) Wettst., a known adaptogen herb, on stress reactivity and mood [23]. This study found a significant effect post-treatment in two of four cognitive measures (improvement in Stroop test and Letter search, but no difference in mental arithmetic or visual tracking). Another placebo-controlled trial investigating two separate acute doses of Bacopa monnieri (L.) Wettst. used a CDB consisting of two serial subtraction tasks and the Bakan Rapid Visual Information Processing task along with a “stress and mental fatigue” visual analogue scale (VAS) and blood pressure monitoring [36]. This study found a significant improvement in cognitive performance at both doses; however, it did not find the treatments to attenuate stress or fatigue induced by the CDB.
A prospective, controlled, three-arm parallel group study compared Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (ES) (a well-known adaptogen herb) to Stress Management Training (SMT) in subjects with symptoms of fatigue and chronic exposure to stress using limited CDR battery testing in conjunction with a number of self-reporting instruments and questionnaires [30]. The cognitive factors included memory, attention, verbal, and visual. This study found that test parameters improved in all three treatment groups (ES, SMT, and a combination of the two).
Four studies examined the effect of Rhodiola rosea L. in participants under circumstances involving stress and fatigue, using other cognitive testing [31,33-35]. Only one of these studies used concomitant mood measures [31], and three out of four tested physical fitness parameters conjunctively [31,34,35]. One randomized, double-blind, placebo-controlled trial used psycho-motoric function testing and a Mental Work Capacity (correction of text) in conjunction with a physical fitness (veloergonomic) test, a self-evaluation test of mental fatigue and a General Wellbeing test (SAM test) [31]. This study found minimal difference in cognitive function (p<0.05 in one of three cognitive tests, the Maze test), and no significant difference in physical fitness, but significant improvement in the self-evaluation of mental fatigue test and SAM test.
Another study examining Rhodiola rosea L. used cognitive measures including reaction time and ability to sustain attention in conjunction with exercise endurance capacity, muscle strength and speed of limb movement [34]. The study found no significant difference in parameters with the exception of an increased time to exhaustion (physical parameter) of p = <0.05. The remaining two randomized, placebo-controlled studies examining Rhodiola rosea L. against a background of fatigue and stress used a range of cognitive measures derived from a known cognitive test battery and employed a Fatigue Index Score to assess the effect [33,35]. Both of these studies found the Total Fatigue Index (TFI) score on cognitive parameters significantly improved post-treatment. One of these studies [35] used additional biological measures (blood pressure and pulse rate) to indicate physiological stress and fatigue, finding a subsequent beneficial effect on these parameters.
A placebo-controlled trial examining Ginkgo biloba L. used cognitive testing including a combined stimulus consisting of mental load (memory test) and static exercise (physical parameter) [37]. Salivary cortisol testing, blood pressure, and heart rate were measured just prior to treatment, and just after mental load and exercise testing. The study found that single administration of Ginkgo biloba L. failed to modify memory performance, however it did prevent a stress-induced rise in salivary cortisol in male subjects. No effect of treatment on salivary cortisol was observed in women. Single administration of Ginkgo biloba L. resulted in a significant inhibition of blood pressure responses to exercise testing, with heart rate responses unchanged.
Mood Measures
Fourteen studies used mood measures which included questionnaires, self-rated instruments, and subjective rating scales. The BL-VAS was the predominantly used mood measure, with seven studies implementing this tool conjunctively with the CDR Battery [23-29]. One study used an alternative VAS [36]. The BL-VAS is a series of 16 analogue scales (composed of 16 pairs of antonyms) designed to assess the mood effects of anxiolytic substances [47]. From the 16 scales, measures are derived from how the participants mark their subjective state. The resultant measures include three factors: “alertness,” “calmness,” and “contentedness” [47].
The BL-VAS was used in five out of the six studies examining Panax ginseng C.A.Mey. with the CDR battery [24-27,29]. No significant main effects were seen across these studies with the exception of one placebo-controlled, randomized trial which found a significant main effect in “calm” rating (but no effect in “alert” or “content” ratings) [27]. Another trial found a significant reduction in “alert” factor post-ginseng treatment, but no significant effect on “calm” or “content” factors [26].
The BL-VAS mood measure was used in one Bacopa monnieri (L.) Wettst. study [23] and found there was a significant main effect post-treatment in absence of induced mental stress (MTF) only. Biological measures (salivary cortisol) used in this study again found a significant main effect of treatment in absence of MTF only. The second Bacopa monnieri (L.) Wettst. study which used VAS found the treatment to have no significant effect on indicators of stress and fatigue [36].
