Abstract
Essential oils (EOs) have risen in popularity over the past decade. These oils function in society as holistic integrative modalities to traditional medicinal treatments, where many Americans substitute EOs in place of other prescribed medications. EOs are found in a multitude of products including food flavoring, soaps, lotions, shampoos, hair styling products, cologne, laundry detergents, and even insect repellents. EOs are complex substances comprised of hundreds of components that can vary greatly in their composition depending upon the extraction process by the producer or the origin of the plant. Thus, making it difficult to determine which pathways in the body are affected. Here, we review the published research that shows the health benefits of EOs as well as some of their adverse effects. In doing so, we show that EOs, as well as some of their individual components, possess antimicrobial, antiviral, antibiotic, anti-inflammatory, and antioxidant properties as well as purported psychogenic effects such as relieving stress, treating depression, and aiding with insomnia. Not only do we show the health benefits of using EOs, but we also indicate risks associated with their use such as their endocrine disrupting properties leading to the induction of premature breast growth in young adolescents. Taken together, there are many positive and potentially negative risks to human health associated with EOs, which make it important to bring awareness to all their known effects on the human body.
Keywords: Endocrine disruptors, prepubertal, gynecomastia, psychological, antimicrobial, anti-inflammatory
Introduction
The essential oil (EO) industry developed into a highly active and successful market over the past decade [1]. Many individuals use essential oil containing commodities regularly, including food flavoring, soaps, lotions, shampoos, hair-styling products, cologne, and laundry detergents [2]. Many people seems to deem essential oils as safe alternatives to more invasive pharmacological forms of treatment due to the concept that they are more “natural.” However, only a modest amount of research has been conducted on essential oils. This leaves the potential beneficial and/or adverse effects unclear, making it necessary to investigate these oils in order to verify their true effects on human health.
There are many methods by which EO exposure can occur including inhalation, ingestion, massage, and skin applications, [3,4]. EOs are known for many of their health effects such as their antibacterial, antibiotic, and antiviral properties [3,5-9]. They are also known for relieving stress and have been used in multiple treatments such as sleep disorders, Alzheimer disease, cardiovascular issues, cancer, and labor pain in pregnancy [3,5-12]. Furthermore, they are also known for their insect repellent properties and antioxidant/anti-inflammatory activity [11,13-15]. Most essential oils are generally safe. The majority of adverse effects are mild, but there have been cases of serious toxic reactions including abortions and pregnancy abnormalities, neurotoxicity, bronchial hyperactivity, hepatotoxicity, prepubertal gynecomastia, and premature thelarche [16-19].
EOs are complex substances, comprised of multi-component mixtures that contains hundreds of chemicals. The oils are typically extracted by steam distillation of plant material [2,20-22]. In an individual oil, up to 400 substances can be identified, or even more when the finest analytical equipment is utilized [20]. In a publication of Contact Dermatitis, 4350 chemicals were found in 91 EOs [23]. The composition of an EO can vary considerably between producers as well as between the same producer. Many of the factors that can change an EO chemical composition includes the species, origin, climate, soil conditions, fertilization, and mode of production. Terpenes are the biggest class of chemicals found in essential oils. This group of chemicals are created from 5-carbon isoprene units. Larger, more sophisticated molecules, can be constructed in biosynthesis from terpenes to make linear-chained chemicals with one or more ring structures [20]. There are several classes of terpenes, however, the most important in essential oils are the monoterpenes and sesquiterpenes [20]. The distinct smell of an EO is produced from these two groups of chemicals. Modification of a terpene or sesquiterpenes, typically from oxidation or rearranging the skeletal structure of the molecule, yields different terpenoids. The oxidation reactions are most important, which create many subgroups such as alcohols, aldehydes, phenols, ethers, and ketones [20,24,25]. Thus, these oils are widely variable in their composition and make it difficult to assess the health effects each time they may be used.
Endocrine Disrupting Activities
According to the United States Environmental Protection Agency, an endocrine disrupting chemical (EDC) is an exogenous agent that interferes with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes [26,27]. An EDC may interfere with hormone action by several mechanisms and can be quite complex. The chemicals may bind to hormone receptors and act directly as an agonist or antagonist, exert indirect agonist or antagonist actions, or may bind to allosteric sites and yield unanticipated effects at very low concentrations [28]. In addition, these chemicals are known to interfere with hormone synthesis, metabolism, transport, and degradation [28].
In previous reports, essential oils have been determined to act as an EDC [16-18]. Essential oils have been demonstrated to act as an agonist to the estrogen receptor alpha (ERα) and antagonist to the androgen receptor (AR) [16-18]. Additionally, these studies have provided support to a suspected link between abnormal breast growth in adolescents, termed prepubertal gynecomastia and premature thelarche, and regular topical exposure to lavender or tea tree oil hygiene commodities [16-18]. Premature thelarche, the most common pubertal disorder in prepubescent girls, which is defined as isolated breast growth before 8 years of age without any other signs of puberty.
Gynecomastia is suspected to have many etiologies. Selected drugs and environmental exposures such as alcohol, heroin, marijuana, amphetamines, antiulcer medications, antibiotics, cancer agents, cardiovascular drugs, and psychoactive drugs have been identified as possible hormonal mimics for the estrogen and androgen receptors [16-18]. The mechanism by which those drugs disrupt the endocrine system is poorly defined but could also involve altering steroidogenesis, with a resultant change in the balance between testosterone and estradiol (E2) levels, increasing proliferation of breast tissue and leading to the onset of gynecomastia [17,29].
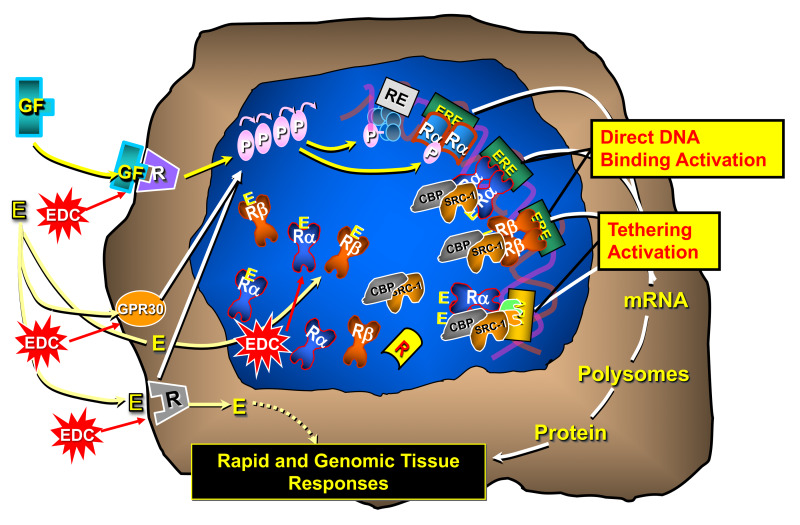
Some EDCs act through nuclear hormonal receptors, while others initiate their effects through different mechanisms [30]. Previous studies have reported that ERα plays a crucial role in mammary gland development using knockout (KO) mouse models. In both aromatase KO mice that lack endogenous estrogen production and ERα knockout mice that lack functional ERα, impaired mammary gland development was exhibited [31]. This supports the view that estrogen-dependent, ERα-mediated actions are critical for mammary gland development and could be the reason for these observations seen in the prepubertal children [32-35]. Figure 1 demonstrates the proposed cellular mechanism of action in which these essential oils produce their biological effects on the human body.
Figure 1.
Proposed Mechanism for EO and components Agonizing and Antagonizing the ERα and/or AR Receptor mechanisms. Estrogen or androgen hormones can elicit biological responses by interaction with cell membrane-based receptor proteins (GPR30 or R) to instigate intracellular agonist signaling mechanisms. The hormones can stimulate agonist activities by interacting with nuclear forms of the receptor proteins to stimulate DNA binding genomic mechanisms of gene regulation (direct or tethering). Nuclear hormone receptors can also be activated in a ligand independent mechanism by other intracellular signaling mechanisms (e.g. Growth Factors). EO acting as endocrine disruptors can alter any of these possible cellular mechanisms.
