Boronic acids can serve as excellent building blocks for the synthesis of a wide range of natural products, pharmaceuticals, and materials.1 However, some of the potentially most useful boronic acids, including 2-heterocyclic,2–4 vinyl,5 and cyclopropyl6 derivatives, are inherently unstable, which can significantly limit their benchtop storage and/or efficient cross-coupling. Many important surrogates have been developed, including trifluoroborate salts,7–10 trialkoxy or trihydroxyborate salts,11,12 diethanolamine adducts,13 sterically bulky boronic esters,14 and boroxines.15 However, none of these can provide air-stable and highly effective substitutes for all three of these challenging boronic acid classes. We herein report that N-methyliminodiacetic acid (MIDA) boronates16,17 represent the first general solution to this problem by virtue of their uniform benchtop stability and remarkable capacity for in situ slow release of unstable boronic acids (Figure 1).
Figure 1.
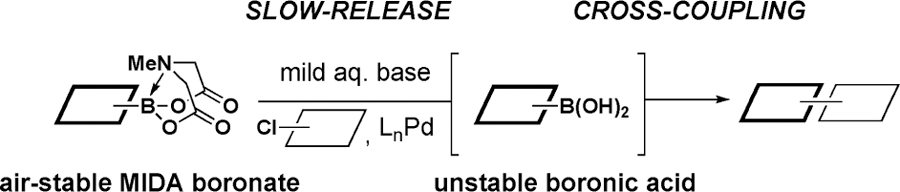
2-Heterocyclic, vinyl, and cyclopropyl boronic acids are known to decompose on the benchtop under air via protodeboronation, oxidation, and/or polymerization.2–6 In addition, these processes are thought to be accelerated in the presence of heat, base, and/or a Pd catalyst, causing the in situ decomposition of unstable boronic acids to compete with their cross-coupling.2 This latter challenge is exacerbated in couplings with slower-reacting halides, such as unactivated aryl chlorides.2 We hypothesized that both of these problems might be solved if we could achieve rate-controlled in situ hydrolysis of air-stable MIDA boronates, thereby promoting “slow release” of the corresponding unstable boronic acids from bench-stable building blocks.
The hydrolysis of MIDA boronates with aqueous NaOH is fast, typically requiring <10 min at 23 °C.17 In contrast, we discovered that K3PO4 in 5:1 dioxane/H2O at 60 °C promotes the continuous release of boronic acids over ∼3 h.18 Remarkably, aryl, heteroaryl, alkenyl, and alkyl MIDA boronates all behave similarly. Moreover, this release rate can be adjusted from 24 h to 30 min by varying the temperature from 23 to 100 °C.18
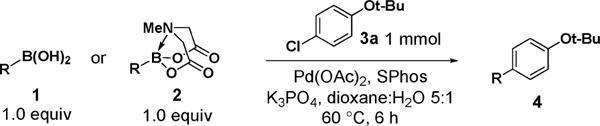
Having verified this capacity for slow release, we systematically compared the benchtop stability and cross-coupling efficiency of freshly prepared boronic acids 1a–h18,19 and the corresponding MIDA boronates 2a–h18 (Table 1). The benchtop instability of 2-heterocyclic, vinyl, and cyclopropyl boronic acids has frequently been discussed anecdotally,2–15 yet there is very little quantitative data available. As shown in Table 1, we determined that boronic acids 1a–h all decompose significantly on the benchtop under air over the course of just 15 days (entries 1–8).18 In fact, with 2-furan, 2-pyrrole, 2-indole, vinyl, and cyclopropyl boronic acids, very little of the original material remains after this time. Alternatively, all of the MIDA boronates 2a–h are indefinitely air-stable, with no decomposition detectable by 1H NMR even after g60 days on the benchtop under air.18
Table 1.