Two placebo-controlled trials used a Health Related Quality of Life (HRQOL) Short-form survey (SF-36) alone [43,45], to assess the effects of Panax ginseng C.A.Mey. [43] after 4 weeks and 8 weeks of treatment and Rhodiola rosea L. [45] on mental and social functioning in healthy individuals. The HRQOL questionnaire is a validated self-reporting measure comprised of a set of questions regarding how one perceives their mental and physical health at that time [48]. The Panax ginseng C.A.Mey. study found significantly higher social functioning in the treatment group at 4 weeks as well as a significantly higher mental component summary score [43]. These changes did not persist to the 8-week evaluation. The Rhodiola rosea L. trial used the SF-36 in conjunction with two other mood measures: The Pines Burnout Scale used to assess fatigue, and the Montgomery-Asberg Depression Rating Scale (MADRS) used to assess symptoms of depression as well as cognitive measures; and biological measures [45]. This study found significantly improved fatigue scores on the Pines Burnout Scale and a tendency towards improved physical health (p = 0.056) on the SF-36, with mental health not significantly changed on this scale.
The general wellbeing (SAM test) was used in another Rhodiola rosea L. study [31] along with a self-evaluation of mental fatigue (and physical and cognitive parameters). The SAM test consists of a 5-point scale assessing general state, degree of activity, mood and motivation to work [31]. The self-evaluation of mental fatigue was a specific Russian designed psychometric test in questionnaire form, where students were asked to evaluate and score signs of fatigue [31]. This study found significant improvements in both the self-evaluation of mental fatigue and the general wellbeing test (though minimal improvements in cognitive testing as outlined previously).
One double-blind, placebo-controlled trial examining Panax ginseng C.A. Mey. used differing mood measures again, measuring three psychological variables: positive effect, negative effect, and total mood disturbance [41]. Positive and negative effect were determined from the 20-item Positive Affect-Negative Affect Scale (PANAS) and total mood disturbance was determined with the 65-item Profile of Mood States Inventory (POMS). In this study no significant effects were found from chronic (60 days) Panax ginseng C.A.Mey. supplementation.
An open-label study investigating Rhodiola rosea L. treatment in life-stress symptoms [42] used Numerical Analogue Scales (NAS) along with five different subjective questionnaires (including MFI-20 and MDMQ also used in the Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. study) [30]. All outcome variables used showed consistent and steady improvement with significant improvement at the 4-week time point.
A trial investigating the effects of Withania somnifera (L.) Dunal in chronically stressed humans used a version of a modified Hamilton anxiety (mHAM-A) scale for stress, along with biological measures [44]. It found significant improvement in wellbeing at both time points (30 and 60 days).
Biological Measures
In total, 10 studies used biological measures including blood pressure, heart rate, and/or salivary or serum cortisol testing. Four of the studies utilizing the CDR battery also measured blood pressure and heart rate [30,35-37]. In three studies biological parameters were improved by treatment [30,35,37], however in one of those studies there was an equal beneficial effect observed in the stress management training group who were not administered an herbal medicine [30]. The type of extract was not specified in this study and it is unknown whether a standardized extract was used. In the fourth study no effect of treatment was observed in blood pressure measurements [36].
Three studies used biological measures as a stand-alone assessment of the effect [38-40]. One randomized, controlled trial assessed the effect of Piper methysticum G.Forst. (kava) and Valeriana officinalis L. (valerian) on physiological and psychological responses to mental stress [38]. The measures used were BP and HR while the subjects were under induced mental stress with a color-word interference task which has been shown to increase blood pressure and heart rate [38]. In the Piper methysticum G.Forst. group a significant beneficial effect was seen on blood pressure (reduction) post-treatment, and in the Valeriana officinalis L. group a significant reduction in BP and HR was also observed.
Another placebo-controlled trial examining Schisandra chinensis (Turcz.) Baill. and Bryonia alba L. utilized salivary cortisol testing against a background of heavy physical exercise [40]. This study found that both Schisandra chinensis (Turcz.) Baill. and Bryonia alba L. significantly decreased plasma and salivary cortisol in well-trained athletes. However, this effect was not observed in beginner athletes.
The Withania somnifera (L.) Dunal trial [44] examined serum cortisol, blood pressure and heart rate along with a mood measure and found all parameters (biological and mood) to be significantly improved after both 30 and 60 days treatment.
