Abstract
Introduction
The COVID-19 pandemic resulted in the suspension of nonemergent surgeries throughout New York. Our tertiary care children’s hospital pivoted towards a brief trial of intravenous (IV) antibiotic therapy in all patients in order to limit operating room (OR) utilization and avoid prolonged hospital stays. We describe our pandemic-based strategy for non-operative management (NOM) of appendicitis but with a limited duration of IV antibiotics.
Methods
We performed a retrospective study of children treated for acute appendicitis at our center from 3/31/2020 to 5/3/2020 during the peak of the New York pandemic. We compared appendicitis volume to similar months in prior years. We evaluated failure of NOM, length of stay, and compared characteristics of children we successfully treated with our expanded NOM protocol to previously published inclusion criteria for NOM.
Results
45.5% of children (25/55) with acute appendicitis underwent NOM. Of the 30 who underwent surgery, 13 had complicated appendicitis while 17 had simple appendicitis. Three patients were COVID-positive, although none had respiratory symptoms. The majority of patients presenting with acute appendicitis (78.2%) did not meet previously published criteria for NOM.
Conclusions
We treated a similar volume of children with acute appendicitis during the pandemic compared to prior years. We applied non-operative management to nearly half our patients, even as we expanded inclusion criteria for NOM to reduce OR utilization, but limited the duration of the antibiotic trial to avoid prolonged hospital stays.
Type of study
Retrospective study.
Level of evidence
IV.
Key words: COVID-19, Appendicitis, Pandemic, SARS-cov-2
As the COVID-19 pandemic spread across the U.S., hospitals country-wide suspended nonemergent surgeries in order to reserve capacity for the expected surge of patients. As the dedicated tertiary children's hospital for the largest integrated health system in New York, we were in the epicenter of the pandemic and particularly affected. We suspended elective operations in mid-March and as such, rapid changes to our clinical approaches were required to treat nonemergent pediatric surgical conditions such as appendicitis.
Acute appendicitis remains the most common pediatric surgical condition [1]. Nonoperative management (NOM) has been shown to be effective in select patients with acute appendicitis, be it simple or perforated. Protocols for NOM for presumed simple appendicitis vary, with differing inclusion and exclusion criteria [2,3]. Failure rates at one year for such NOM range from 0 to 29% [4,5]. Multiple studies have demonstrated the safety and efficacy of the NOM approach; however, equivalency or superiority has not been definitively demonstrated in the literature [6]. Larger scale multicenter trials are ongoing [7] and operative management for appendicitis (via appendectomy) is still considered the standard of care.
Prior to the COVID-19 pandemic, at our center, we favored operative management of acute and perforated appendicitis. The majority of children underwent a single incision transumbilical laparoscopic-assisted appendectomy (TULA), which in combination with perioperative rectus sheath block, allowed the majority of children with simple appendicitis to be discharged promptly postoperatively [8]. Less than 10 patients per year with perforation are treated nonoperatively and undergo an interval appendectomy. These are typically children with large phlegmons or radiologically drainable abscesses. We perform 550–600 appendectomies for acute appendicitis per year.
Once elective operations were suspended during the peak of the COVID-19 pandemic in New York, procedures were categorized by tiers. Only emergent operations were permitted to proceed. While we were able to operate on patients for appendicitis, we shifted to a brief trial of antibiotics alone for all patients with acute appendicitis in an effort to conserve resources, minimize nonemergent surgical procedures, and at certain intervals to allow for COVID testing to result. We also modified our use of intravenous antibiotics in an attempt to reduce length of stay, as our goal was to safely minimize the amount of the time that patients were in the hospital.
We performed a retrospective study of children with acute appendicitis whose management was modified to conform to the contingencies of the COVID-19 pandemic at our center. We aimed to assess the results of a NOM strategy with a limited course of intravenous (IV) antibiotics for all patients with appendicitis, whether complex or simple. We further wished to explore the characteristics of patients we treated with NOM and compare them to established protocols [4]. We report our experience attempting to safely manage children with acute appendicitis while also minimizing healthcare resource utilization (operating room use and hospital stays) during a pervasive modern-era pandemic.