Benchtop Stability and Cross-Coupling Efficiency of Boronic Acids and the Corresponding MIDA Boronates
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | % remaining after benchtop storage under aira | 4 | % isolation yield from cross-couplingc | ||
| 1 (15 days) | 2 (60 days) | 1 | 2 | |||
| 1 |  |
7 | >95b |  |
68 | 94 |
| 2 | 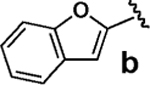 |
88 | >95 | 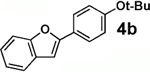 |
50 | 92 |
| 3 | 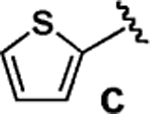 |
80 | >95 | 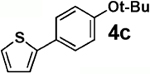 |
37 | 94 |
| 4 | 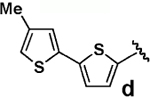 |
80 | >95b | 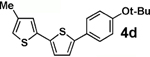 |
45 | 96 |
| 5 | 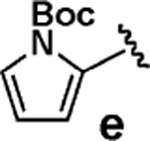 |
<5 | >95 | 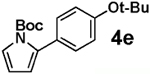 |
61 | 90 |
| 6 | 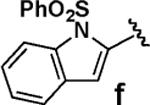 |
<5 | >95 | 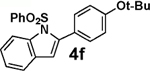 |
14 | 93 |
| 7d | 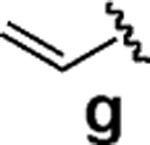 |
5 | >95b | 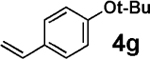 |
79 | 98 |
| 8d | 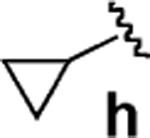 |
31 | >95 | 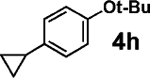 |
95 | 96 |
Freshly prepared boronic acids 1 and MIDA boronates 2 were stored as solids on the benchtop under air for 15 and 60 days, respectively.18
Stored for 107 days.
Reaction conditions: 1.0 equiv of 3a (1 mmol), 1.0 equiv of 1 (freshly prepared, >95% pure) or 2, 5 mol % Pd(OAc)2, 10 mol % SPhos, 7.5 equiv of K3PO4, 0.07 M in 5:1 dioxane/H2O, 60 °C, 6 h.
Cross-couplings were run at 100 °C.
We next tested the cross-coupling efficiency of freshly prepared boronic acids 1a–h19 with aryl chloride 3a using Pd(OAc)2/SPhos2,20 as the catalyst and K3PO4 as the base. Only very low to moderate yields (14–68%) were observed for the 2-heterocyclic derivatives 1a–f (entries 1–6), consistent with our observations that boronic acid decomposition kinetically competes with cross-coupling.2,18 In stark contrast, all of the corresponding MIDA boronates 2a–f coupled under identical conditions with aryl chloride 3a in uniformly excellent yields (90–96%) using in each case only 1 equiv of MIDA boronate.21 In many cases, the improvement in yield using 2 versus 1 is striking [e.g., 92 vs 50% with 2-benzofuran (entry 2), 94 vs 37% with 2-thiophene (entry 3), and 93 vs 14% with 2-indole (entry 6)]. In addition, vinyl MIDA boronate (2g) was significantly more effective than freshly prepared 1g (entry 7).5c,8
Consistent with our hypothesis that these increases in yield are attributable to in situ slow release of the corresponding boronic acids, no significant differences in yields were observed for 1a (64%) vs 2a (59%) under fast-release conditions, i.e., using aqueous NaOH as base.18 Moreover, the high yield observed for 2a under slow-release conditions was replicated via syringe-pump-mediated addition of freshly prepared 1a over the course of 3 h.18 It is noteworthy that cyclopropyl boronic acid (1h) prepared immediately prior to the reaction can be as effective as MIDA boronate 2h (entry 8), suggesting that benchtop decomposition of 1h may be in large part responsible for the challenges frequently encountered with this boronic acid.6a
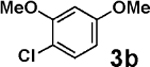
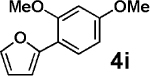
Encouraged by these results, we explored the scope of this slow-release method and found that even some of the most challenging aryl and heteroaryl chlorides can be efficiently coupled with MIDA boronates 2a–h (Table 2). For example, the highly deactivated (electron-rich and sterically hindered) compound 2,4-dimethoxychlorobenzene (3b) represents an exceptionally difficult cross-coupling partner for unstable 2-heterocyclic boronic acids. Nonetheless, just 1.2 equiv of the corresponding MIDA boronates promoted this coupling in generally excellent to outstanding yields (entries 1, 5, 9, 12, and 14). Because of the great importance of polyheterocyclic scaffolds in pharmaceuticals, similar cross-couplings with inexpensive and readily available heteroaryl chlorides would also be highly valuable. We explored this possibility with 3d–i and found that the 2-heterocyclic MIDA boronates are highly effective in such couplings (entries 3, 4, 6–8, 10, 11, 13, and 15). Even electronically deactivated heteroaryl chlorides such as 3f–h were coupled to 2b in good to excellent yields (entries 6–8).
