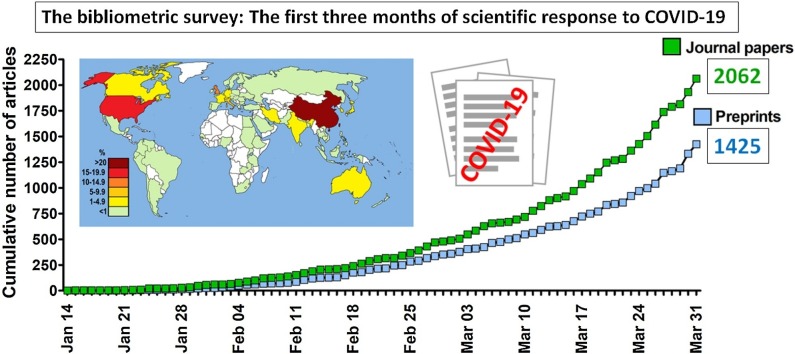
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Emerging infectious disease, Bibliometric analysis, Academia
Highlights
-
•
A bibliometric study on the first 3 months of research on COVID-19 was performed.
-
•
A total of 2062 articles in 578 journals and 1425 preprints were identified.
-
•
The scientific contributions from 73 countries were seen.
-
•
The highest number of original research emerged from China.
-
•
The scientific response to COVID-19 is unprecedented.
Abstract
Here we present the results of a bibliometric survey of peer-reviewed and pre-print papers published in the English language on issues related to COVID-19 within the first three months since a cluster of a severe acute respiratory disease of unknown etiology was officially confirmed by the Chinese Center for Disease Control and Prevention on 31 December 2019. A systematic search using PubMed/Medline and Scopus databases and preprint servers was performed. The articles were classified according to their type, subject and country of origin. Up to 31 March 2020, a total of 2062 papers published in 578 peer-reviewed journals and 1425 preprints posted mostly on medRxiv (55.4 %), were identified. The mean number of published journal papers and preprints per day in the considered period was 27 and 12, respectively, and reached a maximum of 51 and 46 per day in March, respectively. The identified articles, journal papers and preprints, mostly covered the epidemiology of COVID-19 (35.7 %), clinical aspects of infection (21.0 %), preventative measures (12.8 %), treatment options (12.5 %), diagnostics (12.2 %), mathematical modeling of disease transmission and mitigation (9.6 %), and molecular biology and pathogenesis of SARS-CoV-2 (8.7 %). The majority of the journal papers were commentaries (38.5 %), reviews (33.6 %) and original research (21.3 %), while preprints predominantly presented original results (89.8 %). Chinese scientists contributed the highest share of original research and were responsible for 32.9 % journal papers and 43.9 % preprints published in the considered period. A high number of contributions was also seen from the United States, the United Kingdom, and Italy. The benefits and potential risks of such a massive publication output are discussed. The scientific response seen during the first 3 months of the COVID-19 outbreak is a demonstration of the capabilities of modern science to react rapidly to emerging global health threats by providing and discussing the essential information for understanding the etiological factor, its spread, preventative measures, and mitigation strategies.
1. Introduction
The novel infectious respiratory disease COVID-19 caused by betacoronavirus SARS-CoV-2 (provisionally known as 2019-nCoV) emerged at the end of 2019 in Wuhan, China, and later spread to other Asian countries as well as Africa, Australia, Europe, North and South America. The World Health Organization first declared a Public Health Emergency of International Concern on January 30, 2020 [1], and later announced a pandemic on March 11, 2020 [2]. The evolving situation received worldwide mass media coverage, accompanied by a spread of misinformation mostly through social media, and eventually triggered a backlash of global fear and panic [[3], [4], [5], [6]]. However, at the same time, an unprecedented response of the scientific community was seen. On 31 December 2019, the Wuhan Municipal Health Commission officially reported a cluster of 27 pneumonia cases of unknown etiology; by 10 January 2020, a complete genome sequence was publicly released by a team of Chinese researchers (Wuhan-Hu-1, GenBank accession number MN908947) [7]. For comparison, when an outbreak of severe acute respiratory syndrome (SARS) emerged in China in November 2002 [8], the full-length sequencing of the genome of its etiological agent, SARS-CoV-1 strain, was not available until April 14, 2003 [9,10]. The genome sequencing of SARS-CoV-2 was a pivotal step to developing diagnostic assays and initiating research on viral molecular biology. The isolation and successful passaging of the coronavirus in cell cultures, first achieved in late January 2020 by Australian researchers, allowed for direct investigations of the viral mechanism of cellular infection followed by experimental studies on target therapeutic agents. An interactive online dashboard hosted and updated daily by Johns Hopkins University was developed to track the daily global COVID-19 situation [11]. At the same time, vaccine development was initiated, and as announced at the beginning of April, nearly eighty vaccine candidates are in the pipeline, five of which are already reported to have entered Phase 1 clinical trials in early April 2020 – the first one, mRNA-1273 developed by US-based Moderna Therapeutics was administrated to volunteers as early as 16 March 2020 [12].