1. Methods
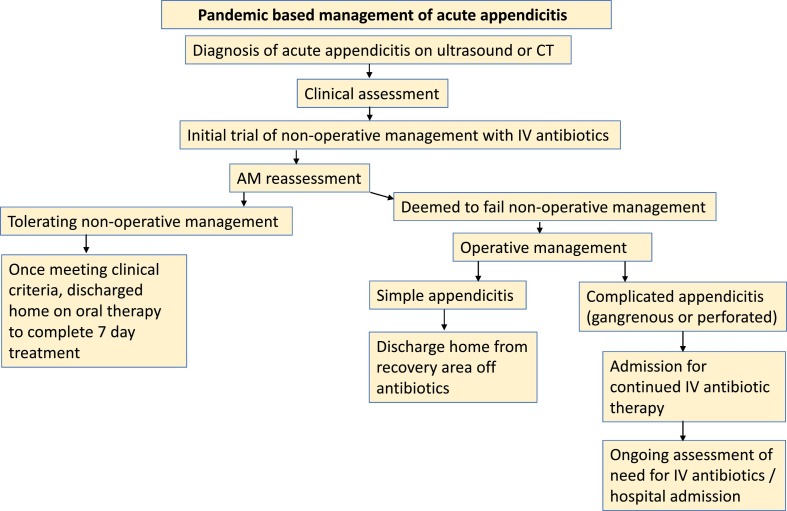
We retrospectively collected data on all children diagnosed with acute appendicitis by our pediatric surgical service from 3/31/2020 to 5/3/2020, the five-week period during the peak of the COVID-19 pandemic in New York. Under our pandemic-based strategy, all patients with acute appendicitis were initiated on intravenous antibiotic therapy for a trial of NOM (Fig. 1 ).
Fig. 1.
Pandemic based management of acute appendicitis.
Children diagnosed with acute appendicitis were assessed by our team in the emergency department, and unless deemed to require immediate operative management (e.g. hemodynamic instability, severe peritonitis), were observed for 12–24 h, regardless of time of presentation, clinical presentation, or imaging characteristics. Children were then reassessed for symptoms such as pain, fever, and emesis. Those with persistent or worsening symptoms were taken to the operating room. Children who had improved were managed nonoperatively and trialed on a diet with a plan for discharge shortly thereafter.
We modified our standard TULA to a three-port appendectomy in response to concerns about aerosolization of the virus [9]. COVID-19 personal protective and other intraoperative precautions were undertaken for all operative cases. COVID-19 testing was available for the majority of the study period, with varying delays for results throughout the pandemic. Children who were operated on with simple appendicitis were discharged from the recovery room, consistent with our established management prior to the pandemic. Children with complicated appendicitis were admitted postoperatively and continued on intravenous antibiotics as per our routine management [10], although they were reassessed earlier for possible discharge.
Time of diagnosis of acute appendicitis was noted as the first time that antibiotics were ordered for patients in the electronic medical record (EMR). Discharge time was recorded as the last discharge order placed for a patient in the EMR. We calculated length of stay (LOS) as the difference between these two times. Ultrasound and computed tomography reports were reviewed for size of appendix and presence of fecalith, phlegmon or abscess. Diagnoses of simple and complicated appendicitis (either perforated or gangrenous) were taken from the brief operative note completed at time of surgery. We included as NOM patients who were discharged from the hospital without an operation, whether they were presumed to have simple or complicated appendicitis. Patient follow-up for the study was censored 6/4/2020.
