Abstract
An increasing body of evidence suggests a protective effect of some psychoactive substances against SARS-CoV-2 (Severe Acute Respiratory Syndrome coronavirus type 2). Recent findings suggest that patients with psychiatric disorders are less affected by SARS-CoV-2 than their caregivers, which may seem surprising given some of the frequent risk factors for an unfavorable course of the disease (e.g., obesity, diabetes, cardiovascular and pulmonary diseases).
We propose here a mixed pharmacoepidemiological and pharmacochemical hypothesis to explain these findings. A number of psychotropic drugs exhibit activities against coronaviruses (Middle East Respiratory Syndrome coronavirus (MERS-CoV), the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV-1) and the Infectious Bronchitis Virus (IBV)) and have been put forward as potentially anti-SARS-CoV-2. These treatments include numerous mee-too drugs (chemically and pharmacologically linked to those which have demonstrated anti-SARS-CoV-2 efficacy) which are frequently prescribed in psychiatric settings. Taken alone or in polypharmacy, these drugs could have a prophylactic anti-SARS-CoV-2 effect, explaining the unexpectedly low proportion of patients with psychiatric disorders and COVID-19. Associated factors such as nicotine can also be considered in the context of a broad chemoprophylactic hypothesis in patients with psychiatric disorders taking different psychoactive substances.
Introduction
The Severe Acute Respiratory Syndrome coronavirus type 2 (SARS-CoV-2) pandemic has sparked a race against time in order to identify curative treatments for this potentially severe and deadly infection. If many treatments are being evaluated, there is also a growing interest regarding several protective substances, which are already part of the drug regimen of many people.
Specifically, a large number of psychotropic drugs and psychoactive substances (e.g., nicotine) have been put forward as potentially effective [1] (see Table 1 ).
Table 1.
Relations between pharmacochemical data, pharmacoepidemiological data and antiviral activities of neurotransmitters modulators (psychotropics).
| Pharmacochemical class | Antiviral activity against |
|||
|---|---|---|---|---|
| SARS-CoV-1 | MERS-CoV | Others viruses | SARS-CoV-2 | |
| Phenothiazines# | ||||
| Chlorpromazine, 2% | [2], [3] | [2], [3] | [3] | [5] |
| Fluphenazine, 1% | [2], [3] | [2], [3] | [3] | |
| Promethazine, 0% | [2], [3] | [2], [3] | ||
| Tiethylperazine ‡ | [2], [3] | [3] | ||
| Triflupromazine ‡ | [2], [3] | [2], [3] | ||
| Cyamemazine, 20% | ||||
| Alimemazine, 14% | ||||
| Levomepromazine, 6% | ||||
| Propericiazine, 1% | ||||
| Pipotiazine, 1% | ||||
| Metopimazine, 0% | ||||
| Mequitazine † | ||||
| Thioxanthenes# | ||||
| Tiotixene ‡ | [2], [3] | [2], [3] | ||
| Flupentixol † | ||||
| Zuclopenthixol † | ||||
| Diphenylbutylpiperidines# | ||||
| Fluspirilene ‡ | [2], [3] | [2], [3] | ||
| Pimozide, 0% | [8] | |||
| Penfluridol † | ||||
| Loperamide,* 0% | [3] | [3] | [3] | |
| Butyrophenones# | ||||
| Haloperidol, 14% | [13], [14] | [8] | ||
| Astemizole † | [2], [3] | [2], [3] | ||
| Pipamperone † | ||||
| Imipramine derivates# (tricyclics antidepressants) | ||||
| Clomipramine, 2% | [2], [3] | [2], [3] | [3] | |
| Amitriptyline, 2% | ||||
| Atropine derivates# (anticholinergics) | ||||
| Benztropine, ‡ | [2], [3] | [2], [3] | [3] | |
| Tropatepine, 19% | ||||
| Biperiden, 2% | ||||
| Trihexyphenidyl, 9% | ||||
| Diphenylbutanamine# (antihistamines) | ||||
| Chlorphenoxamine, ‡ | [2], [3] | [2], [3] | ||
| Hydroxyzine, 9% | ||||
| Cetirizine, 2% | ||||
| Other compounds, 0% @ | ||||
| Others coumpounds | ||||
| Lithium, 4% | [1], [15] | [15] | ||
| Amantadine, 0% | [10]** | [10] | [10] | |
| Paroxetine, 6% | [16] | [9] | ||
| Melatonin † | [17] | [9] | ||
| Nicotine † | [4] | |||
| Cinanserin ‡ | [11] | [1] | ||
| Siramesine ‡ | [8] | |||