The present study aimed to assess the scientific response to the COVID-19 pandemic by performing a bibliometric survey. A systematic search of English language papers indexed in the PubMed/Medline and Scopus databases as well as posted on online preprint servers since the beginning of the outbreak in China till the end of March 2020 was conducted. The number of identified COVID-19 cases on 31 March 2020 was 863,184 with 43,407 reported deaths, and infection confirmed in over 160 countries [13]. The results demonstrated by the present study clearly show that modern science has all the tools to respond to emerging threats to human health by providing fundamental data that is pivotal for their mitigation.
2. Material and methods
The systematic search for English language peer-reviewed papers on SARS-CoV-2 and COVID-19 was performed using PubMed/Medline and Scopus databases. The former is the largest abstract database, frequently accessed by biomedical specialists and other scientists, whereas the latter is an extensive, multidisciplinary search system that currently covers over 41,100 journals. The following keywords were used: ‘SARS-CoV-2’, ‘2019-nCoV’, and ‘COVID-19’. Each identified item was then verified by researchers to ensure it was related to COVID-19 and SARS-CoV-2. Based on content, the papers were classified into one of the following types: (i) original research, (ii) case report, (iii) review, and (iv) commentary. The papers were also grouped according to their subject into (i) molecular biology and pathogenesis of SARS-CoV-2, (ii) origin of SARS-CoV-2, (iii) bioinformatics and computational biology, (iv) infection diagnostics, (v) preventive measures of infection, (vi) COVID-19 treatment options, (vii) clinical aspects of COVID-19, (viii), epidemiology of COVID-19, (ix) mathematical modeling of COVID-19 transmission and mitigation, (x) social distancing and mental health, and (xi) misinformation and panic related to COVID-19. In the case of a paper’s subject lying outside these categories, it was classified as ‘others’. The main country from which each article originated was identified based on the affiliation of the corresponding author(s). If the paper was published as a statement of a scientific society, the country in which such an organization was based was considered. Corrigenda were excluded. A similar bibliometric survey of pre-prints was conducted using arXiv, bioRxiv, medRxiv, and MDPI Preprints servers. If the corresponding author of the preprint paper was not indicated, the affiliation of all the authors was taken into account.
3. Results and discussion
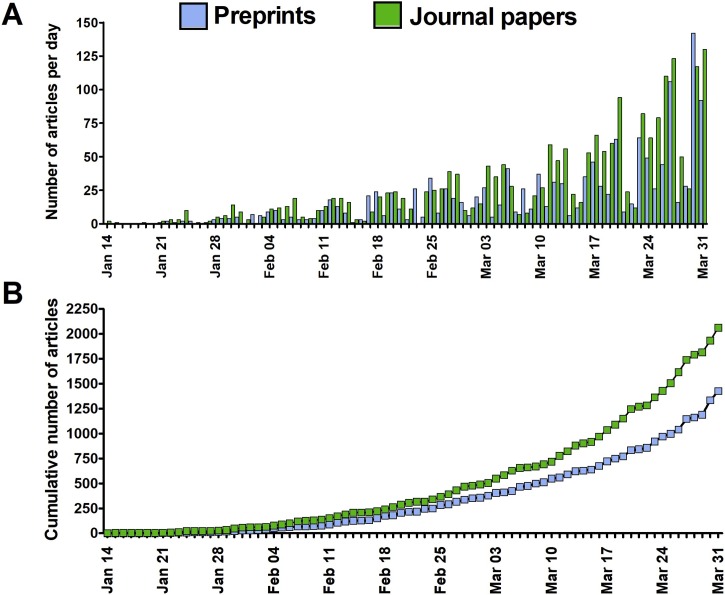
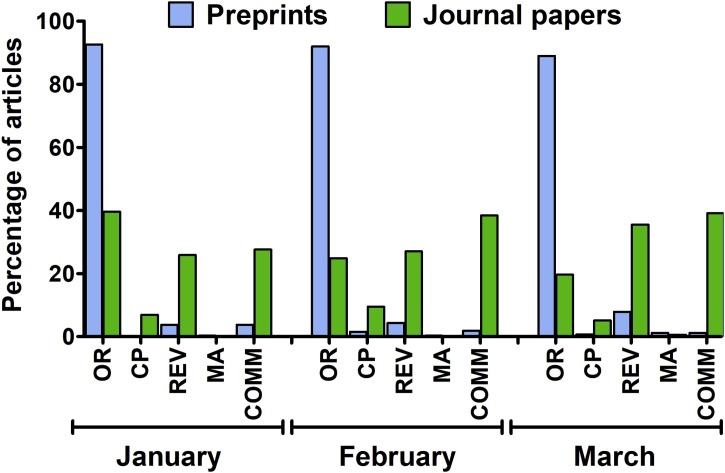
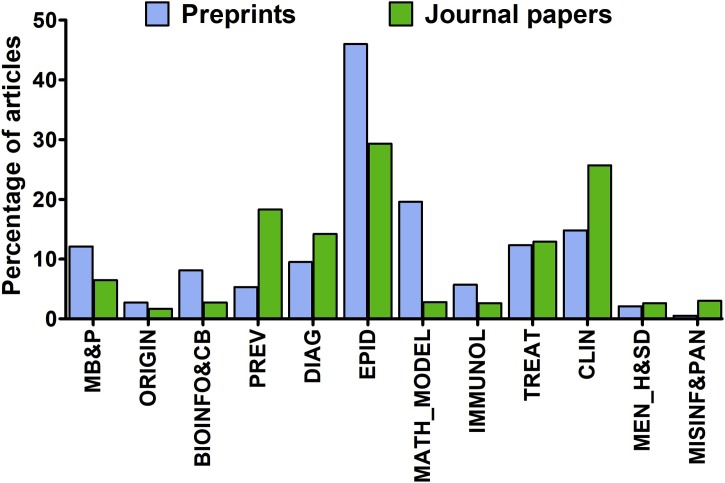