We compared the presenting characteristics of our patients to those assessed for NOM in a large clinical study, in order to assess whether they would have been candidates for NOM outside of a pandemic (Table 1 ) [4]. Historical data were taken from our divisional REDCap (Research Electronic Data Capture) database at Northwell Health [11,12]. We queried all operations performed from 2/1/2017 to 3/30/2020. We included patients 2–17 years of age undergoing operative management of acute appendicitis, excluding interval and incidental appendectomies.
Table 1.
Midwestern Pediatric Surgical Collaborative criteria for nonoperative management of acute appendicitis: inclusion and exclusion criteria.
| Age 7 to 17 years |
| ≤ 48 h of abdominal pain |
| White blood cell count < 18,000 |
| Radiographic evidence based on final radiologic interpretation of nonruptured acute appendicitis on either ultrasound (US) or computed tomography (CT) with an appendiceal diameter ≤ 1.1 cm and without phlegmon, abscess, or fecalith |
| Evaluation by a surgeon confirming clinical suspicion of acute appendicitis |
| Exclusion criteria consisted of diffuse peritonitis, a positive pregnancy test result significant medical or behavioral comorbidities. |
We compared patients presenting during the study period to patients presenting during the same interval during 2017–2019, using Fisher's exact test. For those patients presenting during the study period in 2019, we further calculated LOS as described above. We used the Wilcoxon rank-sum test to compare LOS between groups of patients. We performed univariate and multivariate logistic regression, comparing those patients who underwent operative management to those treated nonoperatively. Statistical analysis was performed using OpenEpi (Atlanta, GA) and SAS 9.4 (Cary, NC). This research was approved by the Northwell Health Institutional Review Board, #20-0391.
2. Results
During the 5-week peak of the COVID-19 pandemic in New York, 55 patients with acute appendicitis were treated at our center (Table 2 ). Median age was 12.4 years (interquartile range [IQR] 7.8–15.4) and no child had severe preexisting comorbid illness. Aside from one child who went promptly to the operating room for severe abdominal pain, all children were initially treated nonoperatively. All three children in our study who tested positive for COVID-19 before and/or during their hospitalization had no respiratory symptoms. Two of these children were discharged from the hospital following NOM, while a third underwent an operation the day following admission. He remained asymptomatic following an operation for perforated appendicitis, and met criteria for discharge by postoperative day 2.
Table 2.
Presenting characteristics of children with acute appendicitis.
| All patientsa |
No operation |
Operation |
||
|---|---|---|---|---|
| Simple |
Complicated |
|||
| N = 55 | N = 25 | N = 17 | N = 13 | |
| Age (in years) | 12.4 (7.8–15.4) | 12.4 (7.8–15.2) | 12.1 (9.7–16.3) | 12.6 (7.1–14.7) |
| Days of symptoms | 1 (1–3) | 1 (1–3) | 1 (1) | 3 (1.5–3) |
| WBC | 15.3 (11.9–20.0) | 14.0 (10.6–17.9) | 18.3 (13.9–20.3) | 16.6 (12.7–21.5) |
| Appendiceal diameterb | 10 (8–12) | 9 (7–11) | 10 (9–12) | 11 (10–13.5) |
| Imaging findings | ||||
| Fecalith | 14 (25.5) | 4 (16) | 6 (35.3) | 4 (30.8) |
| Abscess | 2 (3.6) | 1 (4) | 0 | 1 (7.7) |
| Phlegmon | 3 (5.4) | 1 (4) | 1 (5.9) | 1 (7.7) |
N (%) or median (IQR).
In mm.
Twenty-five children (45.5%) were discharged from the hospital after initial NOM. Overall, median LOS for children treated nonoperatively was 22.5 h (IQR 16.1–29.5). Two children were able to be discharged under 7 h after diagnosis. Among children who were successfully treated with NOM, at least two likely had complicated appendicitis. One child with a phlegmon was discharged home on hospital day 5, while another received a drain for an abscess by our interventional radiologists and was discharged home on oral therapy after about 40 h in the hospital. The only other child treated nonoperatively who was not discharged by hospital day 2 was a younger child who had difficulty tolerating oral antibiotics.