Antimicrobial, Antiviral, and Antibiotic Effects
Essential oils are common natural products that can be used for various medical applications, and in combination with the emergence of antimicrobial resistance, essential oils have been studied as potential antimicrobials agents [36]. These naturally occurring compounds are linked to having bactericidal, virucidal, and fungicidal activity in clinical trials. It has also been suggested that these plant extracts might not only be used to fight cutaneous infections for example, but also serve a role in the preservation of food due to their antimicrobial activity combined with their antioxidant property [36,37]. Table 1 provides a brief summary of certain common essential oils and the organisms targeted.
Table 1. Brief summary of common Essential Oils plant of origin and microorganisms affected by compound extracted. Adapted from [39,45,116].
| Common Name | Plant | Major Essential Oil | Inhibited Microorganisms |
| Thyme | Thymus vulgaris | Thymol | S. aureus, V. parahaemolyticus, C. perfringens |
| Oregano | Origanum vulgare | Carvacrol | Polio virus, Adeno virus, L. monocytogenes |
| Garlic | Allium sativum | Isothiocynate | Candida spp., Enterobacteriaceae |
| Lemon Balm | Melissa officinalis | Linalool, myrcene, camphor | HSV-2, avian influenza virus |
| Cinnamon | Cinnamomum zelancium | Cinnamaldehyde | Enterobacteriaceae, P. mirabilis, S. pyogenes |
| Lavender | Lavandula angustifolia | Linalool, Linalyl acetate | E. coli, M. smegmatis |
Bacterial infections remain a significant cause of mortality in the human population. This has triggered research into the exploration of alternative therapies against bacterial strains as the issue of antibiotic resistance has become more imminent even to the newest antibiotic drugs. The effect of antibacterial activity of essential oils may be bacteriostatic or bactericidal, but is difficult to distinguish these actions therefore activity is commonly measured as the minimum bactericidal concentration (MBC) or the minimum inhibitory concentration (MIC) [38,39]. The mechanism of antibacterial action is facilitated by a succession of biochemical reactions within the bacterial cell that are dependent on the type of chemical constituents present in the essential oil. Due to these compounds being lipophilic, essential oils easily penetrate bacterial cell membranes and have been reported to disrupt critical processes of the cell membrane like nutrient processing, synthesis of structural molecules, emission of growth regulators, energy generation, and influences on the cell-cell communication quorum sensing network [4,39,40]. The list of specific bacteria targeted by the essential oils is expanding and include, but are not limited to, Listeria monocytogenes, Bacillus sphaericus, Enterobacter aerogenes, Escherichia coli O157:H7, P. aeruginosa, S. aureus, S. epidermidis, S. typhi, Shiguella flexneri, and Yersinia enterocolitica [41-44]. Some of the essential oils commonly used come from garlic, ginger, clove, black pepper, green chile, cinnamon, clove, pimento, thyme, oregano, and rosemary [39].
Similarly to the effects on bacteria, essential oils have the ability to enter and interrupt the homeostasis of the fungal cell wall and cytoplasmic membranes, specifically the mitochondria [37,39,45]. One of the mechanisms suggested involves the penetration of essential oils into the mitochondrial membranes and changing the electron flow through the electron transport system, which in return disrupts the lipids, proteins, and nucleic acid contents of the fungal cells [46]. Another proposed mechanism is the depolarization of the mitochondrial membranes that decreases the membrane potential, affecting ion channels to reduce the pH and affect the proton pump leading to fungal cell apoptosis and necrosis [47]. Extracts from plants such as basil, clove, citrus, garlic, fennel, lemongrass, oregano, rosemary, and thyme have demonstrated their significant antifungal activity against a broad range of fungal human pathogens [48]. Some of the fungal pathogens affected include Candida acutus, C. albicans, C. apicola, C. catenulata, C. inconspicua, C. tropicalis, Rhodotorula rubra, Sacharomyces cerevisae, and Trignopsis variabilis, Aspergillus parasiticus, and Fusarium moniliforme [39,41,49].
Since viral infections are still a problem for human health and only a narrow number of drugs are effective, it has prompted researchers to explore new antiviral molecules that can attack these human pathological viruses. Detailed insight on the antiviral action of essential oils still requires more research. Essential oils might interfere with virion envelopment, which is designed for entry into human host cells, synthesis of viral proteins, inhibition of the early gene expression process, glycosylation process of viral proteins, and inhibition of virus replication by hindering cellular DNA polymerase [50-53]. Some of the pathogens targeted include many DNA and RNA viruses, such as herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), dengue virus type 2, Junin virus, influenza virus adenovirus type 3, poliovirus, rhinovirus, and coxsackievirus B1 [39]. Activities of essential oils extracted from Australian tea tree oil, eucalyptus oil, thyme oil, and many other medicinal and aromatic plants have been studied for their effect against viruses [39,45].
Insect Repelling Properties
Arthropod borne infectious diseases are found in zoonotic reservoirs such as birds and mammals. They are transmitted to humans via the bite of infected mosquitoes, midges, flies, fleas, and ticks [54]. Currently, there are few vaccinations available to prevent the transmission of arthropod borne infectious diseases; human transmission prevention relies on arthropod avoidance, insect repellents, and insecticides [54]. Consequently, development of safe and effective therapies against arthropod borne diseases is of utmost importance.
Insect repellents may be synthetic or organic and discourage insects from contact or biting [55]. Among the most commonly used repellents are synthetic repellents such as DEET (N,N-diethyl-3- methylbenzamide, formerly N,N-diethyl-m-toluamide), which has recently raised concerns relating to its environmental, human health, and safety risks [11,56]. Thus, consumers are apprehensive of its use as well as the use of other synthetic repellents [49]. Therefore, plant EOs have been considered as an organic alternative to synthetic repellents such as DEET due to their improved safety and toxicity profiles to humans and the environment [11,57,58]. A comprehensive list of plant EOs exhibiting arthropod repellent properties may be found below in Table 2 [57].
Table 2. Plant essential oils exhibiting arthropod repellence.