Table 2.
Slow-Release Cross-Coupling of Air-Stable 2-Heterocyclic, Vinyl, and Cyclopropyl MIDA Boronates with Aryl and Heteroaryl Chloridesa
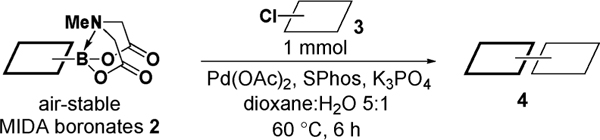 | ||||
|---|---|---|---|---|
| entry | 2 | 3 | 4 | % isolated yield |
| 1 | 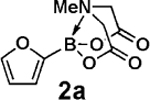 |
 |
 |
99 |
| 2 | 2a | 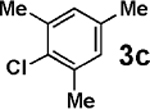 |
 |
97 |
| 3 | 2a | 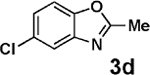 |
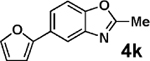 |
99 |
| 4 | 2a | 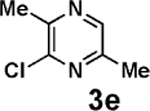 |
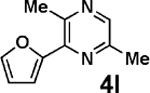 |
91 |
| 5 | 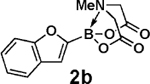 |
3b | 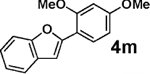 |
94 |
| 6 | 2b | 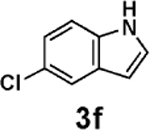 |
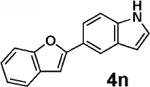 |
94 |
| 7b | 2b | 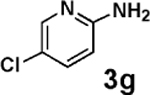 |
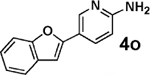 |
85 |
| 8b | 2b | 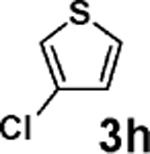 |
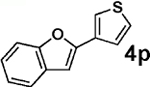 |
85 |
| 9 | 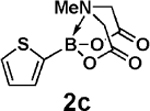 |
3b | 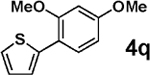 |
98 |
| 10 | 2c | 3d | 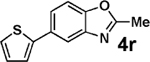 |
99 |
| 11 | 2c | 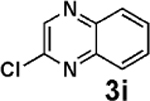 |
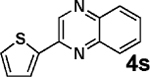 |
97 |
| 12c | 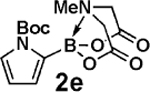 |
3b | 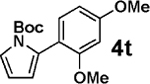 |
81 |
| 13c | 2e | 3d | 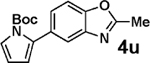 |
98 |
| 14 | 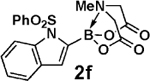 |
3b | 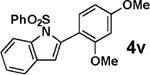 |
97 |
| 15 | 2f | 3d | 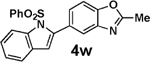 |
93 |
| 16d,e | 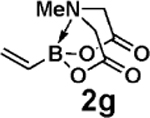 |
3c | 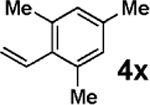 |
91 |
| 17d,e | 2g | 3i | 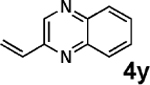 |
87 |
| 18d,e | 2g | 3g | 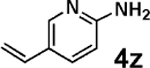 |
76 |
| 19d,e | 2g | 3d | 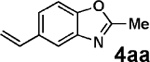 |
96 |
| 20b,d,f | 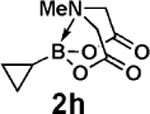 |
3c |  |
79 |
| 21d | 2h | 3b |  |
97 |
General reaction conditions: 1 equiv of aryl halide (1 mmol), 1.2 equiv of MIDA boronate, 5 mol % Pd(OAc)2, 10 mol % SPhos, 7.5 equiv of K3PO4, 0.07 M in 5:1 dioxane/H2O, 60 °C, 6 h.