Prescriptions in French psychiatric hospitals [6]: 58% ofpatients with at least one of these treatments#; 38% of patients with at least one phenothiazine; 16% of patients with at least 1 phenothiazine + 1 anticholinergic; ‡ Not approved for use in France; † No data in Briet et al. [6]; Percentages represent the rate of prescription in French psychiatric hospitals [6]; * No central activity, but able to express morphine-like effect with e.g. quinine [18]; ** Possible antiviral activity; @ Brompheniramine, Chlorphenamine, Dexchlorpheniramine, Diphenhydramine, Doxylamine, Triprolidine, Desloratadine, Loratadine
The effectiveness of these substances has been linked to (i) an activity against different types of coronaviruses, i.e., the Middle East Respiratory Syndrome coronavirus (MERS-CoV), the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV-1) and the Infectious Bronchitis Virus (IBV) [2]; (ii) their utility against other viruses, which suggests a broad antiviral potential (e.g., hepatitis C virus, influenza viruses, Ebola virus or Chikungunya virus) [3]; and (iii) recent epidemiological data indicating a potential prophylactic effect of nicotine [4].
Recent pharmacoepidemiological data are also consistent with the hypothesis of a protective effect of psychoactive drugs in patients with psychiatric disorders. For instance, in Europe, an unexpectedly low proportion of patients with psychiatric disorders and COVID-19 (Coronavirus disease 2019) has been reported, even though these patients often have risk factors for an unfavorable course of the disease (e.g., obesity, diabetes, cardiovascular and pulmonary diseases) [5]. In addition, psychiatric caregivers seem to be more affected by the COVID-19 than psychiatric patients [5]; this has led some groups to consider the drug regimen of psychiatric patients as potentially effective in preventing poor COVID-19 outcomes. Chlorpromazine specifically has been put forward as a potential treatment for COVID-19, owing to its efficacy against other coronaviruses [2], [3], [5].
According to Dyall et al. [2], [3], among the compounds with demonstrated anti-SARS-CoV-1 and/or MERS-CoV activities, the most important therapeutic class is that involved in neurotransmitter systems (along with kinase inhibitors). Neurotransmitter modulators have also been shown to represent 41% and 24% of the substances with such activities, in 2014 and 2017 respectively [2], [3]. Hence psychoactive substances have the potential to become increasingly targeted as anti-SARS-CoV-2 agents.
In this comprehensive article, we propose a hypothesis that integrates the pharmacoepidemiologic and pharmacochemical available data in order to explain the under-representation of symptomatic and/or severe COVID-19 cases among patients with mental health disorders, and to pinpoint possible research and therapeutic options in the future.
Pharmacochemical hypothesis for SARS-CoV-2 prophylaxis with psychoactive compounds
Pharmacoepidemiologic based hypothesis
In France, COVID-19 psychiatric units did not run at full capacity and were not overwhelmed by the severity of the cases. This has raised the question of whether chlorpromazine had a prophylactic effect on COVID-19 in patients with psychiatric disorders [5]. However, this seems unlikely given that chlorpromazine is seldom prescribed in France (2% of hospital prescriptions in mental health in France) [6]. Rather, the data reported by Dyall et al. [2], [3] suggest that the anti-MERS-CoV and SARS-CoV efficacy of chlorpromazine is due to a class effect, since several phenothiazines have proven their effectiveness to this aim (i.e., fluphenazine, promethazine, tiethylperazine and triflupromazine). Thus, the first hypothesis raised here is that phenothiazines as a drug class might play a role in COVID-19 prophylaxis. This could explain the relatively low rates of symptomatic and severe COVID-19 cases in psychiatric settings in France, given that cyamemazine is the most prescribed drug by French hospital psychiatrists (20% of prescriptions) followed by alimemazine, a hypnotic phenothiazine (14% of prescriptions) [6]. Chlorpromazine is highly concentrated in the lungs and its antiviral activity seems in part due to an interference with clathrin-mediated endocytosis but also to additional post-entry effects on viral replication, which could both be shared by the other phenothiazines [2], [3], [5].