Of those 30 patients who failed NOM and underwent appendectomy, 17 had simple appendicitis and 13 had complicated appendicitis. Median length of stay for children undergoing appendectomy for simple appendicitis was 17.4 h (IQR 13.5–25.0) and 5 days (IQR 3–6) for complicated appendicitis. There was no difference in length of stay between children with simple appendicitis treated operatively and children who were successfully treated nonoperatively (P = 0.88). Two patients (15%) with complicated appendicitis met the newly revised criteria for discharge by postoperative day 2.
The majority of children presenting to our institution during the pandemic period did not meet previously published guidelines for NOM (78.2%). Of the 25 children who were successfully managed nonoperatively, only eight met all the Midwestern Pediatric Surgery consortium guidelines for NOM. The most common criteria not met among those 23 children were duration of pain (N = 6), elevated WBC (N = 4), and presence of a fecalith (N = 3). Eight of the 12 children (66.7%) who met guidelines were successfully discharged following NOM, while 4 children underwent a simple appendectomy for failure to improve promptly. In seeking to determine risk factors for failure of NOM, we found that increasing appendiceal diameter on presentation was associated with increased odds of operative management (Table 3 ), even after controlling for other presenting characteristics (P = 0.03).
Table 3.
Multivariate logistic regression for odds of proceeding to operative management during COVID-19 pandemic.
| Univariate OR (CI) | P value | Multivariate OR (CI) | P value | |
|---|---|---|---|---|
| Fecalith | 2.50 (0.67–9.38) | 0.17 | 2.02 (0.33–9.52) | 0.38 |
| Age (in years) | 1.02 (0.90–1.16) | 0.76 | 0.99 (0.84–1.17) | 0.87 |
| WBC | 1.12 (1.00–1.27) | 0.06 | 1.12 (0.98–1.29) | 0.11 |
| Appendiceal diameter | 1.42 (1.06–1.89) | 0.02 | 1.45 (1.04–2.02) | 0.03 |
| Days of symptoms | 0.88 (0.64–1.20) | 0.41 | 0.92 (0.64–1.34) | 0.67 |
OR, odds ratio; CI, 95% confidence interval.
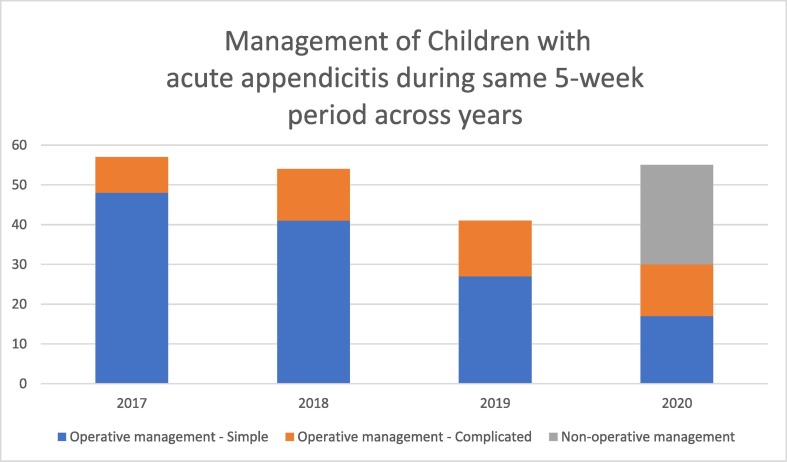
Compared to the same period in prior years, we treated a similar number of patients with acute appendicitis during the COVID-19 pandemic (Fig. 2 ). The proportion of children with complicated appendicitis was also comparable (P = 0.21). Three children treated nonoperatively returned to the hospital after discharge, of whom 1 met published criteria for NOM and 2 did not. One received additional treatment for pain while the other two patients underwent appendectomy and were discharged postoperatively following a simple appendectomy. In comparing only those patients undergoing an appendectomy for simple appendicitis, our LOS increased during the COVID-19 pandemic, from a median 11.1 h (IQR 6.9–17.4) to 22.5 h (IQR 16.1–29.5, P = 0.03).
