Abstract
Objective: Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time. Methods: We observed 1,819 people who completed 5,876 cannabis self-administration sessions using the ReleafApp™ between 06/07/2016 and 07/08/2019, with the goal of measuring real-time effects of consuming Cannabis flower for treating symptoms of depression. Results: On average, 95.8% of users experienced symptom relief following consumption with an average symptom intensity reduction of –3.76 points on a 0-10 visual analogue scale (SD = 2.64, d = 1.71, p <.001). Symptom relief did not differ by labeled plant phenotypes (“C. indica,” “C. sativa,” or “hybrid”) or combustion method. Across cannabinoid levels, tetrahydrocannabinol (THC) levels were the strongest independent predictors of symptom relief, while cannabidiol (CBD) levels, instead, were generally unrelated to real-time changes in symptom intensity levels. Cannabis use was associated with some negative side effects that correspond to increased depression (e.g. feeling unmotivated) in up to 20% of users, as well as positive side effects that correspond to decreased depression (e.g. feeling happy, optimistic, peaceful, or relaxed) in up to 64% of users. Conclusions: The findings suggest that, at least in the short term, the vast majority of patients that use cannabis experience antidepressant effects, although the magnitude of the effect and extent of side effect experiences vary with chemotypic properties of the plant.
Keywords: depression, Cannabis, marijuana, antidepressants, cannabidiol, tetrahydrocannabinol, stress, C. sativa
Significance
Few studies have measured how the breadth of common and commercially available Cannabis flower affects symptoms of depression in real-time. Using a mobile software app for collecting the largest database of individual-level cannabis administration sessions in the United States, we found that Cannabis flower is an effective and fast acting antidepressant medication. Tetrahydrocannabinol (THC) levels are the strongest independent predictors of symptom relief among the product characteristics that are generally available to the public, while cannabidiol (CBD) levels, instead, were generally unrelated to real-time changes in depressive symptom intensity levels. Future research should focus on how THC and CBD interact with other cannabinoids, terpenes, and flavonoids synergistically to alleviate symptoms of depression in vivo.
Among the most pressing epidemics faced by Western societies and future generations worldwide [1-3], depression is also a core feature of numerous other health conditions, including cancer, substance use disorders, anxiety disorders, schizophrenia, dementia, diabetes, cardiovascular disease, chronic pain, and other physical disabilities [4-9]. Firstline treatments for depression remain cognitive behavioral therapy and pharmaceuticals with the most commonly prescribed pharmaceutical medications being sedatives (e.g. benzodiazepines), antidepressants (e.g. monoamine oxidase inhibitors, tricyclics antidepressants, selective serotonin-reuptake inhibitors, selective norepinephrine reuptake inhibitors), antihistamines, and anticonvulsant medicines, with many adolescent and adult suffers also seeking relief through alcohol and illicit drugs [10-12]. However, depression is not a specifically approved condition under any state-regulated medical cannabis program, except as treatment for depression among pediatric patients with terminal illness in Delaware (http://pdaps.org/datasets/medical-marijuana-patient-related-laws-1501600783).
The extant literature has shown mixed finding on the association between cannabis use and symptoms of depression, with unclear conclusions as to the direction of causality [13-16]. For example, while a consistent relationship exists between adolescent cannabis use and greater risk for depression and anxiety in adulthood [17-20], several studies among healthy people and people with psychological diagnoses have shown that current cannabis users are no more likely than non-users to be suicidal [21-23]. Legal access to cannabis is associated with a reduction in suicide rates at the population level [24,25] and a substantial proportion of medical cannabis patients report using cannabis to self-medicate for depression [26,27]. However, several clinical studies using synthetic analogues (e.g. tetrahydrocannabinol (THC) agonists) have failed to find antidepressant effects [28-34]. Unfortunately, there remains very little research on the actual effects of common and commercially available cannabis products such as whole, dried natural flower, on mood and behavioral motivations more generally. As a result of federal regulatory barriers to conducting clinical research on potential medicinal applications of the plant in the United States, the extant research has been mostly limited to measurements of synthetic analogue therapies or cannabis-derived formulates neither widely used nor generalizable to the extensive range of cannabis-based products used by millions of people every day [35,36].
This is the largest study to measure how different types of Cannabis flower, the most prevalent type of product used in the US, affect depression-related symptoms in actual time, as consumed in the patients’ natural environments. We operationalize this research question using the mobile software application, ReleafApp™ (Releaf App, 2019), which was designed to help patients navigate the variable nature of cannabis-based products available to the general public. The app records cannabis usage at the session-level, including product types, routes of administration, labeled phenotypes, and major cannabinoid contents, along with a wide array of symptoms, real-time changes in symptom severity levels, and experienced side effects of the cannabis used, allowing us to analyze at the cannabis-session level, how these Cannabis flower product characteristics affected patient depression severity and associated side effects. A recent study using a similar app among a smaller sample of mostly Canadian respondents showed that cannabis users report dramatic (around 50%) reductions in depression symptom intensity [38]. However, the app relied on retrospective (rather than prospective) reporting and used a respondent incentive system that rewards users for repeated engagement with the app, which could lead to confounding motivations for using the app and potentially weaken the validity of the study’s findings. The current study, instead, measures how Cannabis flower affects depressive symptom intensity levels in real time among respondents with no extrinsic motivations for using the tool other than to obtain feedback on the Cannabis they are using therapeutically. Based on widespread anecdotal patient reporting patterns, we hypothesized that THC potency levels will be positively correlated with elevations in users’ mood.