Using 1.5 equiv of MIDA boronate.
Using 0.5 mmol of aryl halide, 0.6 mmol of MIDA boronate (1.2 equiv)
At 100 °C.
Reaction time 2 h.
Reaction time 24 h.
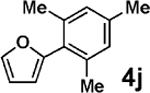
Vinylation of aryl and heteroaryl halides can provide styrene-like building blocks for a wide range of small molecules and materials.5c Thus, the development of a highly effective, nontoxic, environmentally friendly, and air-stable vinyl metal species has long been an important goal.5c Remarkably, vinyl MIDA boronate 2g embodies all of these favorable properties and efficiently coupled even with the highly deactivated aryl chloride 3c (entry 16) as well as a variety of heteroaryl chlorides (entries 17–19). Finally, 2h coupled with highly deactivated aryl chlorides 3c and 3b (entries 20–21).
As a final example, the 2-pyridyl subunit appears with remarkable frequency in biologically active small molecules. However, the corresponding boronic acid is notoriously unstable4,22 and difficult to cross-couple,4,7,11a,23 particularly with aryl chlorides.11a Currently available surrogates either are not air-stable11a,b,12 or cannot be isolated in chemically pure form.13
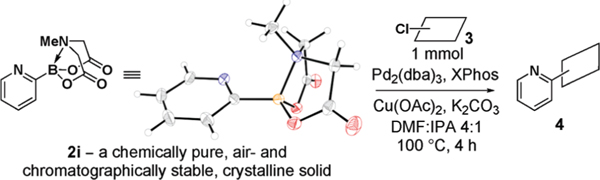
In contrast, 2-pyridyl MIDA boronate (2i) is isolable as a chemically pure and air-stable solid (X-ray structure shown in Table 3, 1H NMR spectra showed no decomposition after 60 days on the benchtop under air). Consistent with a relatively lower rate of transmetalation for 2-pyridylboranes,11a conditions like those used in Tables 1 and 2 were not generally effective for couplings with 2i. However, driven by the hypothesis that in-situ-generated 2-pyridyl boronic esters would be more stable than their boronic acid counterparts, we explored a variety of alcohol-containing solvent mixtures and found that DMF/IPA was advantageous. Moreover, it has been demonstrated that the addition of CuI13,11b or CuCl14c can promote cross-couplings with other 2-pyridylboranes. We therefore surveyed a series of copper salts and found that the inexpensive and nontoxic Cu(OAc)2 was especially beneficial. As shown in Table 3, under these modified slow-release conditions, air-stable 2-pyridyl MIDA boronate (2i) can be cross-coupled with a variety of aryl and heteroaryl chlorides (entries 1–5). The capacity to effectively cross-couple two different 2-substituted heterocycles is a notable advantage of this methodology (Table 3, entries 3–5; also see Table 2, entries 4 and 11).
Table 3.
Slow-Release Cross-Coupling of Air-Stable 2-Pyridyl MIDA Boronate 2i with Aryl and Heteroaryl Chloridesa
 | |||
|---|---|---|---|
| entry | 3 | 4 | % isolation yield |
| 1 | 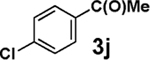 |
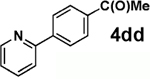 |
72 |
| 2 | 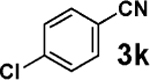 |
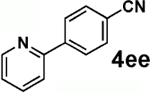 |
60 |
| 3 | 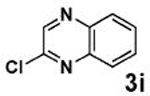 |
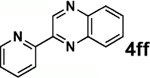 |
79 |
| 4 | 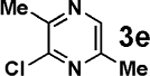 |
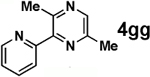 |
52 |
| 5 | 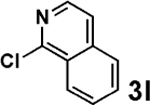 |
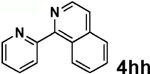 |
74 |
Reaction conditions: 1.0 equiv of aryl halide 3 (1 mmol), 1.5 equiv of MIDA boronate 2i, 1.5 mol % Pd2(dba)3, 6 mol % XPhos, 50 mol % Cu(OAc)2, 5 equiv of K2CO3, 0.1 M in 4:1 DMF/IPA, 100 °C, 4 h.