A total of 2062 peer-reviewed English-language papers related to SARS-CoV-2 and COVID-19 published in 578 journals were identified. The first two articles were published on 14 January 2020 [14,15]. The majority of papers - 76.8 % - were made available throughout March 2020 (Fig. 1 ) and as much as 60.0 % were published after WHO declared the epidemiological situation as pandemic on March 11. The mean number of articles per day published in the considered period was 22.6 and increased from 1.9 per day in January, through 14.5 per day in February, and up to 51.1 per day in March. The highest number of articles was published by The British Journal of Medicine (n = 131), The Lancet (n = 91) and the Journal of Medical Virology (n = 78). Commentaries and review articles were most prevalent, constituting 38.5 and 33.6 %, respectively, followed by original research (21.3 %) and case reports (6.1 %). Original research papers were, however, the most prevalent during the first month of response to the COVID-19 outbreak (Fig. 2 ). During the considered period essential data regarding the taxonomical classification of SARS-CoV-2 [16], its natural origin [17], mechanism of cellular entry [18,19] and routes of transmission [[20], [21], [22]], estimation of basic reproduction number [23,24], the clinical presentation of COVID-19 [[25], [26], [27]] and potential drug treatment options [28,29] were published. Papers reporting on over 80 clinical trials on drug repositioning launched in China as a possible treatment of SARS-CoV-2 infection, as well as the result of the first clinical trial, were also published [[30], [31], [32], [33]]. Overall, ten meta-analyses (constituting 0.5 % of all published papers) were published with the first one, reporting on the clinical characteristics of SARS-CoV-2 infection, made available on 28 February in the Journal of Medical Virology [34]. When classified by subject, the majority of identified articles were related to epidemiology, clinical aspects, prevention and diagnostics (Fig. 3 ). Additionally, 2.3 % of papers pertaining to epidemiology, symptomatology, and treatment of children were identified. Other subjects discussed by published articles were related to economic impacts (2.7 %), COVID-19 discrimination (0.68 %), policy issues (0.63 %), and impacts on education and research (0.29 %). Contributions from authors located in 63 countries were noted, with most of the papers originating from researchers in China (32.9 %), the United States (18.0 %), the United Kingdom (12.2 %) and Italy (7.0 %). Among these four, Chinese researchers had the greatest share in original research papers, 57.7 % - compared to 9.3 % for the USA, 3.2 % for Italy and 2.9 % for the UK.
Fig. 1.
The daily (A) and cumulative (B) total number of English-language peer-reviewed articles and preprints published on SARS-CoV-2 (2019-nCoV) and COVID-19 till 31 March 2020.
Fig. 2.