Fig. 2.
Management of children with acute appendicitis during same 5-week period across years.
3. Discussion
COVID-19 fundamentally changed the way we functioned as a hospital and delivered patient care. Wards of the children's hospital were condensed as both elective and emergency care across pediatric specialties decreased and many of these wards were converted to adult wards to accommodate the overflow of adult COVID-19 patients. There were numerous considerations and limitations to take into account while delivering patient care, attempting to limit hospital stay while also limiting operations. COVID-19 testing was intermittently available at times with rapid turnaround, and unavailable at other times owing to shortages ranging from reagents to nasal swabs. We were further constrained with limited bed availability and, at one point, a shortage of oral liquid antibiotics.
Our volume of acute appendicitis during the pandemic was not significantly different from prior years. We speculated that we might see fewer patients with appendicitis during the pandemic, and that those we did see would present later in their disease course, as families wished to avoid hospital settings. Such a decrease in hospital utilization was seen in other settings during the pandemic, such as adults presenting with ST-segment elevation [13]. Meanwhile, the lay press has reported anecdotally that hospitals were seeing fewer non-COVID emergency patients, including those with surgical conditions [14]. Instead, we saw a similar number of cases of acute appendicitis during the pandemic as compared to prior years.
In considering only those patients who underwent appendectomy and were found to have simple appendicitis, our LOS was higher during the COVID-19 pandemic than during the same time period in 2019 (P < 0.01). However, both for patients we successfully treated nonoperatively and patients undergoing simple appendectomy, hospital stay was shorter than has been described in other series, where the median hospital stay was 37 h for NOM.
Outside the pandemic, we strive to offer patients with simple appendicitis a prompt operation, and are able to discharge the majority of patients following a short observation period after anesthesia. During the COVID-19 pandemic, we accepted this longer length of stay as we strove to limit operative resources. These were nuanced decisions taken on an individual patient basis, and could depend on the most urgent stressors on the hospital at different periods. By mid-May, the strain on our resources had diminished to where we reverted to TULA for the majority of patients.
The majority of patients we treated did not meet published guidelines for NOM of acute appendicitis, yet we were still successful in around 45% of patients. This is far lower than other series, where failure rates were below 30% with different NOM protocols. In treating each patient we were compelled to limit both time in hospital and the need for operative management. As such, we made decisions to promptly operate on patients with simple appendicitis who we deemed likely to fail NOM, so as to shorten their hospital course.
Unlike the published Midwestern Pediatric Surgery collaborative protocol, patients did not receive 48 h of IV antibiotics at the start of their treatment, as our aim was to minimize resource utilization and hospitalization time during the pandemic. The long-term efficacy of our treatment within the pandemic is not known, as our patient population and treatment strategy differed so markedly from published series and we have such short follow-up at this time. A high failure rate has been described in children with fecaliths treated nonoperatively [15]. Many patients will not develop recurrent symptoms following NOM under routine circumstances; within our cohort asymptomatic patients are being followed in clinic and via telehealth visits [16].
None of the children with COVID-19 in our cohort had respiratory symptoms, consistent with the generally mild disease course seen in children [17]. Although this cohort was treated prior to reports of Multisystem Inflammatory Syndrome in Children or Kawasaki-like disease in children associated with COVID-19 [18], we do not believe any such children are included in this study. Two children with COVID-19 completed NOM, while one underwent appendectomy for complicated appendicitis and had an unremarkable postoperative course. The safety of anesthesia in patients with minimal symptoms who test positive for COVID-19 is unclear; however, early results suggest there may be an increase in perioperative complications [19,20]. The overall safety of laparoscopy to the operating room team is also not yet completely known [9].