Pharmacochemical based hypothesis
The second hypothesis is that a common pharmacochemical determinant could be involved in a number of psychotropic drugs, which have anti-coronavirus effects. The treatments identified as having an anti-coronavirus effect by Dyall et al. [2], [3] can also lead to a more general pharmacochemical hypothesis of anti-SARS-CoV-2 chemoprophylaxis in patients with psychiatric disorders.
The compounds identified by Dyall et al. [2], [3] are indeed chemically related to each other as they belong to the same pharmacochemical class and are also often prescribed in mental health settings (for the French settings, see Table 1) [6]. On the other hand, most of the psychotropic drugs reported in Table 1 share a close structural relationship: a polycyclic part (blue) – a spacer (black) – a tertiary amine (red) (see Table 2 ). These common patterns could explain the anti-coronavirus effects of a large number of psychotropic drugs. By focusing on these patterns, different pharmacochemical classes can be identified as derivative forms; for instance, phenothiazines, tioxanthenes, diphenylbutylpiperidines, atropinics, butyrophenones, imipramine and diphenylbutanamine exhibit (i) a chemical structure sharing a certain degree of similarity as well as (ii) anti-coronavirus effects (see Table 2).
Table 2.
Structure-activity relationship (SAR) between some psychotropic drugs and perspectives with their potential anti-coronavirus efficacy.
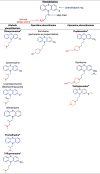 |
 |
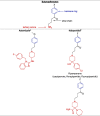 |
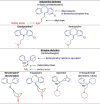 |
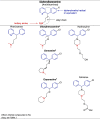 |
Integrating pharmacoepidemiologic and pharmacochemical data: The chemoprophylaxis hypothesis
Another hypothesis is based on a potential multifactorial chemoprophylaxis in mental health patients. According to this hypothesis, anti-SARS-CoV-2 prophylaxis could arise from:
-
(1)
Several antiviral psychotropic agents. A large multicenter study carried out in France (n = 7278 prescriptions in 34 French psychiatric facilities) [6] found a high percentage of patients (58%) with at least one treatment from the aforementioned pharmacological classes (drugs with demonstrated anti-SARS-CoV and/or MERS-CoV activity and mee-too drugs); 38 and 16% of them had respectively at least a prescription of phenothiazine, or a phenothiazine and an anticholinergic drug.
-
(2)
Several antiviral psychotropic agents + nicotine or tobacco use, as tobacco and nicotine substitutes are widely used in mental health settings [7].
Discussion and conclusion
Overall, anti-SARS-CoV-2 chemoprophylaxis activity seems to be linked to several psychotropic drugs which are structurally-related, such as (1) phenothiazine derivates (e.g., chlorpromazine and cyamemazine), tioxanthene derivates (e.g., flupentixol and zuclopenthixol), (2) diphenylbutylpiperidine derivates (e.g., pimozide and penfluridol), (3) atropinic derivates with anticholinergic drugs (e.g., benztropine, trihexyphenidyl or biperiden), (4) butyrophenones derivates (e.g., haloperidol and pipamperone), (5) imipramine derivates (e.g., clomipramine and amitryptiline), and (6) diphenylbutanamine derivates (e.g., antihistamine drugs, such as hydroxyzine) (see Table 2).
However, anti-SARS-CoV-2 chemoprophylaxis cannot be attributed to psychotropic drugs in general, since a number of psychotropic drugs, such as olanzapine and valproic acid, have been found to be ineffective [8]. Moreover, anti-SARS-CoV-2 chemoprophylaxis is not solely mediated by structure–activity relationship similarity as some drugs, which do not share structure–activity relationships seem to have an anti-SARS-CoV-2 effect. This is the case, for instance, of lithium, paroxetine, nicotine, melatonin, cinanserin, siramesine, amantadine and other anandamide derivatives [1], [4], [8], [9], [10], [11].