The distribution of particular types of English-language peer-reviewed articles (n = 2062) and preprints (n = 1425) published on SARS-CoV-2 (2019-nCoV) and COVID-19 between January and March 2020. OR – original research; CP – case reports; REV – review; MA – meta-analysis; COMM – commentary.
Fig. 3.
The subject area of English-language peer-reviewed articles (n = 2062) and preprints (n = 1425) published on SARS-CoV-2 (2019-nCoV) and COVID-19; MB&P – molecular biology and pathogenicity of SARS-CoV-2; ORIGIN – origin of SARS-CoV-2; BIOINFO&CB – bioinformatics and computational biology; PREV – preventive measures in COVID-19; DIAG – diagnostics of infection; EPID – epidemiology of COVID-19; MATH_MODEL – mathematical modeling of COVID-19 transmission and mitigation; IMMUNOL – immunology of COVID-19; TREAT – treatment of COVID-19, CLIN – clinical issues related to COVID-19, MEN_H&SD – mental health effects of social distancing; MISINF&PAN – misinformation and panic associated with COVID-19.
The bibliometric survey conducted by the present study highlights the profound activity of the scientific community and its pivotal role in elucidating the nature of the COVID-19 outbreak and its mitigation. For comparison, the outbreak of Severe Acute Respiratory Syndrome (SARS) that started in November 2002, affected over 8000 individuals, and whose last cases, associated with exposures during laboratory procedures, were reported in May 2004 [35,36], resulted in the publication of 2466 papers in the 2002–2004 period indexed in the PubMed/Medline database and searchable using the keyword 'SARS'. In turn, a search of the same database with the key term 'MERS', relating to the Middle East Respiratory Syndrome, whose first cases were reported in September 2012, affected nearly 2500 subjects and revealed a high mortality rate of 34.4 % [37,38], yields 5563 papers published in the 2012–2019 period. Furthermore, the number of publications devoted to COVID-19 and considered in the present bibliometric study accounts for 23.4 % of all PubMed/Medline-indexed papers related to the Ebola virus (total of 8802 articles searchable with the key term ‘Ebola’ till the end of 2019), whose first outbreaks date back to 1976 [39]. One should, however, note that Ebola virus outbreaks were mostly limited to Western and Central Africa countries with isolated cases recorded in the USA, UK, Italy and Spain [40]. In turn, the vast majority of MERS cases have been associated with the Arabian Peninsula, although as a result of travel, its etiological agent MERS-CoV was also exported across the Middle East, Asia, North Africa and Europe and reported by 27 countries to date [41,42]. Nevertheless, the response to COVID-19, seen during only the first 3 months since it was reported by Chinese officials, marks the unprecedented advances and capabilities of modern science for a joint reaction to an emerging health threat. This reaction has also been supported by initiatives such as those of the Wellcome Trust, a research-charity based in London, whose statement on ensuring access to data and research findings on COVID-19 has gathered over 100 signatories, including leading publishers [43]. Consequently, various publishers (e.g., Elsevier, Springer Nature, Taylor and Francis) and journals (e.g., The British Medical Journal, Journal of the American Medical Association, Nature, New England Journal of Medicine, The Lancet) have made content related to COVID-19 freely available to the public and created continuously updated article collections and resources [44].
Online publication of a preprint, an early version of a scientific article, enables the dissemination of findings, commentaries and critical reviews on an openly accessible platform in advance of the often slow and laborious peer-review process [45]. Under a rapidly evolving epidemiological scenario, preprints provide a possibility to swiftly report results related to therapeutic strategies and preventative measures, which may have a significant value for effective containment. Up to 31 March 2020, 1425 items related to SARS-CoV-2 and COVID-19 had been posted on arXiv, bioRxiv medRxiv and MDPI Preprints servers were identified (Fig. 1). The first preprint was posted on 19 January 2020 and provided a mathematical model for simulations of SARS-CoV-2 transmission, later published in Infectious Diseases of Poverty on 28 February 2020 [46]. The majority of identified preprints (75.3 %) were posted in March with 58.4 % of all considered preprints posted after WHO declared a pandemic on March 11. The mean number of items made available per day in the considered period was 11.8 and increased from 0.9 and 11.2 per day in January and February, respectively, up to 46.0 per day in March. The majority of identified preprints were posted on medRxiv (55.4 %), followed by arXiv (16.6 %), bioRxiv (16.1 %) and MDPI Preprints (11.8 %). The vast majority of preprints (89.8 %) reported original research results with case reports and commentaries having the lowest share (0.8–1.4 %) throughout the considered period (Fig. 2). The most frequently covered subjects were related to the epidemiology of COVID-19, modeling its further transmission, treatment options, and clinical manifestation of the disease (Fig. 3). Overall, preprints originated from authors located in 56 countries with researchers from China (43.9 %), the United States (20.1 %) and the United Kingdom (5.8 %) having the greatest contribution in the number of posted items. The preprints reporting original research data originated mostly from China (42.4 %) and to lesser extent from the United States (15.8 %), the United Kingdom (4.5 %), and Italy (4.2 %).