Methods
Study Design
The study design was approved by the Institutional Review Board at the University of New Mexico. Under an investigator confidentiality agreement, de-identified user-level data were provided by the owner of the Releaf App, MoreBetter, Ltd. The Releaf App is a mobile software application designed to help users manage cannabis consumption by facilitating recording of real-time changes in symptom intensity and side effects. In each user-administered session, the patient first specified the symptoms to be treated and recorded a battery of potentially available (e.g. labeled) product characteristics. Specifically, users record product type (flower, concentrate, pill, tincture, topical, and edible), species (C. sativa, C. indica, or hybrid), combustion method (joint, pipe, and vape), and THC and CBD content. While some of these product characteristics, such as the distinction between “C. sativa” and “C. indica” plant strains are largely believed to be baseless by the modern scientific community [39], we included them because they still tend to play a major role in patient purchasing decisions. Once the product characteristics are entered the patient is prompted to specify a starting symptom intensity level (on a 0-10 on a visual analogue scale), record any cannabis consumption, update the symptom intensity level, select from a range of side effects (referred to as “feelings” in the user interface), and end the session. The patient could update the symptom intensity level at any time throughout the session. The Releaf App included 47 side effects and a patient can select multiple side effects in the same session. We restricted the study sample to treatment sessions with a depression symptom reported, starting symptom intensity levels greater than 0, and ending symptom intensity levels reported within 4 hours, the estimated range of time that inhaled cannabinoids produce maximum psychotropic effects and are concentrated in the plasma [40-42]. The analyzed sample only included sessions that reported using dried flower product, which is the most common and homogeneous product type. The resulting analysis sample included 5,876 sessions completed by 1,819 users between 06/06/2016 and 07/08/2019.
Study Outcomes
The objective of the study is to estimate changes in symptom intensity (symptom relief) and the prevalence of side effects following Cannabis use. We also examined whether these effects varied by product characteristics. The main outcome of interest, symptom relief, is measured as the difference between ending symptom intensity level and the starting symptom intensity level. The resulting variable ranged between -10 (maximum symptom relief) and 9 (maximum symptom exacerbation). In other words, the maximum symptom relief would be experienced by a user with a starting symptom level of 10 and an ending symptom level of 0, while the maximum symptom exacerbation would come from a user with a starting symptom level of 1 and an ending symptom level of 10. On average, 95.8% of users reported symptom relief, 2.1% reported symptom worsening, and 2.1% reported no change in symptom intensity level. We categorized the 47 possible side effects as 17 negative side effects, 19 positive side effects, and 11 context-specific side effects. Context-specific side effects are not unambiguously positive or negative. For example, users can record feeling “high,” however, it is unclear whether the user viewed being “high” as a positive or negative side effect. Similarly, increased appetite could be a positive or negative outcome depending on the user’s baseline health state (see Supplemental Table S1 in Appendix A). We measured the onset of these side effects using dummy variables as well as continuous variables. For each category of side effects, the dummy variable indicated whether the user reported any of the side effects in the category while the continuous variable measures the proportion of total side effects in that category that the user selected. As shown in Supplemental Table S1, the most commonly reported negative side effects were Dry Mouth (reported in 33% of the user sessions) and feeling Foggy (27%), the most frequent positive side effects were feeling Relaxed (64%) and Peaceful (57%), and the most common context-specific side effects were feeling High (53%) and Tingly (31%).
Statistical Analysis
The change in symptom intensity level from cannabis use was measured using means comparison and fixed effects regression models, controlling for starting symptom level. To estimate the effects of product characteristics on symptom relief and the prevalence of side effects, we used fixed effects models to regress the outcome of interest on different product characteristics recorded by the user. The fixed effects model was estimated by comparing the outcomes of the same user in different sessions, rather than comparing across users. Therefore, fixed effects models are free from the concern that users differ in unobserved time-invariant characteristics that may affect symptom relief, e.g. sex or pre-app cannabis use experience. Starting symptom level was included in all regressions because higher starting symptom intensity levels suggested larger possible symptom relief. Standard errors were clustered at the user level to account for heteroscedasticity and user-level arbitrary correlation. Analyses were conducted using Stata 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and alpha values less than p = .05 were considered significant.
We conducted several robustness checks including continuous rather than categorical measures of THC and CBD, maximum symptom relief (minimum symptom level reported, rather than ending symptom level, minus starting symptom level), and symptom relief reported within 1, 2, and 3 hours, rather than 4.
Results
Table 1 presents descriptive statistics for the product characteristics, the starting and ending symptom intensity levels, and the prevalence of side effects. The sample size changes due to non-reporting of product characteristics. On average, users with depression reported a starting symptom intensity of 5.85 and an ending symptom of 2.08, leading to a decrease in symptom intensity of 3.76 (SD=2.64, d=1.71, p<.001). Table 2 shows the results from regressing the change in symptom intensity level on product characteristics. Each column represents a separate regression with different product characteristics included. In all columns, a higher starting symptom intensity level is associated with greater symptom relief. Column 1 shows the effect of labeled plant phenotype. Results show no statistical difference in symptom relief between products labeled “C. indica,” “C. sativa,” or “Hybrid.” Column 2 suggests that symptom relief does not vary with combustion method. Column 3 shows that higher THC levels are associated with greater symptom relief while higher CBD levels are not statistically significant predictors of symptom relief. In column 4, all product characteristics are included in the regression. Results suggest that when labeled plant phenotype and combustion method are controlled for, THC levels are the strongest independent predictor of symptom relief while CBD levels are not statistically significant predictors1. Figure 1 presents adjusted symptom relief by THC and CBD level.