| Essential oil used as repellant | Animal species repelled | |||
| Plant source | Plant family | Part used | Order | Scientific name |
| Mentha piperita | Lamiaceae | Fresh leaves | Diptera | Anopheles annularis |
| Mentha piperita | Lamiaceae | Fresh leaves | Diptera | Anopheles culicifacies |
| Mentha piperita | Lamiaceae | Fresh leaves | Diptera | C. quinquefasciatus |
| Z. piperitum | Rutaceae | Dried fruits | Diptera | A. aegypti |
| Pimpinella anisum | Umbelliferae | Seed | Diptera | Culex pipiens |
| O. basilicum | Lamiaceae | Dried foliage | Diptera | Culex pipiens |
| Eucalyptus camaldulensis | Mirtaceae | Dried fruits | Diptera | Culex pipiens |
| Baccharis spartioides | Compositae | N.I. | Diptera | A. aegypti |
| Aloysia citriodora | Verbenaceae | N.I. | Diptera | A. aegypti |
| Eucalyptus maculate citriodon | Mirtaceae | Leaves | Diptera | Mansonia |
| Croton pseudopulchellus | Annonaceae | N.I. | Diptera | A. gambiae |
| Mkilua fragrans | Annonaceae | N.I. | Diptera | A. gambiae |
| Endostemon tereticaulis | Labiateae | N.I. | Diptera | A. gambiae |
| Ocimum forskolei | Labiateae | N.I. | Diptera | A. gambiae |
| Ocimum fischeri | Labiateae | N.I. | Diptera | A. gambiae |
| Plectranthus longipes | Labiateae | N.I. | Diptera | A. gambiae |
| Conyza newii | Compositae | N.I. | Diptera | A. gambiae |
| Tarchonanthus camphoratus | Compositae | N.I. | Diptera | A. gambiae |
| Tetradenia riparia | Labiateae | N.I. | Diptera | A. gambiae |
| Lippia javanica | Verbenaceae | N.I. | Diptera | A. gambiae |
| Lippia ukambensis | Verbenaceae | N.I. | Diptera | A. gambiae |
| Plectranthus marrubioides | Labiatae | N.I. | Diptera | A. gambiae |
| C. citratus | Poaceae | Fresh aerial parts | Diptera | A. aegypti |
| O. selloi | Lamiaceae | Leaves | Diptera | A. braziliensis |
| O. basilicum | Lamiaceae | Leaves | Diptera | Anopheles stephensi |
| O. basilicum | Lamiaceae | Leaves | Diptera | A. aegypti |
| O. basilicum | Lamiaceae | Leaves | Diptera | C. quinquefasciatus |
| Rosmarinus officinalis | Lamiaceae | Shoot | Diptera | Anopheles stephensi |
| Rosmarinus officinalis | Lamiaceae | Shoot | Diptera | A. aegypti |
| Rosmarinus officinalis | Lamiaceae | Shoot | Diptera | C. quinquefasciatus |
| Cinnamomum zeylanicum | Lauraceae | Bark | Diptera | Anopheles stephensi |
| Cinnamomum zeylanicum | Lauraceae | Bark | Diptera | A. aegypti |
| Cinnamomum zeylanicum | Lauraceae | Bark | Diptera | C. quinquefasciatus |
| C. citratus | Graminae | N.I. | Diptera | Culex. quinquefasciatus |
| Zingiber officinalis | Zingiberaceae | Rhizomes | Diptera | C. quinquefasciatus |
| Moschosma polystachyum | Lamiaceae | Fresh leaves | Diptera | C. quinquefasciatus |
| Solanum xanthocarpum | Solanaceae | Fresh leaves | Diptera | C. quinquefasciatus |
| Curcuma longa L. | Zingiberaceae | Rhizomes | Diptera | A. dirus |
| C. winterianus | Poaceae | Leaves | Diptera | Cx. quinquefasciatus |
| O. americanum | Lamiaceae | Leaves | Diptera | Cx. quinquefasciatus |
| Z. limonella | Rutaceae | Leaves | Diptera | C. quinquefasciatus |
| Z. limonella | Rutaceae | Leaves | Diptera | A. dirus |
| Pogostemon cablin | Lamiaceae | Commercial | Diptera | A. aegypti |
| Pogostemon cablin | Lamiaceae | Commercial | Diptera | C. quinquefasciatus |
| Pogostemon cablin | Lamiaceae | Commercial | Diptera | A. dirus |
| Syzygium aromaticum | Myrtaceae | Commercial | Diptera | A. aegypti |
| Syzygium aromaticum | Myrtaceae | Commercial | Diptera | C. quinquefasciatus |
| Syzygium aromaticum | Myrtaceae | Commercial | Diptera | A. dirus |
| Z. limonella | Rutaceae | Leaves | Diptera | A. aegypti |
| C. nardus | Poaceae | Leaves | Diptera | A. aegypti |
| E. globulus | Myrtaceae | Commercial product | Diptera | A. albopictus |
| D. caryophyllum | Caryophyllaceae | Flowers | Diptera | A. aegypti |
| D. caryophyllum | Caryophyllaceae | Flowers | Ixodida | A. aegypti |
| Nigella sativa | Ranunculaceae | Dried fruits | Coleoptera | T. castaneum |
| Trachyspermum ammi | Umbelliferae | Dried fruits | Coleoptera | T. castaneum |
| Anethum graveolens | Umbelliferae | Dried fruits | Coleoptera | T. castaneum |
| B. salicifolia | Asteraceae | Aerial parts | Coleoptera | T. castaneum |
| Artemisia annua | Asteraceae | Root | Coleoptera | T. castaneum |
| Perilla frutesncens | Labiatae | Leaves | Coleoptera | L. serricorne |
| Thymus vulagris | Labiatae | Leaves, Flower, and Stems | Coleoptera | L. serricorne |
| Satureia hortensis | Labiatae | Spike | Coleoptera | L. serricorne |
| Mentha piperita | Labiatae | Leaves | Coleoptera | L. serricorne |
| Cinnamomum cassia | Lauraceae | Bark | Coleoptera | L. serricorne |
| Litsea cubeba | Lauraceae | Fruit | Coleoptera | L. serricorne |
| Perilla frutesncens | Labiatae | Leaves | Coleoptera | L. serricorne |
| Laurus nobilis | Lauraceae | Immature fruits | Coleoptera | Acanthoscelides obtectus |
| Rosmarinus officinalis | Labiatae | Flowering shoots | Coleoptera | Acanthoscelides obtectus |
| E. globulus | Myrtaceae | Leaves | Coleoptera | Acanthoscelides obtectus |
| Juniperus oxycedrus | Cupressaceae | Leaves | Coleoptera | Acanthoscelides obtectus |
| Lavandula hybrida | Labiatae | Whole flowering plants | Coleoptera | Acanthoscelides obtectus |
| Mentha microphylla | Labiatae | Whole flowering plants | Coleoptera | Acanthoscelides obtectus |
| Mentha viridis | Labiatae | Whole flowering plants | Coleoptera | Acanthoscelides obtectus |
| Apium graveolens | Umbelliferae | Stems and leaves | Coleoptera | Acanthoscelides obtectus |
| O. basilicum | Lamiaceae | Fresh leaves | Coleoptera | Callosobruchus maculatus |
| Artemisia vulgaris | Asteraceae | Fresh leaves | Coleoptera | T. castaneum |
| Ruta graveolens | Rutaceae | N.I. | Lepidoptera | C. pomonella (larvae) |
| Allium sativum | Alliaceae | N.I. | Lepidoptera | C. pomonella (larvae) |
| Pogostemom cablin | Laminaceae | N.I. | Lepidoptera | C. pomonella (larvae) |
| Tanacetum vulgare | Asteraceae | N.I. | Lepidoptera | C. pomonella (larvae) |
| Mentha pulegium | Labiatae | Dried leaves | Phthiraptera | P. humanus capitis |
| Calocedrus macrolepis | Cupressaceae | Heartwood | Isoptera | Coptotermes formosanus |
| Cryptomeria japonica | Cupressaceae | Sapwood | Isoptera | Coptotermes formosanus |
| Chamaecyparis obtuse | Cupressaceae | Leaves | Isoptera | Coptotermes formosanus |
| Rosmarinus officinalis | Lamiaceae | N.I. | Thysanoptera | Thrips tabaci |
N.I. information not available. Table adapted from Luz Stella Nerio; Repellent activity of essential oils: A review [57].
The components of EOs that have been shown to give them repellent activity are monoterpenoids, sesquiterpenes, and alcohols [11,13,59]. Monoterpene repellent compounds include a-pinene, cineole, eugenol, limonene, terpinolene, citronellol, citronellal, camphor, and thymol [57,60-63]. β-caryophyllene is a sesquiterpene with repellent activity [57,64]. Phytol, phenylethyl alcohol, β-citronellol, cinnamyl alcohol, geraniol, and α-pinene are all alcohols with strong repellent activity [57,65]. These constituents have shown repellent activity against mosquitoes, specifically Aedes aegypti and Anopheles gambiae, as well as ticks including Ixodes ricinus [57]. The combination of EOs from different plants is believed to lead to a synergistic activity, increasing the effectiveness of EOs as insect repellents when compared to individually isolated components [11]. This synergistic phenomenon has been observed when combining monoterpenes with sesquiterpenes [11,66]. Some EO plant combinations lead to a decrease in activity when compared to their individual use. This emphasizes the importance of examining and researching the minor constituents of EOs and their effect on repellency [11].
There are a multitude of plant EOs with repellent properties as seen in Table 2. EOs are highly volatile compounds that exert their activity while in their vapor phase [11,67,68]; meaning their activity typically does not last long requiring frequent reapplication for a short protection time [11]. Scientists are currently developing means to retain the active components on the skin for longer periods of time [11]. Some current advances increasing repellency duration include cream-based formulations, polymer mixtures, microencapsulated extended release, fixative agents like vanillin, nanoparticle fabrication, and polymeric repellent patches [11,15,69,70].
Due to the above-mentioned use, the main concern regarding safety and toxicity of plant EOs is skin irritation. Other negative side effects noted have been asthma, contact dermatitis, headache, increased bleeding, eye-irritation, neurotoxicity, genotoxicity, and immunotoxicity [11]. Citronella use has been banned in Europe and Canada since 2006 due to lack of safety information and the presence of methyl eugenol [11]. Methyl eugenol has shown carcinogenic traits in animal studies with no data available in human studies [11,71]. The US National Toxicology Program did state that methyl eugenol is “reasonably anticipated to be a human carcinogen” [11]. Clove oil also contains methyl eugenol and has yet to be evaluated for carcinogenic properties. It is used not only in insect repellents, but in food, cosmetics, and medicines as well [11].