In summary, several highly advantageous features collectively make MIDA boronates an outstanding platform for the preparation and utilization of organoboranes in organic synthesis.17 These include reversibly attenuated reactivity toward anhydrous cross-coupling conditions, compatibility with a wide range of synthetic reagents, air stability, solubility in many common organic solvents, monomeric constitution, and compatibility with silica gel chromatography.17 We now report that MIDA boronates also possess the highly enabling capacity for in situ slow release of the corresponding unstable boronic acids. This remarkably general solution has transformed a wide range of unstable boronic acids into air-stable and highly effective cross-coupling partners, many of which are now commercially available.24
Supplementary Material
Acknowledgment.
We gratefully acknowledge the NSF (CA-REER 0747778), Bristol-Myers Squibb, and Sigma-Aldrich for fund-ing. D.M.K. is an NIH CBTG Fellow, E.P.G. is a Seemon Pines Fellow, and M.D.B. is a Sloan Research Fellow, Beckman and Amgen YI, and Dreyfus New Faculty Awardee.
Footnotes
Supporting Information Available: Procedures, spectral data, and spectra for all new compounds and crystallographic data for 2i (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Hall DG Boronic Acids; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- (2).In a recent systematic study, even the powerful diaryldialkylphosphine ligands developed by Buchwald and coworkers failed to promote efficient couplings of 2-substituted furan, thiophene, and pyrrole boronic acids with unactivated aryl chlorides: Billingsley K; Buchwald SL J. Am. Chem. Soc 2007, 129, 3358. [DOI] [PubMed] [Google Scholar]
- (3).(a) For some examples of coupling of 2-heterocyclic boronic acids with activated aryl and acid chlorides, see: ref 2.; (b) Fleckenstein CA; Plenio H. J. Org. Chem 2008, 73, 3236. [DOI] [PubMed] [Google Scholar]; (c) Burns MJ; Fairlamb IJS; Kapdi AR; Sehnal P; Taylor RJK Org. Lett 2007, 9, 5397. [DOI] [PubMed] [Google Scholar]; (d) Xin B; Zhang Y; Cheng K J. Org. Chem 2006, 71, 5725. [DOI] [PubMed] [Google Scholar]; (e) Pagano N; Maksimoska J; Bregman H; Williams DS; Webster RD; Xue F; Meggers E Org. Biomol. Chem 2007, 5, 1218. [DOI] [PubMed] [Google Scholar]; (f) There is a single report of coupling of excess 2-thiophene and 2-furan boronic acid with the simple unactivated aryl chloride chlorobenzene: Li J-H; Zhu Q-M; Xie Y-X Tetrahedron 2006, 62, 10888. [Google Scholar]; (g) For some representative examples of coupling of 2-heterocyclic boronic acids with aryl iodides and bromides, see: Takimiya K; Kunugi Y; Toyoshima Y; Otsubo T J. Am. Chem. Soc 2005, 127, 3605. [DOI] [PubMed] [Google Scholar]; (h) Thomas SW III; Venkatesan K; Mueller P; Swager TM J. Am. Chem. Soc 2006, 128, 16641. [DOI] [PubMed] [Google Scholar]; (i) Collis GE; Burrell AK; Blandford EJ; Officer DL Tetrahedron 2007, 63, 11141. [Google Scholar]; (j) Qin P; Zhu H; Edvinsson T; Boschloo G; Hagfeldt A; Sun L J. Am. Chem. Soc 2008, 130, 8570. [DOI] [PubMed] [Google Scholar]; (k) Maeda H; Haketa Y; Nakanishi T J. Am. Chem. Soc 2007, 129, 13661. [DOI] [PubMed] [Google Scholar]
- (4).Tyrrell E; Brookes P Synthesis 2003, 469.