Table 1. Descriptive statistics.
| Proportion of Sessions / Means | Std. Dev. | Minimum | Maximum | |
| Panel A: Labeled Plant Phenotype (5,182 symptom-sessions, 1,662 users) | ||||
| Hybrid | 50% | 0.50 | 0 | 1 |
| C. indica | 26% | 0.44 | 0 | 1 |
| C. sativa | 24% | 0.43 | 0 | 1 |
| Panel B: Combustion Method (5,513 symptom-sessions, 1,700 users) | ||||
| Joint | 15% | 0.36 | 0 | 1 |
| Pipe | 51% | 0.50 | 0 | 1 |
| Vape | 33% | 0.47 | 0 | 1 |
| Panel C: THC (2,305 symptom-sessions, 748 users) | ||||
| THC % | 18.61 | 7.90 | 0 | 35 |
| THC < 10% | 17% | 0.37 | 0 | 1 |
| THC 10-19 | 35% | 0.48 | 0 | 1 |
| THC 20-35% | 48% | 0.50 | 0 | 1 |
| Panel D: CBD (1,267 symptom-sessions, 528 users) | ||||
| CBD % | 6.17 | 7.03 | 0 | 35 |
| CBD < 1% | 31% | 0.46 | 0 | 1 |
| CBD 1-9% | 38% | 0.49 | 0 | 1 |
| CBD 10-35% | 31% | 0.46 | 0 | 1 |
| Panel E: Outcome and Control Variables (5,876 symptom-sessions, 1,819 users) | ||||
| Starting Symptom Level | 5.85 | 2.33 | 1 | 10 |
| Ending Symptom Level | 2.08 | 2.07 | 0 | 10 |
| Symptom Change | -3.76 | 2.64 | -10 | 9 |
| Panel F: Side Effects (4,493 sessions, 1,482 users) | ||||
| Any Negative Side Effect | 72% | 0.45 | 0 | 1 |
| % of Negative Side Effects | 13% | 0.15 | 0 | 1 |
| Any Positive Side Effect | 95% | 0.22 | 0 | 1 |
| % of Positive Side Effects | 30% | 0.20 | 0 | 1 |
| Any Context-Specific Side Effect | 86% | 0.34 | 0 | 1 |
| % of Context-Specific Side Effects | 24% | 0.19 | 0 | 1 |
Notes: The sample includes sessions treating depression. Nineteen positive, seventeen negative, and eleven context-specific side effects were available for selection. The % of Side Effects represent the average number of side effects experienced out of the total number of possible side effects within each category.
Table 2. Effects of flower characteristics on depression relief.
| Outcome = Symptom Change (Ending - Starting Symptom) | ||||
| (1) | (2) | (3) | (4) | |
| Panel A: Labeled Plant Phenotype, omitted category = Hybrid | ||||
| C. indica | -0.001 | 0.213 | ||
| (0.071) | (0.187) | |||
| C. sativa | -0.040 | -0.121 | ||
| (0.066) | (0.183) | |||
| Panel B: Combustion Method, omitted category = Joint | ||||
| Pipe | 0.110 | -0.069 | ||
| (0.149) | (0.283) | |||
| Vape | -0.007 | -0.091 | ||
| (0.187) | (0.328) | |||
| Panel C: THC and CBD, omitted categories = THC<10% and CBD<1% | ||||
| THC 10-19% | -0.424 | -0.325 | ||
| (0.220) | (0.208) | |||
| THC 20-35% | -0.549* | -0.505* | ||
| (0.272) | (0.250) | |||
| CBD 1-9% | 0.227 | 0.155 | ||
| (0.157) | (0.178) | |||
| CBD 10-35% | 0.291 | 0.364 | ||
| (0.211) | (0.234) | |||
| Starting Symptom Level | -0.741** | -0.742** | -0.659** | -0.648** |
| (0.031) | (0.029) | (0.049) | (0.052) | |
| Constant | 0.540** | 0.487* | 0.350 | 0.286 |
| (0.181) | (0.213) | (0.367) | (0.374) | |
| Number of sessions | 5,182 | 5,513 | 1,020 | 915 |
| R2 | 0.389 | 0.392 | 0.373 | 0.373 |
| Number of users | 1,662 | 1,700 | 436 | 387 |
Notes: Columns 1-4 represent separate equations regressing change in symptom intensity level on different types of product characteristics, comparing each product type to an omitted category. All regressions are estimated using a fixed effects model. Unstandardized coefficients are shown with standard errors, clustered at the individual user level, in parentheses. ** p<0.01, * p<0.05
Figure 1.
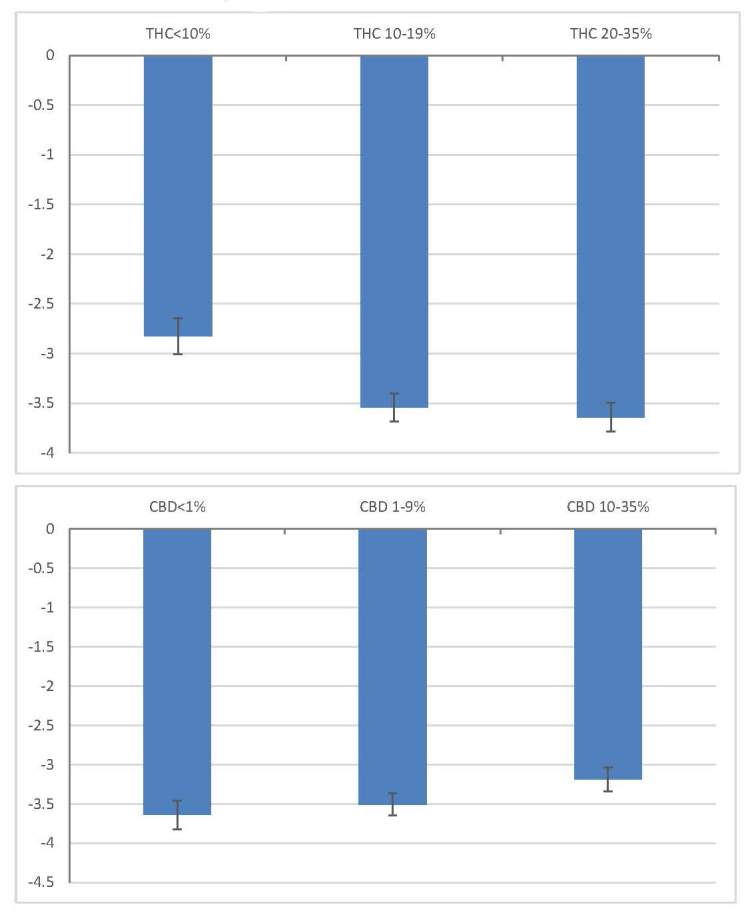
Adjusted symptom relief by THC and CBD levels.