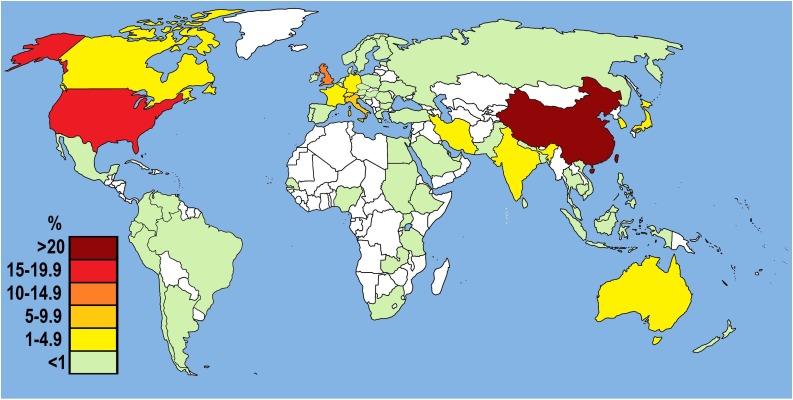
Fig. 4 shows the contributions by country of corresponding authors of papers in peer-reviewed journals and preprints published between January and March 2020. Scientists from 73 countries made contributions, with Chinese researchers having a 37.3 % share in total output. Authors based in the United States and Canada generated 9.4 and 2.3 % of all papers and preprints, respectively. Member States of the European Union with the United Kingdom were responsible for 22.6 % of published articles. The Middle East countries had a 3% share with decidedly the highest number of articles found for Iran (50). Publications from South America accounted for 1.7 % of total papers and preprints, with the highest number originating from Brazilian (30 articles) and Colombian (18 articles) researchers. In turn, African countries had a 0.5 % share in the total number of published papers and preprints; the highest number of these items originated from Nigeria and South Africa – 6 and 5, respectively.
Fig. 4.
The distribution of corresponding author(s) of English-language peer-reviewed articles (n = 2062) and preprints (n = 1425) published on SARS-CoV-2 (2019-nCoV) and COVID-19 between January and March 2020.
Although the history of preprints dates back to the 1960s [47], they have become increasingly distributed in an electronic form since the early 1990s. The COVID-19 pandemic was the first epidemiological event to have experienced such an enormous research response also involving the publication of preprints. The popularity of preprints is a result of growing global access to the Internet and the development of online publishing [48]. During the SARS epidemic in 2002–2004, bioRxiv, medRxiv and MDPI preprint platforms were not available, while only four preprints on this outbreak were posted on arXiV – as compared to 237 articles published on the same server during the first three months of SARS-CoV-2 spread.
There is no doubt that under a pandemic scenario, online preprints are an important medium of dissemination of critical data essential for understanding and forecasting the epidemiological dynamics, implementation of containment strategies, and effective diagnostics and therapies. This is further highlighted by the contrasting difference in the percentage of original research published as peer-reviewed papers and posted as preprints between January and March 2020 (Fig. 2). One must, however, acknowledge that they increase the risk of making false information public as they are posted without any independent quality control. Considering the daily coverage that COVID-19 receives from global mass media, erroneous data and conclusions can be rapidly replicated outside scientific sources and lead to public misinformation and/or a backlash of panic. This is despite clear warnings announced by preprint websites during the COVID-19 pandemic that all published items constitute preliminary reports that should not be relied on to guide clinical practice or health-related behaviors and should not be reported in news media as established information.
All in all, the emphasis should be placed on the responsibility of the entire scientific community for a critical assessment of posted preprints during a pandemic or other global threats in the future. This process can be facilitated, inter alia, through a commentary section available for each published item. To support this, the journal Nature and the Wellcome Trust have launched Outbreak Science Rapid PREreview, an open-source platform for a rapid review of preprints related to emerging outbreaks that allows researchers with an ORCID identification number to rate preprints by answering yes-or-no questions and optionally to express their comments [49]. The COVID-19 pandemic has seen at least one good example of such a rapid reaction when a preprint describing structural and genetic features of the spike glycoprotein of SARS-CoV-2, in which the authors unjustifiably and incautiously suggested that the similarities between the novel coronavirus and HIV are “uncanny” and “unlikely to be fortuitous” [50]. Due to the number of researchers commenting on the limitations of the conducted analysis, which led to a pure coincidence in reported similarities and inappropriate conclusions potentially implying that the SARS-CoV-2 was engineered, the authors decided to withdraw the preprint before it received larger attention from major news media. Nevertheless, it has fueled harmful conspiracy theories according to which a novel coronavirus strain was purposely engineered as a biological weapon [51].