Plant EOs as insect repellents are of high interest due to their overall improved safety profile when compared to their synthetic counterparts such as DEET. The synergism observed when combining different plant EOs and the experimentation with things such as extended release formulations are extending the repellent activity of plant EOs. More research should be conducted noting plant EOs minor constituents and their contribution to repellency. Research is lacking in the health risks associated with plant EOs as insect repellents. Plant EOs are overall promising alternatives to synthetic compounds demonstrating a need for increased focus in the field of multiomics for their improvement and development.
Anti-Inflammation and Antioxidant Properties
Inflammation is the body’s response to noxious stimuli such as infection or tissue injury; the response depends on biological, chemical, and mechanisms [72-74]. EOs such as chamomile, eucalyptus, rosemary, lavender, millefolia, have been found to mediate the inflammatory response [14]; they have the ability to influence antioxidant activity, signaling cascades, cytokines, regulatory transcription factors, and the expression of pro-inflammatory genes [14]. The three main anti-inflammation properties of EOs include inhibition of arachidonic metabolism, cytokine production, and pro-inflammatory gene expression [14].
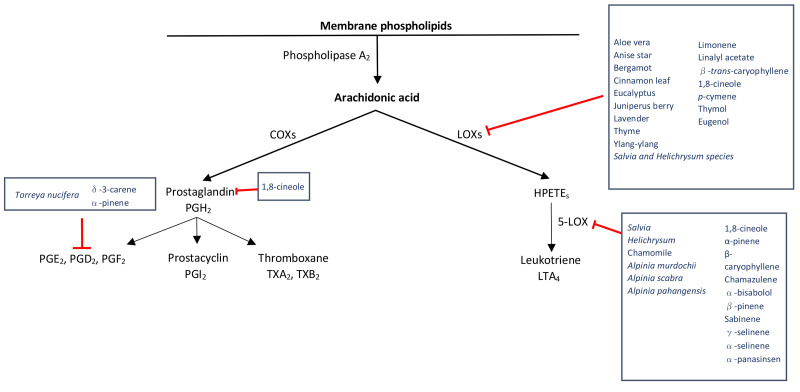
Arachidonic acid is released by the cell membrane via phospholipase A2 as part of the inflammatory response and further metabolized through either the cyclooxygenase (COX) or lipoxygenase (LOX) pathway [14]. The COX pathway produces prostaglandins (PGs) and thromboxane A2 while the LOX pathway produces leukotrienes (LTs) [14]. Inhibiting either pathway leads to a reduction in inflammation via reduction of PGs, thromboxane A2, and LTs, key inflammatory mediators. Aloe vera, anise star, bergamot, cinnamon leaf, eucalyptus, juniperus berry, lavender, thyme, and ylang-ylang, are all EOs containing limonene, linalyl acetate, β-trans-caryophyllene, 1,8-cineole, p-cymene, thymol, and eugenol which inhibit the LOX pathway [14,75]. EOs of the Salvia and Helichrysum species express 1,8-cineole, α-pinene and β-caryophyllene inhibiting 5-lipoxegenase [14,76,77]. Chamomile’s constituents chamazulene and α-bisabolol inhibit 5-lipoxegenase [14,78]. Alpinia murdochii, Alpinia scabra, and Alpinia pahangensis also inhibit 5-lipoxynease via their main components β-pinene, α-pinene, sabinene, γ-selinene, α-selinene, and α-panasinsen [14,79]. A common component of EOs, 1,8-Cineole, inhibits both LTs and PGs affecting both pathways of arachidonic acid metabolism [14,80]. Torreya nucifera contains δ-3-carene and α-pinene, selectively inhibiting the COX-2 pathway and PGE2 production [14,81]. Figure 2 organizes and summarizes EOs and their components effects on arachidonic acid metabolism.
Figure 2.
Schematic representation of the inhibition of arachidonic acid metabolism via EOs and their constituents. COX= cyclooxygenase, LOX= lipoxygenase, HPETE= hydroperoxyeicosatetraenoic acid. Figure adapted from Chapter 7, Pathogenesis and Progression of Multiple Sclerosis: The Role of Arachidonic Acid–Mediated Neuroinflammation [118].
The innate and adaptive immune response generates cytokines; cytokines play a major role in immune and inflammatory processes of the body [82]. Significant pro-inflammatory cytokines include interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6, and IL-8 [14]. Cytokine signaling via lipopolysaccharide (LPS) found on Gram-negative bacterial cell walls, lipoteichoic acid (LTA) found on Gram-positive cell walls, and peptidoglycan leads to inflammation, tissue destruction, and loss of function [14,83,84]. EOs inhibit the (LPS)-induced secretion of IL-1β and TNF-α, including Cheistocalyx operculatus [14,85]. Tea tree oil’s main constituent terpinen-4-ol prevents the production of cytokines TNF-α, IL-1β, IL-8, IL-10, and PGE2 via LPS [14,86]. Tea tree oil also prevents production of pro-inflammatory cytokine IL-2 while increasing the production of anti-inflammatory cytokines IL-4 and IL-10 [14]. Taxandria fragrans components 1,8-cineole, α-pinene, and linalool inhibit TNF-α and IL-6 [14,87]. Components of the EO Cinnamomum osmophloeum 1,8-cineole, santoline, spathulenol and caryophyllene oxide decrease production of IL-1β and IL-6 [14]. Rosmarinus officinalis EOs constituents 1,8-cineole, α-pinene, camphor, and p-cymene inhibit IL-6 production [14,88]. Cinnamomum osmophloeum EO contains cinnamaldehyde which obstructs IL-1β and TNF-α production [14]. Cordia verbenacea EO reduces TNF-α levels via components such as (-)-trans caryophyllene and α-humelene. IL-1β levels reduce TNF-α levels and are also affected by α-humelene [14,89-91]. Cryptomeria japonica oil inhibits IL-1β, IL-6, and TNF-α with components kaurene, elemol, γ-eudesmol, and sabinene [14,92]. Artemisia fukudo and its constituents α-Thujone, β-thujone, camphor, and caryophyllene inhibits TNF-α, IL-1β, and IL-6 [75]. Both eugenol from Syzygium aromaticum and citral from Cymbopogon citratus decrease secretion of IL-1β and IL-6 [14]. Eugenol also prevents secretion of TNF-α and PGE2 [14]. Cinnamomum insularimontanum inhibits TNF-α through the action of citral. Myristicin from nutmeg oil inhibits TNF-α release [14]. Pterodon emarginatus oil contains trans-Caryophyllene, β-elemene, and germacrene reducing IL-1 and TNF-α levels. Thyme containing p-cymene and thymol and oregano containing carvacrol inhibit IL-1β and IL-6 [93]. All of the above mentioned EOs and EO constituents act as antagonists to pro-inflammatory cytokine activity. A summary of those constituents and their activities may be found in Table 3.
Table 3. Representation of essential oils and their constituents that inhibit pro-inflammatory cytokine production.