Notes: Adjusted symptom relief refers to covariate-adjusted change in symptom intensity level, obtained from a fixed effects model controlling for labeled plant phenotype, combustion method, and starting symptom level. CBD levels are controlled for in the THC figure while THC levels are controlled for in the CBD figure. 95% confidence intervals are plotted.
Table 3 provides regressions of side effect outcomes on product characteristics, including all product characteristics. Labeled plant phenotype is not associated with the prevalence of side effects. Relative to joint, vaping is associated with less reporting of positive and context-specific side effects. Higher THC levels are associated with decreased reporting of positive side effects.
Table 3. Effects of product characteristics on side effects.
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Negative | % of Negative | Positive | % of Positive | Context-Specific | % of Context-Specific | |
| C. indica | 0.039 | 0.001 | 0.007 | -0.011 | -0.043 | -0.002 |
| (0.068) | (0.014) | (0.021) | (0.017) | (0.045) | (0.016) | |
| C. sativa | 0.088 | 0.011 | -0.012 | 0.005 | -0.006 | -0.001 |
| (0.089) | (0.012) | (0.022) | (0.021) | (0.051) | (0.021) | |
| Pipe | -0.119 | 0.009 | -0.050 | -0.001 | -0.114 | -0.032 |
| (0.092) | (0.026) | (0.031) | (0.027) | (0.076) | (0.031) | |
| Vape | -0.025 | 0.005 | -0.102* | -0.014 | -0.214** | -0.040 |
| (0.123) | (0.026) | (0.046) | (0.028) | (0.079) | (0.031) | |
| THC 10-19% | 0.067 | 0.025 | -0.059* | 0.013 | 0.082 | 0.030 |
| (0.088) | (0.018) | (0.029) | (0.018) | (0.054) | (0.021) | |
| THC 20-35% | 0.001 | 0.014 | -0.056* | 0.009 | 0.079 | 0.029 |
| (0.077) | (0.019) | (0.023) | (0.020) | (0.051) | (0.021) | |
| CBD 1-9% | -0.047 | 0.003 | -0.030 | -0.022 | -0.170* | -0.012 |
| (0.099) | (0.015) | (0.030) | (0.027) | (0.073) | (0.021) | |
| CBD 10-35% | -0.116 | 0.005 | -0.077 | -0.049 | -0.174* | -0.020 |
| (0.077) | (0.015) | (0.040) | (0.030) | (0.087) | (0.024) | |
| Starting Symptom Level | 0.019 | 0.005 | -0.002 | -0.012** | -0.001 | 0.002 |
| (0.013) | (0.004) | (0.005) | (0.004) | (0.011) | (0.004) | |
| Constant | 0.631** | 0.057 | 1.120** | 0.371** | 1.083** | 0.213** |
| (0.161) | (0.041) | (0.056) | (0.046) | (0.121) | (0.044) | |
| Number of sessions | 757 | 757 | 757 | 757 | 757 | 757 |
| R2 | 0.023 | 0.015 | 0.028 | 0.032 | 0.047 | 0.014 |
| Number of users | 318 | 318 | 318 | 318 | 318 | 318 |
Notes: All regressions are estimated using a fixed effects model. C. indica and C. sativa are relative to Hybrid, THC categories are relative to THC between 0 and 10%, and CBD categories are relative to 0% CBD, and Pipe and Vape are relative to Joint. Unstandardized coefficients are shown with standard errors, clustered at the individual user level, in parentheses. ** p<0.01, * p<0.05.
Table 4 presents results from our robustness checks. In column 1, THC and CBD levels are coded as continuous variables, confirming that higher THC levels are the strongest independent predictor of symptom relief. In column 2, maximum symptom relief during the session is used as the outcome, rather than ending minus starting symptom level, and the same pattern of results exists with increased statistical significance. As in the main results, higher THC levels are associated with greater symptom relief, but unlike in the main results, maximum symptom relief is lower for sessions involving products labeled as “C. indica” rather than “Hybrid.” Columns 3-5 restrict the analysis samples to sessions that ended within 1-3 hours. Results are generally similar to the main results, except that higher CBD levels are weakly associated with less symptom relief among users who ended sessions within 1 or 2 hours.