Discussion
A network meta-analysis was initially intended for this review to identify domains that have been used to measure the effect and outcome of adaptogenic herbal medicines in human studies. However, due to the significant heterogeneity in clinical studies of adaptogens this was not possible. As such, a critical review was conducted to ascertain the major domains used in clinical research on adaptogens. A critical review methodology was chosen to go beyond mere description and to include a degree of analysis identifying the most important aspects of the field [49].
The review identified relevant consistencies in outcome measures used, finding three broad categories of measurement including cognitive measures, mood measures, and biological measures. Despite these broader consistencies, significant heterogeneity in choice of measurement tools was identified in each of these areas. Individual studies had used modified and varying selections of tests included in those measurement tools, in particular varying and minimized selections of tests from the CDR battery to measure cognitive function. Even when similar measurement tools had been employed, they were used and analyzed in differing ways, often resulting in contradictory results between those studies. For example, studies examining Rhodiola rosea L. where CDR battery derived testing had been used in each study (although a differing selection of tests from the battery between studies). Two studies utilized a Total Anti-fatigue Index (TAFI) as their method of analysis and found significant benefits of treatment [33,35]; whereas other Rhodiola rosea L. studies [31,34] using tailored cognitive testing had used either Students t-test or repeated measures ANOVA as methods of analysis finding little or no benefit of treatment on cognitive function, in stark contrast.
The cognitive tests selected varied between studies, producing an additional type of heterogeneity, and there was a vast diversity between dosing regime and timing amongst studies, with differing (and in some cases contradictory) results between doses, and in one study differing results between sexes [37]. Many of the studies examining adaptogens had utilized only a narrow selection of cognitive tests, with the average number of cognitive tests used per study being five (out of a possible 16 tests across five categories (described in Table 2) in a complete CDR battery test panel [46]).
Furthermore, significant diversity in results was identified within categories of outcome measures. Mood measures had particular diversity, where the most commonly used mood measure (BL-VAS) reported little or no effect in studies, and yet alternative validated mood measures used in other studies reported significant effects. Such heterogeneity suggests there has not been sufficient consistency in domains used to measure adaptogenic activity to capture the potential clinical outcomes, and that the domains used may have been too narrowly focused. Within the collective heterogeneity of the data, the individual studies appear to have used measures more narrowly focused than could be expected to capture a multi-system action such as adaptogenic activity. For example, a proportion of the studies tested the effect of adaptogens on cognitive function only, and although the overall findings were significant, to test this effect alone is drastically insufficient to provide insight into a class of herb which has a non-specific stress-protective effect across multiple body systems [2].
The biological parameters tested in the human studies (salivary cortisol, blood pressure, and heart rate) were narrow in comparison to the wide range of hormones and key mediators of stress and homeostasis identified in laboratory work [2]. The body of laboratory literature on adaptogens investigates their mode of action [50], molecular mechanisms, proteins, and key signaling pathways associated with stress-protective effects of adaptogens [6,10], biological activity [7] and implications in stress resistance [16,51]. Yet this laboratory-based knowledge is not well-reflected in domains used to measure adaptogenic activity in the clinical studies reviewed.
One factor which may have contributed to the diversity of measures used and subsequent heterogeneous results is the diversity of views around the concept and definition of adaptogen. Many adaptogen herbs were traditionally documented as tonics prior to the adaptogenic concept being formally codified by Lazarev in 1947. These include Panax ginseng C.A.Mey. and Schisandra chinensis (Turcz.) Baill., which are listed as tonics in the State Pharmacopoeia of the USSR [4]. The first known literature to define the action of plant adaptogens also refers to these plants as “tonic plants” [5]. Modern phytotherapy texts now differentiate the two phytotherapeutic actions and highlight key differences, such as tonics considered to be “revitalizing” herbs and adaptogens considered to “improve response to stress” [52]. Wagner, Nörr [9] discuss the conundrum of differentiating between tonics and adaptogens where both concepts have overlapping features (such as improving performance) yet distinct differences (where tonics ameliorate a lack of tonus in an organism or organ), yet neither concept has been clearly defined. Such common misunderstanding, general confusion and competing – often vague – definitions of adaptogen may be contributing factors to the diverse array of methods which have been used to measure adaptogenic activity in clinical research.
Interestingly, of the 24 papers reviewed nearly 70% of papers predated 2006. While laboratory and theoretical data on adaptogens has increased in the last 10 years, it appears clinical trials have plateaued. Reasons for this are unknown, however it could be that as laboratory research evolves so too does the understanding of the complexity of the inter-systems activity of adaptogens, highlighting the need for more diverse methods of measurement which have not yet been identified. Identifying appropriate methods to capture adaptogenic activity may assist in remedying the lack of up-to-date research on adaptogens.