To speed up the process of publication in a scientific journal, one can submit results presented in the form of a Letter to the Editor, which, depending on the journal’s policy, may or may not undergo a peer-review. However, even if the former is the case, the manner in which such letters are prepared can often facilitate a swift review and publication. Therefore, this method of reporting was often practiced in the considered period of January-March 2020 to publish original information, e.g., clinical characteristics of the first COVID-19 cases in various geographical locations [52,53], detection of SARS-CoV-2 in induced sputum of patients considered to be clinically cured and revealing multiple negative throat swabs [54], the ineffectiveness of a symptom-based screening process in detecting SARS-CoV-2 infection in evacuated travelers [55]. In selected cases, the publication timeline of such papers was very short. For example, a letter in the New England Journal of Medicine, that reported asymptomatic transmission of SARS-CoV-2 in Germany was published on 30 January 2020. This must have been submitted very shortly before publication if one considers that the authors describe patients tested on 28 January [56]. One should also note that in response to COVID-19, selected journals, such as the Royal Society of Open Science, decided to maximally shorten the peer-review process and recruited a team of reviewers willing to provide their reports on submitted manuscripts within 24−48 h [57]. An extraordinary timeline of peer-review was seen in the case of a manuscript reporting the results of an open-label non-randomized clinical trial of hydroxychloroquine and azithromycin as a treatment for COVID-19 - it was submitted to the International Journal of Antimicrobial Agents on 16 March 2020, accepted a day later and published online on 20 March [33]. Both aforementioned articles (a correspondence in the New England Journal of Medicine, as well as clinical trial on COVID-19 treatment), have received critical comments clearly pointing out that pressure to review and publish quickly during an epidemic also results in a more uncertain quality of the data made public [58,59]. It must, however, be stated that with such scientific activity as seen in the case of COVID-19, the reassessment of biased and inaccurate information could be provided in a similarly swift manner thereby slowing the escalation of impacts. Nevertheless, the rigorous peer-review standards and editorial practices are the core to ensure high quality research and best way to avoid the dissemination of erroneous, inaccurate or inconclusive data [60].
The COVID-19 outbreak has revealed the potential of modern global science to respond rapidly and on multiple levels – by providing and discussing the data essential for epidemiological models, implementation of preventative measures, clinical procedures, treatment, and vaccine development as well as accessory information crucial for understanding the impacts of a pandemic on the human population. The rush to report and publish in journals and in the form of preprints preceding peer-review increases the risk of biased or unreliable information being disseminated. This, however, must be considered as a side-effect that is difficult to avoid (also under any other circumstances than pandemic) and can be counteracted by responsible attention from the scientific community providing critical comments and assessments. Weighing the pros and cons, it is beyond any doubt that a rapid joint-response of researchers to emerging health threats is the core of their alleviation. This also highlights the role that can be played by science in minimizing the effects of other future threats, including the climate crisis, if only the voice of researchers is acknowledged by political leaders and stakeholders.