Table 4. Robustness checks.
| (1) | (2) | (3) | (4) | (5) | |
| THC & CBD continuous | Max symptom relief | 1 hour relief | 2 hours relief | 3 hours relief | |
| C. indica | 0.194 | 0.318* | 0.356 | 0.343 | 0.223 |
| (0.189) | (0.141) | (0.200) | (0.210) | (0.185) | |
| C. sativa | -0.108 | 0.046 | 0.111 | 0.075 | -0.077 |
| (0.184) | (0.164) | (0.208) | (0.237) | (0.190) | |
| Pipe | -0.082 | -0.068 | -0.070 | -0.090 | -0.082 |
| (0.287) | (0.328) | (0.282) | (0.269) | (0.281) | |
| Vape | -0.094 | -0.114 | -0.141 | 0.018 | -0.028 |
| (0.318) | (0.340) | (0.338) | (0.285) | (0.323) | |
| THC 10-19% | -0.202 | -0.484 | -0.428 | -0.312 | |
| (0.156) | (0.259) | (0.232) | (0.208) | ||
| THC 20-35% | -0.324 | -0.604* | -0.592* | -0.516* | |
| (0.186) | (0.294) | (0.293) | (0.253) | ||
| CBD 1-9% | 0.189 | 0.352 | 0.352 | 0.133 | |
| (0.182) | (0.213) | (0.202) | (0.169) | ||
| CBD 10-35% | 0.313 | 0.522 | 0.524* | 0.324 | |
| (0.236) | (0.277) | (0.245) | (0.221) | ||
| THC % | -0.025 | ||||
| (0.013) | |||||
| CBD % | 0.009 | ||||
| (0.011) | |||||
| Starting Symptom Level | -0.646** | -0.690** | -0.637** | -0.687** | -0.648** |
| (0.052) | (0.048) | (0.053) | (0.051) | (0.052) | |
| Constant | 0.503 | 0.160 | 0.261 | 0.382 | 0.305 |
| (0.418) | (0.337) | (0.386) | (0.389) | (0.380) | |
| Number of sessions | 915 | 915 | 849 | 897 | 914 |
| R2 | 0.369 | 0.432 | 0.379 | 0.409 | 0.379 |
| Number of users | 387 | 387 | 378 | 384 | 387 |
Notes: All regressions are estimated using a fixed effects model. C. indica and C. sativa are relative to Hybrid, and Pipe and Vape are relative to Joint. In column 1, continuous THC and CBD levels are used. In columns 2-5, THC categories are relative to THC between 0 and 10%, and CBD categories are relative to 0% CBD. Unstandardized coefficients are shown with standard errors, clustered at the individual user level, in parentheses. ** p<0.01, * p<0.05.
Discussion
The current study supports and expands upon the recent work by Cuttler et al. (2018) [38] by measuring which types of Cannabis flower characteristics among common product alternatives are associated with relief from depression. Rather than including a wide range of cannabis products (e.g. topical and edibles) and treating THC and CBD potency levels as continuous measures, we focused exclusively on Cannabis flower and allowed the effects of THC and CBD to vary nonlinearly, comparing across ordinal categories (e.g. low, medium, and high) to best represent the restricted range of potency levels in which Cannabis flower “strains” are typically marketed and to reflect recent findings in the literature that suggest that the increasing THC can have opposite effects depending on the baseline level [43] or that the effect may plateau at high levels, at least for pain conditions [44]. Both the current study and Cuttler et al. (2018) [38] showed that common and commercially available Cannabis products used by millions of people every day affect self-reported depression intensity. Also similar, while some user sessions resulted in the manifestation of experiences that contribute to depression and low mood (e.g. feeling unmotivated), both studies showed the vast majority of people reported an overall symptom intensity reduction (over 95% in the current study), with an average reduction of three to four points on a standard 0 to 10 scale. The mobile software technology helps solve the challenge of measuring and informing the patient as to the pharmacodynamic effects of an inherently variable ethnomedicinal product that was once alive and that, even across cloned batches, can show chemotypic differences. Not only does plant heterogeneity limit the value of randomized controlled trials using a specific cannabis formulate, but it also forces patients to constantly experiment with fundamentally new products. By enabling prospective, real-time recording of cannabis administration sessions across the vast range of commonly available and chemotypically distinct products and consumption methods used by the general population, the current study made it possible to measure whether consumption of Cannabis flower products immediately affected changes in depression symptom intensity levels and which types of common product characteristics are associated with the strongest effects. The findings suggest benefits from patient-directed, cannabis therapy as an antidepressant treatment; however, effectiveness and side effect manifestation vary across different types of Cannabis flower with different cannabinoid potency levels.
In addition to the similarity across studies in the apparent general clinical effectiveness of using Cannabis flower for reducing feelings of depression, differences existed in the associations between the product characteristics and the extent of symptom relief. Although Cuttler et al. (2018) [38] reported that cannabis products with high CBD / low THC values statistically predicted the greatest improvements in feelings of depression, it is unclear which types of products (e.g. topicals vs edibles) such values would apply. Instead, we found that Cannabis flower with relatively higher THC potency levels emerged as the strongest independent predictor of symptom relief and experienced side effects, but that the benefit of higher THC appears to plateau to some extent such that products with THC ranges of 10-19% and 20-35% offer greater relief than products with THC less than 10%, but increasing from 10-19% to 20-35% does not convey additional benefits in terms of symptom relief. In contrast, flower with CBD potency levels greater than 1.0% were generally not predictive of changes in depressive symptom levels and may even reduce depression symptom relief reported within short treatment windows, e.g. less than 2 hours to ending effect. Understanding these effects is challenging given the complexity of the etiology of depression which involves much more than a mere neurochemical imbalance. Dysregulation of the hypothalamic-pituitary-arenal (HPA) axis, cancer, pathogens, gut dysbiosis (microbial imbalance), nutritional deficiencies, degenerative neurological conditions, endocannabinoid system disruption, immune system dysregulation, chronic pain, substance abuse, prescription medications, abuse, social isolation, conflict, death, trauma, family history, and chronic stress may all be contributing factors of depression [45,46].