Although cognitive testing holds promise as a useful measurement tool in conjunction with other tools, in order to gain a thorough indication of cognitive effects a more comprehensive and standardized set of cognitive tests may need to be implemented. Moreover, cognitive enhancement is only one potential facet of adaptogenic activity which appears to exhibit activity across multiple body systems [2]. Therefore, tests measuring the effects on individual body systems may only be relevant to adaptogens when used alongside additional tools to measure other parameters in line with the current understandings of the action incorporating pharmacological, traditional, and expert clinician perspectives. In short, the heterogeneity of the collective data makes it difficult to draw conclusions on the effect and efficacy of adaptogens based on current research, or even how these effects may be best measured. Nevertheless, the significant heterogeneity uncovered highlights the need for more research studies on adaptogens, and more consistency in those studies.
This review has some limitations, including the overall quality and reporting of the studies as assessed with the Jadad Scale (and the required use of the simple Jadad scale itself, given the heterogeneity of studies) and the deficit in current data with nearly 70% of studies predating 2006. The clinical data have a number of shortcomings including study design and methods of analysis (described earlier within the results). Further, the review included human studies only, meaning there is a substantial body of animal studies missing from this picture. However, the purpose of the paper is to determine domains used to measure adaptogenic activity in humans, in order to open pathways for the translation of theory into practical applications. Recently, attempts have been made to develop and evaluate combination products that are specifically marketed as adaptogenic, though have not been included in our study because – although often marketed as adaptogenic – they have not been formally assessed or verified in accordance with traditional texts or official pharmacopeia. However, domains used to evaluate these appear to follow similar domains to the individual herbal medicines assessed in our study (i.e. stress or fatigue scores, cortisol-focused biological studies) [53].
In summary, three broad areas of outcome measures have been used to measure the outcome and effect of adaptogens, which include cognitive measures, mood measures, and biological measures. Significant heterogeneity amongst studies was identified, making it difficult to compare the outcome measures and effects and derive definitive conclusions on the action of adaptogens or the most appropriate way to measure them capturing the holistic activity in humans. Individually, these studies give some level of information regarding the action and efficacy of certain herbal medicines in stress related conditions; however, collectively, the level of heterogeneity could be seen to render each individual study redundant based on the differing results found depending on the methods, outcome measures and methods of analysis used. Comprehensive cognitive testing holds promise as a measurement tool when used with additional measures relevant to the scope of adaptogenic activity as it is understood to date. Those additional measures need clarification. A key area of focus for future research on adaptogens is on the development of a standardized battery of tests designed for capturing the broad-spectrum multi-system activity of adaptogens. Standardization in measures as well as in methods of analysis of studies is crucial for the interpretation, reliability and clinical relevance of adaptogen research. This data provides evidence of the need for further research to develop appropriate measures and methods of analysis suitable to adaptogenic herbal medicines, in order to bridge the gap between traditional understanding and use, and modern evidence.
Acknowledgments
This manuscript is modified from original work completed as a component of an Honours program with Endeavour College of Natural Health.
Glossary
- ASRP
Adaptive Stress-response Signaling Pathway
- BL-VAS
Bond-Lader Visual Analogue Scale
- BP
Blood Pressure
- CDB
Cognitive Demand Battery
- CDR
Cognitive Drug Research
- EMA
European Medicines Agency
- ES
Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.
- FDA
Food and Drug Administration
- HR
Heart Rate
- HRQOL SF
Health Related Quality of Life Short Form
- HSP
Heat Shock Protein
- MADRS
Montgomery-Asberg Depression Rating Scale
- MFI
Multi-dimensional Fatigue Inventory
- mHAM-A
modified Hamilton Anxiety Scale
- MTF
Multi-tasking Framework
- NAS
Numerical Analogue Scales
- NO
Nitric Oxide
- NPY
Neuropeptide Y
- PANAS
Positive Affect-Negative Affect Scale
- POMS
Profile of Mood States Inventory
- SAM
Self-Assessment Manikin
- SMT
Stress Management training
- STAI
State-trait Anxiety Inventory
- TAFI
Total Anti-fatigue Index
- TFI
Total Fatigue Index
- USSR
Union of Soviet Socialist Republics
- VAS
Visual Analogue Scale
Appendix A.