| Cytokine | Main Sources | Function | Essential Oil Cytokine Inhibition |
| IL-1β | Macrophages, monocytes | Pro-inflammation, proliferation, apoptosis, differentiation | Cheistocalyx operculatus, tea tree oil, terpinen-4-ol, 1,8-cineole, santoline, spathulenol, caryophyllene oxide, Cinnamomum osmophloeum, thyme, cinnamaldehyde, α-humelene, thymol, Cryptomeria japonica, sabinene, citral, kaurene, elemol,γ-eudesmol, eugenol, α-Thujone, β-thujone, camphor, caryophyllene, Artemisia fukudo, Cymbopogon citratus, lemongrass, Syzygium aromaticum, β- elemene, Pterodon emarginatus, germacrene, trans- caryophyllene, p-cymene, oregano, carvacrol |
| IL-6 | Macrophages, T-cells, adipocyte | Pro-inflammation, differentiation, cytokine production | Taxandria fragrans, 1,8-cineole, thyme, α-pinene, linalool, santoline, camphor, spathulenol, caryophyllene oxide, Rosmarinus officinalis, p-cymene, Cryptomeria japonica, sabinene, Citral, kaurene, elemol,γ-eudesmol, eugenol, α-Thujone, β-thujone, caryophyllene, Artemisia fukudo, Cymbopogon citratus, Syzygium aromaticum, thymol, p-cymene, oregano, carvacrol |
| IL-8 | Macrophages, epithelial cells, endothelial cells | Pro-inflammation, chemotaxis, angiogenesis | Tea tree oil, terpinen-4-ol |
| TNF-α | Macrophages, NK cells, CD4+ lymphocytes, adipocyte | Pro-inflammation, cytokine production, apoptosis, anti-infection, cell proliferation | Cheistocalyx operculatus, tea tree oil, terpinen-4-ol, Taxandria fragrans, 1,8-cineole, α-pinene, linalool, Citral, Cinnamomum osmophloeum, eugenol, cinnamaldehyde, Cordia verbenacea, α-humelene, (-)-trans-caryophyllene, Cryptomeria japonica, sabinene, kaurene, elemol,γ-eudesmol, α-Thujone, β-thujone, camphor, caryophyllene, Artemisia fukudo, Cinnamomum insularimontanum, myristicin, nutmeg oil, germacrene, Pterodon emarginatus, β- elemene, trans- caryophyllene |
Table adapted from Linlin Chen: Inflammatory responses and inflammation-associated diseases in organs [117].
Nitric oxide (NO) is a free radical produced either enzymatically or non-enzymatically. Enzymatic production of NO via NO synthase is a redox reaction that breaks down L-arginine to L-citrulline and NO; the reaction requires oxygen and NADPH [94]. Non-enzymatically, NO is produced from nitrite under acidic conditions such as ischemia [94]. NO modulates the inflammatory response by regulating transcription factors such as NF-κB, AP-1, Jak-STAT, bacterial transcription factors, in addition to monitoring the levels of neutrophils, and eosinophils [94]. EOs Teucrium brevifolia and Teucrium montbretia directly inhibit NO production thus inhibiting the inflammatory response; spathulenol, δ-cadinene, carvacrol, 4-vinyl guaiacol, and caryophyllene oxide are their constituents [14]. Fortunella japonica and Citrus sunki, both containing limonene, also inhibit NO production and inflammation [14]. Origanum ehrenbergii oil with thymol and p-cymene exhibits NO inhibition, along with citrus peel and Distichoselinum tenuifolium, composed of myrcene [95]. EOs Cryptomeria japonica, Abies koreana, Farfugium japonicum, Illicium anisatum, Juniperus oxycedrus, Cinnamomum insularimontanum, and Juniperus oxycedrus and constituents 1-undecene, 1-nonene, β-caryophyllene, 1,8-cineole were all found to inhibit NO production [14]. Regulation of NO and inflammation via inhibition of NF-kB transcription has been observed by EOs including Pimpinella, Artemisia fukudo, Cleistocalyx operculatus, Juniperus oxycedrus [14]. Their constituents include α-thujone, β-thujone, camphor, caryophyllene, anethole, eugenol, α-pinene, and isoeugenol [14].
Psychological Effects
Generalized Anxiety Disorder (GAD) is characterized by persistent and excessive worry with associated psychic and somatic symptoms [96]. GAD is a common condition that can lead to significant personal and social impairment [97]. Current treatment modalities for GAD include cognitive behavioral therapy, as well as medical therapy primarily with benzodiazepines or antidepressants [98]. Essential oils represent a potential new treatment category for GAD. Animal models have demonstrated anxiolytic properties in certain essential oils including Lavendula angustifolia, Citrus sinensis, and Citrus aurantium subspecies bergamia [99]. These properties have been demonstrated to be replicable in human clinical trials [100-102]. The method of administration also appears to play a role in the effectiveness of these products, with the three most common administration routes being inhalation, oral, and topical. Anosmia models have been used in experimental animal studies that show the anxiolytic effects of lavender still occurs even if the olfactory receptors have been disabled [103]. Studies are beginning to elucidate the mechanism of action of essential oils. Many essential oils exert their central nervous system pharmacological properties through interactions with serotonin receptors, the GABAergic system, and voltage-gated Na+ channels [104]. Inhalation of bergamot (Citrus bergamia) oil could regulate the blood pressure and heart rate of healthy volunteers [105]. Lemon essence has been studied in palliative care patients and was shown to increase heart rate, diastolic blood pressure, and respiratory rate in both conscious and unconscious patients, while lavender oil was found to have opposing effects [106]. Interestingly, some essential oils have been associated with worsened anxiety symptoms. Specifically, lemon essence was shown to worsen nociceptive and anxiety responses in rats [107].
One challenge to studying the effects of essential oils has been isolating the active compounds. Harvesting essential oils from their natural reservoirs presents a challenge in ensuring standardization of components as chemical composition can vary based on numerous factors including, geographical location and timing of harvest [108]. In a study of Satureja oil, varying chemical compositions were isolated from members of the same genus of plant, which led to significant changes in anxiolytic effects [109]. Another challenge has been the inherent bias present through inhalation methods, as adequate blinding is difficult to achieve due to the recognizable nature of many essential oils. However, oral essential oil products like Silexan have been used in randomized double-blind studies and demonstrated statistically significant anxiolytic activity [110]. Silexan has even been shown to be as effective in reducing anxiety symptoms as paroxetine and lorazepam, with additional improvement in comorbid depression and impaired sleep [111].
Depression is an extremely prevalent mental health disorder characterized by decreased mood, loss of interest, hopelessness, and impaired social function [112]. Traditional antidepressant medication functions through neurotransmitter modulation, but many patients do not experience complete remission of symptoms with monotherapy alone [113]. There have been many studies researching other natural products as alternative antidepressant therapies, specifically St John’s Wart. St John’s Wart is superior to placebo in improving depression symptoms and not significantly different from antidepressant medication [114]. Essential oils represent a potential additional treatment modality for depression [115]. Lavender oil specifically has been shown to ameliorate the depression-like behavior induced by the chronic administration of corticosterone [115].
Concluding Remarks
EOs have a variety of effects on human health. As it has been demonstrated in many studies, these oils have many psychological effects such as reducing anxiety, treating depression, and even aid with falling asleep. Additionally, they have also been shown to possess antimicrobial, antiviral, antioxidant, anti-inflammatory properties and used as an alternative to synthetic insect repellents. As there are many proven health benefits to essential oils, there are also adverse effects. It has been shown that certain essential oils and their components contain EDCs which appear to have enhanced breast growth in prepubertal children. Taken together, there has been a great amount of research performed in the essential oil field but considering their multitude of components and the spectrum of possible activities there is still a vast amount unknown about their true effects on human health.
Glossary
- AR
androgen receptor
- COX
cyclooxygenase
- DEET
N,N-diethyl-3-methylbenzamide, formerly N,N-diethyl-m-toluamide
- EDC
endocrine disrupting chemical
- EO
essential oil
- ERα
estrogen receptor alpha
- GAD
generalized anxiety disorder
- HSV
herpes simplex virus
- IL
interleukin
- KO
knock out
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- LT
leukotrienes
- LTA
lipoteichoic acid
- MBC
minimum bactericidal concentration
- MIC
minimum inhibitory concentration
- NO
nitric oxide
- PG
prostaglandins
- TNF
tumor necrosis factor.
Funding
Our research support was provided by the Division of Intramural Research of NIEHS to KSK through 1ZIAES070065.
Author Contributions
JTR, BCS, TRN, and KDC wrote the original manuscript draft. JTR, BCS, TRN, KDC, YL, and KSK edited the manuscript.
References
- Essential Oils Market Size , Share & Trends Analysis Report By Application (Cleaning & Home, Medical, Food & Beverages, Spa & Relaxation), By Product, By Sales Channel, And Segment Forecasts, 2019 - 2025. Grand View Research; 2019.