Given this, we argue that the chemoprophylactic hypothesis better explains the myriad of findings that have arisen in the last months, suggesting an anti-SARS-CoV-2 effect of psychotropic drugs and psychoactive substances. Another potential explanation is that related to a multifactorial origin, i.e., based on the additive or synergistic efficacy of several psychoactive compounds.
To better understand the relationship between psychiatric disorders, psychoactive compounds, and COVID-19, we have first to determine if patients with psychiatric disorders are indeed less infected than their caregivers and the general population. Indeed, it is also possible that patients with psychiatric disorders have similar seroprevalence rates but rarely develop symptomatic and severe COVID-19 forms. To acknowledge both possibilities and further elucidate whether psychotropic drugs have an antiviral activity or not, SARS-CoV-2 seroprevalence studies are warranted.
In addition, COVID-19 pathophysiology is still poorly understood, especially in terms of its potential deleterious effects on immune response [12]. Whether antiviral agents limit viral replication in the first stages of the infection and prevent its severe outcomes, is still unknown.
Perspectives
Further, docking – molecular modeling – could be a useful option in order to further understand the link between psychotropics classes with anti-coronavirus effects and the different SARS-CoV-2 proteins.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- 1.Javelot H, Llorca PM, Meyer G, Fossati P, Haffen E. Challenges for psychotropics in the context of the SARS-Cov-2 pandemic. Encephale. 2020 Apr 23. pii: S0013-7006(20)30077-4. https://doi.org/10.1016/j.encep.2020.04.009. [DOI] [PMC free article] [PubMed]
- 2.Dyall J., Coleman C.M., Hart B.J. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyall J., Gross R., Kindrachuk J. Middle east respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77(18):1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farsalinos K., Niaura R. J.Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep. 2020 doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plaze M, Attali A, Petit AC, et al. Repurposing of chlorpromazine in COVID-19 treatment: the reCoVery study. Encephale. 2020 Apr 29. pii: S0013-7006(20)30079-8. https://doi.org/10.1016/j.encep.2020.04.010.
- 6.Briet J., Javelot H., Heitzmann E., Weiner L., Lameira C., D’Athis P., Corneloup M., Vailleau J.-L. The anticholinergic impregnation scale: towards the elaboration of a scale adapted to prescriptions in French psychiatric settings. Therapies. 2017;72(4):427–437. doi: 10.1016/j.therap.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Kagabo R., Gordon A.J., Okuyemi K. Smoking cessation in inpatient psychiatry treatment facilities: a review. Addict Behav Rep. 2020;11 doi: 10.1016/j.abrep.2020.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon D.E., Jang G.M., Bouhaddou M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abreu G.E.A., Aguilar M.E.H., Covarrubias D.H., Durán F.R. Amantadine as a drug to mitigate the effects of COVID-19. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L., Gui C., Luo X., Yang Q., Günther S., Scandella E. Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J Virol. 2005;79(11):7095–7103. doi: 10.1128/JVI.79.11.7095-7103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wunderlich V., Fey F., Sydow G. Antiviral effect of haloperidol on Rauscher murine leukemia virus. Arch Geschwulstforsch. 1980;50(8):758–762. [PubMed] [Google Scholar]
- 14.De Voss J.J., Sui Z., DeCamp D.L., Salto R., Babé L.M., Craik C.S. Haloperidol-based irreversible inhibitors of the HIV-1 and HIV-2 proteases. J Med Chem. 1994;37(5):665–673. doi: 10.1021/jm00031a017. [DOI] [PubMed] [Google Scholar]
- 15.Bou K.R. Lithium chloride combination with rapamycin for the treatment of COVID-19 pneumonia. Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbahesh H., Gerlach T., Saletti G., Rimmelzwaan G.F. Response modifiers: tweaking the immune response against influenza a virus. Front Immunol. 2019;10:809. doi: 10.3389/fimmu.2019.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeque A.J., Wandel C., He H., Shah S., Wood A.J. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68(3):231–237. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]