Early antidepressants, primarily monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants, targeted dopamine receptors in the brain, and were effective for reducing depressive symptoms, but only after several weeks to effect and with potentially severe negative side effects, including sedation, substantial weight gain, and deadly food interactions [47]. Selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs), which target serotonin and norepinephrine generation rather than dopamine levels, can also carry intolerable side effects (e.g. sedation, agitation, anxiety, suicidal ideation, anorgasmia, and demotivation) and also have times-to-effect of as many as 5 to 6 weeks, making them of little use in the short run to many dangerously ill individuals at risk of harming themselves or others [48]. More recent research has furthermore documented significant withdrawal symptoms associated with cessation of SSRI use [49]. As a result, individuals who are nonresponsive to or who cannot tolerate selective SSRIs and SNRIs are frequently prescribed older or modified versions of the older antidepressants that enhance dopamine neurotransmission (e.g. MAOIs, dopamine receptor agonists) [50]. Newer versions of some products, e.g. EMSAM, a transdermal version of the monoamine oxidase inhibitor selegiline, carry lower risks including reduced food interactions, but still involve significant risks and relatively long times-to-effect, when they are effective, which appear to vary by person and with medication-specific tolerances [51,52]. When comparing these drawbacks to the relatively minor negative side effects and quick acting effects reported by the Cannabis users in the current study, it is understandable why some patients might choose to replace or augment their conventional pharmaceutical antidepressant treatment with medical Cannabis when given the legal opportunity to do so.
Our results indicate that THC in particular is positively correlated with an immediate reduction in the intensity of depressive feelings. Animal studies have shown that, like conventional antidepressants, THC affects dopamine, serotonin, and norepinephrine generation [53,54]. The differences in time-to-effect and relative side effect prevalence across products and users could arise from other antidepressant and synergistic compounds in the plant beyond THC or CBD, and prior research suggests there may be several thousand cannabis chemotypes, each with its own unique symphony of compounds [55]. In addition to the mood-elevating effects of THC, several terpenoids are known to exhibit antidepressant properties independent from cannabinoids. At least 200 terpenoids are reported to be present within Cannabis, and while their yield is usually less than 1% in dried flower, they may constitute as much as 10% of the total trichome content [56]. Only limited research exists on the effects of specific terpenoids. The terpenoid, D-limonene, in particular, appears likely to have antidepressant effects in humans and typically composes more than 50% of commercially available citrus oils [57]. One study found that citrus oil-based fragrance either eliminated the need for or substantially reduced the required dosage of antidepressants [58]. A later study using mice found that, when compared to the SSRI fluoxetine, 400 mg/kg of lemon essential oil was shown to reduce immobility time in mice that were subjected to a tail suspension test (TST). The dose was shown to significantly increase the levels of serotonin, norepinephrine, and dopamine in the mouse prefrontal cortex (PFC), striatum, and hippocampus. The results also indicated no evidence of negative side effects across all dosage levels received by the mice [59].
The relative predominance of specific cannabinoids, flavonoids, and terpenoids can also vary considerably from one plant to another according to combustion method. For example, vaporizing Cannabis flower differs from other forms of combustion by allowing the user to select the precise temperature required to target the desired compounds, while pipe use and joints require full combustion of the plant material. In addition to preserving cannabinoid, flavonoid, and terpenoid contents that evaporate at lower temperatures than THC and CBD, vaporizing rather than combusting the plant material could preserve THC levels as well. Although some research has shown that THC rate of delivery is comparable between vaporization and combustion, the process of combustion may destroy as much as 20-30% of the THC [60,61]. Combustion can also result in the pyrolytic degradation of hundreds of therapeutic terpenoids and phenolic compounds, such as flavonoids [62,63], which are conventionally believed to contribute to the enhanced “entourage” effect of whole flower rather than what is experienced from using THC or CBD isolates. Compounds found in the Cannabis plant have demonstrable anti-microbial, anti-oxidant, analgesic, neuroprotective, anxiolytic, and anti-inflammatory effects [64]. Certain combinations of both cannabinoid and noncannabinoid components, therefore, may increase the therapeutic effects of THC and reduce unwanted side effects, making vaporization a more optimized delivery mechanism as compared to combustion using pipes and joints [55,65]. Likewise, because vaporization permits lower consumption temperatures than is possible with pipes and joints, vaporization also should be a safer method of administration. The normal process of combustion generates substantially more carbon monoxide, ammonia, nitric oxide, hydrogen cyanide, polynuclear aromatic hydrocarbons (PAHs), and carcinogens such that vaporization may be an attractive delivery option in terms of harm reduction [62,63]. The lack of a difference by combustion methods in our results suggests that either users were vaping at temperatures comparable to those required for full combustion, that some terpenoids are still sufficiently present at higher temperatures to elicit antidepressant effects, or that terpenoid effects are not required for cannabis to have an antidepressant effect, controlling for THC and CBD levels. Future research would benefit from tracking vaping temperatures along with overall product heating method, as well as the direct examination of the effects of terpenes found in the Cannabis plant on depression in humans.