- de Groot AC, Schmidt E. Essential Oils, Part I: Introduction. Dermatitis. 2016;27(2):39-42. doi: 10.1097/DER.0000000000000175. [DOI] [PubMed] [Google Scholar]
- Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy. Syst Rev. 2015:601–11. [Google Scholar]
- Svoboda KP, Deans SG. Biological Activities of Essential Oils from Selected Aromatic Plants. 1995: International Society for Horticultural Science (ISHS), Leuven, Belgium; 10.17660/ActaHortic.1995.390.28 [DOI] [Google Scholar]
- Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K. Effect of aromatherapy on patients with Alzheimer's disease. Psychogeriatrics. 2009;9(4):173-9. doi: 10.1111/j.1479-8301.2009.00299.x [doi]. [DOI] [PubMed] [Google Scholar]
- Lai TK, Cheung MC, Lo CK, Ng KL, Fung YH, Tong M, et al. Effectiveness of aroma massage on advanced cancer patients with constipation: a pilot study [doi]. Complement Ther Clin Pract. 2011. February;17(1):37–43. 10.1016/j.ctcp.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Perry N, Perry E. Aromatherapy in the management of psychiatric disorders: clinical and neuropharmacological perspectives. CNS drugs. 2006;20(4):257-80. doi: 2041 [pii]. 10.2165/00023210-200620040-00001 [DOI] [PubMed] [Google Scholar]
- Shiina Y, Funabashi N, Lee K, Toyoda T, Sekine T, Honjo S, et al. Relaxation effects of lavender aromatherapy improve coronary flow velocity reserve in healthy men evaluated by transthoracic Doppler echocardiography. Int J Cardiol. 2008;129(2):193-7. doi: S0167-5273(07)01261-2 [pii]. 10.1016/j.ijcard.2007.06.064 [DOI] [PubMed] [Google Scholar]
- Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database Systematic Rev. 2011;(7):CD009215. doi(7):CD009215. doi: 10.1002/14651858.CD009215 [doi]. [DOI] [PubMed] [Google Scholar]
- Hwang E, Shin S. The effects of aromatherapy on sleep improvement: a systematic literature review and meta-analysis [doi]. J Altern Complement Med. 2015. February;21(2):61–8. 10.1089/acm.2014.0113 [DOI] [PubMed] [Google Scholar]
- Lee MY. Essential Oils as Repellents against Arthropods. BioMed Res Int. 2018. October;2018:6860271–9. 10.1155/2018/6860271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand L. Integrative and complementary therapies for patients with advanced cancer [doi]. Ann Palliat Med. 2014. July;3(3):160–71. 10.3978/j.issn.2224-5820.2014.07.01 [DOI] [PubMed] [Google Scholar]
- Sritabutra D, Soonwera M. Repellent activity of herbal essential oils against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.). Asian Pac J Trop Dis. 2013. August;3(4):271–6. [Google Scholar]
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010. December;15(12):9252–87. 10.3390/molecules15129252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songkro S, Jenboonlap M, Boonprasertpon M, Maneenuan D, Bouking K, Kaewnopparat N. Effects of glucam P-20, vanillin, and fixolide on mosquito repellency of citronella oil lotions. J Med Entomol. 2012. May;49(3):672–7. 10.1603/ME11141 [DOI] [PubMed] [Google Scholar]
- Diaz A, Luque L, Badar Z, Kornic S, Danon M. Prepubertal gynecomastia and chronic lavender exposure: report of three cases [doi]. J Pediatr Endocrinol Metab. 2016. January;29(1):103–7. 10.1515/jpem-2015-0248 [DOI] [PubMed] [Google Scholar]
- Henley DV, Lipson N, Korach KS, Bloch CA. Prepubertal gynecomastia linked to lavender and tea tree oils. New Engl J Med. 2007;356(5):479-85. doi: 356/5/479 [pii]. 10.1056/NEJMoa064725 [DOI] [PubMed] [Google Scholar]
- Ramsey JT, Li Y, Arao Y, Naidu A, Coons LA, Diaz A, et al. Lavender Products Associated With Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities [doi]. J Clin Endocrinol Metab. 2019. November;104(11):5393–405. 10.1210/jc.2018-01880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand R, Young R. Essential Oil Safety - E-Book: A Guide for Health Care Professionals: Elsevier Health Sciences; 2013. [Google Scholar]
- de Groot AC, Schmidt E. Essential Oils, Part III: Chemical Composition. Dermatitis. 2016;27(4):161-9. doi: 10.1097/DER.0000000000000193 [doi]. [DOI] [PubMed] [Google Scholar]
- Lawless J. The Encyclopedia of Essential Oils: The Complete Guide to the Use of Aromatic Oils In Aromatherapy, Herbalism, Health, and Well Being: Red Wheel Weiser; 2013. [Google Scholar]
- Rhind JP, Pirie D. Essential Oils: A Handbook for Aromatherapy Practice. Jessica Kingsley Publishers; 2012. [Google Scholar]
- de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition [doi]. Contact Dermat. 2016. September;75(3):129–43. 10.1111/cod.12591 [DOI] [PubMed] [Google Scholar]
- Schiller C, Schiller D. 500 Formulas for Aromatherapy: Mixing Essential Oils for Every use. New York: Sterling Pub.; 1994. 128 pp. [Google Scholar]
- Wildwood C. The Encyclopedia of Aromatherapy: Inner Traditions/Bear; 1996. [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop [doi]. Environ Health Perspect. 1996. August;104 Suppl 4:715–40. 10.1289/ehp.96104s4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society [doi]. Endocrinology. 2012. September;153(9):4097–110. 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement [doi]. Endocr Rev. 2009. June;30(4):293–342. 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein GD. Gynecomastia [doi]. N Engl J Med. 1993. February;328(7):490–5. 10.1056/NEJM199302183280708 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease [doi]. J Clin Invest. 2006. March;116(3):561–70. 10.1172/JCI27987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanos T, Rojo L, Echeverria P, Brisken C. ER and PR signaling nodes during mammary gland development. Breast Cancer Res. 2012;14(4):210. doi: 10.1186/bcr3166 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt LM, Kuperwasser C. Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy [doi]. J Mammary Gland Biol Neoplasia. 2015. June;20(1-2):9–25. 10.1007/s10911-015-9337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice [doi]. Endocrinology. 2000. August;141(8):2982–94. 10.1210/endo.141.8.7609 [DOI] [PubMed] [Google Scholar]
- Chauchereau A, Savouret JF, Milgrom E. Control of biosynthesis and post-transcriptional modification of the progesterone receptor [doi]. Biol Reprod. 1992. February;46(2):174–7. 10.1095/biolreprod46.2.174 [DOI] [PubMed] [Google Scholar]
- Tekmal RR, Liu YG, Nair HB, Jones J, Perla RP, Lubahn DB, et al. Estrogen receptor alpha is required for mammary development and the induction of mammary hyperplasia and epigenetic alterations in the aromatase transgenic mice. J Steroid Biochem Mol Biol. 2005;95(1-5):9-15. doi: S0960-0760(05)00174-3 [pii]. 10.1016/j.jsbmb.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Deyno S, Mtewa AG, Abebe A, Hymete A, Makonnen E, Bazira J, et al. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complementary Ther Med. 2019;47:102224. doi: S0965-2299(19)31268-3 [pii]. 10.1016/j.ctim.2019.102224 [DOI] [PubMed] [Google Scholar]
- Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Diaz J, Gil A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients. 2019;11(11): 10.3390/nu11112786. doi: E2786 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review [doi]. Int J Food Microbiol. 2004. August;94(3):223–53. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid Based Complement Alternat Med. 2016;2016:3012462. doi: 10.1155/2016/3012462 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussalah M, Caillet S, Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes [doi]. J Food Prot. 2006. May;69(5):1046–55. 10.4315/0362-028x-69.5.1046 [DOI] [PubMed] [Google Scholar]
- Arora DS, Kaur J. Antimicrobial activity of spices. Int J Antimicrob Agents. 1999;12(3):257-62. doi: S0924-8579(99)00074-6 [pii]. 10.1016/S0924-8579(99)00074-6 [DOI] [PubMed] [Google Scholar]
- Elgayyar M, Draughon FA, Golden DA, Mount JR. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms [doi]. J Food Prot. 2001. July;64(7):1019–24. 10.4315/0362-028x-64.7.1019 [DOI] [PubMed] [Google Scholar]