In conclusion, we found we could apply NOM to nearly half of patients presenting with acute appendicitis. We successfully did this with an abbreviated observation period and expanded inclusion criteria, thereby limiting operations and reducing length of stay as much as possible.
Management of any disease process requires a variety of personnel and materials, and during a pandemic these may be in varying supply at different times. We were able to safely deliver care throughout the pandemic, as material resource availability, COVID-19 testing capacity, and bed constraints shifted on a daily and even hourly basis. We hope our experience will never be repeated here or elsewhere, but if it should, we hope that this report may help other practitioners in the future.
Footnotes
Declaration of interest: none
Funding source: none
Acknowledgments: The Northwell COVID-19 Research Consortium
References
- 1.Somme S., Bronsert M., Morrato E., et al. Frequency and variety of inpatient pediatric surgical procedures in the United States. Pediatrics. 2013;132(6):e1466–e1472. doi: 10.1542/peds.2013-1243. [DOI] [PubMed] [Google Scholar]
- 2.Minneci P.C., Sulkowski J.P., Nacion K.M., et al. Feasibility of a nonoperative management strategy for uncomplicated acute appendicitis in children. J Am Coll Surg. 2014;219(2):272–279. doi: 10.1016/j.jamcollsurg.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maita S.A.B., Svensson J.F., Wester T. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261–269. doi: 10.1007/s00383-019-04610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minneci P.C., Mahida J.B., Lodwick D.L., et al. Effectiveness of patient choice in nonoperative vs surgical management of pediatric uncomplicated acute appendicitis. JAMA Surg. 2016;151(5):408–415. doi: 10.1001/jamasurg.2015.4534. [DOI] [PubMed] [Google Scholar]
- 5.Georgiou R., Eaton S., Stanton M.P., et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139(3) doi: 10.1542/peds.2016-3003. [DOI] [PubMed] [Google Scholar]
- 6.Lopez M.E., Wesson D.E. Nonoperative treatment of appendicitis—reply. JAMA Pediatrics. 2017;171(11):1127. doi: 10.1001/jamapediatrics.2017.2943. [DOI] [PubMed] [Google Scholar]
- 7.Minneci P.C., Hade E.M., Lawrence A.E., et al. Multi-institutional trial of non-operative management and surgery for uncomplicated appendicitis in children: design and rationale. Contemp Clin Trials. 2019;83:10–17. doi: 10.1016/j.cct.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney C., Kallis M., El-Shafy I.A., et al. Ultrasound-guided bilateral rectus sheath block vs. conventional local analgesia in single port laparoscopic appendectomy for children with nonperforated appendicitis. J Pediatr Surg. 2018;53(3):431–436. doi: 10.1016/j.jpedsurg.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Francis N.D.J., Cho E., et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. 2020;34:2327–2331. doi: 10.1007/s00464-020-07565-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen T.M.C., Kvasnovsky C., Rich B.S., et al. Academic Surgical Conference; Houston, Texas: 2020. Minimizing the spectrum and duration of antibiotics in children with complicated appendicitis. [Google Scholar]
- 11.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz H.M. The New York Times; 2020. Where have all the heart attacks gone? [Google Scholar]
- 15.Mahida J.B.L.D., Nacion K.M., et al. High failure rate of nonoperative management of acute appendicitis with an appendicolith in children. J Pediatr Surg. 2016;51:908–911. doi: 10.1016/j.jpedsurg.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Hall N.J., Eaton S., Stanton M.P., et al. Active observation versus interval appendicectomy after successful non-operative treatment of an appendix mass in children (CHINA study): an open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2(4):253–260. doi: 10.1016/S2468-1253(16)30243-6. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 18.Verdoni L.M.A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;10(1016):S0140–S6736. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R., Zhang Y., Huang L., et al. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67(6):655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;100331 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]