- (5).(a) Matteson DS J. Am. Chem. Soc 1960, 82, 4228. [Google Scholar]; (b) Peyroux E; Berthiol F; Doucet H; Santelli M Eur. J. Org. Chem 2004, 1075.; (c) For an excellent review of the advantages and challenges of vinylation via cross-coupling, see: Denmark SE; Butler CR Chem. Commun 2009, 20. [DOI] [PMC free article] [PubMed]
- (6).(a) Cyclopropyl boronic acid decomposes under air primarily via protode-boronation: Todd RC; Josyula KVB; Gorr K; Priebe K; Gao P Abstr. Pap.sAm. Chem. Soc 2007, 233, ORGN780.; (b) For couplings with aryl bromides, see: Wallace DJ; Chen C Tetrahedron Lett 2002, 43, 6987. [Google Scholar]; (c) For couplings with activated aryl and acid chlorides, see: Lemhadri M; Doucet H; Santelli M Synth. Commun 2006, 36, 121. [Google Scholar]; (d) Chen H; Deng M-Z Org. Lett 2000, 2, 1649. [DOI] [PubMed] [Google Scholar]
- (7).(a) Trifluoroborate salts typically possess outstanding air-stability (see ref 10). However, some 2-heterocyclic derivatives (e.g., 2-pyridyl potassium trifluoroborate) can be problematic. See: Molander GA; Biolatto BJ Org. Chem 2003, 68, 4302. [DOI] [PubMed] [Google Scholar]; (b) Molander GA; Canturk B; Kennedy LE J. Org. Chem 2009, 74, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Also see refs 2, 11a, and 3e.
- (8).(a) Potassium vinyltrifluoroborate can be coupled to activated aryl chlorides, but these conditions were not successful with unactivated aryl chlorides: Molander GA; Brown AR J. Org. Chem 2006, 71, 9681. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA; Rivero MR Org. Lett 2002, 4, 107. [DOI] [PubMed] [Google Scholar]
- (9).Potassium cyclopropyl trifluoroborate is air-stable and can be coupled efficiently to a variety of unactivated aryl and heteroaryl chlorides: Molander GA; Gormisky PE J. Org. Chem 2008, 73, 7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) For excellent reviews on the use of trifluoroborate salts in organic synthesis, see: Molander GA; Ellis N Acc. Chem. Res 2007, 40, 275. [DOI] [PubMed] [Google Scholar]; (b) Darses S; Genet J-P Chem. Rev 2008, 108, 288. [DOI] [PubMed] [Google Scholar]; (c) Stefani HA; Cella R; Vieira AS Tetrahedron 2007, 63, 3623. [Google Scholar]
- (11).(a) Lithium triisopropyl 2-pyridylborate salts can be coupled to unactivated aryl chlorides, but the lack of air stability of these reagents is an important limitation: Billingsley KL; Buchwald SL Angew. Chem., Int. Ed 2008, 47, 4695. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) For additional studies with preformed borate salts, see ref 12 and: Yamamoto Y; Takizawa M; Yu X-Q; Miyaura N Angew. Chem., Int. Ed 2008, 47, 928. [DOI] [PubMed] [Google Scholar]; (c) Cammidge AN; Goddard VHM; Gopee H; Harrison NL; Hughes DL; Schubert CJ; Sutton BM; Watts GL; Whitehead AJ Org. Lett 2006, 8, 4071. [DOI] [PubMed] [Google Scholar]; (d) O’Neill BT; Yohannes D; Bundesmann MW; Arnold EP Org. Lett 2000, 2, 4201. [DOI] [PubMed] [Google Scholar]
- (12).(a) As pointed out by Molander and coworkers (ref 7), a previously described moderate-yield coupling between an aryl bromide and 2-pyridyl methylboronic ester in fact likely employed the air-sensitive 2-pyridyl trimethylborate salt: Sindkhedkar MD; Mulla HR; Wurth MA; Cammers-Goodwin A Tetrahedron 2001, 57, 2991. [Google Scholar]; (b) The same seems to be the case in: Fernando SRL; Maharoof USM; Deshayes KD; Kinstle TH; Ogawa MY J. Am. Chem. Soc 1996, 118, 5783. [Google Scholar]