Author Contributions
SEG: Conceptualization, Methodology, Search strategy, data extraction, data analysis, Writing: original draft, Writing: review and editing, Project administration. JW: Conceptualization, Methodology, Search strategy, Writing: review and editing, Project administration. DC: Conceptualization, Search strategy, Writing: review and editing. ANS: Conceptualization, Writing: review and editing.
References
- Panossian AG. Adaptogens in mental and behavioral disorders. Psychiatr Clin North Am. 2013. March;36(1):49–64. [DOI] [PubMed] [Google Scholar]
- Panossian A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci. 2017. August;1401(1):49–64. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Reflection paper on the adaptogenic concept In: Agency EM. London: European Medicines Agency; 2008. [Google Scholar]
- Shikov AN, Pozharitskaya ON, Makarov VG, Wagner H, Verpoorte R, Heinrich M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J Ethnopharmacol. 2014. July;154(3):481–536. [DOI] [PubMed] [Google Scholar]
- Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9(1):419–30. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress—protective activity. Pharmaceuticals (Basel). 2010. January;3(1):188–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian A, Wagner H. Adaptogens. HerbalGram. 2011;(90):52–63. [Google Scholar]
- Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol. 2009. September;4(3):198–219. [DOI] [PubMed] [Google Scholar]
- Wagner H, Nörr H, Winterhoff H. Plant adaptogens. Phytomedicine. 1994. June;1(1):63–76. [DOI] [PubMed] [Google Scholar]
- Panossian A, Seo EJ, Efferth T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine. 2018. November;50:257–84. [DOI] [PubMed] [Google Scholar]
- European Scientific Cooperative on Phytotherapy ESCOP monographs: The scientific foundation for herbal medicinal products. United Kingdom: European Scientific Cooperative on Phytotherapy; 2003. [Google Scholar]
- World Health Organization WHO monographs on selected medicinal plants. Geneva: World health Organization; 1999. [Google Scholar]
- Shikov AN, Pozharitskaya ON, Makarov VG, Yang WZ, Guo DA. Oplopanax elatus (Nakai) Nakai: chemistry, traditional use and pharmacology. Chin J Nat Med. 2014. October;12(10):721–9. [DOI] [PubMed] [Google Scholar]
- Shikov AN, Pozharitskaya ON, Makarov VG. Aralia elata var. mandshurica (Rupr. & Maxim.) J.Wen: an overview of pharmacological studies. Phytomedicine. 2016. November;23(12):1409–21. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Regulations on statements made for dietary supplements concerning the effect of the product on the structure or function of the body. In: Services DoHaH, editor. USA: Federal Register; 1998. [PubMed]
- Panossian A. Adaptogens: tonic herbs for fatigue and stress. Altern Complement Ther. 2003;9(6):327–31. [Google Scholar]
- Panossian A, Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005. October;19(10):819–38. [DOI] [PubMed] [Google Scholar]
- Clarivate Analytics EndNote. X8.2 ed. Boston2018.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A Product From the ESRC Methods Programme Version 1. 2006:b92.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996. February;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- Moher D, Sampson M, Campbell K, Beckner W, Lepage L, Gaboury I, et al. Assessing the quality of reports of randomized trials in pediatric complementary and alternative medicine. BMC Pediatr. 2002;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow J, Nienhuis C. Medicinal plants for primary dysmenorrhoea: A systematic review. Complement Ther Med. 2018. April;37:13–26. [DOI] [PubMed] [Google Scholar]
- Benson S, Downey LA, Stough C, Wetherell M, Zangara A, Scholey A. An acute, double-blind, placebo-controlled cross-over study of 320 mg and 640 mg doses of Bacopa monnieri (CDRI 08) on multitasking stress reactivity and mood. Phytother Res. 2014. April;28(4):551–9. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Haskell CF, Wesnes KA, Scholey AB. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacol Biochem Behav. 2004. November;79(3):401–11. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Scholey AB, Wesnes KA. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav. 2002. April;75(5):739–51. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Scholey AB, Wesnes KA. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr Neurosci. 2001;4(4):295–310. [DOI] [PubMed] [Google Scholar]
- Reay JL, Scholey AB, Kennedy DO. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010. August;25(6):462–71. [DOI] [PubMed] [Google Scholar]
- Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, et al. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl). 2010. October;212(3):345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunram-Lea SI, Birchall RJ, Wesnes KA, Petrini O. The effect of acute administration of 400mg of Panax ginseng on cognitive performance and mood in healthy young volunteers. Curr Top Nutraceutical Res. 2005;3(1):65–74. [Google Scholar]
- Schaffler K, Wolf OT, Burkart M. No benefit adding eleutherococcus senticosus to stress management training in stress-related fatigue/weakness, impaired work or concentration, a randomized controlled study. Pharmacopsychiatry. 2013. July;46(5):181–90. [DOI] [PubMed] [Google Scholar]
- Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000. April;7(2):85–9. [DOI] [PubMed] [Google Scholar]
- D’Angelo L, Grimaldi R, Caravaggi M, Marcoli M, Perucca E, Lecchini S, et al. A double-blind, placebo-controlled clinical study on the effect of a standardized ginseng extract on psychomotor performance in healthy volunteers. J Ethnopharmacol. 1986. Apr-May;16(1):15–22. [DOI] [PubMed] [Google Scholar]
- Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Rhodiola rosea in stress induced fatigue—a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000. October;7(5):365–71. [DOI] [PubMed] [Google Scholar]
- De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. 2004. June;14(3):298–307. [DOI] [PubMed] [Google Scholar]
- Shevtsov VA, Zholus BI, Shervarly VI, Vol’skij VB, Korovin YP, Khristich MP, et al. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003. March;10(2-3):95–105. [DOI] [PubMed] [Google Scholar]
- Downey LA, Kean J, Nemeh F, Lau A, Poll A, Gregory R, et al. An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother Res. 2013. September;27(9):1407–13. [DOI] [PubMed] [Google Scholar]
- Jezova D, Duncko R, Lassanova M, Kriska M, Moncek F. Reduction of rise in blood pressure and cortisol release during stress by Ginkgo biloba extract (EGb 761) in healthy volunteers. J Physiol Pharmacol. 2002. September;53(3):337–48. [PubMed] [Google Scholar]
- Cropley M, Cave Z, Ellis J, Middleton RW. Effect of kava and valerian on human physiological and psychological responses to mental stress assessed under laboratory conditions. Phytother Res. 2002. February;16(1):23–7. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Neri I, Tarabusi M. Eleutherococcus senticosus reduces cardiovascular stress response in healthy subjects: A randomized, placebo-controlled trial. Stress Health. 2002;18(1):11–7. [Google Scholar]
- Panossian AG, Oganessian AS, Ambartsumian M, Gabrielian ES, Wagner H, Wikman G. Effects of heavy physical exercise and adaptogens on nitric oxide content in human saliva. Phytomedicine. 1999. March;6(1):17–26. [DOI] [PubMed] [Google Scholar]
- Cardinal BJ, Engels HJ. Ginseng does not enhance psychological well-being in healthy, young adults: results of a double-blind, placebo-controlled, randomized clinical trial. J Am Diet Assoc. 2001. June;101(6):655–60. [DOI] [PubMed] [Google Scholar]
- Edwards D, Heufelder A, Zimmermann A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms—results of an open-label study. Phytother Res. 2012. August;26(8):1220–5. [DOI] [PubMed] [Google Scholar]
- Ellis JM, Reddy P. Effects of Panax ginseng on quality of life. Ann Pharmacother. 2002. March;36(3):375–9. [DOI] [PubMed] [Google Scholar]
- Auddy B, Hazra J, Mitra A, Abedon B, Ghosal S. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: A double blind, randomized, placebo-controlled trial. J Am Nutraceut Assoc. 2008;11(1):50–6. [Google Scholar]
- Olsson EM, von Schéele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009. February;75(2):105–12. [DOI] [PubMed] [Google Scholar]
- Wesnes KA. The value of assessing cognitive function in drug development. Dialogues Clin Neurosci. 2000. September;2(3):183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–8. [Google Scholar]
- Centers for Disease Control and Prevention Health-related quality of life (hrqol): U.S. Department of Health and Human Services; 2016. [cited 2018 6/4/18]. Available from: https://www.cdc.gov/hrqol/concept.htm
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009. June;26(2):91–108. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G, Wagner H. Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine. 1999. October;6(4):287–300. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G, Kaur P, Asea A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine. 2009. June;16(6-7):617–22. [DOI] [PubMed] [Google Scholar]
- Bone K, Mills S. Principles and practice of phytotherapy, modern herbal medicine. 2nd ed. Sydney (NSW): Elsevier; 2013. [Google Scholar]
- Hovhannisyan A, Nylander M, Wikman G, Panossian A. Efficacy of adaptogenic supplements on adapting to stress: A randomized, controlled trial. J Athl Enhancement. 2015;4(4). http://dx.doi.org/10.4172/2324-9080.1000205 [Google Scholar]