4. Conclusions
The present bibliometric study clearly shows the capabilities of modern science to respond to an emerging health crisis, and most likely, to any other future threats. Using a broad range of scientific journals as well as different online preprint servers, over 3400 manuscripts were published within the first three months of the outbreak of COVID-19 in Wuhan, China. The journal papers and preprints covered all essential aspects related to understanding the epidemic, its etiological factor, transmission routes, diagnostics, preventive measures, treatment options, and clinical manifestation of the disease. The scientific community contributed by generating original results and also by reviewing the existing literature and providing commentaries on different aspects of the ongoing outbreak. Chinese, US, UK, and Italian researchers were the most active in the considered period, although journal papers and preprints were contributed by corresponding authors affiliated in over 60 and 50 countries, respectively. As highlighted, such a massive inflow of manuscripts/preprints and pressure to review and publish quickly during an epidemic may, in certain instances, result in a more uncertain quality of the data made public. The present bibliometric survey underlines that science plays a key role in response to emerging global threats – a notion that has to be considered and acknowledged by political leaders regarding other future risks, be they health-related or not, to the human population.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. (Lond. Engl.) 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzymski P., Nowicki M. Preventing COVID-19 prejudice in academia. Science. 2020;368(6484):1313. doi: 10.1126/science.abb4870. [DOI] [PubMed] [Google Scholar]
- 4.COVID-19: fighting panic with information. Lancet. 2020;395(10224):537. doi: 10.1016/S0140-6736(20)30379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manderson L., Levine S. COVID-19, risk, fear, and fall-out. Med. Anthropol. 2020:1–4. doi: 10.1080/01459740.2020.1746301. [DOI] [PubMed] [Google Scholar]
- 6.Mian A., Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89. doi: 10.1186/s12916-020-01556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y.-Z. Novel 2019 coronavirus genome. Virological. 2020 http://virological.org/t/novel-2019-coronavirus-genome/319 (Accessed 12 April 2002 2020) [Google Scholar]
- 8.Hawkey P.M., Bhagani S., Gillespie S.H. Severe acute respiratory syndrome (SARS): breath-taking progress. J. Med. Microbiol. 2003;52(Pt 8):609–613. doi: 10.1099/jmm.0.05321-0. [DOI] [PubMed] [Google Scholar]
- 9.CDC . 2003. CDC Lab Sequences Genome of New Coronavirus.https://www.cdc.gov/media/pressrel/r030414.htm (Accessed 12 April 2020 2020) [Google Scholar]
- 10.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 11.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 13.2020. Worldometer.https://www.worldometers.info/coronavirus/ (Accessed 25 April 2020) [Google Scholar]
- 14.Bogoch I.I., Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J. Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., V. Coronaviridae Study Group of the International Committee on Taxonomy of The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C.-w., Liu X.-f., Jia Z.-f. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Lou J., Bai Y., Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am. J. Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D., Li H., Lu X.X., Xiao H., Ren J., Zhang F.R., Liu Z.S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr.: WJP. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B., Wang X., Li Q., Bragazzi N.L., Tang S., Xiao Y., Wu J. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (Lond. Engl.) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia Ja., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.J., Zhong N.S. Clinical characteristics of Covid-19 in China. Reply. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 28.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 29.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-a possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Publica. 2020;44 doi: 10.26633/RPSP.2020.40. e40-e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T., He Y., Xu W., Ma A., Yang Y., Xu K.-F. Clinical trials for the treatment of coronavirus disease 2019 (COVID-19): a rapid response to urgent need. Sci. China Life Sci. 2020;63(5):774–776. doi: 10.1007/s11427-020-1660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q., Wang Y. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020;92:540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sun K., Chen J., Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit. Health. 2020;2:e201–e208. doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim P.L., Kurup A., Gopalakrishna G., Chan K.P., Wong C.W., Ng L.C., Se-Thoe S.Y., Oon L., Bai X., Stanton L.W., Ruan Y., Miller L.D., Vega V.B., James L., Ooi P.L., Kai C.S., Olsen S.J., Ang B., Leo Y.-S. Laboratory-acquired severe acute respiratory syndrome. N. Engl. J. Med. 2004;350(17):1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 36.Sampathkumar P., Temesgen Z., Smith T.F., Thompson R.L. SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin. Proc. 2003;78(7):882–890. doi: 10.4065/78.