The current study does have limitations. Importantly, this study did not include a control placebo group, that is, individuals who did not use Cannabis to treat their depression or any Cannabis consumption sessions not tracked in the app, potentially resulting in selection bias. People who choose to use Cannabis to treat their depression may be those most likely to benefit from it or those for whom conventional treatments are less effective. The direction of the bias for app use is not as clear. Not using the app could be simply a matter of no knowing about the app or a dislike of app-based technologies, which would introduce noise into our analysis, or it could arise from an intolerance or dissatisfaction with Cannabis, making our results an overestimate of expected population effects. Alternatively, not using the app or stopping app use could arise from satisfaction with existing cannabis use and the lack of a need to explore other product options. Although our study extended the literature by incorporating a wider range of product characteristics than had been previously examined, we still were not able to include the full range of characteristics of products available (e.g. terpene profiles, vaping temperatures) and did not include non-flower cannabis products. We also did not account for factors beyond those time-invariant characteristics captured by the user fixed effects, including time-varying user demographics, cannabis experience, whether or not respondents had a diagnosis of depression, or the concomitant use of medications other than Cannabis. The current cannabis industry in the US is also wrought with product mislabeling [66,67], and black market products without labels may result in people guessing the product characteristics rendering the need for further product validation in future studies. Lastly, because we are focused on short-term effects and do not have every single Cannabis use session of the patients documented in our data, we do not know the long-term effects of Cannabis use. For example, Cuttler et al. (2018) [38] found a correlation between repeated use of their data recording system and increased baseline depression levels, however, it is unclear whether this may be a function of the effects of using Cannabis or a sample selection bias among respondents that used the system repeatedly, for example, people using the app to experiment with unfamiliar products, which could be less effective than the treatment approach the patient typically uses.
In conclusion, almost all patients in our sample experienced symptom relief from using Cannabis to treat depression and with minimal evidence of serious side effects in the short run. However, use of Cannabis does have well-established clinical drawbacks, including the potential for dependence and addiction, alterations in judgment, and an increased risk of behavioral accidents [68,69]. In addition, there is a well-established association between adolescent use of Cannabis and increased risk of depression in adulthood [20,70], but directionality of this relationship remains elusive. Likewise, the positive effects found in this paper along with the negative long-term associations documented in the prior literature support the importance of supplementing our analysis of short-term Cannabis symptom relief with causal studies of the longer term benefits and risks from using commercially available Cannabis.
One of the most clinically relevant findings from this study was the widely experienced relief from depression within 2 hours or less. Because traditional antidepressants have times-to-effect in weeks, short-term Cannabis use might be a solution to these delays in treatment or could be used to treat acute episodes associated with suicidal behavior and other forms of violence. Such short-term or acute episode treatment approaches would fill an important gap in existing clinical methods while avoiding the addiction and dependence risks of long-term Cannabis use. Given the significant costs to our society from depression, the research herein offers hope for new avenues of treatment. Future research on Cannabis and depression is needed, directly comparing the short- and long-term treatment effectiveness and side effect severity of Cannabis use with conventional antidepressant treatment, in conjunction with conventional treatment approaches, and in the presence of clinically discouraged behaviors, such as alcohol consumption.
Acknowledgments
We thank all the donors to the University of New Mexico Medical Cannabis Research Fund for supporting this research.
Glossary
- THC
Tetrahydrocannabinol
- CBD
cannabidiol
Appendix A.
Author Contributions
JMV, SSS, and XL conceived the study. FB, KK, BH independently designed and developed the ReleafApp™ and server infrastructure as part of their effort to help create an education tool for medical cannabis patients. XL conducted the analyses. JMV, SSS, JD, and XL drafted the manuscript. All authors contributed substantially to its intellectual content and revision.
Funding
This research was supported in part by student scholarships provided by the University of New Mexico Medical Cannabis Research Fund, mcrf.unm.edu.
Footnotes
1Similar findings were obtained if the omitted categories are C. indica (instead of Hybrid), Pipe (instead of Joint), and high THC/CBD categories (instead of low THC/CBD categories).
References
- Liu Y, Collins C, Wang K, Xie X, Bie R. The prevalence and trend of depression among veterans in the United States. J Affect Disord. 2019. February;245:724–7. [DOI] [PubMed] [Google Scholar]
- Patalay P, Gage SH. Changes in millennial adolescent mental health and health-related behaviours over 10 years: a population cohort comparison study. Int J Epidemiol. 2019. October;48(5):1650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2018. June;48(8):1308–15. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001. June;24(6):1069–78. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003. November;163(20):2433–45. [DOI] [PubMed] [Google Scholar]
- Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014. October;79(2):184–90. [DOI] [PubMed] [Google Scholar]
- Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: A systematic review and meta-analysis. J Affect Disord. 2017. October;221:36–46. [DOI] [PubMed] [Google Scholar]
- Samsom JN, Wong AH. Schizophrenia and depression co-morbidity: what we have learned from animal models. Front Psychiatry. 2015. February;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008. January;21(1):14–8. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008. October;455(7215):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health The NICE Guideline on the Treatment and Depression the Treatment and Management of Depression. The British Psychological Society and The Royal College of Psychiatrists; 2010. [Google Scholar]