- Ramos-Nino ME, Clifford MN, Adams MR. Quantitative structure activity relationship for the effect of benzoic acids, cinnamic acids and benzaldehydes on Listeria monocytogenes [doi]. J Appl Bacteriol. 1996. March;80(3):303–10. 10.1111/j.1365-2672.1996.tb03224.x [DOI] [PubMed] [Google Scholar]
- Sakagami Y, Kaikoh S, Kajimura K, Yokoyama H. Inhibitory Effect of Clove Extract on Vero-Toxin Production by Enterohemorrhagic Escherichia coil O 157: H7. Biocontrol Sci. 2000;5(1):47–9. 10.4265/bio.5.47 [DOI] [Google Scholar]
- Akhtar MS. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues in Biological Sciences and Pharmaceutical Research. 2014;2:1–7. [Google Scholar]
- Arnal-Schnebelen B, Hadji-Minaglou F, Peroteau JF, Ribeyre F, de Billerbeck VG. Essential oils in infectious gynaecological disease: a statistical study of 658 cases. 2004. pp. 192–7. [Google Scholar]
- Yoon HS, Moon SC, Kim ND, Park BS, Jeong MH, Yoo YH. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP [doi]. Biochem Biophys Res Commun. 2000. September;276(1):151–6. 10.1006/bbrc.2000.3445 [DOI] [PubMed] [Google Scholar]
- Kivanc M, Akgul A, Dogan A. Inhibitory and stimulatory effects of cumin, oregano and their essential oils on growth and acid production of Lactobacillus plantarum and Leuconostoc mesenteroides. Int Journal Food Microbiol. 1991;13(1):81-5. doi: 0168-1605(91)90140-K [pii]. 10.1016/0168-1605(91)90140-K [DOI] [PubMed] [Google Scholar]
- Juglal S, Govinden R, Odhav B. Spice oils for the control of co-occurring mycotoxin-producing fungi [doi]. J Food Prot. 2002. April;65(4):683–7. 10.4315/0362-028x-65.4.683 [DOI] [PubMed] [Google Scholar]
- Armaka M, Papanikolaou E, Sivropoulou A, Arsenakis M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antiviral Research. 1999;43(2):79-92. doi: S0166-3542(99)00036-4 [pii]. [DOI] [PubMed] [Google Scholar]
- Benencia F, Courrèges MC. In vitro and in vivo activity of eugenol on human herpesvirus [pii]. Phytother Res. 2000. November;14(7):495–500. 10.1002/1099-1573(200011)14:7;2-8 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hayashi T, Ujita K, Takaishi Y. Characterization of antiviral activity of a sesquiterpene, triptofordin C-2 [doi]. J Antimicrob Chemother. 1996. April;37(4):759–68. 10.1093/jac/37.4.759 [DOI] [PubMed] [Google Scholar]
- Pusztai R, Hohmann J, Rédei D, Engi H, Molnár J. Inhibition of human cytomegalovirus IE gene expression by dihydro-beta-agarofuran sesquiterpenes isolated from Euonymus species. In Vivo. 2008. Nov-Dec;22(6):787–92. [PubMed] [Google Scholar]
- Diaz JH, Tm DF. Chemical and Plant-Based Insect Repellents: Efficacy, Safety, and Toxicity. Wilderness Environ Med. 2016. March;27(1):153–63. 10.1016/j.wem.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Debboun M, Strickman D. Insect repellents and associated personal protection for a reduction in human disease. Med Vet Entomol. 2013. March;27(1):1–9. 10.1111/j.1365-2915.2012.01020.x [DOI] [PubMed] [Google Scholar]
- Bulbuli K. Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and Docking studies. J Appl Pharm Sci. 2013: 10.7324/JAPS.2013.30202 [DOI] [Google Scholar]
- Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010. January;101(1):372–8. 10.1016/j.biortech.2009.07.048 [DOI] [PubMed] [Google Scholar]
- Tong F, Bloomquist JR. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: culicidae). J Med Entomol. 2013. July;50(4):826–32. 10.1603/ME13002 [DOI] [PubMed] [Google Scholar]
- Sathantriphop S, Achee NL, Sanguanpong U, Chareonviriyaphap T. The effects of plant essential oils on escape response and mortality rate of Aedes aegypti and Anopheles minimus. J Vector Ecol. 2015. December;40(2):318–26. 10.1111/jvec.12170 [DOI] [PubMed] [Google Scholar]
- Amer A, Mehlhorn H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res. 2006. September;99(4):478–90. 10.1007/s00436-006-0184-1 [DOI] [PubMed] [Google Scholar]
- Jaenson TG, Pålsson K, Borg-Karlson AK. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J Med Entomol. 2006. January;43(1):113–9. 10.1093/jmedent/43.1.113 [DOI] [PubMed] [Google Scholar]
- Park BS, Choi WS, Kim JH, Kim KH, Lee SE. Monoterpenes from thyme (Thymus vulgaris) as potential mosquito repellents. J Am Mosq Control Assoc. 2005. March;21(1):80–3. 10.2987/8756-971X(2005)21[80:MFTTVA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang YC, Lee EH, Lee HS, Lee DK, Ahn YJ. Repellency of aromatic medicinal plant extracts and a steam distillate to Aedes aegypti. J Am Mosq Control Assoc. 2004. June;20(2):146–9. [PubMed] [Google Scholar]
- Gillij YG, Gleiser RM, Zygadlo JA. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol. 2008. May;99(7):2507–15. 10.1016/j.biortech.2007.04.066 [DOI] [PubMed] [Google Scholar]
- Tunón H, Thorsell W, Mikiver A, Malander I. Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia. 2006. June;77(4):257–61. 10.1016/j.fitote.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010. November;17(13):1061–6. 10.1016/j.phymed.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Gnankiné O, Bassolé IH. Essential Oils as an Alternative to Pyrethroids’ Resistance against Anopheles Species Complex Giles (Diptera: culicidae). Molecules. 2017. September;22(10):1321. 10.3390/molecules22101321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam J, Zaman K, Duarah S, Raju PS, Chattopadhyay P. Mosquito repellents: an insight into the chronological perspectives and novel discoveries. Acta Trop. 2017. March;167:216–30. 10.1016/j.actatropica.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Benelli G, Caselli A, Canale A. Nanoparticles for mosquito control: Challenges and constraints. Journal of King Saud University - Science. 2017;29(4):424-35. doi: 10.1016/j.jksus.2016.08.006. [DOI] [Google Scholar]
- Misni N, Nor ZM, Ahmad R. Repellent effect of microencapsulated essential oil in lotion formulation against mosquito bites. J Vector Borne Dis. 2017. Jan-Mar;54(1):44–53. [PubMed] [Google Scholar]
- Tan KH, Nishida R. Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci. 2012;12(56):56. 10.1673/031.012.5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lavor ÉM, Fernandes AW, de Andrade Teles RB, Leal AE, de Oliveira Júnior RG, Gama E Silva M, et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxid Med Cell Longev. 2018. December;2018:6468593–23. 10.1155/2018/6468593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013. March;13(3):159–75. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008. July;454(7203):428–35. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Moon JY, Kang JY, Kim GO, Lee NH, Hyun CG. Neolitsea sericea essential oil attenuates LPS-induced inflammation in RAW 264.7 macrophages by suppressing NF-kappaB and MAPK activation. Nat Prod Commun. 2010. August;5(8):1311–6. 10.1177/1934578X1000500835 [DOI] [PubMed] [Google Scholar]
- Kamatou GP, van Zyl RL, van Vuuren SF, Viljoen AM, Figueiredo AC, Barroso JG, et al. Chemical Composition, Leaf Trichome Types and Biological Activities of the Essential Oils of Four Related Salvia Species Indigenous to Southern Africa. J Essent Oil Res. 2006;18 sup1:72–9. 10.1080/10412905.2006.12067125 [DOI] [Google Scholar]
- Lourens AC, Reddy D, Başer KH, Viljoen AM, Van Vuuren SF. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol. 2004. December;95(2-3):253–8. 10.1016/j.jep.2004.07.027 [DOI] [PubMed] [Google Scholar]
- Kamatou G, Kamatou G, Viljoen A, Viljoen A. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J Am Oil Chem Soc. 2010;87(1):1–7. 10.1007/s11746-009-1483-321350591 [DOI] [Google Scholar]
- Syamsur DR. Essential oils and biological activities of three selected wild Alpinia species. University of Malaya; 2009. [Google Scholar]
- Yoon YJ. Torreya nucifera Essential Oil Inhibits Skin Pathogen Growth and Lipopolysaccharide-Induced Inflammatory Effects. Int J Pharmacol. 2009;5(1):37–43. 10.3923/ijp.2009.37.43 [DOI] [Google Scholar]
- Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003. March;97(3):250–6. 10.1053/rmed.2003.1432 [DOI] [PubMed] [Google Scholar]
- Ge Y, Huang M, Yao YM. Autophagy and proinflammatory cytokines: interactions and clinical implications. Cytokine Growth Factor Rev. 2018. October;43:38–46. 10.1016/j.cytogfr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71(1):635–700. 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder NW, Morath S, Alexander C, Hamann L, Hartung T, Zähringer U, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003. May;278(18):15587–94. 10.1074/jbc.M212829200 [DOI] [PubMed] [Google Scholar]
- Dung NT, Bajpai VK, Yoon JI, Kang SC. Anti-inflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food Chem Toxicol. 2009. February;47(2):449–53. 10.1016/j.fct.2008.11.033 [DOI] [PubMed] [Google Scholar]
- Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000. November;49(11):619–26. 10.1007/s000110050639 [DOI] [PubMed] [Google Scholar]
- Hammer KA, Carson CF, Dunstan JA, Hale J, Lehmann H, Robinson CJ, et al. Antimicrobial and anti-inflammatory activity of five Taxandria fragrans oils in vitro. Microbiol Immunol. 2008. November;52(11):522–30. 10.1111/j.1348-0421.2008.00070.x [DOI] [PubMed] [Google Scholar]
- Juhás Š, Bukovská A, Čikoš Š, Czikková S, Fabian D, Koppel J. Anti-Inflammatory Effects of Rosmarinus officinalis Essential Oil in Mice. Acta Vet Brno. 2009;78(1):121–7. 10.2754/avb200978010121 [DOI] [Google Scholar]
- Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, et al. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol. 2007. August;569(3):228–36. 10.1016/j.ejphar.2007.04.059 [DOI] [PubMed] [Google Scholar]
- Medeiros R, Passos GF, Vitor CE, Koepp J, Mazzuco TL, Pianowski LF, et al. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br J Pharmacol. 2007. July;151(5):618–27. 10.1038/sj.bjp.0707270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos GF, Fernandes ES, da Cunha FM, Ferreira J, Pianowski LF, Campos MM, et al. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J Ethnopharmacol. 2007. March;110(2):323–33. 10.1016/j.jep.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Kim SS, Oh TH, Lee NH, Hyun CG. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Pol J Microbiol. 2009;58(1):61–8. [PubMed] [Google Scholar]
- Bukovská A, Cikos S, Juhás S, Il’ková G, Rehák P, Koppel J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediators Inflamm. 2007;2007:23296–9. 10.1155/2007/23296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005. August;4(4):471–9. 10.2174/1568010054526359 [DOI] [PubMed] [Google Scholar]
- Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules. 2017. June;22(6):989. 10.3390/molecules22060989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins JT. Generalized anxiety disorder. Epidemiology, impact of comorbidity, and natural history [doi]. Postgrad Med. 1999. November;106(6 Suppl):3–9. 10.3810/pgm.11.1999.suppl1.1 [DOI] [PubMed] [Google Scholar]
- Altunoz U, Kokurcan A, Kirici S, Bastug G, Ozel-Kizil ET. Clinical characteristics of generalized anxiety disorder: older vs. young adults [doi]. Nord J Psychiatry. 2018. February;72(2):97–102. 10.1080/08039488.2017.1390607 [DOI] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017. June;19(2):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa DP, de Almeida Soares Hocayen P, Andrade LN, Andreatini R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models [doi]. Molecules. 2015. October;20(10):18620–60. 10.3390/molecules201018620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag E, Samancioglu S, Ozden D, Bakir E. Effects of aromatherapy on sleep quality and anxiety of patients [doi]. Nurs Crit Care. 2017. March;22(2):105–12. 10.1111/nicc.12198 [DOI] [PubMed] [Google Scholar]
- Pimenta FC, Alves MF, Pimenta MB, Melo SA, de Almeida AA, Leite JR, et al. Anxiolytic Effect of Citrus aurantium L. on Patients with Chronic Myeloid Leukemia [doi]. Phytother Res. 2016. April;30(4):613–7. 10.1002/ptr.5566 [DOI] [PubMed] [Google Scholar]
- Shirzadegan R, Gholami M, Hasanvand S, Birjandi M, Beiranvand A. Effects of geranium aroma on anxiety among patients with acute myocardial infarction: A triple-blind randomized clinical trial. Complement Ther Clinical Pract. 2017;29:201-6. doi: S1744-3881(17)30430-9 [pii]. 10.1016/j.ctcp.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Chioca LR, Antunes VD, Ferro MM, Losso EM, Andreatini R. Anosmia does not impair the anxiolytic-like effect of lavender essential oil inhalation in mice [doi]. Life Sci. 2013. May;92(20-21):971–5. 10.1016/j.lfs.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Heinbockel T. Essential Oils and Their Constituents Targeting the GABAergic System and Sodium Channels as Treatment of Neurological Diseases. Molecules. 2018;23(5): 10.3390/molecules23051061. doi: E1061 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Kuchta K, Kimura M, Rauwald HW, Kamei T, Imanishi J. Effects of bergamot (Citrus bergamia (Risso) Wright & Arn.) essential oil aromatherapy on mood states, parasympathetic nervous system activity, and salivary cortisol levels in 41 healthy females. Forschende Komplementarmedizin (2006). 2015;22(1):43-9. doi: 10.1159/000380989 [doi]. [DOI] [PubMed] [Google Scholar]
- Goepfert M, Liebl P, Herth N, Ciarlo G, Buentzel J, Huebner J. Aroma oil therapy in palliative care: a pilot study with physiological parameters in conscious as well as unconscious patients [doi]. J Cancer Res Clin Oncol. 2017. October;143(10):2123–9. 10.1007/s00432-017-2460-0 [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Lariviere WR, Fiorenzani P, Sacerdote P, Aloisi AM. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats [doi]. Brain Res. 2004. March;1001(1-2):78–86. 10.1016/j.brainres.2003.10.063 [DOI] [PubMed] [Google Scholar]
- Zhang N, Yao L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review [doi]. J Agric Food Chem. 2019. December;67(50):13790–808. 10.1021/acs.jafc.9b00433 [DOI] [PubMed] [Google Scholar]
- Soto-Vásquez MR, Alvarado-García PA. Aromatherapy with two essential oils from Satureja genre and mindfulness meditation to reduce anxiety in humans [doi]. J Tradit Complement Med. 2016. June;7(1):121–5. 10.1016/j.jtcme.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Müller WE, Volz HP, Möller HJ, Dienel A, et al. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: a randomized, double-blind, placebo controlled trial [doi]. Int Clin Psychopharmacol. 2010. September;25(5):277–87. 10.1097/YIC.0b013e32833b3242 [DOI] [PubMed] [Google Scholar]
- Kasper S, Muller WE, Volz HP, Moller HJ, Koch E, Dienel A. Silexan in anxiety disorders: Clinical data and pharmacological background. World J Biol Psychiatry. 2018;19(6):412-20. doi: 10.1080/15622975.2017.1331046 [doi]. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-66. doi: S0140-6736(17)32802-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137-51. doi: nrn1846 [pii]. 10.1038/nrn1846 [DOI] [PubMed] [Google Scholar]
- Maher AR, Hempel S, Apaydin E, Shanman RM, Booth M, Miles JN, et al. St. John’s Wort for Major Depressive Disorder: A Systematic Review. Rand Health Q. 2016. May;5(4):12. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vidana D, Po KK, Fung TK, Chow JK, Lau WK, So PK, et al. Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats. Neuroscience Lett. 2019;701:180-92. doi: S0304-3940(19)30142-9 [pii]. 10.1016/j.neulet.2019.02.042 [DOI] [PubMed] [Google Scholar]
- Kozics K, Buckova M, Puskarova A, Kalaszova V, Cabicarova T, Pangallo D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules. 2019;24(24): 10.3390/molecules24244570. doi: E4570 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017. December;9(6):7204–18. 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo S. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane: Codon Publications; 2017. Chapter 7 p. [PubMed] [Google Scholar]