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry H., Bakhrebah M.A., Abdulaal W.H., Zamzami M.A., Baothman O.A., Hassan M.A., Zeyadi M., Helmi N., Alzahrani F., Ali A., Zakaria M.K., Kamal M.A., Warsi M.K., Ahmed F., Rasool M., Jamal M.S. Middle East respiratory syndrome: pathogenesis and therapeutic developments. Future Virol. 2019;14(4):237–246. doi: 10.2217/fvl-2018-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Costa V.G., Moreli M.L., Saivish M.V. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch. Virol. 2020;165:1517–1526. doi: 10.1007/s00705-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malvy D., McElroy A.K., de Clerck H., Günther S., van Griensven J. Ebola virus disease. Lancet. 2019;393(10174):936–948. doi: 10.1016/S0140-6736(18)33132-5. [DOI] [PubMed] [Google Scholar]
- 40.Feldmann H., Sprecher A., Geisbert T.W. Ebola. N. Engl. J. Med. 2020;382(19):1832–1842. doi: 10.1056/NEJMra1901594. [DOI] [PubMed] [Google Scholar]
- 41.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle east respiratory syndrome. Lancet (Lond. Engl.) 2020;395(10229):1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleibtreu A., Bertine M., Bertin C., Houhou-Fidouh N., Visseaux B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV) Med. Mal. Infect. 2020;50(3):243–251. doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellcome Trust . 2020. Sharing Research Data and Findings Relevant to the Novel Coronavirus (COVID-19) Outbreak.https://wellcome.ac.uk/coronavirus-covid-19/open-data (Accessed 25 April 2020) [Google Scholar]
- 44.Calling all coronavirus researchers: keep sharing, stay open. Nature. 2020 doi: 10.1038/d41586-020-00307-x. https://www.nature.com/articles/d41586-020-00307-x (Accessed 25 April 2020) [DOI] [PubMed] [Google Scholar]
- 45.Fry N.K., Marshall H., Mellins-Cohen T. In praise of preprints. Microbiology. 2019;165(5):489–491. doi: 10.1099/mic.0.000785. [DOI] [PubMed] [Google Scholar]
- 46.Chen T.-M., Rui J., Wang Q.-P., Zhao Z.-Y., Cui J.-A., Yin L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty. 2020;9(1):24. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobb M. The prehistory of biology preprints: a forgotten experiment from the 1960s. PLoS Biol. 2017;15(11) doi: 10.1371/journal.pbio.2003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maslove D.M. Medical preprints—a debate worth having. JAMA. 2018;319(5):443–444. doi: 10.1001/jama.2017.17566. [DOI] [PubMed] [Google Scholar]
- 49.Johansson M.A., Saderi D. Open peer-review platform for COVID-19 preprints. Nature. 2020;579:29. doi: 10.1038/d41586-020-00613-4. [DOI] [PubMed] [Google Scholar]
- 50.Pradhan P., Pandey A.K., Mishra A., Gupta P., Tripathi P.K., Menon M.B., Gomes J., Vivekanandan P., Kundu B. 2020. Uncanny Similarity of Unique Inserts in the 2019-nCoV Spike Protein to HIV-1 gp120 and Gag. bioRxiv, 2020.01.30.927871. [Google Scholar]
- 51.Tyler D. 2020. Coronavirus Contains "HIV Insertions", Stoking Fears Over Artificially Created Bioweapon.https://www.zerohedge.com/geopolitical/coronavirus-contains-hiv-insertions-stoking-fears-over-artificially-created-bioweapon (Accessed 22 April 2020 2020) [Google Scholar]
- 52.Van Cuong L., Giang H.T.N., Linh L.K., Shah J., Van Sy L., Hung T.H., Reda A., Truong L.N., Tien D.X., Huy N.T. The first Vietnamese case of COVID-19 acquired from China. Lancet Infect. Dis. 2020;20(4):408–409. doi: 10.1016/S1473-3099(20)30111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mousavi S.H., Shah J., Giang H.T.N., Al-Ahdal T.M.A., Zahid S.U., Temory F., Paikan F.M., Karimzadeh S., Huy N.T. The first COVID-19 case in Afghanistan acquired from Iran. Lancet Infect. Dis. 2020;20:657–658. doi: 10.1016/S1473-3099(20)30231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han H., Luo Q., Mo F., Long L., Zheng W. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect. Dis. 2020;20:655–656. doi: 10.1016/S1473-3099(20)30174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., Behrens P., Böddinghaus B., Götsch U., Naujoks F., Neumann P., Schork J., Tiarks-Jungk P., Walczok A., Eickmann M., Vehreschild M.J.G.T., Kann G., Wolf T., Gottschalk R., Ciesek S. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 2020;382(13):1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Academia Europaea . 2020. Call for Reviewers and Submissions – Rapid Review Network for COVID-19.https://www.ae-info.org/ae/Acad_Main/News/Rapid%20Review%20Network%20for%20COVID-19 (Accessed 25 April 2020) [Google Scholar]
- 58.Kupferschmidt K. 2020. Study Claiming New Coronavirus Can be Transmitted by People Without Symptoms Was Flawed.https://www.sciencemag.org/news/2020/02/paper-non-symptomatic-patient-transmitting-coronavirus-wrong (Accessed 25 April 2020) [Google Scholar]
- 59.Taccone F.S., Gorham J., Vincent J.-L. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir. Med. 2020;8:539–541. doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rzymski P., Nowicki M., Mullin G.E., Abraham A., Rodríguez-Román E., Petzold M.B., Bendau A., Kant Sahu K., Ather A., Naviaux A.-F., Janne P., Gourdin M., Delanghe J.R., Ochs H.D., Talmadge J.E., Garg M., Hamblin M.R., Rezaei N. Quantity does not equal quality: scientific principles cannot be sacrificed. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106711. [DOI] [PMC free article] [PubMed] [Google Scholar]