- Green KM, Zebrak KA, Fothergill KE, Robertson JA, Ensminger ME. Childhood and adolescent risk factors for comorbid depression and substance use disorders in adulthood. Addict Behav. 2012. November;37(11):1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010. June;160(3):511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Trim RS, Roesch SC, Mrnak-Meyer J, Tate SR, Brown SA. Comorbid depression and substance use disorder: longitudinal associations between symptoms in a controlled trial. J Subst Abuse Treat. 2012. October;43(3):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvet AL, Hasin D. The natural history of substance use disorders. Curr Opin Psychiatry. 2016. July;29(4):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit N, Shoval G, Shlosberg D, Feingold D, Lev-Ran S. The association between cannabis use and suicidality among men and women: A population-based longitudinal study. J Affect Disord. 2016. November;205:216–24. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002. November;325(7374):1212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler MS, Vasilenko SA, Lanza ST. Age-varying associations between substance use behaviors and depressive symptoms during adolescence and young adulthood. Drug Alcohol Depend. 2015. December;157:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010. May;67(5):440–7. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2019. April;76(4):426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naji L, Rosic T, Dennis B, Bhatt M, Sanger N, Hudson J, et al. The association between cannabis use and suicidal behavior in patients with psychiatric disorders: an analysis of sex differences. Biol Sex Differ. 2018. June;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G, Bagge CL, Orozco R. A literature review and meta-analyses of cannabis use and suicidality. J Affect Disord. 2016. May;195:63–74. [DOI] [PubMed] [Google Scholar]
- Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: A population based longitudinal study. Psychiatry Res. 2017. May;251:225–34. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Rees DI, Sabia JJ. Medical marijuana laws and suicides by gender and age. Am J Public Health. 2014. December;104(12):2369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos BJ, Kubrin CE, Newark C. Medical Marijuana Laws and Suicide. Arch Suicide Res. 2019;14:1-14. [DOI] [PubMed] [Google Scholar]
- Webb CW, Webb SM. Therapeutic benefits of cannabis: a patient survey. Hawaii J Med Public Health. 2014. April;73(4):109–11. [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, Finnell JS, Mischley LK. A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis Cannabinoid Res. 2016. June;1(1):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin J, Post RM, Goodwin FK. 9 -Tetrahydrocannabinol in depressed patients. Arch Gen Psychiatry. 1973. March;28(3):345–8. [DOI] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004. August;10(4):434–41. [DOI] [PubMed] [Google Scholar]
- Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008. January;336(7637):199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008. March;9(3):254–64. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012. May;13(5):438–49. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005. September;65(6):812–9. [DOI] [PubMed] [Google Scholar]
- Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008. February;9(2):164–73. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences E and M. The Health Effects of Cannabis and Cannabinoids The Current State of Evidence and Recommendations for Research. The Health Effects of Cannabis and Cannabinoids; 2017. [PubMed] [Google Scholar]
- Stith S, Vigil JM. Federal barriers to Cannabis research. Science. 2016;352:1182. [DOI] [PubMed] [Google Scholar]
- Releaf App | Cannabis Treatment Tracking & Research [Internet]. [cited 2019 Jun 10]. Available from: https://releafapp.com/
- Cuttler C, Spradlin A, McLaughlin RJ. A naturalistic examination of the perceived effects of cannabis on negative affect. J Affect Disord. 2018. August;235:198–205. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Russo EB, Smith KM. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2018. March;84(4):225–33. [DOI] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018. November;9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–60. [DOI] [PubMed] [Google Scholar]
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007. August;4(8):1770–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress [Internet]. Drug Alcohol Depend. 2017. August;177:136–44. [cited 2019 Jun 10] Available from: https://linkinghub.elsevier.com/retrieve/pii/S037687161730220X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Vigil JM, Stith SS, Brockelman F, Keeling K, Hall B. The effectiveness of self-directed medical cannabis treatment for pain. Complement Ther Med. 2019. October;46:123–30. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic Staff Depression (major depressive disorder) - Symptoms and causes - Mayo Clinic. Mayo Clinic; 2018. [Google Scholar]
- Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018. January;67:230–45. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004. July;10(4):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother Psychosom. 2016;85(5):270–88. [DOI] [PubMed] [Google Scholar]
- Wilson E, Lader M. A review of the management of antidepressant discontinuation symptoms. Ther Adv Psychopharmacol. 2015. December;5(6):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveanu RV, Nemeroff CB. Etiology of Depression: Genetic and Environmental Factors. Psychiatric Clinics of North America; 2012. [DOI] [PubMed] [Google Scholar]
- Asnis GM, Henderson MA. EMSAM (deprenyl patch): how a promising antidepressant was underutilized. Neuropsychiatr Dis Treat. 2014. October;10:1911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Chen JJ. Transdermal selegiline for the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2007;3(5):527–37. [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature. 2016. November;539(7629):369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Borysenko M, Kumar MS. Effect of delta 9-THC on brain and plasma catecholamine levels as measured by HPLC. Brain Res Bull. 1985. January;14(1):85–90. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Russo EB. Cannabis and Cannabis Extracts. J Cannabis Ther. 2005 [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011. August;163(7):1344–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINS GDSO ZAGO HB, COSTA AV, ARAUJO JUNIOR LM DE, CARVALHO JR DE. CHEMICAL COMPOSITION AND TOXICITY OF CITRUS ESSENTIAL OILS ON Dysmicoccus brevipes (HEMIPTERA: PSEUDOCOCCIDAE). Rev Caatinga. 2017 [Google Scholar]
- Komori T, Fujiwara R, Tanida M, Nomura J, Yokoyama MM. Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation. 1995. May-Jun;2(3):174–80. [DOI] [PubMed] [Google Scholar]
- Hao CW, Lai WS, Ho CT, Sheen LY. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: use of the tail suspension test. J Funct Foods. 2013 [Google Scholar]
- Fehr KO, Kalant H. Analysis of Cannabis Smoke Obtained under Different Combustion Conditions. Can J Physiol Pharmacol. 2011. [DOI] [PubMed] [Google Scholar]
- Harder S, Rietbrock S. Concentration-effect relationship of delta-9-tetrahydrocannabiol and prediction of psychotropic effects after smoking marijuana. Int J Clin Pharmacol Ther. 1997. April;35(4):155–9. [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008. February;21(2):494–502. [DOI] [PubMed] [Google Scholar]
- Gieringer D, St. Laurent J, Goodrich S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J Cannabis Ther. 2004 [Google Scholar]
- Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci. 2016. February;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollastro F, Minassi A, Fresu LG. Cannabis Phenolics and their Bioactivities. Curr Med Chem. 2017. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA -. JAMA. 2015. June;313(24):2491–3. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJ, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA -. JAMA. 2017. November;318(17):1708–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008. June;178(13):1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria B, Degenhardt L, Hall W, Lynskey M. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010. May;29(3):318–30. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014. March;44(4):797–810. [DOI] [PubMed] [Google Scholar]