- (13).(a) N-phenyldiethanolamine 2-pyridylboronate can be prepared as a structurally undefined complex containing variable quantities of isopropyl and N-phenyldiethanolamine groups and a stoichiometric quantity of lithium: Hodgson PB; Salingue FH Tetrahedron Lett 2004, 45, 685. [Google Scholar]; (b) Jones NA; Antoon JW; Bowie AL; Borak JB; Stevens EP J. Heterocyclic Chem 2007, 44, 363. [Google Scholar]; (c) For solid-supported diethanolamine adducts, see: Gravel M; Thompson KA; Zak M; Bérubé C; Hall DG J. Org. Chem 2002, 67, 3. [DOI] [PubMed] [Google Scholar]; (d) A solid-supported diethanolamine-bound 2-pyridyl reagent has also been reported: Gros P; Doudouh A; Fort Y Tetrahedron Lett 2004, 45, 6239. [Google Scholar]
- (14).(a) Lightfoot AP; Twiddle SJR; Whiting A SynLett 2005, 3, 529. [Google Scholar]; (b) Yang DX; Colletti SL; Wu K; Song M; Li GY; Shen HC Org. Lett 2009, 11, 381. [DOI] [PubMed] [Google Scholar]; (c) 2-Pyridyl pinacol boronic esters can be coupled to aryl bromides in the presence of CuCl. However, although there is a single reported example, this approach has not been shown to be generally effective with aryl chlorides: Deng JZ; Paone DV; Ginnetti AT; Kurihara H; Dreher SD; Weissman SA; Stauffer SR; Burgey BS Org. Lett 2009, 11, 345. [DOI] [PubMed] [Google Scholar]
- (15).(a) Perkins JR; Carter RG J. Am. Chem. Soc 2008, 130, 3290. [DOI] [PubMed] [Google Scholar]; (b) Kerins F; O’Shea DF J. Org. Chem 2002, 67, 4968. [DOI] [PubMed] [Google Scholar]; (c) Cioffi CL; Spencer WT; Richards JJ; Herr RJ J. Org. Chem 2004, 69, 2210. [DOI] [PubMed] [Google Scholar]
- (16).Mancilla T; Contreras R; Wrackmeyer BJ Organomet. Chem 1986, 307, 1. [Google Scholar]
- (17).(a) Gillis EP; Burke MD J. Am. Chem. Soc 2007, 129, 6716. [DOI] [PubMed] [Google Scholar]; (b) Lee SJ; Gray KC; Paek JS; Burke MD J. Am. Chem. Soc 2008, 130, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gillis EP; Burke MD J. Am. Chem. Soc 2008, 130, 14084. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Uno BE; Gillis EP; Burke MD Tetrahedron 2009, 65, 3130. [Google Scholar]; (e) Ballmer SG; Gillis EP; Burke MD Org. Synth 2009, submitted.; (f) Gillis EP; Burke MD Aldrichimica Acta 2009, in press. [PMC free article] [PubMed]
- (18). See the Supporting Information (SI).
- (19). All of the boronic acids used in this study were freshly prepared in >95% purity via hydrolysis of the corresponding MIDA boronates (see the SI).
- (20).Martin R; Buchwald SL Acc. Chem. Res 2008, 41, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).To effectively promote both in situ release and cross-coupling of the boronic acids, the best results were achieved using 7.5 equiv of K3PO4. Interestingly, the cleaved MIDA2− appears to have no deleterious effect on these reactions, even though it is known to be a ligand for Pd(II): Smith BB; Sawyer DT Inorg. Chem 1968, 7, 1526. [Google Scholar]
- (22).Fischer FC; Havinga E Recl. TraV. Chim. Pays-Bas 1974, 93, 21. [Google Scholar]
- (23).(a) For selected examples of coupling of 2-pyridyl boranes with aryl bromides and iodides, see: Deshayes K; Broene RD; Chao I; Knobler CB; Diedrich FJ Org. Chem 1991, 56, 6787. [Google Scholar]; (b) Mandolesi SD; Vaillard SE; Podestá JC; Rossi RA Organometallics 2002, 21, 4886. [Google Scholar]; (c) Bouillon A; Lancelot J-C; Sopkova de Oliveira Santos J; Collot V; Bovy PR; Rault S Tetrahedron 2003, 59, 10043 For examples with 2-pyridyl pinacol boronic esters, see refs 14b, c and 23c. [Google Scholar]
- (24). Sigma-Aldrich: 2a (701017), 2b (701106), 2f (697443), 2g (704415), and 2h (697311).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


